Abstract
Lipopolysaccharide (LPS) of Burkholderia cepacia was purified by the conventional phenol-water extraction method (preparation BcLPS-1), followed by enzymatic treatments with DNase, RNase, trypsin, and proteinase K (preparation BcLPS-2), and finally by deoxycholate-phenol-water extraction (preparation BcLPS-3). Cells of LPS-hyporesponsive C3H/HeJ mice were activated by both the BcLPS-1 and the BcLPS-2 preparations but barely activated by BcLPS-3. When LPS-responsive C3H/HeN mice were used as targets, endotoxic activities such as lethal toxicity to galactosamine-sensitized mice, mitogenicity to spleen cells, and activation of macrophages to induce tumor necrosis factor alpha and interleukin-6 (IL-6) were strongly exhibited even by highly purified BcLPS-3 at levels comparable to those of the highly active enterobacterial LPS of Salmonella enterica serovar Abortus-equi (SaeLPS), used as the control. The ability of BcLPS-3 to activate murine macrophages for induction of IL-1β was, however, much weaker than that of SaeLPS. Both accumulation of pro-IL-1β protein and expression of IL-1β mRNA in macrophages by stimulation with BcLPS-3 were much weaker than by stimulation with SaeLPS. These results indicate that LPS of B. cepacia has the potential to play a role as a pathogenic factor with strong activity comparable to that of usual enterobacterial LPS, but unlike the latter, this LPS has a relative lack of ability in the activation of murine macrophages to induce IL-1β. The lack of IL-1β-inducing ability appears to be caused by incomplete signal transduction somewhere in the upstream step(s) of IL-1β gene transcription.
Burkholderia cepacia is an aerobic glucose-nonfermenting gram-negative bacillus that possesses flagella and formerly belonged to the genus Pseudomonas (37). Bacteria of this species are intrinsically resistant to many antibiotics and active as opportunistic pathogens in hospitalized patients and other compromised hosts (8, 26). Especially among patients with cystic fibrosis, infection by this organism makes possible the development of serious pneumonia and sepsis with a high mortality rate, i.e., “cepacia syndrome” (9). Lipase, protease, extracellular toxic complex, and lipopolysaccharide (LPS) released from the bacteria have been suggested as candidates for the virulence factors which cause pathological changes, such as accumulation of polymorphonuclear leukocytes and proteinaceous exudate in bronchial and alveolar lumina, injury and necrosis of bronchial epithelium, and disorganization of alveolar structure. The specific crucial trigger of the pneumonia and sepsis in B. cepacia infection, however, remains to be clarified (16, 20, 31, 34, 39).
Among these four candidates, we were interested in LPS, a major component of the outer membrane in gram-negative bacteria, since it is assumed to play a central role in the induction of various pathophysiological responses, such as fever, disseminated intravascular coagulation, and shock in hosts suffering from gram-negative bacterial infections (22, 27). LPSs of members of the Enterobacteriaceae, such as Escherichia coli and Salmonella, have been investigated intensively as typical LPSs with high endotoxic activity. Structurally, LPSs are composed of an O-polysaccharide, an outer core saccharide, an inner core saccharide, and lipid A regions. The part of the inner core region adjacent to the lipid A moiety in usual LPS is constructed of one to three molecules of 3-deoxy-d-manno-octulosonic acid (KDO) and is well conserved among various bacterial species. In the case of LPS extracted from B. cepacia, KDO was not detectable by the conventional method (17, 33) but was detectable after hydrolyzing the LPS with strong acid (32). This suggests that the KDO portion of the inner core region in LPS of this organism is replaced by some molecules which provide acid resistance. Recently, we revealed that B. cepacia LPS contains a d-glycero-d-talo-2-octulosonic acid (KO) by which KDO is replaced to form a KO-KDO structure in the inner core region rather than the KDO-KDO structure of usual LPS (11). As for the biological activities of an LPS with such a unique structure, the activities of Limulus amoebocyte lysate activation, tumor necrosis factor alpha (TNF-α) induction, and neutrophil activation have been reported to be stronger than those of LPS from Pseudomonas aeruginosa, a key opportunistic pathogen isolated from patients with cystic fibrosis, and similar to those of a typical LPS from E. coli (10, 30, 38). Based on those results, the authors suggested a pathogenic role of B. cepacia LPS in inflammatory diseases. The LPS preparations used in those studies were purified only by the conventional method of phenol-water extraction (36), without further purification.
In the present study, we aimed to characterize the biological activity of B. cepacia LPS itself in more detail. We found that preparations of B. cepacia LPS obtained by the phenol-water extraction method are not sufficiently purified to be free of some trace contaminants which induce the response of LPS-hyporesponsive cells of C3H/HeJ mice. We also found that introduction of the deoxycholate (DOC)-phenol-water extraction method (18) in a further purification step was effective in removing such contaminants. This highly purified B. cepacia LPS indicated strong biological activity, comparable to that of a typical LPS of Salmonella enterica serovar Abortus-equi (SaeLPS) but with the exception of weak induction of interleukin-1-β(IL-1β) from murine macrophages.
MATERIALS AND METHODS
Mice and cell culture.
C3H/HeN (LPS-responsive) and C3H/HeJ (LPS-hyporesponsive) mice were purchased from Clea Japan, Inc. (Tokyo, Japan), and used at ages of 8 to 14 weeks. For cell culture, RPMI 1640 medium (Flow Laboratories, Inc., Rockville, Md.) supplemented with 10 mM HEPES, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 0.2% NaHCO3, and 2.5 to 10% heat-inactivated fetal calf serum (FCS-RPMI) was used. All cells were cultured in a humidified chamber at 37°C with 5% CO2. Murine peritoneal macrophages were prepared by conventional method. Peritoneal exudate cells taken from mice that had received 2 ml of thioglycolate broth (Difco Laboratories, Detroit, Mich.) intraperitoneally 4 days in advance were washed and resuspended in 5% FCS-RPMI. A 0.5-ml aliquot of the cell suspension (106/ml) was dispensed into each well of a 48-well culture plate (Sumitomo Bakelite Co. Ltd., Tokyo, Japan) and cultured for 3 h. Nonadherent cells were washed off, and the remaining adherent cells were used as macrophages. A murine macrophage-like cell line, RAW 264.7, obtained from the American Type Culture Collection (ATCC) (Manassas, Va.) and maintained in our laboratory was also used.
LPS preparations.
B. cepacia GIFU 645 used in the present study is a type strain of B. cepacia, the same strain as ATCC 25416 or NCTC 10743, and belongs to genomovar I. The bacterial cells obtained by culture with nutrient broth no. 2 (Oxoid Ltd., Basingstoke, United Kingdom) at 35°C for 24 h were heat killed (100°C for 30 min), and LPS was extracted in the aqueous phase by the conventional phenol-water extraction method (36). The aqueous phase was centrifuged to obtain LPS as precipitate; this first LPS preparation was referred to as BcLPS-1. For further purification, BcLPS-1 was treated with 1 μg of DNase/ml plus 1 μg of RNase/ml (both enzymes were from bovine pancreas; Sigma Chemical Co., St. Louis, Mo.) at 30°C for 90 min at pH 7.5, followed by the addition of 1 μg of trypsin/ml from porcine pancreas (Wako Pure-Chemical Industries Ltd., Osaka, Japan) and incubation at 30°C for 90 min. Finally, 1 μg of proteinase K/ml from fungi (Wako Pure Chemical Industries Ltd.) was added and incubated for an additional 90 min. After these enzymatic treatments, the solution was dialyzed against distilled water and lyophilized to obtain the second preparation, BcLPS-2. This preparation was then treated with 45% phenol containing triethylamine and sodium DOC, and LPS extracted in the aqueous phase was precipitated with ethanol by the DOC-phenol-water extraction method (18) to obtain the third preparation, BcLPS-3. These three preparations were examined for biological activities. SaeLPS, purified as described elsewhere (7), was kindly donated by C. Galanos (Max-Planck-Institut für Immunbiologie, Freiburg, Germany) and used as a control enterobacterial LPS.
Lethal toxicity.
Lethal toxicity of LPS preparations to galactosamine (GalN)-sensitized mice was examined according to the established method (6). Groups of 6 to 10 mice were administered GalN (Sigma Chemical Co.) intraperitoneally at 18 mg/mouse and LPS preparations intravenously at doses of serial 10-fold dilutions. Survival of the mice was observed for 3 days, and results were expressed as 50% lethal doses calculated by the method of Kärber (12).
Mitogenic activity.
Mouse spleen cells suspended in 10% FCS-RPMI were plated on a 96-well round-bottom microplate (Nunc, Roskilde, Denmark) at a density of 4 × 105 cells/well and cultured in the presence of LPS preparations for 48 h. The culture was pulse-labeled with 1 μCi of [3H]thymidine (ICN Biomedicals, Inc., Irvine, Calif.) per well for the last 4 h, and radioactivity (counts per minute) incorporated into the cells was measured by a TopCount microplate scintillation counter (Packard Japan Co. Ltd., Tokyo, Japan). Results were expressed as the stimulation index, which is the ratio of counts per minute in a test sample to that in a negative control sample (absence of stimulants).
Macrophage stimulation and cytokine assay.
Murine peritoneal macrophages were cultured in the presence or absence of LPS preparations with 5% FCS-RPMI. Culture supernatants obtained at 4 h were assayed for activity of TNF-α, and those obtained at 48 h were assayed for activity of IL-6 and for amount of IL-1β. In some experiments, adherent RAW 264.7 cells were also used as murine macrophages in a manner similar to peritoneal macrophages.
Activity of TNF-α was determined by a cytotoxic assay against actinomycin D-treated L929 cells (28). Briefly, L929 cells were cultured with 5% FCS-RPMI in a 96-well flat-bottom culture plate (Nunc) for 3 h, and actinomycin D (Sigma Chemical Co.) was added at a final concentration of 1 μg/ml with serial dilutions of test samples. Viable cells in the overnight culture were stained with crystal violet, and absorbance of the blue color extracted with 30% acetic acid was measured at 540 nm by a Biomek 1000 spectrophotometer (Beckman Instruments, Palo Alto, Calif.). The activity of TNF-α (in units per milliliter) was calculated from the dilution factor of test samples necessary for 50% cell lysis, with correction by an internal standard of recombinant human TNF-α in each assay.
IL-6 activity was determined by a proliferation assay of IL-6-dependent mouse hybridoma MH60.BSF2 cells (19), a kind gift from N. Nishimoto of Osaka University (Osaka, Japan). Briefly, the cells were cultured for 3 days in a 2.5% FCS-RPMI supplemented with 5 × 10−5 M 2-mercaptoethanol in a 96-well flat-bottom culture plate (Nunc), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma Chemical Co.) was added for the last 4 h of the culture for formation of formazan blue crystals by viable cells (23). The supernatant was removed, and precipitated formazan crystals were dissolved with isopropanol solution containing 5% formic acid to measure the absorbance at 540 nm. IL-6 activity (in units per milliliter) was calculated from the dilution factor of test samples required to induce 50% cell growth, with correction by an internal standard of recombinant human IL-6 in each assay.
The amount of IL-1β was determined by a specific sandwich enzyme-linked immunosorbent assay. A 96-well EIA/RTA plate (Costar, Cambridge, Mass.) was coated with a purified goat antibody to mouse IL-1β (R&D Systems, Minneapolis, Minn.). To the plate incubated with test samples and standard solutions (mouse IL-1β enzyme-linked immunosorbent assay standard; Endogen, Woburn, Mass.), biotinylated monoclonal antibody against mouse IL-1β (Endogen) was added as a detection antibody. Streptavidin-horseradish peroxidase conjugate (PharMingen, San Diego, Calif.) was added for the development of an enzyme reaction with a substrate solution of TMBZ (0.01% 3,3′,5,5′-tetramethylbenzidine and 0.03% H2O2 in 0.11 M acetate buffer, pH 5.5). The color reaction was stopped by adding 1.8 M H2SO4, and absorbance was measured at 450 nm. Quantification of IL-1β (in picograms per milliliter) was performed based on a standard IL-1β curve in each assay.
Detection of pro-IL-1β.
Macrophages were stimulated with LPS for 20 to 24 h, and culture supernatant was removed. The cells were washed and lysed in lysis buffer (1% Triton X-100, 20 mM Tris-HCl, [pH 8.0], 137 mM NaCl, 10% glycerol, 1 mM Na3VO4, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 20 μM leupeptin, 0.15 U of aprotinin per ml) by three freeze-and-thaw cycles. The cell lysate obtained after removal of insoluble materials by centrifugation was adjusted for protein content at 30 μg and separated on sodium dodecyl sulfate–10% polyacrylamide gel according to the method of Laemmli (15). Proteins separated on the running gel were transferred to a polyvinylidene difluoride membrane (Nihon Millipore Ltd., Tokyo, Japan), and pro-IL-1β protein (>30 kDa) was analyzed using biotinylated anti-mouse IL-1β monoclonal antibody (Endogen) and ECL Western blotting reagent (Amersham Life Science, Amersham, United Kingdom) according to the manufacturers'-instructions.
Detection of IL-1β mRNA by reverse transcribed (RT)-PCR.
Total RNA of macrophages stimulated with LPS for 6 h was extracted by the acid guanidinium thiocyanate-phenol-chloroform method (4). Synthesis of cDNA from the total RNA (1 μg) was performed by priming with 200 ng of random hexamer, 1.25 mM deoxynucleoside triphosphate, and 60 U of SuperScript II reverse transcriptase (Gibco BRL Life Technologies, Inc., Gaithersburg, Md.) at 38°C for 1 h. The sequences of the primers used in PCR for mouse IL-1β were 5′-GCAACTGTTCCTGAACTCAA-3′ and 5′-GACGTGATGTCCGAGGCTC-3′, and those for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 5′-CCTCAACTACATGGTCTA-3′ and 5′-TGAAACCGTAACACCTTCC-3′. PCR was carried out at 94°C for 1 min, 63°C for 1 min, and 72°C for 2 min as 1 cycle, and 25 and 27 cycles were performed for IL-1β and GAPDH, respectively. The PCR products were analyzed after electrophoresis on a 2% agarose gel by ethidium bromide staining.
RESULTS
Mitogenic activity of BcLPS preparations to mouse spleen cells.
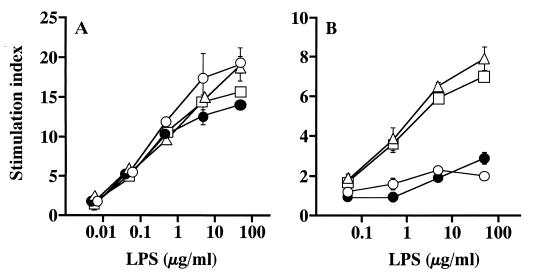
All three preparations of BcLPS exhibited strong mitogenic activity to C3H/HeN spleen cells in a manner similar to that of the control SaeLPS (Fig. 1A); i.e., significant activities detected at 0.05 μg/ml increased dose dependently up to the highest concentration tested (50 μg/ml). Mitogenic activity to spleen cells of C3H/HeJ mice, which are hyporesponsive to LPS due to defective functional Toll-like receptor 4 (TLR4) production (25), was then examined. As shown in Fig. 1B, BcLPS-1 and -2 exhibited significant mitogenic activity to C3H/HeJ spleen cells at concentrations over 0.5 μg/ml. In contrast, the activity of BcLPS-3 was much weaker and only marginal activity was detectable at the highest concentration tested (50 μg/ml), just like activity of the control SaeLPS. These results indicate that BcLPS-3 inevitably requires TLR4 as well as purified usual LPS for the transduction of mitogenic signals, while BcLPS-1 and -2 have a contaminating factor(s) which induces mechanisms other than the TLR4 pathway for the transduction of signals.
FIG. 1.
Mitogenic activity of BcLPS preparations to murine spleen cells. Spleen cells of C3H/HeN (A) and C3H/HeJ (B) mice were cultured with BcLPS-1 (▵), BcLPS-2 (□), BcLPS-3 (●), or the control SaeLPS (○) at the indicated concentrations for 48 h. The cells were pulse-labeled with [3H]thymidine for the last 4 h of culture, and radioactivity (counts per minute) in the harvested cells was measured. Results are expressed as a stimulation index, which is the ratio of count per minute in a test sample to that in a negative control (absence of stimulants). Data are means ± standard errors of the means for triplicate samples. A representative result obtained from three independent experiments is shown.
Activity of BcLPS preparations to induce TNF-α production from murine peritoneal macrophages.
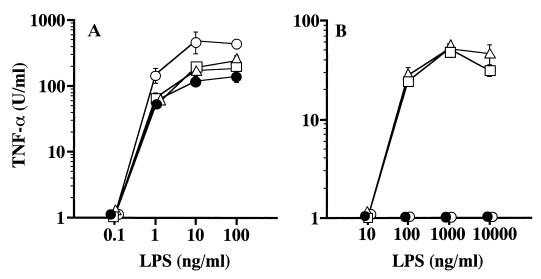
Potency of the LPS preparations to activate murine peritoneal macrophages for induction of TNF-α was then investigated. When the macrophages of C3H/HeN mice were used as target cells, all three BcLPS preparations exhibited strong, similar activities and induced TNF-α production at concentrations over 1 ng/ml, as did the control SaeLPS, although the amounts of induced TNF-α were somewhat smaller than those of the control SaeLPS (Fig. 2A). In the case of C3H/HeJ macrophages used as target cells, BcLPS-1 and -2 induced significant amounts of TNF-α at concentrations over 100 ng/ml, while BcLPS-3 and the control SaeLPS induced no detectable amounts of TNF-α even at the highest concentration tested (10 μg/ml) (Fig. 2B).
FIG. 2.
Induction of TNF-α from murine peritoneal macrophages by BcLPS preparations. Peritoneal macrophages obtained from C3H/HeN (A) and C3H/HeJ (B) mice were cultured in the presence of BcLPS-1 (▵), BcLPS-2 (□), BcLPS-3 (●), or the control SaeLPS (○) at the indicated concentrations. Culture supernatants obtained at 4 h after LPS stimulation were assayed for determination of TNF-α induction. Data are means ± standard errors of the means for triplicate samples. A representative result obtained from three independent experiments is shown.
These results, together with those of the mitogenic activity shown in Fig. 1, indicate that BcLPS-1 and -2 contain some minor contaminants which play a role in activating TLR4-deficient C3H/HeJ cells and that such contaminants have been removed in the BcLPS-3 preparation. In the following experiments, BcLPS-3 was used as the purified BcLPS preparation.
Lethal toxicity of BcLPS-3 to GalN-sensitized mice.
Groups of 6 to 10 mice aged 11 to 14 weeks were given GalN (18 mg/mouse) intraperitoneally and then LPS intravenously at doses of serial 10-fold dilutions. Survival of the mice was observed for 3 days to estimate the lethal toxicity of LPS. The 50% lethal dose of BcLPS-3 to C3H/HeN mice was calculated to be 3 ng/mouse, which was somewhat lower than that of the control SaeLPS, 10 ng/mouse. This indicates that the lethal toxicity of BcLPS-3 to C3H/HeN mice is very strong, comparable to or somewhat stronger than that of the control SaeLPS. In contrast, BcLPS-3 as well as the control LPS exhibited no lethal toxicity to C3H/HeJ mice even at the highest dose tested (10,000 ng/mouse). These results indicate that activation of signaling pathways through TLR4 is indispensable for expression of the lethal toxicity of BcLPS-3 as well as usual LPS.
Potency of BcLPS-3 to activate murine macrophages for induction of cytokines.
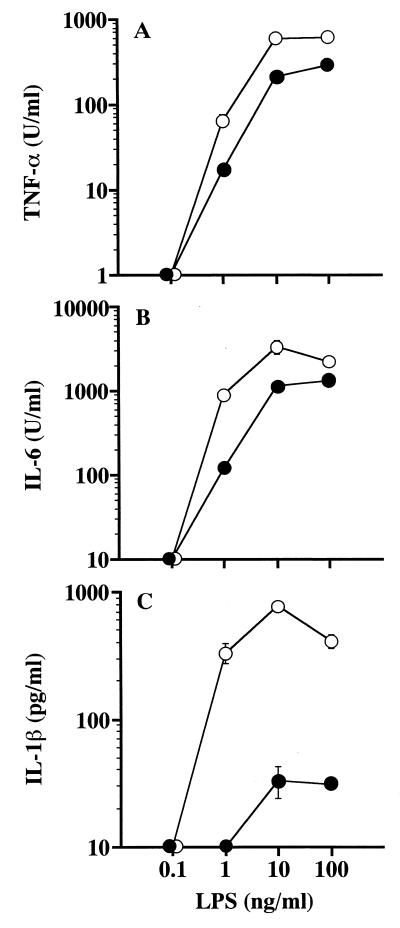
Peritoneal macrophages of C3H/HeN mice were stimulated with BcLPS-3, and production of cytokines TNF-α, IL-6, and IL-1β in the culture supernatant was measured. Significant amounts of TNF-α and IL-6 were produced upon stimulation with BcLPS-3 at concentrations over 1 ng/ml, in a manner similar to that of the control LPS, although the amounts of cytokines produced by BcLPS-3 were somewhat lower than those produced by the control LPS (Fig. 3A and B). In contrast, BcLPS-3 exhibited weak potency in the induction of IL-1β while the control SaeLPS exhibited strong potency in induction of this cytokine (Fig. 3C). Only small amounts of IL-1β were detected upon stimulation with BcLPS-3 at 10 and 100 ng/ml. However, the control LPS induced significant amounts of IL-1β at concentrations over 1 ng/ml, similar to the induction of the other two cytokines.
FIG. 3.
Stimulation of C3H/HeN peritoneal macrophages with BcLPS-3 to induce cytokines. Peritoneal macrophages obtained from C3H/HeN mice were cultured in the presence of BcLPS-3 (●) or the control SaeLPS (○) at the indicated concentrations. Culture supernatants recovered at 4 h after LPS stimulation were assayed for determination of TNF-α induction (A), and those recovered at 48 h were assayed for determination of IL-6 (B) and IL-1β (C) induction Data are means ± standard errors of the means for triplicate samples. A representative result obtained from three independent experiments is shown.
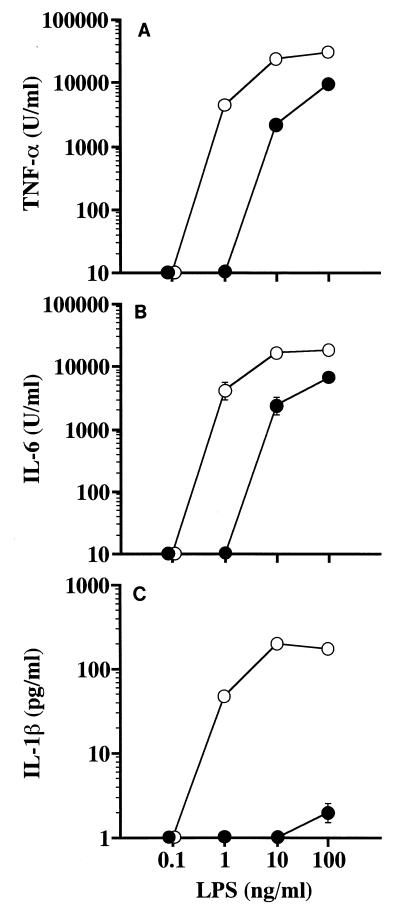
A murine macrophage cell line, RAW 264.7, was then used to further investigate the potency of BcLPS-3 to induce those cytokines. As shown in Fig. 4A and B, large amounts (over 1,000 U/ml) of TNF-α and IL-6 were produced by RAW 264.7 cells upon stimulation with BcLPS-3 at concentrations over 10 ng/ml, although a concentration about 1 order of magnitude higher than that of the control LPS was required to induce similar amounts of these cytokines. Only marginal amounts of IL-1β were produced by RAW 264.7 cells upon stimulation with BcLPS-3 at 100 ng/ml, while significantly larger amounts were induced by the control LPS at concentrations over 1 ng/ml, similar to the induction of the other two cytokines (Fig. 4C).
FIG. 4.
Stimulation of the murine macrophage cell line RAW 264.7 with BcLPS-3 to induce cytokines. RAW 264.7 cells were cultured in the presence of BcLPS-3 (●) or the control SaeLPS (○) at the indicated concentrations. Culture supernatants recovered at 48 h after LPS stimulation were assayed for determination of TNF-α (A), IL-6 (B), and IL-1β (C) induction. Data are means ± standard errors of the means for triplicate samples. A representative result obtained from three independent experiments is shown.
These results reveal the unique character of BcLPS; i.e., it has a much reduced ability to induce IL-1β, while it has abilities comparable to those of usual enterobacterial LPS for other activities, such as induction of TNF-α and IL-6, mitogenic activity, and lethal toxicity.
Production of pro-IL-1β in murine macrophages upon stimulation with BcLPS-3.
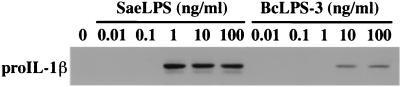
We then investigated the underlying mechanisms in the weak ability of BcLPS to induce IL-1β. It was clarified that mature IL-1β is released from cells after proteolytic cleavage of precursor pro-IL-1β by the action of IL-1β-converting enzyme (2, 14). After LPS stimulation of peritoneal macrophages of C3H/HeN mice, the culture supernatant was removed and the cells were washed. Accumulation of pro-IL-1β (>30 kDa) in the cell lysate was detected by immunoblotting analysis. Low-molecular-weight bands corresponding to mature IL-1β (<20 kDa) were scarcely detected in these lysates of washed cells, as was shown in numerous previous reports. High-density bands corresponding to pro-IL-1β protein were detected in the lysates upon with stimulation of the control LPS at concentrations over 1 ng/ml, while only low-density bands were detectable in the lysates upon stimulation of BcLPS-3 at 10 and 100 ng/ml as shown in Fig. 5. These results correlate well with the results of IL-1β-inducing potency shown in Fig. 3C and indicate that, as early as the step of intracellular production of pro-IL-1β protein, signals coming from BcLPS-3 stimulation were hardly transduced.
FIG. 5.
Production of pro-IL-1β in murine peritoneal macrophages upon LPS stimulation. Peritoneal macrophages of C3H/HeN mice were stimulated for 20 to 24 h with the indicated concentrations of SaeLPS or BcLPS-3. After removal of the culture supernatant, cells were washed and lysed by three freeze-and-thaw cycles in the lysis buffer. The cell lysates containing 30 μg of total proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins on the gel were transferred to a polyvinylidene difluoride membrane, and pro-IL-1β (>30 kDa) was detected by immunoblotting analysis with biotin-labeled anti-mouse IL-1β monoclonal antibody.
Expression of IL-1β mRNA in murine macrophages upon stimulation with BcLPS-3.
As one of the upstream processes of pro-IL-1β synthesis, expression of IL-1β mRNA in those cells was then investigated by the RT-PCR method. As shown in Fig. 6, the bands originating from IL-1β mRNA were clearly detected in the cells stimulated with SaeLPS at concentrations over 1 ng/ml, while only weak bands were detected in cells stimulated with BcLPS-3 at the corresponding concentrations. These results suggest that signals for IL-1β production upon stimulation with BcLPS-3 are down-regulated before the step of IL-1β gene transcription.
FIG. 6.
Expression of IL-1β mRNA in murine peritoneal macrophages upon LPS stimulation. Peritoneal macrophages of C3H/HeN mice were exposed to the indicated concentrations of SaeLPS or BcLPS-3 for 6 h. Total RNA extracted from the cells was analyzed for mRNA expression of IL-1β and control GAPDH by RT-PCR. PCR products of IL-1β and GAPDH obtained by 25 and 27 cycles, respectively, were subjected to agarose gel electrophoresis and visualized by ethidium bromide staining.
DISCUSSION
In the present study, we show that preparations of B. cepacia LPS purified by phenol-water extraction, (BcLPS-1) and further by enzymatic treatments (BcLPS-2) have the potency to activate the cells of LPS-hyporesponsive C3H/HeJ mice (Fig. 1 and 2). Such potency was reduced to negligible levels in the preparation of BcLPS-3 obtained after further purification by the DOC-phenol-water extraction method (18). Since this method was developed for the elimination of trace endotoxin protein from LPS preparations, BcLPS-1 and -2 in this study were assumed to contain trace amounts of proteins, such as endotoxin protein (35) and lipid A-associated protein (21), that induce the response of LPS-hyporesponsive cells even with very slight contamination of LPS. In previous reports of biological activities of B. cepacia LPS (10, 30, 38), LPS preparations used were obtained by the phenol-water extraction method without further purification and their potency to activate C3H/HeJ cells was not tested. Those LPS preparations were purified to no further level than that of BcLPS-1, suggesting that they probably were contaminated with trace amounts of proteins, such as endotoxin protein. When using such preparations, it is problematic to assign a biological activity to LPS itself free from the effect of such protein contaminants. Highly purified preparations of B. cepacia LPS, such as BcLPS-3, have not been used for biological investigations before the present study.
It is important to clarify the biological activity of B. cepacia LPS itself for a better understanding of the role of LPS in diseases with B. cepacia infection. We used BcLPS-3 for biological investigations and found that it has strong endotoxic activity, comparable to that of a representative enterobacterial LPS (SaeLPS) in activities such as lethal toxicity to GalN-sensitized mice, mitogenic activity, and macrophage activation activities for induction of TNF-α and IL-6. These results suggest that B. cepacia LPS, independently of contaminating proteins, has the ability to play a pathogenic role in the sepsis-like cepacia syndrome that is as strong as those of highly active enterobacterial LPSs. The above-mentioned B. cepacia LPS preparations in previous studies (10, 30, 38) were obtained from different strains, and all of them were reported to exhibits strong endotoxic activity, comparable to that of the control enterobacterial LPS. Considering the results of the present study with those of the previous reports, LPS of B. cepacia through a wide variety of strains may have potential as a pathogenic factor with strong endotoxic activity, although biological studies using more preparations of purified B. cepacia LPS without reactivity to HeJ cells are required to confirm this potential.
It is interesting that B. cepacia LPS, unlike the enterobacterial LPS, exhibited only slight activity in the induction of IL-1β, while it showed strong activity, comparable to that of the enterobacterial LPS, in the induction of TNF-α and IL-6, as well as in mitogenic activity and lethal toxicity. The selective lack of IL-1β-inducing ability observed in B. cepacia LPS has not been reported in biological studies of any other LPS. A typical chemical characteristic of B. cepacia LPS revealed by chemical analysis of LPS obtained from the strain used in the present study is the unusual presence of KO, instead of the usual KDO, as a side chain of KDO linking the lipid A backbone and polysaccharide chain of LPS (11). The presence of this KO-KDO structure in the inner core region is suggested to form an acid-resistant linkage of lipid A to the polysaccharide chain. This unique chemical structure, however, does not seem to be directly related to the expression of the above-mentioned unique biological character of the LPS, since similarly purified LPS of Burkholderia pseudomallei exhibited IL-1β-inducing activity comparable to that of enterobacterial LPS (data not shown) despite possession of the KO-KDO structure in its inner core region. To elucidate the part of the chemical structure in B. cepacia LPS responsible for the expression of this unique biological activity, further chemical studies, including precise determination of the entire structure of the LPS, are necessary.
Recently, some reports have indicated that nitric oxide (NO) has the potency to prevent induction of IL-1β (13, 24, 29). In the experiments on macrophage activation in the present study, we measured induction of NO in addition to the cytokines and found that BcLPS-3 induced no larger amounts of NO than the control SaeLPS did (data not shown). This indicates that selective inhibition of IL-1β induction in a feedback fashion by excessive production of NO is not the action mechanism of the B. cepacia LPS. Furthermore, prevention of IL-1β induction by NO was indicated to be caused by inhibition of IL-1β-converting enzyme activity, which catalyzes the processing of pro-IL-1β to form active IL-1β and release it to the outside of the cells (13). In such a case, large amounts of pro-IL-1β protein are accumulated in the cells and small amounts of IL-1β are released from the cells. In the present study, only small amounts of pro-IL-1β were detected in the cell lysate obtained after stimulation with B. cepacia LPS, in good correlation with the amounts of extracellular IL-1β (Fig. 3 and 5). These results indicate that signal transduction for IL-1β production in response to the B. cepacia LPS is down-regulated in some steps before pro-IL-1β synthesis is completed.
Selective inhibition of IL-1β gene expression by gamma interferon (IFN-γ) in activated RAW 264.7 cells has been reported (5). In the experiments, IFN-γ was exogenously added to a culture of RAW 264.7 cells stimulated with LPS plus TNF-α, and a suppressive effect of IFN-γ on IL-1β gene expression was shown. In our experiments using RAW 264.7 cells (Fig. 4), no detectable amounts of IFN-γ were present in the culture supernatant upon stimulation with either BcLPS-3 or SaeLPS (data not shown), indicating no participation of IFN-γ in the mechanism of the weak IL-1β induction by the B. cepacia LPS.
Expression of IL-1β mRNA in murine macrophages by stimulation with BcLPS-3 was clearly weaker than that with SaeLPS (Fig. 6), suggesting that the lack of IL-1β induction by B. cepacia LPS is caused by down-regulation at the transcriptional step of the IL-1β gene or the upstream signaling pathways. Stimulation of macrophages by LPS induces activation of a number of different second-messenger pathways, including the mitogen-activated protein kinase and NF-κB cascades, which activate nuclear transcription factors to induce their DNA-binding ability to regulatory sequences in promoter regions of target genes such as cytokine genes. Although virtually nothing is known about the signaling pathways that affect the regulation of LPS-induced IL-1β gene transcription, important roles of p38 mitogen-activated protein kinase through activation of CCAATT/enhancer binding protein (C/EBP)/NFIL-6 transcription factors (1) and of the newly identified mitogen- and stress-activated kinase-1 through activation of cyclic AMP-responsive element-binding protein (CREB) (3) have recently been indicated. B. cepacia LPS may have incomplete potency to transduce some parts of the signals leading to activation of such factors as C/EBP/NFIL-6 and CREB, which are essential for gene transcription of IL-1β but not for those of TNF-α, IL-6, and some other LPS mediators, and may be a useful tool for investigation of the LPS signals selectively required for IL-1β induction.
In the present study, we have demonstrated that preparations of B. cepacia LPS at the purification levels of the conventional phenol-water extraction method and further enzymatic treatments still contain trace amounts of contaminants which exhibit endotoxin protein-like activity and that introduction of the DOC-phenol-water extraction method in a further purification step is effective in eliminating such contaminants. By using such a highly purified B. cepacia LPS, the strong endotoxic potency of this LPS, comparable to that of highly active enterobacterial LPS, was confirmed, and the unique characteristic of this LPS, a relative lack of ability to induce IL-1β from murine macrophages, was found. The weak ability of this LPS to induce IL-1β was suggested to be caused by incomplete signal transduction somewhere in the upstream step(s) of IL-1β gene transcription.
REFERENCES
- 1.Baldassare J J, Bi Y, Bellone C J. The role of p38 mitogen-activated protein kinase in IL-1β transcription. J Immunol. 1999;162:5367–5373. [PubMed] [Google Scholar]
- 2.Black R A, Kronheim S R, Cantrell M, Deeley M C, March C J, Prickett K S, Wignall J, Conlon P J, Cosman D, Hopp T P, Mochizuki D Y. Generation of biologically active interleukin-1β by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- 3.Caivano M, Cohen P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1β in RAW264 macrophages. J Immunol. 2000;164:3018–3025. doi: 10.4049/jimmunol.164.6.3018. [DOI] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Chujor C S N, Klein L, Lam C. Selective inhibition of interleukin-1β gene expression in activated RAW 264.7 macrophages by interferon-γ. Eur J Immunol. 1996;26:1253–1259. doi: 10.1002/eji.1830260611. [DOI] [PubMed] [Google Scholar]
- 6.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanos C, Lüderitz O, Westphal O. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-pyrexal) Zentbl Bakteriol Hyg I Abt 1 Orig A. 1979;243:226–244. [PubMed] [Google Scholar]
- 8.Golgman D A, Klinger J D. Pseudomonas cepacia biology, mechanisms of virulence, epidemiology. J Pediatr. 1986;108:806–812. doi: 10.1016/s0022-3476(86)80749-1. [DOI] [PubMed] [Google Scholar]
- 9.Govan J R W, Nelson J W. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 10.Hughes J E, Stewart J, Barclay G R, Govan J R W. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia cepacia. Infect Immun. 1997;65:4281–4287. doi: 10.1128/iai.65.10.4281-4287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isshiki Y, Kawahara K, Zähringer U. Isolation and characterisation of disodium (4-amino-4-deoxy-β-l-arabinopyranosyl)-(1→8)-(d-glycero-α-d-talo-oct-2-ulopyranosylonate)-(2→4)-(methyl-3-deoxy-d-manno-oct-2-ulopyranoside)onate from the lipopolysaccharide of Burkholderia cepacia. Carbohydr Res. 1998;313:21–27. doi: 10.1016/s0008-6215(98)00179-7. [DOI] [PubMed] [Google Scholar]
- 12.Kärber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Arch Exp Pathol Pharmakol. 1931;162:480–487. [Google Scholar]
- 13.Kim Y M, Talanian R V, Li J, Billiar T R. Nitric oxide prevents IL-1β and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β-converting enzyme) J Immunol. 1988;161:4122–4128. [PubMed] [Google Scholar]
- 14.Kostura M J, Tocci M J, Limjuco G, Chin J, Cameron P, Hillman A G, Chartrain N A, Schmidt J A. Identification of a monocyte specific pre-interleukin 1β convertase activity. Proc Natl Acad Sci USA. 1989;86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lonon M K, Woods D E, Straus D C. The effects of purified 25 kDa lipase from a clinical isolate of Pseudomonas cepacia in the lungs of rats. Curr Microbiol. 1992;25:89–93. doi: 10.1007/BF01570965. [DOI] [PubMed] [Google Scholar]
- 17.Manniello J M, Heymann H, Adair F W. Isolation of atypical lipopolysaccharides from purified cell walls of Pseudomonas cepacia. J Gen Microbiol. 1979;112:397–400. doi: 10.1099/00221287-112-2-397. [DOI] [PubMed] [Google Scholar]
- 18.Manthey C L, Vogel S N. Elimination of trace endotoxin protein from rough chemotype LPS. J Endotoxin Res. 1994;1:84–91. [Google Scholar]
- 19.Matsuda T, Hirano T, Kishimoto T. Establishment of an interleukin 6 (IL-6)/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL-6 monoclonal antibodies. Eur J Immunol. 1987;18:951–956. doi: 10.1002/eji.1830180618. [DOI] [PubMed] [Google Scholar]
- 20.McKevitt A I, Bajaksouzian S, Klinger J D, Woods D E. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect Immun. 1989;57:771–778. doi: 10.1128/iai.57.3.771-778.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison D C, Betz S J, Jacobs D M. Isolation of a lipid A bound polypeptide responsible for ‘LPS-initiated’ mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976;144:840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 23.Mosman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assay. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Obermeier F, Gross V, Schölmerich J, Falk W. Interleukin-1 production by mouse macrophages is regulated in a feedback fashion by nitric oxide. J Leukoc Biol. 1999;66:829–836. doi: 10.1002/jlb.66.5.829. [DOI] [PubMed] [Google Scholar]
- 25.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Bridwell D, Alejos E, Silva M, Galanos C, Freudenberg C, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutation in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 26.Prince A. Antibiotic resistance of Pseudomonas species. J Pediatr. 1986;108:830–834. doi: 10.1016/s0022-3476(86)80753-3. [DOI] [PubMed] [Google Scholar]
- 27.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, Padova F D, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 28.Ruff M R, Gilfford G E. Purification and physiological characterization of rabbit tumor necrosis factor. J Immunol. 1980;125:1671–1677. [PubMed] [Google Scholar]
- 29.Schroeder R A, Cai C, Kuo P C. Endotoxin-mediated nitric oxide synthesis inhibits IL-1β gene transcription in ANA-1 murine macrophages. Am J Physiol. 1999;277:C523–C530. doi: 10.1152/ajpcell.1999.277.3.C523. [DOI] [PubMed] [Google Scholar]
- 30.Shaw D, Poxton I R, Govan J R W. Biological activity of Burkholderia (Pseudomonas) cepacia lipopolysaccharide. Immunol Med Microbiol. 1995;11:99–106. doi: 10.1111/j.1574-695X.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 31.Straus D C, Lonon M K, Hutson J C. Inhibition of rat alveolar macrophage phagocytic function by a Pseudomonas cepacia lipase. J Med Microbiol. 1992;37:335–340. doi: 10.1099/00222615-37-5-335. [DOI] [PubMed] [Google Scholar]
- 32.Straus D C, Lonon M K, Woods D E, Garner C W. 3-Deoxy-d-manno-2-octulosonic acid in the lipopolysaccharide of various strains of Pseudomonas cepacia. J Med Microbiol. 1990;33:265–269. doi: 10.1099/00222615-33-4-265. [DOI] [PubMed] [Google Scholar]
- 33.Straus D C, Lonon M K, Woods D E, Garner C W. Production of an extracellular toxic complex by various strains of Pseudomonas cepacia. J Med Microbiol. 1989;30:17–22. doi: 10.1099/00222615-30-1-17. [DOI] [PubMed] [Google Scholar]
- 34.Straus D C, Woods D E, Lonon M K, Garner C W. The importance of extracellular antigens in Pseudomonas cepacia infection. J Med Microbiol. 1988;26:269–280. doi: 10.1099/00222615-26-4-269. [DOI] [PubMed] [Google Scholar]
- 35.Sultzer B M, Goodman G W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ.lymphocytes. J Exp Med. 1976;144:821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westphal O, Lüderitz O, Bister F. Über die Extraction von Bakterien mit Phenol/Wasser. Z Naturforsch Teil B. 1952;7:148–155. [Google Scholar]
- 37.Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 38.Zughaier S M, Ryley H C, Jackson S K. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect Immun. 1999;67:1505–1507. doi: 10.1128/iai.67.3.1505-1507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zughaier S M, Ryley H C, Jackson S K. A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect Immun. 1999;67:908–913. doi: 10.1128/iai.67.2.908-913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]