Abstract
The enriched nutritional and functional properties of inulinase with wide attention are considered commercial/industrial food enzymes. It can be produced by many microorganisms such as yeasts, fungi, and bacteria. Nocardiopsis is a genus under Actinomycetes, which has biotechnologically important microorganisms. This study aims to isolate and identify marine Actinomycetes Nocardiopsis species and to evaluate the antibacterial potential of the inulinase enzyme obtained from it. Marine actinobacteria (Nocardiopsis sp.) were isolated from sediment samples on YM agar. The isolate was identified by biochemical analysis of cell walls (amino acid and sugar). Enzyme screening assay was performed with temperature and pH influence in the production inulinase enzyme production. Antibacterial activity and minimal inhibitory activity of inulinase enzyme were performed with Staphylococcus, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrobial testing revealed that with higher concentrations of inulinase enzyme, the zone of inhibition of bacterial growth increased, and the minimum inhibitory concentration of inulinase enzyme that prevented the growth of bacteria was close to the standard tetracycline. Inulinase enzyme obtained from Nocardiopsis species shows good antibacterial activity against Staphylococcus aureus, K. pneumoniae, and P. aeruginosa in comparison to the standard, tetracycline.
Keywords: Actinomycetes, antibacterial, inulinase, Nocardiopsis, novel enzyme
INTRODUCTION
The inulinase enzyme is considered commercially important food enzyme with wide attention. Its systemic name is 1-beta-D-fructan fructanohydrolase. Inulin is a naturally occurring water-soluble polysaccharide and it is one of the most frequently used carbohydrates like starch.[1] It is a nondigestible carbohydrate called fructans and has wide applications in the food industry as dietary fiber and fat and sugar replacer. Inulin consumption enhances the absorption of minerals and prevents gut problems like constipation. It also stimulates the immune system.[2] Inulinases target the β-2,1glycosidic linkage of inulin and break the inulin by hydrolysis to produce fructose, inulo-oligosaccharides, and glucose.[3] Inulinase can be of two types; endoinulinase, which hydrolyzed lengthy chains into tiny and exoinulinase will obtain the fructose through the hydrolysis.[4] It has various industrial applications in the production of protein, mannitol, sorbitol, citric acid, lactic acid, cell oil, and biofuel. Furthermore, it improves digestion, lowering cholesterol, bone health, blood sugar control, and immunomodulation activity.[1,5] Nocardiopsis is a genus under the order Actinomycetes. The organisms which are under the order Actinomycetes have huge importance in agriculture through the decomposition of organic matter in the soil system.[6] Nocardiopsis species are Gram-positive, aerobic, catalase positive, and nonacid-fast organisms will form both aerial and substrate mycelium with a long spore chain.[7,8,9,10] Most of the species are halophilic or halotolerant which is associated with the production of various metabolites.[11] Especially the species of Nocardiopsis are economically resourceful and biotechnologically significant compounds which have various therapeutic activities. In addition, diverse enzymes, such as chitinase, β-glucanases, xylanase, amylase, cellulose, protease, and inulinase, have been reported by the species of Nocardiopsis[12] with potential agriculture and food application. Natural products remain to be the most propitious source of antibiotics.[13] Previously, our team has had extensive experience in working on various research projects across multiple disciplines involving the use of various compounds obtained from natural products and evaluating their biological importance.[14,15,16,17,18,19,20,21,22,23] In industries, microbes like actinobacteria have been used for high yield of enzyme inulinase due to easy growth and high yield in a short time.[24,25,26,27] Furthermore, marine actinobacteria have extensive bioactive applications such as antibacterial, antioxidant, anticancer, antifungal, antiviral, insecticidal, and antidiabetics has drawn global attention in the past several decades.[28] In the phylum of actinobacteria, the species of Streptomyces, Nocardiopsis, Nocardia, Marinospora, Rhodococcus, Micromonospora, and Salinospora are well known to contribute to the maximum level of potentially bioactive compounds in the commercial.[29] Further, our team has extensive knowledge and research experience that has translated into high-quality publications (30–49). This study aims to isolate and identify marine Actinomycetes Nocardiopsis species and to evaluate the antibacterial potential of the inulinase enzyme obtained from it against a few important bacterial strains.
MATERIALS AND METHODS
Sample collection and preparation
Samples were collected from the Gulf of Mannar biosphere reserve, Tamil Nadu. Samples were collected by van veen grab in the offshore region, and the collected sediment materials were processed as per the previous reference.[8]
Isolation of actinobacteria
Marine actinobacteria were isolated on Kuster's agar medium prepared with seawater. The medium was supplemented with antibiotics (10 μg/ml of cycloheximide and nalidixic acid) to inhibit the contamination of bacteria and fungus. A serially diluted sediment sample was inoculated on KUA medium and incubated for a week at ambient temperature. Well-grown distinct pure cultures were used for further studies.
Identification of marine actinobacteria
Identification of marine actinobacteria was made by certain characteristic features of bacteria. These include aerial mass color (white), spore chain morphology (long chain), and the absence of melanoid, reverse side, and soluble pigment.
Chemotaxonomical characteristics
Whole-cell amino acid analysis and sugar pattern were analyzed through the hydrolysis of strain with the respective procedure. The actinobacterial isolate was well cultivated and harvested through the centrifuge and rinsed twice with sterile distilled water then part of the samples was hydrolyzed with 6N Hcl for amino acid analysis, remaining samples were hydrolyzed with 1N H2 SO4 for sugar pattern. The hydrolyzed samples were placed on the TLC plate to notice the presence of amino acid and sugars in the cell wall of the strain.
Carbon source utilization
The carbon source utilization of each strain was analyzed with different carbon sources with the proper method given by International Streptomyces Project (Shirling and Gottlieb, 1966).
Inulinolytic potential
The isolate was spotted on Czapek-Dox agar supplemented with 1% of inulin and incubated for 24 h in room temperature. After growth, the plates were treated with lugol's iodine solution for 3 to 5 min and rinsed with sterile distilled water and incubated for an hour at room temperature. The colorless clear zone around the isolate indicated the hydrolysis of inulin.
Inulinase enzyme assay
Well-grown culture was centrifuged, and the aqueous phase has been considered the source of enzyme. The substrate inulin was prepared (2%) with phosphate buffer (pH 7). An equal volume of enzyme source (1ml) and substrate (1ml) was added and kept at 50°C for half an hour. Then, the reaction was stopped by the addition of 2 ml of DNS solution, then allowed it to boil for 15 min. After the color developing from red to brown, the absorbance was measured at 540 nm.
Impact of pH and temperature
The potential strain was inoculated in Czapek-Dox broth at a pH range from 6 to 8. Similarly, the culture medium was prepared and incubated at various temperatures ranging from 25°C, 30°C, 35°C, 40°C, and 50°C. After incubation, the enzyme production was measured by the previous method, and the cell growth was measured at 600 nm.
Antibacterial and minimal inhibitory concentration
Antibacterial potential of the Inulinase was done by following the method of disc diffusion. The 5mm diameter discs were used for the assay and different concentration such as 50, 100, 150, 200 and 250 μg/ml of Inulinase samples with oral antibiotic tetracycline and DMSO as a negative control. Further, the plates were kept in incubator for 1 day and maintained the room temperature. The zone of inhibition was considered as better results and measured the zone and calculated the activities. The Minimal Inhibitory Concentration (MIC) of the Inulinase was analyzed using different concentrations such as 50, 100, 150, 200 and 250 μg/m of melanin with tetracycline (Standard), DMSO was negative control. The bacterial suspension (in test tubes) was kept in incubator for 1 day in room temperature and optical density was analyzed.
RESULTS
Assessment of the antibacterial potential of inulinase
The different concentrations of inulinase were loaded in media containing Staphylococcus, Klebsiella pneumonia, and Pseudomonas aeruginosa; the plates were incubated for 24 h at room temperature. After the incubation time, the zone of inhibition of bacterial growth was measured.
In this study, inulinase-producing marine actinobacteria Nocardiopsis was isolated from sediment sample. The isolate was identified through cell wall amino acid, sugar pattern, and micro-morphology.
The aerial mass color and spore chain morphology are recorded in Figure 1. Cell wall sugars and amino acids released on hydrolysis are given in Table 1. Assimilation of carbon source for metabolic activity of actinobacteria is given in Table 2. The enzyme production was confirmed in various pH and temperatures to optimize the enzyme production up to 147 IU/mg. The temperature and pH have an impact on enzyme production on the Czapek-Dox medium [Tables 3 and 4]. Enhancing the temperature improved the production of enzyme production with lower stability. Finally, antibacterial testing was done by incorporating the enzyme in different concentrations into the media plates containing each bacteria, and the zone of inhibition was measured. Table 5 reveals that, with increasing concentrations of Inulinase enzyme, the zone of inhibition of bacterial growth increased for all the bacteria, providing a higher range of inhibition in Klebsiella pneumoniae and P. aeruginosa when compared to Staphylococcus sp and minimum inhibitory concentration of Inulinase enzyme that prevented the growth of the bacterial strains was very close to that of the standard tetracycline [Table 6].
Figure 1.
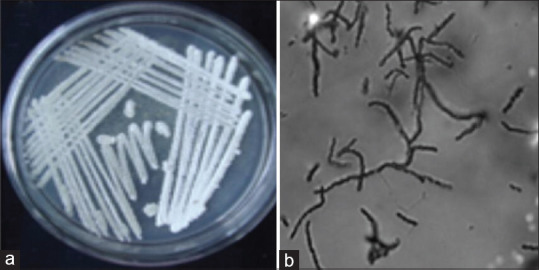
Aerial mass colour (white) and long spore chain morphology obtained for Nocardiopsis sp
Table 1.
Cell wall sugars and amino acids released upon hydrolysis
| Cell wall amino acids LL-DAP |
MesoDAP | Glycine | Cell wall sugar Arabinose |
Galactose | Cell wall type | Index |
|---|---|---|---|---|---|---|
| - | + | - | - | ± | I | Nocardiopsis |
LL-DAP: LL-diaminopimelic acid
Table 2.
Assimilation of carbon source
| Color of aerial mycelium | White |
|---|---|
| Melanoid pigment | - |
| Reverse side pigment | - |
| Soluble pigment | - |
|
| |
| Spore chain | Long chain |
|
| |
| Assimilation of carbon source | |
|
| |
| Arabinose | + |
| Xylose | + |
| Inositol | + |
| Mannitol | - |
| Fructose | + |
| Rhamnose | - |
| Sucrose | + |
| Raffinose | - |
Table 3.
Effect of temperature on enzyme production
| Temperature | IU/ml±SE |
|---|---|
| 25 | 9.27±2.1 |
| 30 | 12.08±2.9 |
| 35 | 14.25±2.4 |
| 40 | 10.36±2.7 |
| 50 | 8.21±2.2 |
SE: Standard error
Table 4.
Effect of pH on enzyme production
| pH | IU/ml±SE |
|---|---|
| 6 | 8.24±2.4 |
| 6.5 | 11.35±1.9 |
| 7 | 16.38±2.8 |
| 7.5 | 12.07±2.5 |
| 8 | 10.25±2.2 |
SE: Standard error
Table 5.
Zone of inhibition exhibited by different concentrations of inulinase
| Concentration (µg/ml) | Staphylococcus | Klebsiella pneumoniae | Pseudomonas aeruginosa |
|---|---|---|---|
| 50 | 3±1.8 | 5±1.7 | 4±1.6 |
| 100 | 5±2.1 | 11±1.9 | 9±1.2 |
| 150 | 9±1.9 | 16±1.6 | 13±1.9 |
| 200 | 13±2.4 | 20±1.7 | 22±1.8 |
| 250 | 18±2.1 | 23±1.2 | 26±1.7 |
| 300 | 25±2.4 | 27±1.9 | 31±1.4 |
Table 6.
Minimum inhibitory concentration of inulinase that prevents the growth of bacteria in relation to the positive control
| 0 | 10 | 20 | 30 | 40 | 50 | MIC (µg/ml) | |
|---|---|---|---|---|---|---|---|
| Staphylococcus | + | + | + | + | − | + | 40 |
| Positive control | + | + | − | − | − | − | 20 |
| Klebsiella pneumoniae | + | + | + | − | − | − | 30 |
| Positive control | + | + | − | − | − | − | 20 |
| Pseudomonas aeruginosa | + | + | + | − | − | − | 30 |
| Positive control | + | + | − | − | − | − | 20 |
MIC: Minimum inhibitory concentration
DISCUSSION
A similar study done by Aziz et al. purified the inulinase enzyme from K. pneumoniae and assessed its antibacterial potential. They found that inulinase obtained from K. pneumoniae exhibited broad-spectrum bioactivity against microbial pathogens and also observed that inulinase had increased the activity of ceftazidime against bacteria when a combination between this enzyme and the antibiotic was used.[30] Muslim et al., in their study, purified inulinase enzyme from Staphylococcus aureus S3, and their findings, showed that anti-staphylococcal activity of ceftazidime antibiotic was increased in the presence of inulinase enzyme, and the inulinase may serve as a useful adjuvant for the treatment of Staphylococcal infections in combination with this antibiotic.[31] Vimal et al., in their study, assessed the antimicrobial potential of different extracts and compounds derived from Nocardiopsis species and found that the petroleum ether extract exhibited antibacterial activity against many Gram-positive as well as Gram-negative bacteria, ethyl acetate extract showed good antifungal activity, chloroform extract was very effective against yeasts, and the compounds present within these extract showed broad-spectrum antimicrobial activity.[32] Siddharth and Rai, in their study, isolated 4-bromophenol and Bis (2-Ethylhexyl) phthalate from genus Nocardiopsis, and derived their antibacterial and antioxidant activities and concluded that marine actinobacteria are promising sources of therapeutically important bioactive metabolites.[33]
Bennur et al., in their study, assessed the biological properties of secondary metabolites of Nocardiopsis, namely Nocardiopyrone (A and B), and found that Nocardiopyrone A was active against P. aeruginosa, Enterobacter aerogenes, and Escherichia coli. Nocardiopyrone B was active against P. aeruginosa, E. aerogenes, E. coli, S. aureus, and Candida albicans. Many studies have found the antibacterial and antimicrobial potential of different strains of Nocardiopsis such as Nocardiopsis sp. TFS65-07,[34] N. dassonvillei (MAD08).[35,36] Many studies have also reported that Nocardiopsis species produce polyketide-based structures and peptides that display anticancer and antitumor activities.[37]
CONCLUSION
Inulinase enzyme obtained from Nocardiopsis species shows good antibacterial activity against S. aureus, K. pneumoniae, and P. aeruginosa in relation to the standard tetracycline. Further studies could be done to evaluate the antimicrobial potential of inulinase against other microorganisms.
Financial support and sponsorship
Golden sand property developers, Chennai, Tamil Nadu.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Saveetha Dental College and Saveetha Institute of Medical and Technical Sciences for their kind support to utilize the facilities for the study.
REFERENCES
- 1.Das D, Ramananda BM, Selvaraj R. Review of inulinase production using solid-state fermentation. Ann Microbiol. 2019;69:201–9. [Google Scholar]
- 2.Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR, et al. Inulin: Properties, health benefits and food applications. Carbohydr Polym. 2016;147:444–54. doi: 10.1016/j.carbpol.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Neagu C, Bahrim C. Inulinases a versatile tool for biotechnology. Innovative Romanian Food Biotechnol. 2011;9:1–11. [Google Scholar]
- 4.Singh RS, Singh RP. Production of fructooligosaccharides from inulin by endoinulinases and their prebiotic potential. Food Technol Biotechnol. 2010;48:435. [Google Scholar]
- 5.Mohan A, Flora B, Girdhar M. Inulinase: An important microbial enzyme in food industry. In: Singh J, Sharma D, Kumar G, Sharma NR, editors. Microbial Bioprospecting for Sustainable Development. Singapore: Springer Singapore; 2018. pp. 237–48. [Google Scholar]
- 6.Bhatti AA, Haq S, Bhat RA. Actinomycetes benefaction role in soil and plant health. Microb Pathog. 2017;111:458–67. doi: 10.1016/j.micpath.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Kroppenstedt RM, Evtushenko LI. The family Nocardiopsaceae. In: Martin Dworkin, Stanley Falkow, Eugene Rosenberg, Karl-Heinz Schleifer, Erko Stackebrandt., editors. The Prokaryotes: A Handbook on the Biology of Bacteria. Vol. 3. Singapore: Springer; 2006. pp. 754–95. [Google Scholar]
- 8.Kamala K, Sivaperumal P, Gobalakrishnan R, Swarnakumar NS, Rajaram R. Isolation and characterization of biologically active alkaloids from marine actinobacteria Nocardiopsis sp. NCS1. Biocatal Agric Biotechnol. 2015;4:63–9. [Google Scholar]
- 9.Sivaperumal P, Kamala K, Rajaram R. Biosorption of long half-life radionuclide of strontium ion (Sr) by marine Actinobacterium Nocardiopsissp. 13H. Geomicrobiol J. 2018;35:300–10. [Google Scholar]
- 10.Sivaperumal P, Kamala K, Rajaram R. Adsorption of cesium ion by marine actinobacterium Nocardiopsis sp. 13H and their extracellular polymeric substances (EPS) role in bioremediation. Environ Sci Pollut Res Int. 2018;25:4254–67. doi: 10.1007/s11356-017-0818-0. [DOI] [PubMed] [Google Scholar]
- 11.Bennur T, Kumar AR, Zinjarde S, Javdekar V. Nocardiopsis species: Incidence, ecological roles and adaptations. Microbiol Res. 2015;174:33–47. doi: 10.1016/j.micres.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Elleuche S, Schröder C, Sahm K, Antranikian G. Extremozymes –Biocatalysts with unique properties from extremophilic microorganisms. Curr Opin Biotechnol. 2014;29:116–23. doi: 10.1016/j.copbio.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Bull AT, Stach JE. Marine actinobacteria: New opportunities for natural product search and discovery. Trends Microbiol. 2007;15:491–9. doi: 10.1016/j.tim.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Chandrasekaran R, Gnanasekar S, Seetharaman P, Keppanan R, Arockiaswamy W, Sivaperumal S. Formulation of Carica papaya latex-functionalized silver nanoparticles for its improved antibacterial and anticancer applications. Mol Liq. 2016;219:232–8. [Google Scholar]
- 15.Shree MK, Arivarasu L, Rajeshkumar S. Cytotoxicity and antimicrobial activity of chromium picolinate mediated zinc oxide nanoparticle. [Last accessed on 2021 May 21];Pharm Res Int. 2020 32:28–32. https://journaljpri.com/index.php/JPRI/article/view/30726. [Google Scholar]
- 16.Paul RP, Roy A, Maajida Aafreen M, Shanmugam R. Antibacterial activity of white pepper oleoresin mediated silver nanoparticles against oral pathogens. J Evol Med Dent Sci. 2020;9:2352–6. [Google Scholar]
- 17.Pranati T, Anitha R, Rajeshkumar S, Lakshmi T. Preparation of silver nanoparticles using nutmeg oleoresin and its antimicrobial activity against oral pathogens. Res J Pharm Technol. 2019;12:2799–803. [Google Scholar]
- 18.Vignesh S, Anitha R, Rajesh Kumar S, Lakshmi T. Evaluation of the Antimicrobial activity of Cumin oil mediated silver nanoparticles on Oral microbes. Res J Pharm Technol. 2019;12:3709. [Google Scholar]
- 19.Sunar S, Rajeshkumar S, Roy A, Lakshmi T. Preparation of herbal formulation and it's application on nanoparticles synthesis and antibacterial activity. Int J Res Pharm Sci. 2019;10:2177–2180. [Google Scholar]
- 20.Labh AK, Rajeshkumar S, Roy A, Santhoshkumar J, Lakshmi T. Herbal formulation mediated synthesis of silver nanoparticles and its antifungal activity against Candida albicans. Indian J Public Health Res Dev. 2019;10:3454. [Google Scholar]
- 21.Kandhan TS, Roy A, Lakshmi T, Rajeshkumar S. Green synthesis of Rosemary oleoresin mediated silver nanoparticles and its effect on Oral pathogens. Res J Pharm Technol. 2019;12:5379. [Google Scholar]
- 22.Pranati T, Roy A, Rajeshkumar S, Lakshmi T. Antibacterial activity of tamarind fruit pulp mediated selenium nanoparticles isolated from wounds. Plant Cell Biotechnology and Molecular Biology. 2020;21:69–77. [Google Scholar]
- 23.Pawar R, Mohandass C, Sivaperumal E, Sabu E, Rajasabapathy R, Jagtap T. Epiphytic marine pigmented bacteria: A prospective source of natural antioxidants. Braz J Microbiol. 2015;46:29–39. doi: 10.1590/S1517-838246120130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill PK, Manhas RK, Singh P. Hydrolysis of inulin by immobilized thermostable extracellular exoinulinase from Aspergillus fumigatus. J Food Eng. 2006;76:369–75. [Google Scholar]
- 25.Gao L, Chi Z, Sheng J, Ni X, Wang L. Single-cell protein production from Jerusalem artichoke extract by a recently isolated marine yeast Cryptococcus aureus G7a and its nutritive analysis. Appl Microbiol Biotechnol. 2007;77:825–32. doi: 10.1007/s00253-007-1210-7. [DOI] [PubMed] [Google Scholar]
- 26.Gao L, Chi Z, Sheng J, Wang L, Li J, Gong F. Inulinase-producing Marine Yeasts: Evaluation of their diversity and inulin hydrolysis by their crude enzymes. Microb Ecol. 2007;54:722–9. doi: 10.1007/s00248-007-9231-4. [DOI] [PubMed] [Google Scholar]
- 27.Chi Z, Chi Z, Zhang T, Liu G, Yue L. Inulinase-expressing microorganisms and applications of inulinases. Appl Microbiol Biotechnol. 2009;82:211–20. doi: 10.1007/s00253-008-1827-1. [DOI] [PubMed] [Google Scholar]
- 28.Manivasagan P, Kang KH, Sivakumar K, Li-Chan EC, Oh HM, Kim SK. Marine actinobacteria: An important source of bioactive natural products. Environ Toxicol Pharmacol. 2014;38:172–88. doi: 10.1016/j.etap.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Manivasagan P, Venkatesan J, Sivakumar K, Kim SK. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol Res. 2014;169:262–78. doi: 10.1016/j.micres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Aziz SN, Aziz RN, Falih MS, Al-Sallami KJ, Ghadi HH, Ali AM, et al. Purification of inulinase from Klebsiella pneumoniae and study the antibacterial effect of combination of inulinase and ceftazidime. Glob J Public Health Med. 2019;1:70–6. [Google Scholar]
- 31.Muslim SN, Ali ANM, Hussein SN, Salman IM. Improving the antibacterial activity of ceftazidime by inulinase purified from Staphylococcus aureus. Journal of Biology, Agriculture and Healthcare. 2015;5:84–89. [Google Scholar]
- 32.Vimal V, Rajan BM, Kannabiran K. Antimicrobial activity of marine actinomycete Nocardiopsis sp. VITSVK 5 (FJ973467) Asian J Med Sci. 2009;1(2):57–63. [Google Scholar]
- 33.Siddharth S, Rai RV. Isolation and characterization of bioactive compounds with antibacterial, antioxidant and enzyme inhibitory activities from marine-derived rare actinobacteria Nocardiopsis sp. SCA21. Microb Pathog. 2019;137:103775. doi: 10.1016/j.micpath.2019.103775. [DOI] [PubMed] [Google Scholar]
- 34.Engelhardt K, Degnes KF, Kemmler M, Bredholt H, Fjaervik E, Klinkenberg G, et al. Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl Environ Microbiol. 2010;76:4969–76. doi: 10.1128/AEM.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvin J, Shanmughapriya S, Gandhimathi R, Seghal Kiran G, Rajeetha Ravji T, Natarajaseenivasan K, et al. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl Microbiol Biotechnol. 2009;83:435–45. doi: 10.1007/s00253-009-1878-y. [DOI] [PubMed] [Google Scholar]
- 36.Dusane DH, Damare SR, Nancharaiah YV, Ramaiah N, Venugopalan VP, Kumar AR, et al. Disruption of microbial biofilms by an extracellular protein isolated from epibiotic tropical marine strain of Bacillus licheniformis. PLoS One. 2013;8:e64501. doi: 10.1371/journal.pone.0064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsujibo H, Sakamoto T, Miyamoto K, Kusano G, Ogura M, Hasegawa T, et al. Isolation of cytotoxic substance, kalafungin from an alkalophilic actinomycete Nocardiopsis dassonvillei subsp. prasina. Chem Pharm Bull (Tokyo) 1990;38:2299–300. doi: 10.1248/cpb.38.2299. [DOI] [PubMed] [Google Scholar]


