Airways have a mucus lining that protects the lungs from the deleterious effects of exposure to inhaled particles, pathogens, and gases. Airway mucus is a gel in which the polymer network consists of heavily glycosylated mucins and the swelling agent is water. Glycosylation extends the mucin polypeptide in solution, and cross-linking of mucin polymers imparts to the airway mucus hydrogel its solid structure and adhesive tack. The relatively low elasticity of the airway mucus gel in health is tuned to enable ease of transport by the mucociliary escalator. Airway mucus gel pathology is a feature of multiple lung diseases, and relatively few treatments target this pathology. Among mechanisms of pathologic mucus gel formation, abnormalities in mucin gene expression are important and potentially treatable.
Mucins have the gene symbol MUC followed by a number, and MUC5AC and MUC5B are the principal secreted gel-forming mucins in airway mucus. In contrast, MUC5B and MUC7 mucins predominate in saliva, and MUC5AC and MUC6 mucins predominate in gastric secretions. MUC5AC is secreted exclusively by goblet cells in the airway epithelium, and MUC5B is secreted by both goblet cells and mucus cells in submucosal gland (SMG) acini (Figure 1A) (1). The genes encoding MUC5AC and MUC5B are located on chromosome 11p15, and their genomic structure consists of a single large exon encoding a complex multidomain protein. Although the protein structures of MUC5AC and MUC5B are broadly similar, there are differences in how the mucin domains are organized (Figure 1B). Deletion of muc5ac in mice does not cause a noticeable airway phenotype, except that muc5ac-deficient mice are protected from allergen-induced airway mucus plugging and airway hyperresponsiveness (2). In contrast, deletion of muc5b results in airway infection with Staphylococcus aureus, impaired mucus transport, and accumulation of neutrophils, eosinophils, and macrophages (3). These murine studies reveal a distinct role for MUC5B in host defense of the lungs.
Figure 1.
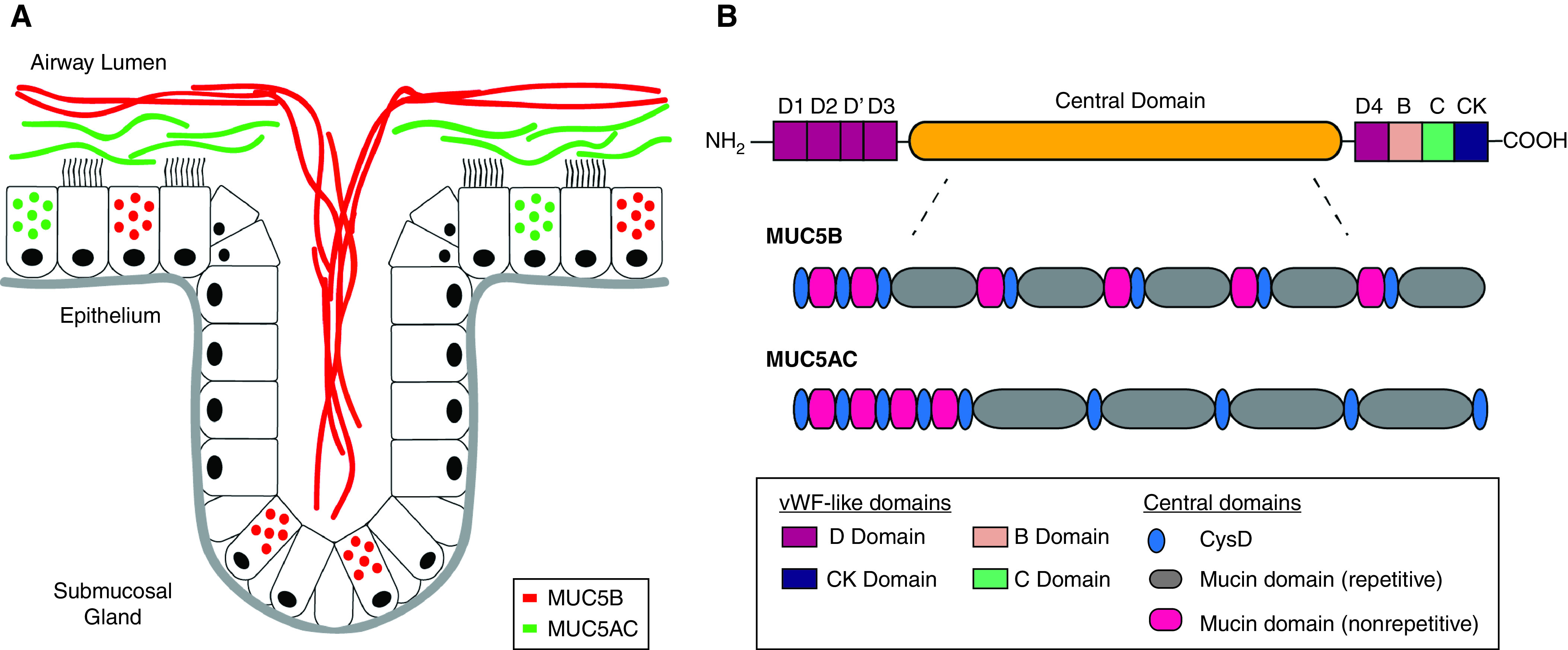
MUC5AC and MUC5B in the airway: cellular origins and protein domain architecture. (A) Schematic representation of mucin-secreting cells in the airway in health. MUC5B is secreted by mucus cells in submucosal glands (SMGs) and goblet cells in the surface epithelium, and MUC5AC is secreted only by goblet cells. Goblet cells and SMG mucus cells secrete mucins under basal conditions and are regulated by neurohumoral signals that include neurotransmitters, cytokines, and purinergic stimuli. Available evidence suggests that MUC5AC and MUC5B form distinct gels in the mucus layer (10, 11). Pathologic increases in MUC5AC protein secretion can result in immobilized MUC5AC-rich gels because of mucin tethering to the epithelial cell surface (11). MUC5B-rich gels emerge from SMG ducts onto the airway surface in the form of mucus strands that play a key role in particle clearance from the airways (14). (B) Schematic representation of the complex multidomain organization of MUC5AC and MUC5B. At the N and C termini of both mucins, there are cysteine-rich domains that are similar to structural domains (“D domains”) in vWF (von Willebrand factor). D domains are important for disulfide-mediated polymerization of the vWF blood glycoprotein, and they are also important for polymerization of mucins. The central domain, which has repetitive and nonrepetitive amino acid sequences, comprises serine-, threonine-, and proline-rich mucin domains interspersed with regions rich in cysteine residues (termed cys domains). The serine and threonine residues in the mucin domains are the sites of attachment of O-linked glycans. The cys domains may be sites of disulfide-mediated cross-linking (as distinct from disulfide-mediated polymerization). Compared with MUC5B, the central domain of MUC5AC contains two additional Cys-D domains in the central glycosylated region. Redrawn and modified by permission from Reference 15.
In this issue of the Journal, Costain and colleagues (pp. 761–768) report on a family with hereditary MUC5B deficiency because of a genetic variant in MUC5B that results in a truncated MUC5B mRNA and a failure to translate MUC5B protein (4). Two sisters with MUC5B deficiency had histories of repeated S. aureus airway infections since childhood, and they both had impaired mucociliary transport, low lung function, and bronchiectasis. Interestingly, cytologic analysis of sputum from the MUC5B-deficient patients revealed neutrophilia and increased numbers of apoptotic macrophages. All of these findings closely mirror the phenotype of muc5b−/− mice and provide strong support for a critical role for MUC5B in controlling infections in the airways and maintaining immune homeostasis in the lungs.
The mechanisms by which MUC5B selectively protects the airways from infection are unclear. One possibility is that MUC5B directly inhibits microbial growth or survival. Although MUC6 inhibits growth of Helicobacter pylori and MUC5AC inhibits survival of Trichuris muris (5, 6), neither MUC5AC nor MUC5B inhibits growth of S. aureus (3). Another possibility is that MUC5B is a scaffold for antimicrobial molecules. Sialylation and sulfation of mucin glycans impart negative charges that promote electrostatic interactions with cationic proteins, and epitopes presented by mucin glycans in the mucus gel allow interactions with lectins. It is not known if MUC5B has specific glycan features that generate a mucin interactome peculiarly well suited to airway defense. Finally, it is possible that MUC5B polymers generate mucus gels that more efficiently clear particles from the airways. MUC5AC forms tightly organized networks with a high degree of branching and is secreted by goblet cells as wispy threads (7, 8). In contrast, MUC5B is secreted from SMG ducts in the form of filaments that bind to inhaled particles to facilitate their clearance. Thus, despite similar protein architecture, MUC5B and MUC5AC appear to form specific types of mucus gel structures that may have specific particle clearance functions. It is possible that cells that secrete MUC5B (goblet cells and SMG mucus cells) or MUC5AC (goblet cells) determine the mucin-specific mucus gel structure. Recent studies in pigs that lack airway SMGs show that their airway surface liquid has a reduced ability to kill S. aureus and other bacteria (9). SMGs produce multiple antimicrobial peptides and also regulate mucus gel hydration and pH, but it may be that MUC5B secretion by mucus cells in SMGs is a critical component of SMG-mediated bacterial defense. So evidence is accumulating that MUC5B forms gel structures important for microbial defense, but too much MUC5B may not be a good thing. For example, whereas chronic upregulation of MUC5AC is associated with airway mucus plugging and airflow obstruction (10, 11), upregulation of MUC5B (through a gene polymorphism in its promotor) is associated with pulmonary fibrosis (12). The mechanism of MUC5B-mediated lung fibrosis is not well understood, but the association has emphasized the multiple ways in which mucins are involved in mucociliary clearance, inflammatory cell turnover, and epithelial mesenchymal signaling.
The clinical phenotype of the MUC5B-deficient patients described by Costain and colleagues informs knowledge of lung biology and contains surprises about oral biology. For example, the complete absence of MUC5B did not preclude lung development and permitted a TLC in the proband of 82% predicted at 20 years of age. This patient had the benefit of repeated courses of antibiotics, but complete absence of secreted gel-forming mucins in the airways of the lungs is unlikely to be compatible with survival from childhood. Thus, the presence of MUC5AC protein in these MUC5B-deficient patients must have provided significant backup mucin-related host defense in the lungs. A surprising finding is the absence of an oral or a dental phenotype given that MUC5B and MUC7 mucins confer to saliva its critical lubricant and desiccation barrier functions (13). The absence of a salivary phenotype in MUC5B-deficient patients reveals that MUC7 is the key salivary mucin for maintenance of oral and dental health.
In summary, Costain and colleagues’ description of a family with hereditary MUC5B deficiency uncovers a genetic airway disease that can now be grouped with cystic fibrosis and primary ciliary dyskinesia in the list of genetic causes of suppurative lung disease. The prominent clinical phenotype of MUC5B-deficient patients proves the distinct and nonredundant role of MUC5B as an airway mucin and should prompt ongoing research to more fully understand how this enigmatic complex protein regulates lung health. Such understanding has potential to point to MUC5B-focused treatment strategies for lung disease.
Footnotes
Supported by NIH/NHLBI grants HL080414, HL107202, and HL128191.
Originally Published in Press as DOI: 10.1164/rccm.202201-0064ED on February 11, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, et al. Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med . 2019;199:715–727. doi: 10.1164/rccm.201804-0734OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun . 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature . 2013;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costain G, Liu Z, Mennella V, Radicioni G, Goczi AN, Albulescu A, et al. Hereditary mucin deficiency caused by biallelic loss of function of MUC5B. Am J Respir Crit Care Med . 2022;205:761–768. doi: 10.1164/rccm.202106-1456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science . 2004;305:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- 6. Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med . 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carpenter J, Wang Y, Gupta R, Li Y, Haridass P, Subramani DB, et al. Assembly and organization of the N-terminal region of mucin MUC5AC: indications for structural and functional distinction from MUC5B. Proc Natl Acad Sci U S A . 2021;118:e2104490118. doi: 10.1073/pnas.2104490118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, et al. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci U S A . 2017;114:6842–6847. doi: 10.1073/pnas.1703228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ostedgaard LS, Price MP, Whitworth KM, Abou Alaiwa MH, Fischer AJ, Warrier A, et al. Lack of airway submucosal glands impairs respiratory host defenses. eLife . 2020;9:1–25. doi: 10.7554/eLife.59653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med . 2016;194:1296–1299. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest . 2016;126:2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med . 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frenkel ES, Ribbeck K. Salivary mucins protect surfaces from colonization by cariogenic bacteria. Appl Environ Microbiol . 2015;81:332–338. doi: 10.1128/AEM.02573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer AJ, Pino-Argumedo MI, Hilkin BM, Shanrock CR, Gansemer ND, Chaly AL, et al. Mucus strands from submucosal glands initiate mucociliary transport of large particles. JCI Insight . 2019;4:124863. doi: 10.1172/jci.insight.124863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol . 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]


