Abstract
Mycobacterium tuberculosis grows within the phagocytic vacuoles of macrophages, where it encounters a moderately acidic and possibly nutrient-restricted environment. Other mycobacterial species encounter acidic conditions in soil and aquatic environments. We have evaluated the influence of pH and divalent cation levels on the growth of M. tuberculosis and seven other mycobacterial species. In a defined medium, the growth of M. tuberculosis was very restricted by acidic pH. Higher levels of Mg2+ were required for growth of M. tuberculosis in mildly acidic media (pH 6.0 to 6.5) compared to pH 7.0 medium. The divalent cations Ca2+, Zn2+, or Mn2+ could not replace Mg2+ during growth at pH 6.25, but Ca2+ could at least partially substitute for Mg2+ during growth at pH 7.0. Among eight species of mycobacteria tested, there was a diversity of growth rates in media with acidic pH and low Mg2+ levels. M. tuberculosis was the most restricted in growth at pH 6.0, and all of this growth required elevated levels of Mg2+. M. kansasii and M. smegmatis also grew very poorly in acidic media with limiting Mg2+. M. fortuitum, M. marinum, M. scrofulaceum, M. avium, and M. chelonae grew at pH 6.0 in an unrestricted manner. These results demonstrate that M. tuberculosis is unique among the mycobacteria in its extreme sensitivity to acid and indicate that M. tuberculosis must acquire sufficient Mg2+ in order to grow in a mildly acidic environment such as within the phagosome of macrophages.
M. tuberculosis grows within the phagocytic vacuoles of macrophages (2), where it is exposed to a relatively hostile environment. The interior of the phagosome may be limited in nutrients (11, 12) as well as acidic in pH. Although M. tuberculosis limits acidification of phagosomes by exclusion of the vesicular proton ATPase (19, 20), the vacuole is moderately acidic with the pH measured at values between 6.1 and 6.5 (14, 17, 19, 20).
An acidic environment is one of the most stressful conditions encountered by living cells. In order for enzymes and proteins to function normally, bacteria must maintain an internal pH close to pH 7 (4). The importance of maintaining a constant internal pH is underscored by the multiple systems utilized by bacteria during growth under acidic conditions. These include transport systems which exchange protons for cations, systems which transport protons out of cells in association with ATP hydrolysis, and the production of cytoplasmic macromolecules which function as internal buffers (5, 10). Although mycobacterial species have been reported to grow under acidic conditions in a complex media (7, 18), their response to pH stress in a defined medium has not been examined. The stress induced by acidic pH is known to be more severe in a nutrient-limited environment (10). In addition, nothing is known about specific factors which may facilitate the growth of mycobacteria under acidic conditions.
During our study of M. tuberculosis genes that are required for growth within macrophages, we noticed an association between bacterial growth in acidic medium and the requirement for Mg2+. Our experiments demonstrated that an M. tuberculosis mutant in the mgtC gene was attenuated for growth in media with reduced Mg2+ levels but only when the pH of the media was mildly acidic (6). The mgtC mutant of M. tuberculosis was also attenuated for growth in human macrophages and for growth in vivo in the lungs and spleens of mice. This suggested that during infection of the host M. tuberculosis grows in a moderately acidic environment with limiting levels of Mg2+. We therefore wanted to study the relationship between the growth of mycobacteria, including virulent M. tuberculosis, in acidic media and relate this to the requirement for Mg2+ or other divalent cations. In this report we demonstrate that M. tuberculosis is very restricted for growth by acid pH by using a defined medium and that any growth in moderately acidic media requires elevated levels of Mg2+. The requirement for Mg2+ during growth in acidic medium could not be replaced with the divalent cations Ca2+, Mn2+, or Zn2+. We also observed a great diversity among eight mycobacterial species in their ability to grow in media with acidic pH and low levels of Mg2+, with M. tuberculosis being among the most restricted for growth under these conditions.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study include M. tuberculosis Erdman (ATCC 35801), M. smegmatis (ATCC 607), M. chelonae subsp. chelonae (ATCC 35752), M. fortuitum subsp. peregrinum (ATCC 14467), M. kansasii (clinical isolate), M. scrofulaceum (clinical isolate), M. marinum (ATCC 927), and M. avium (clinical isolate). The bacteria were routinely grown in Middlebrook 7H9 supplemented with ADC and 0.05% Tween 80 (15).
Growth assays under variable pH levels and divalent cation concentrations.
Growth of mycobacteria under conditions of variable pH and divalent cation levels was studied using a defined medium (Sauton medium) that was modified so that the pH and divalent cation concentrations could be varied. Sauton medium (1) was modified by omitting the magnesium sulfate, which was the sole Mg2+ source, and replacing the sulfate with 28 mM potassium sulfate. To limit clumping during growth, 0.05% Tween 80 was added along with 10% ADC (15). Media were buffered with either 100 mM morpholineethanesulfonic acid (MES) or 100 mM morpholinepropanesulfonic acid (MOPS) (16), adjusted to the appropriate pH, filter sterilized, and then supplemented with various levels of magnesium chloride, calcium chloride, manganese chloride, or zinc chloride. For each assay, bacteria were grown to an optical density at 580 nm (OD580) of 0.3 to 0.5 in Middlebrook 7H9, washed once in saline, and diluted 1/4,000 in 10 ml of the appropriate modified Sauton medium. Cultures were incubated at 37°C with 5% CO2 for 3 to 4 weeks. The assays were terminated before bacterial clumping was evident. Growth was monitored by measuring the OD580. Data are expressed as the mean and standard error of the mean from triplicate flasks.
RESULTS
The growth of M. tuberculosis is very restricted by acidic pH.
A modified Sauton medium was used to evaluate the influence of pH and divalent cation concentration on the growth of mycobacteria. The Sauton medium was chosen because it is a defined medium in which divalent cation levels can be readily manipulated. To modify the Sauton medium, the sole divalent cation source (magnesium sulfate) was omitted and was replaced by potassium sulfate (as the sulfate source). Various levels of magnesium chloride, calcium chloride, zinc chloride, or manganese chloride were then added. In addition, all media were buffered with either 100 mM MES or 100 mM MOPS (16) to prevent the pH from changing during the course of the experiment.
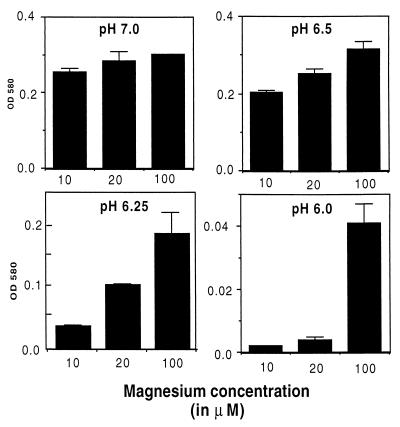
The growth of M. tuberculosis in media buffered to pH values between pH 7.0 and 6.0 were examined. This pH range was chosen because it falls within the pH range measured in macrophage phagosomes which contain M. tuberculosis (14, 17, 19, 20). The extent of growth at day 24 is shown in Fig. 1. In media with moderate (100 μM) levels of Mg2+, M. tuberculosis grew well at pH 7.0 and 6.5. As the pH dropped below 6.5, the amount of bacterial growth declined. At pH 6.25, there was a moderate but significant decrease in growth. At pH 6.0, growth of M. tuberculosis was almost completely absent in the 24-day culture.
FIG. 1.
M. tuberculosis requires elevated levels of Mg2+ for growth in acidic media. Growth of M. tuberculosis in modified Sauton media buffered to the indicated pH and containing various levels of Mg2+ is shown. Growth was measured at day 24 by measuring the OD580. Data are expressed as the mean and standard error of the mean from three cultures.
M. tuberculosis requires elevated levels of Mg2+ to grow in acidic media.
We went on to examine the requirement for Mg2+ during growth of M. tuberculosis in both neutral and acidic media. At neutral pH (pH 7.0), the growth rate of M. tuberculosis was similar in cultures with low (10 and 20 μM) and moderate (100 μM) levels of Mg2+. In mildly acidic media (pH 6.5), M. tuberculosis grew significantly better in the cultures containing higher levels (100 μM) of Mg2+ (OD580 0.315) compared to medium with 10 μM Mg2+ (OD580 0.203). In the more acidic media the requirement for Mg2+ became more pronounced. At pH 6.25, there was fourfold more growth in the culture with 100 μM Mg2+ compared to the culture with 10 μM Mg2+. The culture with 20 μM Mg2+ produced intermediate levels of growth. At pH 6.0, the only measurable growth of M. tuberculosis was in the culture containing 100 μM Mg2+. Thus, M. tuberculosis requires higher levels of Mg2+ for growth at acidic pH compared to growth at neutral pH.
Other divalent cations cannot replace Mg2+ for growth at acid pH.
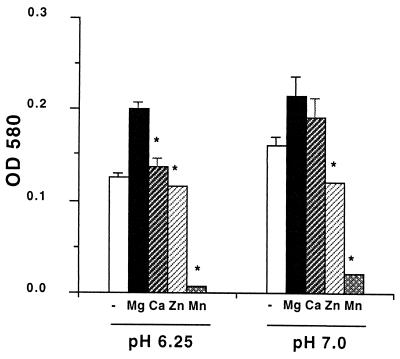
We next examined whether divalent cations other Mg2+ could facilitate the growth of M. tuberculosis in acidic media. Modified Sauton media containing 10 μM Mg2+ and buffered to either pH 6.25 or 7.0 served as the base media for these assays. We had previously determined that 10 μM Mg2+ was sufficient Mg2+ for growth of M. tuberculosis at pH 7.0 (Fig. 1). Aliquots of the base media were supplemented with 100 μM concentrations of the divalent cations Mg2+, Ca2+, Zn2+, or Mn2+. Growth of M. tuberculosis in the base medium at pH 6.25 or 7.0 was compared to growth in media supplemented with each of the divalent cations. The amount of growth attained at day 19 is presented in Fig. 2. At pH 6.25, the addition of Mg2+ (100 μM) increased the growth from an OD580 of 0.126 in the base medium to an OD580 of 0.200 with the Mg2+. The addition of Ca2+ (100 μM) to the culture produced a slight increase in growth compared to the base medium from OD580 0.126 to 0.137. However, growth in the Ca2+ culture was significantly less than the level attained with the addition of Mg2+ (P < 0.05). Zn2+ had no effect on growth, while 100 μM Mn2+ was inhibitory. At pH 7.0, the addition of Ca2+ enhanced the growth of M. tuberculosis to a level close to that with Mg2+. The addition of Zn2+ or Mn2+ was inhibitory at pH 7.0. Higher levels (1,000 μM) of Ca2+, Zn2+, or Mn2+ or lower (50 μM) levels of Zn2+ or Mn2+ were unable to replace Mg2+ during growth at pH 6.25 (data not shown). Thus, M. tuberculosis specifically requires Mg2+ for maximum growth at pH 6.25.
FIG. 2.
Other divalent cations cannot replace Mg2+ during growth of M. tuberculosis at pH 6.25. Growth of M. tuberculosis in modified Sauton media buffered to the indicated pH and supplemented with 100 μM Mg2+, Ca2+, Mn2+, or Zn2+ is shown. Growth was measured at day 19. Data are expressed as the mean and standard error of the mean from three cultures. ∗, P < 0.05 compared to growth in the Mg2+ supplemented culture.
The limited growth of M. tuberculosis at pH 6.0 is unique among eight species of mycobacteria.
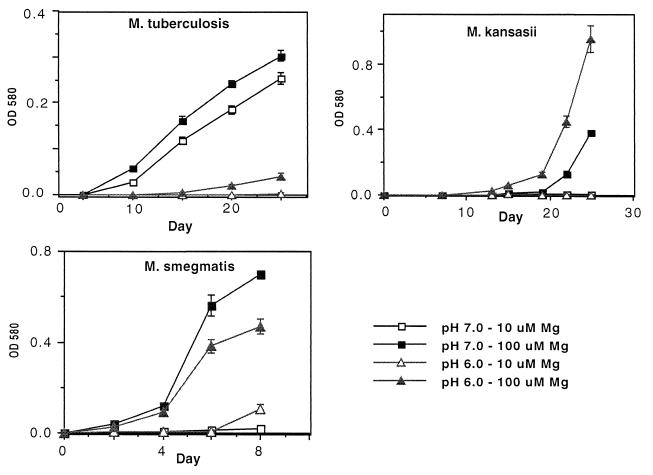
We went on to compare the growth rate of M. tuberculosis with mycobacteria that may encounter acidic conditions in soil or aquatic environments. The mycobacteria were grown in media buffered to pH 6.0 or 7.0 and containing either 10 or 100 μM Mg2+. As we had previously observed, M. tuberculosis grew very poorly at pH 6.0 and required the higher level of Mg2+ for the limited growth that was observed (Fig. 3). Among eight mycobacterial species, M. tuberculosis was the most restricted for growth at pH 6.0 (Fig. 3 and 4). M. smegmatis and M. kansasii were also limited in growth in the pH 6.0 medium with low Mg2+ (10 μM) (Fig. 3). However, M. smegmatis and M. kansasii also failed to grow in the pH 7.0 medium with low Mg2+ (10 μM), indicating that they require higher concentrations of Mg2+ for maximum growth at neutral as well as acid pH.
FIG. 3.
M. tuberculosis, M. smegmatis, and M. kansasii require higher concentrations of Mg2+ for growth in acidic medium. Growth of mycobacterial species in modified Sauton media buffered to the indicated pH and containing 10 or 100 μM Mg2+ is shown. Data are expressed as the mean and standard error of the mean from three cultures.
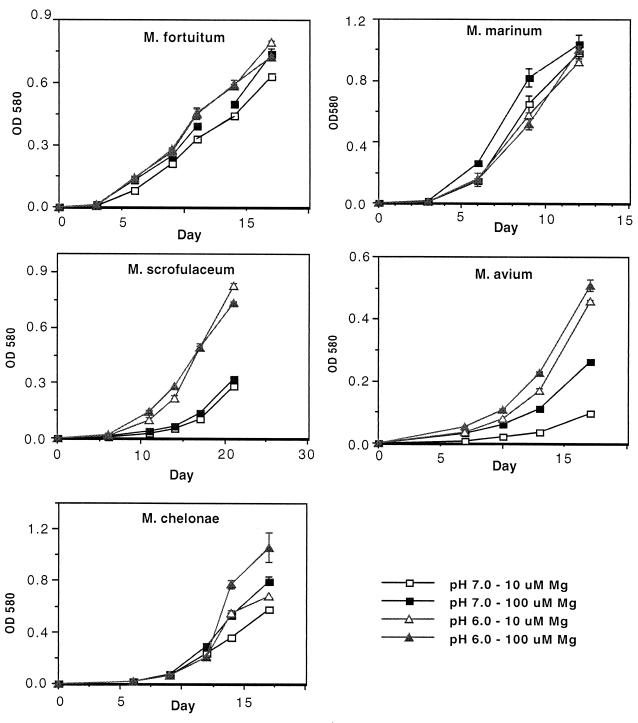
FIG. 4.
Growth curves of mycobacteria which do not require higher concentrations of Mg2+ for growth in acidic medium. Growth of mycobacterial species in modified Sauton media buffered to the indicated pH and containing 10 or 100 μM Mg2+ is shown. Data are expressed as the mean and standard error of the mean from three cultures.
The five additional mycobacterial species exhibited a diversity of growth patterns in acidic media, but none required the higher level of Mg2+ in order to grow at pH 6.0 (Fig. 4). M. marinum and M. fortuitum grew equally well at pH 7.0 and 6.0, with no restriction on growth by low Mg2+. M. avium and M. scrofulaceum grew better in the acidic medium (pH 6.0) than at pH 7.0 and were not dependent upon increased Mg2+ for growth at pH 6.0. M. chelonae grew best at pH 6.0, and this growth was partially enhanced by higher levels of Mg2+. A summary of these results is presented in Table 1. M. tuberculosis was the most limited for growth at pH 6.0, while M. tuberculosis, M. kansasii, and M. smegmatis required higher levels of Mg2+ for growth at pH 6.0.
TABLE 1.
Growth of mycobacterial species in modified Sauton media
| Species | Growth at pH 6.0 | Elevated levels of Mg2+ required for growth at pH 6.0 |
|---|---|---|
| M. tuberculosis | Poor | Yes |
| M. marinum | Good | No |
| M. kansasii | Good | Yes |
| M. avium | Good | No |
| M. smegmatis | Good | Yes |
| M. chelonae | Good | Partial |
| M. fortuitum | Good | No |
| M. scrofulaceum | Good | No |
DISCUSSION
This study has demonstrated that in an environment which contains only simple nutrients, M. tuberculosis is very restricted in growth by acid pH and requires higher concentrations of Mg2+ for growth at a pH of 6.5 or lower. Using a defined medium (Sauton) containing moderate levels of Mg2+ (100 μM), the growth of M. tuberculosis was reduced by a pH of 6.25 and was almost completely absent at pH 6.0. The sensitivity of M. tuberculosis to a moderate acidic pH correlates with observations made with M. tuberculosis-infected macrophages. Mycobacteria containing phagosomes exhibit limited fusion with late endosomes and lysosomes. This results in the exclusion of the vacuolar ATPase from the phagosome membrane which limits acidification of the vacuole (8, 19). Nevertheless, the pH of mycobacteria containing vacuoles is mildly acidic and has been measured at pH values between 6.1 and 6.5 (14, 17, 19). Thus, the pH within the phagosome is slightly above the pH level (pH 6.0) which excluded growth of M. tuberculosis in the modified Sauton medium and is within the pH range that required higher levels of Mg2+ for growth. Gomes et al. recently suggested that the sensitivity of M. tuberculosis to acidic pH contributes to the control of infection (13). Using a coinfection with Coxiella burnetii, these authors demonstrated that M. tuberculosis could not grow in acidified vacuoles. On the other hand, M. avium grew in the acidified macrophage vacuoles, which coincides with our observation that M. avium grew well in the pH 6.0 defined medium. Thus, the limited growth of M. tuberculosis in defined medium with acid pH parallels the observation that M. tuberculosis fails to grow in acidified vacuoles of macrophages.
Growth of M. tuberculosis in acidic media was even more restricted when the level of Mg2+ was low. The requirement for Mg2+ during growth in acidic media was specific for Mg2+ and could not be replaced by the divalent cations Ca2+, Mn2+, or Zn2+. The need for Mg2+ was observed in the presence of millimolar levels of K+ and Na+ which are normal components of the modified Sauton medium. K+ and Na+ transport systems are associated with an electroneutral exchange for protons which contributes to pH homeostasis in bacteria (5). We have identified one of the M. tuberculosis genes required for growth under low-Mg2+, low-pH conditions. An M. tuberculosis mutants in the mgtC gene failed to grow in mildly acidic medium (pH 6.25) with limiting levels of Mg2+ (20 μM) (6). Furthermore, the mgtC mutant was attenuated for growth in human macrophages as well as for virulence in mice. These data suggest that the virulence of M. tuberculosis is dependent upon the growth of the bacteria in a mildly acidic low-Mg2+ environment. The concentration of Mg2+ in phagosomes has not been measured directly; however, it has been estimated to be in the low micromolar range (12). Thus, a limitation in Mg2+ within the phagosome would intensify the sensitivity of M. tuberculosis to moderately acidic pH.
Among diverse mycobacterial species, there was a spectrum of tolerance to acid pH and low levels of Mg2+. M. tuberculosis was the most restricted for growth at pH 6.0. Nontuberculosis mycobacterial species, which may grow in soil or aquatic environments, were much more acid tolerant and in fact M. kansasii, M. scrofulaceum, M. avium, and M. chelonae grew better at pH 6.0 than at pH 7.0. Previous studies investigating the sensitivity of mycobacteria to acid pH have reported growth at a broad range of pH values (7, 18). These studies reported that the optimum growth of M. tuberculosis in Dubos medium was between pH 5.8 and pH 6.5, with growth observed at as low as pH 5.4 (18). The disparity in results between these studies and those presented here may be explained by the influence of the different media used since the sensitivity to extremes in pH can be masked in complex media (5). Dubos medium contains caseinate extract, while Sauton medium contains no protein extract. Our results suggest that in a environment containing simple nutrients, mycobacteria are more sensitive to acid.
The role of Mg2+ in the adaptation of M. tuberculosis to growth under moderately acidic conditions is unknown. Mg2+ may be participating directly in the maintenance of a neutral internal pH. The Mg2+-dependent proton-translocating ATP synthase is reported to play a role in acid tolerance in bacteria (3). Homologues of the ATP synthase genes are present in the M. tuberculosis genome (9). Conversely, Mg2+ may not be directly involved in maintenance of a neutral internal pH. Instead, the Mg2+ transport systems of M. tuberculosis may function less efficiently under acidic conditions, and the bacteria may therefore require more Mg2+ in the surrounding environment for adequate uptake. Alternatively, under acidic conditions, greater amounts of Mg2+ may be required to perform the normal cellular functions of Mg2+ such as stabilization of membranes and acting as cofactors for enzymes. Ongoing studies to identify the function of the MgtC protein in M. tuberculosis should provide additional information on mechanisms involving Mg2+ which facilitate growth of M. tuberculosis under acidic conditions.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI40075 and AI37005 and by undergraduate research funds from U.S. grants to A.K.
We thank E. Bearer, J. Fierer, S. Reed, and S. Sabat for mycobacterial strains used in this study.
REFERENCES
- 1.Allen B W. Mycobacteria: general culture methodology and safety considerations. In: Parish T, Stoker N, editors. Mycobacteria protocols. Totowa, N.J: Humana Press; 1998. pp. 15–30. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong J A, Hart P D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bearson S, Bearson B, Foster J W. Acid stress responses in enterobacteria. FEMS Microbiol Lett. 1997;147:173–180. doi: 10.1111/j.1574-6968.1997.tb10238.x. [DOI] [PubMed] [Google Scholar]
- 4.Booth I R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth I R. The regulation of intracellular pH in bacteria. Novartis Found Symp. 1999;122:19–28. doi: 10.1002/9780470515631.ch3. [DOI] [PubMed] [Google Scholar]
- 6.Buchmeier N, Blanc-Potard A, Ehrt S, Piddington D, Riley L, Groisman E. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 2000;35:1375–1382. doi: 10.1046/j.1365-2958.2000.01797.x. [DOI] [PubMed] [Google Scholar]
- 7.Chapman J S, Bernard J S. The tolerances of unclassified mycobacteria. I. Limits of pH tolerance. Am Rev Respir Dis. 1962;86:582–583. doi: 10.1164/arrd.1962.86.4.582. [DOI] [PubMed] [Google Scholar]
- 8.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 10.Dilworth M, Glenn A. Problems of adverse pH and bacterial strategies to combat it. Novartis Found Symp. 1999;122:4–14. doi: 10.1002/9780470515631.ch2. [DOI] [PubMed] [Google Scholar]
- 11.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-del Portillo F, Foster J W, Maguire M E, Finlay B B. Characterization of the micro-environment of Salmonella typhimurium-containing vacuoles within MDCK epithelial cells. Mol Microbiol. 1992;6:3289–3297. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 13.Gomes M S, Paul S, Moreira A, Appleberg R, Rabinovitch M, Kaplan G. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect Immun. 1999;67:3199–3206. doi: 10.1128/iai.67.7.3199-3206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackam D J, Rotstein O D, Zhang W, Gruenheid S, Gros P, Grinstein S. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J Exp Med. 1998;188:351–364. doi: 10.1084/jem.188.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs W R, Jr, Kalpana G V, Cirillo J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Frey J, Slonczewski J, Foster J. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol. 1995;177:4097–4104. doi: 10.1128/jb.177.14.4097-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh Y K, Straubinger R M. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization on phagosomal pH and phagosome-lysosome interaction. Infect Immun. 1996;64:319–325. doi: 10.1128/iai.64.1.319-325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portaels F, Pattyn S. Growth of Mycobacteria in relation to the pH of the medium. Ann Microbiol. 1982;133B:213–221. [PubMed] [Google Scholar]
- 19.Sturgill-Koszycki, Schlesinger P, Chakraborty P, Haddix P, Collins H, Fok A, Allen R, Gluck S, Heuser J, Russell D. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;163:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 20.Xu S, Cooper A, Sturgill-Koszycki S, Heyningen T v, Chatterjee D, Orme I, Allen P, Russell D. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]