Abstract
To define the role of nontypeable Haemophilus influenzae (NTHI) lipooligosaccharide (LOS) in the induction of proinflammatory cytokine gene expression during otitis media, we compared the abilities of formalin-killed NTHI strain 2019 and its LOS htrB and rfaD mutants to stimulate human middle ear epithelial (HMEE) cell cytokine and chemokine gene expression and production in vitro. Strain DK-1, an rfaD gene mutant, expresses a truncated LOS consisting of only three deoxy-d-manno-octulosonic acid residues, a single heptose, and lipid A. Strain B29, an isogenic htrB mutant, possesses an altered oligosaccharide core and an altered lipid A. HMEE cells were incubated with formalin-killed NTHI 2019, B29, or DK-1. The supernatants and the cells were collected at 2, 4, 8, and 24 h after stimulation. Expression of genes for the cytokines tumor necrosis factor alpha (TNF-α), interleukin lβ (IL-1β), and IL-6 and for the chemokines macrophage inflammatory protein 1β (MIP-1β), monocyte chemotactic peptide 1 (MCP-1), and IL-8 was quantitated by real-time PCR. NTHI B29 did not significantly stimulate any cytokine or chemokine mRNA expression in HMEE cells. In striking contrast, NTHI 2019 induced up to 105-, 139-, and 187-fold increases in HMEE cell expression of IL-1β, TNF-α, and MIP-1β, respectively (P < 0.01 [2019 versus B29]). NTHI 2019 also induced upregulation of IL-8, IL-6, and MCP-1 mRNA expression (by 26-, 44-, and 14-fold, respectively [P < 0.05 {2019 versus B29}]). The significant induction of cytokine genes was confirmed by quantitating the secretion of cytokines in culture supernatants with an enzyme-linked immunosorbent assay. There were no significant differences in mRNA expression of IL-8, IL-6, and MCP-1 between the 2019- and DK-1-treated groups. The low levels of gene transcripts observed after incubation of HMEE cells with B29 indicate that products of the disrupted NTHI htrB LOS gene may play a major role in induction of these particular inflammatory mediators.
Considerable evidence indicates that endotoxin or its subcomponent lipopolysaccharide (LPS) or lipooligosaccharide (LOS), each an integral component of the outer membrane of gram-negative bacteria, is refractile and present in a large percentage of middle ear effusions from children with otitis media (OM) (10, 14). Endotoxin is a potent stimulant of proinflammatory reactions of the host's innate immune response (22). The inflammatory cytokines are thought to be of central importance in the pathogenesis and regulation of proliferation, chemotaxis, and activation of inflammatory cells during the course of middle ear infections (21, 29). A previous report from our laboratory indicates that there is a significant correlation between endotoxin, tumor necrosis factor alpha (TNF-α), and interleukin 1β (IL-1β) concentrations in middle ear effusions from children with OM (35). Recent experimental studies, moreover, demonstrate that formalin-killed nontypeable Haemophilus influenzae (NTHI) stimulates the release of IL-1β, IL-6, IL-8, and TNF-α from the middle ear mucosa in the guinea pig OM model (30). It has been proposed that the initial interaction of endotoxin with middle ear epithelium leads to the production and release of inflammatory cytokines in the middle ear (2, 5, 8). This is followed by recruitment of neutrophils, eruption of a cytokine-mediated inflammatory cascade, and neutrophil activation, resulting in the release of inflammatory molecules which participate directly in middle ear inflammation (27, 37). To date, reports on the effects of NTHI LOS on induction of cytokine gene expression by human cells have focused primarily on stimulation of monocytes (34) and bronchial and tracheal epithelial cells (7, 16, 24). The effects of NTHI LOS on cytokine and chemokine gene expression by cultured human middle ear epithelial (HMEE) cells have never been reported.
Major advances have also been made recently in identifying the molecular mechanisms underlying LPS or endotoxin recognition and signaling for LPS-induced cell activation. It is believed that membrane-bound CD14 (mCD14), a glycosyl phosphatidylinositol-anchored protein expressed on myeloid cells, binds to LPS and initiates its signaling (33). Toll-like receptors (TLRs) have been identified as the signaling molecules and may act as transmembrane coreceptors with CD14 in LPS activation of different cell populations (15). The potential role of NTHI LOS in the activation of HMEE cells via these pathways has also not been explored.
It is now possible to define the role of NTHI LOS in the pathogenesis of OM by using mutants with various LOS gene disruptions as tools. A recent study from our laboratory indicates that disruption of the NTHI htrB and rfaD LOS genes does not impact the ability of NTHI to colonize the nasopharynx; however, it induces marked differences in the abilities of the two LOS mutants to induce OM and survive in the middle ear (9). In this study, the relative contributions of disruption of NTHI htrB and rfaD LOS genes to proinflammatory cytokine gene expression by HMEE cells were quantitated by a novel, highly accurate, and reproducible real-time PCR amplification technique. Our data demonstrate that NTHI LOS htrB gene products play an important role in the induction of proinflammatory cytokine genes in cultured HMEE cells.
MATERIALS AND METHODS
Bacteria.
The following NTHI strains were obtained from Michael A. Apicella, University of Iowa College of Medicine, and were used in this study. All have been previously described (17, 18, 25).
NTHI strain 2019 is the parental isolate (wild type) and grows on chocolate agar, as well as on brain heart infusion agar supplemented with Fildes enrichment (sBHI), at 30 to 37°C.
Strain B29 is an isogenic htrB mutant generated by shuttle mutagenesis using a mini-Tn3. This mutant exhibits phenotypic changes in both the lipid A moiety and the core oligosaccharide. B29 contains a chloramphenicol resistance gene and can be differentiated from the parent and from strain DK-1 by its ability to grow on sBHI containing 100 μg of chloramphenicol/ml. It has been described in detail previously (18).
DK-1 is an rfaD gene mutant. This strain's LOS is truncated and consists only of 2-keto-3-deoxyoctulosonic acid and lipid A. It is devoid of other core oligosaccharides. DK-1 contains a kanamycin resistance cassette and can thus be differentiated from the parent and from strain B29 by its ability to grow on sBHI containing 15 μg of kanamycin/ml. It has been described in detail previously (25).
Preparation of formalin-killed NTHI strains.
The use of whole, formalin-killed bacterial cells as a source of endotoxin was developed in our laboratory over 15 years ago and is widely used (26, 30). NTHI strains were grown on sBHI agar, with or without antibiotics as indicated above, overnight in a CO2 incubator at 37°C. The bacteria were harvested, washed in sterile phosphate-buffered saline, and killed by incubation with 0.3% formalin at room temperature for 24 h, as previously reported (26). Killed NTHI strains were washed three times in sterile phosphate-buffered saline and suspended in cell culture medium at a concentration of 2 × 107 CFU per milliliter for the experiment.
Cell culture.
Two primary cultures of HMEE cells were established from middle ear biopsy specimens taken near the orifice of the eustachian tube from a 19-year-old patient and a 34-year-old patient undergoing acoustic neuroma surgery at The Ohio State University Medical Center, Columbus, Ohio. The biopsy specimens were minced and washed in ice-cold Hanks' balanced salt solution containing 10 U of penicillin/ml, 10 μg of streptomycin/ml, 500 ng of amphotericin B (Fungizone)/ml, and 5% fetal bovine serum and were subsequently digested with 0.01% trypsin–0.1 M EGTA for 10 min at room temperature. The minced biopsy suspensions were then placed in a 25-cm2 Corning tissue culture flask and cultured in modified minimal essential medium α (MEMα) (Gibco BRL, Gaithersburg, Md.) buffered with 155 mg of NaHCO3 and 3.6 g of HEPES/liter supplemented with 5 mg of insulin/liter, 2 mg of transferrin/liter, 500 μg of hydrocortisone/liter, 25 ng of epithelial growth factor/ml, 10 μg of streptomycin/ml, 5 U of penicillin/ml, 10% fetal bovine serum, and 250 ng of amphotericin B/ml at 37°C. All medium additives were from Collaborative Research (Boston, Mass.).
At first, cell cultures were maintained in growth medium containing 48 mg of d-valine (Gibco BRL)/liter, instead of l-valine, to prevent growth of fibroblasts. The medium was changed every 3 days; when the epithelial cells from the primary explant reached confluency, the medium was changed to that containing l-valine. The cells were serially passed, characterized, and stored in liquid nitrogen.
Characterization of HMEE cells.
Anti-cytokeratin polypeptide 8, 18, and 19 antibody (Vector Laboratories, Burlingame, Calif.) probes were used to immunocytochemically characterize the cells. Monoclonal antivimentin (clone V9; Sigma-Aldrich, St. Louis, Mo.), which labels vimentin in fibroblast cells, was used to rule out fibroblast contamination.
mRNA expression of TLR2, TLR3, TLR4, and mCD14 genes was examined in cultured HMEE cells by means of reverse transcription (RT)-PCR. Total cellular RNA was extracted by using an RNeasy Mini Kit (Qiagen, Valencia, Calif.), and the first cDNAs were synthesized with the SuperScript preamplification system (Gibco BRL). The primers and PCR conditions for each amplified gene have been published previously (4, 38). The primers used for TLR2 were 5′-GCC AAA GTC TTG ATT GAT TGG-3′ and 5′-TTG AAG TTC TCC AGC TCC TG-3′. The primers used for TLR3 were 5′-AAA TTG GGC AAG AAC TCA CAG-3′ and 5′-GTG TTT CCA GAG CCG TGC TAA-3′. The primers used for TLR4 were 5′-TGG ATA CGT TTC CTT ATA TG-3′ and 5′-GAA ATG GAG GCA CCC CTT C-3′. Expression of human mCD14 mRNA was assessed by RT-PCR with the primers 5′-GGT GCC GCT GTG TAG GAA AGA-3′ and 5′-GGT CCT CGA GCG TCA GTT CCT-3′. The PCR products for each gene were confirmed by DNA sequencing, which was performed by the Neurobiotechnology Center of The Ohio State University.
Stimulation of HMEE cells.
HMEE cells were seeded at a concentration of 1.5 × 105 cells per 150-cm2 tissue flask (Costar Corp., Cambridge, Mass.) in freshly supplemented MEMα and grown to approximately 80% confluency. The cells were washed, and the medium was replaced with serum-free MEMα containing 250 μg of bovine serum albumin/ml. The cells were grown overnight and were 90% confluent by the next day. The growth medium was replaced with fresh serum-free medium containing 250 μg of bovine serum albumin/ml. Cell cultures were incubated either with formalin-killed NTHI parent strain 2019 or with formalin-killed B29 or DK-1 LOS mutant cells (2 × 107 CFU/ml). This challenge dose has been used previously (7). The experiment included an unstimulated negative control flask and a positive control flask incubated with TNF-α (20 ng/ml; Sigma-Aldrich), as described for a previous study that used cytokines in positive controls (7). Supernatants from HMEE cell cultures were removed from each culture, and host cell total RNA was isolated after 2, 4, 8, and 24 h of incubation at 37°C.
Quantitation of HMEE cell cytokine transcripts by real-time PCR.
Real-time PCR is a novel method that allows for rapid, accurate, and precise quantitation of gene transcripts (12, 13). Real-time PCR assays were performed, with modifications, as part of this study to specifically quantitate human cytokine (TNF-α, IL-1β, and IL-6) and chemokine (monocyte chemotactic peptide 1 [MCP-1], macrophage inflammatory protein 1β [MIP-1β], and IL-8) transcripts. Total cellular RNA extraction and the first cDNA synthesis were performed as described above. Each cDNA sample was then used as a template for a PCR amplification mixture containing forward and reverse primers (450 nM [each]), 6-carboxyfluorescein-labeled probe (125 nM) for the target cytokines, forward and reverse primers for 18S rRNA (100 nM [each]), 2,7-dimethoxy-4,5-dichloro-6-carboxyfluorescein-labeled probe (100 nM) for 18S rRNA (internal control), and 2× TaqMan Universal PCR Master Mix (Perkin-Elmer Applied Biosystems, Foster City, Calif.). PCR amplifications for the target cytokine and internal control 18S rRNA were performed in a single well of a capped 96-well optical plate. Reaction mixtures were subjected to the following amplification scheme: 1 cycle at 50°C for 2 min (AmpErase uracil-N-glycosylase deactivation) and 1 cycle at 95°C for 10 min (AmpliTaq Gold activation), followed by 40 cycles at 95°C for 15 s (denaturation) and 60°C for 1 min (annealing and extension). Real-time PCR data were analyzed by using Sequence Detection software, version 1.6, included with the 7700 Sequence Detector (Perkin-Elmer Applied Biosystems). Final quantitation was derived using the comparative CT (threshold cycle) method (see below) and was reported as the fold difference relative to a calibrator cDNA (negative control, nonstimulated HMEE cells) prepared in parallel with the experimental cDNAs. All calculations were performed with the Microsoft Excel software program (Microsoft, Redmond, Wash.). The primers and probes for TNF-α, IL-1β, IL-6, and MIP-1β used in this study are commercially available and were obtained from Perkin-Elmer Applied Biosystems. The primers and probes for IL-8 and MCP-1 were designed by using Primer Express software, version 1.0 (Perkin-Elmer Applied Biosystems). The primers for IL-8 were 5′-GCG CCA ACA CAG AAA TTA TTG TAA-3′ and 5′-TTA TGA ATT CTC AGC CCT CTT CAA-3′, and the probe for IL-8 was 5′-TTC TCC ACA ACC CTC TGC ACC CAG TT-3′ (GenBank accession no. M28130) (23). The primers for MCP-1 were 5′-TCG CTC AGC CAG ATG CAA T-3′ and 5′-CCA CAA TGG TCT TGA AGA TCA CA-3′, and the probe for MCP-1 was 5′-TCA CCA GCA GCA AGT GTC CCA AAG AA-3′ (GenBank accession no. M37719) (31). These primers and probes were synthesized by Perkin-Elmer Applied Biosystems.
Comparative CT method.
The comparative CT method has been described previously (11). Briefly, the fractional cycle at which reporter fluorescence generated by cleavage of the probe passes a fixed threshold above baseline is defined as the threshold cycle (CT). 18S rRNA was used as an internal control to distinguish true target-negative results from PCR inhibition and to normalize for differences in the amounts of total nucleic acid added to a reaction mixture. Detection of multiple target cDNAs in the sample tube was achieved by labeling probes with different and distinguishable reporter dyes. For relative quantitation, values are expressed relative to a reference sample, called the calibrator (negative control in this study), and relative quantitation was calculated by the comparative CT method. First, the CT for the target amplicon and the CT for the internal control were determined for each sample. Differences in the CT for the target and the CT for the internal control, called ΔCT, were calculated to normalize for the differences in the amounts of total nucleic acid added to each reaction mixture. Then, the ΔCT for each experimental sample was subtracted from the ΔCT of the calibrator. This difference is termed the ΔΔCT. Finally, the amount of target normalized to an internal control and relative to the calibrator was calculated by the equation 2−ΔΔCT. Thus, all experimental results are expressed as fold differences relative to the calibrator.
ELISA.
Cell supernatants were centrifuged at 500 × g and frozen at −70°C. Cytokine and chemokine concentrations in culture supernatants were measured by commercial enzyme-linked immunosorbent assay (ELISA) kits (Quantikine; R&D Systems, Minneapolis, Minn.), according to the manufacturer's instructions.
Statistical analysis.
The arithmetic mean fold increase ± the standard error of the mean (SEM) of cytokine or chemokine mRNA expression over the corresponding value for resting cells, based on three replicates per experiment, was calculated. Statistical analyses were performed with Student's t test; a P value of <0.05 was used as the level of significance for all analyses.
RESULTS
Primary culture of HMEE cells.
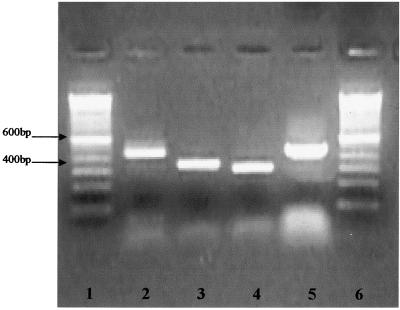
A primary explant culture from HMEE cells is shown in Fig. 1. These cells were serially passaged up to 15 times. The growth kinetics of HMEE cells exhibited a characteristic sigmoid curve. The cumulative population doubling level (PDL) of these primary cells was 25.4 before termination of growth at 57 days in culture. The average PDL per passage of the primary culture of HMEE cells was 2.1. Immunocytochemical staining positively identified cytokeratins 8, 18, and 19 in both HMEE primary cell cultures, with strong staining for cytokeratin 18 (Fig. 2); vimentin was not detected in these cells (data not shown), indicating that they were of epithelial origin. We successfully amplified mRNA of mCD14, TLR2, TLR3, and TLR4 from the two primary cultures of HMEE cells (Fig. 3).
FIG. 1.
Phase-contrast light micrograph, showing epithelial cell outgrowth, of a primary explant from human middle ear mucosa after 10 days in culture (magnification, ×67).
FIG. 2.
Immunohistochemical detection of cytokeratin 18 in HMEE cells at a cumulative PDL of 16.4, 4 days postseeding (magnification, ×109).
FIG. 3.
mRNA expression of mCD14 and TLRs in HMEE cells. Lanes 1 and 6, 100-bp DNA ladder; lane 2, mCD14; lane 3, TLR2; lane 4, TLR3; lane 5, TLR4.
Kinetics of cytokine and chemokine production after NTHI stimulation.
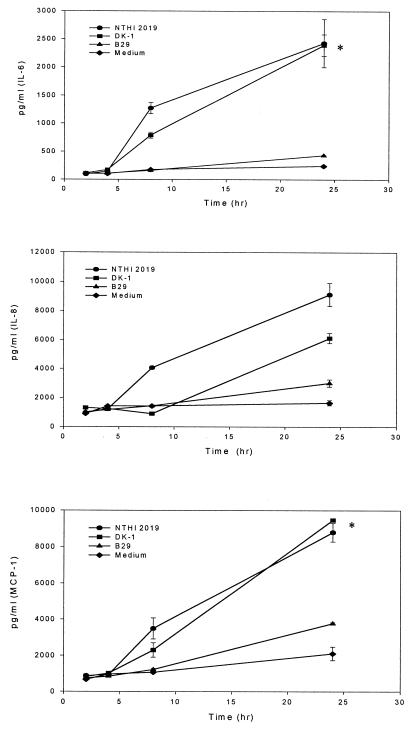
The cytokine and chemokine concentrations in the supernatants of HMEE cells maintained with culture medium only (control group) were used as measures of constitutive production by unstimulated HMEE cells. Under these conditions, low levels of IL-6, IL-8, and MCP-1 activities were detected in the supernatants (Fig. 4). However, no secreted IL-1β, MIP-1β, or TNF-α was detected in culture supernatants from unstimulated cells at any time during this experiment.
FIG. 4.
Secretion of cytokines by HMEE cells stimulated with NTHI 2019, DK-1, or B29. Results are mean concentrations of IL-6, IL-8, and MCP-1 (± SEM) in cell supernatants from two duplicate wells in ELISAs from three experiments. ✻, P < 0.05 (NTHI 2019 or DK-1 versus B29).
To investigate the kinetics of cytokine and chemokine induction in HMEE cells exposed to NTHI strain 2019, B29, or DK-1, the amounts of cytokines and chemokines released at 2, 4, 8, and 24 h after stimulation were compared (Fig. 4). By 8 h after stimulation with NTHI 2019, significantly elevated levels of IL-6 were found in the supernatants. The concentrations of IL-6, IL-8, and MCP-1 peaked at 24 h after stimulation with NTHI 2019 or DK-1. In contrast, the total amounts of IL-6, IL-8, and MCP-1 induced by B29 were approximately 10-, 3-, and 4-fold lower, respectively, than those induced by NTHI 2019. There were no significant differences between the concentrations of IL-6, IL-8, and MCP-1 induced by B29 and those for the control group. DK-1 also induced significant secretion of IL-6, IL-8, and MCP-1. These results corroborate the gene expression data (see below) and indicate that secretion of IL-6, IL-8, and MCP-1 is strongly induced by NTHI 2019 or DK-1 but not by B29.
Cytokine and chemokine gene expression in HMEE cells after NTHI stimulation.
In order to define the roles of the NTHI htrB or rfaD gene products in the induction of proinflammatory cytokine transcription in HMEE cells and to examine whether the NTHI-stimulated secretion of cytokines and chemokines by HMEE cells was due to release from internal stores or due to de novo synthesis, gene expression was evaluated by real-time PCR analysis. Stimulation of HMEE cells with TNF-α (20 ng/ml) (the positive control) showed increases in peak mRNA responses with TNF-α (4,300-fold) and MIP-1β (1,164-fold) after 2 h of coincubation, with IL-1β (199-fold) and IL-8 (62-fold) after 4 h of coincubation, and with IL-6 (76-fold) and MCP-1 (28-fold) after 24 h of coincubation.
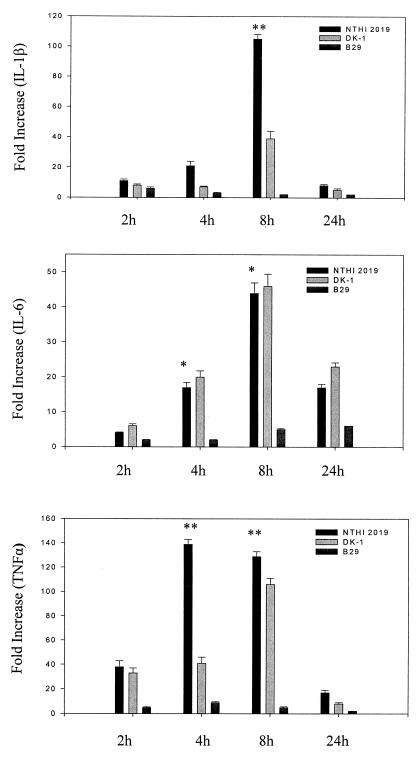
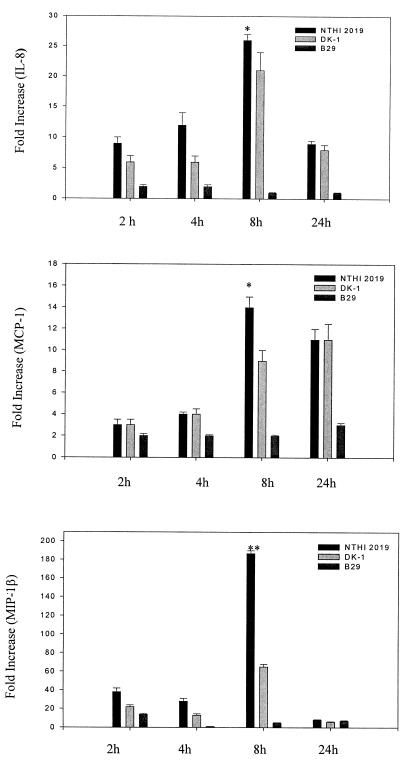
Quantification of the amounts of mRNA transcripts induced revealed that TNF-α, IL-1β, and MIP-1β genes were strongly induced by NTHI 2019 (105- to 187-fold increases over control levels), while IL-8, MCP-1, and IL-6 genes were intermediately induced by NTHI 2019 (14- to 44-fold increases) (Fig. 5 and 6). Significant increases in TNF-α, IL-1β, and MIP-1β mRNA expression were evident as soon as 2 to 4 h after stimulation of HMEE cells with formalin-killed NTHI 2019, and expression continued to increase even further for 6 h. A peak value was evident at 8 h, with a decrease at 24 h. However, expression of cytokine and chemokine genes was either not induced at all or induced at a reduced level by B29. In addition, DK-1 induced 65-, 39-, and 41-fold increases in MIP-1β, IL-1β, and TNF-α mRNA expression, respectively, increases which were approximately three times lower than those induced by NTHI 2019. Moreover, at 8 h after stimulation, there were no significant differences between the levels of IL-8, MCP-1, and IL-6 mRNA expression in HMEE cells treated with NTHI 2019 and the corresponding levels in cells treated with DK-1 (Fig. 5 and 6).
FIG. 5.
NTHI 2019 stimulates greater cytokine gene expression by HMEE cells. Results are mean fold increases in IL-1β, IL-6, and TNF-α transcript levels (± SEM) for three separate experiments. ✻✻, P < 0.01 (NTHI 2019 versus B29); ✻, P < 0.05 (NTHI 2019 versus B29).
FIG. 6.
NTHI 2019 stimulates greater chemokine gene expression by HMEE cells. Results are mean fold increases in IL-8, MCP-1, and MIP-1β transcript levels (± SEM) for three separate experiments. ✻✻, P < 0.01 (NTHI 2019 versus B29); ✻, P < 0.05 (NTHI 2019 versus B29).
DISCUSSION
Previous data indicate that 77% of middle ear effusions from patients with OM exhibit bacterial cells by Gram staining; however, only 52% or less are culture positive (19). The use of formalin-killed NTHI was developed over 15 years ago to simulate this clinical window, i.e., when the acute inflammatory phase has resolved and bacterial pathogens have been killed by either antibiotics or host immune mechanisms, and it is a widely used method of inducing sterile OM (26).
In this study, IL-6, IL-8, and MCP-1 were secreted constitutively by HMEE cells. Our results indicate that HMEE cell interactions with intact, killed NTHI bacterial cells stimulate early mRNA expression of the proinflammatory cytokines TNF-α, IL-1β, IL-6, IL-8, MCP-1, and MIP-1β by 2 to 4 h after stimulation. This early gene expression precedes an increased secretion of IL-6, IL-8, and MCP-1 within 24 h of stimulation. IL-1β, MIP-1β, and TNF-α mRNA transcripts were detected, but their products (proteins) were not detectable in culture supernatants. The reason for this is not entirely clear, but the most apparent explanation is that the cultured cells have been dedifferentiated and the secretory machinery required for excretion is no longer present. There is no question that these particular inflammatory mediators have been identified as being produced in vivo. Recent reports, moreover, indicate that IL-1β transcription does not always correlate with translation and that a nonsecreted form of TNF-α is produced by normal human breast epithelial cells in vitro (3, 7). TNF-α was not detected in cell supernatants during the present study; however, it was detected by Western blotting in nuclear extracts of HMEE cells after NTHI 2019 stimulation (data not shown). This suggests that HMEE cells may produce a nonsecreted form of TNF-α during the early phase of NTHI LOS stimulation in vitro. The rapid induction and kinetics of HMEE cell cytokine and chemokine gene expression subsequent to exposure with killed NTHI suggest that the middle ear epithelium might be the first site of proinflammatory cytokine production during the course of OM. The results from this study are in line with several publications reporting the upregulation of IL-1β, IL-6, and TNF-α expression during experimental induced OM (21, 30). Moreover, our data correlate well with studies showing induction of IL-6, IL-8, IL-1β, and MCP-1 from human tracheal epithelial cells in response to stimulation with NTHI LOS (7).
The present data demonstrate that HMEE cells exposed to the NTHI 2019 parent strain express significantly higher levels of mRNA and release higher levels of both cytokines and chemokines than those exposed to its LOS mutant B29. These results suggest that NTHI LOS htrB gene products are responsible for the induction of proinflammatory cytokine gene expression in HMEE cells after stimulation with killed NTHI cells. The chemokine most strongly induced by NTHI 2019 is MIP-1β, which belongs to the CC chemokine subfamily, is chemotactic primarily for monocytes and T cells, and activates T cells and macrophages (6). The cytokines most strongly induced by NTHI 2019 were TNF-α and IL-1β. TNF-α contributes to the acute phase of the inflammatory response, primes the immune system for rapid activity (36), and promotes the release of other cytokines. The DK-1 rfaD mutant, expressing a truncated oligosaccharide and lipid A, induced three times lower mRNA expression of IL-1β, MIP-1β, and TNF-α than did NTHI 2019, which suggests a partial role for rfaD gene products in the induction of these cytokines in HMEE cells.
NTHI 2019 and DK-1 moderately induced IL-6, IL-8, and MCP-1. IL-8 belongs to the CXC chemokine subfamily, is chemotactic primarily for neutrophils (and for T cells, NK cells, endothelial cells, basophils, and eosinophils), and stimulates neutrophil degranulation, adhesion, and microbicidal activity (32). IL-6 has been shown to be essentially protective in infection, which is linked to its capacity to induce the release of acute-phase protein (6). Slower kinetics of induction of MCP-1 are consistent with its newly discovered anti-inflammatory properties (39). MCP-1, in addition to its role in chemotaxis, also downregulates production of proinflammatory cytokines (39), which is beneficial at the later, rather than early, stages of the NTHI-induced inflammatory process. There were no significant differences between the mRNA expression and secretion of IL-6, IL-8, and MCP-1 in the NTHI 2019 group and those of the DK-1 group, suggesting that the products of rfaD do not play a significant role in induction of these cytokine genes by HMEE cells.
The NTHI htrB mutant did not significantly stimulate cytokine or chemokine expression in HMEE cells, thus providing additional direct evidence for the role of NTHI htrB products in the induction of proinflammatory cytokines in HMEE cells. It is noteworthy that the abilities of NTHI 2019 and each LOS mutant to induce cytokine and chemokine gene expression are closely related to their virulence in the chinchilla OM model in vivo (9). These data corroborate earlier findings of Nichols et al., who originally described the reduced ability of NTHI B29 to induce TNF-α production in vitro, that the host's TNF-α expression is attenuated in response to NTHI htrB mutant strains (24).
Data from this study suggest that the middle ear epithelium itself appears to play a key role in upregulation of the host immune defense by recognizing and subsequently responding to invading pathogenic threats by secretion of proinflammatory cytokines. Our results show for the first time that mRNAs of mCD14, TLR2, TLR3, and TLR4 are expressed in HMEE cells. Recent attention has focused on putative LPS receptors found on the surfaces of cells, the relationship of these receptors to LPS-induced signal transduction, and the role of each in development of the proinflammatory response. LPS elicits several immediate proinflammatory responses in peripheral blood leukocytes via several recently described signaling pathways: CD14, TLRs, serine-threonine kinases, and the NF-κB transcription factor (1, 20, 28). Until recently, however, little was known about how the NTHI LOS signal is transduced across the plasma membrane in HMEE cells. As the basic understanding of NTHI LOS signal transduction grows, novel therapies that can effectively improve the outcome of NTHI OM may arise.
In conclusion, the significant decline in gene transcript levels observed with the NTHI B29 mutant indicates that the product of the NTHI LOS htrB gene plays a major role in induction of these particular inflammatory mediators. The experiments described here contribute to our understanding of the role of various LOS gene disruptions in OM pathogenesis and may thus lead to new targets for future protection and intervention strategies.
ACKNOWLEDGMENTS
This study was supported in part by grant 5 R01 DC00090-28, from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, to T.F.D.
We thank Kathy Holloway for manuscript preparation.
REFERENCES
- 1.Baeuerle P A. Pro-inflammatory signaling: last pieces in the NF-κB puzzle? Curr Biol. 1998;8:R19–R22. doi: 10.1016/s0960-9822(98)70010-7. [DOI] [PubMed] [Google Scholar]
- 2.Ball S S, Prazma J, Dais C G D, Triana R J, Pillsbury H C. Role of tumor necrosis factor and interleukin-1 in endotoxin-induced middle ear effusions. Ann Otol Rhinol Laryngol. 1997;106:633–639. doi: 10.1177/000348949710600803. [DOI] [PubMed] [Google Scholar]
- 3.Basolo F, Conalde P G, Fiore L, Calvo S, Toniolo A. Normal breast epithelial cells produce interleukins 6 and 8 together with tumor necrosis factor: defective IL6 expression in mammary carcinoma. Int J Cancer. 1993;55:926–930. doi: 10.1002/ijc.2910550609. [DOI] [PubMed] [Google Scholar]
- 4.Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H C, Podolsky D K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–997. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 5.Catanzaro A, Ryan A, Batcher S, Wasserman S I. The response to human rIL-1, rIL-2 and rTNF in the middle ear of guinea pigs. Laryngoscope. 1991;101:271–275. doi: 10.1288/00005537-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cavaillon J M. Pathophysiological role of pro- and anti-inflammatory cytokines in sepsis. Sepsis. 1999;2:127–140. [Google Scholar]
- 7.Clemans D L, Bauer R J, Hanson J A, Hobbs M V, St. Geme III J W, Marrs C F, Gilsdorf J R. Induction of proinflammatory cytokines from human respiratory epithelial cells after stimulation by nontypeable Haemophilus influenzae. Infect Immun. 2000;68:4430–4440. doi: 10.1128/iai.68.8.4430-4440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMaria T F, Murwin D M. Tumor necrosis factor during experimental lipopolysaccharide-induced otitis media. Laryngoscope. 1997;107:369–372. doi: 10.1097/00005537-199703000-00017. [DOI] [PubMed] [Google Scholar]
- 9.DeMaria T F, Apicella M A, Nichols W A, Leake E R. Evaluation of the virulence of nontypeable Haemophilus influenzae lipooligosaccharide htrB and rfaD mutants in the chinchilla model of otitis media. Infect Immun. 1997;65:4431–4435. doi: 10.1128/iai.65.11.4431-4435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMaria T F, Prior R B, Briggs B R, Lim D J, Birck H G. Endotoxin in middle ear effusions from patients with chronic otitis media with effusion. J Clin Microbiol. 1984;20:15–17. doi: 10.1128/jcm.20.1.15-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehniger T A, Shah M H, Turner M J, Van Deusen J B, Whitman S P, Cooper M A, Suzuki K, Wechser M, Goodsaid F, Caligiuri M A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 12.Gibson U E, Heid C A, Williams P M. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 13.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 14.Iino Y, Kaneko Y, Takasaka J. Endotoxin in middle ear effusions tested with Limulus assay. Acta Oto-Laryngol. 1985;100:42–50. doi: 10.3109/00016488509108586. [DOI] [PubMed] [Google Scholar]
- 15.Ingalls R R, Heine H, Lien E, Yoshimura A, Golenbock D. Lipopolysaccharide recognition, CD14, and lipopolysaccharide receptors. Infect Dis Clin N Am. 1999;13:341–353. doi: 10.1016/s0891-5520(05)70078-7. [DOI] [PubMed] [Google Scholar]
- 16.Khair O A, Devalia J L, Abdelaziz M M, Sapsford R J, Tarraf H, Davies R J. Effect of Haemophilus influenzae endotoxin on the synthesis of IL-6, IL-8, TNF-α and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur Respir J. 1994;7:2109–2116. doi: 10.1183/09031936.94.07122109. [DOI] [PubMed] [Google Scholar]
- 17.Lee N G, Sunshine M G, Apicella M A. Molecular cloning and characterization of the nontypeable Haemophilus influenzae 2019 rfaE gene required for lipopolysaccharide biosynthesis. Infect Immun. 1995;63:818–824. doi: 10.1128/iai.63.3.818-824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee N G, Sunshine M G, Engstrom J J, Gibson B W, Apicella M A. Mutation of the htrB locus of H. influenzae nontypeable strain 2019 is associated with modifications of lipid A and phosphorylation of the lipo-oligosaccharide. J Biol Chem. 1995;270:27151–27159. [PubMed] [Google Scholar]
- 19.Liu Y S, Lim D S, Lang R W, Birck H G. Chronic middle ear effusions: immunochemical and bacteriological investigations. Arch Otolaryngol. 1975;101:278–286. doi: 10.1001/archotol.1975.00780340010003. [DOI] [PubMed] [Google Scholar]
- 20.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 21.Melhus A, Ryan A F. Expression of cytokine genes during pneumococcal and nontypeable Haemophilus influenzae acute otitis media in the rat. Infect Immun. 2000;68:4024–4031. doi: 10.1128/iai.68.7.4024-4031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison D C, Ulevitch R F. The effects of bacterial endotoxin on host mediation systems. Am J Pathol. 1978;93:527–617. [PMC free article] [PubMed] [Google Scholar]
- 23.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 24.Nichols W, Raetz C R, Clementz T, Smith A L, Hanson J A, Ketterer M R, Sunshine M, Apicella M A. htrB of H. influenzae: determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. J Endotoxin Res. 1997;4:163–172. [Google Scholar]
- 25.Nichols W A, Gibson B W, Melaugh W, Lee N-G, Sunshine M, Apicella M A. Identification of the ADP-l-glycero-d-manno-heptose-6-epimerase (rfaD) and heptosyltransferase II (rfaF) biosynthesis genes from nontypeable Haemophilus influenzae 2019. Infect Immun. 1997;65:1377–1386. doi: 10.1128/iai.65.4.1377-1386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okazaki N, DeMaria T F, Briggs B R, Lim D J. Experimental otitis media with effusion induced by nonviable Hemophilus [sic] influenzae: cytologic and histologic study. Am J Otolaryngol. 1984;5:80–92. doi: 10.1016/s0196-0709(84)80026-5. [DOI] [PubMed] [Google Scholar]
- 27.Patel J, Chonmaitree T, Schmalsteig F. Effect of modulation of polymorphonuclear leukocyte migration with anti-CD18 antibody on pathogenesis of experimental otitis media in guinea pigs. Infect Immun. 1993;61:1132–1135. doi: 10.1128/iai.61.3.1132-1135.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock F L, Hardiman G, Timans J C, Kastelein R A, Bazan J F. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato K, Liebeler C L, Quartey M K, Le C T, Giebink G S. Middle ear fluid cytokine and inflammatory cell kinetics in the chinchilla otitis media model. Infect Immun. 1999;67:1943–1946. doi: 10.1128/iai.67.4.1943-1946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato K, Kwana M, Nonomura N, Nakano Y. Course of IL-1β, IL-6, IL-8, and TNF-α in the middle ear fluid of guinea pig otitis media model induced by nonviable H. influenzae. Ann Otol Rhinol Laryngol. 1999;108:599–563. doi: 10.1177/000348949910800606. [DOI] [PubMed] [Google Scholar]
- 31.Shyy Y-J, Li Y-S, Kolattukudy P E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990;169:346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- 32.Storgaard M, Larsen K, Blegvad S, Nodgaard H, Ovesen T, Anderson P L, Obet N. Interleukin-8 and chemotactic activity of middle ear effusions. J Infect Dis. 1997;175:474–477. doi: 10.1093/infdis/175.2.474. [DOI] [PubMed] [Google Scholar]
- 33.Tobias P S, Soldau K, Gregner J A, Mintz D, Ulevitch R J. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 34.van Furth A M, Verhard-Seijmonsbergen E M, Langermans J A, van Dissel J T, van Furth R. Anti-CD14 monoclonal antibodies inhibit the production of tumor necrosis factor alpha and interleukin-10 by human monocytes stimulated with killed and live Haemophilus influenzae or Streptococcus pneumoniae organisms. Infect Immun. 1999;67:3714–3718. doi: 10.1128/iai.67.8.3714-3718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willet D N, Rezaee R P, Billy J M, Tighe M B, DeMaria T F. Relationship of endotoxin to tumor necrosis factor-α and interleukin-1β in children with otitis media with effusion. Ann Otol Rhinol Laryngol. 1998;7:28–33. doi: 10.1177/000348949810700106. [DOI] [PubMed] [Google Scholar]
- 36.Wright S D. Multiple receptors of endotoxin. Curr Opin Immunol. 1991;3:83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]
- 37.Yamanaka N, Somekawa Y, Suzuki T, Kataura A. Immunologic and cytologic studies in otitis media with effusions. Acta Otolaryngol. 1987;104:481–486. doi: 10.3109/00016488709128278. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F X, Kirschning C J, Mancinelli R, Xu X P, Jin Y, Faure E, Mantovani A, Rothe M, Uzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 39.Zisman D A, Kunkel S L, Strieter R M, Tsai W C, Bucknell K, Wilkowski J, Standiford T J. MCP-1 protects mice in lethal endotoxemia. J Clin Investig. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]