Abstract
Background
SARS-CoV-2 invades human cells and leads to COVID-19 by direct associating with angiotensin converting enzyme 2 (ACE2) receptors, the level of which may be increased by treatment with angiotensin-converting enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs). This meta-analysis aimed to explore the impact of ACEI/ARB treatment on the clinical outcomes of patients with COVID-19 infections among population in the East-Asia region.
Methods
We collected clinical data published from January 2000 to May 2022 in the English databases including PubMed, Embase, and the Cochrane Library. Two reviewers independently screened and identified studies that met the prespecified criteria. Review Manager 5.3 software was used to perform the meta-analysis.
Results
A total of 28 articles were included in this analysis. The results showed that patients who were prescribed with ACEI/ARB had a shorter duration of hospital stay [MD = -2.37, 95%CI (-3.59, -1.14), P = 0.000 2] and a lower mortality rate [OR = 0.61, 95% CI (0.52, 0.70), P<0.000 01] than patients who were not on ACEI/ARB. Furthermore, there was no statistically significant difference in disease severity [OR = 0.99, 95% CI (0.83, 1.17), P = 0.90] between individuals receiving ACEI/ARB or not.
Conclusions
This meta-analysis suggested that the use of ACEI/ARB was not associated with adverse clinical outcomes in East-Asian Covid-19 patients and a reduced mortality and shorter duration of hospital stay among East-Asian population (especially for female subjects) was found. Thus, ACEI/ARB should be continued in patients infected by Covid-19.
Introduction
Coronavirus-infected pneumonia COVID-19 or SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) [1] has caused a worldwide pandemic with more than 500 million confirmed infections and over 6 million deaths have been reported as of 1st May 2022 [2]. The pandemic has placed overwhelming pressure on the medical systems globally and has dealt a huge blow to world economies.
Similar to SARS (severe acute respiratory syndrome), the pathogenesis of COVID-19 is closely related to angiotensin converting enzyme 2 (ACE2). It was reported that ACE2 is considered the functional receptor for novel SARS-CoV-2 coronavirus entry into host cells, causing lung, blood vessel, immune and other damage of human beings [3]. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are renin-angiotensin aldosterone system (RAAS) inhibitors, which are widely used for patients with hypertension (HT) and cardiovascular diseases (CVDs). There have been concerns that the use of ACEI/ARB might cause a higher ACE/ACE2 expression and increase the risk of COVID-19 infection and mortality, thus resulting in a worse clinical outcome [4].
To date, some studies and meta-analyses have already explored the correlation between ACEI/ARB and COVID-19, yet they are mostly some small observational studies, or are based on the global population, or analyzed data only up to the early of 2021. The results are still controversial. Considering the relative higher expression of ACE2 in the Asian population compared to other populations [5], the question has been raised of what is the real relationship between ACEI/ARB and COVID-19 in the East-Asian population, and whether such relationship will turn out to be as the same as the global one?
In this study, systematic literature search and meta-analysis aimed to determine a potential association between the prescription of ACEI/ARB and the clinical prognosis COVID-19 in the East-Asian population.
Methods
Search strategy
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [6]. The systematic review protocol was registered via PROSPERO (https://www.crd.york.ac.uk/PROSPERO/; CRD42022322682). The literature search was conducted on the databases of PubMed, Embase, and the Cochrane Library, from January 2000 to May 2022. Using keywords and Medical Subject Headings (MeSH) terms, such as "novel coronavirus pneumonia", "NCP", "2019-nCoV", "COVID-19", "coronavirus disease 2019", "SARS-CoV-2", "angiotensin converting enzyme inhibitors", "angiotensin receptor blockers", "ACEI”, “ARB ". We limited the search condition to human beings. And research published in languages other than English was not included. Search was not limited to any particular diagnosis or reason for prescription of ACEI/ARB.
Literature inclusion and exclusion criteria
Inclusion criteria: (1) study design: cohort study; (2) study population: East-Asian adult (>18 years) hospitalized patients with confirmed COVID-19 infection, diagnosed by clinical laboratory or imaging results; (3) groups: the clinical prognosis of the experimental group (treated with ACEI/ARBs) were compared with the control group (not treated with ACEI/ARBs); (4) assessment of outcomes: mortality, severe infection of COVID-19 (including admission to intensive care unit (ICU), mechanical ventilation use or progressing to severe pneumonia), as well as the duration of hospital stay. Exclusion criteria: (1) literature with insufficient or incorrect data that cannot be analyzed; (2) manuscripts that did not meet the inclusion criteria; (3) repeated publications; (4) articles with only abstracts.
Literature screening and data extraction and quality evaluation
In this study, two reviewers (N.H. and Q.Y.) independently carried out literature screening and identified studies that met the prespecified criteria. Any discrepancy was resolved through discussion by the third independent reviewer (F.F.) to reach a consensus. In terms of quality evaluation, two reviewers independently conducted methodological quality evaluation of the included studies according to the Newcastle-Ottawa Scale (NOS) [7], a 9-point scale ranging from 0 (high risk of bias) to 9 (low risk of bias). According to the NOS score, the studies were divided into high quality (> 7 points), medium quality (3–6 points), and low quality (< 3 points).
Statistical analysis
In this study, Review Manager 5.3 statistical software was used to conduct a meta-analysis of the included literature. Mean Difference (MD) and 95% confidence interval were used for continuous variables, and Odds Ratio (OR) and 95% confidence interval (95% CI) were used for binary variables. If the heterogeneity test P≤0.1 or I2>50%, it indicates that the index is statistically different between the studies, and the Random Effects Model (Random) is used to merge. If the heterogeneity test P>0.1 and I2 <50%, it indicates that there is no statistical difference in this indicator between studies, and the Fixed Effects Model (Fixed) is used for merging. Publication bias was checked by funnel chart analysis.
Results
Literature search results
A preliminary screening based on the search strategy was conducted and identified 1,869 relevant studies. After removing duplicates, 413 papers were deleted. The remaining 1456 articles were screened by reading the titles and abstracts, and 71 articles were retrieved for full-paper review. Finally, 28 articles met the eligibility criteria mentioned above and were included in our meta-analysis (Fig 1). The included researches are all observational studies.
Fig 1. Screening flow chart for the systematic review and metaanalysis.

It includes selecting and exclusion reasons which followed Preferred Reporting Items for Systematic Review and Metaanalysis (PRISMA) statement.
These included articles evaluated a total of 14,963 East-Asian COVID-19 patients, including 7,533 who were prescribed with ACEI/ARBs and 7,430 who were not. All studies had explored the impact of the use of ACEI/ARBs on the prognosis of East-Asian COVID-19 patients. In terms of the clinical prognosis, 23 studies considered mortality as the indicator; 17 studies used the severity of COVID-19 as the indicator; 9 studies employed the duration of hospital stay as the evaluation indicator. We choose death as the most severe clinical outcome. The basic characteristics and quality evaluation of the included studies are shown in Table 1.
Table 1. Basic characteristics of the included studies.
| Study | Region | Research type | Population | Number | ACEI/ARB | Non-ACEI/ARB | Clinical Outcome | NOS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Total | ||||||||||
| Hu J2020 [13] | China | Prospective cohort study | HT | 149 | 65 | 84 | ① | ② | ③ | 4 | 1 | 1 | 6 |
| Huang Z2020 [9] | China | Prospective cohort study | HT | 50 | 20 | 30 | ① | ② | ③ | 4 | 1 | 2 | 7 |
| Tan N-D2020 [8] | China | Retrospective cohort study | HT | 100 | 31 | 69 | ① | ② | ③ | 4 | 1 | 3 | 8 |
| Zhang Y2020 [10] | China | Retrospective cohort study | HT | 75 | 28 | 47 | ① | ② | ③ | 4 | 1 | 3 | 8 |
| Pan W2020 [24] | China | Retrospective cohort study | HT | 282 | 41 | 241 | ② | ③ | 4 | 1 | 3 | 8 | |
| Wang T2021 [11] | China | Retrospective cohort study | HT | 93 | 35 | 58 | ① | ② | ③ | 4 | 1 | 1 | 6 |
| Wang W2021 [12] | China | Retrospective cohort study | HT | 67 | 22 | 45 | ① | ② | 4 | 1 | 2 | 7 | |
| Huang L2021 [23] | China | Retrospective cohort study | Whole a | 152 | 38 | 114 | ② | ③ | 4 | 2 | 2 | 8 | |
| Gao C2020 [17] | China | Retrospective cohort study | HT | 710 | 183 | 527 | ② | ③ | 4 | 2 | 3 | 9 | |
| Li J2020 [18] | China | Retrospective cohort study | HT | 362 | 115 | 247 | ② | ③ | 4 | 1 | 3 | 8 | |
| Li X2020 [19] | China | Retrospective cohort study | Whole b | 545 | 42 | 503 | ② | 4 | 1 | 2 | 7 | ||
| Liu Y2020 [20] | China | Retrospective cohort study | HT | 78 | 22 | 56 | ② | 4 | 1 | 2 | 7 | ||
| Meng J2020 [21] | China | Retrospective cohort study | HT | 42 | 17 | 25 | ② | ③ | 4 | 1 | 3 | 8 | |
| Yang G2020 [14] | China | Retrospective cohort study | HT | 126 | 43 | 83 | ① | ② | ③ | 4 | 1 | 3 | 8 |
| Yuan Y2020 [15] | China | Retrospective cohort study | HT | 232 | 108 | 124 | ① | 4 | 2 | 2 | 8 | ||
| Chen Y2020 [16] | China | Retrospective cohort study | HT | 71 | 32 | 39 | ① | ③ | 4 | 1 | 3 | 8 | |
| Meng X2021 [28] | China | Retrospective cohort study | HT | 259 | 73 | 186 | ③ | 4 | 1 | 3 | 8 | ||
| Wang HY2021 [29] | China | Retrospective cohort study | HT | 590 | 141 | 449 | ③ | 4 | 1 | 3 | 8 | ||
| Zhang P2020 [30] | China | Retrospective cohort study | HT | 1128 | 188 | 940 | ③ | 4 | 2 | 3 | 9 | ||
| Park J2021 [26] | Korea | Retrospective cohort study | HT | 1885 | 1098 | 787 | ② | 4 | 1 | 3 | 8 | ||
| Matsuzawa Y2020 [25] | Japan | Retrospective cohort study | HT | 39 | 21 | 18 | ② | ③ | 4 | 1 | 2 | 7 | |
| Hwang JM2020 [33] | Korea | Retrospective cohort study | Whole c | 103 | 13 | 90 | ③ | 4 | 1 | 3 | 8 | ||
| Bae S2020 [32] | Korea | Retrospective cohort study | HT or CVDs d | 864 | 359 | 505 | ③ | 4 | 2 | 2 | 8 | ||
| Kim JH2021 [27] | Korea | Retrospective cohort study | HT | 1290 | 682 | 608 | ② | ③ | 4 | 1 | 2 | 7 | |
| Lee J2021 [35] | Korea | Retrospective cohort study | HT | 1609 | 1041 | 568 | ③ | 4 | 1 | 2 | 7 | ||
| Kang SH2021 [34] | Korea | Retrospective cohort study | HT | 1044 | 782 | 262 | ③ | 4 | 1 | 1 | 6 | ||
| Seo J2021 [36] | Korea | Retrospective cohort study | HT | 1644 | 1217 | 427 | ③ | 4 | 1 | 3 | 8 | ||
| Bae JH2021 [31] | Korea | Retrospective cohort study | HT | 1374 | 1076 | 298 | ③ | 4 | 1 | 2 | 7 | ||
①duration of hospital stay; ②severity; ③mortality.
HT, hypertension; CVDs, cardiovascular diseases.
# a: HT 36%, CVDs 15%; b: HT 30.3%, CVDs 6.2%; c: HT 55.3%, CVDs 12%; d: HT 98.9%, CVDs 33%.
Meta-analysis results
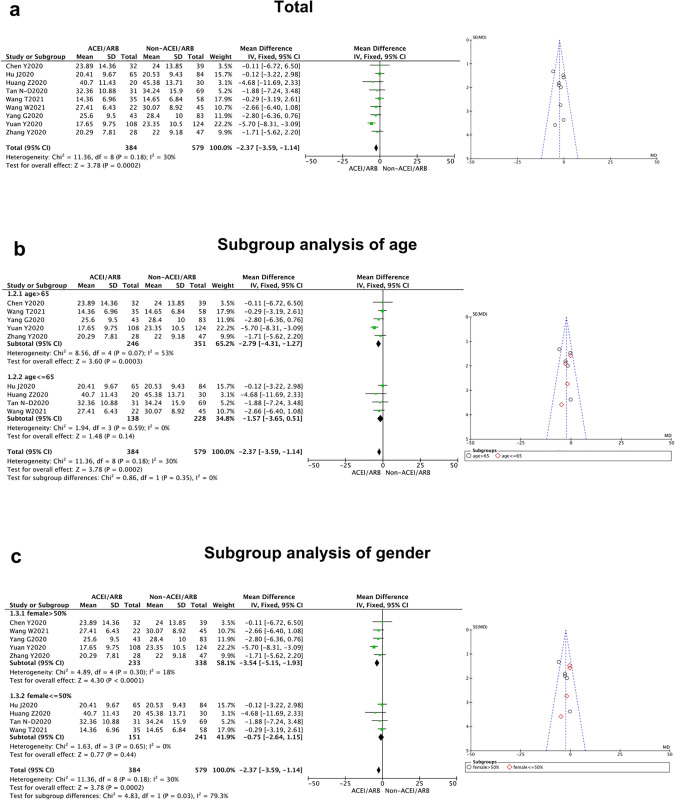
ACEI/ARB usage could shorter duration of hospital stay of COVID-19
Nine studies estimated the impact of ACEI/ARB treatment on the duration of hospital stay in East-Asian patients with COVID-19 [8–16]. The duration of hospital stay of COVID-19 patients treated with ACEI/ARB was significantly shorter than those who did not receive ACEI/ARB treatment [MD = -2.37, 95% CI(-3.59, -1.14), P = 0.000 2], and there was no significant heterogeneity between studies (P = 0.18, I2 = 30%) (Fig 2, Table 2).
Fig 2. Duration of hospital stay of East-Asian Covid-19 patients treated with ACEI/ARB vs. untreated subjects.
The duration of hospital stay data of MD and 95% CI from 9 studies were pooled in this meta-analysis and the result of metaanalysis was described as a forest plot. (a) The whole group analysis; (b)The subgroup analysis of age;(c) The subgroup analysis of gender.
Table 2. Clinical outcomes of East-Asian Covid-19 patients treated with ACEI/ARB vs. untreated subjects (all studies).
| Outcomes | No. of Studies | Pooled OR/MD (95% CI) | P for Heterogeneity | I2 | P | P for subgroup differences | Model Used |
|---|---|---|---|---|---|---|---|
| Duration of hospital stay | 9 [8–16] | -2.37(-3.59, -1.14) | 0.18 | 30% | 0.0002 | Fix | |
| (Mean age) | 0.35 | ||||||
| >65 y | 5 | -2.79(-4.31, -1.27) | 0.07 | 53% | 0.0003 | ||
| < = 65 y | 4 | -1.57(-3.65, 0.51) | 0.59 | 0% | 0.14 | ||
| (Female sex) | 0.03 | ||||||
| >50% | 5 | -3.54(-5.15, -1.93) | 0.30 | 18% | <0.000 01 | ||
| < = 50% | 4 | -0.75 (-2.64, 1.15) | 0.65 | 0% | 0.44 | ||
| Severity | 17 [8–13, 17–27] | 0.99 (0.83,1.17) | 0.15 | 27% | 0.90 | Fix | |
| (Mean age) | 0.21 | ||||||
| >65 y | 7 | 0.85(0.63, 1.14) | 0.05 | 52% | 0.27 | ||
| < = 65 y | 10 | 1.07 (0.87, 1.32) | 0.54 | 0% | 0.53 | ||
| (Female sex) | 0.20 | ||||||
| >50% | 4 | 1.21(0.85, 1.72) | 0.26 | 25% | 0.29 | ||
| < = 50% | 13 | 0.93(0.76, 1.13) | 0.18 | 27% | 0.46 | ||
| Mortality | 23 [8–11, 13, 16–18, 21–25, 27–36] | 0.61 (0.52,0.70) | 0.19 | 20% | <0.000 01 | Fix | |
| (Mean age) | 0.66 | ||||||
| >65 y | 12 | 0.59(0.49, 0.71) | 0.09 | 38% | <0.000 01 | ||
| < = 65 y | 11 | 0.63(0.49, 0.81) | 0.48 | 0% | 0.0004 | ||
| (Female sex) | 0.31 | ||||||
| >50% | 6 | 0.65(0.53, 0.79) | 0.34 | 12% | <0.0001 | ||
| < = 50% | 17 | 0.56(0.44, 0.70) | 0.15 | 26% | <0.000 01 |
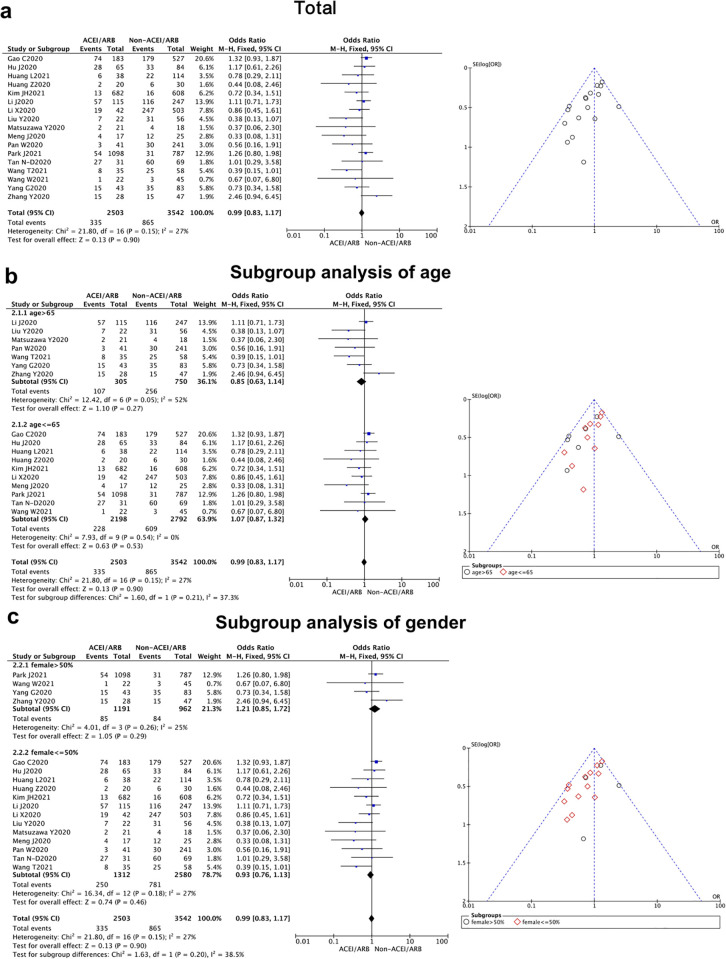
ACEI/ARB usage would not further worsen the severity of COVID-19
A total of 17 articles compared whether the use of ACEI/ARB affect the severity of COVID-19 [8–13, 17–27]. Our study showed that there was no significant difference in the severity of coronavirus disease between the two groups [OR = 0.99, 95% CI (0.83, 1.17), P = 0.90] (Fig 3, Table 2). Our research result indicated that the use of ACEI/ARB will not further worsen the severity of this pneumonia. There was no statistical heterogeneity among the studies (P = 0.15, I2 = 27%), so a fixed-effect model can be used to combine results. A funnel chart test found that the distribution of the funnel chart was basically symmetrical, showing an inverted funnel shape, suggesting that the publication bias of this part of study was low (Fig 3).
Fig 3. Severity of East-Asian Covid-19 patients treated with ACEI/ARB vs. untreated subjects.
The severity data of OR and 95% CI from 17 studies were pooled in this meta-analysis and the result of meta-analysis was described as a forest plot. (a) The whole group analysis; (b) The subgroup analysis of age; (c) The subgroup analysis of gender.
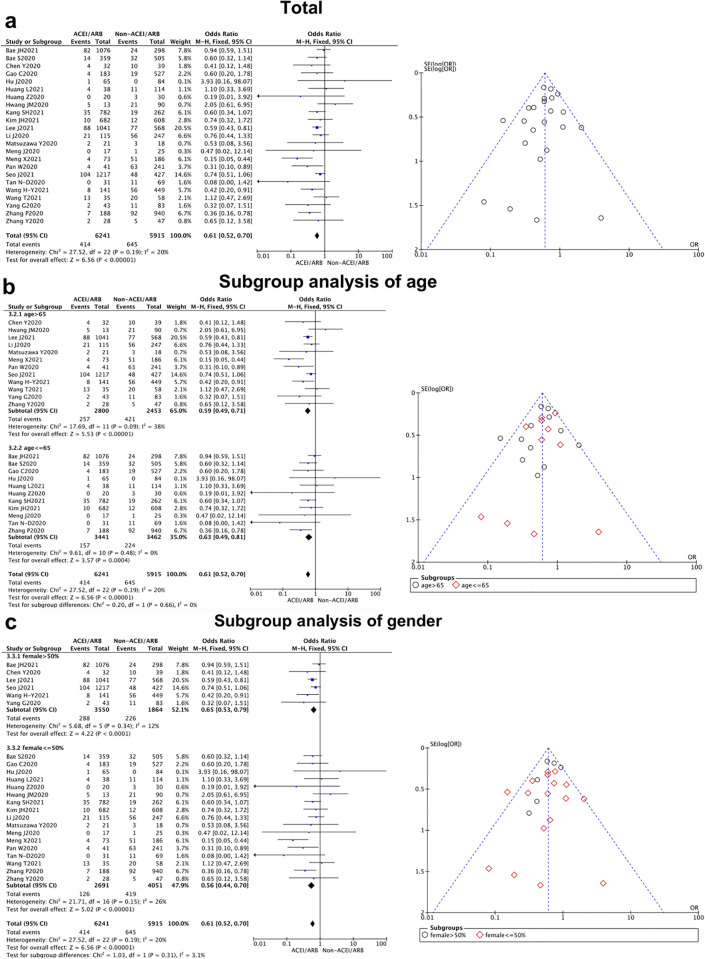
ACEI/ARB usage could decrease the mortality of COVID-19
Of the literature included in our analysis, 23 articles compared the mortality of COVID-19 patients treated with and without ACEI/ARB [8–11, 13, 16–18, 21–25, 27–36]. The result of analysis pointed out that there was a statistically significant difference in mortality between whether ACEI/ARB were used or not [OR = 0.61, 95% CI (0.52, 0.70), P<0.000 01] (Fig 4, Table 2). The mortality of COVID-19 patients who were prescribed with ACEI/ARB was lower than that of patients who were not. The fixed effect model was used to combine studies as no statistical difference was found between groups(P = 0.19, I2 = 20%). The publication bias analysis of related studies on the impact of ACEI/ARB on the mortality of COVID-19 patients is shown in Fig 4. The scattered points of the funnel chart are concentrated on both sides of the invalid line, in an inverted funnel shape, and the distribution on both sides is basically symmetrical, indicating that there is low publication bias.
Fig 4. Mortality of East-Asian Covid-19 patients treated with ACEI/ARB vs. untreated subjects.
All-cause mortality data of OR and 95% CI from 23 studies were pooled in this meta-analysis and the result of meta-analysis was described as a forest plot. (a) The whole group analysis; (b) The subgroup analysis of age; (c) The subgroup analysis of gender.
Was the effect of ACEI/ARB influenced by age or gender?
When we separated the studies by age and gender for subgroup analysis, a significant difference could be found in the duration of hospital stay between the female and male patients (P = 0.03), while no statistical difference was revealed from the age subgroups analysis (P = 0.35). It showed East-Asian female patients could benefit further from ACEI/ARB prescription with a shorter duration of hospital stay. In addition, we performed the same subgroup analysis in other clinical outcomes (severity and mortality), and no meaningful differences could be detected between the elder and younger patients (P = 0.21 for severity, P = 0.66 for mortality), as well as female vs. male subgroups (P = 0.20 for severity, P = 0.31 for mortality) (Figs 2–4).
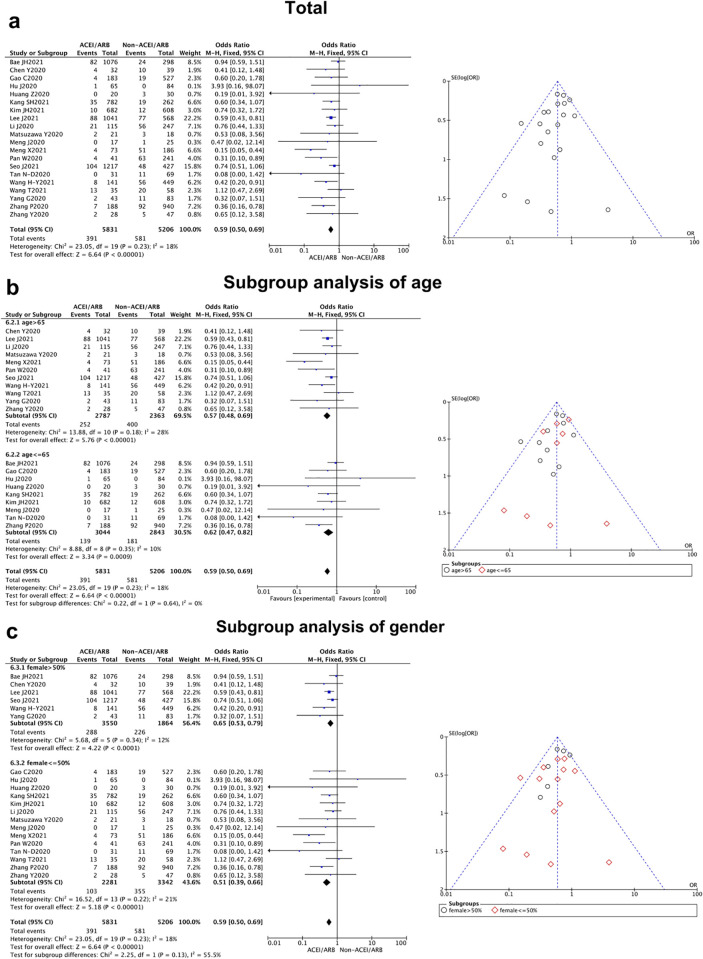
The effect of ACEI/ARB is more obvious in hypertension population
ACEI/ARB is one of the most widely used anti-hypertensive medications. 24 of the included studies were on hypertension patients and the remaining 4 studies included other patients. We had also conducted subgroup analysis to explore the impact of ACEI/ARB among hypertension patients. The subgroup analysis revealed a more obvious reductions in mortality among patients in the hypertension subgroup who were receiving ACEI/ARB [OR = 0.59, 95% CI (0.50, 0.69), P<0.000 01] (Fig 5, Table 3). Additionally, for the outcome of severity in the hypertension subgroup, the result indicated a non-statistically significant difference between whether ACEI/ARB was used or not (Fig 6, Table 3). Analysis of mortality in the CVDs subgroup showed a similar fall in mortality but it was not significant (S1 Table).
Fig 5. Mortality of East-Asian Covid-19 patients treated with ACEI/ARB vs. untreated subjects (HT subgroup).
All-cause mortality data of OR and 95% CI from 20 studies were pooled in this meta-analysis and the result of meta-analysis was described as a forest plot. (a) The whole group analysis; (b) The subgroup analysis of age; (c) The subgroup analysis of gender.
Table 3. Clinical outcomes of East-Asian Covid-19 patients treated with ACEI/ARB vs. untreated subjects (HT subgroup).
| Outcomes | No. of Studies | Pooled OR (95% CI) | P for Heterogeneity | I2 | P | P for subgroup differences | Model Used |
|---|---|---|---|---|---|---|---|
| Severity | 15 [8–13, 17, 18, 20–22, 24–27] | 1.01 (0.84,1.21) | 0.09 | 34% | 0.93 | Fix | |
| (Mean age) | 0.17 | ||||||
| >65 y | 7 | 0.85(0.63, 1.14) | 0.05 | 52% | 0.27 | ||
| < = 65 y | 8 | 1.12 (0.89, 1.40) | 0.44 | 0% | 0.33 | ||
| (Female sex) | 0.24 | ||||||
| >50% | 4 | 1.21(0.85, 1.72) | 0.26 | 25% | 0.29 | ||
| < = 50% | 11 | 0.95(0.77, 1.17) | 0.10 | 38% | 0.60 | ||
| Mortality | 20 [8–11, 13, 16–18, 21, 22, 24, 25, 27–31, 34–36] | 0.59 (0.50,0.69) | 0.23 | 18% | <0.000 01 | Fix | |
| (Mean age) | 0.64 | ||||||
| >65 y | 11 | 0.57(0.48, 0.69) | 0.18 | 28% | <0.000 01 | ||
| < = 65 y | 9 | 0.62(0.47, 0.82) | 0.35 | 10% | 0.0009 | ||
| (Female sex) | 0.13 | ||||||
| >50% | 6 | 0.65(0.53, 0.79) | 0.34 | 12% | <0.0001 | ||
| < = 50% | 14 | 0.51(0.39, 0.66) | 0.22 | 21% | <0.000 01 |
Fig 6. Severity of East-Asian Covid-19 patients treated with ACEI/ARB vs. untreated subjects (HT subgroup).
The severity data of OR and 95% CI from 15 studies were pooled in this meta-analysis and the result of meta-analysis was described as a forest plot. (a) The whole group analysis; (b) The subgroup analysis of age; (c) The subgroup analysis of gender.
Discussion
This meta-analysis systematically evaluated the impact of ACEI/ARB on the clinical prognosis of COVID-19 patients in East-Asia. The result of the study showed that ACEI/ARB would not aggravate the severity of a new COVID-19 infection in East-Asian patients, nor would it prolong the duration of hospital stay of East-Asian patients with COVID-19, and that the use of ACEI/ARB can reduce the risk of death to a certain extent, especially those with hypertension.
Further, the results of this study have potentially important implications for clinical practice. Recently, there has been controversy regarding the use of RAAS system inhibitors during the COVID-19 pandemic. On the one hand, some experts believed that the application of ACEI/ARB would accelerate the spread of COVID-19 and increase the severity of new COVID-19 induced pneumonia, so the drugs should be stopped [37]. On the other hand, some researchers believed that RAAS system inhibitors would not only not worsen the clinical prognosis of COVID-19, but also reduce the mortality rate, so they should continue to be used [38].
Multiple studies have shown that individuals with hypertension and other cardiovascular diseases are more likely to be infected with COVID-19, and further had the worse clinical outcomes [39]. Research has demonstrated that the pathophysiology of COVID-19 is similar to SARS: the novel coronavirus enters cell by binding to the ACE2 receptor of the host-cell. The ACE2 receptor is highly expressed in the human body, such as in lung tissues, the digestive system, vascular endothelial cells and vascular smooth muscle cells. The spike protein of SARS-CoV-2 mainly binds to ACE2 in human’s respiratory and lung tissues as a cellular receptor, leading to a series of pathological changes and causing damage to the respiratory system. ACEI/ARB are widely used in the treatment of hypertension and cardiovascular diseases. Animal experiments have shown that ACEI/ARB could inhibit ACE while increasing ACE2 receptors [40]. Thus, some experts hypothesize that these medications may increase the susceptibility to viruses by increasing the expression of ACE2 receptor, thereby speeding up the spread of COVID-19 [41]. However, our results do not support this statement. Although some studies have reported that RAAS system inhibitors may increase the expression of ACE2 receptors, the results of these tests are not consistent. Most of them are animal tests, and a small number of human tests are studies with small sample sizes. Animal studies about the effect of ACEI/ARB on ACE2 are mostly focused on heart and kidney tissues [42]. And human studies mainly detect circulating ACE2 or soluble ACE2 receptor levels in the urine or plasma, and these indicators may not truly reflect the ACE2 levels in other tissues and organs [43, 44]. In 2017, a human study by Masato et al. evaluated the expression of Urinary and Plasma ACE2 receptors in 605 patients who used RAAS system inhibitors. They found the use of RAAS system inhibitors is not an independent predictor of the Urinary and Plasma ACE2 levels, and their levels could also be influenced by other factors like high blood pressure, liver dysfunction and so on [45]. We know that SARS-CoV-2 enters the human body mainly through the ACE2 receptor of respiratory tract. The concentration of medication in different organs and tissues is different, and the expression and distribution of enzymes and receptors in different organs and tissues are significantly different as well. Thus, ACEI/ARB may not have the similar effect in lung tissue as in other tissues or organs. Additionally, a recent study finds that ACE2 levels are not increased in the respiratory tract in patients taking long term ACEI and ARBs [46]. But further studies are required to confirm this finding and its relevance to the action of ACEI/ARBs in reducing mortality. As noted above many other organs and systems are potentially involved and outcome may be determined more by these than the infection in the lung per se.
It has pointed out that the current evidence does not support the harmful effects of RAAS system inhibitors on COVID-19 infection, and it is recommended not to discontinue RAAS system inhibitors [47]. In fact, the evidence of its benefits is substantial. Our meta-analysis also confirmed this opinion. Some researchers even advocate the continued use of RAAS system inhibitors, because the RAAS system is a key target for the treatment of acute lung injury [48]. ACE2 is not only the receptor to help SARS-CoV-2 entering into cell but also will be down-regulate by this virus, subsequently reducing the increased level of angiotensin II which may play a great role of organ injury in COVID-19. Nevertheless, ACEI/ARBs could inhibit the activity of angiotensin II, as well as increase the expression of ACE2 to offset the reduction of ACE2 in lung tissue caused by the virus, thereby play a protective role [49]. More notably, some previous researchers have demonstrated in patients with pneumonia and acute respiratory distress syndrome that ACEI/ARB treatment could improve clinical outcomes [50]. Recently, some studies also found ACEI/ARB can significantly reduce COVID-19 caused pulmonary inflammation by inhibiting the levels of IL-6, C-reactive protein and calcitonin [14, 21].
At the same time, RAAS system inhibitors have been proven to be effective in protecting the cardiovascular and renal system [51]. Discontinuation of ACEI/ARB will increase the chance of decompensation in high-risk patients, because the specific benefits of ACEI/ARB in slowing down ventricular remodeling cannot be replaced by other drugs. It is in consistent with our meta-analysis result that the CVDs subgroup prescribed with ACEI/ARB showed an observable decline in mortality. Although such decline was not significant, probably because of small numbers. And this question can be explored by future research. In addition, the proper prescription for hypertension requires frequent adjustment of dosages and good management of adverse reactions. Changing prescription would increase the times of hospital visiting, thereby increasing the patients’ exposure time, and finally raising the risk of COVID-19 infection.
Currently, as the sample size of clinical studies on COVID-19 is not large enough, the results of meta-analysis would more truly reflect the reality through combining multiple clinical trials, adjusting weight and model according to the research design, sample size and results. The main advantages of our meta-analysis are large samples and comprehensive searches to provide more accurate and reliable analyses.
In addition, the meta-analyses reported so far have mostly been multiracial analysis; there are few studies targeting the East-Asian population alone. Different races have different numbers of ACE2 receptors, which leads to different responses to medication. Studies have shown that the expression of ACE2 is relatively high among Asian women and young people [5]. Therefore, a meta-analysis specifically targeted at the East-Asian population is particularly important for the guidance of prescription of ACEI/ARB.
Additionally, we investigated whether age and gender might be relevant, ACE2 receptor density is comparatively high in Asian women and a younger population. Our meta-analysis of clinical studies finding was consistent with the result revealed from the basic studies at the molecular level that Asian females with higher ACE2 expression have the better clinical outcome [5]. Based on that, gender may be the possible reason that influences the clinical outcome, especially in duration of hospital stay. However, we could not find any statistical difference in severity and mortality between subgroups of elder vs. younger and female vs. male patients. It illustrated that East-Asian COVID-19 patients prescribed with ACEI/ARB had lower mortality, regardless of whether they were elder or younger, female or male.
However, this meta-analysis still has some limitations. Firstly, the included articles are all observational. Observational studies could only reflect correlations but cannot clarify the causal relationship. Therefore, it is impossible to confirm the causal relationship between ACEI/ARB treatment and the clinical prognosis of COVID-19 patients. Further research, such as large randomized controlled clinical trials, are still needed to confirm our results. Second, most studies lack specific data of RAAS inhibitor dosage, and different dosages may cause different results, which may affect our results. Third, as most of the included literature is retrospective studies, recall bias is inevitable, which may affect the reliability of the conclusions.
Conclusions
Currently, millions of patients in the world have used ACEI/ARB, and the COVID-19 epidemic is still raging, especially due to the recent outbreak of novel coronaviruses of Delta and Omicron. Therefore, there is an urgent need for medication guidance for ACEI/ARB. Our result shows that for East-Asian COVID-19 patients, the use of ACEI/ARB will not increase the severity of COVID-19, and the use of ACEI/ARB can significantly reduce duration of hospital stay (especially for female population) and all-cause mortality of COVID-19 patients. These results indicate that COVID-19 patients should continue to receive treatment with ACEI/ARB, especially among the patients who are currently using ACEI/ARB. However, it still needs further animal experiments and large prospective studies to confirm these results, and to explore the possible protective mechanism of ACEI/ARB.
The results of our research provide new additional information to develop guidelines for the use of ACEI/ARB treatment during the ongoing COVID-19 epidemic, and may help to control the spread of the virus and improve the clinical outcome of COVID-19 patients.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020; 76:71–6. doi: 10.1016/j.ijsu.2020.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO coronavirus disease (COVID-19) dashboard 2020 [cited 2022 1 May]. https://covid19.who.int/.
- 3.Azevedo RB, Botelho BG, Hollanda JVG, Ferreira LVL, Junqueira de Andrade LZ, Oei S, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021; 35(1):4–11. doi: 10.1038/s41371-020-0387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020; 5(7):802–10. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Jiang Q, Xia X, Liu K, Yu Z, Tao W, et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020; 19(7). doi: 10.1111/acel.13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009; 339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses: The Ottawa Hospital Research Institute; 2019 [cited 2022 February 1]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 8.Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations Between Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blocker Use, Gastrointestinal Symptoms, and Mortality Among Patients With COVID-19. Gastroenterology. 2020; 159(3):1170–2 e1. doi: 10.1053/j.gastro.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z, Cao J, Yao Y, Jin X, Luo Z, Xue Y, et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020; 8(7):430. doi: 10.21037/atm.2020.03.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Sha T, Wu F, Hu H, Chen Z, Li H, et al. Hypertension in Patients Hospitalized with COVID-19 in Wuhan, China. Int Heart J. 2021; 62(2):337–43. doi: 10.1536/ihj.20-323 [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Tang R, Ruan H, Chen R, Zhang Z, Sang L, et al. Predictors of fatal outcomes among hospitalized COVID-19 patients with pre-existing hypertension in China. Clin Respir J. 2021; 15(8):915–24. doi: 10.1111/crj.13382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Zhao X, Wei W, Fan W, Gao K, He S, et al. Angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) may be safe for COVID-19 patients. BMC Infect Dis. 2021; 21(1):114. doi: 10.1186/s12879-021-05821-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Zhang X, Zhang X, Zhao H, Lian J, Hao S, et al. COVID-19 is more severe in patients with hypertension; ACEI/ARB treatment does not influence clinical severity and outcome. J Infect. 2020; 81(6):979–97. doi: 10.1016/j.jinf.2020.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, et al. Effects of Angiotensin II Receptor Blockers and ACE (Angiotensin-Converting Enzyme) Inhibitors on Virus Infection, Inflammatory Status, and Clinical Outcomes in Patients with COVID-19 and Hypertension: A Single-Center Retrospective Study. Hypertension. 2020; 76(1):51–8. doi: 10.1161/HYPERTENSIONAHA.120.15143 [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Liu D, Zeng S, Wang S, Xu S, Wang Y, et al. In-hospital use of ACEI/ARB is associated with lower risk of mortality and critic illness in COVID-19 patients with hypertension. J Infect. 2020; 81(5):816–46. doi: 10.1016/j.jinf.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care. 2020; 43(7):1399–407. doi: 10.2337/dc20-0660 [DOI] [PubMed] [Google Scholar]
- 17.Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020; 41(22):2058–66. doi: 10.1093/eurheartj/ehaa433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020; 5(7):825–30. doi: 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020; 146(1):110–8. doi: 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Huang F, Xu J, Yang P, Qin Y, Cao M, et al. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020; 03.20:20039586. doi: 10.1101/2020.03.20.20039586 [DOI] [Google Scholar]
- 21.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020; 9(1):757–60. doi: 10.1080/22221751.2020.1746200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guang Yang ZT, Ling Zhou, Min Yang, Lang Peng, Jinjin Liu, Jingling Cai, et al. Effects Of ARBs And ACEIs On Virus Infection, Inflammatory Status And Clinical Outcomes In COVID-19 Patients With Hypertension: A Single Center Retrospective Study. Hypertension. 2020; 76(4). doi: 10.1161/HYPERTENSIONAHA.120.15143 [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Chen Z, Ni L, Chen L, Zhou C, Gao C, et al. Impact of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers on the Inflammatory Response and Viral Clearance in COVID-19 Patients. Front Cardiovasc Med. 2021; 8:710946. doi: 10.3389/fcvm.2021.710946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan W, Zhang J, Wang M, Ye J, Xu Y, Shen B, et al. Clinical Features of COVID-19 in Patients With Essential Hypertension and the Impacts of Renin-angiotensin-aldosterone System Inhibitors on the Prognosis of COVID-19 Patients. Hypertension. 2020; 76(3):732–41. doi: 10.1161/HYPERTENSIONAHA.120.15289 [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa Y, Ogawa H, Kimura K, Konishi M, Kirigaya J, Fukui K, et al. Renin-angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020; 43(11):1257–66. doi: 10.1038/s41440-020-00535-8 [DOI] [PubMed] [Google Scholar]
- 26.Park J, Lee SH, You SC, Kim J, Yang K. Effect of renin-angiotensin-aldosterone system inhibitors on Covid-19 patients in Korea. PLoS One. 2021; 16(3):e0248058. doi: 10.1371/journal.pone.0248058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Baek YH, Lee H, Choe YJ, Shin HJ, Shin JY. Clinical outcomes of COVID-19 following the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among patients with hypertension in Korea: a nationwide study. Epidemiol Health. 2021; 43:e2021004. doi: 10.4178/epih.e2021004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng X, Liu Y, Wei C, Zhang K, Zhang Y, Zhong M, et al. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers improved the outcome of patients with severe COVID-19 and hypertension. Sci China Life Sci. 2021; 64(5):836–9. doi: 10.1007/s11427-020-1813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HY, Peng S, Ye Z, Li P, Li Q, Shi X, et al. Renin-angiotensin system inhibitor is associated with the reduced risk of all-cause mortality in COVID-19 among patients with/without hypertension. Front Med. 2021:1–9. doi: 10.1007/s11684-021-0850-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020; 126(12):1671–81. doi: 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae JH, Choi SK, Kim NH, Lee J, Kim SG. Use of Renin-Angiotensin-Aldosterone System Inhibitors and Severe COVID-19 Outcomes in Patients with Hypertension: A Nationwide Cohort Study. Diabetes Metab J. 2021; 45(3):430–8. doi: 10.4093/dmj.2020.0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae S, Kim JH, Kim YJ, Lim JS, Yun SC, Kim YH, et al. Effects of Recent Use of Renin-Angiotensin System Inhibitors on Mortality of Patients With Coronavirus Disease 2019. Open Forum Infect Dis. 2020; 7(11):ofaa519. doi: 10.1093/ofid/ofaa519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang JM, Kim JH, Park JS, Chang MC, Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurological Sciences. 2020; 41(9):2317–24. doi: 10.1007/s10072-020-04541-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang SH, Lee DH, Han KD, Jung JH, Park SH, Dai AM, et al. Hypertension, renin-angiotensin-aldosterone-system-blocking agents, and COVID-19. Clin Hypertens. 2021; 27(1):11. doi: 10.1186/s40885-021-00168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Jo SJ, Cho Y, Lee JH, Oh IY, Park JJ, et al. Effects of renin-angiotensin system blockers on the risk and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with hypertension. Korean J Intern Med. 2021; 36(Suppl 1):S123–s31. doi: 10.3904/kjim.2020.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo J, Son M. Update on association between exposure to renin-angiotensin-aldosterone system inhibitors and coronavirus disease 2019 in South Korea. Korean J Intern Med. 2021; 36(Suppl 1):S114–s22. doi: 10.3904/kjim.2020.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am J Respir Crit Care Med. 2020; 201(11):1380–8. doi: 10.1164/rccm.202002-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The International Society of Hypertension. A statement from the International Society of Hypertension on COVID-19. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/.
- 39.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020; 55(5). doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004; 43(5):970–6. doi: 10.1161/01.HYP.0000124667.34652.1a [DOI] [PubMed] [Google Scholar]
- 41.Garami A. Is there a magic bullet to save COVID-19 patients? We can give it a try! BMJ British Medical Journal. 2020. [Google Scholar]
- 42.Klimas J, Olvedy M, Ochodnicka-Mackovicova K, Kruzliak P, Cacanyiova S, Kristek F, et al. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med. 2015; 19(8):1965–74. doi: 10.1111/jcmm.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariana CP, Ramona PA, Ioana BC, Diana M, Claudia RC, Stefan VD, et al. Urinary angiotensin converting enzyme 2 is strongly related to urinary nephrin in type 2 diabetes patients. Int Urol Nephrol. 2016; 48(9):1491–7. doi: 10.1007/s11255-016-1334-8 [DOI] [PubMed] [Google Scholar]
- 44.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015; 47(4):693–705. doi: 10.1007/s00726-014-1889-6 [DOI] [PubMed] [Google Scholar]
- 45.Furuhashi M, Sakai A, Tanaka M, Higashiura Y, Mori K, Koyama M, et al. Distinct Regulation of U-ACE2 and P-ACE2 (Urinary and Plasma Angiotensin-Converting Enzyme 2) in a Japanese General Population. Hypertension. 2021; 78(4):1138–49. doi: 10.1161/HYPERTENSIONAHA.121.17674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee IT, Nakayama T, Wu CT, Goltsev Y, Jiang S, Gall PA, et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat Commun. 2020; 11(1):5453. doi: 10.1038/s41467-020-19145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heart Failure Society of America; American College of Cardiology; American Heart Association. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19- American College of Cardiology.
- 48.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005; 436(7047):112–6. doi: 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020; 382(25):2441–8. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Choi SM, Lee J, Park YS, Lee CH, Yim JJ, et al. Effect of Renin-Angiotensin System Blockage in Patients with Acute Respiratory Distress Syndrome: A Retrospective Case Control Study. Korean J Crit Care Med. 2017; 32(2):154–63. doi: 10.4266/kjccm.2016.00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, et al. Initiation, Continuation, or Withdrawal of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers and Outcomes in Patients Hospitalized With Heart Failure With Reduced Ejection Fraction. J Am Heart Assoc. 2017; 6(2). doi: 10.1161/JAHA.116.004675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.