Abstract
Social housing improves the well-being of monkeys in research settings; however, little is known about factors influencing the long-term stability of established, full-contact pairs. Archival data were examined to determine whether sex, age, weight, duration pair housed, familiarity, social interruptions, room changes, or sedation events predicted eventual separation of pairs for social incompatibility (n = 80) or for nonsocial reasons (e.g., research or health needs) (n = 1143). Using a logistic regression model (Wald X2(8) = 42.325, p < .001), three significant factors were identified. Pairs in which partners had known prior familiarity in group housing were less likely to experience social incompatibility (p = .034). Pairs housed together longer (p < .001) and who staff had temporarily separated through the placement of a cage divider to reduce physical contact were more likely to require permanent separation for social incompatibility (p < .001); additional analysis revealed that dividers were often placed for social reasons, suggesting early signs of social instability. Findings may be useful for primate caregivers when making decisions about managing social partners.
Keywords: Social housing, primate, pair stability, compatibility, familiarity
Introduction
For nonhuman primates housed in research settings, access to a compatible social partner promotes increased physical activity and species-typical behavior, decreased abnormal and anxious behaviors (DiVincenti & Wyatt, 2011), and can buffer the effects of stressors in the laboratory environment (Gilbert & Baker, 2011; Hennessy, Chun, & Capitanio, 2017; Kikusui, Winslow, & Mori, 2006; Kiyokawa & Hennessy, 2018). While living in large social groups is the most beneficial social arrangement (Gottlieb, Coleman, & Prongay, 2017; Novak, 2004), housing monkeys in pairs allows researchers needed access to the animals while also providing some social contact (Hannibal, Bliss-Moreau, Vandeleest, McCowan, & Capitanio, 2017). Housing nonhuman primates in social pairs is a common practice in research settings in the United States and elsewhere (Jennings, Prescott, & Joint Working Group on Refinement (Primates), 2009; DiVincenti & Wyatt, 2011; Rennie & Buchanan-Smith, 2006).
Successful social housing continues to be a focus of facilities that house nonhuman primates and is required to meet the mandate set forth in the Guide for the Care and Use of Laboratory Animals (National Research Council (NRC), 2011), which is used globally for accreditation by AAALAC International. Earlier studies have investigated factors that influence the success of initial social introductions and have found that individual monkey characteristics, including sex, age, and temperament, pair characteristics like familiarity and weight disparity, and environmental factors contribute to the establishment of compatible pairs (Capitanio, Blozis, Snarr, Steward, & McCowan, 2017; Coleman, 2017; DiVincenti & Wyatt, 2011; Rox, Vliet, Sterk, Langermans, & Louwerse, 2019; Truelove, Martin, Perlman, Wood, & Bloomsmith, 2017b). However, even after a successful introduction, some pairs will later require permanent separation due to social incompatibility as indicated by fighting or resource monopolization. Some of the factors that can contribute to a shorter pair tenure (i.e., the number of days pair housed) include maturation into adulthood, changes in pair member rank, and conspecific composition within a dyad’s homeroom (Hannibal et al., 2017). Although it has been shown that sex, kinship, temperament, and relationship quality can influence the longevity of peer relationships in young, group housed rhesus (Weinstein & Capitanio, 2012), there is little information on factors that predict long-term social stability in established pairs.
To address this gap in the literature, we used archival data from Yerkes National Primate Research Center (YNPRC) to investigate factors that could predict long-term social stability in established (i.e., living in full contact for a minimum of 30 days) rhesus macaque pairs. Prior research has demonstrated relationships between the success of social introductions and factors such as sex, age, weight differences between pair members, and social familiarity. In rhesus macaque introductions, success rates are higher among younger pairs and are generally lower for males than females once individuals enter adulthood (Truelove et al., 2017b). For adult rhesus males, those with a weight disparity at introduction are more likely to succeed than potential pairs with more similar weights (Capitanio et al., 2017; Doyle, Baker, & Cox, 2008), as the visual size difference may serve to establish a quick clear-cut dominance without physical contact (e.g., Preuschoft & van Schaik, 2000); these findings have not been shown to be true for other age classes of rhesus (Maguire-Herring, Stonemetz, Lynch, & Fahey, 2013; West et al., 2009). It is unknown whether an increasingly greater weight disparity between pair members over time, suggesting food competition (e.g., one animal is not permitted access to food by a cagemate), contributes to social instability. Individuals with prior familiarity while group housed may have an advantage over unfamiliar individuals at introduction because they already have had the opportunity to observe and interact with their partner (Higham et al., 2011). During a dyadic introduction, unfamiliar individuals who establish a clear-cut dominance relationship and those who have prior familiarity (i.e., those previously housed in the same group) behave similarly, whereas pairs with unfamiliar individuals without an obvious dominance order show less grooming and more displacement activities, indicative of social tension (Schino, Maestripieri, Turillazzi, & Scucchi, 1990). Additionally, an absence of aggression has been reported between previously paired young animals when reunited after long-term separation, illustrating familiarity’s role in pair reintroduction (Erwin & Flett, 1974; Erwin, Maple, Willott, & Mitchell, 1974). We hypothesized that these factors could also impact long-term social stability, positing that: (1) adults, particularly males, would be more likely to be separated for social reasons and (2) pairs are more likely to experience social incompatibility if they have been pair housed for a longer duration; (3) paired individuals who develop greater weight disparities over time (indicating possible food monopolization) would be more likely to be separated for social reasons; and (4) paired individuals with social familiarity, such as previously living in the same large social group, would be less likely to be separated for social reasons than those without prior familiarity.
In addition to individual and pair characteristics, environmental variables like management and research-related practices may also play a role in the social dynamics of captive primates. Prior research has shown that some common laboratory events such as relocations and sedation for veterinary or research procedures can be positively correlated with cortisol concentrations, a measurement of stress (Lutz et al., 2016). Further, relocation to a novel environment can elicit both behavioral and physiological changes that indicate stress (e.g., elevated cortisol, sleep disturbance, increased abnormal behavior, chronic diarrhea; Gottlieb et al., 2018), as can separation from a social partner (e.g., Capitanio, Kyes, & Fairbanks, 2006; Truelove, Martin, Perlman, & Bloomsmith, 2017a). Given the impact of these common events on the health and well-being of individuals, we further hypothesized that: (5) pair housed rhesus macaques experiencing a relocation or frequent sedations over a short period of time would be more likely to be separated for social reasons than those not experiencing such events; and (6) temporary social separations sometimes used in the management of captive primates would disrupt long-term social stability.
Materials and methods
Nonhuman animals and housing
Data were compiled on 2,446 rhesus macaque monkeys housed in full contact pairs for at least 30 days at YNPRC between 2007 and 2017. Each of the 1,223 pairs were same sex: 46% female (n = 567), 54% male (n = 656). Monkey ages at introduction ranged from 5 days to 25 years, with an average age at introduction of 4.3 years (SD = 3.7 years). The number of days a pair was housed together ranged from 30 days to 8.2 years (M = 350 days, SD = 351 days). Eighty pairs (6.5%) were separated due to social incompatibility while 1,143 pairs (93.5%) remained socially compatible until they were eventually separated for nonsocial reasons. Common reasons for separation due to social incompatibility included socially inflicted wounding, frequent aggression, or resource (e.g., food, space) monopolization. Nonsocial reasons for permanent separation included health concerns like illness or weight loss, and separations required due to Institutional Animal Care and Use Committee (IACUC) approved research protocols.
Dyads were housed in two, adjoining standard primate cages appropriate for their size and weight (each cage 23 ¾ in x 27 in x 30 in or 33.5 in x 27 in x 32 in). The animals were on a 12:12 hr light cycle, fed a diet of commercial monkey chow (LabDiet 5037, Purina Mills International, St Louis, MO) and a variety of additional produce and foraging items in accordance with the institution’s standard operating procedures, and received water ad libitum. Animal care and use procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and United States Department of Agriculture and Animal and Plant Health Inspection Service (2020) and were approved by the IACUC of Emory University (Atlanta, Ga); the primate center is fully AAALAC-accredited.
Data collection and analyses
Archival data were sourced from YNPRC databases including veterinary, behavioral, and housing records spanning from 2007–2017. For this analysis, we examined data on same sex rhesus macaque pairs who had been housed together in full contact for a minimum of 30 days. We excluded rhesus pairs who were still living together at the time of data compilation because they did not yet have a separation reason, as well as individuals housed in protected contact (i.e., in adjacent caging separated with a perforated divider, permitting limited physical contact but preventing entry by one animal into the other’s cage). Multiple pairing attempts between the same two individuals were also excluded from the analysis.
The data were analyzed using SAS software, version 9.4 of the SAS System for Windows (Copyright © 2019 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA). We ran a logistic regression analysis to investigate the impact of individual characteristics, pair characteristics, and environmental stressor variables on the long-term compatibility of animals (i.e., whether the animals were eventually separated due to social incompatibility or whether they remained compatible until they were separated for nonsocial reasons).
Prior to running the logistic regression, we checked for problematic multicollinearity between the variables. All individual variable correlations were less than 0.8, tolerance levels were above 0.4, and variance inflation factors (VIF) were below 2.5 (see Table 1), indicating that multicollinearity was not a major concern in our model. The logistic regression model was computed between eight independent variables and reason of separation (whether social or nonsocial) with an alpha level of 0.05. The independent variables included sex (male/female), pair age (sum of individual animal ages at introduction), prior familiarity (whether individuals in a dyad were known to have been previously housed together as part of a group or whether they had no or unknown social familiarity), and total days pair housed. A weight difference index was also calculated for each pair to examine how weight difference changed over the duration of the pairing. This was calculated by subtracting the weight difference of the paired individuals at introduction from the weight difference at their separation and dividing by the number of weeks pair housed; a positive value indicated that the weight difference between the individuals grew larger during their pairing and a negative value indicated that the individual weights became more similar. The final independent variables examined environmental events that occurred during the 30 days prior to permanent separation, including whether the pair experienced a social interruption (defined as a temporary interruption in partner access through separation by a cage divider with or without perforation), whether they experienced a room move (defined as relocating to a different room in the facility), and the number of sedation events (calculated as the sum of sedation events for both animals).
Table 1.
Summary of logistic regression analysis for variables predicting a social separation in rhesus macaque pairs (n =1,223) housed in a research setting.
| Variable | B | SE | WALD | SIG | EXP(B) | 95% ODDS CI | TOL | VIF | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | −0.265 | 0.274 | 0.933 | 0.334 | 0.767 | 0.449 | 1.313 | 0.766 | 1.305 |
| Pair age | 0.002 | 0.018 | 0.011 | 0.916 | 1.002 | 0.967 | 1.038 | 0.758 | 1.320 |
| Prior Familiarity | −0.585 | 0.276 | 4.590 | 0.034 | 0.557 | 0.324 | 0.957 | 0.979 | 1.021 |
| Days pair housed | 0.001 | <0.001 | 20.220 | <0.001 | 1.001 | 1.001 | 1.002 | 0.978 | 1.022 |
| Weight difference index | −3.304 | 3.106 | 1.131 | 0.288 | 0.037 | <0.001 | 16.189 | 0.992 | 1.008 |
| Social interruptions | 1.041 | 0.290 | 12.867 | <0.001 | 2.833 | 1.604 | 5.004 | 0.992 | 1.008 |
| Room move | −0.650 | 0.481 | 1.827 | 0.176 | 0.522 | 0.204 | 1.339 | 0.977 | 1.024 |
| Veterinary procedures | −0.028 | 0.046 | 0.370 | 0.543 | 0.972 | 0.890 | 1.064 | 0.985 | 1.015 |
In addition to this analysis, a post-hoc Pearson’s chi-squared test (α = 0.05) was run on the 131 pairs who experienced a temporary (≤ 30 day) social interruption. This analysis served to examine the relationship between the reason for the temporary social interruption (social or nonsocial) and the reason for eventual permanent separation (social or nonsocial).
Results
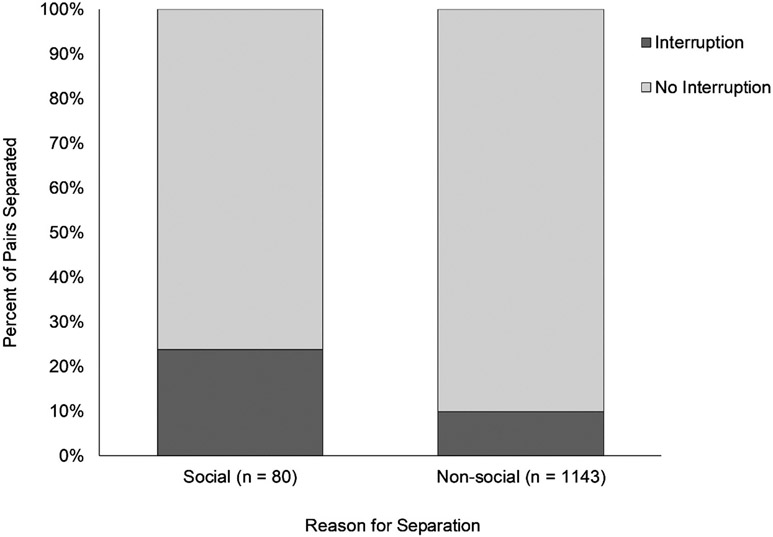
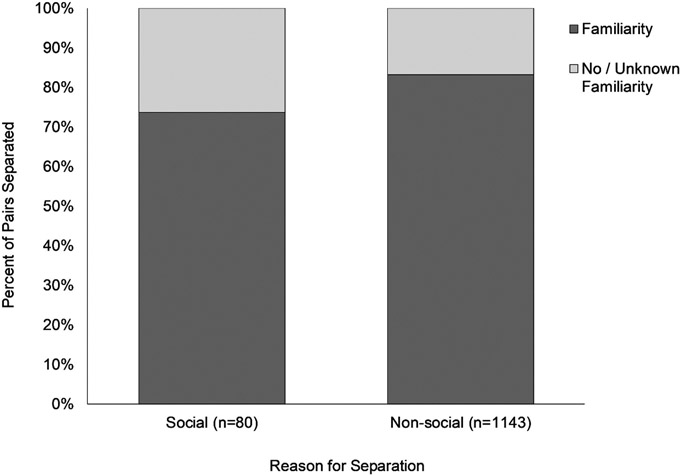
Our overall logistic regression model was significant, Wald X2(8) = 42.325, p < .001. Of our eight variables, three predicted whether the monkeys would require separation due to social reasons (see Table 1). Pairs that eventually required separation due to social incompatibility had been housed together for more days (M = 552) than those separated due to nonsocial reasons (M = 336). Pairs separated because of social incompatibility were more likely to have experienced an interruption in access to their social partner in the 30 days preceding their permanent separation as compared with pairs who remained together until separated for other reasons (see Figure 1). Pairs who were separated due to social incompatibility were more likely to have no or unknown prior social familiarity (see Figure 2).
Figure 1.
The percent of pairs separated for social or nonsocial reasons stratified by whether they experienced a social interruption (i.e., temporary placement of a cage divider) in their last 30 days housed as pairs.
Figure 2.
The percent of pairs separated for social or nonsocial reasons stratified by whether they had prior social familiarity prior to being pair housed.
The post-hoc chi-square test compared the reason for the temporary social interruption and the reason for eventual permanent social separation (i.e., social or nonsocial). We found that of pairs who experienced a social interruption (n = 131), those eventually separated due to social incompatibility were more likely to have had a cage divider placed because of social reasons (63%) than were stable social pairs (2%), X2 (1) = 64.10, p < .001.
Discussion
Our logistic regression model indicated that three variables were significant predictors for separation due to social incompatibility in rhesus macaques living in a research facility: the length of time they were pair housed, whether the pair had prior familiarity, and whether cagemates experienced a temporary interruption in access to their partner (i.e., by staff placement of a cage divider). These findings are in partial support of our hypotheses regarding these variables (hypotheses 2, 4, 6). However, our results did not support our hypotheses that age and sex (1), weight (3), or frequent sedation events or a relocation (5) would influence social stability.
The longer pairs were housed together, the more at-risk they were of being separated due to social incompatibility. In our sample, the majority of animals separated for nonsocial reasons were separated due to research protocol needs (n = 869 of 1143 pairs); therefore, the timing of research needs may have affected this finding. However, with longer pair housing durations, the dynamic relationship between a dyad will be subject to more potential disruptions caused by events such as new conspecifics in their homeroom, as well as alterations to pair interactions as a result of maturation, and individuals’ rank change over time (Hannibal et al., 2017). This finding highlights the need for continued monitoring of social pairs beyond the initial introduction (Truelove et al., 2017b).
Our data suggest that social familiarity may predict the long-term compatibility of monkeys when pair housed and suggests that, when possible, primate managers should identify social partners with prior familiarity from other social settings when selecting monkeys for pairing. These findings are consistent with previous research which has shown that social familiarity plays a role in regulating macaque social behavior (Marler, 1976), particularly when new individuals are introduced to an established group. During these times, some degree of familiarity can blunt the aggressive response of rhesus macaques to strangers (Bernstein, Gordon, & Rose, 1974) shown to develop at young ages and even in the absence of adults (Singh & Gupta, 1980). This is likely occurring in dyads, too; in the absence of kinship, dominance rank guides the interactions of individuals (Snyder-Mackler et al., 2016). Having lived in a group previously, familiar animals will have already established their rank based on matriline membership within that context. Reliance on the display of submissive behaviors to reinforce established rank, shown to be associated with lower risk of wounding after the first 30 days of newly introduced pairs (Pomerantz & Baker, 2017), and engaging in affiliative behaviors (Snyder-Mackler et al., 2016), both of which serve to inhibit aggression (Marler, 1976), may be underlying drivers for why pairs with social familiarity are less likely to be later separated for social reasons. Our findings highlight the importance of prior familiarity in the social stability of established dyads and how it might be used specifically to choose potential partners when pair formation of macaques is required. When possible, those managing primate pairs should select partners with histories of living in the same social group, even if the quality of the relationship between those two monkeys is unknown. If primates are being acquired from a vendor or breeding facility, inquiries should be made about shared group membership.
Temporary cage dividers may be placed between social partners for either social or nonsocial (health or research) reasons. For example, pairs experiencing social difficulties are sometimes managed through short-term protected contact. It is possible that these short-term separations negatively impact the pair’s relationship. We found that the placement of temporary cage dividers was more often due to social (63.2%) rather than nonsocial reasons (36.8%) in pairs eventually separated due to social incompatibility, suggesting that these pairs were already showing early signs of social instability rather than the interruptions causing the instability of the pair. Previous research has shown detrimental effects of separation on the welfare of long-term pair housed macaques (Truelove et al., 2017a), although the type (e.g., mother-infant or peer) and quality of the relationship (e.g., tense or friendly) will impact the severity of the response to a separation (see Capitanio et al., 2006 for review). Given the complexity of social separations and the many interacting factors associated with an archival, applied data set such as ours, further research could focus on more controlled studies investigating the effects of best management practices of temporary separations on rhesus macaque pairs housed in research settings.
In contrast to our predictions, there were no significant relationships found in our data between the reason for final separation (social or nonsocial) and sex, pair age, or weight, contrasting with previous research which found that these factors can influence successful introductions in macaques (Capitanio et al., 2017; Doyle et al., 2008; Truelove et al., 2017b). This suggests that once a pair is successfully introduced, these factors may play a limited role in their long-term success and primate managers should focus on them primarily when considering the success of the initial introduction, not long-term stability. For future consideration, given the importance of individual temperament in the formation of friendship in young rhesus and its influence in the maintenance of these relationships (Weinstein & Capitanio, 2008, 2012), integrating temperament data into pair housing analyses could provide insight into its influence on the long-term stability of relationships in older animals. Determining whether early rearing history and introduction methodology, two factors also known to influence the success of macaque introductions (Truelove et al., 2017b), also have a hand in long-term compatibility is another avenue to pursue to better understand how to manage pairs.
Additionally, we found no significant effect of the occurrence of a room move or the number of sedation events on the long-term stability of social pairs. Generally, pairs were sedated for research-related procedures rather than for clinical or social related accesses. We limited the assessment of relocation and sedations to 30 days prior to separation (because we needed to control for shorter duration pairings of those nearer 30 days), so it is possible that different timeframes could have varying outcomes. Further, we expected that any possible negative effects on social stability these events might lead to would be evidenced nearer them (i.e., individuals experience a stressful event or series of them and soon after the pair is separated for social reasons). This reinforces the idea that having a social partner may function as a buffer from the stress of such events (Gilbert & Baker, 2011; Gottlieb et al., 2018; Hennessy et al., 2017; Kiyokawa & Hennessy, 2018), and it appears these common events in the research setting may occur without endangering the stability of rhesus macaque pairs.
Conclusion
The goal of any social housing program is to provide primates living in captivity with beneficial social interactions that promote psychological well-being. Whenever possible, providing the opportunity for complex social interaction in large groups is recommended to maximize psychological well-being; however, pair housing offers a compromise to achieving research goals while permitting social contact between individuals. When compared with single housing, pair housing of macaques results in more physical activity, a more complete expression of species-typical social behaviors, and a reduction in abnormal behaviors (DiVincenti & Wyatt, 2011). Social introductions carry potential risk to the animals involved because of possible injury or stress, they require specialized housing facilities, and involve considerable effort to arrange and monitor (e.g., Pomerantz & Baker, 2017; Ruhde, Baker, Russell-Lodrigue, Blanchard, & Bohm, 2020; Truelove et al., 2017b; Worlein et al., 2017). Given these costs, one goal of the primate manager is to establish social partnerships that endure. Our findings show that the majority of successful introductions at our facility (93.5%) resulted in a social pair that was able to remain together until they were separated due to research requirements or health concerns; this should reassure primate managers that the time and effort put into establishing these pairs is worthwhile. Doing so allows the monkeys to live in compatible social housing in which they can experience the benefits of pairing for longer durations. Additional research should be undertaken to develop the best practices for the use of temporary cage dividers in the management of social pairs. To further ensure long-term compatibility, primate managers should pair monkeys who are previously familiar with one another whenever possible.
Acknowledgments
We thank Thurman Johnson and Adonis Khupe-Ayema for assistance with gathering of historical data. We thank Jaine Perlman for assistance with logistics coordination and for project feedback. The Yerkes Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Funding
This project was funded by the NIH Office of Research Infrastructure Programs/OD P51OD011132 to the Yerkes National Primate Research Center.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bernstein IS, Gordon TP, & Rose RM (1974). Factors influencing the expression of aggression during introductions to rhesus monkey groups. In Holloway RL (Ed.), Primate aggression, territoriality, and xenophobia: A comparative perspective (pp. 211–240). New York, NY: Academic Press. [Google Scholar]
- Capitanio JP, Blozis SA, Snarr J, Steward A, & McCowan BJ (2017). Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting. American Journal of Primatology, 79(1), e22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, & Fairbanks LA (2006). Considerations in the selection and conditioning of Old World monkeys for laboratory research: Animals from domestic sources. ILAR Journal, 47(4), 294–306. [DOI] [PubMed] [Google Scholar]
- Coleman K (2017). Individual differences in temperament and behavioral management. In Schapiro SJ (Ed.), Handbook of primate behavioral management (pp. 95–114). Boca Raton, FL: CRC Press. [Google Scholar]
- DiVincenti L Jr., & Wyatt JD (2011). Pair housing of macaques in research facilities: A science-based review of benefits and risks. Journal of American Association of Laboratory Animal Science, 50(6), 856–863. [PMC free article] [PubMed] [Google Scholar]
- Doyle LA, Baker KC, & Cox LD (2008). Physiological and behavioral effects of social introduction on adult male rhesus macaques. American Journal of Primatology, 70(6), 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin J, & Flett M (1974). Responses of rhesus monkeys to reunion after long-term separation: Cross-sexed pairings. Psychological Reports, 35(1), 171–174. [Google Scholar]
- Erwin J, Maple T, Willott J, & Mitchell G (1974). Persistent peer attachments of rhesus monkeys: Responses to reunion after two years of separation. Psychological Reports, 34, 1179–1183. [Google Scholar]
- Gilbert MH, & Baker KC (2011). Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. Journal of Medical Primatology, 40(2), 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D, Coleman K, & Prongay K (2017). Behavioral management of Macaca species (except Macaca fascicularis). In Schapiro SJ (Ed.), Handbook of primate behavioral management (pp. 279–304). Boca Raton, FL: CRC Press. [Google Scholar]
- Gottlieb DH, Rosso LD, Sheikhi F, Gottlieb A, McCowan B, & Capitanio JP (2018). Personality, environmental stressors, and diarrhea in Rhesus macaques: An interactionist perspective. American Journal of Primatology, 80(12), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, & Capitanio J (2017). Laboratory rhesus macaque social housing and social changes: Implications for research. American Journal of Primatology, 79(1), e22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Chun K, & Capitanio JP (2017). Depressive-like behavior, its sensitization, social buffering, and altered cytokine responses in rhesus macaques moved from outdoor social groups to indoor housing. Social Neuroscience, 12(1), 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Hughes KD, Brent LJ, Dubuc C, Engelhardt A, Heistermann M, & Maestriperi D (2011). Familiarity affects the assessment of female facial signals of fertility by free-ranging male rhesus macaques. Proceedings of the Royal Society B, 278(1723), 3452–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M, Prescott MJ, Buchanan-Smith HM, Gamble MR, Gore M, Hawkins P, … Buist D, & Joint Working Group on Refinement (Primates). (2009). Refinements in husbandry, care and common procedures for non-human primates: Ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Laboratory Animals, 43(1_suppl), 1–47. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1476), 2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y, & Hennessy MB (2018). Comparative studies of social buffering: A consideration of approaches, terminology, and pitfalls. Neuroscience and Biobehavioral Reviews, 86, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CK, Coleman K, Worlein JM, Kroeker R, Menard MT, Rosenberg K, … Novak MA (2016). Factors influencing alopecia and hair cortisol in rhesus macaques (Macaca mulatta). Journal of Medical Primatology, 45(4), 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire-Herring V, Stonemetz KM, Lynch LJ, & Fahey MA (2013). The effect of weight on the compatibility of isosexual pairs of captive rhesus macaques (Macaca mulatta). American Journal of Primatology, 75(S1), 77. [Abstract]. [Google Scholar]
- Marler P (1976). On animal aggression: The roles of strangeness and familiarity. American Psychologist, 31(3), 239–246. [DOI] [PubMed] [Google Scholar]
- National Research Council. (2011). Guide for the Care and Use of Laboratory Animals: (8th). Washington, DC: The National Academies Press. 10.17226/12910 [DOI] [Google Scholar]
- Novak M (2004). Housing for captive nonhuman primates: The balancing act. In National Research Council (US) Institute for Laboratory Animal ResearchDevelopment of science-based guidelines for laboratory animal care: Proceedings of the November 2003 international workshop (pp. 79–85). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Pomerantz O, & Baker KC (2017). Higher levels of submissive behaviors at the onset of the pairing process of rhesus macaques (Macaca mulatta) are associated with lower risk of wounding following introduction. American Journal of Primatology, 79(8), e22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoft S, & van Schaik CP (2000). Dominance and communication. In Aureli F & De Waal FBM (Eds.), Natural conflict resolution (pp. 77–105). Berkeley: University of California Press. [Google Scholar]
- Rennie AE, & Buchanan-Smith HM (2006). Refinement of the use of non-human primates in scientific research. Part II: Housing, husbandry and acquisition. Animal Welfare, 15(3), 215–238. [Google Scholar]
- Rox A, Vliet AH, Sterk EH, Langermans JA, & Louwerse AL (2019). Factors determining male introduction success and long-term stability in captive rhesus macaques. Plos One, 14(7), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhde AA, Baker KC, Russell-Lodrigue KE, Blanchard JL, & Bohm RP (2020). Trio housing of adult male rhesus macaques (Macaca mulatta): Methodology and outcome predictors. Journal of Medical Primatology, 49(4), 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Maestripieri D, Turillazzi PG, & Scucchi S (1990). Social tension in familiar and unfamiliar pairs of long-tailed macaques. Behaviour, 113(3–4), 264–272. [Google Scholar]
- Singh SD, & Gupta BS (1980). Xenophobic reactions of free-ranging rhesus infant groups raised in natural habitat. Primates, 21(4), 492–497. [Google Scholar]
- Snyder-Mackler N, Kohn JN, Barreiro LB, Johnson ZP, Wilson ME, & Tung J (2016). Social status drives social relationships in groups of unrelated female rhesus macaques. Animal Behaviour, 111, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truelove MA, Martin AL, Perlman JE, & Bloomsmith MA (2017a). Two methods of social separation for paired adolescent male rhesus macaques. Journal of the American Association for Laboratory Animal Science, 56(6), 729–734. [PMC free article] [PubMed] [Google Scholar]
- Truelove MA, Martin AL, Perlman JE, Wood JS, & Bloomsmith MA (2017b). Pair housing of macaques: A review of partner selection, introduction techniques, monitoring for compatibility, and methods for long-term maintenance of pairs. American Journal of Primatology, 79(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture and Animal and Plant Health Inspection Service (2020). USDA animal care: Animal welfare act and animal welfare regulations. Retrieved July 20, 2020, from https://www.aphis.usda.gov/animal_welfare/downloads/AC_BlueBook_AWA_508_comp_version.pdf
- Weinstein TA, & Capitanio JP (2008). Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Animal Behaviour, 76(2), 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein TA, & Capitanio JP (2012). Longitudinal stability of friendships in rhesus monkeys (Macaca mulatta): Individual-and relationship-level effects. Journal of Comparative Psychology, 126(1), 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AM, Leland SP, Collins MW, Welty TM, Wagner WL, & Erwin JM (2009). Pair-formation in laboratory rhesus macaques (Macaca mulatta): A retrospective assessment of a compatibility testing procedure. American Journal of Primatology, 71(S1), 41. [Abstract]. [Google Scholar]
- Worlein JM, Kroeker R, Lee GH, Thom JP, Bellanca RU, & Crockett CM (2017). Socialization in pigtailed macaques (Macaca nemestrina). American Journal of Primatology, 79(1), e22556. [DOI] [PMC free article] [PubMed] [Google Scholar]