Abstract
Organoids, which are multicellular clusters with similar physiological functions to living organs, have gained increasing attention in bioengineering. As organoids become more advanced, methods to form complex structures continue to develop. There is evidence that the extracellular microenvironment can regulate organoid quality. The extracellular microenvironment consists of soluble bioactive molecules, extracellular matrix, and biofluid flow. However, few efforts have been made to discuss the microenvironment optimal to engineer specific organoids. Therefore, this review article examines the extent to which engineered extracellular microenvironments regulate organoid quality. First, we summarize the natural tissue and organ's unique chemical and mechanical properties, guiding researchers to design an extracellular microenvironment used for organoid engineering. Then, we summarize how the microenvironments contribute to the formation and growth of the brain, lung, intestine, liver, retinal, and kidney organoids. The approaches to forming and evaluating the resulting organoids are also discussed in detail.
Impact statement
Organoids, which are multicellular clusters with similar physiological function to living organs, have been gaining increasing attention in bioengineering. As organoids become more advanced, methods to form complex structures continue to develop. This review article focuses on recent efforts to engineer the extracellular microenvironment in organoid research. We summarized the natural organ's microenvironment, which informs researchers of key factors that can influence organoid formation. Then, we summarize how these microenvironmental controls significantly contribute to the formation and growth of the corresponding brain, lung, intestine, liver, retinal, and kidney organoids. The approaches to forming and evaluating the resulting organoids are discussed in detail, including extracellular matrix choice and properties, culture methods, and the evaluation of the morphology and functionality through imaging and biochemical analysis.
Keywords: microfabrication, biomaterials, organoid, hydrogel
Introduction
Engineered cell clusters with similar cellular organization and physiological function to tissues and organs of living organisms, also known as “organoids,” have been gaining more attention in biomaterial and bioengineering research. Organoids create a platform to better understand and regulate the biomolecular and cellular activities responsible for development, homeostasis, and regeneration. In the past, tissue explants excised from human or animal models, called “ex vivo tissue,” were cultured in a controlled extracellular environment for both fundamental and applied bioscience studies. However, these model systems have drawbacks due to imaging limitations, source availability, rapid phenotype loss, and profound differences between species.1 Cell clusters have also emerged as a promising alternative to ex vivo tissues; however, these simple cell aggregates called “spheroids” do not reproduce the delicate ultrastructure and function of their corresponding tissues or organs.
In contrast to ex vivo tissue or spheroids, organoids are generated from pluripotent and multipotent stem cells, progenitor cells, and precursor cells for self-assembly. These cells differentiate to lineage-committed tissue cells and reproduce the developmental niche during the differentiation process. These cell clusters can be molded into various forms such as spheres,2,3 hollow tubes,4,5 and sheets,6,7 all of which can further integrate to form partial or entire organs.
In the early years of organoid engineering, the focus was to control the self-organization of cells to reproduce the ultrastructure of target organs. Recently, the new focus is to establish the physiological functionality of engineered organoids. It is common to induce aggregation between cells in a suspended state. However, this cell culture method disregards the important factor of cell–matrix interactions modulated by extracellular microenvironments. According to developmental biology studies, these microenvironmental factors significantly affect the structure and physiological function of the resulting organs.8 In addition, the cell–matrix interactions regulate the cell–cell interactions and cellular response to soluble factors.9
Accordingly, efforts are increasingly made to design advanced culture platforms of organoid that recapitulate the multifaceted extracellular microenvironment in a bottom-up approach. In this regard, individual or aggregated cells are cultured on a two-dimensional (2D) substrate or in a three-dimensional (3D) matrix that reproduces the in vivo microenvironment. Target extracellular matrices (ECMs) are provisional matrices resulting from injuries, connective tissues of an embryo, or mature tissues. Consequently, cells cultured in a proper platform yield functional organoids, including the optic cup,10 brain,11 intestine,12,13 liver,14 heart,3 and kidney.15
The extracellular microenvironment is engineered by orchestrating an ECM along with soluble factors. Common ECM materials used to develop organoids include Matrigel and collagen. Their popularity stems from their ease of access, implementation in 2D and 3D cell culture platforms, historic reliability for cell culture applications, biocompatibility with cells and biochemicals, and tunability of the elastic modulus. Because they are natural ECM components or can easily be modified with natural proteins, they can be tailored to a variety of different organoid environments. For example, Matrigel can be found in retinal, liver, intestine, lung, and kidney organoid experiments despite an elastic modulus range from 1.8 to 20 kPa. These ECM materials are coated on various microfluidic platforms or substrates made with polydimethylsiloxane (PDMS) to recapitulate in vivo extracellular environments. PDMS is a silicone elastomer that is thermally stable, optically transparent, and biocompatible, thus widely used for cell and organoid culture.16 This review article will explain the applications of these common biomaterials, specific to organoid culture.
This review article outlines extracellular microenvironment variables which factor into the choice of extracellular microenvironments used for organoid formation and growth. In particular, we focus on the extent to which extracellular chemical and mechanical environments modulate cellular organization and subsequent morphology and physiological function of organoids. The role of extracellular microenvironments will be explained with the formation of the brain, lung, intestine, liver, eye, kidney, and cardiovascular organoids. Overall, this review article will significantly serve to advance the development of biological tools useful to improving human health.
Controlling Extracellular Microenvironment for Brain Organoid Assembly
Increasing efforts have been made to advance brain organoids by recapitulating the physiological function of brain. For example, neural developmental events which usually take months to mature in in vivo models can be studied within weeks using brain organoids.17 The bioelectrical activity of brain organoids should shift from random bursts of spontaneous activity like in developing embryos to coordinated synchronized activity. Since a brain consists of multiple neuronal cells, including neurons, glia, and oligodendrocytes, pluripotent or multipotent stem cells are frequently the starting point for a bottom-up approach to form brain organoids. To date, most studies are focused on creating morphologically similar tissue to the specific regions of the brain, like the cortex or hippocampus, or attempt to recreate a brain disease, like microencephaly.18–20
The brain is one of the softest tissues in the body with an elastic modulus ranging between 1.8 and 2.3 kPa according to measurement using magnetic resonance elastography.21 The brain has an intricate structure relating to different functions in behavior and movement. The brain also has a unique blood–brain barrier (BBB) that allows for specific molecules to pass the vascular wall. This barrier keeps the brain free from contaminants circulating in the blood stream and hinders the ability of drugs to reach the internal brain tissue for disease treatment. As such, much of the research on brain organoids is divided into three directions: (1) growth of morphologically correct tissue to understand function and synaptic activity, (2) recapitulation of the BBB, and (3) development of the strategies to treat neurodegenerative diseases and acute/traumatic brain injury. This section will discuss how the extracellular microenvironment controlled with biomaterials contributes to these efforts.
Engineering of brain organoids
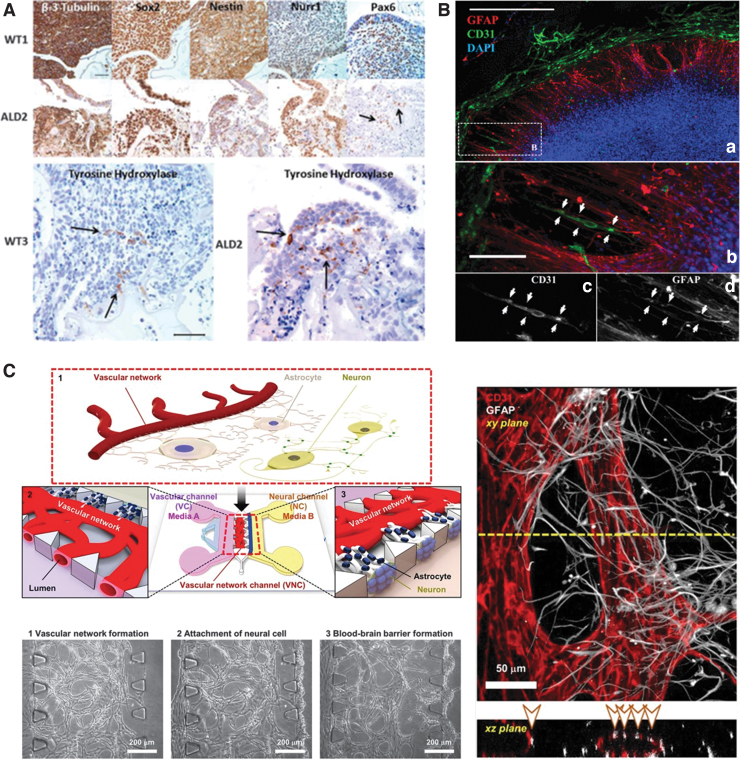
Due to the brain's structural complexity and the heterogeneity of the cells involved with making neural networks, many researchers use pluripotent or multipotent stem cells to create brain organoids along with biomaterials designed to mimic the multiple chemical and physical environments of the brain. For example, Lindborg et al. cultured cerebral organoids from pluripotent stem cells in a hyaluronan and chitosan hydrogel.18 Hyaluronan was chosen due to its recognition as an integral part of the ECM in developing brains. The hydrogels were made by first lyophilizing the chitosan base into leaflets, mixing with dry hyaluronan, and finally hydrating the powders with a hydration fluid (Cell-Mate3D). The modulus of the hydrogels was measured to be between 9 and 13 kPa, slightly stiffer than the normal brain tissue. From human induced pluripotent stem cells (iPSCs) incorporated into the hyaluronan and chitosan hydrogel, brain organoids were formed with distinct regions of forebrain, midbrain, and hindbrain as confirmed with protein and gene expression (Fig. 1A). Human iPSCs used herein were derived from six patients: three normal control subjects and three from patients with cerebral adrenoleukodystrophy, a genetic brain disorder causing breakdown of the myelin sheath. The resulting organoids are being used to develop an in vitro model of adrenoleukodystrophy.
FIG. 1.
Cerebral organoid models. (A) Immunohistochemical staining of cerebral organoids derived from control (WT1, WT3) or an adrenoleukodystrophy patient's (ALD2) iPSCs. Reproduced with permission.18 Copyright 2016, Wiley. (B) Vascular networks of brain organoids cultured in polyethylene glycol-norbornene hydrogels modified with cRGDs. (a) neural construct after 21 days. Endothelial cells (CD31) are stained in green, glial cells (GFAP) are stained in red, and cell nuclei (DAPI) are stained in blue. (b) Zoom of the boxed region shown in A to illustrate association and alignment for a capillary tubule and radially oriented glial cells (arrows). The cells in B are shown as single channel grayscale images for (c) CD31 and (d) GFAP. Reproduced with permission.19 Copyright 2015, National Academy of Sciences. (C) Scheme of the microfluidic platform for a neurovascular unit, including the BBB and phase contrast and immunofluorescent images of the vascular network formed in the microfluidic chip. Endothelial cells (CD31) are stained in red, and glial cells (GFAP) are stained in white. Reproduced with permission.23 Copyright 2017, Springer Nature. BBB, blood–brain barrier; cRGD, cyclic RGD peptide; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; iPSCs, induced pluripotent stem cells. Color images are available online.
Schwartz et al. used human embryonic stem cell-derived progenitor cells, mesenchymal stem cells, and microglia/macrophage precursors to create a brain organoid used to test for neural toxicity.19 They modified collagen with cysteines to crosslink 8-arm poly(ethylene glycol) (PEG)-norbornene. Cyclic arginylglycylaspartic acid (RGD) peptides that act as cell adhesion epitopes were then added as a 2 mM solution. The various cell types were seeded in order of appearance during in vivo brain development: radial glia was formed first with GABAergic and glutaminergic neurons layering outward. Vascular cells were introduced to the organoids on day 9, a relatively early time point in their neural construct's development, which allowed for dispersive capillary formation throughout all neuronal layers (Fig. 1B). Control organoids, which lacked vascular cells, had detectable amounts of vascular endothelial growth factor (VEGF); however, this amount was not necessary to form interconnected vasculature. They characterized the construct's ability to accurately predict toxicity by running a gene expression profile using 31 control compounds such as food additives or pharmaceuticals with no neurotoxicity and 39 toxin compounds.19
Recapitulation of the BBB formation
The brain is also characterized by the BBB, one of the most restrictive barriers of the body. The barrier consists of the capillary basement membrane separated from the neurons by astrocytes and endothelial cells which create tight junctions.20 This structure reduces the fenestrations that are present in other capillaries around the body. Interestingly, various transporters such as amino acids, glucose, and waste export allow certain molecules and immune cells to cross the BBB, facilitated by the astrocytes and the central nervous system. Chemical cues from the body, like histamine, travel to the astrocytic end-feet, which surround the endothelial cells of the blood vessel, allowing for homeostasis of capillary permeability in the brain.22
Bang et al. recreated this tight physical barrier in a microfluidic chip.23 By sequentially adding human umbilical vein endothelial cells and then rat cortical neurons into channels of the PDMS microfluidic chip (Fig. 1C), endothelial cells, neurons, and astrocytes orchestrate to form a BBB. Astrocytes' end-feet are in contact with capillaries like the native BBB. A fibrinogen was injected into the channel with human umbilical vein endothelial cells and thrombin to activate formation of a provisional fibrin matrix appropriate to support vasculogenesis.
The neurons were also in direct contact with the astrocytes which formed the relevant protective mechanical environment found in the brain. The resulting BBB exhibited comparable permeability, which was evaluated with molecular diffusivity. For instance, diffusion coefficients of fluorescein isothiocyanate-dextran molecules in vivo (i.e., 0.45 × 10−6 cm/s for 20 kDa molecules and 0.36 × 10−6 cm/s for 70 kDa molecules) are similar to that of in vivo models (0.24 × 10−6 cm/s for 20 kDa molecules and 0.15 × 10−6 cm/s for 70 kDa molecules).
Extracellular Microenvironment Control for Lung Organoid Assembly
The lungs are responsible for transmitting oxygen (O2) from the air into the blood at the alveolar-capillary interface.24 In addition to supporting this phase boundary, the lungs undergo dynamic and constant motion with each breath.25 During inhalation, the intrapleural pressure decreases, causing the alveoli to expand and fill with external air. Therefore, from a design perspective, lung organoids should exhibit elasticity and mechanical resilience through repeated expansion. By doing so, organoids should be able to undergo nonuniform radial strain that causes around a 50% change in surface area and Poisson ratios of 0.41–0.45 without fracture.26–28 The pleural membrane should also withstand high tensions, ranging from 103 to 104 dyne/cm in dogs, which is similar to that of humans.29 This tension increases with the membrane surface area.
In native tissue, these mechanical properties are attained by an epithelial cell layer at the alveoli surface that allows for rapid gas diffusion, surrounded by an elastic basement membrane. The consistent mechanical stretching of the epithelial cell layer is important for the overall function of the lung, playing a role in phospholipid production, organ growth, cellular viability, and signaling.27,30–32 Since the epithelium and underlying tissue in the lungs are constantly undergoing dynamic changes, the overall elastic and shear moduli of this region vary.
Recreating the lung's mechanical microenvironment
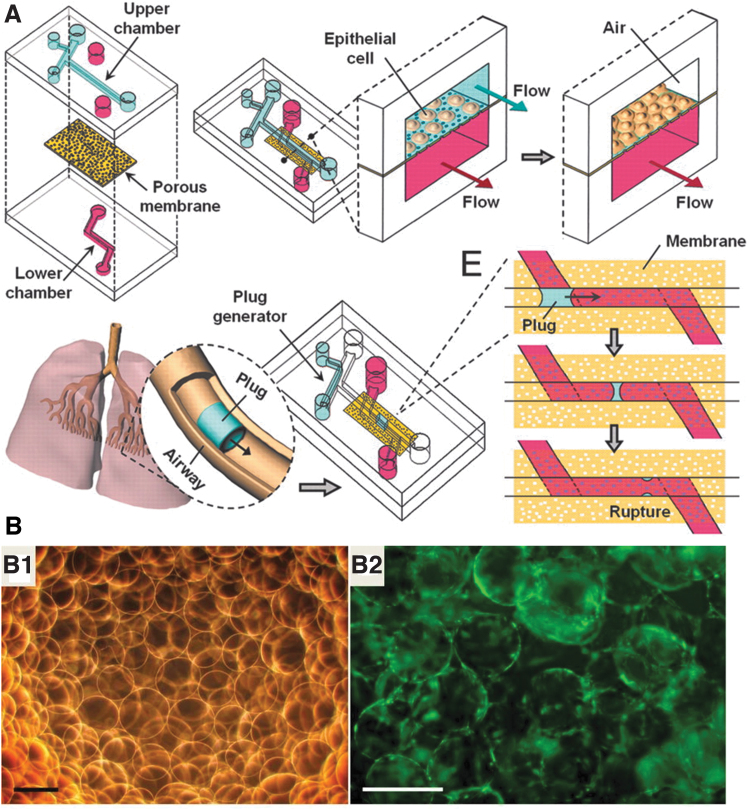
Since the lungs are mechanically dynamic, lung organoid cultures should be designed to be equally dynamic to support and simulate the complex physical environment in which the cells reside. Researchers often accomplish this goal by culturing cells on devices that can mechanically oscillate to simulate the strains caused by respiration.27 Similarly, researchers have simulated the alveolar-capillary interface by engineering multichanneled microfluidic chips.33,34 For instance, a study by Huh et al. formed two chambers within a PDMS mold separated by a porous polyester membrane to simulate this interface (Fig. 2A). Small airway epithelial cells were cultured on this membrane with microchannels of 300 μm diameter to mimic the gaps caused by conducting airways and bronchioles. The cell culture media was flown past the cells with the physiologically relevant speed of 1.5 mm/s.
FIG. 2.
Lung organoid models. (A) Compartmentalized microfluidic airway systems for lung organoid culture. Reproduced with permission.33 Copyright 2007, National Academy of Sciences. (B) (B1) Bright-field image of alginate beads and (B2) fluorescence image of human lung fibroblast spheres after 24 h in the bioreactor. Fibroblasts are stained in green. Reproduced with permission.37 Copyright 2016, The American Physiological Society. Color images are available online.
Interestingly, these devices can reproduce the secretory activity of native cells.35 The epithelial cells at the interface differentiated in response to the air/liquid interface. This platform was used to experimentally test the injuring effects of lung collapse and subsequent reopening by propagating a liquid plug through the microchannels.34 A follow-up study coated the PDMS membrane with fibronectin or collagen, cultured the epithelial cells with human pulmonary microvascular endothelial cells, and applied 10% cyclic strains to mimic the native tissue. This cell culture platform increased lung surfactant production, viability, and various biofunctional markers compared to the control without any applied cyclic strain. For example, intercellular cell adhesion molecule-1 expression was increased by around three times, and reactive O2 species production was increased by around four times relative to the amount produced without strain.36
Small molecule determinants of morphological development
Rather than mimicking the mechanical environment of the lung, some researchers have instead simulated the fluctuations in O2 content to simulate bronchopulmonary dysplasia.37 Human fetal lung fibroblasts were cultured on alginate beads that were coated with collagen. The cell-gel constructs were suspended in Tris-buffered dopamine hydrochloride using a rotating bioreactor for 96 h, then matured in an incubator that oscillated its O2 gas content between 10% and 70% every 24 h (Fig. 2B). This gas content aimed to replicate the gas content in diseased patients and resulted in upregulation in biomarkers such as α-smooth muscle actin and the Notch pathway compared to cells cultured in normoxic conditions (∼20%). Ultimately, the human fetal lung fibroblasts, which were cultured on the collagen-coated alginate beads in hypoxic conditions, formed lung organoids which had similar structural changes as what was seen in infants with bronchopulmonary dysplasia. Thus, the biomaterial substrate accurately mimicked the disease-state environment.
Success has also been found by engrafting mouse fetal pulmonary cells below the renal capsule by Mondrinos et al.38 These cells were cultured in a hydrogel made from 1.2 mg/mL collagen type I mixed with tenascin-C, an ECM glycoprotein. The hydrogels improved the circulation of nutrients to the organoids and engraftment, resulting in vascularization into the gels by endothelial cells. Differentiation and organoid formation were further promoted by media supplemented with fibroblast growth factors (FGFs) and modulated by concentrations of tenascin-C, VEGF, and sonic hedgehog compared to serum-free control medium.
Overall, lung organoid culture presents interesting mechanical challenges to sufficiently mimic the native environment. Lung tissue undergoes constant changes in transpulmonary pressure, which imposes cyclic changes in the elastic modulus experienced by the cells. To address these cyclic changes in elastic modulus, lung cells deposit an elastic ECM so that they can withstand repeated mechanical deformations. As such, researchers have developed technologies, such as oscillating bioreactors, to recapture the mechanically dynamic environment of native lungs during culture. Cell-adherent scaffolds like collagen or Matrigel were used to support the growth and guide the organization of mature lung organoids. However, since the lungs are a dynamic organ, scaffolds should be rigid and flexible enough to sustain large deformations without fracture. Future studies in engineering functional lung organoids will likely combine rigid scaffolds with oscillating bioreactors.
Extracellular Microenvironment Control for Intestine Organoid Assembly
The intestinal epithelium consists of a monolayer of epithelial cells folded to form villus and crypt domains.39 The microstructure of the intestine has an important role in providing increased surface area for nutrient absorption. Besides, epithelial cells exhibit active self-renewal in which cells are replaced every 4–5 days.40 Thus, the majority of intestinal organoid engineering studies have focused on controlling the pathways that promote differentiation and self-renewal of the intestinal stem cells. In the epithelium, Lgr5 intestinal stem cells are populated at the intestinal crypts where the differentiation and self-renewal are controlled by the bone morphogenic protein (BMP) and Wnt pathways.41–43 Thus, studies are focused on regulating BMP and Wnt pathways in the intestinal cells. Due to the importance of controlling the genetic pathways, studies have introduced various methods to genetically stimulate the cells.12,13,44,45 These engineered intestinal organoids hold valuable therapeutic potential for scrutinizing the intestinal in vitro and screening drugs for intestinal diseases.
Macroscale manipulation of biomaterial shape to facilitate the intestinal organoid formation
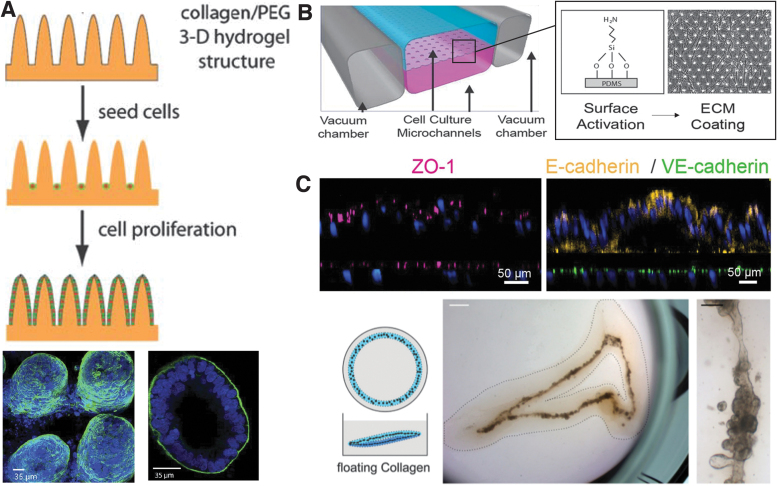
In addition to these methods, researchers used a simpler robust method by manipulating the material where the intestinal organoids were formed. Due to the geometric characteristic of the intestine, which consists of a villus and crypt region, certain topographical features can support intestinal organoid formation. Several studies demonstrated that epithelial cells assemble into a villi-like morphology geometry on pillar arrays made of collagen or collagen-polyethylene glycol diacrylate (collagen-PEGDA), which act as cell adhesion substrate.46–48 For instance, Sung et al. fabricated a PDMS mold to engineer a collagen and PEG-based substrate with a villi-like topography46 (Fig. 3A). Human colon carcinoma cells (Caco-2) cultured on both the control collagen-only substrate, as well as the collagen-PEGDA substrate, formed a villi epithelium. Caco-2 cells were chosen for their widespread use in in vitro drug absorption studies. Wang et al. also reported that a collagen substrate with micro-sized pillars supports intestinal organoid formation compared to control conditions of culturing crypts in wells without Matrigel or in Matrigel overlaying the microwells.49 By having both ridges and grooves on the substrate, the engineered organoids showed both crypts and villi.
FIG. 3.
Intestinal organoid models. (A) Schematic image of 3D hydrogel for intestinal organoid with villi-like topography and confocal images of Caco-2 cells on 3D hydrogel. Actin filaments are stained in green, and nuclei are stained in blue. Reproduced with permission.46 Copyright 2011, Royal Society of Chemistry (B) The scheme of the intestine-on-a-chip platform cross-sectioned and confocal immunofluorescence images showing vertical cross-sections of the intestine chip. Tight junction protein (ZO-1) is stained in pink, E-cadherin is stained in yellow, VE-cadherin is stained in green, and cell nuclei are stained in blue. Reproduced with permission.52 Copyright 2018, Springer Nature. (C) Intestinal organoids form in floating collagen hydrogel rings. The scale bar represents 1 mm in the middle brightfield image and 100 μm on the right brightfield image. Reproduced with permission.57 Copyright 2017, Company of Biologists. 3D, three-dimensional. Color images are available online.
A concave microwell also produced a 3D colonic epithelium.49 A PDMS microwell filled with Matrigel assembled the intestinal organoid. In the well, the crypts isolated from mice successfully formed an epithelial tissue that resembles the proliferative and nonproliferative zones of the intestine in vivo. These results suggest that controlled topographical features of the substrate allow for the formation of an organoid morphologically similar to the intestine in vivo.
Intestinal villi and crypts are exposed to fluid flow, which influences the rate at which absorption of drugs or nutrients enter the body. Many animal models cannot accurately predict the uptake of molecules in the human gut because of differences in metabolism and transport phenomena. Thus, there is a need for an in vitro human intestinal model. For example, microfluidic systems are increasingly used to reproduce fluid flow of the natural interstitial fluid.50–52 Kasendra et al. used a PDMS microfluidic chip with two vacuum chambers on either side of a stacked cell culture channel52 (Fig. 3B). The chip was fabricated using soft lithography of the PDMS portion, and the porous membrane that separates the gut epithelium channel from a culture media channel was fabricated using a silicon wafer with a micropillar array.53 Interestingly, duodenal organoids resulting within this microfluidic chip resembled the human duodenum more closely than static 2D studies of Caco-2 cells on Transwell plates. The Transwell plates were coated with the same mixture of collagen and Matrigel as for the chip device.
Focus on biomaterial chemical composition
The chemical composition of a substrate is another factor to support intestinal organoid formation. Conventional methods of engineering intestinal organoids focused on using Matrigel.44–47 Recently, various biological materials such as alginate, collagen, and PEG are being used to grow intestinal organoids in vitro because of their cell compatibility and workability in forming complex substrate structures. In particular, type I collagen gel, the most abundant matrix protein in the intestine, supports long-term maintenance of the intestinal organoids.54–56 In addition, embedding the proliferating intestinal organoids in a type I collagen gel allowed the cells to form hollow tube structures.57 Interestingly, while the organoids formed in the Matrigel only case formed single budding cysts, the intestinal stem cells embedded in the collagen gel formed a continuous tube, similar to the natural intestine (Fig. 3C).
Intestinal villi and crypts are exposed to fluid flow, which influences the rate at which absorption of drugs or nutrients enter the body. Many animal models cannot accurately predict the uptake of molecules in the human gut because of differences in metabolism and transport phenomena. Thus, there is a need for an in vitro human intestinal model. For example, microfluidic systems are increasingly used to reproduce fluid flow of the natural interstitial fluid.50–52 Kasendra et al., used a PDMS microfluidic chip with two vacuum chambers on either side of a stacked cell culture channel52 (Fig. 3B). The chip was fabricated using soft lithography of the PDMS portion, and the porous membrane that separates the gut epithelium channel from a culture media channel was fabricated using a silicon wafer with a micropillar array.53 Interestingly, duodenal organoids resulting within this microfluidic chip resembled the human duodenum more closely than static 2D studies of Caco-2 cells on Transwell plates. The Transwell plates were coated with the same mixture of collagen and Matrigel as for the chip device.
The intestine is a dynamic organ that undergoes acute pulsatile motion. Future studies may utilize oscillatory tensile force bioreactors, such as those used in lung organoid culture, to elicit more in vivo-like behavior. Alternatively, these dynamic conditions could be reproduced by controlling fluid flow in a microfluidic chip used to engineer the intestinal organoid.
Manipulation of Extracellular Microenvironments for Liver Organoid Assembly
Primary in filtration and aiding in digestion, the liver is one of the organs which provides nutrition to the body by aiding in the breakdown of food. The liver also protects the body by filtering and destroying harmful materials. The capacity and efficiency for liver function stem from the organization of tissue at the cellular level. Efforts have been made to engineer liver organoids with physiological functionality by understanding the mechanical properties and permeability of normal and diseased livers. For instance, elastic moduli vary from <6 kPa in a healthy liver, to 8–12.5 kPa in early fibrotic liver disease, and >20 kPa in high liver cirrhosis.58 In addition, liver disease elevates the blood pressure gradient to higher than 10 mm Hg, leading to a rapid decrease in the production of proteins and filtration, eventually resulting in death from internal bleeding, liver failure, or hepatocarcinoma.58
Mixed biomaterial matrices to tune mechanical and chemical properties
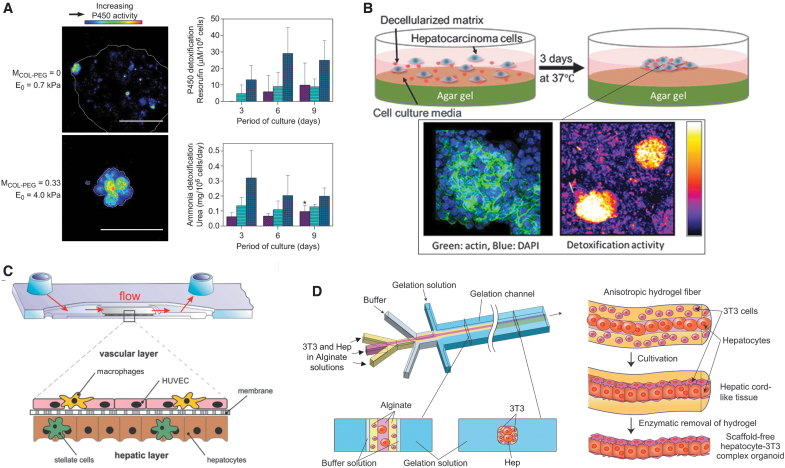
Since the liver organoids were grown from primary or stem cells cultured in Matrigel, there has been a push toward growing these miniature organs in various environments mimicking normal and diseased liver tissue using synthetic and natural ECMs with controlled matrix stiffness and bioactive molecules.14,59 For instance, the HepG2 liver cancer cells grown in collagen gels tailored to have an elastic modulus of 4–7 kPa using PEG disuccinimidyl ester cross-linker suppressed malignancy. In particular, cells retained hepatocyte-like detoxification activity and formed the lobule-like lumen in the gel (Fig. 4A). In contrast, the same cells cultured in a softer collagen gel with an elastic modulus of 0.7 kPa showed minimal detoxification activity.
FIG. 4.
Liver organoid models. (A) The effect of hydrogel modulus on detoxification activities of 3D hepatocarcinoma cell spheroids, analyzed with cytochrome P450 activity. Reproduced with permission.59 Copyright 2011, Elsevier. (B) The schematic image of formation process of a hepatic cluster with decellularized matrix and immunohistochemical image and detoxification assay of hepatic cluster. Actin filaments are stained in green, and cell nuclei are stained in blue. Reproduced with permission.61 Copyright 2018 American Chemical Society. (C) The cross-sectional scheme of the biochip liver model. Reproduced with permission.63 Copyright 2015, Elsevier. (D) Schematic illustrations of the microfluidic system for hydrogel microfiber incorporated hepatocytes and 3T3 cells and formation process of hepatocyte-3T3 complex micro-organoids in the microfiber Reproduced with permission.64 Copyright 2012, Elsevier. Color images are available online.
In addition, Klaas et al. demonstrated that cells in a diseased or tumorigenic liver could restore activities to form a liver organoid in matrices with proper chemical composition.60 In this study, cells isolated from mild and severe fibrotic livers were cultured on dishes coated with fibronectin, type I collagen, or type IV collagen. Cells cultured on the type IV collagen exhibited enhanced proliferation compared to cells cultured on the fibronectin or type I collagen, where a large fraction of cells lost hepatic phenotypes. In addition, decellularized matrix produced by bone marrow-derived mesenchymal stem cells could serve to make HepG2 liver cancer cells form hepatic clusters retaining detoxification activities (Fig. 4B).61
Using hepatic stromal cells
Separately, efforts have been made to recapitulate complex liver tissue in which hepatocytes and supporting endothelial cells such as fibroblasts, stellate cells, and Kupffer cells are organized to form a liver sinusoid-like structure using a microfluidic system.62 For example, Rennert et al. seeded liver cells and endothelial cells on opposite sides of the cyclic olefin copolymers-TOPAS® biochip.63 Then, this microfluidic system reproduced the layering of the cells around a liver sinusoid structure (Fig. 4C). Because nonparenchymal cells support the function of the liver, cells were introduced into the media flowing through the chip. By doing so, cells formed the supportive stroma. Overall, the microfluidic system increased retention of CYP3A4 expression, commonly lost through prolonged cultures of liver cells, and exhibited a significantly higher synthesis of albumin and urea than static hepatocyte cultures. To create large liver organoids, liver organoids were also vascularized by hollow endothelial lumen. For instance, certain approaches used biodegradable scaffolds made from alginate and cocultured with vascular endothelial cells and fibroblasts (Fig. 4D).64–66
The future of liver organoids points toward creating larger tissues that could be implantable into humans to replace excised tissues from cancer or disease. While the introduced studies were mostly successful in forming vasculature in the organoid, there are still limitations in recapitulating the full function of the natural liver. These limitations can be solved by culturing vascularized liver organoids in microenvironments that better model the liver. For example, Sasaki et al. used a layer-by-layer approach coupled with ECM molecules.67 They coated the human liver cells and dermal fibroblasts with fibronectin and gelatin and then added human umbilical vein endothelial cells to create a densely vascularized tissue. The tissues were then implanted into the subcutaneous space of immunocompromised mice. After 21 days, the newly formed vasculature from the liver organoid was engrafted to the host vasculature, making the organoid active to produce human serum albumin.
Manipulation of Extracellular Microenvironments for Retinal Organoid Assembly
The eye is one of the most complex and compact organs. It originates from the optic vesicle connected to a bilayer optic cup with a neural retinal cell layer enclosed by a retinal pigment epithelium. The front of the eye consists of dynamic, light-focusing structures, including the lens enclosed by the ciliary body and iris with the cornea surrounding the entire structure.68 While solutions such as glasses and laser eye surgery can correct improper focusing of light onto the retinal cells in the back of the eye, few solutions exist for repairing function of the optic cup itself where retinal cells capture light and transmit signals through the optic nerve.69 Therefore, as a first step toward total eye reformation, researchers have developed culture methods to form optic cup organoids in vitro.
Mimicking retinal developmental stages
Interestingly, retinal cells possess a substantial ability to self-aggregate and organize in vitro, leading researchers to mimic developmental processes.70,71 For example, Sasai and coworkers have developed culture methods with embryonic stem cells that guide the stem cells' differentiation into distinct neural retina and retinal pigment epithelium layers without the need for external force.10,72 These processes use low adhesion 96-well plates for the initial cell differentiation and aggregation. The cultures were then embedded in 2% v/v Matrigel and grown in serum-free growth factor-reduced media charged with 40% O2/5% carbon dioxide. Although both mouse and human cells form organoids with this technique, the resulting organoids present differences in size, relative amounts of rods and cones, and speed of growth.73
Moreover, during organogenesis, the initially spherical cell cluster undergoes extensive reorganization, eventually forming a spatially organized multilayered cup without any external forces. To underlie the mechanism, the Sasai and Eiraku laboratories combined an in vitro and an in silico approach to model the 3D mechanical forces that deform the overall structure at a single cell layer.74 They found that the deformation is driven primarily by cellular proliferation at precise locations, causing a mechanical instability and buckling of the hollow cell sphere. Understanding how stem cells mechanically form organoids was further explored by Lowe et al. from the perspective of the cytoskeleton and cellular adhesions.75 They cultured human embryonic stem cells suspended within a Matrigel construct. The construct was then broken apart to form floating fragments. Around 5 days, the cells formed cysts and gradually expressed retinal progenitor eye-field markers such as SIX3, RAX, and PAX6. Upon further growth of the cysts on tissue culture multiwell plates, the intrinsic programming for the stem cells served to form retinal organoids by modulating actomyosin forces through Rho kinase activation.76,77
Völkner et al. have established a protocol for retinal organoid formation that exhibits high organoid yields and cell diversity. This method does not rely on the evagination of optic vesicle structures. Instead, the researcher manually cut the developing organoid into three equal pieces.78 By trisectioning the organoids in an unbiased manner, each will potentially contain eye field-determined neuroepithelium, thus allowing for the growth of three organoids from each starting aggregate. According to analysis of PAX6 reporter expression, which is a major regulator of neurogenesis, the organoids recaptured a similar expression level as native mouse retina.
Alternative Matrigel extracellular microenvironment to facilitate nutrient transport
While Matrigel is the most widely used material to culture retinal organoids, researchers also have cultured organoids through a combination of poly(2-hydroxyethyl methacrylate) coated plates followed by culture in a rotating culture chamber at progressively faster rotation speeds.79 The rotation speed is optimized such that the organoids are kept in a stationary suspension, also known as simulated microgravity. In this rotating vessel environment, the organoids had improved proliferation and differentiation into ganglion and S-cone photoreceptors compared to organoids cultured in static vessels. Other researchers have also found that culturing cells in similar stirred or shaken bioreactors improves the yield of photoreceptor cells.80,81 The better cell survival is due to an enhanced nutrient, O2 transport, and waste removal in dynamics cultures, allowing for larger cell organoids to form by combating the usually poor transport within cell clusters.
Despite its relative complexity of design and function, retinal organoids exhibit a substantial ability to reorganize and form eye-like structures semiautonomously. Most optic cups and retinal organoids can be formed on Matrigel and rotating bioreactors. The organoids present cell signaling pathways crucial for cell reorganization. However, while organoids can be formed to mimic some of the components of the eye, researchers are yet to assemble a single organoid using the diverse cell types found at all layers of the eye. Future work in this field will require a detailed knowledge of the biological mechanisms behind the cellular organization, as well as proper mechanical supports and localized differentiation of different cell populations. Biomaterials would be well suited to this end by providing supports of physiological relevance while allowing transport of cell signaling molecules to induce organoid formation.
Manipulation of Extracellular Microenvironments for Kidney Organoid Assembly
In the body, the kidney filters wastes, controls blood pressure, regulates fluid, and balances the pH. Nephrons, the kidney's functional units, are responsible for serving these roles.82 The kidney undergoes complex developmental stages that require reciprocal interaction between endoderm and mesoderm.83 Therefore, researchers have focused on both recapitulating the environment necessary for the endoderm and mesoderm to interact and induce the formation of functional nephrons. In this regard, kidney organoids hold potential applications in modeling renal diseases and drug screenings, as well as testing the toxicity of chemicals.84
Similar to other organoid engineering strategies, methods to supply biological molecules such as basic FGF, retinoic acid, and BMP are commonly used to control the differentiation lineage of renal organoids from human embryonic stem cells to ureteric bud progenitor cells.15,85,86 By providing growth factors at different time points, the researchers were able to develop kidney organoids similar to the developmental process. The predominantly used material to support kidney organoid growth is Matrigel.87–90 The Matrigel served to increase the number of adhered cells; however, Matrigel forms variable organoids. Because of its tumorigenic origins, the relative concentrations of dissolved growth factors and extracellular proteins result in high batch-to-batch variability. Thus, efforts are required to identify or synthesize an ECM proper for kidney organoid formation.
A new 3D bioprinting method that directly prints proximal tubule cells into a gelatin-fibrinogen matrix was used to generate a kidney organoid.91 Previous studies focused on recapitulating the renal proximal tubules which are responsible for reabsorption of fluid that is filtered through glomerulus. A fugitive ink, Pluronic® F127, was printed in a tubule shape inside of the gelatin–fibrin gel. The gelatin and fibrin mixture allowed for a controllable gelation time. This structure was connected to a peristaltic pump and covered again with gelatin–fibrin mixture and the fugitive ink. Finally, the two fugitive ink layers were removed by cooling the entire gel to the lower critical solution temperature for the Pluronic which produced an open channel. The epithelial cells grown in these proximal tubules showed enhanced albumin uptake levels and exhibited morphologies similar to the natural proximal tubules. Therefore, this study showed an effective way to mimic the complex intracellular architecture using 3D printed biomaterials.
Conclusion
As organoid development becomes more prevalent in research, differing approaches have developed to improve controllability of the dimensionality, differentiation, and assembly of cell clusters (Table 1). However, the goal of highly functional and morphologically similar counterparts to real-life organs is challenging for conventional cell culture techniques. Researchers have invested tremendous efforts in synthesizing microenvironments that can regulate organoid formation and physiological functions using ECM-mimicking biomaterials mixed or tethered with bioactive molecules. To reproduce the microstructure of tissues, continuous gel matrices, fibrous networks, 3D printed structures, or microfluidic chips are used to advance the quality of organoids. Alternatively, decellularized matrices attained from native tissue or stem cell spheroids are other options used for organoid engineering.
Table 1.
The Functionality of Organoid Depending on Extracellular Environment
| Organoid | Type of cells | Extracellular matrix | External device | Functionality | Reference |
|---|---|---|---|---|---|
| Brain | Human induced pluripotent stem cells | Hyaluronan/chitosan | 3D culture within hydrogel | Distinct regions of forebrain, midbrain, and hindbrain | 17 |
| Human embryonic stem cells | Collagen | 3D culture within hydrogel | Formation of 3D neural constructs with interconnected vascular network | 18 | |
| Astrocyte and Human umbilical vein endothelial cells | Fibrin | Microfluidics | Increasing of BBB permeability | 22 | |
| Lung | Rat pulmonary epithelial cells | Fibronectin/collagen | Microfluidics | Increasing of lung surfactant production, viability, and biofunctional markers | 33 |
| Human fetal lung fibroblasts | Alginate/collagen | 3D microbeads | Mimicking similar structure with bronchopulmonary dysplasia | 36 | |
| Mouse fetal pulmonary cells | Collagen | 3D culture within hydrogel | Increasing of differentiation and organoid formation | 37 | |
| Intestine | Human colon carcinoma cells | Collagen | Microstructure | Morphologically similar structure with in vivo | 45 |
| Mouse crypt | Matrigel | Microstructure | Morphologically similar structure with in vivo | 48 | |
| Human primary crypt | Collagen/Matrigel | Microfluidics | Resembled the human duodenum | 55 | |
| Liver | Human hepatocellular carcinoma cells | Collagen | 3D culture within hydrogel | Retaining hepatocyte-like detoxification activity and lobule-like lumen formation | 58 |
| Human hepatocellular carcinoma cells | Decellularized matrix | 3D culture on nonadherent substrate | Retaining detoxification activities | 60 | |
| Mouse embryonic fibroblasts/Rat hepatocytes | Alginate | Microfluidics | Increasing albumin secretion, urea synthesis, and hepatocyte-specific gene | 63 | |
| Retinal | Human embryonic stem cells | Matrigel | 3D culture within hydrogel | Increasing retinal progenitor eye-field markers | 74 |
| Mouse embryonic stem cells | Matrigel | Rotating culture chamber | Improving proliferation and differentiation into ganglion and S-cone photoreceptors | 78 | |
| Kidney | Human embryonic stem cells | Matrigel | 3D culture within hydrogel | Formation of 3D tubular structure similar to in vivo | 87 |
| Human proximal tubule epithelial cells | Gelatin/fibrin | 3D bioprinting | Enhancement of albumin uptake level and natural proximal tubule morphology | 90 |
3D, three-dimensional; BBB, blood–brain barrier.
However, the results are diversified depending on the cells' emergent behavior mitigated by cell–cell interactions, neovascularization, and other extrinsic factors such as external physical forces. In addition, the extracellular environment is another factor that influences a development and homeostasis of organoids because it changes with stages of development and progression of disease. By reproducing transitory changes of extracellular microenvironment using stimulus-responsive biomaterials, it may be possible to gain insight into how mechanical and chemical cues affect the growth and functionality of organoid cultures over time. In addition, ECMs must be defined better to recapitulate the structure and function of target organs. Furthermore, a high-level microscopic and spectroscopic analysis could also be used to improve the quality and controllability of organoids. These methods would provide an in-depth understanding of the extracellular microenvironment at the molecular and cellular levels.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported partially by the National Science Foundation (STC-EBICS Grant Nos. CBET-0939511, 1932192, and 2032521) and National Research Council of Science and Technology grant and the Korean Government (CAP-17-01-KIST Europe). K.M.S. and J.D.I. were supported by the National Science Foundation Training Grant, Understanding the Brain: Training the Next Generation of Researchers in Engineering and Deciphering of Miniature Brain Machinery under Award No. 1735252. E.K. was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award No. T32EB019944. E.M.K. was supported by the Korean Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea under grant No. HI19C0753.
References
- 1. Shanks, N., Greek, R., and Greek, J.. Are animal models predictive for humans? Philos Ethics Humanit Med 4, 2, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takai, A., Fako, V., Dang, H., et al. . Three-dimensional organotypic culture models of human hepatocellular carcinoma. Sci Rep 6, 21174, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shkumatov, A., Baek, K., and Kong, H.. Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies. PLoS One 9, e94764, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kessler, M., Hoffmann, K., Brinkmann, V., et al. . The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun 6, 8989, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ranga, A., Girgin, M., Meinhardt, A., et al. . Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci U S A 113, E6831, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Irie, Y., Mizumoto, H., Fujino, S., and Kajiwara, T.. Development of articular cartilage grafts using organoid formation techniques. Transplant Proc 40, 631, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Susaimanickam, P.J., Maddileti, S., Kumar Pulimamidi, V., et al. . Generating minicorneal organoids from human induced pluripotent stem cells. Development 144, 2338, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Fisher, O.Z., Khademhosseini, A., Langer, R., and Peppas, N.A.. Bioinspired materials for controlling stem cell fate. Acc Chem Res 43, 419, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kong, H.J., and Mooney, D.J.. Microenvironmental regulation of biomacromolecular therapies. Nat Rev Drug Discov 6, 455, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Eiraku, M.,Takata, N., Ishibashi, H., et al. . Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Lancaster, M.A., Renner, M., Martin, C.A., et al. . Cerebral organoids model human brain development and microcephaly. Nature 501, 373, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spence, J.R., Mayhew, C.N., Rankin, S.A., et al. . Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCracken, K.W., Howell, J.C., Wells, J.M., and Spence, J.R.. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc 6, 1920, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takebe, T., Sekine, K., Enomura, M., et al. . Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Takasato, M., Er, P.X., Becroft, M., et al. . Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16, 118, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Raj M,K., and Chakraborty, S. PDMS microfluidics: a mini review. J Appl Polym Sci 137, 48958, 2020. [Google Scholar]
- 17. Heide, M., Huttner, W.B., and Mora-Bermúdez, F.. Brain organoids as models to study human neocortex development and evolution. Curr Opin Cell Biol 55, 8, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Lindborg, B.A., Brekke, J.H., Vegoe, A.L., et al. . Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med 5, 970, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz, M.P., Hou, Z., Propson, N.E., et al. . Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci U S A 112, 12516, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauvsnes, M.B., Tjensvoll, A.B., Maroni, S.S., et al. . The blood–brain barrier, TWEAK, and neuropsychiatric involvement in human systemic lupus erythematosus and primary Sjögren's syndrome. Lupus 27, 2101, 2018. [DOI] [PubMed] [Google Scholar]
- 21. Kolipaka, A.,Wassenaar, P.A., Cha, S., et al. . Magnetic resonance elastography to estimate brain stiffness: measurement reproducibility and its estimate in pseudotumor cerebri patients. Clin Imaging 51, 114, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abbott, N.J., Rönnbäck, L., and Hansson, E.. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7, 41, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Bang, S., Lee, S.R., Ko, J., et al. . A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci Rep 7, 8083, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Jaegere, A., Van Veenendaal, M.B., Michiels, A., and Van Kaam, A.H.. Lung recruitment using oxygenation during open lung high-frequency ventilation in preterm infants. Am J Respir Crit Care Med 174, 639, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Mead, J.,Takishima, T., and Leith, D.. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28, 596, 1970. [DOI] [PubMed] [Google Scholar]
- 26. Williams, J.L., Chen, J.H., and Belloli, D.M.. Strain fields on cell stressing devices employing clamped circular elastic diaphragms as substrates. J Biomech Eng 113, 377, 1992. [DOI] [PubMed] [Google Scholar]
- 27. Tschumperlin, D.J., and Margulies, S.S.. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol Cell Mol Physiol 275, L1173, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Lai-Fook, S.J., and Hyatt, R.E.. Effects of age on elastic moduli of human lungs. J Appl Physiol 89, 163, 2000. [DOI] [PubMed] [Google Scholar]
- 29. Hajji, M.A., Wilson, T.A., and Lai-Fook, S.J.. Improved measurements of shear modulus and pleural membrane tension of the lung. J Appl Physiol Respir Environ Exerc Physiol 47, 175, 1979. [DOI] [PubMed] [Google Scholar]
- 30. Liu, M., Skinner, S.J.M., Xu, J., et al. . Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch. Am J Physiol 263, L376, 1992. [DOI] [PubMed] [Google Scholar]
- 31. Scott, J.E., Yang, S.Y., Stanik, E., and Anderson, J.E.. Influence of strain on [3H]thymidine incorporation, surfactant-related phospholipid synthesis, and cAMP levels in fetal type II alveolar cells. Am J Respir Cell Mol Biol 8, 258, 1993. [DOI] [PubMed] [Google Scholar]
- 32. Skinner, S.J.M., Somervell, C.E., and Olson, D.M.. The effects of mechanical stretching on fetal rat lung cell prostacyclin production. Prostaglandins 43, 413, 1992. [DOI] [PubMed] [Google Scholar]
- 33. Huh, D., Fujioka, H., Tung, Y.C., et al. . Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci U S A 104, 18886, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bilek, A.M., Dee, K.C., and Gaver, D.P.. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 94, 770, 2003. [DOI] [PubMed] [Google Scholar]
- 35. Yoon, J.H., Gray, T., Guzman, K., Koo, J.S.,and Nettesheim, P.. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix. Am J Respir Cell Mol Biol 16, 724, 1997. [DOI] [PubMed] [Google Scholar]
- 36. Huh, D., Matthews, B.D., Mammoto, A., et al. . Reconstituting organ-level lung functions on a chip. Science 328, 1662, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sucre, J.M.S., Wilkinson, D., Vijayaraj, P., et al. . A three-dimensional human model of the fibroblast activation that accompanies bronchopulmonary dysplasia identifies Notch-mediated pathophysiology. Am J Physiol Cell Mol Physiol 310, L889, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mondrinos, M.J., Jones, P.L., Finck, C.M., and Lelkes, P.I.. Engineering de novo assembly of fetal pulmonary organoids. Tissue Eng Part A 20, 2892, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meneses, A.M.C., Schneeberger, K., Kruitwagen, H.S., et al. . Intestinal organoids-Current and future applications. Vet Sci 3, 31, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Umar, S. Intestinal stem cells. Curr Gastroenterol Rep 12, 340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qi, Z., Li, Y., Zhao, B., et al. . BMP restricts stemness of intestinal Lgr5 + stem cells by directly suppressing their signature genes. Nat Commun 8, 13824, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farin, H.F., Van Es, J.H., and Clevers, H.. Redundant sources of Wnt regulate intestinal stem cells and promote formation of paneth cells. Gastroenterology 143, 1518, 2012. [DOI] [PubMed] [Google Scholar]
- 43. Li, Y.,Liu, Y.,Liu, B., et al. . A growth factor-free culture system underscores the coordination between Wnt and BMP signaling in Lgr5+ intestinal stem cell maintenance. Cell Discov 4, 49, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miyoshi, H., and Stappenbeck, T.S.. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 8, 2471, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crespo, M.,Vilar, E., Tsai, S.Y., et al. . Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med 23, 878, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sung, J.H., Yu, J., Luo, D., Shuler, M.L., and March, J.C.. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 11, 389, 2011. [DOI] [PubMed] [Google Scholar]
- 47. Koppes, A.N., Kamath, M., Pfluger, C.A., et al. . Complex, multi-scale small intestinal topography replicated in cellular growth substrates fabricated via chemical vapor deposition of Parylene C. Biofabrication 8, 35011, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang, Y., Gunasekara, D.B., Reed, M.I., et al. . A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 128, 44, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang, Y., Ahmad, A.A., Sims, C.E., Magness, S.T., and Allbritton, N.L.. In vitro generation of colonic epithelium from primary cells guided by microstructures. Lab Chip 14, 1622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Midwoud, P.M., Merema, M.T., Verpoorte, E., and Groothuis, G.M.M.. A microfluidic approach for in vitro assessment of interorgan interactions in drug metabolism using intestinal and liver slices. Lab Chip 10, 2778, 2010. [DOI] [PubMed] [Google Scholar]
- 51. Workman, M.J., Gleeson, J.P., Troisi, E.J., et al. . Enhanced utilization of induced pluripotent stem cell–derived human intestinal organoids using microengineered chips. Cell Mol Gastroenterol Hepatol 5, 669, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kasendra, M., Tovaglieri, A., Sontheimer-Phelps, A., et al. . Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep 8, 2871, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim, H.J., Huh, D., Hamilton, G., and Ingber, D.E.. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165, 2012. [DOI] [PubMed] [Google Scholar]
- 54. Yui, S., Nakamura, T., Sato, T., et al. . Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5 + stem cell. Nat Med 18, 618, 2012. [DOI] [PubMed] [Google Scholar]
- 55. Jabaji, Z., Brinkley, G.J., Khalil, H.A., et al. . Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One 9, e107814, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Graham, M.F., Diegelmann, R.F., Elson, C.O., et al. . Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology 94, 257, 1988. [DOI] [PubMed] [Google Scholar]
- 57. Sachs, N., Tsukamoto, Y., Kujala, P., Peters, P.J., and Clevers, H.. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development 144, 1107, 2017. [DOI] [PubMed] [Google Scholar]
- 58. Mueller, S. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepatic Med Evid Res 2, 49, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liang, Y., Jeong, J., DeVolder, R.J., et al. . A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials 32, 9308, 2011. [DOI] [PubMed] [Google Scholar]
- 60. Klaas, M., Kangur, T., Viil, J., et al. . The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci Rep 6, 27398, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park, J., Kim, J., Sullivan, K.M., et al. . Decellularized matrix produced by mesenchymal stem cells modulates growth and metabolic activity of hepatic cell cluster. ACS Biomater Sci Eng 4, 456, 2018. [DOI] [PubMed] [Google Scholar]
- 62. Yoon No, D., Lee, K.H., Lee, J., and Lee, S.H.. 3D liver models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip. Lab Chip 15, 3822, 2015. [DOI] [PubMed] [Google Scholar]
- 63. Rennert, K., Steinborn, S., Gröger, M., et al. . A microfluidically perfused three dimensional human liver model. Biomaterials 71, 119, 2015. [DOI] [PubMed] [Google Scholar]
- 64. Yamada, M., Utoh, R., Ohashi, K., et al. . Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials 33, 8304, 2012. [DOI] [PubMed] [Google Scholar]
- 65. Chen, A.A., Thomas, D.K., Ong, L.L., et al. . Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A 108, 11842, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee, J.W., Choi, Y.J., Yong, W.J., et al. . Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 8, 15007, 2016. [DOI] [PubMed] [Google Scholar]
- 67. Sasaki, K., Akagi, T., Asaoka, T., et al. . Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Biomaterials 133, 263, 2017. [DOI] [PubMed] [Google Scholar]
- 68. Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol 93, 61, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sakimoto, T., Rosenblatt, M.I., and Azar, D.T.. Laser eye surgery for refractive errors. Lancet 367, 1432, 2006. [DOI] [PubMed] [Google Scholar]
- 70. Nakagawa, S., Takada, S., Takada, R., and Takeichi, M.. Identification of the laminar-inducing factor: Wnt-signal from the anterior rim induces correct laminar formation of the neural retina in vitro. Dev Biol 260, 414, 2003. [DOI] [PubMed] [Google Scholar]
- 71. Moscona, A.A. Analysis of cell recombinations in experimental synthesis of tissues in vitro. J Cell Comp Physiol 60, 65, 1962. [Google Scholar]
- 72. Hasegawa, Y., Takata, N., Okuda, S., et al. . Emergence of dorsal-ventral polarity in ESC-derived retinal tissue. Dev 143, 3895, 2016. [DOI] [PubMed] [Google Scholar]
- 73. Lubowitz, J.H., Provencher, M.T., and Poehling, G.G.. Stem cells in arthroscopy. Arthroscopy 28, 891, 2012. [DOI] [PubMed] [Google Scholar]
- 74. Okuda, S., Takata, N., Hasegawa, Y., et al. . Strain-triggered mechanical feedback in self-organizing optic-cup morphogenesis. Sci Adv 4, eaau1354, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lowe, A., Harris, R., Bhansali, P., Cvekl, A., and Liu, W.. Intercellular adhesion-dependent cell survival and ROCK-regulated actomyosin-driven forces mediate self-formation of a retinal organoid. Stem Cell Reports 6, 743, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Reichman, S., Terray, A., Slembrouck, A., et al. . From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A 111, 8518, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Amano, M., Nakayama, M., and Kaibuchi, K.. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67, 545, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Völkner, M., Zschätzsch, M., Rostovskaya, M., et al. . Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Rep 6, 525, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. DiStefano, T., Chen, H.Y., Panebianco, C., et al. . Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Rep 10, 300, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ovando-Roche, P., West, E.L., Branch, M.J., et al. . Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther 9, 156, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reichman, S., Slembrouck, A., Gagliardi, G., et al. . Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells 35, 1176, 2017. [DOI] [PubMed] [Google Scholar]
- 82. Montserrat, N., Garreta, E., and Izpisua Belmonte, J.C.. Regenerative strategies for kidney engineering. FEBS J 283, 3303, 2016. [DOI] [PubMed] [Google Scholar]
- 83. Takasato, M., Maier, B., and Little, M.H.. Recreating kidney progenitors from pluripotent cells. Pediatr Nephrol 29, 543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morizane, R., and Bonventre, J.V.. Kidney organoids: a translational journey. Trends Mol Med 23, 246, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xia, Y., Sancho-Martinez, I., Nivet, E., et al. . The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat Protoc 9, 2693, 2014. [DOI] [PubMed] [Google Scholar]
- 86. Morizane, R., and Bonventre, J.V.. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat Protoc 12, 195, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Przepiorski, A., Sander, V., Tran, T., et al. . A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep 11, 470, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yamaguchi, S., Morizane, R., Homma, K., et al. . Generation of kidney tubular organoids from human pluripotent stem cells. Sci Rep 6, 38353, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Freedman, B.S., Brooks, C.R., Lam, A.Q., et al. . Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6, 8715, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Takasato, M., Er, P.X., Chiu, H.S., and Little, M.H.. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc 11, 1681, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Homan, K.A., Kolesky, D.B., Skylar-Scott, M.A., et al. . Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6, 34845, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]