Abstract
Background:
The goals of this study were to characterize urine-derived stem cells obtained from the upper urinary tract (uUSC), induce these cells to differentiate into urothelial and smooth muscle cells, and determine whether they could serve as a potential stem cell source for bladder tissue engineering.
Materials and Methods:
Urine samples were collected from five patients with normal upper urinary tracts during renal pyeloplasty. Cells were isolated from this urine and extensively expanded in vitro.
Results:
The mean population doubling of uUSC was 46.5±7.7. The uUSC expressed surface markers associated with mesenchymal stem cells and pericytes. These cells could differentiate into smooth muscle-like cells that expressed smooth muscle-specific gene transcripts and proteins, including α-smooth muscle actin, desmin, and myosin, when exposed to TGF-β1 and PDGF-BB. In a collagen lattice assay, these myogenic-differentiated uUSC displayed contractile function that was similar to that seen in native smooth muscle cells. Urothelial-differentiated uUSC expressed urothelial-specific genes and proteins such as uroplakin-Ia and -III, cytokeratin (CK)-7, and CK-13.
Conclusions:
uUSC possess expansion and differentiation (urothelial and myogenic) capabilities, and can potentially be used as an alternative cell source in bladder tissue engineering for patients needing cystoplasty.
Introduction
Engineered bladder tissues created with autologous cells seeded on biodegradable scaffolds have been used in patients for reconstructive purposes after cystoplasty.1 These autologous cells were obtained from a small biopsy of the patients' bladder tissue and then expanded to a large amount of cells for the cell-based therapy. However, it may not always be possible to obtain an adequate number of healthy bladder cells for tissue engineering purposes from patients with end-stage bladder diseases (ESBD). In addition, autologous bladder cells from patients with bladder tumors cannot be used, as there is a high potential for contamination of the engineered tissue with tumor cells. To treat these patients, it would be useful to develop an alternative source of cells that could be used to generate smooth muscle and urothelium in an engineered bladder construct.
Several mesenchymal stem cells (MSC) from bone marrow, adipose tissue, and skeletal muscle tissues may serve as potential autologous cell sources for use in urological tissue engineering.2–7 Our recent studies have demonstrated that certain cells derived from human voided urine possess characteristics of stem cells, including the ability to adhere to plastic, clonogenicity, and multipotential differentiation capability. These urine-derived stem cells (USC) display MSC and pericyte surface markers and can differentiate into a variety of cell types, including osteoblasts, chondrocytes, adipocytes, and skeletal myocytes. In particular, these cells can differentiate with high efficiency into urothelial cells (UC) and smooth muscle cells (SMC) in vitro in the presence of urothelial inductive culture conditions and myogenic growth factors,5–7 respectively.
Because the upper urinary tract is usually normal in the patients with ESBD or bladder cancer, we hypothesized that cells obtained from the upper urinary tract may be an alternative source for tissue engineering applications designed to treat the ESBD and bladder cancer. Our goal in the present study was to further characterize USC obtained from the upper urinary tract (uUSC) by their phenotype, expansion, and differentiation potential capability.
Materials and Methods
Cell culture
Upper urinary tract urine samples were obtained from five consenting patients (two male and three female; age range, 17 months–18 years) with ureteropelvic junction obstruction but normal renal pelvic structure and renal function during pyeloplasty surgery. Sterile voided urine samples from healthy donors were also collected for use as controls. After urine samples were centrifuged, cell pellets were resuspended and plated in 24-well tissue culture plates (Becton Dickinson, Franklin Lakes, NJ) using mixed media composed of keratinocyte serum-free medium (KSFM; Invitrogen, Carlsbad, CA) and embryonic fibroblast medium in a 1:1 ratio, as previously described for isolating cells from voided urine.4,7,8 Only cells that attached to culture wells by day 2 after plating were used. The 24-well dishes were scored for cells and only colonies derived from single cells used as an independent clone. These cells displayed a distinctive morphology and rapid proliferation. Unattached cells were washed away when the medium was changed on day 2 after plating. A description of the urine volumes collected and the clones obtained from the samples is given in Table 1. UC and SMC were obtained from ureter tissue discarded from kidney transplantation surgery. The two cell types were cultured in KSFM and Dulbecco's modified Eagle's medium (DMEM; HyClone, Logan, UT) with 10% fetal bovine serum (FBS; HyClone), respectively, as a control.8 Cell morphology (Morph) at each passage, population doublings (PD), cell growth pattern, expression of mesenchymal stem cell surface markers (MSC), bipotential differentiation capability (Diff ), and cell contractility (Contr) were assayed (see Table 1) on at least two independent clones per patient. Use of human urine and ureter tissue was approved by the Wake Forest University Health Sciences Institutional Review Board.
Table 1.
Details of Urine Samples Harvested from the Upper Urinary Tract
| Patient no. | Age | Sex | Volume (mL) | No. of clones | Assays performed |
|---|---|---|---|---|---|
| 1 | 17 months | M | 2.5 | 6 | MSC, PD, Diff, Contr |
| 2 | 14 years | F | 6 | 6 | MSC, PD, Diff, Morph |
| 3 | 18 years | F | 17.5 | 12 | MSC, PD, Diff, Morph |
| 4 | 18 years | M | 4 | 6 | PD, Diff, Contr, Karyo |
MSC, mesenchymal stem cell marker expression; PD, population doubling studies; Diff, bipotential differentiation studies; Contr, collagen lattice assay for contraction; Morph, morphology; Karyo, karyotype analysis.
Cell proliferation assay
To generate a growth curve, uUSC were seeded in triplicate at a density of 500 cells/cm2. Cell counts were manually recorded on days 2, 4, 6, 7, and 8 after plating. Voided USC were used as a control and were handled in the same manner. To calculate doubling time (DT), cell counts were recorded with every passage from p1 onward. Beginning with passage 2, uUSC (1×105) and voided USC (1×105) were regularly plated in 10 cm dishes. Cell cultures were trypsinized when they reached 70%–80% confluence (generally by day 4–6), and the cells were counted manually using a hemocytometer. The PD for estimation of cell growth and DT for quantification of cell growth rate were then calculated using the following formula9:
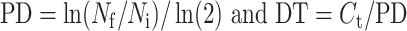 |
where Nf is the final number of cells, Ni is the initial number of cells, and Ct is the culture time. DT and PD for five passages are shown in Table 2 and PD with terminal passages in Table 3.
Table 2.
Comparison of uUSC and Voided USC for Doubling Time and Population Doublings
| Average doubling time (h) with passages (P) | |||||
|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |
| Voided USC | 20.4±5.2 | 19.7±1.9 | 22.4±3.1 | 26.5±4.2 | 28.6±2.9 |
| uUSC | 21.8±3.5 | 19.2±3.1 | 21.7±2.3 | 24.5±3.4 | 29.3±0.7 |
| Population doublings with passage | |||||
|---|---|---|---|---|---|
| Voided USC | 13.0±1.3 | 4.7±0.5 | 4.3±0.7 | 3.1±0.4 | 3.4±0.7 |
| uUSC | 13.8±1.6 | 4.4±0.6 | 3.4±0.7 | 3.8±0.8 | 3.2±0.2 |
| Cumulative PD with total cell number at passage 5 and total duration taken | |||||
|---|---|---|---|---|---|
| Voided USC | 28.5 (i.e., 228.5=3.7×108 cells), 26.6±3.5 days | ||||
| uUSC | 28.6 (i.e., 228.6=4.0×108 cells), 28.3±4.1 days | ||||
Three urine-derived cell clones from each of the three patient samples (n=9) were used for calculating the doubling time and is expressed as mean±standard deviation.
uUSC, urine-derived stem cells obtained from the upper urinary tract.
Table 3.
Comparison of Population Doublings at the Terminal Passages Between uUSC and Voided USC
| Clone no. | uUSC (terminal passages) | Clone no. | Voided USC (terminal passages) |
|---|---|---|---|
| 1B | 56.7 (p14) | 1A2 | 43.3 (p10) |
| 4C | 46.5 (p11) | 2A3 | 36.4 (p8) |
| 7B | 44.9 (p10) | 4B6 | 32.0 (p7) |
| 5A | 37.9 (p8) | 6D4 | 31.9 (p6) |
| Mean±SD | 46.5±7.7 | 35.9±5.3 |
Flow cytometry analysis
Cells undergoing exponential growth was trypsinized, washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (wash buffer), and counted. One million cells in 100 μL of PBS containing 3% bovine serum albumin were incubated with fluorochrome-conjugated antibodies (Table 6) for 30 min on ice. The cells were washed again, resuspended in 100 μL of wash buffer, and passed through a 70 μm filter for flow analysis. For unlabeled surface marker antibodies, a second incubation with a flurochrome-conjugated secondary antibody was used before filtration and flow analysis using FACSCalibur machine (BD Biosciences, Franklin Lakes, NJ).
Table 6.
Details of Antibodies Used for Immunofluorescence Staining and FACS Analysis
| Primary antibody | Source/catalog no. | Antibody dilution |
|---|---|---|
| Desmin | Santa Cruz/sc23879 | 1:100 |
| Myosin heavy chain | Sigma/M7786 | 1:50 |
| Smooth muscle actin | Sigma/A5228 | 1:100 |
| Vimentin (S-20) | Santa Cruz/sc7558 | 1:200 |
| Uroplakin-Ia | Santa Cruz/sc15173 | 1:50 |
| Uroplakin-III | US Biological/U2735-50 | 1:100 |
| AE1/AE3 | Dako/M3515 | 1:200 |
| Cytokeratin-13 | Novocastra/NCL-CK-13 | 1:50 |
| CD31 | Santa Cruz/sc1506 | 1:50 |
| CD34 | Miltenyi Biotec/130-081-002 | 1:50 |
| CD44 | R&D Systems/BBA10 | 1:50 |
| CD73 | Becton Dickinson/#550257 | 1:50 |
| CD105 | R&D Systems/MAB10971 | 1:50 |
| CD133 | Abcam/ab19898 | 1:50 |
| SSEA-4 | R&D Systems/MAB1435 | 1:50 |
| STRO-1 | R&D Systems/MAB1038 | 1:50 |
| CD146 | Abcam/ab75769 | 1:100 |
| NG2 | Abcam/ab50009 | 1:100 |
| PDGF-rβ | Abcam/ab32570 | 1:100 |
| CD44-FITC | BD Pharmingen/560977 | 1:5 |
| CD105-PerCP-Cy | BD Pharmingen/560819 | 1:20 |
| CD146-PE | BD Pharmingen/561013 | 1:5 |
| CD73-PE | BD Pharmingen/561014 | 1:5 |
| CD90-FITC | BD Pharmingen/555595 | 1:5 |
| CD45-FITC | BD Pharmingen/560976 | 1:5 |
| CD34-FITC | BD Pharmingen/560942 | 1:5 |
| CD31-FITC | BD Pharmingen/560984 | 1:5 |
| CD117 | Santa Cruz/sc13508 | 1:10 |
| CD133 | Abcam/ab16518 | 1:10 |
| SSEA4-FITC | BD Pharmingen/560126 | 1:5 |
| STRO-1 | Abcam/ab102969 | 1:10 |
FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP-Cy, peridinin chlorophyll protein-cyanine.
Karyotype analysis
Cultured cells were analyzed for chromosomal stability at various passages as describe.4 Briefly, the cultured cells were treated with a hypotonic solution and fixed using methanol-acetic acid solution. The metaphase spreads on the glass slides were digested using trypsin and followed by Giemsa stain to generate G bands along each chromosome. Standard cytogenetic analysis was carried out on the captured images and karyotyping performed using the CytoVision®, software.
Urothelial and smooth muscle differentiation of uUSC
A single clone of uUSC (p5) plated at a density of 1000 cells/cm2 was used for differentiation into two lineages, SMC (mesoderm) and UC (endoderm), after culture in specific medium for 14 days. For myogenic differentiation, equal volumes of DMEM (containing 10% FBS) and embryonic fibroblast medium (containing TGF-β1 [2.5 ng/mL], PDGF-BB [5.0 ng/mL], and 10% FBS) were used. For uroepithelial differentiation, DMEM containing 10% FBS was mixed with KSFM at a 1:4 ratio and 30 ng/mL epidermal growth factor to the mix. The differentiation medium was replaced every third day. All growth factors were purchased from R&D Systems (Minneapolis, MN).
RNA analysis
Reverse transcriptase–polymerase chain reaction
Urothelial- and myogenic-differentiated uUSC were assessed by semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of SMC transcripts, including desmin, myosin heavy chain (MHC), α-smooth muscle actin (ASMA), and UC transcripts, including uroplakin (Up)-Ia, Up-III, cytokeratin (CK)-7, and CK-13 (see Table 5). For RNA extraction experiments, uUSC were cultured in urothelial and myogenic differentiation media in 10 cm dishes for 14 days. Five micrograms of RNA, extracted using Trizol (Invitrogen) reagent, was used for cDNA synthesis using the Superscript II RT enzyme (Invitrogen) according to the manufacturer's instructions. Briefly, RNA was incubated with random hexamers, nucleotides, RT enzyme, and reaction buffer in 20 μL volume for synthesis of cDNA. One tenth of the reaction volume was taken for PCR using specific primer pairs (Table 5).
Table 5.
Details of Primers Used for Gene Expression Analysis Along with Their Expected Product Size
| Semiquantitative primers (RT-PCR) | ||||
|---|---|---|---|---|
| Target gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Amplicon (bp) | |
| Desmin | CCATCGCGGCTAAGAACATT | TCGGAAGTTGAGGGCAGAGTA | 440 | |
| MHC | GGACGACCTGGTTGTTGATT | GTAGCTGCTTGATGGCTTCC | 656 | |
| ASMA | ACCCACAATGTCCCCATCTA | TGATCCACATCTGCTGGAAG | 595 | |
| Up-Ia | ACGTCCTACACCCACCGTGA | ACCCCACGTGTAGCTGTCGAT | 360 | |
| Up-IIIA | ACAAACAGAGGGTGGGAGGACAG | AGAAGGGCAGGGAGCCCAGG | 392 | |
| CK-7 | TGGTGCTGAAGAAGGATGTG | CACGCTGGTTCTTGATGTTG | 405 | |
| CK-13 | GGCTTCCTACCTGGAGAAGG | CGACCACCTGGTTGCTAAAT | 422 | |
| GAPDH | CGGATTTGGTCGTATTGG | TCAAAGGTGGAGGAGTGG | 861 | |
| Quantitative primers (real-time PCR) | ||||
|---|---|---|---|---|
| Target gene | Sequence ID | Exon boundary | Catalog no. | Amplicon (bp) |
| Desmin | NM_001927.3 | 1–2 | Hs01090875 | 86 |
| Myosin | NM_022844.2 | 3–4 | Hs00224610 | 78 |
| Smoothelin | NM_134269.1 | 13–14 | Hs00199489 | 52 |
| Actin | NM_001141945.1 | 1–2 | Hs00909449 | 64 |
| Up-Ia | NM_007000.2 | 2–3 | Hs00199638 | 61 |
| Up-IIIA | NM_006953.3 | 5–6 | Hs00199590 | 58 |
| ZO-1 | NM_175610.2 | 8–9 | Hs01551876 | 64 |
| E-Cad | NM_004360.3 | 3–4 | Hs00170423 | 117 |
| GAPDH | NM_002046.3 | 3–4 | 4333764F | 122 |
MHC, myosin heavy chain; ASMA, alpha smooth muscle actin; Up, Uroplakin; CK, cytokeratin; ZO, Zona Occludens; E-Cad, epithelial-cadherin; GAPDH, glyceraldehyde-3 phosphate dehydrogenase; RT-PCR, reverse transcriptase–polymerase chain reaction.
Real-time PCR
All the components for real-time analysis were obtained from Applied Biosystems (Foster City, CA). Five micrograms of total RNA was converted to cDNA using the High-capacity cDNA Reverse Transcription Kit. The cDNA was diluted five-fold with water and 2 μL was used for amplification using specific primers (Table 5) and 2× Taqman Universal PCR Mix in a 7300 Real-Time PCR system (Applied Biosystems).
Immunofluorescence
The uUSC clones were cultured on eight-well chambered slides (Thermo Scientific, Waltham, MA) for staining with MSCmarkers (CD44, CD73, CD105, CD133, STRO-1, and SSEA-4), pericyte markers (CD146, NG2, and PDGF-rβ), UC markers (Uroplakin Ia and III, AE1/AE3, and CK-7), and SMC markers (desmin, MHC, ASMA, and vimentin) by immunofluoresence. The slides were fixed with 4% paraformaldehyde for 20 min at room temperature, extracted with 0.1% Triton X-100 (for SMC and UC markers alone), and followed by several washes with PBS. Primary antibodies (Table 4) were incubated overnight following a blocking step (serum-free block; Dako, Glostrup, Denmark). To test the specificity of a primary antibody, immunoglobulin fractions from those animals were used on adjacent wells. A secondary antibody conjugated to fluorescein isothiocyanate (FITC; Vector Laboratories, Burlingame, CA) was used to observe hybridization (green) to primary antibody. The nucleus was counterstained red with propidium iodide containing mounting media (Vector Laboratories). All antibodies used in this study are listed in Table 6. Images were captured using a Leica upright microscope (DM 4000B). A constant gain was maintained during capture of images for the control and treatment samples so as to avoid amplifying nonspecific staining signals.
Table 4.
Comparison of uUSC and Voided USC in Cell Surface Markers at Passage 4
| Cell surface marker | uUSC | Voided USC |
|---|---|---|
| CD31 | <1% | ≤1% |
| CD34 | <1% | ≤1% |
| CD44 | 100% | 100.0% |
| CD45 | <1% | ≤1% |
| CD73 | 99% | 99.2% |
| CD90 | 51.5% | 45.8% |
| CD105 | 1.8% | 42.5% |
| CD117 | 11.2% | 2.3% |
| CD133 | 1.5% | 3.66% |
| CD146 | 99% | 98.0% |
| SSSEA-4 | 99.3% | 99% |
| STRO-1 | 4.0% | 3.1% |
Bold indicates significant difference.
In vitro contractility assessment
Cell-embedded collagen lattices were employed to measure contractile forces in the myogenic differentiated uUSC as described previously.10 Chemicals were purchased from Sigma (St. Loius, MO) unless otherwise mentioned. Briefly, an aliquot containing 3×105 uUSC/mL was mixed with soluble type I collagen (1 mg/mL; BD Biosciences) containing NaOH, F-12 nutrient mixture (N3250), and NaHCO3 to create a cell-collagen suspension. An aliquot (250 μL) of the uUSC-collagen suspension was placed onto a 35-mm tissue culture plate (Techno Plastic Products AG, Trasadigen, Switzerland) and allowed to set. Lattices were maintained for 7 days with or without myogenic growth factor containing media. A side by side comparison of uUSC with voided USC and SMC isolated from bladder was also performed. Initial lattice diameter was noted before mechanical release of the cell-collagen lattices for contractile force measurement. The diameter of the cell-collagen lattices was measured after release (10 min), and the relative change in diameter calculated. Each experiment was performed in triplicate. Lattices released in the presence of 5% FBS and agonist (1 μM calcium-ionophore, A23187) in serum-free medium served as positive controls; lattices released under serum-free conditions alone served as negative controls. The percentage of contraction was calculated by as follows: (Du−Dr)/Du×100, where Du and Dr are the diameters of unreleased lattice and released lattice, respectively.
Statistical analysis
For growth characterization and contractility assay, at least three independent clones were used to calculate mean±standard deviation, and the comparison was made between the groups using a two-tailed Student's t-test with unequal variance. Differences were considered significant at p≤0.05.
Results
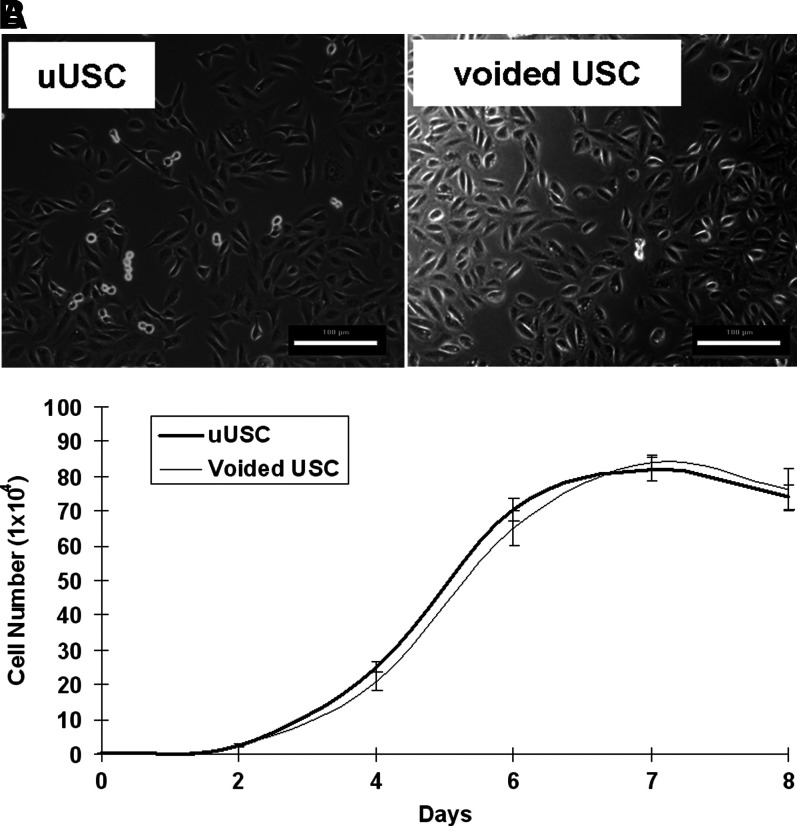
In terms of cell phenotype and viability, most of the cells in the upper urinary tract urine were red blood cells and flat UC that could not attach to the culture plates, and these cells were washed away when the culture medium was changed. Only a few cells from each urine sample could attach and expand. Almost all of these clones could grow well and expand to at least p8 (see Table 1). The uUSC clones were generated from 4 out of 5 urine samples. One urine sample was discarded due to contamination on day 3 of plating. The average urine volume obtained from the upper urinary tract was 7.5±6.8 mL and the samples contained 1.4±0.7 uUSC clones per mL of urine (see Table 1). The cell clones displayed rice grain-like morphology; they were bright and compact during the first 3–7 days in culture, and then they began to form colonies with symmetrical cell clusters with time (Fig. 1A). uUSC could extensively expand in vitro and their DT were stable at 19–22 h for passages below 2, and after which the DT increased to ∼29 for p5 (Table 2). The maximum PD of uUSC was 56.7 (average 46.5±7.7) and the cells were grown to a maximum passage of p14 (Table 3). On the other hand, voided USC displayed a maximum PD of 43.3 (average 35.9±5.3) with a maximum passage of p10. The cell morphology and growth pattern of the expanded uUSC clones at earlier passages (<p4) were similar to those obtained from voided USC (Fig. 1A, B and Table 2). However, uUSC significantly generated more cells (46.5±7.7 PD) compared to voided USC (35.9±5.3 PD) (p<0.001) at terminal passages (Table 3).
FIG. 1.
Morphology and growth of uUSC. (A) A single clone of uUSC shown along with voided USC at passage 2 demonstrating the similarity in morphology of the two cell clones. Scale bar=100 μm. (B) The growth pattern of uUSC was similar to voided USC at passage 5. The average doubling time for both the cells was around 20 h. uUSC, urine-derived stem cells obtained from the upper urinary tract.
Karyotype analysis was performed to test the chromosomal stability of uUSC after serial cultures. The uUSC lines displayed a karyotype of 1× and 1 Y chromosome, as expected for a male donor, and a normal diploid (2n=38) complement of autosomes. At passage 5 of uUSC, no obvious chromosomal rearrangements at metaphase were detected by Giemsa bandings.
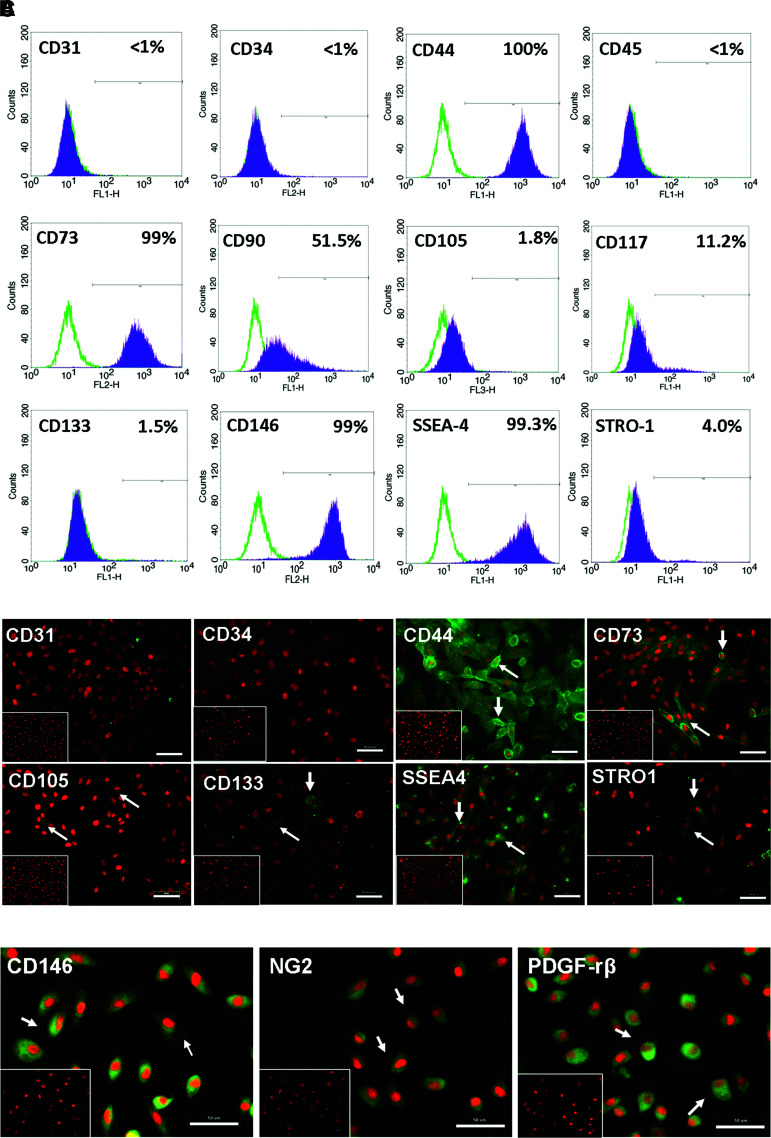
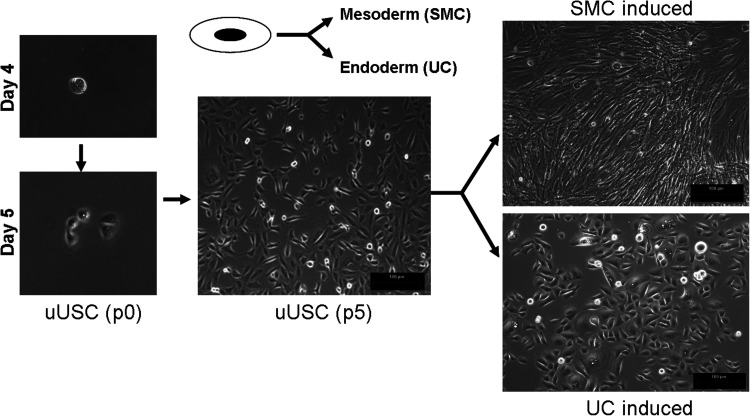
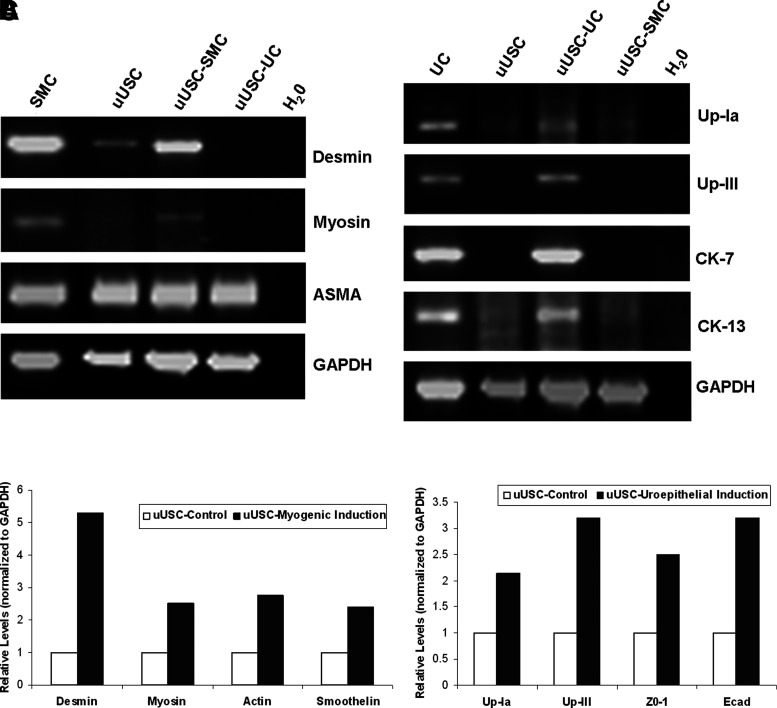
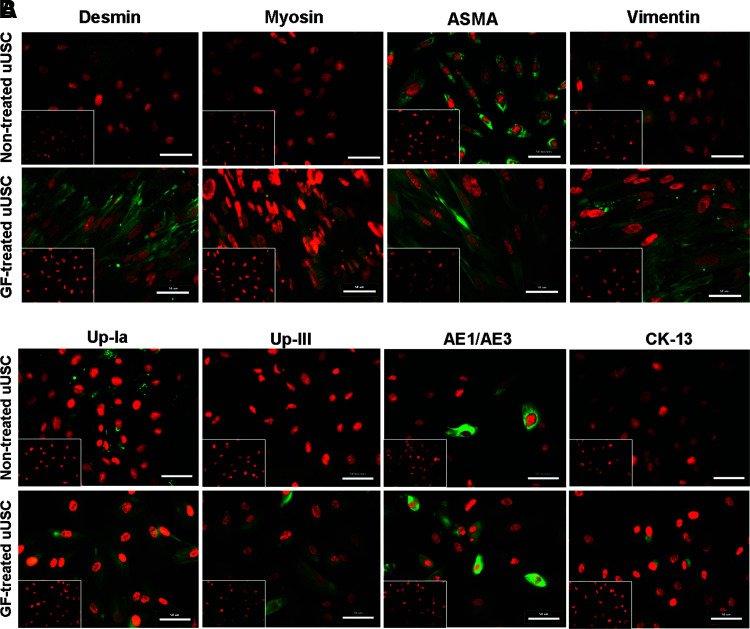
Immunophenotyping for surface antigens by flow cystometry was performed on uUSC (Fig. 2A). These cells strongly expressed CD44, CD73, CD90, CD146, and SSEA-4, except CD105 as previously described for voided USC.4 Further, uUSC were negative for the hematopietic lineage markers CD31, CD34, CD45, and CD133 (Table 4). Similar results were obtained using immunofluorescent staining: uUSC stained strongly positive for the MSC markers CD44, CD73, and SSEA-4; weakly positive for CD105, CD133, and STRO-1; and negative for the hematopoietic stem cell markers CD31 and CD34 (Fig. 2B). Further, these cells also expressed all pericyte membrane markers (CD146, NG2, and PDGF-rβ) (Fig. 2C). To test the bipotential differentiation capability of the uUSC, single clones of uUSC (p5) were subjected to myogenic as well as uroepithelial differentiation conditions for 14 days. A distinct morphology change was evident by day 7 in each culture, and this became even more pronounced on day 14 (Fig. 3). The long, spindle-shaped morphology of stromal cells was evident among the cells in myogenic differentiation medium, and a cuboidal phenotype appeared in the uroepithelial differentiation medium (Fig. 3). The bipotential differentiation capacity of uUSC was confirmed by analysis of the expression of lineage-specific transcripts with semiquantitative RT-PCR (Fig. 4A, B) as well as real-time analysis (Fig. 4C) and by the expression of specific proteins observed by immunofluorescent staining for desmin, myosin, ASMA, and vimentin for myogenic differentiation (Fig. 5A) and uroplakin-Ia (Up-Ia), Up-III, AE1/AE3, and CK-13 for urothelial differentiation (Fig. 5B).
FIG. 2.
(A) Analysis of surface marker expression in uUSC by FACS. A clone of uUSC (p4) was stained with CD markers expressed by mesenchymal as well as hematopeietic stem cells. All MSC markers stained positive, whereas the hematopoitic markers (CD31, 34, and 45) were negative. (B) Immunofluorescence staining for stem cell markers. Immunofluorescent images of uUSC (p3) stained positive (green) for MSC markers (CD44, CD73, CD105, CD133, STRO-1, and SSEA-4) and negative for hematopoietic stem cell markers (CD31 and CD34) are shown. (C) Expression of pericyte markers. Immunofluorescent images of uUSC (p3) stained (green) with pericyte markers (CD146, NG2, and PDGF-rβ). Nucleus was counterstained with propidium iodide (PI, red). Representative cells exhibiting distinct membrane staining are shown by arrows. The inset shows cells stained using control immunoglobulin fraction. Scale bar=50 μm. MSC, mesenchymal stem cell. Color images available online at www.liebertonline.com/tea
FIG. 3.
Morphology changes of differentiated uUSC. A single clone of uUSC differentiated to myogenic and uroepithelial lineage by culture in lineage-specific differentiation medium for 14 days. A distinct change in cell shape from oval (one single cell on day 3 at p0) to ‘rice-grain’-like (on day 5-6 with cell division at p0) (nontreated control) then to that of spindle shape (SMC induced, at p5) and cobblestone-like shape (uroepithelial induced, at p5) was observed. Brightfield image scale bar=100 μm. SMC, smooth muscle cells.
FIG. 4.
Gene expression analysis of differentiated uUSC. Reverse transcriptase–polymerase chain reaction analysis of uUSC (p5) differentiated into (A) myogenic lineage and (B) uroepithelial lineage on day 14. Specific transcripts were amplified with lineage-specific primers only in the differentiated samples. uUSC, nontreated control; uUSC-SMC, differentiated to myogenic lineage for 14 days; uUSC-UC, differentiated to uroepithelial lineage for 14 days and H2O is no template control. RNA from SMC and UC serve as positive controls. (C) Real-time analysis of uUSC for myogenic and urothelial-specific transcripts. A single clone of uUSC (p4) was differentiated into SMC and UC by incubation in specific growth factor containing medium for 12 days. At least a twofold increase in all the transcripts was observed. Up, uroplakin; ZO, Zona Occludens; Ecad, epithelial cadherin; UC, urothelial cells.
FIG. 5.
(A) Immunofluorescent staining of uUSC for smooth muscle-specific markers. uUSC (p6) were differentiated to SMC lineage by culturing in myogenic medium containing TGF-β1 (2.5 ng/mL) and PDGF-BB (5.0 ng/mL) for 14 days. Nontreated uUSC controls are shown in the top row. Upon differentiation, specific staining was observed with desmin, myosin, and vimentin antibodies. (B) Immunofluorescent staining of uUSC for urothelial-specific markers. uUSC(p6) differentiated to urothelial lineage by culturing in epithelial medium containing epidermal growth factor (30 ng/mL) for 14 days. Nontreated uUSC controls are shown in the top row. Upon differentiation, specific staining or enrichment was observed with the urothelial-specific antibodies. Image scale bar=50 μm. The inset shows cells stained using control immunoglobulin fraction. Color images available online at www.liebertonline.com/tea
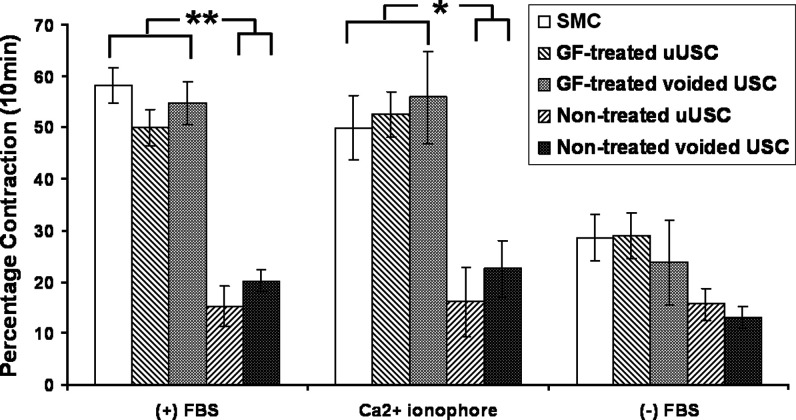
In the contractility analyses, cell-collagen lattices containing myogenic-differentiated uUSC displayed the maximum contraction (50%±4%) in the presence of 5% FBS and a calcium agonist (ionophore) (53%±4%), which was similar to the contraction of control SMC and voided USC that was differentiated in myogenic media. Noninduced uUSC and voided USC did not display any significant contractile activity (Fig. 6). Contraction in the absence of FBS was minimal and was used as a negative control.
FIG. 6.
Contractile function of differentiated uUSC. Contraction of collagen lattices in the presence of serum and calcium ionophore agonist displayed very significant contraction in myogenic-differentiated uUSC (p6) that was similar to the voided USC after induction for 7 days compared to the nontreated controls. Contraction in the absence of serum was used as experimental negative control. SMC were also included in the assay as a positive control. Values (uUSC) are replicates of at least three clones from two different patient samples and were considered significant when p≤0.05 (*) and p≤0.005 (**).
Discussion
The presented data demonstrate that uUSC clones contain small, bright, rice grain-like, compact cells (high nucleus to cytoplasm ratio) that can be extensively expanded. Cells derived from these clones expressed MSC and pericyte surface markers. In addition, a single clone of uUSC could differentiate into both functional SMC (with application of myogenic growth factors) and UC (through culture in epithelial inductive differentiation media). Myogenic-differentiated uUSC displayed similar contractile function characteristics to native SMC in a collagen lattice assay and urothelial differentiation of uUSC expressed the functional gene markers of tight junctions. The advantage of using one single cell clone to differentiate into two cell lineages is the cost effectiveness in further clinical application.
Numerous surgical approaches have been used in treatment of ESBD and muscle invasive bladder cancer. Bladder reconstruction using segments of the small or large intestine has been widely applied. However, the use of bowel segments in the urinary tract can cause several serious complications7 and patients reconstructed in this manner also carry a significantly higher risk of developing intestinal tumor compared to the general population.11 The main reason for this risk is that after reconstruction, the intestinal mucosa is placed in a nonphysiological environment in which it is exposed to urine for a lifetime.
Tissue engineering technology may provide an alternative approach for patients with ESBD who need cystectomy and bladder reconstruction.1 However, obtaining a reliable cell source might be a challenge to those patients with abnormal bladder tissues. An obvious advantage of using a patient's own MSC is in immune compatibility. Bone marrow stem cells have been demonstrated to differentiate into SMC,12 but the efficiency of urothelial differentiation is low with BMSC, as specific growth factors that induce MSC differentiation toward a urothelial phenotype have not yet been defined. Recently, we have successfully isolated stem cells from voided urine,4 and these cells can grow from a single cell clone to a large cell population with a 30 h DT, displaying an average PD of 40. Additionally, these cells express detectable levels of telomerase and display MSC/pericyte markers,13 thereby displaying mesenchymal-like properties, including the ability to differentiate into myogenic and other mesodermal cell lineages, such as osteocytes, chondrocytes, and adipocytes.13–15 However, use of voided USC for the construction of new bladder tissue in ESBD patients carries the potential risk of bladder cancer recurrence, as cancer cells may find their way into the urine while it is stored in the bladder. Thus, we sought a new source of USC—the upper urinary tract.
In chronic bladder diseases, uUSC might be a good cell source for bladder tissue regeneration because the cells from the upper urinary tract are most often normal. In addition, the possible risk of finding ureter, renal pelvic, or kidney cancer in bladder cancer patients can be eliminated with careful screening. For the treatment of ESBD or muscle-invasive bladder cancer, engineered bladder tissue constructed with uUSC as the cell source may have advantage over current surgical procedures, that is, bladder reconstruction using intestinal segments. Using engineered tissue would avoid the complications associated with the use of bowel tissues. Harvesting uUSC from patients who already have a nephrostomy tube in place would be a simple and low-cost approach for obtaining cells, with the option of multiple urine collections if necessary, for engineering bladder tissue.
In this study, we demonstrated that some urine-derived cells from the upper urinary tract possessed characteristics similar to voided USC, including expansion capacity, cell surface markers, chromosomal stability, and potential differentiation to urothelium- and SMC-like cells. We have previously demonstrated that these differentiated uUSC can be cultured on bacterial cellulose polymer scaffolds in vitro.7 Large population of cells could be generated from a single clone. We observed that the average expansion capacity or PD of uUSC was 46.5±7.7 (range 37 to 56 PD, n=5). This implies that a single stem cell from the upper urinary tract, on average, can generate 1.0×1014 cells (246.5) within about 8 weeks. It is known that 1.4×109 cells are required for both SMC and UC to create a tissue-engineered bladder.1 To retain highly efficient bipotential differentiation capacity, we typically use USC below or at passage 5. Under our optimized culture conditions, one cell clone of uUSC could generate about 4×108 cells on p5 within 30 days. Although we have not collected large urine volumes, our data show that 1 mL of urine from the upper urinary tract contains about 1.4±0.7 USC clones. Based on this ratio of cells and urine volume, expansion of the stem cells from upper urinary tract of urine could potentially yield at least 3.8×109 cells from 20 mL. Thus, assuming 50% bipotent differentiation efficiency uUSC can provide an adequate amount of cells to engineer a neo-bladder. Importantly, uUSC are able to give rise to bladder cells, both UC and SMC when grown in specific media cocktails. The myogenic-differentiated uUSC exhibited contractile function and the urothelial-differentiated uUSC expressed the functional markers of tight junction (ZO-1 and E-cadherin), which are essential for forming an impermeable barrier to fluids. Additionally, uUSC do not express SMC and UC gene and protein markers before induction of differentiation, indicating that these cells do not originate from muscle and UC lineages. As uUSC express MSC and pericyte cell surface makers (with no expression of hematopoietic stem cell markers) similar to voided USC4,7,16 with the exception of CD105 and hence are likely to be MSC or pericytes. Further, uUSC are a reliable cell source as cell clones can be obtained from almost every urine sample. Importantly, like voided USC, uUSC appear to be safe as the chromosomal stability after serial cultures are maintained and no teratoma or malignant tissue formation have been observed after implantation in vivo.7–8,13–15,17
Compared to voided USC, uUSC currently seem to have at least two advantages: (1) isolation of more cell clones from a defined volume of upper urinary tract urine and (2) a possible higher PD in uUSC. One plausible reason for these observations could be that the residual time of uUSC in the urine may be shorter, making them relatively fresher than the voided USC. Whether the duration these cells spend in urine affect their robustness or quality needs to be further studied. Also, the tissue of origin for uUSC remains to be established.
Conclusions
uUSC are a novel source of adult autologous stem cells with high expansion potential and UC and smooth muscle differentiation capability. They are identical to the voided USC in terms of the morphology, growth characteristics, CD marker expression profile, and differentiation potential being fairly similar to each other. These cells are safe and easily obtainable, and unlike embryonic stem cells or iPS cells, they do not form teratomas or other tumors in vivo. Hence, uUSC could be used in bladder tissue engineering for patients with the ESBD or malignancy who require cystoplasty.
Acknowledgments
The authors thank Dr. Steve Hodges for urine samples and Dr. Jennifer Olson for her editorial assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Atala A. Bauer S.B. Soker S. Yoo J.J. Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241. doi: 10.1016/S0140-6736(06)68438-9. . [DOI] [PubMed] [Google Scholar]
- 2.Serakinci N. Keith W.N. Therapeutic potential of adult stem cells. Eur J Cancer. 2006;42:1243. doi: 10.1016/j.ejca.2006.01.036. . [DOI] [PubMed] [Google Scholar]
- 3.Furth M.E. Atala A. Stem cell sources to treat diabetes. J Cell Biochem. 2009;106:507. doi: 10.1002/jcb.22000. . [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y. McNeill E. Tian H. Soker S. Andersson K.E. Yoo J.J. Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226. doi: 10.1016/j.juro.2008.07.023. . [DOI] [PubMed] [Google Scholar]
- 5.Tian H. Bharadwaj S. Liu Y. Ma P.X. Atala A. Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 2010;16:1769. doi: 10.1089/ten.tea.2009.0625. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian H. Bharadwaj S. Liu Y. Ma H. Ma P.X. Atala A. Zhang Y. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials. 2010;31:870. doi: 10.1016/j.biomaterials.2009.10.001. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodin A. Bharadwaj S. Wu S. Gatenholm P. Atala A. Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889. doi: 10.1016/j.biomaterials.2010.07.108. . [DOI] [PubMed] [Google Scholar]
- 8.Wu S. Wang Z. Bharadwaj S. Hodges S.J. Atala A. Zhang Y. Implantation of autologous urine derived stem cells expressing vascular endothelial growth factor for potential use in genitourinary reconstruction. J Urol. Jun 16, 2011. [Epub ahead of print]. [DOI] [PubMed]
- 9.Rainaldi G. Pinto B. Piras A. Vatteroni L. Simi S. Citti L. Reduction of proliferative heterogeneity of CHEF18 Chinese hamster cell line during the progression toward tumorigenicity. In Vitro Cell Dev Biol. 1991;27A:949. doi: 10.1007/BF02631122. . [DOI] [PubMed] [Google Scholar]
- 10.Kropp B.P. Zhang Y. Tomasek J.J. Cowan R. Furness P.D., 3rd Vaughan M.B. Parizi M. Cheng E.Y. Characterization of cultured bladder smooth muscle cells: assessment of in vitro contractility. J Urol. 1999;162:1779. . [PubMed] [Google Scholar]
- 11.Austen M. Kalble T. Secondary malignancies in different forms of urinary diversion using isolated gut. J Urol. 2004;172:831. doi: 10.1097/01.ju.0000134890.07434.8e. . [DOI] [PubMed] [Google Scholar]
- 12.Becker C. Jakse G. Stem cells for regeneration of urological structures. Eur Urol. 2007;51:1217. doi: 10.1016/j.eururo.2007.01.029. . [DOI] [PubMed] [Google Scholar]
- 13.Bharadwaj S. Wu S. Atala A. Zhang Y. Characterization of urine derived stem cells obtained from upper urinary tract for cell-based tissue engineering in urology. Tissue Engineering and Regeneration Medicine International Association North American (TERMIS-NA) Annul Meeting; Orlando, Florida. December 5–8;2010 ; . [Google Scholar]
- 14.Bharadwaj S. Wu S. Smith J. Atala A. Zhang Y. Skeletal muscle derived from human urine-derived cells: a potential source for injection therapy for the treatment of stress urinary incontinence. J Urol. 2010;183:e681. . [Google Scholar]
- 15.Bharadwaj S. Wu S. Rohozinski J. Furth M. Lan X. Atala A., et al. Multipotent stem cells from urine for tissue engineered bladder. J Urol. 2010;183:e61. . [Google Scholar]
- 16.Wu S. Liu Y. Bharadwaj S. Atala A. Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317. doi: 10.1016/j.biomaterials.2010.10.006. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S. Bharadwaj S. Liu Y. Hodges S. Atala A. Zhang Y. Urine-derived stem cells for urological injection therapy. Tissue Eng. 2009;6:S86. . [Google Scholar]