Abstract
Surgical sutures are the most well-known surgical biomaterial device for ligating blood vessels. The primary goal of wound closure is to align wound margins to provide a closed and stable environment. Sutures with lesser tensile strength are susceptible to break throughout the healing process due to edema. To evaluate the tensile strength of absorbable and nonabsorbable suture materials after immersion in fruit juices. In this in vitro study, eight samples of commercially available sutures such as black silk and vicryl were divided into two groups: sample -1 were immersed in grape juice and sample -2 were immersed in lemon juice for 1 week. Universal testing machine INSTRON E300 UTM was used to test the tensile strength of various suture materials. The data are statistically analyzed using an independent t-test. The P < 0.05 was considered to be statistically significant. The mean of vicryl suture after immersion in grape and lemon juice was found to be 34.445 and 43.39; the mean value of black silk after immersion in grape and lemon juice was found to be 36.95 and 33.1. The tensile strength of black silk was slightly lower than the vicryl. Independent sample t-test showed that P = 0.561 (>0.05) which is statistically insignificant. Vicryl suture tested to have the highest tensile strength along with excellent knot holding capacity than black silk suture after immersion in fruit juices.
Keywords: Innovative measurement, in vitro study, juice, suture material, tensile strength
INTRODUCTION
A suture material is a biomaterial device, that can be synthetic or natural, that is used to ligate blood vessels and attach tissues together.[1] Wound closure minimizes open spaces, evenly distributes tension along deep suture lines, and maintains tensile strength across the wound.[2] An ideal suture should be easy to apply, have little tissue response, do not support pathogen activity, have high tensile strength, be easy to disinfect, create no adverse response, have no lethal effects, and be absorbed once the function has been achieved.[3] Meanwhile, the suture material must be dispersible in body fluids.[4] It is not likely to create or increase complications in the wound closure line, such as infection, wound dehiscence, and sinus formation.[5] Suture material weakness may lead to premature suture breakage, resulting in poor surgical flap adaptation and secondary intention tissue healing.[6]
In previous research, tensile strength of sutures materials on beverages, hyaluronic acid, artificial saliva versus saline solution, Chlorhexidine and Listerine, human pancreatic juice and bile, ringer's solution were assessed. Kakoei et al. performed an in vivo study in which saliva was used to test the tissue response of 4 suture materials and it was found that saliva contains an abundance of bacterial species that can infiltrate through the suture material and obstruct tissue healing, which is a compelling reason to test tensile strength with a moist oral cavity.[7] In sterile neutral and Escherichia coli-inoculated urine, polyglyconate and polydioxanone suture material had superior tensile strengths property.[8]
Among surgeons, there is no general agreement, and each has his as well as her own choices. Other than scientific facts, training, experience, economic reasons, and personal preferences have also affected suture selection.[9] Suturing in the mouth is usually only needed for a short time. The kinds of tissues implicated, the presence of saliva on a constant basis, high tissue microcirculation, speaking or swallowing movements are some of the reasons that distinguish suturing in dentistry from suturing in other body areas.[10] It is critical to understand which suture material is most able to tolerate a wide range of adverse environments while retaining its fundamental properties. Our research and knowledge have resulted in high-quality publications from our team.[11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]
Therefore, this present study aims at evaluating the tensile strength of surgical absorbable and nonabsorbable suture materials after immersion in fruit juices.
MATERIALS AND METHODS
Collection of suture material
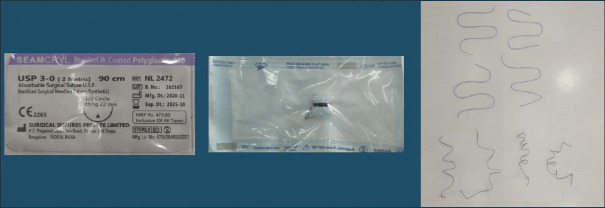
In this in vitro study, we selected two distinct types of suture materials namely absorbable (vicryl) and non-absorbable (silk) with a uniform gauge of 3.0. Four samples of each suture type were kept for the study group and one specimen for the control group. The suture samples were all measured at the same length of 21 cm [Figure 1].
Figure 1.
Collection of commercially available suture material in dentistry (black silk and vicryl)
Postimmersion in fresh juices
The selected suture samples from each group were immersed in two borosilicate containers with grape and lemon juices [Figure 2]. These immersion procedures were carried out for 2 times a day within 10 min. After 1 week of immersion, the sutures were tied with a surgeon knot followed by two square knots around two metal hooks affixed to the universal testing machine's opposite arms with a predetermined distance of 18 cm between the hooks.
Figure 2.

Immersion of suture material in grape and lemon juice
Determination of tensile strength
After 1 week of observation, the tensile strength of suture was determined using an INSTRON E300 UTM Universal Testing Machine with a cross-head speed of 1.0 mm/min and a computer for digital output. The highest force produced to the suture sample in Newtons prior to failure was then measured as Tensile strength (breaking strength) [Figure 3].
Figure 3.

Estimation of tensile strength of black silk and vicryl suture material using INSTRON E3000 UTM Universal testing machine
Statistical analysis
The results were statistically analyzed by the independent t-test. The P < 0.05 was considered to be statistically significant.
RESULTS
The results obtained are recorded and the tensile strength of vicryl and silk suture material after post immersion in lemon and grape juice is shown in Tables 1 and 2. The mean of maximum force was found to be 191.628 N and the mean of tensile strength was found to be 126.121 psi. The mean of vicryl suture material after post immersion in grape and lemon juice was found to be 34.445 and 43.39 and the mean value of black silk suture material after post immersion in grape and lemon juice was found to be 36.95 and 33.1. The tensile strength of black silk was slightly lower than the vicryl. Independent sample t-test showed that the P value was 0.561 (>0.05) which is statistically insignificant.
Table 1.
The tensile strength of different suture material after immersion in fresh juices
| Suture material | Grape juice | Lemon juice |
|---|---|---|
| Vicryl | 34.445 | 43.39 |
| Silk | 36.95 | 33.1 |
Table 2.
Significance among the suture material
| Suture material | Mean | SD | Significance |
|---|---|---|---|
| Vicryl | 22.4 | 8.31 | 0.561 |
| Silk | 18.76 | 13.61 |
Independent sample t-test is used. P≤0.05 is significant. SD: Standard deviation
DISCUSSION
In this present study, we selected vicryl and black silk suture material to evaluate their tensile strength due to their flexibility as well as popularity in oral and maxillofacial surgeries. To discourage knot untying if using synthetic absorbable sutures, the surgeon's knot is advised.[26] Therefore, all the samples were tied with the surgeon's knot, and any loosening or untangling was investigated. In our study, suture materials were immersed in fresh juices such as grape and lemon. These immersion procedures were conducted 2 times a day with a duration of 10 min. The immersion period was short due to spoilage of fresh juice and not storable. In previous studies, sutures were exposed to minimal environmental influences such as beverages, hyaluronic acid, artificial saliva versus saline solution, Chlorhexidine and Listerine, human pancreatic juice and bile that had an impact on their physical and mechanical characteristics.[27] When suture materials come into contact with saliva or other bodily fluids, their physical and functional characteristics may change. Because of the tension caused by edema and tissue tension, low-tensile-strength suture materials are more likely to break during the healing process.[28] As a result, the selection of best suture materials is important for proper wound healing.
In the present findings, the absorbable vicryl suture material shows significantly increased tensile strength in grape and lemon juices compared to nonabsorbable black silk suture material. Similar to our study, Pons-Vicente et al. determined the structural characteristics of silk versus polyester and noticed that silk suture material accumulated more plaque than polyester suture material, causing patients discomfort. Therefore, polyester suture is not the best suture material in situations where there was a lot of tissue response and tensile strength degradation.[29] Another study reported that silk suture exhibited a greater loss in tensile strength related to initial values of 3% intragastric, 0% intra intestinal, 58% bile, and 24% intravesical. The use of silk was recommended in gastric and intestinal surgery as well as wound healing capacity remaining after 5 days.[30]
In the present study, vicryl sutures had the greatest tensile strength as well as excellent knot holding capacity. In contrast to the present finding, vicryl sutures had the highest tensile strength, but this property quickly declined with time.[31] Another study by Ferguson et al. found that vicryl soaked in saliva has lower tensile strength than saline or milk. They supported this assertion by claiming that saliva appears to hasten suture deterioration, resulting in a reduction in tensile strength.[32] In contrast to present findings, vicryl sutures preserved the greatest tensile strength in a therapeutic pH ranges from 5.25 to 10.09 as well as exhibited a greater reduction of tensile strength in both acidic and alkaline medium than Dexon sutures.[33]
The limitations of this study were low sample size and short time frame kept to test the tensile strength of various suture materials. Since it is an in vitro study, it may or may not be completely applicable to the clinical situation due to the potential consequences of comorbidities such as eating habits, malnutrition, chemotherapeutic agents as well as glucocorticoid steroids, alcohol abuse, tobacco smoking, and compromised oral hygiene, all of which may affect the prevailing physiological infirmity. This in vitro study will aid oral surgeons in selecting the best suture materials for their procedures.
CONCLUSION
In the present study, vicryl suture material tested to have the highest tensile strength along with excellent knot holding capacity than black-silk suture materials after immersion in fruit juices (grape and lemon juices). Hence, vicryl suture material VICRYL® (polyglactin 910) can be preferred for the periodontal surgeries due to its retention properties for longer periods compared to black silk as it has less tensile strength.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chu C. Wound Closure Biomaterials and Devices. Florida: CRC Press; 2018. New emerging and experimental materials for wound closure; pp. 347–84. [Google Scholar]
- 2.Islam A, Ehsan A. Comparison of suture material and technique of closure of subcutaneous fat and skin in caesarean section. N Am J Med Sci. 2011;3:85–8. doi: 10.4297/najms.2011.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sewta R. Manual of Surgical Equipment. New Delhi: Jaypee Brothers Medical Publishers; 2010. Suture material; p. 43. [Google Scholar]
- 4.Bindra V, Paul B. Practical Manual of Obstetrics and Gynecology for Residents and Fellows. New Delhi: Jaypee Brothers Medical Publishers; 2011. Wound healing, sutures and surgical knots; p. 251. [Google Scholar]
- 5.Vijayalakshmi B, Ganapathy D. Medical management of cellulitis. Res J Pharm Technol. 2016;9:2067. [Google Scholar]
- 6.Fraunhofer JA, von von Fraunhofer JA, Storey RJ, Masterson BJ. Tensile properties of suture materials. Biomaterials. 1988;9:324–7. doi: 10.1016/0142-9612(88)90027-0. [DOI] [PubMed] [Google Scholar]
- 7.Kakoei S, Baghaei F, Dabiri S, Parirokh M, Kakooei S. A comparative in vivo study of tissue reactions to four suturing materials. Iran Endod J. 2010;5:69–73. [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg CB, Davidson EB, Bellmer DD, Morton RJ, Payton ME. Evaluation of the tensile strengths of four monofilament absorbable suture materials after immersion in canine urine with or without bacteria. Am J Vet Res. 2004;65:847–53. doi: 10.2460/ajvr.2004.65.847. [DOI] [PubMed] [Google Scholar]
- 9.Celeste C. Equine Wound Management. Newyork: Wiley; 2016. Selection of suture materials, suture patterns, and drains for wound closure; pp. 173–99. [Google Scholar]
- 10.Varghese SS, Thomas H, Jayakumar ND, Sankari M, Lakshmanan R. Estimation of salivary tumor necrosis factor-alpha in chronic and aggressive periodontitis patients. Contemp Clin Dent. 2015;6:S152–6. doi: 10.4103/0976-237X.166816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eapen BV, Baig MF, Avinash S. An assessment of the incidence of prolonged postoperative bleeding after dental extraction among patients on uninterrupted low dose aspirin therapy and to evaluate the need to stop such medication prior to dental extractions. J Maxillofac Oral Surg. 2017;16:48–52. doi: 10.1007/s12663-016-0912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy A, Sherlin HJ, Ramalingam K, Natesan A, Premkumar P, Ramani P, et al. Glandular odontogenic cyst: Report of two cases and review of literature. Head Neck Pathol. 2009;3:153–8. doi: 10.1007/s12105-009-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dua K, Wadhwa R, Singhvi G, Rapalli V, Shukla SD, Shastri MD, et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev Res. 2019;80:714–30. doi: 10.1002/ddr.21571. [DOI] [PubMed] [Google Scholar]
- 14.Abdul Wahab PU, Senthil Nathan P, Madhulaxmi M, Muthusekhar MR, Loong SC, Abhinav RP. Risk factors for post-operative infection following single piece osteotomy. J Maxillofac Oral Surg. 2017;16:328–32. doi: 10.1007/s12663-016-0983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thanikodi S, Singaravelu D, Kumar Devarajan C, Venkatraman V, Rathinavelu V. Teaching learning optimization and neural network for the effective prediction of heat transfer rates in tube heat exchangers. Therm Sci. 2020;24(1 Part B):575–81. [Google Scholar]
- 16.Subramaniam N, Muthukrishnan A. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: A prospective analysis in a tertiary care dental hospital. J Investig Clin Dent. 2019;10:e12454. doi: 10.1111/jicd.12454. [DOI] [PubMed] [Google Scholar]
- 17.Kumar SP, Girija AS, Priyadharsini JV. Targeting NM23-H1-mediated inhibition of tumour metastasis in viral hepatitis with bioactive compounds from Ganoderma lucidum: A computational study. Indian J Pharm Sci. 2020;82:300–5. [Google Scholar]
- 18.Manickam A, Devarasan E, Manogaran G, Priyan MK, Varatharajan R, Hsu CH, et al. Score level based latent fingerprint enhancement and matching using SIFT feature. Multimed Tools Appl. 2019;78:3065–85. [Google Scholar]
- 19.Ravindiran M, Praveenkumar C. Status review and the future prospects of CZTS based solar cell –A novel approach on the device structure and material modeling for CZTS based photovoltaic device. Renew Sustain Energy Rev. 2018;94:317–29. [Google Scholar]
- 20.Vadivel JK, Govindarajan M, Somasundaram E, Muthukrishnan A. Mast cell expression in oral lichen planus: A systematic review. J Investig Clin Dent. 2019;10:e12457. doi: 10.1111/jicd.12457. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Karunakaran T, Veeraraghavan VP, Mohan SK, Li S. Sesame inhibits cell proliferation and induces apoptosis through inhibition of STAT-3 translocation in thyroid cancer cell lines (FTC-133) Biotechnol Bioprocess Eng. 2019;24:646–52. [Google Scholar]
- 22.Mathivadani V, Smiline AS, Priyadharsini JV. Targeting epstein-barr virus nuclear antigen 1 (EBNA-1) with murraya koengii bio-compounds: An in-silico approach. Acta Virol. 2020;64:93–9. doi: 10.4149/av_2020_111. [DOI] [PubMed] [Google Scholar]
- 23.Happy A, Soumya M, Venkat Kumar S, Rajeshkumar S, Sheba RD, Lakshmi T, et al. Phyto-assisted synthesis of zinc oxide nanoparticles using cassia alata and its antibacterial activity against Escherichia coli. Biochem Biophys Rep. 2019;17:208–11. doi: 10.1016/j.bbrep.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prathibha KM, Johnson P, Ganesh M, Subhashini AS. Evaluation of salivary profile among adult type 2 diabetes mellitus patients in South India. J Clin Diagn Res. 2013;7:1592–5. doi: 10.7860/JCDR/2013/5749.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paramasivam A, Vijayashree Priyadharsini J. Novel insights into m6A modification in circular RNA and implications for immunity. Cell Mol Immunol. 2020;17:668–9. doi: 10.1038/s41423-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busse B. Wound Management in Urgent Care. Germany: Springer; 2016. Selecting materials for wound closure; pp. 19–23. [Google Scholar]
- 27.Asher M. Manual on Vaginal Surgery. New Delhi: Jaypee Brothers Medical Publishers; 2013. Suture materials, needles and knots; p. 8. [Google Scholar]
- 28.Hermann JB. Tensile strength and knot security of surgical suture materials. Plast Reconstr Surg. 1972;49:110. [PubMed] [Google Scholar]
- 29.Pons-Vicente O, López-Jiménez L, Sánchez-Garcés MA, Sala-Pérez S, Gay-Escoda C. A comparative study between two different suture materials in oral implantology. Clin Oral Implants Res. 2011;22:282–8. doi: 10.1111/j.1600-0501.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 30.Karabulut R, Sonmez K, Turkyilmaz Z, Bagbanci B, Basaklar AC, Kale N. An in vitro and in vivo evaluation of tensile strength and durability of seven suture materials in various ph and different conditions: An experimental study in rats. Indian J Surg. 2010;72:386–90. doi: 10.1007/s12262-010-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasanthan A, Satheesh K, Hoopes W, Lucaci P, Williams K, Rapley J. Comparing suture strengths for clinical applications: A novel in vitro study. J Periodontol. 2009;80:618–24. doi: 10.1902/jop.2009.080490. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson RE, Jr, Schuler K, Thornton BP, Vasconez HC, Rinker B. The effect of saliva and oral intake on the tensile properties of sutures: An experimental study. Ann Plast Surg. 2007;58:268–72. doi: 10.1097/01.sap.0000245071.98517.8c. [DOI] [PubMed] [Google Scholar]
- 33.Chu CC. A comparison of the effect of pH on the biodegradation of two synthetic absorbable sutures. Ann Surg. 1982;195:55–9. doi: 10.1097/00000658-198201001-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]