Abstract
Binge drinking during adolescence is highly prevalent despite increasing evidence of its long-term impact on behaviors associated with modulation of behavioral flexibility by the medial prefrontal cortex (mPFC). In the present study, male and female rats underwent adolescent intermittent ethanol (AIE) exposure by vapor inhalation. After aging to adulthood, retrograde bead labelling and viral tagging were used to identify populations of neurons in the prelimbic region (PrL) of the mPFC that project to specific subcortical targets. Electrophysiological recording from bead-labelled neurons in PrL slices revealed that AIE did not alter the intrinsic excitability of PrL neurons that projected to either the NAc or the BLA. Similarly, recordings of spontaneous inhibitory and excitatory post-synaptic currents revealed no AIE-induced changes in synaptic drive onto either population of projection neurons. In contrast, AIE exposure was associated with a loss of dopamine receptor 1 (D1), but no change in dopamine receptor 2 (D2), modulation of evoked firing of both populations of projection neurons. Lastly, confocal imaging of proximal and apical dendritic tufts of viral-labelled PrL neurons that projected to the nucleus accumbens (NAc) revealed AIE did not alter the density of dendritic spines. Together, these observations provide evidence that AIE exposure results in disruption of D1 receptor modulation of PrL inputs to at least two major subcortical target regions that have been implicated in AIE-induced long-term changes in behavioral control.
Keywords: Prelimbic cortex, Adolescence, Alcohol, Dopamine 1 receptor, Pyramidal neuron, Development
1. Introduction
Alcohol is the most commonly abused drug among adolescents where its pattern of use often occurs through repeated episodes of heavy binge drinking [1]. In addition to the risks posed to personal safety, underage drinking is associated with negative outcomes in adulthood that include an increased risk of developing alcohol use disorder [2,3], learning and memory deficits [4–6], and attentional deficits [7,8]. These changes are accompanied by accelerated declines in gray matter volume [9,10], slowed growth of white matter volume [10,11], and altered brain activation during cognitive tasks [12,13].
The medial prefrontal cortex (mPFC), which mediates executive functioning and behavioral flexibility, exhibits a delayed developmental trajectory that continues well into early adulthood [14]. During adolescent development, the mPFC undergoes significant structural and functional changes that parallel maturation of its executive function [14–16]. While the heightened plasticity of the adolescent mPFC reflects its ability to undergo experience-dependent changes during this critical period of continued development, this may also render it particularly vulnerable to environmental insults, including those associated with repeated episodes of heavy binge drinking [17–20]. Dopamine (DA) neurotransmission in the mPFC critically modulates cognitive function and behavioral flexibility [21,22]. Of particular interest, the developmental changes that occur in the mPFC during adolescence involve striking alterations in the DA system that include increased innervation by DA fibers [23,24], increased DA tone [25], and maturation of DA receptor expression and function [26–29]. Gamma aminobutyric acid (GABA) signaling onto deep layer pyramidal neurons in the mPFC also undergoes developmental changes during adolescence that is characterized by the emergence of a GABAA - mediated tonic current [30], and increased sIPSC frequency that occur in parallel with maturation of mPFC interneurons [31]. During adolescence, glutamatergic neurotransmission in the medial prefrontal cortex undergo structural and functional refinement due to ongoing pruning of excitatory synapses and dendritic spines [32–35] as well as continued maturation of AMPA and NMDA receptor trafficking and expression [36,37]. Additionally, the intrinsic excitability of deep layer pyramidal neurons in the mPFC also matures during adolescence and is accompanied by increases in hyperpolarization-activated current (Ih ) [38,39]. Previous studies in animal models have shown that alcohol exposure during adolescence disrupts DA neurotransmission in the adult mPFC while also altering GABA and glutamatergic signaling [27,30,40]. Furthermore, changes in intrinsic excitability following adolescent alcohol exposure are accompanied by reduced Ih in the adult mouse [38]. These findings suggest that mPFC intrinsic excitability, DA, glutamate, and GABA systems are vulnerable to lasting dysregulation following adolescent alcohol misuse.
Pyramidal cells in the mPFC, which are its principal output neurons, project to a number of subcortical regions. Recent research has highlighted the importance of defining the specific circuits through which information passes from the mPFC to subcortical structures for behavioral control. For example, ensembles of neurons projecting from the infralimbic (IfL) cortex (ventral area of the rodent mPFC) to the nucleus accumbens (NAc) core promote cocaine self-administration. In contrast, ensembles of neurons projecting from the IfL cortex to the NAc shell promote extinction of cocaine self-administration [41]. In addition, neurons in the prelimbic cortex (PrL) cortex (dorsal area of the rodent mPFC) projecting to the basolateral amygdala (BLA) and the NAc bidirectionally modulate threat avoidance, with activation of PrL-BLA neurons facilitating active avoidance while activation of PrL-NAc neurons reduces active avoidance [42]. These findings highlight how assessing the circuit specificity of long-lasting changes in mPFC function following adolescent alcohol abuse would be beneficial for understanding how those changes might contribute to long-term alterations in behavioral control associated with AIE. Improved understanding of the vulnerability of different mPFC circuits to persistent changes following adolescent alcohol exposure may also aid in the development of improved treatments for the enduring effects of adolescent alcohol use on cognition. The goal of the present study was to determine the effect of AIE on select populations of pyramidal neurons in the PrL that projected to either the NAc (PrLNAc ) or the BLA (PrLBLA ). The PrLNAc and PrLBLA projections were selected for investigation as these circuits undergo substantial refinement during adolescence [29,43,44], and the adult behaviors they modulate have been shown to be impacted by AIE exposure leading to reductions of behavioral flexibility [45–47].
2. Materials & methods
2.1. Animals
Long-Evans dams with litters were obtained from Envigo (Indianapolis, IN). At postnatal day (PD) 21 the rat pups were weaned, pair housed, and assigned either to the control (Air) or experimental (AIE) group. Rats from each litter were evenly divided between the Air (n = 21) and AIE (n = 22) treatment conditions. Rats were housed in a temperature and humidity-controlled environment on a 12 hr/12hr light/dark cycle with lights off from 09:00-21:00 each day. All rats were provided with Teklad 2918 (Envigo) chow and water ad libitum. All procedures were carried out in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (2011) and were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
2.2. Adolescent intermittent ethanol-exposure
Adolescent intermittent ethanol exposure was carried out via vapor inhalation as previously described [48,49]. For animals used in the electrophysiology studies, rats underwent 8 cycles of binge-like intermittent ethanol vapor exposure beginning at postnatal day (PD) 28 and continuing through PD 54. For consistency with our previous investigation of the effects of AIE on basal dendritic spine density, animals used in the apical dendritic spine imaging study were subjected to 5 cycles of AIE with vapor exposure ending at PD 44. Each cycle was composed of two days of ethanol exposure separated by two days of no ethanol exposure. The litter-matched Air control animals were treated identically except they were not exposed to ethanol vapor. On ethanol exposure days, the rats were placed in the vapor chambers at 18:00 and removed from the chambers at 08:00 the following day. After removal from the vapor chambers the level of intoxication of each rat was scored using a 5-point behavioral intoxication scale [45,48]. This involved scoring motoric behavior according to the following 5-point scale: 1 = No signs of intoxication; 2 = Slightly intoxicated (slight motor impairment); 3 = Moderately intoxicated (obvious motor impairment but able to walk); 4 = Highly intoxicated (dragging abdomen, loss of righting reflex); 5 = Extremely intoxicated (loss of righting reflex and loss of eye blink reflex). On the last day of each cycle, tail vein blood was collected and the blood ethanol concentration (BEC) was measured using an Analox alcohol analyzer (AM1, Analox Instruments, Stourbridge, GBR). Following the last exposure cycle, rats remained pair housed in the vivarium until they were sacrificed in adulthood to obtain slices for experimental use.
2.3. Retrograde labeling of prelimbic neurons
Beginning 7 days after completion of the AIE-exposure paradigm, rats underwent stereotaxic surgery. In brief, the animals were maintained under isoflurane (2-3%) throughout the surgery and injected with ketorolac (2 mg/kg, sc) prior to beginning the procedure. A Nanoject II (Drummond Scientific Company, Broomall, PA, USA) under stereotaxic control was used to inject 500 nl red retrobeads IX (Lumafluor Inc., Durham, NC, USA) into the BLA of the left hemisphere (from bregma: −2.7 mm AP, −5.1 mm ML, −8.8 mm DV) and 500 nl green retrobeads IX (Lumafluor Inc.) into the NAc of the right hemisphere (from bregma: +1.8 mm AP, +1.5 mm ML, −7.0 mm DV; Fig. 1) of the same animal. The beads were injected over the course of 6 min with the microinjector remaining in place for an additional 5 min following completion of the injection. After the final injection the injector was withdrawn, and the surgical site was sutured closed. Postoperative analgesia was provided using topical lidocaine, ketorolac (2 mg/kg, sc), and dexamethasone (2 mg/kg, sc). After the surgery a minimum of 3 weeks was given for the rats to recover and for retrograde transport of the retrobeads to occur.
Fig. 1.
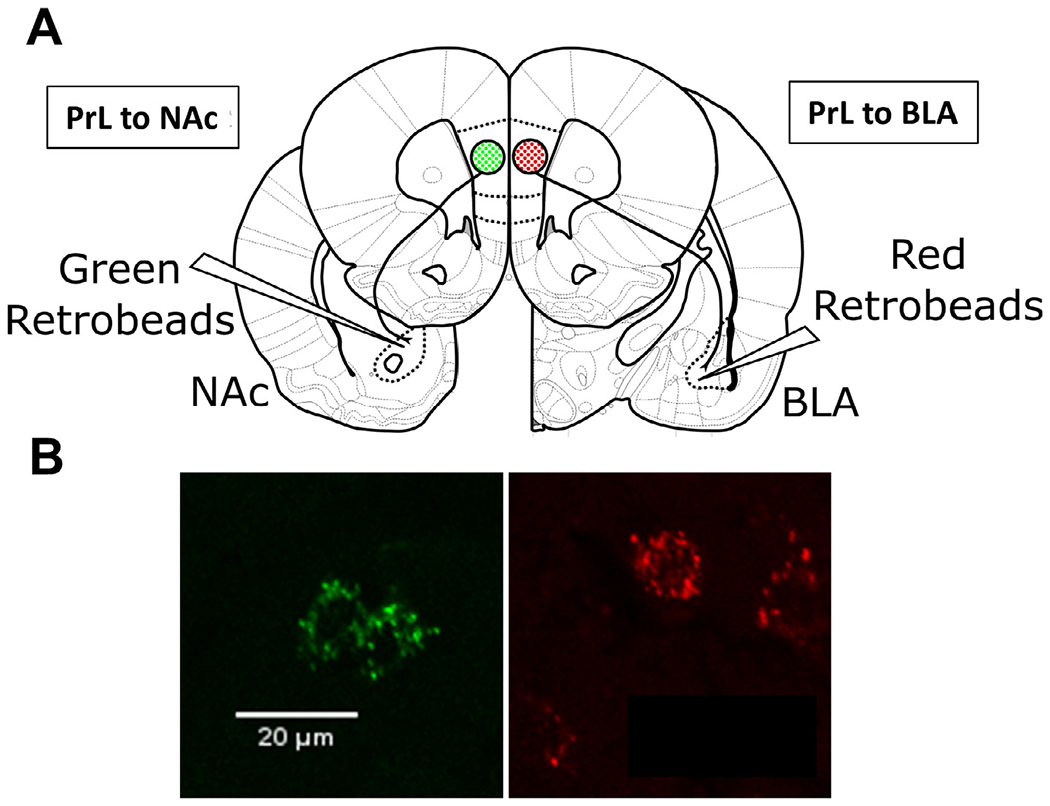
Retrobead labelling and injection location. (A) Schematic depicting the injection site of green fluorescent retrobeads in the NAc of the right hemisphere and the injection site of red fluorescent retrobeads in the NAc of the left hemisphere. (B) Representative images of neurons labeled with green retrobeads (left panel) and red retrobeads (right panel) in the PrL.
2.4. Electrophysiological recordings
Acute slices were obtained from Air control (n = 16, 8 male and 8 female) and AIE exposed (n = 16, 8 male and 8 female) rats for electrophysiological recordings beginning at PD 90 and current and voltage clamp experiments were performed as described previously [27,50]. In brief, rats were anesthetized, and the brain was rapidly removed and placed into an ice-cold sucrose dissection solution containing (in mM): 200 sucrose, 1.9 KCl, 1.2 Na2 HPO4, 33 NaHCO3, 10 d-glucose, 2.5 C5H9NO3S, 5 ascorbic acid, 10 MgCl2, and 0.5 CaCl2. Following slice preparation using a Leica vibratome (VT1000 S, Wetzlar, DEU) slices (300 μm thick) were incubated for 30 min at 34°C before being incubated for an additional 30 min at room temperature in incubation aCSF containing (in mM): 92 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 25 d-glucose, 20 HEPES, 5 ascorbic acid, 10 MgCl2 , and 0.5 CaCl2. After incubation slices were transferred to a submerged recording chamber held at 34°C and constantly perfused with recording aCSF containing (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 10 d-glucose, 0.4 ascorbic acid, 1.3 MgCl2, and 2 CaCl2. Each of these solutions had an osmolarity of 300-310 mOsm, was pH adjusted (7.3-7.43), and was continuously aerated with 95% O2 /5% CO2.
Recordings were made with a Multiclamp 700B amplifier (Molecular Devices, San Jose, CA, USA) connected to a Windows-PC running Axograph X software through an Instrutech ITC-18 digital to analog converter (HEKA Instruments, Holliston, MA, USA). Borosilicate glass electrodes were pulled on a Sutter Instruments P97 micropipette puller (Novato, CA, USA). Tip resistances ranged from 2 to 4 MΩ. All recordings were obtained from visually identified retrobead-labelled pyramidal neurons in layer V of the PrL ipsilateral to the retrobead injection site. Cells were identified using a Zeiss Axio Examiner.A1 microscope (Oberkochen, DEU) equipped with a DIC filter as well as filters for visualizing red and green retrobeads. All internal solutions were adjusted to pH 7.4 and 285 mOsm. Series and input resistance were measured at the beginning and end of each recording. If the series resistance changed by more than 10% during the experiment, the series resistance exceeded 16 MΩ, or the membrane resistance was not at least 10-times greater than the series resistance then the recording was not included in the analysis.
For current clamp recordings, electrodes were filled with an internal solution containing (in mM): 125 potassium gluconate, 20 KCl, 10 HEPES, 1 EGTA, 2 MgCl2, 2 Na2-ATP, 0.3 Tris-GTP, 10 phosphocreatine. To analyze intrinsic excitability 1 s current steps were applied in 20 pA increments ranging from −100 to +200 pA or until the cell fired 6–10 action potentials. Once the current step at which 6-10 action potentials were evoked had been identified for a neuron, this current step was used to compare firing properties at baseline and following bath application of 5 μM of the DA receptor agonists SKF 38393 and quinpirole. All recordings were analyzed for the number of spikes evoked in response to the injected current. Recordings were digitized at 10 kHz and filtered at 4 kHz.
For voltage clamp recordings, electrodes were filled with an internal solution containing (in mM): 135 CsCl, 2 MgCl2, 10 HEPES, 1 EGTA, 4 NaCl, 2 Na2-ATP, 0.3 Tris-GTP, 10 phosphocreatine, 1 QX-314-Cl. Cells were held at −70 mV and sIPSC or sEPSC were recorded in the presence of 10 μM CNQX and 50 μM dl-APV or 100 μM picrotoxin, respectively. Spontaneous events were recorded for 5 min in each cell and detected offline using a template matching algorithm. The ratio of sEPSC/sIPSC amplitude and frequency (events/s) were calculated within each animal using the average frequency and amplitude of each event type for that animal. Recordings were digitized at 10 kHz and filtered at 4.2 kHz.
2.5. Viral vector infusion and immunohistochemistry
To label PrL neurons that project to the NAc for dendritic spine analysis, adult Air control (n = 5) and AIE exposed (n = 6) rats underwent stereotaxic injection of viral vectors as previously described [51]. In brief, animals were maintained under isoflurane (2-3%) throughout the surgery and injected with ketorolac (2 mg/kg, sc) prior to beginning the procedure. All rats received bilateral microinjection (0.75 μl/hemisphere) of a retrograde adeno-associated virus expressing Cre under direction of the human synapsin promoter (AAVrg-hSyn-Cre) obtained from Addgene (Cambridge, MA, USA) into the nucleus accumbens core (from bregma: + 1.8 mm AP, ± 1.5 mm ML, −7.0 mm DV). Rats then received bilateral microinjection (0.75 μl/hemisphere) of Cre-dependent mCherry labeling vector (AAV2–hSyn–DIO–mCherry) obtained from Addgene into the prelimbic cortex (from bregma: +2.8 mm AP, ±0.6 mm ML, −3.8 mm DV). For both the prelimbic cortex and nucleus accumbens core microinfusions, injections were performed over a period of 5 min (0.15 μl/min) using a Nanoject II and injectors were left in place for 10 min to facilitate diffusion away from the injection site, then slowly retracted. Following surgical recovery, rats were housed in the animal vivarium for 4-6 weeks to allow sufficient viral expression, after which time the rats were euthanized and the brains perfusion fixed with 4% PFA in PBS. The extracted brain was left in PFA at 4°C until it sank to the bottom of the beaker, and then stored in 30% sucrose in 1 x PBS at 4°C.
Coronal sections of the prelimbic cortex (AP + 2.5–4.2 mm from bregma) were sliced at 100 μm using a Leica vibratome, collected in 0.1 M PBS containing 0.01% sodium azide, and stored at 4°C before being processed for IHC. A previously described immunoamplification procedure was used to enhance the visualization of the virally expressed mCherry protein [51]. Sections were blocked with 0.1 M PBS plus 2% Triton X-100 (PBST) and 2% normal goat serum (NGS) for 2 hr at room temperature. Sections were then incubated in chicken anti-mCherry (1:2000; LS Biosciences #LS-C204825, Seattle, WA, USA RRID:AB_2716246) at 4°C overnight with vigorous shaking. Sections were then washed 3 × 10 min in PBST, and then incubated with goat anti-chicken Alexa Fluor®594 conjugated secondary antisera (1:1000, Abcam, Cambridge, GBR) diluted in 2% PBST with 2% NGS for five hr at room temperature. Sections were then washed 3 × 10 min and then mounted on Superfrost Plus slides using ProLong® gold Antifade (Thermo Fisher Scientific; Waltham, MA, USA). Slides were stored at 4°C until imaging.
2.6. Confocal imaging and dendritic spine morphology analyses
Confocal Z-series data sets were acquired using a Zeiss LSM880 confocal microscope. Thirty to fifty μm segments of dendrites at either the proximal apical region, (approximately 100-200 μm from the cell body) or at the apical tuft region (located after the third branch point within 10 μm of the end of the dendrite) were selected for imaging. Overall imaging parameters including laser power, gain and pinhole, were set empirically prior to imaging and were held constant for the remainder of the experiment. Images were acquired using a 63 × oil immersion objective (1.4 N.A.) with a 1024 × 512 frame size, 3.5 × digital zoom and 0.1 μm step size. One dendritic spine segment was imaged per region (proximal apical vs. apical tuft), per neuron. Three to five neurons were imaged per animal. Following imaging and analysis, animal averages were computed for each region, and data were analyzed. Spine head density and head diameter values at proximal and apical tuft regions were compared between Air control and AIE exposed animals using unpaired t-tests.
2.7. Statistical analyses
Statistical analyses were carried out using Stata 15.1 (StataCorp LLC, College Station, TX, USA) and GraphPad Prism (Dotmatics, Boston, MA, USA). Data were assessed for normality using the Wilks-Shapiro test and checked for outliers using the IQR rule. Repeated measures analyses used the Greenhouse-Geisser correction for sphericity. Post-hoc tests were corrected for multiple comparisons using the Bonferroni method. In instances where the same measures were recorded from multiple neurons in the same animal the values obtained were averaged as the experimental unit for this study was the individual animal. Intrinsic excitability data was analyzed using mixed model analysis of variance (ANOVA) with AIE exposure and sex as between-subject variables and the current step as a within-subject variable. Data related to spontaneous events was analyzed using a two-way ANOVA with AIE exposure and sex as between-subject variables. The pharmacology data in which quinpirole and SKF 38393 were bath applied was first analyzed using a one sample t-test to verify that the agonists had altered evoked firing in the Air control group. After this was confirmed, the data were analyzed using a two-way ANOVA with AIE exposure and sex as between-subject variables. For dendritic spine density and head diameter analyses, potential differences between Air and AIE groups were evaluated using unpaired t-tests. All values reported are mean ± SEM. For purposes of statistical significance, p ≤ 0.05 was considered significant.
3. Results
The adolescent alcohol exposure model utilized involves a well-characterized vapor inhalation procedure designed to simulate repeat episodes of heavy binge-like alcohol exposure. Behavioral intoxication and blood ethanol concentration (BEC) were assessed at the end of each exposure cycle. For rats that underwent acute slice electrophysiology the average intoxication score using the 5-point rating scale was 2.4 ± 0.1 for male rats and 2.3 ± 0.1 for female rats, which represents a moderate level of intoxication. The corresponding BEC values were 248.0 ± 10.9 mg/dl for the male rats and 229.3 ± 11.9 mg/dl for the female rats. There was no significant difference between male and female rats for the average intoxication score (Wilcoxon rank-sum test: z = 1.877, p = 0.0605) or BEC level (Wilcoxon rank-sum test: z = 1.356, p = 0.1751). For rats that underwent imaging for dendritic spine density, the average intoxication score was 2.0 ± 0.1. The corresponding BEC values were 256.3 ± 10.9.
3.1. Projection-specific analysis of intrinsic excitability
Electrophysiology assessment of the projection-specific effects of AIE involved recording from fluorescent bead-labelled neurons in acute mPFC slices obtained from adult Air control and AIE exposed rats (Fig. 1). A neuron retrogradely labelled with green beads indicated it projected to the NAc of the right hemisphere, while retrograde labelling with red beads indicated it projected to the BLA in the left hemisphere. As expected, the red and green labelled neurons represented distinct cell populations as dual labelled neurons were rarely observed. For all electrophysiological experiments, the values for each animal represented recordings obtained from 1-4 cells that were averaged to provide a single value for each rat. Table 1 provides a comparison of the biophysical properties of cells in each treatment group, which shows there were no significant differences between groups for any of the parameters (resting membrane potential, membrane resistance, number of action potentials evoked at baseline, and rheobase).
Table 1.
Biophysical properties of pyramidal neurons across treatment condition and sex.
| Condition | Sex (n) | Vm (mV) | Rm (MΩ) | Evoked APs at Baseline | Rheobase (mV) |
|---|---|---|---|---|---|
| AIR: PrL-BLA | Female (7) | −68.0 ± 1.6 | 84.0 ± 8.5 | 9.9 ± 0.8 (100 – 260 pA) | 83 ± 9 |
| Male (8) | −67.7 ± 2.1 | 80.5 ± 8.2 | 9.3 ± 0.8 (60 – 220 pA) | 90 ± 15 | |
| AIE: PrL-BLA | Female (8) | −68.9 ± 2.3 | 84.9 ± 9.7 | 7.5 ± 0.9 (80 – 240 pA) | 80 ± 16 |
| Male (8) | −66.0 ± 1.0 | 92.5 ± 10.9 | 8.8 ± 1.0 (60 – 220 pA) | 45 ± 9 | |
| AIR: PrL-NAc | Female (8) | −68.6 ± 0.4 | 75.0 ± 8.0 | 8.1 ± 0.6 (100 – 220 pA) | 108 ± 12 |
| Male (8) | −67.7 ± 1.9 | 82.3 ± 5.8 | 6.8 ± 0.9 (100 – 220 pA) | 88 ± 17 | |
| AIE: PrL-NAc | Female (8) | −68.5 ± 1.6 | 78.8 ± 4.5 | 9.0 ± 1.1 (80 – 240 pA) | 70 ± 16 |
| Male (8) | −67.0 ± 1.5 | 88.8 ± 12.2 | 6.6 ± 1.4 (120 – 300 pA) | 93 ± 24 |
The first set of studies examined the effects of AIE on the intrinsic excitability of the PrL projection neurons. Analysis of current evoked firing of PrLNAc neurons (i.e., recordings from green bead-labelled cells) revealed there was no main effect of AIE exposure or sex on intrinsic excitability (main effect AIE: F(1, 28) = 0.09, p = 0.7618; main effect sex: F(1, 28) = 0.00, p = 0.9750; Fig. 2A–D). There were also no significant interactions between AIE exposure, sex, and the injected current (AIE x sex interaction: F(1, 28) = 0.06, p = 0.8154; AIE x current interaction: F(1, 280) = 1.23, p = 0.3039; sex x current interaction: F(10, 280) = 0.62, p = 0.5723; AIE x sex x current interaction: F(10, 280) = 1.67, p = 0.1905). Increasing the amount of current injected increased the number of evoked events (F(10, 280) = 62.58, p < 0.0001, partial η2 = 0.6909 [0.6236, 0.7249]).
Fig. 2.
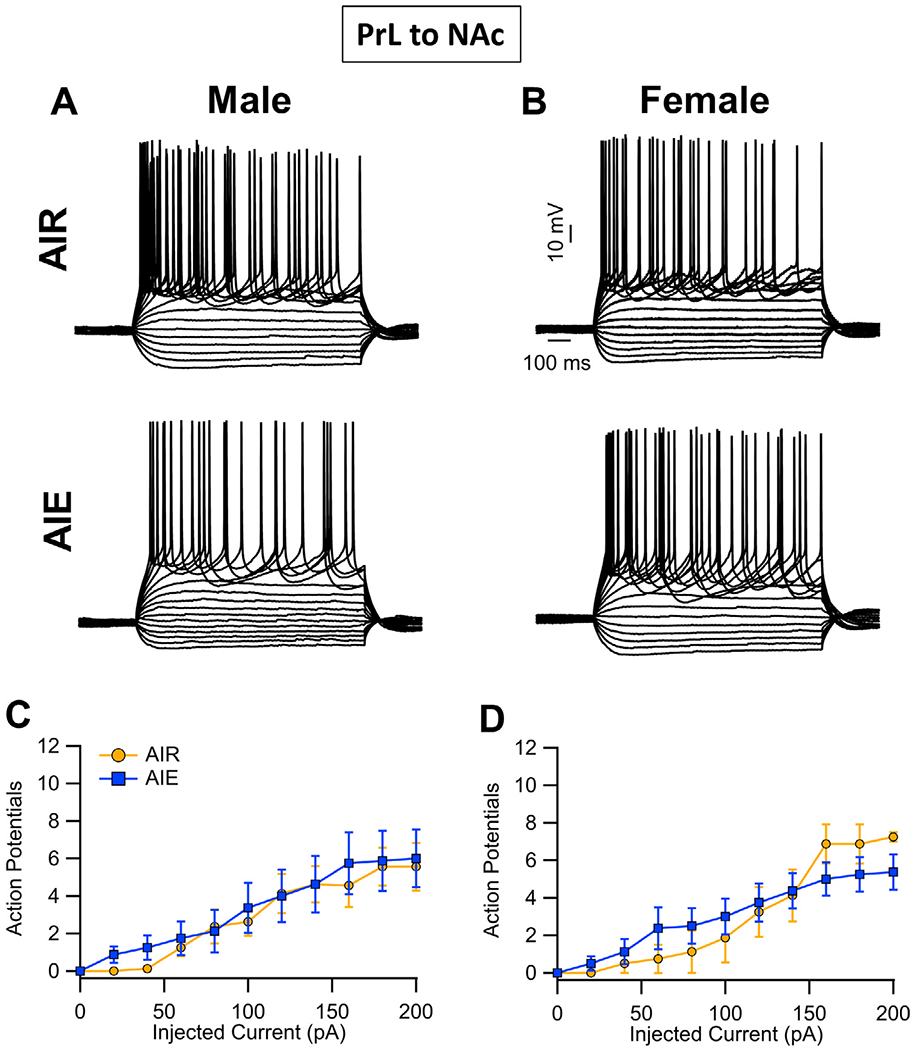
Intrinsic excitability of PrL neurons that project to the NAc (PrLNAc). (A,B) Representative traces showing the input-output relationship between firing frequency and injected current in PrLNAc cells in slices obtained from adult male and female Air control and AIE exposed rats. (C,D) Quantification of the evoked firing data revealed that AIE exposure did not alter the intrinsic excitability of PrL NAc neurons in either male or female rats. Data represent the mean ± SEM, n = 8 rats/group.
Analysis of the recordings from PrLBLA neurons (i.e., recordings from red bead-labelled neurons) revealed there was no effect of AIE on intrinsic excitability within this circuit (main effect AIE: F(1, 28) = 0.60, p = 0.4456; Fig. 3A–D). This analysis further indicated there were no sex differences or significant interactions (main effect sex: F(1, 28) = 1.64, p = 0.2110; AIE x sex interaction: F(1, 28) = 0.24, p = 0.6274; AIE x current interaction: F(10, 280) = 1.13, p = 0.3306; sex x current interaction: F(10, 280) = 0.85, p = 0.4280; AIE x sex x current interaction: F(10, 280) = 0.19, p = 0.8230). Increasing the amount of current injected increased the number of evoked events (F(10, 280) = 87.31, p < 0.0001, partial η2 = 0.7572 [0.7028, 0.7843]).
Fig. 3.
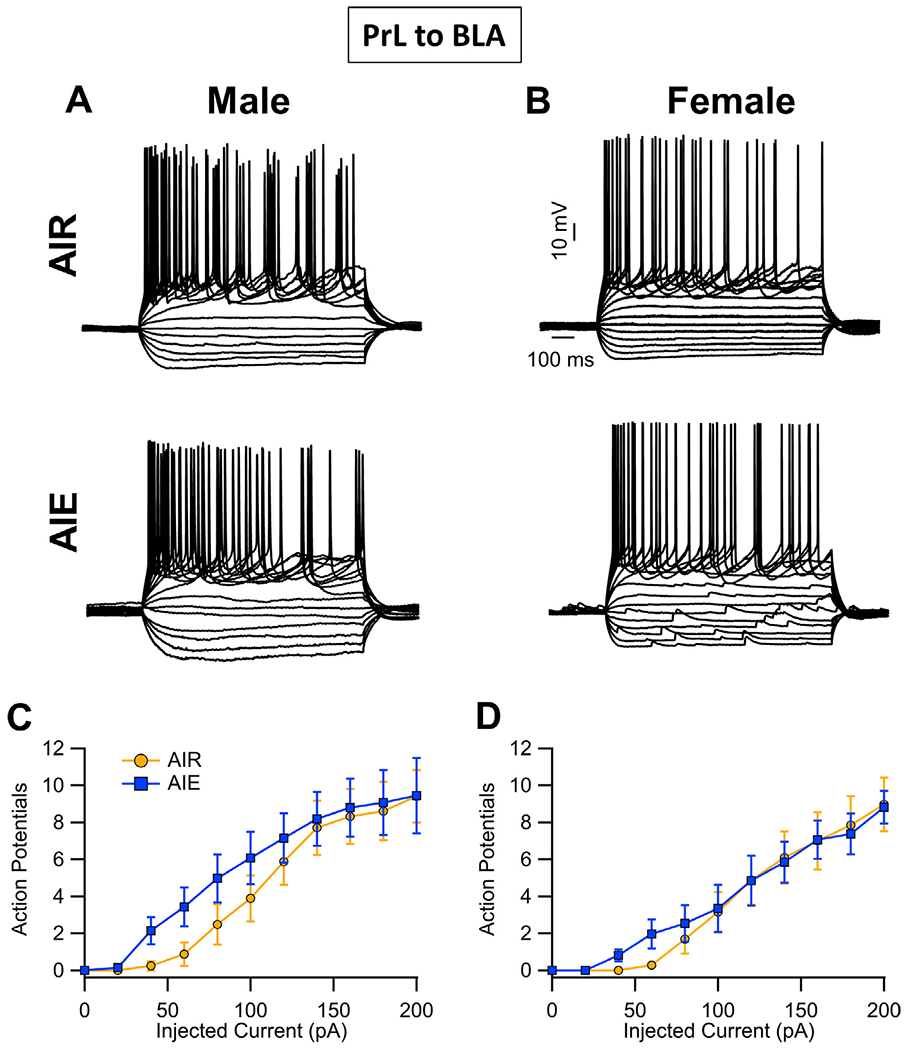
Intrinsic excitability of PrL neurons that project to the BLA (PrL BLA). (A,B) Representative traces showing the input-output relationship between firing frequency and injected current in PrL BLA cells in slices obtained from adult male and female Air control and AIE exposed rats. (C,D) Quantification of the evoked firing data revealed that AIE exposure did not alter the intrinsic excitability of PrL BLA neurons in either male or female rats. Data represent the mean ± SEM, n = 8 rats/group.
3.2. Projection-specific analysis of D1 and D2 modulation of excitability
It is well known that dopamine receptor 1 (D1) and dopamine receptor 2 (D2) bidirectionally modulate the activity of pyramidal cells in the mPFC, with D1 agonism increasing evoked firing and D2 agonism reducing evoked firing [52–54]. It has also been reported that AIE exposure attenuates D1 enhancement of firing without altering D2 inhibition of firing of pyramidal neurons in the adult mPFC [27]. Therefore, the next set of studies examined the effects of AIE on D1 and D2 modulation of evoked firing of PrL NAc and PrL BLA projecting neurons.
For enhancement of firing of PrL NAc neurons in response to bath application of the D1 agonist SKF 38393 (5 μM) (Fig. 4A & C), analysis revealed that AIE exposure significantly attenuated agonist enhanced firing (main effect AIE: F(1, 28) = 6.76, p = 0.0147, partial η2 = 0.1946 [0.0073, 0.4193]). There were no sex-dependent effects of SKF 38393 (main effect sex: F(1, 28) = 0.13, p = 0.7230) nor was there a significant interaction between sex and AIE exposure (AIE x sex interaction: F(1, 28) = 0.30, p 0.5875). In contrast, inhibition of evoked firing produced by D2 receptor agonism with quinpirole (Fig. 4B and D) was not altered by AIE exposure and was not sex dependent (main effect AIE: F(1,28) = 2.23, p = 0.1467; main effect sex: F(1,28) = 0.15, p = 0.6983; AIE x sex interaction: F(1,28) = 1.05, p = 0.3140).
Fig. 4.
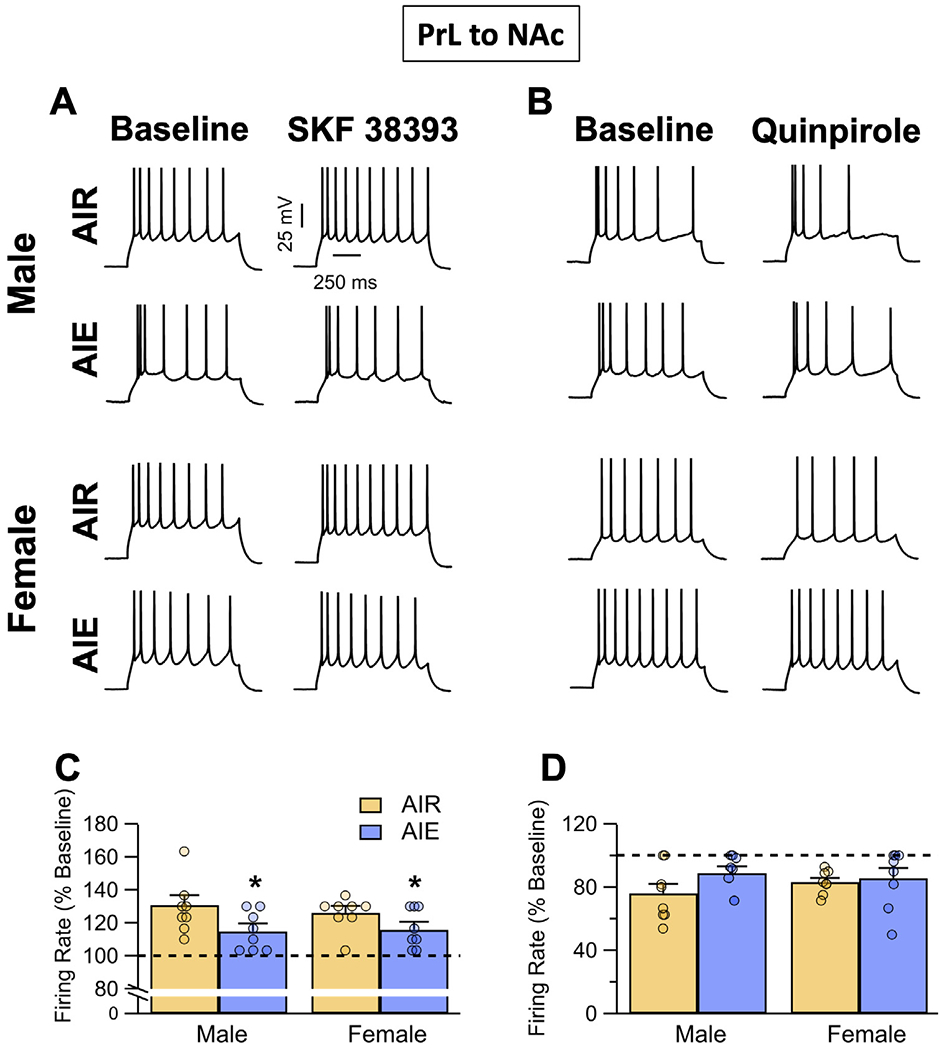
Dopamine receptor modulation of evoked firing of PrL neurons that project to the NAc (PrL NAc). (A) Representative traces showing the effects of the D1 agonist SKF 38393 (5 μM) on evoked firing of PrL NAc cells in slices obtained from adult male and female Air control and AIE exposed rats. (B) Representative traces showing the effects of the D2/D4 agonist quinpirole on evoked firing of PrL NAc cells in slices obtained from adult male and female Air control and AIE exposed rats. (C) Enhancement of evoked firing in response to bath application of SKF 38393 was significantly attenuated by AIE exposure in slices obtained from both male and female rats. (D) Inhibition of evoked firing in response to bath application of quinpirole (5 μM) was not significantly altered by AIE exposure in either male or female rats. Dashed lines indicate baseline firing. Data represent the mean ± SEM. * indicates significant difference compared to respective Air controls; p ≤ 0.05; n = 8 rats/group.
For enhancement of evoked firing of PrLBLA neurons by D1 agonism (Fig. 5A and C), analysis revealed that AIE exposure reduced SKF 38393 enhanced firing (main effect AIE: F(1, 27) = 14.21, p = 0.0008, partial η2 = 0.3448 [0.0757, 0.5491]). The effect of SKF 38393 on evoked firing was greater in female than in male rats (main effect sex: F(1, 27) = 9.83, p = 0.0041, partial η2 = 0.2669 [0.0320, 0.4863]), but there was no significant interaction between sex and AIE exposure (AIE x sex interaction: F(1, 27) = 0.00, p = 0.9859). In comparison, the inhibition of evoked firing of PrLBLA neurons produced by D2 receptor agonism with quinpirole (Fig. 5B and D) was not altered by AIE nor was it sex-dependent (main effect AIE: F(1,27) = 0.01, p = 0.9164; main effect sex: F(1,27) = 2.59, p = 0.1193; AIE x sex interaction: F(1,27) = 1.94, p = 0.1745).
Fig. 5.
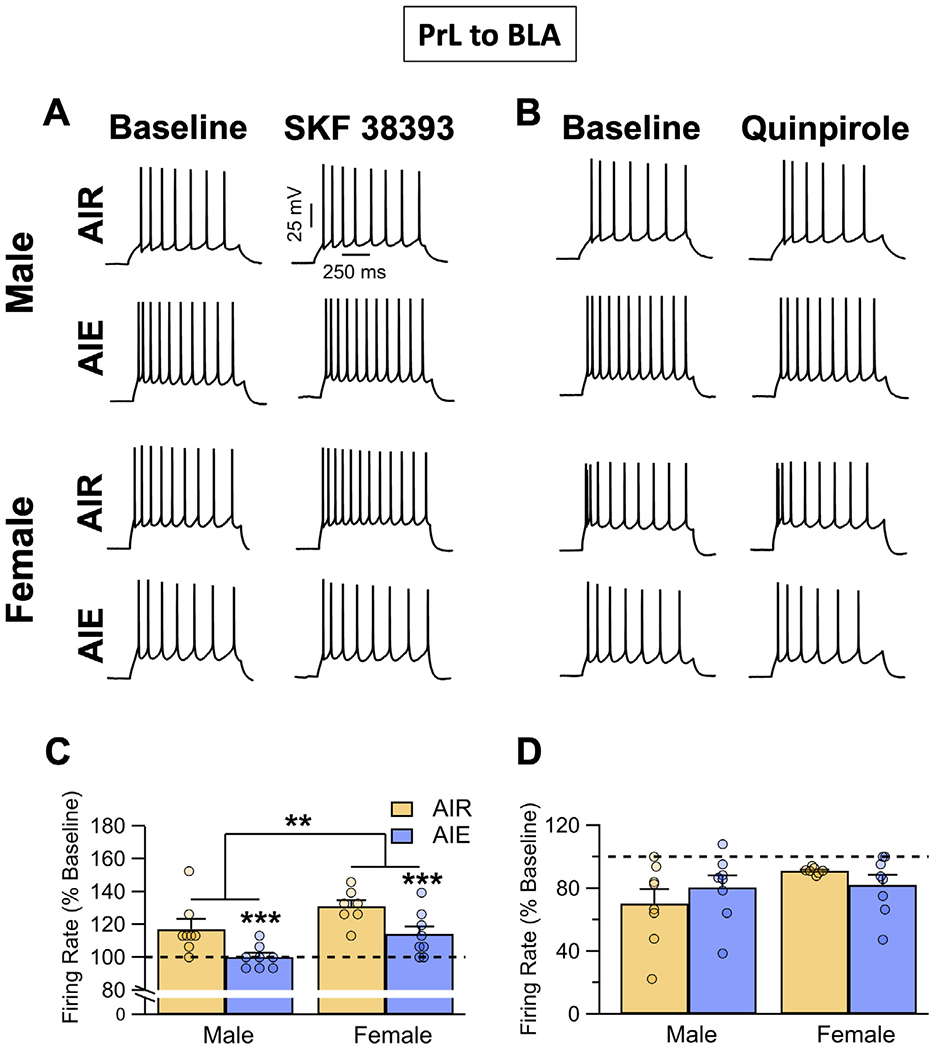
Dopamine receptor modulation of evoked firing of PrL neurons that project to the BLA (PrL BLA). (A) Representative traces showing the effects of the D1 agonist SKF 38393 (5 μM) on evoked firing of PrL BLA cells in slices obtained from adult male and female Air control and AIE exposed rats. (B) Representative traces showing the effects of the D2/D4 agonist quinpirole on evoked firing of PrL BLA cells in slices obtained from adult male and female Air control and AIE exposed rats. (C) Enhancement of evoked firing in response to bath application of SKF 38393 observed in the Air controls was significantly attenuated by AIE exposure in slices obtained from both male and female rats. (D) Inhibition of evoked firing in response to bath application of quinpirole (5 μM) was not significantly altered by AIE exposure in either male or female rats. Dashed lines indicate baseline firing. Data represent the mean ± SEM. **, indicates significant group difference between males versus females, p ≤ 0.01; ***, indicates significant difference compared to respective Air controls; p ≤ 0.001; n = 7-8 rats/group.
Not all PrLNAc and PrLBLA projecting neurons express D1 [55–57]. To confirm that the observed reduction in SKF 38393 modulation of evoked firing following AIE was due to changes in D1 function, D1-Cre rats were injected with retrograde Cre-dependent viruses to label D1 PrLNAc and PrLBLA projecting neurons. Recordings were then performed from visually identified D1-Cre PrLNAc and PrLBLA projecting neurons ipsilateral to the injection site. Analysis of recordings from PrLNAc and PrLBLA projecting D1 expressing neurons revealed that AIE exposure reduced enhancement of evoked firing by D1 agonist SKF 38393 (Supplemental Fig. 1A and B).
3.3. Projection-specific analysis of synaptic drive
To assess effects of AIE exposure on synaptic drive of the PrLNAc neurons, voltage-clamp recordings of sIPSCs and sEPSCs from green bead labelled cells in the PrL region of the slice were obtained. Analysis of the frequency of sIPSCs (Fig. 6A and C) revealed no significant effect of AIE exposure or sex (main effect AIE: F(1,28) = 2.72, p = 0.1105; main effect sex: F(1,28) = 2.40, p = 0.1328; AIE x sex interaction: F(1,28) = 0.68, p = 0.4179). The amplitude of sIPSCs (Fig. 6A and D) was also not affected by either AIE exposure or sex (main effect AIE: F(1,28) = 0.58, p = 0.4511; main effect sex: F(1,28) = 1.04, p = 0.3176; AIE x sex interaction: F(1,28) = 1.41, p = 0.2446). For sEPSCs, analysis revealed their frequency (Fig. 6B and E) was not altered by AIE exposure or sex (main effect AIE: F(1,28) = 0.00, p = 0.9469; main effect sex: F(1,28) = 1.58, p = 0.2188; AIE x sex interaction: F(1,28) = 1.58, p = 0.2186). While the amplitude of sEPSCs (Fig. 6B and F) was also not significantly impacted by either AIE exposure or sex alone (main effect AIE: F(1,28) = 0.00, p = 0.9627; main effect sex: F(1,28) = 0.00, p = 0.9842), the analysis did reveal a significant AIE exposure x sex interaction (AIE x sex interaction: F(1,28) = 5.16, p = 0.0310, partial h2 = 0.1556 [0.0000, 0.3813]).
Fig. 6.
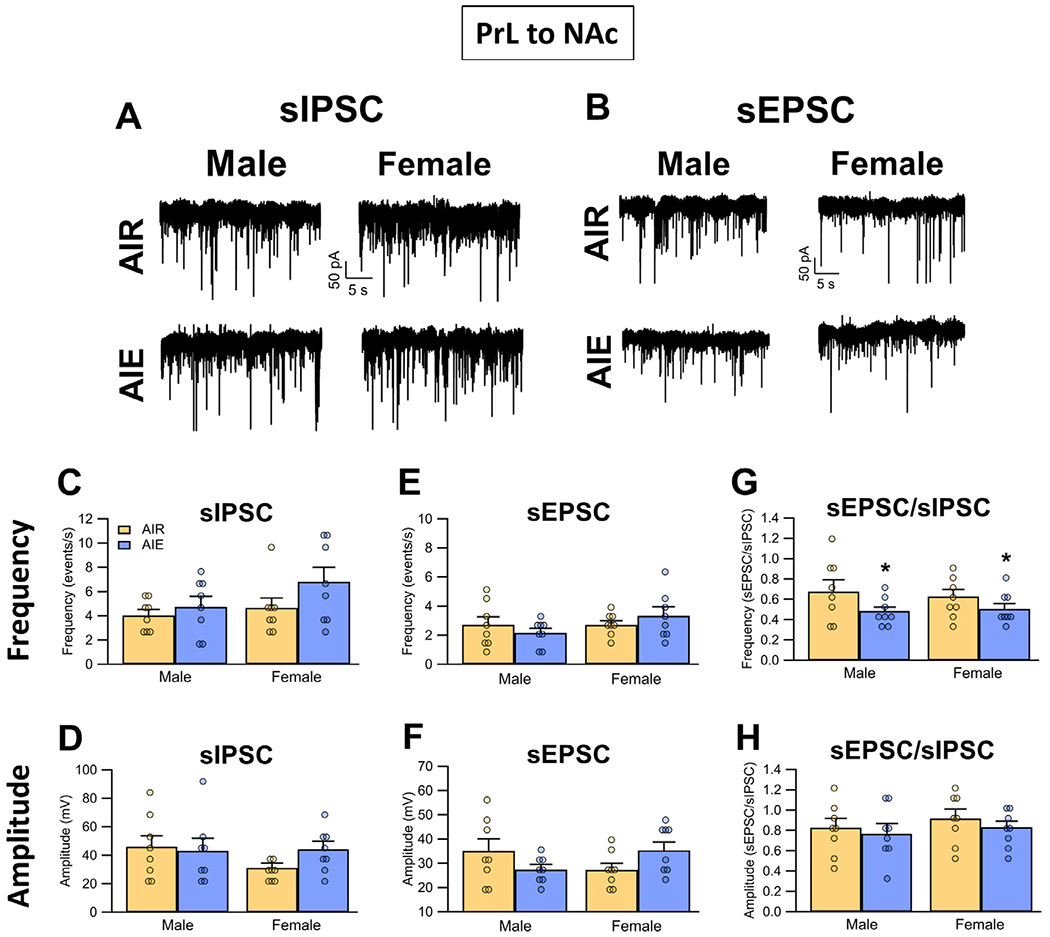
Spontaneous excitatory and inhibitory inputs to PrL neurons that project to the NAc (PrL NAc). (A) Representative traces of sIPSCs recorded from PrL NAc neurons of Air and AIE exposed adult male and female rats. (B) Representative traces of sEPSCs recorded from PrL NAc neurons of Air and AIE exposed adult male and female rats. When compared across treatment and sex, there were no differences in either the frequency (C) or amplitude (D) of sIPSCs. Similarly, there were no differences in either the frequency (E) or amplitude (F) of sEPSCs. (G) Examination of the sEPSC/sIPSC ratios (as an index of the excitatory-inhibitory balance of the synaptic inputs) for frequency revealed a significant AIE-induced decrease in both male and female rats. (H) There were no differences in the ratio of the current amplitudes as a function of either treatment or sex. Data represent the mean ± SEM. *, indicates significant difference compared to respective Air controls; p ≤ 0.05; n = 8 rats/group.
For assessment of the excitatory/inhibitory balance of synaptic inputs onto the PrLNAc neurons, comparisons of the ratio of the frequency of sEPSCs and sIPSCs (Fig. 6G) revealed that AIE exposure significantly reduced this ratio (main effect AIE: F(1,28) = 4.39, p = 0.0454, partial h2 = 0.1355 [0.0000, 0.3605]). The effect of AIE exposure on the frequency ratio was not sex-dependent, nor was there an effect of sex alone (main effect sex: F(1,28) = 0.03, p = 0.8541; AIE x sex interaction: F(1,28) = 0.21, p = 0.6481). Comparison of the ratio of the amplitude of sEPSCs and sIPSCs (Fig. 6H) revealed there was no effect of either AIE exposure or sex (main effect AIE: F(1,28) = 0.66, p = 0.4236; main effect sex: F(1,28) = 0.79, p = 0.3816; AIE x sex interaction: F(1,28) = 0.02, p = 0.8935).
To examine the effects of AIE on the inhibitory and excitatory synaptic inputs to the PrLBLA neurons, sIPSCs and sEPSCs were again obtained under voltage-clamp conditions. Analysis of the sIPSCs revealed that there was no significant effect of sex or AIE, nor was there an interaction between sex and AIE exposure on either the frequency (Fig. 7A and C) (main effect AIE: F(1, 28) = 1.09, p = 0.3058; main effect sex: F(1, 28) = 0.73, p = 0.4006; interaction AIE x sex: F(1,28) = 0.08, p = 0.7765) or amplitude (Fig. 7A and D) (main effect AIE: F(1, 28) = 3.44, p = 0.0742; main effect sex: F(1, 28) = 2.02, p = 0.1663; interaction AIE x sex: F(1, 28) = 0.00, p = 0.9842) of sIPSCs. Analysis of the sEPSC recordings also revealed the frequency of sEPSCs (Fig. 7B and E) was not affected by sex or AIE exposure (main effect AIE: F(1, 28) = 0.23, p = 0.6372; main effect sex: F(1, 28) = 0.00, p = 0.9712; interaction AIE x sex: F(1, 28) = 0.15, p = 0.6996). While there was no effect of AIE exposure on sEPSC amplitude (Fig. 7B and F) (main effect AIE: F(1, 28) = 0.11, p = 0.7423; AIE x sex interaction: F(1,28) = 0.67, p = 0.4201), the amplitude of sEPSCs was reduced in female compared to male rats (main effect sex: F(1, 28) = 6.52, p = 0.0164, partial η2 = 0.1889 [0.0057, 0.4140]). For the excitatory/inhibitory synaptic balance of inputs to PrLBLA neurons, analysis indicated there was no difference in either sEPSC/sIPSC frequency (Fig. 7G) (main effect AIE: F(1, 28) = 1.45, p = 0.2391; main effect sex: F(1, 28) = 0.07, p = 0.7955; interaction AIE x sex: F(1, 28) = 0.33, p = 0.5675) or amplitude ratio (Fig. 7H) (main effect AIE: F(1, 28) = 2.39, p = 0.1332; main effect sex: F(1, 28) = 1.67, p = 0.2068; interaction AIE x sex: F(1, 28) = 0.63, p = 0.4339).
Fig. 7.
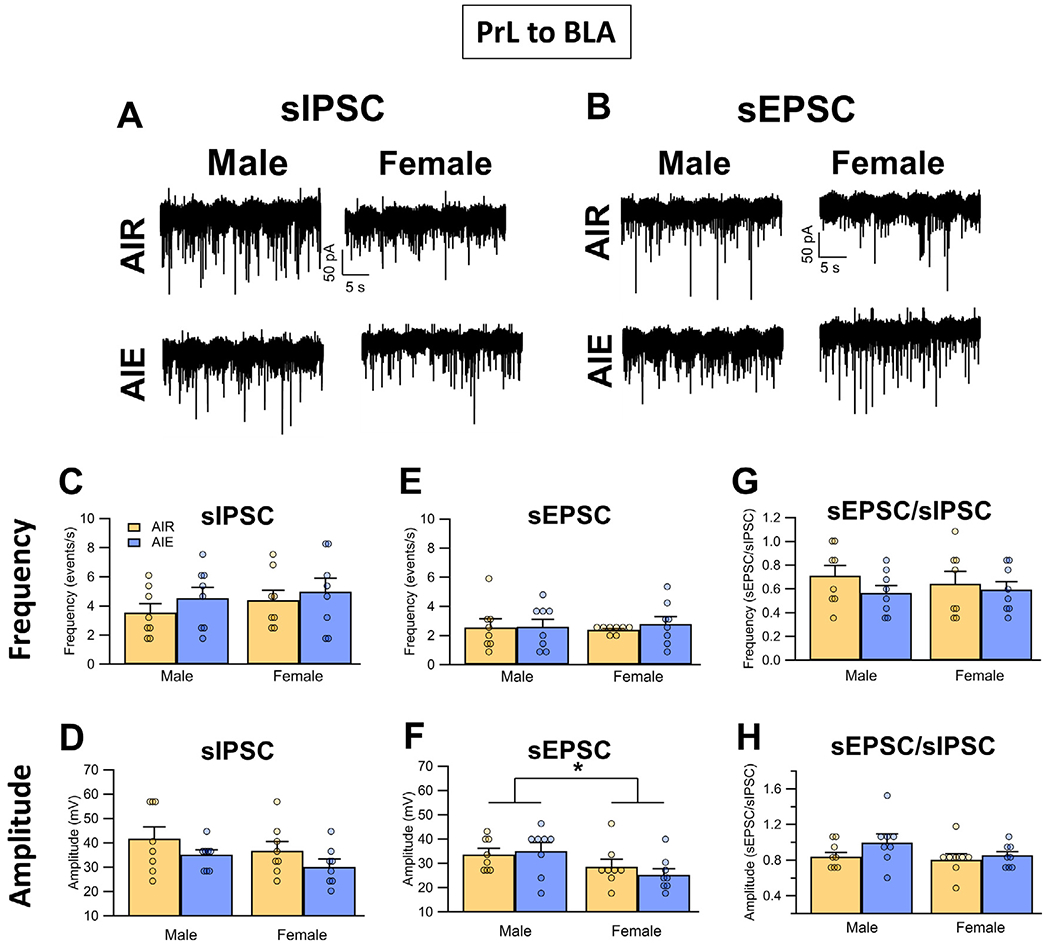
Spontaneous excitatory and inhibitory inputs to PrL neurons that project to the BLA (PrL BLA). (A) Representative traces of sIPSCs recorded from PrL BLA neurons of Air and AIE exposed adult male and female rats. (B) Representative traces of sEPSCs recorded from PrL BLA neurons of Air and AIE exposed adult male and female rats. There were no differences in either the frequency (C) or amplitude (D) of sIPSCs across either treatment or sex. While AIE also had no effect on either the frequency (E) or amplitude (F) of the sEPSC in either male or female rats, the amplitudes of the sEPSC were significantly reduced in female rats compared to male rats. Examination of the sEPSC/sIPSC ratios as an index of excitatory-inhibitory balance of synaptic inputs revealed no differences in either frequency (G) or amplitude (H) of the currents. Data represent the mean ± SEM. *, indicates significant group difference between males and females; p ≤ 0.05; n = 8 rats/group.
3.4. Projection-specific analysis of dendritic spines
Using a diolistic labelling approach, we previously demonstrated that exposure of male rats to AIE resulted in increases in the density of dendritic spines on pyramidal neurons in the adult mPFC. One limitation of diolistic labeling is the incomplete filling of the apical dendrite, which is particularly evident at the apical tuft. Accordingly, this limitation restricted our previous analyses of the effects of AIE to the dendritic spines of basal dendrites. Of particular interest for the present study is that the majority of the DA inputs in the mPFC occur in the superficial layers of the cortex, and are thus associated with apical dendrites of deep-layer pyramidal projection neurons. Therefore, the next set of studies utilized a viral labelling approach that results in complete filling of the entire length of the apical dendrite [51]. Because changes in synaptic drive following AIE were only observed in PrLNAc neurons, dendritic spine density was only examined in PrLNAc and not PrLBLA neurons. As illustrated in Fig. 8A, this approach involved injection of a retrograde cre expression virus into the NAc and a cre-dependent mCherry reporter virus into the PrL to specifically label the PrLNAc projection neurons. Animals remained in the housing colony for four to six weeks after surgery to allow adequate viral expression. Following transcardial perfusion, slices were obtained and processed for immunoamplification of the mCherry reporter construct prior to confocal imaging of the proximal (Fig. 8B) and tuft (Fig. 8C ) regions of the apical dendrites of PrLNAc labeled neurons. As shown in Fig. 8D, analysis of the z-stack confocal data sets revealed that AIE had no effect on either dendritic spine density or the head diameter at either the proximal or tuft regions of the apical dendrite.
Fig. 8.
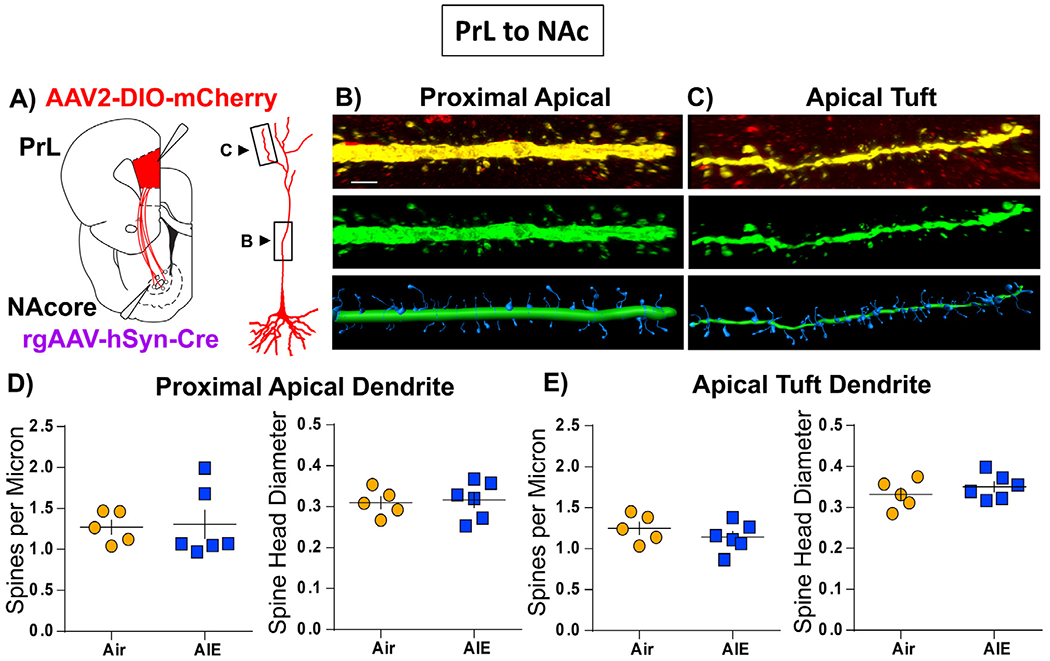
Dendritic spine morphometric analyses in PrL neurons that project to the NAc (PrL NAc ). (A) Surgery schematic depicting localization of retrograde Cre virus and Cre-dependent mCherry viral vector infusion sites. A pyramidal neuron is also depicted with boxes around the areas of the apical dendrite where spine segments were imaged (B,C) Representative images of proximal apical (B) and apical tuft dendritic segments (C). Top panel shows viral labeling in red with the dendrite of interest highlighted in yellow. The center panel shows the isolated dendrite pseudo colored in green. The bottom panel provides the digital spine analyses images with the shaft region shown in green and the spine region shown in blue. Dendritic spine density and head diameter data for the proximal apical segments (D) and the apical tuft segments (E). No significant differences were observed in spine density or head diameter at either the proximal or tuft regions of the apical dendrite when comparing Air (yellow circles) and AIE (blue squares) groups (unpaired t-tests). Data represent the mean ± SEM. n = 5-6 rats/group.
4. Discussion
In humans and preclinical animal models, adolescent alcohol exposure results in persistent deficits in behavioral flexibility [58–62]. These changes are linked, at least in part, to alterations in prefrontal function [17,63]. We previously demonstrated that deep-layer pyramidal neurons in the PrL cortex of adult male rats that had been exposed to alcohol during adolescence exhibited a number of alterations that included morphological changes observed as increases in dendritic spine density, and functional alterations that included reductions in D1 modulation of excitability [27]. A key aspect of the mPFC that relates to its function is the diversity of subcortical projections that participate in a diverse array of behaviors. Accordingly, the present study investigated the effect of AIE on the structure and function of two separate populations of PrL subcortical projection neurons. The principal finding of this study was that AIE reduces D1 modulation of both PrLNAc and PrLBLA excitability.
In the present study, the intrinsic excitability of PrLNAc and PrLBLA neurons in adult male and female rats was assessed by measuring the input-output relationship between injected current and firing rate using whole-cell current-clamp recordings. No changes in the intrinsic excitability of PrLNAc and PrLBLA neurons were found following AIE exposure. Previous studies examining the effects of adolescent alcohol exposure on the intrinsic excitability of PrL neurons have reported mixed results. In mice, adolescent voluntary binge drinking was reported to enhance the intrinsic excitability of PrL neurons [38], while studies in rats have demonstrated both enhanced [40] and unchanged intrinsic excitability of PrL neurons [27,40]. Several methodological and experimental differences between these studies likely help explain why some studies have observed increased excitability following AIE while other have not. These differences include the use of rats versus mice, differences in the alcohol exposure paradigms (passive vapor exposure versus voluntary drinking), housing conditions (single versus pair housed), and age of the animals at the time of testing. In considering only studies which have been performed in rats, it is notable that differences in intrinsic excitability were apparent only when currents much larger than those used in the present study were injected [40]. As such, the present findings are consistent with previous observations of recordings obtained from non-specific populations of pyramidal neurons from rats in that they demonstrate that across the levels of current injected in the present study, the intrinsic excitability of PrLNAc and PrLBLA pyramidal neurons is largely unaltered in adulthood following AIE.
In addition to assessing the effects of AIE on intrinsic excitability, whole-cell voltage-clamp recordings were performed to determine the effects of AIE on synaptic drive onto these two populations of projection neurons. For these studies, both the amplitude and frequency of postsynaptic currents were measured. The results of these studies revealed that synaptic input onto PrLNAc projection neurons, but not onto the PrLBLA projection neurons, was altered in slices obtained from adult AIE exposed rats. In PrLNAc neurons, the ratio of the frequency of sEPSC/sIPSC was reduced in AIE animals relative to Air controls. While AIE also produced a sex-dependent effect on the amplitude of sEPSCs onto PrLNAc neurons, the significance of this effect is unclear as post-hoc analysis did not reveal any differences in sEPSC amplitude among the various experimental conditions. It is notable that these findings suggest a slight shift toward increased inhibition of PrLNAc projection neurons following AIE, especially given that the E/I balance onto pyramidal neurons in the mPFC is thought to shift toward inhibition during adolescence [64]. As such, it is interesting that while we observed a difference in the ratio of sEPSC/sIPSC frequencies onto PrLNAc projection neurons, AIE did not significantly alter the frequency of sIPSC or sEPSC relative to Air controls. This may suggest that subtle changes in the release probability or excitability of both GABA and glutamate neurons account for this effect.
Dopamine in the mPFC plays a critical role in modulating neural network activity and directing the efficient flow of information that underlies cognition, including appropriate decision-making and behavioral flexibility. Dopamine receptor 1 is expressed on both pyramidal neurons and interneurons in the mPFC [65–67]. The subtypes of interneurons expressing D1 in the mPFC may be species dependent with robust D1 expression reported in parvalbumin containing interneurons from macaques [68] and rats [69], but not mice [55]. In projection neurons, D1 expression is greatest in neurons projecting intratelencephalically [55,67] with expression reported in a subset of both PrLNAc and PrLBLA projecting neurons [55–57,70,71]. As AIE has previously been shown to alter DA neurotransmission in the mPFC of male rats, the current study investigated the effects of AIE on D1 and D2 receptor modulation of PrLNAc and PrLBLA neuron excitability. We observed that D1 mediated enhancement of excitability was significantly attenuated in both populations of projection neurons in slices obtained from adult rats compared to the Air controls. Furthermore, this AIE-induced attenuation was observed in both male and female rats, with the only difference being that D1 activation enhanced excitability to a greater degree in PrLBLA neurons from female compared to male rats. Moreover, recordings from D1 expressing PrLNAc and PrLBLA projecting neurons from D1-Cre rats support the conclusion that AIE reduces D1 mediated enhancement of excitability. In contrast to the effect of AIE on D1 modulation of firing, the inhibitory effect of D2 activation on either PrLNAc or PrLBLA excitability was not altered by AIE exposure. Thus, these results broaden our understanding of the impact of AIE exposure on dopamine receptor function in the PrL by demonstrating that D1 modulation of both PrLNAc and PrLBLA projection neuron excitability is reduced in the adult following AIE.
Information flowing from the PrL cortex to the NAc or BLA modulates distinct subsets of behavior. Prelimbic neurons projecting to the NAc are critically involved in motivation and reward [72–74] while prelimbic neurons projecting to the BLA are involved in fear and anxiety [75–77]. An appropriate balance between D1 and D2 receptor activity in the mPFC critically controls information processing, and alterations of the balance of D1/D2 signaling negatively impact cognitive control. Consistent with this, infusions of D1 antagonists in the PrL cortex impair the acquisition of conditioned fear [78], reinstatement of drug seeking [79], set-shifting [80], and flexibility in response to changes in reward value [81]. Moreover, blockade of D1 increases impulsivity during a delayed reward task [82,83] and decreases loss tolerance during a probabilistic discounting task [84]. Prelimbic D2 blockade similarly reduces behavioral flexibility [81,85], and increases impulsivity [83]. However, in contrast to the effects of D1 antagonism on risk taking, D2 antagonism increases risky choice.
Based upon our observation of a selective reduction of D1 modulation of excitability, it is reasonable to suggests that AIE exposure results in an alteration in the balance of D1/D2 receptor modulation of signaling in PrLNAc and PrLBLA circuits. This reduction of D1 modulation of excitability within PrLNAc and PrLBLA circuitry may contribute to AIE-induced deficits in cognitive and behavioral control. Adolescent alcohol exposure produces persistent deficits in set-shifting and reversal learning [48,62,86] which are reflective of a broader reduction in behavioral flexibility [17]. Reduced D1 function on PrLNAc projection neurons may contribute to this deficit. Evidence for this comes from research indicating that optogenetic manipulation of PrLNAc neurons bidirectionally modulates strategy shifting [87] while D1 receptor antagonists infused in the PrL reduce behavioral flexibility [80,81]. Additionally, loss of D1 receptor modulation of activity of PrLBLA projection neurons may contribute to the induction of anxiety- [88–90] and depression-like [91–93] behaviors, and disinhibition observed following AIE [48,94,95]. This is supported by observations that infusion of D1 antagonists into the PrL increases impulsivity [82,83], as does functional disconnection of the PrL cortex and the BLA [96]. In addition, optogenetic stimulation of PrLBLA terminals of D1 expressing neurons reduces anxiety- and depression-like behavior [97]. Therefore, it is resonable to suggest that AIE-induced alterations of D1 receptor modulation of excitability may contribute to behavioral deficits in a projection-specific manner. For example, attenuation of D1 function on PrLNAc neurons may contribute to reduced behavioral flexibility following AIE, while loss of D1 function on PrLBLA neurons may contribute to increased anxiety- and depression-like behavior as well as induction of impulsivity/disinhibition.
Loss of D1 function on PrLNAc neurons may also contribute to the lack of an observed effect of AIE on apical dendritic spine density. Dopamine receptor 1 is highly expressed on the head and neck region of dendritic spines [98,99], where D1 activation has been linked to increased dendritic spine density [100–102]. Thus, if AIE increases dendritic spine density by reducing pruning of dendritic spines, loss of D1 function could potentially counteract the effect of decreased pruning on dendritic spine density through a reduction in spinogenesis. Dendritic spine density and morphology are intimately associated with synaptic efficiency and play a key role in regulating neuronal signaling. Dendritic spines are plastic, displaying activity and experience-dependent changes in size and structure that reflect changes in neuronal connectivity and function. During development, the overall density of dendritic spines in the mPFC reaches its peak near the onset of puberty and then progressively declines throughout adolescence before stabilizing in adulthood [32,33]. Spine morphology also changes during adolescence, which is characterized by a decrease in the number of immature thin spines with a corresponding increase in the number of mature spines that is thought to reflect increasing stability of synaptic connectivity [35]. In the present study, we measured dendritic spine density in layer V PrLNAc neurons from male rats. This circuit was selected as AIE-exposure alters functional connectivity between the mPFC and NAc [103,104]. Following AIE exposure, we found no difference in spine density or head density at either proximal or tuft regions of apical dendrites. This finding is similar to that of a previous study which found no change in the density of dendritic spines on apical dendrites of layer II/III pyramidal neurons in the mPFC of mice following adolescent alcohol exposure [105]. Despite this, the present findings were somewhat surprising as we had previously found that the density of spines on basal dendrites of layer V pyramidal neurons in the mPFC of male rats was elevated following AIE [27]. The lack of observed changes in spine density or head diameter of apical dendritic spines on PrLNAc neurons may suggest that either apical dendritic spines are more resilient to adolescent ethanol exposure or that PrLNAc spine density is spared following AIE. One caveat to this interpretation is the difference in methodology between the prior study and the current one. The present study utilized a viral approach to label dendrites of PrLNAc neurons whereas the earlier study used diolistic labelling. As mentioned previously, the viral approach provides better labelling of spines on apical dendrites as well as enabling a projection specific analysis [51].
5. Conclusions
The present study demonstrates a loss of D1 modulation of neuronal excitability in pyramidal cells of the PrL projecting to the NAc and BLA in adult rats that had been subjected to AIE exposure. It also demonstrates that these effects are not sex dependent. Despite a reduction in D1 modulation of excitability following AIE, no effect of AIE on intrinsic excitability was observed. While synaptic input to PrLNAc and PrLBLA neurons was largely unaltered by AIE in adulthood, the ratio of sEPSC/sIPSC frequency was reduced in PrLNAc neurons following AIE. Lastly, AIE exposure did not impact dendritic spine density or head diameter at either the proximal or tuft region of the apical dendrite of adult PrLNAc neurons. Together, these findings provide further evidence that adolescent alcohol exposure disrupts the normal development of the mPFC, which may contribute to alterations in behavioral flexibility and cognitive functioning in adulthood.
Supplementary Material
Acknowledgments
The authors wish to thank the members of the Chandler lab for assistance with carrying out the adolescent intermittent ethanol vapor procedure.
Funding
This work was supported by NIH grants AA019967 (LJC), AA027706 (LJC), T32 AA007474 (JDO), F31 AA027951 (JDL) and R01 DA054154 (MDS).
Footnotes
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CRediT authorship contribution statement
J. Daniel Obray: Visualization, Methodology, Project administration, Formal analysis, Data curation, Writing – original draft, Visualization, Methodology, Project administration. Justine D. Landin: Visualization, Methodology, Supervision, Visualization, Methodology. Dylan T. Vaughan: Formal analysis. Michael D. Scofield: Visualization, Methodology, Writing – original draft, Visualization, Methodology. L. Judson Chandler: Visualization, Methodology, Writing – original draft, Visualization, Methodology.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.addicn.2022.100044.
Data availability
Data will be made available on request.
References
- [1].SAMHSA, Report to Congress on the prevention and reduction of underage drinking, Substance Abuse and Mental Health Services Administration (SAMHSA), US Department of Health and Human Services (HHS), 2018. https://www.stopalcoholabuse.gov/media/ReportToCongress/2018/report_main/stop_act_rtc.pdf. [Google Scholar]
- [2].Guttmannova K, Bailey JA, Hill KG, Lee JO, Hawkins JD, Woods ML, Catalano RF, Sensitive periods for adolescent alcohol use initiation: predicting the lifetime occurrence and chronicity of alcohol problems in adulthood, J. Stud. Alcohol. Drugs 72 (2) (2011) 221–231, doi: 10.15288/jsad.2011.72.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Davis CN, Slutske WS, Piasecki TM, Martin NG, Lynskey MT, Comparing the potential causal influence of two indicators of early alcohol use on later alcohol use disorder symptoms, J. Abnorm. Psychol 129 (3) (2020) 256–265, doi: 10.1037/abn0000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hanson KL, Cummins K, Tapert SF, Brown SA, Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment, Psychol. Addict. Behav 25 (1) (2011) 127–142, doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mota N, Parada M, Crego A, Doallo S, Caamano-Isorna F, Rodriguez Holguin S, Cadaveira F, Corral M, Binge drinking trajectory and neuropsychological functioning among university students: a longitudinal study, Drug Alcohol. Depend. 133 (1) (2013) 108–114, doi: 10.1016/j.drugalcdep.2013.05.024. [DOI] [PubMed] [Google Scholar]
- [6].Nguyen-Louie TT, Tracas A, Squeglia LM, Matt GE, Eberson-Shumate S, Tapert SF, Learning and memory in adolescent moderate, binge, and extreme-binge drinkers, Alcohol Clin. Exp. Res 40 (9) (2016) 1895–1904, doi: 10.1111/acer.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nguyen-Louie TT, Matt GE, Jacobus J, Li I, Cota C, Castro N, Tapert SF, Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults, Alcohol Clin. Exp. Res 41 (12) (2017) 2082–2092, doi: 10.1111/acer.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tapert SF, Granholm E, Leedy NG, Brown SA, Substance use and withdrawal: neuropsychological functioning over 8 years in youth, J. Int. Neuropsychol. Soc 8 (7) (2002) 873–883, doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- [9].Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, Brown SA, Colrain IM, Baker FC, Prouty D, De Bellis MD, Clark DB, Nagel BJ, Chu W, Park SH, Pohl KM, Sullivan EV, Altered brain developmental trajectories in adolescents after initiating drinking, Am. J. Psychiatry 175 (4) (2018) 370–380, doi: 10.1176/appi.ajp.2017.17040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A, Brain development in heavy-drinking adolescents, Am. J. Psychiatry 172 (6) (2015) 531–542, doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Luciana M, Collins PF, Muetzel RL, Lim KO, Effects of alcohol use initiation on brain structure in typically developing adolescents, Am. J. Drug Alcohol. Abuse 39 (6) (2013) 345–355, doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wetherill RR, Squeglia LM, Yang TT, Tapert SF, A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking, Psychopharmacology (Berl.) 230 (4) (2013) 663–671, doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cservenka A, Jones SA, Nagel BJ, Reduced cerebellar brain activity during reward processing in adolescent binge drinkers, Dev. Cogn. Neurosci 16 (2015) 110–120, doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Klune CB, Jin B, DeNardo LA, Linking mPFC circuit maturation to the developmental regulation of emotional memory and cognitive flexibility, Elife 10 (2021) e64567, doi: 10.7554/eLife.64567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Selemon LD, A role for synaptic plasticity in the adolescent development of executive function, Transl. Psychiatry 3 (3) (2013) e238, doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Best JR, Miller PH, A developmental perspective on executive function, Child Dev 81 (6) (2010) 1641–1660, doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS, Vetreno RP, Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings, Alcohol. Clin. Exp. Res 43 (9) (2019) 1806–1822, doi: 10.1111/acer.14154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spear LP, Effects of adolescent alcohol consumption on the brain and behaviour, Nat. Rev. Neurosci 19 (4) (2018) 197–214, doi: 10.1038/nrn.2018.10 . [DOI] [PubMed] [Google Scholar]
- [19].Spear LP, The adolescent brain and age-related behavioral manifestations, Neurosci. Biobehav. Rev 24 (4) (2000) 417–463, doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [20].Crews F, He J, Hodge C, Adolescent cortical development: a critical period of vulnerability for addiction, Pharmacol. Biochem. Behav 86 (2) (2007) 189–199, doi: 10.1016/j.pbb.2006.12.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Floresco SB, Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions, Front. Neurosci 7 (2013) 62, doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Robbins TW, Arnsten AF, The neuropsychopharmacology of fronto-executive function: monoaminergic modulation, Annu. Rev. Neurosci 32 (2009) 267–287, doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Willing J, Cortes LR, Brodsky JM, Kim T, Juraska JM, Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats, Dev. Psychobiol 59 (5) (2017) 583–589, doi: 10.1002/dev.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosenberg DR, Lewis DA, Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis, J. Comp. Neurol 358 (3) (1995) 383–400, doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- [25].Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, Hiyama H, Huang B, Kohda K, Noda Y, O’Donnell P, Nakajima K, Sawa A, Nabeshima T, Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits, Neuron 65 (4) (2010) 480–489, doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tarazi FI, Baldessarini RJ, Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain, Int. J. Dev. Neurosci 18 (1) (2000) 29–37, doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- [27].Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, Glover EJ, Floresco SB, Crews FT, Krishnan HR, Pandey SC, Chandler LJ, Binge-like alcohol exposure during adolescence disrupts dopaminergic neurotransmission in the adult prelimbic cortex, Neuropsychopharmacology 42 (5) (2017) 1024–1036, doi: 10.1038/npp.2016.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cullity ER, Madsen HB, Perry CJ, Kim JH, Postnatal developmental trajectory of dopamine receptor 1 and 2 expression in cortical and striatal brain regions, J. Comp. Neurol 527 (6) (2019) 1039–1055, doi: 10.1002/cne.24574. [DOI] [PubMed] [Google Scholar]
- [29].Brenhouse HC, Sonntag KC, Andersen SL, Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence, J. Neurosci 28 (10) (2008) 2375–2382, doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Centanni SW, Burnett EJ, Trantham-Davidson H, Chandler LJ, Loss of delta-GABAA receptor-mediated tonic currents in the adult prelimbic cortex following adolescent alcohol exposure, Addict. Biol 22 (3) (2017) 616–628, doi: 10.1111/adb.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Du X, Serena K, Hwang WJ, Grech AM, Wu YWC, Schroeder A, Hill RA, Prefrontal cortical parvalbumin and somatostatin expression and cell density increase during adolescence and are modified by BDNF and sex, Mol. Cell. Neurosci 88 (2018) 177–188, doi: 10.1016/j.mcn.2018.02.001. [DOI] [PubMed] [Google Scholar]
- [32].Koss WA, Belden CE, Hristov AD, Juraska JM, Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats, Synapse 68 (2) (2014) 61–72, doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- [33].Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I, Extraordinary neoteny of synaptic spines in the human prefrontal cortex, Proc. Natl. Acad. Sci. U. S. A 108 (32) (2011) 13281–13286, doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rakic P, Bourgeois J-P, Goldman-Rakic PS, Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness, in: Van Pelt J, Corner MA, Uylings HBM, Lopes Da Silva FH (Eds.), Progress in Brain Research, Elsevier, 1994, pp. 227–243. [DOI] [PubMed] [Google Scholar]
- [35].Barfield ET, Sequeira MK, Parsons RG, Gourley SL, Morphological responses of excitatory prelimbic and orbitofrontal cortical neurons to excess corticosterone in adolescence and acute stress in adulthood, Front. Neuroanat 14 (2020) 45, doi: 10.3389/fnana.2020.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Murphy KM, Tcharnaia L, Beshara SP, Jones DG, Cortical development of AMPA receptor trafficking proteins, Front. Mol. Neurosci 5 (2012) 65, doi: 10.3389/fnmol.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Insel TR, Miller LP, Gelhard RE, The ontogeny of excitatory amino acid receptors in rat forebrain–I. N-methyl-D-aspartate and quisqualate receptors, Neuroscience 35 (1) (1990) 31–43, doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- [38].Salling MC, Skelly MJ, Avegno E, Regan S, Zeric T, Nichols E, Harrison NL, Alcohol consumption during adolescence in a mouse model of binge drinking alters the intrinsic excitability and function of the prefrontal cortex through a reduction in the hyperpolarization-activated cation current, J. Neurosci 38 (27) (2018) 6207–6222, doi: 10.1523/JNEUROSCI.0550-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang SS, Li YC, Coley AA, Chamberlin LA, Yu P, Gao WJ, Cell-type specific development of the hyperpolarization-activated current, Ih, in prefrontal cortical neurons, Front. Synaptic Neurosci 10 (2018) 7, doi: 10.3389/fnsyn.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Galaj E, Guo C, Huang D, Ranaldi R, Ma YY, Contrasting effects of adolescent and early-adult ethanol exposure on prelimbic cortical pyramidal neurons, Drug Alcohol. Depend 216 (2020) 108309, doi: 10.1016/j.drugalcdep.2020.108309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Warren BL, Kane L, Venniro M, Selvam P, Quintana-Feliciano R, Mendoza MP, Madangopal R, Komer L, Whitaker LR, Rubio FJ, Bossert JM, Caprioli D, Shaham Y, Hope BT, Separate vmPFC ensembles control cocaine selfadministration versus extinction in rats, J. Neurosci 39 (37) (2019) 7394–7407, doi: 10.1523/JNEUROSCI.0918-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Diehl MM, Iravedra-Garcia JM, Moran-Sierra J, Rojas-Bowe G, Gonzalez-Diaz FN, Valentin-Valentin VP, Quirk GJ, Divergent projections of the prelimbic cortex bidirectionally regulate active avoidance, Elife 9 (2020) e59281, doi: 10.7554/eLife.59281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Arruda-Carvalho M, Wu WC, Cummings KA, Clem RL, Optogenetic examination of prefrontal-amygdala synaptic development, J. Neurosci 37 (11) (2017) 2976–2985, doi: 10.1523/JNEUROSCI.3097-16.2017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H, Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat, J. Comp. Neurol 518 (14) (2010) 2693–2709, doi: 10.1002/cne.22359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barker JM, Bryant KG, Osborne JI, Chandler LJ, Age and sex interact to mediate the effects of intermittent, high-dose ethanol exposure on behavioral flexibility, Front. Pharmacol 8 (2017) 450, doi: 10.3389/fphar.2017.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chandler LJ, Vaughan DT, Gass JT, Adolescent alcohol exposure results in sex-specific alterations in conditioned fear learning and memory in adulthood, Front. Pharmacol. (2022) 837657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Varlinskaya EI, Hosova D, Towner T, Werner DF, Spear LP, Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood, Behav. Brain Res 378 (2020) 112292, doi: 10.1016/j.bbr.2019.112292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ, Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood, Neuropsychopharmacology 39 (11) (2014) 2570–2583, doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nentwig TB, Starr EM, Chandler LJ, Glover EJ, Absence of compulsive drinking phenotype in adult male rats exposed to ethanol in a binge-like pattern during adolescence, Alcohol 79 (2019) 93–103, doi: 10.1016/j.alcohol.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ, Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex, J. Neurosci 34 (10) (2014) 3706–3718, doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Siemsen BM, Giannotti G, McFaddin JA, Scofield MD, McGinty JF, Biphasic effect of abstinence duration following cocaine self-administration on spine morphology and plasticity-related proteins in prelimbic cortical neurons projecting to the nucleus accumbens core, Brain Struct. Funct 224 (2) (2019) 741–758, doi: 10.1007/s00429-018-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tseng KY, O’Donnell P, Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms, J. Neurosci 24 (22) (2004) 5131–5139, doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gorelova NA, Yang CR, Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons In vitro, J. Neurophysiol 84 (1) (2000) 75–87, doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- [54].Gulledge AT, Jaffe DB, Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex, J. Neurosci 18 (21) (1998) 9139–9151, doi: 10.1523/jneurosci.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Anastasiades PG, Boada C, Carter AG, Cell-Type-Specific D1 dopamine receptor modulation of projection neurons and interneurons in the prefrontal cortex, Cereb. Cortex 29 (7) (2019) 3224–3242, doi: 10.1093/cercor/bhy299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Han SW, Kim YC, Narayanan NS, Projection targets of medial frontal D1DR-expressing neurons, Neurosci. Lett 655 (2017) 166–171, doi: 10.1016/j.neulet.2017.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vander Weele CM, Siciliano CA, Matthews GA, Namburi P, Izadmehr EM, Espinel IC, Nieh EH, Schut EHS, Padilla-Coreano N, Burgos-Robles A, Chang BJ, Kimchi EY, Beyeler A, Wichmann R, Wildes CP, Tye KM, Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli, Nature 563 (7731) (2018) 397–401, doi: 10.1038/s41586-018-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Salas-Gomez D, Fernandez-Gorgojo M, Pozueta A, Diaz-Ceballos I, Lamarain M, Perez C, Sanchez-Juan P, Binge drinking in young university students is associated with alterations in executive functions related to their starting age, PLoS One 11 (11) (2016) e0166834, doi: 10.1371/journal.pone.0166834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gil-Hernandez S, Mateos P, Porras C, Garcia-Gomez R, Navarro E, Garcia-Moreno LM, Alcohol binge drinking and executive functioning during adolescent brain development, Front. Psychol 8 (2017) 1638, doi: 10.3389/fpsyg.2017.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Acheson SK, Bearison C, Risher ML, Abdelwahab SH, Wilson WA, Swartzwelder HS, Effects of acute or chronic ethanol exposure during adolescence on behavioral inhibition and efficiency in a modified water maze task, PLoS One 8 (10) (2013) e77768, doi: 10.1371/journal.pone.0077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Contreras A, Polin E, Miguens M, Perez-Garcia C, Perez V, Ruiz-Gayo M, Morales L, Del Olmo N, Intermittent-excessive and chronic-moderate ethanol intake during adolescence impair spatial learning, memory and cognitive flexibility in the adulthood, Neuroscience 418 (2019) 205–217, doi: 10.1016/j.neuroscience.2019.08.051. [DOI] [PubMed] [Google Scholar]
- [62].Vetreno RP, Bohnsack JP, Kusumo H, Liu W, Pandey SC, Crews FT, Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: restoration with voluntary exercise, Addict. Biol 25 (2) (2020) e12731, doi: 10.1111/adb.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dannenhoffer CA, Robertson MM, Macht VA, Mooney SM, Boettiger CA, Robinson DL, Chapter Four - Chronic alcohol exposure during critical developmental periods differentially impacts persistence of deficits in cognitive flexibility and related circuitry, in: Bell RL, Rahman S (Eds.), International Review of Neurobiology, Academic Press, 2021, pp. 117–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Caballero A, Orozco A, Tseng KY, Developmental regulation of excitatory-inhibitory synaptic balance in the prefrontal cortex during adolescence, Semin. Cell Dev. Biol 118 (2021) 60–63, doi: 10.1016/j.semcdb.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Santana N, Artigas F, Laminar and cellular distribution of monoamine receptors in rat medial prefrontal cortex, Front. Neuroanat 11 (2017) 87, doi: 10.3389/fnana.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, Kleinman JE, Akil M, Postnatal alterations in dopaminergic markers in the human prefrontal cortex, Neuroscience 144 (3) (2007) 1109–1119, doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- [67].Co M, Hickey SL, Kulkarni A, Harper M, Konopka G, Cortical Foxp2 supports behavioral flexibility and developmental dopamine D1 receptor expression, Cereb. Cortex 30 (3) (2020) 1855–1870, doi: 10.1093/cercor/bhz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Muly EC, Szigeti K, Goldman-Rakic PS, D1Receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization, J. Neurosci 18 (24) (1998) 10553–10565, doi: 10.1523/jneurosci.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Le Moine C, Gaspar P, Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes, Mole. Brain Res 58 (1-2) (1998) 231–236, doi: 10.1016/s0169-328x(98)00118-1. [DOI] [PubMed] [Google Scholar]
- [70].Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ, Medial prefrontal D1 dopamine neurons control food intake, Nat. Neurosci 17 (2) (2014) 248–253, doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Green SM, Nathani S, Zimmerman J, Fireman D, Urs NM, Retrograde labeling illuminates distinct topographical organization of D1 and D2 receptorpositive pyramidal neurons in the prefrontal cortex of mice, eNeuro 7 (5) (2020), doi: 10.1523/ENEURO.0194-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Moschak TM, Carelli RM, An opposing role for prelimbic cortical projections to the nucleus accumbens core in incubation of craving for cocaine versus water, Drug Alcohol Depend. 228 (2021) 109033, doi: 10.1016/j.drugalcdep.2021.109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stefanik MT, Kupchik YM, Kalivas PW, Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior, Brain Struct. Funct 221 (3) (2016) 1681–1689, doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].James MH, McGlinchey EM, Vattikonda A, Mahler SV, Aston-Jones G, Cued reinstatement of cocaine but not sucrose seeking is dependent on dopamine signaling in prelimbic cortex and is associated with recruitment of prelimbic neurons that project to contralateral nucleus accumbens core, Int. J. Neuropsychopharmacol 21 (1) (2018) 89–94, doi: 10.1093/ijnp/pyx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Grossman YS, Fillinger C, Manganaro A, Voren G, Waldman R, Zou T, Janssen WG, Kenny PJ, Dumitriu D, Structure and function differences in the prelimbic cortex to basolateral amygdala circuit mediate trait vulnerability in a novel model of acute social defeat stress in male mice, Neuropsychopharmacology 47 (3) (2022) 788–799, doi: 10.1038/s41386-021-01229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Arruda-Carvalho M, Clem RL, Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding, J. Neurosci 34 (47) (2014) 15601–15609, doi: 10.1523/JNEUROSCI.2664-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C, Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression, Nature 505 (7481) (2014) 92–96, doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- [78].Stubbendorff C, Hale E, Cassaday HJ, Bast T, Stevenson CW, Dopamine D1-like receptors in the dorsomedial prefrontal cortex regulate contextual fear conditioning, Psychopharmacology (Berl.) 236 (6) (2019) 1771–1782, doi: 10.1007/s00213-018-5162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].See RE, Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse, Int. J. Neuropsychopharmacol 12 (3) (2009) 431–436, doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ragozzino ME, The effects of dopamine D(1) receptor blockade in the prelimbicinfralimbic areas on behavioral flexibility, Learn. Mem 9 (1) (2002) 18–28, doi: 10.1101/lm.45802 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Winter S, Dieckmann M, Schwabe K, Dopamine in the prefrontal cortex regulates rats behavioral flexibility to changing reward value, Behav. Brain Res 198 (1) (2009) 206–213, doi: 10.1016/j.bbr.2008.10.040. [DOI] [PubMed] [Google Scholar]
- [82].Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, Spijker S, van Gaalen MM, Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats, Cereb. Cortex 20 (5) (2010) 1064–1070, doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- [83].Pardey MC, Kumar NN, Goodchild AK, Cornish JL, Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex, J. Psychopharmacol 27 (2) (2013) 203–212, doi: 10.1177/0269881112465497. [DOI] [PubMed] [Google Scholar]
- [84].St Onge JR, Abhari H, Floresco SB, Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making, J. Neurosci 31 (23) (2011) 8625–8633, doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT, Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting, Neuropsychopharmacology 31 (2) (2006) 297–309, doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- [86].Coleman LG Jr., He J, Lee J, Styner M, Crews FT, Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice, Alcohol Clin. Exp. Res 35 (4) (2011) 671–688, doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cui Q, Li Q, Geng H, Chen L, Ip NY, Ke Y, Yung WH, Dopamine receptors mediate strategy abandoning via modulation of a specific prelimbic cortex-nucleus accumbens pathway in mice, Proc. Natl. Acad. Sci. U. S. A 115 (21) (2018) E4890–E4899, doi: 10.1073/pnas.1717106115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Pandey SC, Sakharkar AJ, Tang L, Zhang H, Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood, Neurobiol. Dis 82 (2015) 607–619, doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kyzar EJ, Zhang H, Sakharkar AJ, Pandey SC, Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood, Addict. Biol 22 (5) (2017) 1191–1204, doi: 10.1111/adb.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Vetreno RP, Yaxley R, Paniagua B, Crews FT, Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction, Addict. Biol 21 (4) (2016) 939–953, doi: 10.1111/adb.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT, Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology, Neuroscience 199 (2011) 333–345, doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Slawecki CJ, Thorsell A, Ehlers CL, Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models, Ann. N. Y. Acad. Sci 1021 (1) (2004) 448–452, doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- [93].Lee KM, Coehlo MA, Solton NR, Szumlinski KK, Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge-drinking in adolescent, But Not Adult, Mice, Front. Psychol 8 (2017) 1128, doi: 10.3389/fpsyg.2017.01128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gilpin NW, Karanikas CA, Richardson HN, Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats, PLoS One 7 (2) (2012) e31466, doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Desikan A, Wills DN, Ehlers CL, Ontogeny and adolescent alcohol exposure in Wistar rats: open field conflict, light/dark box and forced swim test, Pharmacol. Biochem. Behav 122 (2014) 279–285, doi: 10.1016/j.pbb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Churchwell JC, Morris AM, Heurtelou NM, Kesner RP, Interactions between the prefrontal cortex and amygdala during delay discounting and reversal, Behav. Neurosci 123 (6) (2009) 1185–1196, doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS, Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects, Nat. Commun 10 (1) (2019) 223, doi: 10.1038/s41467-018-08168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bordelon-Glausier JR, Khan ZU, Muly EC, Quantification of D1 and D5 dopamine receptor localization in layers I, III, and V of Macaca mulatta prefrontal cortical area 9: coexpression in dendritic spines and axon terminals, J. Comp. Neurol 508 (6) (2008) 893–905, doi: 10.1002/cne.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS, Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain, J. Neurosci 15 (12) (1995) 7821–7836, doi: 10.1523/jneurosci.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Li J, Gu J, Wang B, Xie M, Huang L, Liu Y, Zhang L, Xue J, Guo F, Zhang L, Zhang L, Activation of dopamine d1 receptors regulates dendritic morphogenesis through Rac1 and RhoA in prefrontal cortex neurons, Mol. Neurobiol 51 (3) (2015) 1024–1037, doi: 10.1007/s12035-014-8762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Shinohara R, Taniguchi M, Ehrlich AT, Yokogawa K, Deguchi Y, Cherasse Y, Lazarus M, Urade Y, Ogawa A, Kitaoka S, Sawa A, Narumiya S, Furuyashiki T, Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice, Mol. Psychiatry 23 (8) (2018) 1717–1730, doi: 10.1038/mp.2017.177. [DOI] [PubMed] [Google Scholar]
- [102].Wu M, Minkowicz S, Dumrongprechachan V, Hamilton P, Kozorovitskiy Y, Ketamine rapidly enhances glutamate-evoked dendritic spinogenesis in medial prefrontal cortex through dopaminergic mechanisms, Biol. Psychiatry 89 (11) (2021) 1096–1105, doi: 10.1016/j.biopsych.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL, Shih YI, Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood, Addict. Biol 23 (2) (2018) 810–823, doi: 10.1111/adb.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Liu W, Crews FT, Adolescent intermittent ethanol exposure enhances ethanol activation of the nucleus accumbens while blunting the prefrontal cortex responses in adult rat, Neuroscience 293 (2015) 92–108, doi: 10.1016/j.neuroscience.2015.02.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, Bergstrom HC, Holmes A, Chronic ethanol during adolescence impacts corticolimbic dendritic spines and behavior, Alcohol Clin. Exp. Res 41 (7) (2017) 1298–1308, doi: 10.1111/acer.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


