Abstract
Background:
Breast arterial calcification (BAC), which may be detected during screening mammography, is hypothesized to be a noninvasive imaging marker that may enhance cardiovascular risk assessment.
Materials and Methods:
In this systematic review and meta-analysis, we sought to assess the association between BAC and coronary artery disease (CAD) by conducting a meta-analysis. We conducted a literature search of PubMed, Scopus, Cochrane library, ClinicalTrials.gov, and conference proceedings, from inception through December 24, 2019. The outcome of interest was the presence of CAD in patients with BAC. This was reported as crude and adjusted odds ratio (OR).
Results:
A total of 18 studies comprising 33,494 women (mean age of 60.8 ± 3.7 years, 25% with diabetes, 57% with hypertension, and 21% with history of tobacco smoking) were included in the current meta-analysis. The prevalence of BAC among study participants was 10%. There was a statistically significant association between BAC and CAD (unadjusted OR 2.14; 95% confidence interval [CI] 1.63–2.81, p < 0.001, I2 = 76.5%). Moreover, adjusted estimates were available from 10 studies and BAC was an independent predictor of CAD (OR 2.39; 95% CI 1.68–3.41, p < 0.001, I2 = 61.7%). In the meta-regression analysis, covariates included year of publication, age, hypertension, diabetes mellitus, and history of tobacco smoking. None of these study covariates explained the heterogeneity across studies.
Conclusions:
BAC detected as part of screening mammography is a promising noninvasive imaging marker that may enhance CAD risk prediction in women. The clinical value of BAC for cardiovascular risk stratification merits further evaluation in large prospective studies.
Keywords: women, coronary artery disease, breast arterial calcification, mammography
Introduction
Coronary artery disease (CAD) is the leading cause of mortality and morbidity among women worldwide.1 Although women suffer similar cardiovascular morbidity and mortality as men, significantly fewer preventive therapies are employed in women and delays in the diagnosis of CAD remain major barriers to the optimal management of women with or at risk for cardiovascular disase.2 These observations can be in part explained by an underestimation of atherosclerotic cardiovascular disease (ASCVD) risk in women using traditional risk factors or contemporary risk calculators provided by the American College of Cardiology (ACC) and American Heart Association (AHA).3 A large proportion of cardiovascular events occur in women whose 10-year estimated ASCVD risk is <7.5%.4 Therefore, improving ASCVD risk assessment in women remains an important unmet need.
Current cancer screening guidelines recommend consideration of mammography in women starting at the age of 40 years, and nearly two-thirds of U.S. women ≥40 years have undergone a mammography within the past 2 years.5,6 Breast arterial calcification (BAC) can be detected on mammography and has emerged as a potentially useful noninvasive marker to enhance cardiovascular risk assessment.7 However, observational studies have yielded conflicting results regarding the association of BAC with CAD.8–13 In this systematic review and meta-analysis, we aimed to clarify the association between BAC and CAD.
Materials and Methods
Search strategy
We conducted a literature search of PubMed, Cochrane library, Scopus, clinicaltrials.gov, and conference proceedings, from inception through December 31, 2019. Our search strategy is presented in Supplementary Table S1. We utilized the “related articles” function in PubMed to find relevant articles that were missed by the initial search. In addition, reference lists of included studies were hand searched to find additional relevant articles that were missed by the primary search. Our search and meta-analysis were conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) Statement 2015.14 The protocol of this study was registered at the International prospective register of systematic reviews (PROSPERO) database.
Study selection
Titles and abstracts of the studies, which were retrieved by the initial search, were screened by two authors (S.R. and K.O). The full texts of relevant articles were reviewed to determine if they met the inclusion criteria. Any discrepancies were resolved by a third author (M.O).
Inclusion criteria
To be included in this analysis, the study had to (1) include patients in whom BAC was detected by screening mammogram and (2) report the association between BAC and CAD.
Outcomes and definitions
The outcome of interest was CAD. CAD was defined by either clinical diagnosis (e.g., history of myocardial infarction, unstable angina, and revascularization) or by imaging evidence of CAD (>50% stenosis of an epicardial coronary artery on invasive coronary angiography or coronary computed tomography angiography or abnormal results on stress testing).
Quality assessment
Two authors (S.R and K.O) assessed the quality of the included studies using the Modified Newcastle-Ottawa scale for assessment of observational studies (Supplementary Table S2).
Statistical analysis
Effect estimates were extracted from each study in the form of odds ratio (OR) and 95% confidence intervals (CIs). These measures of association were directly extracted from the article or calculated based on the available data presented in the results. Whenever available, adjusted ORs were extracted from the studies. The effect measures were pooled together using the random effect model to account for between-study variation. Heterogeneity between studies was explored by Cochran Q statistic (p < 0.05) and I-squared (I2) statistic. All statistical tests were two-sided and p-values <0.05 were considered significant. Sensitivity analyses were performed by removing one study at a time to assess the effect of each on the overall effect size (leave-one-out analysis). Funnel plots were examined for publication bias and potential sources of heterogeneity were investigated using meta-regression. We also conducted a sensitivity analysis, in which we only included studies that reported adjusted ORs to account for hidden confounders in the relationship between BAC and CAD. All statistical analyses were performed using Stata Statistical Software version 15.1 (StataCorp. 2017; StataCorp, LLC, College Station, TX).
Results
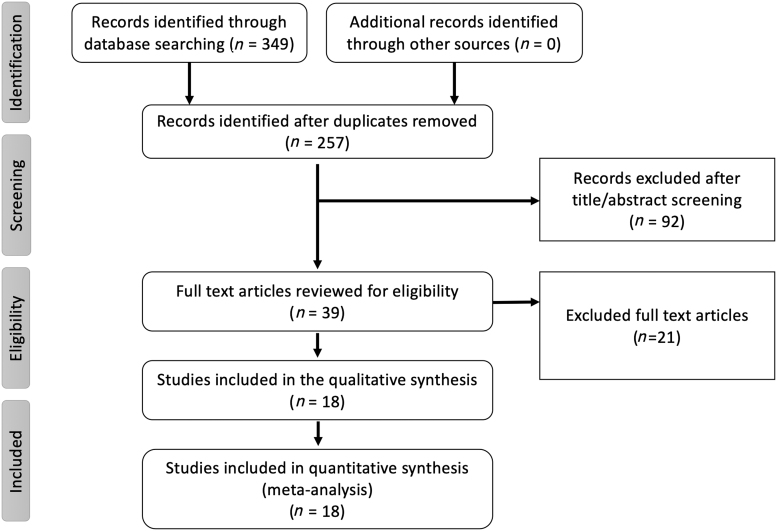
The initial database search retrieved 349 articles. After excluding duplicates, a total of 257 articles were screened for eligibility by reading the title and abstract of the study. A total of 39 articles were then screened using the predetermined inclusion criteria to assess eligibility. Funnel plots for the outcomes were examined for asymmetry (Supplementary Fig. S1). Details of the study selection process are reported following the PRISMA guidelines along with the PRISMA checklist (Fig. 1 and Supplementary Table S3).
FIG. 1.
Flowchart for the systematic review and meta-analysis as per the PRISMA. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
A total of 18 studies8–13,15–26 including 33,494 women (mean age of 60.8 ± 3.7 years, 25% with diabetes, 57% with hypertension, and 21% with history of tobacco smoking) met the inclusion and exclusion criteria. The prevalence of BAC among included participants was 10%. Detailed baseline characteristics of the included studies are shown in Table 1.
Table 1.
Baseline Characteristics of the Studies Included in the Systematic Review and Meta-Analysis
| |
N | BAC (%) | Age (mean) |
HTN (%) |
DM (%) |
HLD (%) |
CKD (%) |
Smoking (%) |
BMI (mean) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year)Ref. | BAC | Control | BAC | Control | BAC | Control | BAC | Control | BAC | Control | BAC | Control | BAC | Control | ||
| Kataoka et al. (2006)11 | 1590 | 16 | 65.8 | 62.7 | 10.4 | 11.7 | 2.4 | 1.9 | — | — | — | — | — | — | 25.9 | 26.3 |
| Newallo et al. (2015)12 | 204 | 20.6 | 52.5 | 74 | 33.3 | 62.3 | 5.4 | 45.6 | 32.9 | |||||||
| Karm et al. (2015)18 | 198 | — | 72 | 61 | 80.2 | 87.8 | 39 | 28.4 | 78 | 82 | — | — | — | — | — | — |
| Fiuza Ferreira et al. (2007)16 | 131 | 40 | 61 | 88 | 27 | 67 | — | — | 15 | |||||||
| Henkin et al. (2003)10 | 319 | 41 | 64.3 | 59.9 | 83.2 | 65.6 | — | — | 83.2 | 82.4 | — | — | 12 | 28.4 | ||
| Moshyedi et al. (1995)21 | 182 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Oliveira et al. (2009)22 | 80 | 41 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Penugonda et al. (2010)23 | 94 | 61 | 68.9 | 63.2 | 72 | 73 | 21 | 23 | 53 | 54 | 9 | 11 | 16 | 38 | 28.1 | 27.9 |
| Topal et al. (2007)26 | 123 | 40 | 68.85 | 52.34 | 82 | 70 | 33 | 23 | — | — | — | — | 6 | 13 | — | — |
| Zgheib et al. (2010)13 | 172 | 57 | 72 | 60.4 | 70 | 52 | 49 | 36 | 66 | 63 | 23 | 30 | — | — | ||
| Abou-Hassan et al. (2015)8 | 202 | 58 | 61.5 | 53.9 | — | — | 69 | 40 | — | — | 100 | 100 | 25 | 21 | — | — |
| Iribarren et al. (2004)17 | 12761 | 3 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Kemmeren et al. (1998)19 | 12239 | — | 60.1 | 57.5 | 6.4 | 3.5 | 14.2 | 28.8 | ||||||||
| Schnatz et al. (2011)25 | 1449 | — | 56.3 | 31 | 4.7 | 34.9 | — | — | 7.4 | — | — | |||||
| McLenachan et al. (2019)20 | 405 | 23 | 58 | 33 | 8 | — | — | — | — | — | — | 30 | ||||
| Crystal et al. (2000)15 | 865 | — | 65 | 54 | 52 | 25 | 13 | 5 | 32 | 25 | 9 | 30 | ||||
| Dale et al. (2008)9 | 819 | 10.5 | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Rotter et al. (2008)24 | 819 | 14 | 70 | 54 | 52 | 57 | 11 | 4 | 47 | 33 | — | — | 4 | 9 | — | — |
BAC, breast arterial calcification; BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; HLD, hyperlipidemia; HTN, hypertension; N, total number of patients.
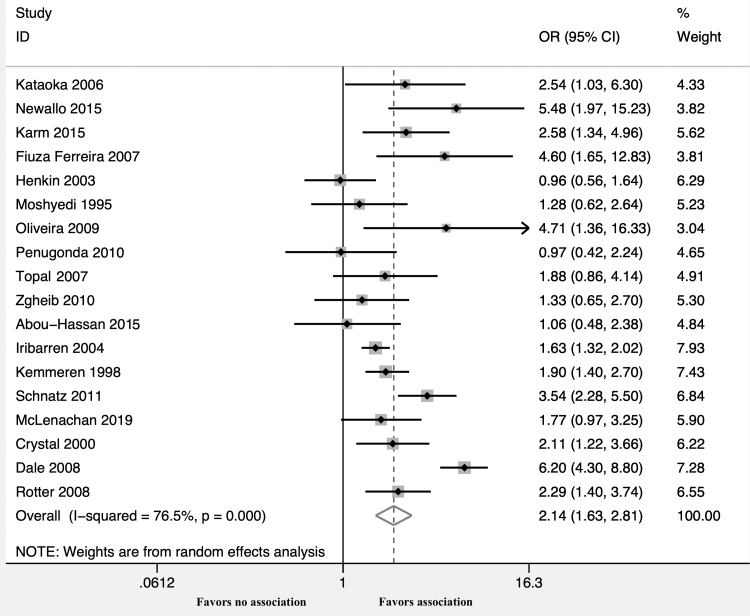
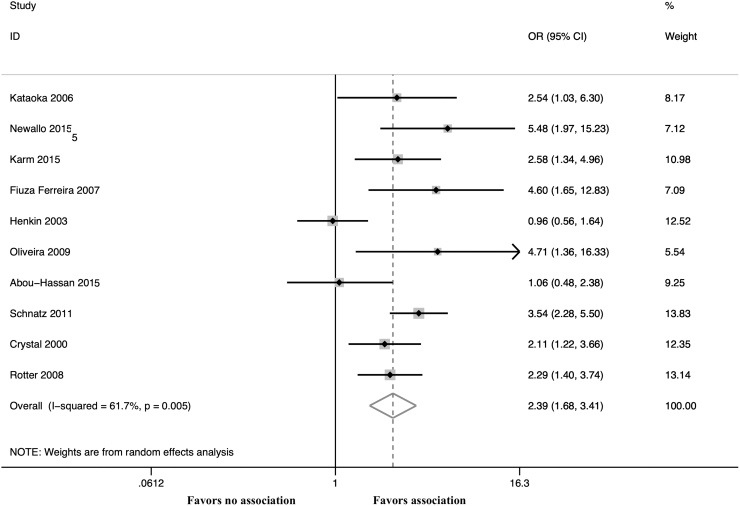
The unadjusted OR calculated from these 18 studies demonstrated a statistically significant association between BAC and CAD (OR 2.14; 95% CI 1.63–2.81, p < 0.001, I2 = 76.5%) (Fig. 2). Moreover, adjusted estimates were available from 10 studies8,10–12,15,16,18,22,24,25 and BAC was an independent predictor of CAD even after covariate adjustment (OR 2.39; 95% CI 1.68–3.41, p < 0.001, I2 = 61.7%) (Fig. 3 and Supplementary Table S4). A leave-one-out sensitivity analysis was performed by removing one study at a time. This analysis did not demonstrate a difference in the pooled ORs.
FIG. 2.
Forest plot comparing CAD in patients with versus without BAC. BAC, breast arterial calcification; CAD, coronary artery disease; OR, odds ratio.
FIG. 3.
Forest plot comparing CAD in patients with versus without BAC using adjusted estimates.
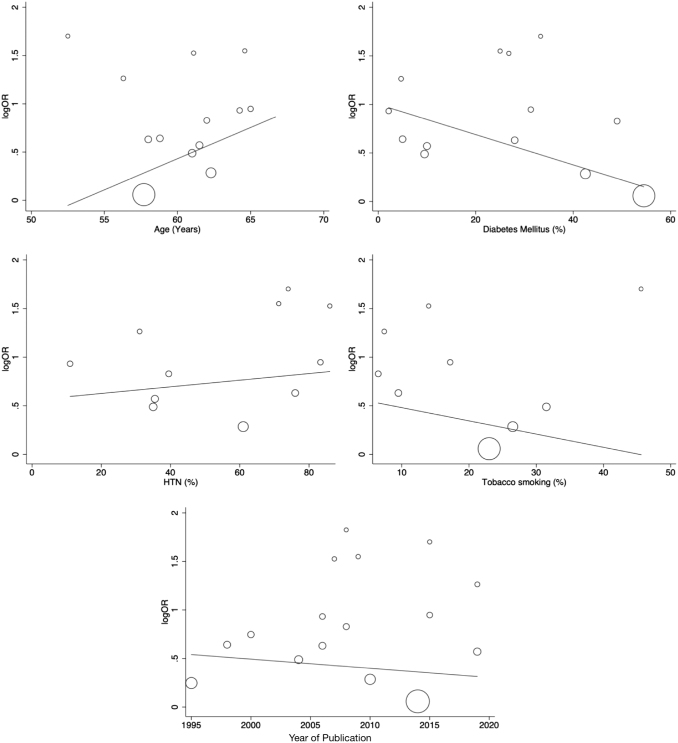
Owing to the heterogeneity detected in the meta-analysis, we performed meta-regression to investigate the contribution of study-level covariates, including year of publication, age, hypertension, diabetes mellitus, and history of tobacco smoking. Although we observed a trend suggesting that heterogeneity may be explained by specific covariates such as diabetes mellitus, tobacco smoking, and age, none of these covariates were found to be statistically significant in explaining the heterogeneity among the studies (Fig. 4 and Supplementary Table S5).
FIG. 4.
Meta-regression analysis for study-level covariates on the association of BAC and CAD.
Discussion
This meta-analysis assessed the association between BAC and CAD among 33,494 women from 18 observational studies. There are three key findings from this study. First, the overall prevalence of BAC in the participants was 10%. Importantly, there was a statistically significant association between BAC and CAD (OR 2.14; 95% CI 1.63–2.81). Moreover, in the meta-analysis of adjusted estimates, BAC continued to show a statistically significant association with CAD, even after controlling for additional possible confounders, further suggesting the potential independent clinical relevance of BAC.
Atherosclerotic risk is underestimated in many women using the traditional risk factor approach or contemporary risk calculators such as the ACC/AHA ASCVD Risk Calculator.3 There is increased recognition of the need to evaluate sex-specific risk factors in assessing risk for CAD in women.27 It is also known that the prevalence of obstructive CAD is lower in women than men, yet women have increased morbidity and mortality from CAD.28 This observation is likely due to underestimation of risk and suboptimal preventive measures, including underutilization of statin therapy and behavioral risk factor modification in women. Improved models to assess sex-specific risks are needed to address the current discrepancies in care. Identification of computed tomography-derived coronary artery calcium (CAC) is a well-validated guideline-directed modality to help refine risk in patients in whom risk uncertainty exists.27 The presence of CAC, in women considered to be at otherwise low risk based on the Framingham risk score, was predictive of future coronary events in the Multi-Ethnic Study of Atherosclerosis.29 Although computed tomography-derived CAC is a well-validated predictive marker of atherosclerotic risk, it is underutilized to refine risk estimates.30 In contrast, nearly two-thirds of U.S. women ≥40 years have undergone a mammogram within the past 2 years.5 Given national guidelines for breast cancer screening with mammography, assessment of BAC to further enhance ASCVD risk assessment, if validated, would not require any additional expense or radiation exposure.
Although conflicting data concerning the strength of association between BAC and CAD exist, most studies to date have suffered from small sample sizes, their observational nature, and varying methods for detecting and defining CAD. The MINERVA (Multiethnic Study of Breast Arterial Calcium Gradation and Cardiovascular Risk) study, a 5-year prospective multiethnic observational cohort, hopes to address some of these shortcomings to investigate the ability of BAC to predict cardiovascular outcomes.31 Given the current absence of large well-constructed prospective studies, we undertook this systematic review and meta-analysis to synthesize the currently available data across similarly conducted studies to provide insight on the relation between BAC and CAD. Prior meta-analyses have suggested similar association between BAC and CAD; however, this analysis is different than the previous studies.32,33 First, we conducted analysis for adjusted ORs reported by the primary studies to account for confounders, which was not done in the previous studies. Second, we used meta-regression techniques to explore explanation for heterogeneity in the included studies. After applying these advanced analytic techniques, the conclusion from this analysis support BAC detected as part of screening mammography as a promising noninvasive imaging marker that may enhance CAD risk prediction in women. The clinical value of BAC for cardiovascular risk stratification merits further evaluation in large prospective studies.
This analysis has limitations that need to be acknowledged. First, this was a study-level meta-analysis, which lacked individual patient level data. Second, the currently included studies were all observational in nature, which introduce many biases related to study design.
Conclusion
Based on currently available data, there is a statistically significant association between BAC and CAD. Whether the addition of BAC to standard quantitative risk assessment algorithms will enhance risk prediction remains unknown. Further evaluation of BAC in well-designed prospective studies will define the potential value of this noninvasive imaging marker in cardiovascular risk assessment.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Supplementary Material
References
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal NR, Patel HN, Mehta LS, et al. Sex differences in ischemic heart disease: Advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes 2018;11:e004437. [DOI] [PubMed] [Google Scholar]
- 3. Kavousi M, Desai CS, Ayers C, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: A meta-analysis. JAMA 2016;316:2126–2134. [DOI] [PubMed] [Google Scholar]
- 4. Yeboah J, Polonsky TS, Young R, et al. Utility of nontraditional risk markers in individuals ineligible for Statin therapy according to the 2013 American College of Cardiology/American Heart Association Cholesterol guidelines. Circulation 2015;132:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siu AL. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164:279–296. [DOI] [PubMed] [Google Scholar]
- 6. Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015;314:1599–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quispe R, Al-Rifai M, Di Carlo PA, et al. Breast arterial calcium: A game changer in women's cardiovascular health? JACC Cardiovasc Imaging 2019;12:2538–2548. [DOI] [PubMed] [Google Scholar]
- 8. Abou-Hassan N, Tantisattamo E, D'Orsi ET, O'Neill WC. The clinical significance of medial arterial calcification in end-stage renal disease in women. Kidney Int 2015;87:195–199. [DOI] [PubMed] [Google Scholar]
- 9. Dale PS, Mascarhenas C, Richards M, Mackie G. Mammography as a screening tool for coronary artery disease. J Surg Res 2008;148:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Henkin Y, Abu-Ful A, Shai I, Crystal P. Lack of association between breast artery calcification seen on mammography and coronary artery disease on angiography. J Med Screen 2003;10:139–142. [DOI] [PubMed] [Google Scholar]
- 11. Kataoka M, Warren R, Luben R, et al. How predictive is breast arterial calcification of cardiovascular disease and risk factors when found at screening mammography? AJR Am J Roentgenol 2006;187:73–80. [DOI] [PubMed] [Google Scholar]
- 12. Newallo D, Meinel FG, Schoepf UJ, et al. Mammographic detection of breast arterial calcification as an independent predictor of coronary atherosclerotic disease in a single ethnic cohort of African American women. Atherosclerosis 2015;242:218–221. [DOI] [PubMed] [Google Scholar]
- 13. Zgheib MH, Buchbinder SS, Abi Rafeh N, et al. Breast arterial calcifications on mammograms do not predict coronary heart disease at coronary angiography. Radiology 2010;254:367–373. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crystal P, Crystal E, Leor J, Friger M, Katzinovitch G, Strano S. Breast artery calcium on routine mammography as a potential marker for increased risk of cardiovascular disease. Am J Cardiol 2000;86:216–217. [DOI] [PubMed] [Google Scholar]
- 16. Fiuza Ferreira EM, Szejnfeld J, Faintuch S. Correlation between intramammary arterial calcifications and CAD. Acad Radiol 2007;14:144–150. [DOI] [PubMed] [Google Scholar]
- 17. Iribarren C, Go AS, Tolstykh I, Sidney S, Johnston SC, Spring DB. Breast vascular calcification and risk of coronary heart disease, stroke, and heart failure. J Womens Health (Larchmt) 2004;13:381–389; discussion 390–392. [DOI] [PubMed] [Google Scholar]
- 18. Karm D, Marks DS, Wein M, Kong AL. Benign arterial calcification on screening mammogram: A marker for coronary artery disease? J Womens Health (Larchmt) 2015;24:795–800. [DOI] [PubMed] [Google Scholar]
- 19. Kemmeren JM, van Noord PA, Beijerinck D, Fracheboud J, Banga JD, van der Graaf Y. Arterial calcification found on breast cancer screening mammograms and cardiovascular mortality in women: The DOM Project. Doorlopend Onderzoek Morbiditeit en Mortaliteit. Am J Epidemiol 1998;147:333–341. [DOI] [PubMed] [Google Scholar]
- 20. McLenachan S, Camilleri F, Smith M, Newby DE, Williams MC. Breast arterial calcification on mammography and risk of coronary artery disease: A SCOT-HEART sub-study. Clin Radiol 2019;74:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moshyedi AC, Puthawala AH, Kurland RJ, O'Leary DH. Breast arterial calcification: Association with coronary artery disease. Work in progress. Radiology 1995;194:181–183. [DOI] [PubMed] [Google Scholar]
- 22. Oliveira EL, Freitas-Junior R, Afiune-Neto A, Murta EF, Ferro JE, Melo AF. Vascular calcifications seen on mammography: An independent factor indicating coronary artery disease. Clinics (Sao Paulo) 2009;64:763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Penugonda N, Billecke SS, Yerkey MW, Rebner M, Marcovitz PA. Usefulness of breast arterial calcium detected on mammography for predicting coronary artery disease or cardiovascular events in women with angina pectoris and/or positive stress tests. Am J Cardiol 2010;105:359–361. [DOI] [PubMed] [Google Scholar]
- 24. Rotter MA, Schnatz PF, Currier AA Jr., O'Sullivan DM. Breast arterial calcifications (BACs) found on screening mammography and their association with cardiovascular disease. Menopause 2008;15:276–281. [DOI] [PubMed] [Google Scholar]
- 25. Schnatz PF, Marakovits KA, O'Sullivan DM. The association of breast arterial calcification and coronary heart disease. Obstet Gynecol 2011;117:233–241. [DOI] [PubMed] [Google Scholar]
- 26. Topal U, Kaderli A, Topal NB, et al. Relationship between the arterial calcification detected in mammography and coronary artery disease. Eur J Radiol 2007;63:391–395. [DOI] [PubMed] [Google Scholar]
- 27. Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. J Am Coll Cardiol 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 29. Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: The multi-ethnic study of atherosclerosis (MESA). Arch Intern Med 2007;167:2437–2442. [DOI] [PubMed] [Google Scholar]
- 30. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iribarren C, Sanchez G, Husson G, et al. MultIethNic Study of BrEast ARterial Calcium Gradation and CardioVAscular Disease: Cohort recruitment and baseline characteristics. Ann Epidemiol 2018;28:41..e12–47.e12. [DOI] [PubMed] [Google Scholar]
- 32. Lee SC, Phillips M, Bellinge J, Stone J, Wylie E, Schultz C. Is breast arterial calcification associated with coronary artery disease?-A systematic review and meta-analysis. PLoS One 2020;15:e0236598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hendriks EJ, de Jong PA, van der Graaf Y, Mali WP, van der Schouw YT, Beulens JW. Breast arterial calcifications: A systematic review and meta-analysis of their determinants and their association with cardiovascular events. Atherosclerosis 2015;239:11–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.