Abstract
Tumors were characterized as nonhealing wounds by Virchow in 1858 and Dvorak in 1986. Since then, researchers have analyzed tumors from a new perspective. The parallels between tumorigenesis and physiological wound healing can provide a new framework for developing antitumor therapeutics. One commonality between tumors and wounds is the involvement of the stromal environment, particularly adipose stromal/stem cells (ASCs). ASCs exhibit dual functions, in which they stimulate tumor progression and assist in tissue repair and regeneration. Numerous studies have focused on the role of ASCs in cancer and wound healing, but none to date has linked age, cancer, and wound healing. Furthermore, very few studies have focused on the role of donor-specific characteristics of ASCs, such as age and their role in facilitating ASC behavior in cancer and wound healing. This review article is designed to provide important insights into the impact of donor age on ASC tumor and wound response and their role in facilitating ASC behavior in cancer and wound healing.
Keywords: tumor microenvironment (TME), adipose-derived stromal/stem cells (ASCs), wound healing, keloid scars
Introduction
Parallels between cancer & wound healing
Aging is notably characterized as a progressive, generalized impairment of physiological function, resulting in a poor response to environmental challenges and expanding risk of disease and death. Although susceptibility to chronic diseases such as cancer and impaired wound healing increases with age, the potency of wound and cancer response appears to be far greater in younger individuals [1,2]. Younger individuals experience more aggressive cancers with lower survival rates in both breast and colorectal cancers [3–6].
Tumors, which can be characterized as an unconventional nonhealing wound, hijack the body's wound response to create an ideal environment for tumor growth and progression. Furthermore, all of the primary pathways activated in wound healing are also active in cancers [7–10]. Chang et al. revealed that the gene expression pattern of serum-treated fibroblasts exhibiting a wound healing response parallels that of human carcinomas [11].
The physiological wound healing response occurs via a tightly regulated series of overlapping phases involving numerous cell types, tissues, secretory factors, and proteolytic enzymes. In breast, lung, and gastric carcinomas, molecular features that characterize the wound-like phenotype are observed at an early clinical stage, persist during treatment, and predict the risk of metastasis and death [11–13]. Riss et al. have shown that 77% of genes expressed in a model of renal repair and regeneration were also expressed in renal cancer. Many of these genes are active pathways common to cancer and repair, including cell proliferation, growth, metabolism, and defense [14].
In a study by Groessl et al., cancer-associated fibroblasts (CAFs) from breast cancer biopsies displayed a wound healing signature [15]. Multiple sources have emphasized that inflammation, a critical phase of the wound healing response, is a key component of cancer progression [7,16,17]. An overabundance of specific inflammatory cell types, as discussed in detail below, results in the transformation of both an acute wound into a chronic wound, and noncancerous tissue into tumors [18–20]. Similar to a wound, tumors also secrete various trophic factors that aid in the recruitment of multiple cell types, including mesenchymal stromal/stem cells (MSCs).
Stromal contribution to cancer and wound healing
MSCs are derivatives of mesenchymal tissues, including bone marrow (bone marrow MSC, BMSCs) and adipose tissue (adipose-derived stromal/stem cells, ASCs). In response to damaged tissues and organs, these cells secrete cytokines, chemokines, and growth factors to mediate the physiological wound healing response [21–23]. The therapeutic effect of MSCs during wound repair is attributed to the release of these trophic factors, which promote angiogenesis, cell recruitment, differentiation, proliferation, and extracellular matrix (ECM) formation [24–26].
Kilroy et al. characterized the cytokine profile of MSCs, showing that the use of the proinflammatory ligand lipopolysaccharide, an immune-stimulating glycolipid produced by gram-negative bacteria, resulted in MSCs not only releasing angiogenic [hepatocyte growth factor (HGF)], vascular endothelial growth factor (VEGF), and hematopoietic [IL-7, granulocyte macrophage-colony stimulating factor (GM-CSF)] cytokines but also secreting a number of proinflammatory cytokines (IL-6, IL-8, IL-11, TNF-α) [27].
The paracrine behavior of MSCs also poses both an immunomodulatory and immunosuppressive effect that impacts the wound healing process. As a response to both injury and inflammation, the release of prostaglandin E2 (PGE2) by MSCs results in the upregulation of interleukin 10 (IL-10) and a decrease in both tumor necrosis factor-alpha (TNF-α) and interleukin 12 (IL-12) secretion from dendritic cells, leading to a shift from a more proinflammatory Th1 subtype to an anti-inflammatory Th2 subtype [28]. MSCs attenuate the release of inflammatory mediators from macrophages and modulate the proliferation, differentiation, and immunoglobulin secretion of B cells [28–30]. The release of cytokines, which both support and delay tissue recovery, poses many questions concerning the role of MSCs in wound healing and tumorigenesis.
Evidence has shown that MSCs not only aid in the healing of conventional wounds but are also linked to nonhealing tumor wounds. As observed in wound healing, MSCs respond to tumors in a similar manner by homing into the tumor site to mediate the physiological wound healing response. Both newly recruited and resident MSCs work together to accelerate the tumor “wound healing” [31]. Similar to their role in physiological wound healing, MSCs assist in regulating tumor-associated immune responses.
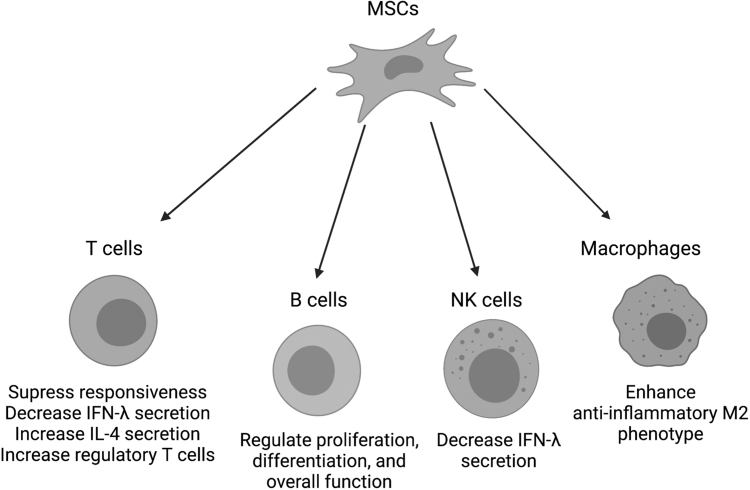
After instruction by the tumor microenvironment (TME), MSCs secrete cytokines and chemokines that exaggerate tumor-associated inflammation via the recruitment of multiple immune cell types [32–36] (Fig. 1). In addition to inducing inflammation, MSCs can also suppress the adaptive immune response in the TME through the release of effector molecules, such as nitric oxide (NO) and indoleamine 2,3-dioxygenase (IDO) [37–41].
FIG. 1.
MSC paracrine function regulates immune cell function. MSCs secrete cytokines and growth factors that modulate immune cell response to tissue damage by altering lymphocyte and leukocyte behavior. T cells experience a reduction in responsiveness, increase in IL-4, and increase in regulatory T cell phenotype. Both T cells and NK cells exhibit a decrease in IFN-γ secretion. MSCs impact overall function of B cells, including proliferation and differentiation, and drive macrophages toward the anti-inflammatory M2 phenotype. MSC, mesenchymal stromal/stem cell.
Physiological Wounds Versus Tumor Wounds
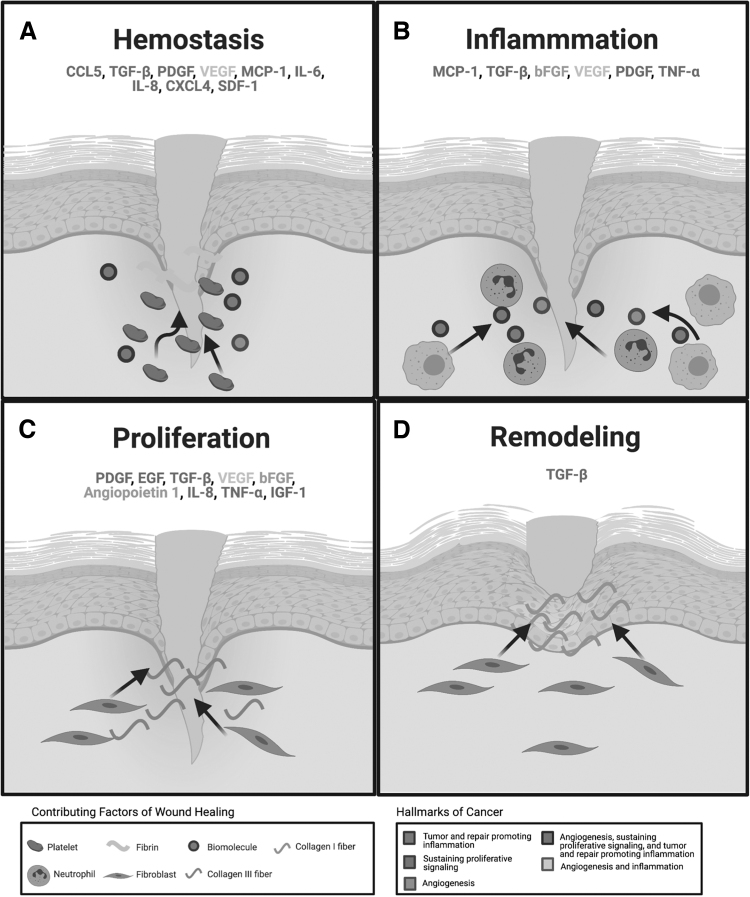
Wound healing involves a dynamic series of overlapping phases involving numerous cell types, tissues, cytokines, chemokines, growth factors, and proteolytic enzymes [1]. Cellular activities, including proliferation, migration, and ECM synthesis, are tightly regulated during the phases of wound healing: hemostasis (coagulation), inflammation, proliferation (formation of granulation tissue), and tissue remodeling (scar formation) [42,43] (Fig. 2).
FIG. 2.
Cytokines released during the phases of acute wound healing are also present during cancer hallmark acquisition. (A) Tissue injury activates platelet recruitment to the site of injury, where a temporary fibrin clot stops blood vessel hemorrhage. Platelets release a heterogeneous mix of growth factors and cytokines (PDGF, TGF-β, IL-8, SDF-1, CXCL4, bFGF, and VEGF) that aid in the repair process. (B) The inflammatory phase begins with the influx of neutrophils followed by macrophages to the wound bed. Neutrophils begin the phagocytosis of debris in the wound and release chemokines (MCP-1 and CCL5) that recruit macrophages to the wound. With the reduction of neutrophils around days 2–4, macrophages become the dominant inflammatory cells in the wound. They not only protect the wound from foreign microorganisms, but they also release growth factors, chemokines, and cytokines (VEGF, bFGF, PDGF, and TNF-α) that aid in wound repair. (C) Around days 3–10, fibroblasts are recruited to the wound, where they contribute to the formation of a temporary ECM. (D) Several months to years after injury, cells leave the wound or undergo apoptosis. ECM is broken down by MMPs and metalloproteinase tissue inhibitors (TIMPs). Type III collage that was deposited during the proliferation phase is degraded and replaced by a more permanent Type I collagen. Growth factors and cytokines highlighted in the 4 phases of wound healing are also actively involved in the hallmarks of cancer outlined by Hanahan and Weinberg [10]. Parallels in secreted factors of wound healing are observed in the following cancer hallmarks: tumor and repair promoting inflammation, sustaining proliferative signaling, and angiogenesis. The color scheme linking the association between the hallmarks of cancer and specific paracrine factors can be referenced using the figure legend. This figure was created using BioRender.com. ECM, extracellular matrix; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor beta; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor; MCP-1, monocyte chemoattractant protein 1.
Hemostasis is characterized by fibrin clot formation, platelet activation, and the release of inflammatory mediators. The release of numerous cytokines, chemokines, and growth factors, including platelet-derived growth factor (PDGF), transforming growth factor alpha-1 (TGF-α1), and transforming growth factor beta-2 (TGF-β2), promotes the migration of inflammatory cells such as leukocytes, neutrophils, and macrophages to the wound site. The inflammatory phase, characterized by the infiltration of neutrophils and macrophages, is crucial in supplying growth factor and cytokine signals that are responsible for cell migration and subsequent tissue repair [18,44].
Neutrophils release a variety of inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-8, and monocyte chemoattractant protein 1 (MCP-1), which not only amplify the inflammatory response but also stimulate the release of VEGF and IL-8 to further enhance the repair response. Macrophages are described as key regulators in the inflammatory and repair response because of their roles in debris removal, promotion, and conclusion of inflammation, and secretion of cytokines and growth factors for the recruitment and activation of other cells involved in the repair process. The proliferative phase entails the replacement of temporary fibrin matrix with granulation tissue via fibroblast-driven ECM deposition.
Growth factors produced by remaining inflammatory cells and migrating epidermal and dermal cells maintain cell proliferation and initiate cell migration to the wound bed. In response to the hypoxic wound environment, a robust angiogenic response is initiated and sustained by the production of VEGF, fibroblast growth factor 2 (FGF2), and PDGF by platelets and resident cells. In the final phase of remodeling, ECM components undergo multiple steps of degradation and synthesis to restore normal tissue architecture.
The vascularization process initiated during the previous phase provides a favorable environment for continued epidermal and dermal cell migration and proliferation. This leads to wound reepithelialization and restoration of epidermal integrity. Fibroblasts proliferate within the wound and synthesize ECM, which is initially composed of collagen III, fibronectin (FN1), fibrin, and hyaluronic acid.
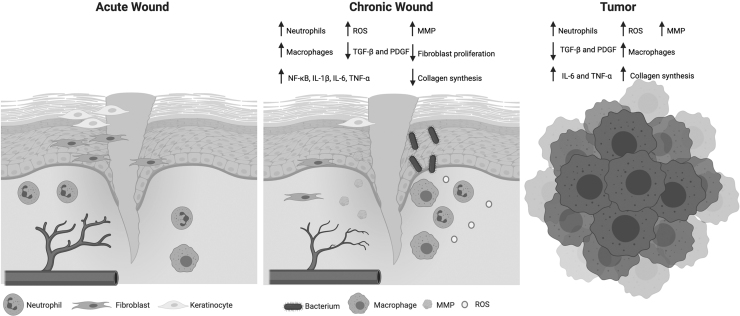
The initial matrix is then substituted with ECM, mainly composed of collagen I. After wound contraction and matrix remodeling, fibroblasts undergo apoptosis and leave a relatively acellular scar that is comparable to the unwounded skin. The previous overview of the wound healing process strictly applies to acute wounds. Unlike acute wounds, chronic wounds exhibit prolonged or excessive inflammation, the inability of dermal and epidermal cells to respond to reparative stimuli, and persistent infections (Fig. 3).
FIG. 3.
Chronic wounds exhibit similarities to tumors. Acute wounds exhibit adequate angiogenesis that aids in fibroblast proliferation, reepithelialization, and neutrophil infiltration. On the contrary, chronic wounds exhibit poor angiogenesis, elevated neutrophil infiltration, persistent bacterial infections, and decreased fibroblast proliferation. Higher infiltration of inflammatory cells leads to the excessive secretion of inflammatory markers, which leads to growth factor and ECM degradation. The chronic inflammation also prevents macrophage polarization from an inflammatory to anti-inflammatory phenotype, which prevents resolution of the wound and maintains it in a nonhealing state. Similar to chronic wounds, tumors also exhibit higher neutrophil and macrophage infiltrate, increased levels of proinflammatory cytokines, and ECM degradation as a result of inflammation. Unlike chronic wounds, tumors increase collagen synthesis. This figure was created using BioRender.com.
Chronic wounds, including diabetic, pressure, and vascular ulcers, are all characterized by a chronically inflamed wound bed and failure to heal [45]. Although the differences between ECM composition of acute and chronic wounds is both minimal and controversial, studies have shown that chronic wounds are characterized by prolonged or poor expression of fibronectin, chondroitin sulfate, and tenascin. This leads to impaired cell proliferation and migration [45].
Glycation of matrix proteins also contributes to matrix instability and disrupts interactions between collagen and its binding partners [45]. Not only is glycation involved in pathologies such as diabetes, renal failure, inflammation, and cancer, but it is also an endogenous aging mechanism that induces injury to the ECM and contributes to aging and aging-related diseases [46,47]. In chronic wounds, excessive recruitment of inflammatory cells that produce various reactive oxygen species (ROS) results in the damage of ECM structural elements and cell membranes, leading to premature cell senescence [45]. Increased levels of matrix metalloproteinases (MMPs) also contribute to the degradation of ECM and inhibition of new ECM deposition [48]. In conjunction with proinflammatory cytokines, ROS induces the production of enzymes that degrade and inactivate ECM components and growth factors necessary for normal cell function.
Although chronic wounds exhibit an observed increase in growth factors compared to acute wounds, both their quality and bioavailability are compromised in chronic wounds. In addition to excessive inflammation, chronic wounds also display impaired angiogenesis and neovascularization, which subsequently leads to insufficient oxygen and nutrient supply to cells within the wound bed. Similar to a physiological wound, tumors activate the same multistep process to aid in growth and progression.
Normal tissues are comprised of two components, the parenchyma and stroma. The stroma, a mixture of fixed tissue cells, inflammatory cells, blood vessels, matrix proteins, and proteoglycans, provides support to the parenchyma. Tumor composition is analogous to normal tissue, organized into the parenchyma (malignant cells) and stroma. Solid tumors such as carcinomas or sarcomas attain semblance to healing wounds by exploiting the host vascularized tissue stroma for survival, growth, and metastasis [49–53].
Troester et al. have shown that a wound response signature is activated in histologically normal tissue of breast cancer patients [13]. The overlapping phases of blood clotting, inflammation, ECM alterations, angiogenesis, and tissue remodeling are expressed in normal tissue adjacent to breast cancer and in tumors, further solidifying similarities between tumors and physiological wounds [7,54]. Of particular interest is the role of the inflammatory phase in both wounds and tumors. The inflammatory phase not only entails an infiltration of cells into the wound but also requires the secretion of numerous cytokines, chemokines, and growth factors to maintain the healing response.
The inflammatory nature of the TME is fostered by inflammatory cell infiltration and their secretory profile [55–57]. In chronic wounds, the inflammatory phase remains in over-drive, preventing triggers necessary for macrophages to move to the next phase. One type of tumor-like growth that embodies the nature of malignant tumors and chronic wounds is keloid scars.
Keloid scars are characterized as benign human tumors without malignant potential that exhibit elevated matrix deposition and chronic inflammation [58]. Fibroblasts in keloids exhibit an altered phenotype of intrinsic or growth factor-stimulated collagen, fibronectin, elastin, and proteoglycan accumulation [59]. Previously, keloids were characterized by an overabundance of disorganized type I and III collagen bundles; however, current studies have shown that new keloid lesions demonstrate elevated matrix protein expression of collagens (COL1A1, COL6A1, COL10A1, COL11A1, COL12A1), FN1, and fibrillin-2 (FBN2) compared to nonlesional tissue [60–62]. Many of these same matrix components are observed to be elevated in triple-negative breast cancer (TNBC) compared to matched nondiseased breast adipose tissue.
TNBC is void of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2, characterizing it as a more aggressive breast cancer type.
TNBC matrix composition mirrors that of keloid scars with elevated protein levels of COL6A1, COL11A1, COL12A1, FN1, and FBN2 and COL1A1 [60]. Another link between keloids and cancer progression is plasminogen activator inhibitor 1 (PAI-1). In in vitro 3D culture, keloid fibroblasts show elevated collagen accumulation and altered fibrin degradation. PAI-1, a major inhibitor of plasminogen activators that are responsible for the conversion of plasminogen to plasmin, is thought to be linked with poor fibrin degradation observed in keloids [22]. For proper repair in the wound healing process, the temporary fibrin matrix must be degraded and replaced by fibroblast-synthesized collagen.
In addition to organ fibrosis, PAI-1 also plays a role in tumor progression. Not only is it highly expressed in tumor biopsies but it also is prognostic for disease progression and relapse in certain cancer types, one being breast cancer [63]. It has been shown to induce tumor vascularization, promote cell dissemination, and tumor metastasis [63]. Keloid-derived fibroblasts are elevated in expression of TGFβ-1 and TGF-β2 [61,64].
TGFβ has profound effects on PAI-1 upregulation in tissue and organ fibrosis. TGFβ increases the production of ECM molecules, such as the stimulation of collagen, by cells and slows down their removal by upregulating protease inhibitors (TIMP1 and PAI-1) and downregulating protease (MMP1 and uPA) expression [59]. Elevated TGFβ-1 in hepatocellular carcinoma and breast, lung, and prostate cancer patients correlates with poor outcomes [65].
In breast cancer, TGFβ-1 exhibits a dual role as both a tumor suppressor and oncogenic driver. In early stages of breast cancer, TGFβ-1 shows tumor suppressive effects by inhibiting epithelial cell cycle progression and promoting apoptosis. However, in the late stages of breast cancer, TGFβ-1 is correlated with increased tumor progression, higher cell motility, cancer invasiveness, and metastasis [66]. Similar to TNBC, keloid scarring has a higher prevalence in African Americans and in individuals between the age of 10 and 30 [67,68]. Age and race appear to be common factors between keloid scarring and more aggressive cancer subtypes such as TNBC.
Data indicate that breast cancer in young women (<40 years old) has a poorer prognosis and higher mortality rate compared to breast cancers diagnosed in older women [69,70]. Furthermore, young African American women (<40 years old) are more likely to be diagnosed at younger ages with a more aggressive TNBC subtype [71,72]. The commonalities between keloid scarring and TNBC may offer more insight into the role of age, stem cells, and more aggressive tumors.
The TME
The TME is a heterogeneous network consisting of a diverse cell population and ECM components. The cellular and extracellular network communicates with the tumor cells, creating an intricate signaling system. The “seed and soil” hypothesis initially put forth in 1889 suggests that the host microenvironment (the soil) is required for optimal growth of the tumor cells (the seed) [73]. This hypothesis has driven a shift from a primary focus on the tumor cells instead to the characterization and analysis of the interactions between the tumor stroma and the tumor cells. The communication between the host stroma and tumor cells drastically impacts tumor growth and progression. Dvorak describes tumors as “wounds that do not heal” and emphasizes the similarities of normal wound healing to tumor stroma generation [74].
Similar to the phases of wound healing, tumor stromal generation exhibits neoangiogenesis, infiltration of fibroblasts and immune cells, and remodeling of the ECM (Table 1). Tumors recruit supporting cells from the local host stroma, which promote ECM remodeling, cellular migration, neoangiogenesis, invasion, drug resistance, and evasion of immunosurveillance through the production of growth factors, chemokines, and cytokines [75]. The supporting cells include fibroblasts, myofibroblasts, endothelial cells, adipocytes, MSCs, and various immune cells [76–78].
Table 1.
Paracrine Factors Observed in Wound Healing and Tumorigenesis
| Paracrine factor | Role in wound healing | Role in tumorigenesis | ASC-mediated cancer type | Differentially expressed with age |
|---|---|---|---|---|
| CCL5 | Macrophage recruitment | Immune cell recruitment, stimulates angiogenesis, modulates ECM, tumor cell proliferation, enhances tumor cell migration and invasiveness | Breast cancer [80] | Unknown |
| GDF11 | Activates fibroblasts in hemostasis and proliferation phases, activates platelets in clot formation, increases angiogenesis/cell proliferation/cell migration/ECM production | Induces tumor suppressive and oncogenic properties | N/A | Unknown |
| IL-6 | Regulates chronic inflammation, macrophage recruitment, macrophage polarization | Chronic inflammatory environment can lead to tumor development, enhances migration and invasiveness of tumor cells | Breast Cancer [81,82] | ✓ |
| IL-8 | Stimulates angiogenesis, granulocyte recruitment | EMT, neutrophil migration | Breast cancer [81,82] | Unknown |
| LEP | Stimulates angiogenesis, cell proliferation, and differentiation, keratinocyte migration | Increases TNF-α and ROS production, increases MCP-1 expression, increases endothelial cell proliferation and migration, increases tumor cell invasion and metastasis | ER+ breast cancer [82] | ✓ |
| MCP-1 | Macrophage recruitment | Involved in tumor progression and metastasis | Bladder cancer, Breast cancer [81], Melanoma [83] | Unknown |
| PAI-1 | Keratinocyte migration, fibroblast migration and fibroblast-myofibroblast differentiation | Tumor cell migration and metastasis, macrophage migration and polarization, inhibits fibrinolysis, cell adhesion | Breast cancer [80], Colon cancer [80] | Unknown |
| PDGF | Stimulates chemotaxis, proliferation, and new gene expression in macrophages and fibroblasts | ECM remodeling | Breast cancer [84] | ✓ |
| SDF-1 | Chemotaxis of ASCs | SDF-1-CXCR4 expression associated with EMT phenotype, contribution to hormone independence via SDF-1-CXCR4-ER-α crosstalk | ER+ breast cancer [81] | ✓ |
| TGF-β1 | ECM production and remodeling | Promotes EMT, increases tumor cell motility and metastasis, ECM remodeling | Breast cancer [81] | ✓ |
| TNF-α | Upregulated in inflammatory phase, ASCs stimulate macrophages to secrete TNF-α | Development of tissue architecture for tumor growth and metastasis, EMT with long-term exposure to TNF-α | ER+ breast cancer [80], Breast cancer [84] | ✓ |
| VEGF | Stimulates collagen deposition, angiogenesis, and epithelization | Stimulates angiogenesis, enhances tumor cell invasiveness and migration | Melanoma [83], Breast cancer [81,84] | ✓ |
N/A indicates that no information was found in the literature that supports the role of GDF11 in ASC-mediated cancer. The ✓ indicates that patient age plays a role in the expression of the specified paracrine factor.
ASC, adipose stromal/stem cell; ECM, extracellular matrix; EMT, epithelial to mesenchymal transition; ROS, reactive oxygen species; LEP, leptin; MCP-1, monocyte chemoattractant protein 1; PDGF, platelet-derived growth factor; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
The infiltration of the cellular recruits is in response to tumor-driven inflammation [79]. Changes in the TME mirror the process of chronic inflammation, which initiates with ischemia followed by interstitial and cellular edema, the appearance of immune cells, growth of blood vessels, and tissue repair [79].
Chronic inflammation aids in shaping the TME and has been referred to as a host reaction to the tumor; however, it is more appropriate to characterize this response as a tumor-promoting reaction [79]. The hypoxic environment of the tumor stimulates an inflammatory phenotype in the infiltrating cells and favors specific inflammatory cells, including phagocytic macrophages and granulocytes [79]. In contrast, wound-associated hypoxia drives angiogenesis and neovascularization at the site of injury [45].
Human ASCs exhibit increased proliferation in hypoxic conditions and with increased antioxidants. Furthermore, antioxidants were found to increase the expression of stemness genes and the differentiation potential of ASCs [85]. It has been well documented that physiological levels of ROS are important for proper differentiation of stem cells, especially for vasculogenesis (new blood vessel formation) [86].
The careful balance of antioxidants and ROS influence stem cell activities by mitigating oxidative stress through the neutralization of free radicals, increasing the expression of antioxidant enzymes, influencing differentiation fate of precursor stem cells, increasing genomic stability, improving stem cell adhesion, and the ability to manipulate stem cell proliferation with varied antioxidant concentrations [87]. Similar to wound healing, tumors experience hypoxia and ROS.
Early in tumor development, tumor cells activate hypoxia-responsive genes, which in turn stimulate the influx of inflammatory cells into the TME. Hypoxia activates the nuclear factor-kappa B (NF-κB) signaling pathway, which plays a key role in signaling of cancer cells and tumor-infiltrating leukocytes [88–90].
NF-κB activation leads to the secretion of TNF-α, a proinflammatory cytokine involved in systemic inflammation, and other pro-inflammatory cytokines that drive the expression of cytokine genes responsible for cell proliferation. In response to the proinflammatory cytokine cascade, tumor and stromal cells produce a wide array of biological mediators that help maintain the cell proliferation and differentiation, matrix remodeling, neoangiogenesis, and cell migration/recruitment necessary for tumor growth.
MSCs, one of the supporting cell populations of the tumor stroma, are of particular interest in the development and progression of breast cancer because of their release of cytokines and growth factors that enhance the inflammatory nature of tumors. Due to the role of hypoxia in ROS and wound healing, it may be suggested that ROS and hypoxia alter stem cell function in the TME. To date, the role of patient age has not been evaluated with respect to stem cell response to hypoxic conditions in the tumor.
Once recruited to the TME, ASCs also undergo phenotypic changes into a more aggressive CAF cell type. CAFs, an alpha-smooth muscle actin (α-SMA), tenascin-C, nestin, neural/glial antigen 2 (NG2), and PDGFR-α positive cell population, have been shown to increase tumor aggressiveness through cytokine-mediated activities [80,91]. There are no studies to date validating the role of age in ASC-CAF transition and tumor stroma integration.
Some MSCs have a natural proclivity to induce inflammation because their tissue of origin, such as adipose tissue or bone marrow, actively participates in physiologic and pathologic processes, including immunity and inflammation. As one of the body's largest endocrine organs, adipose tissue releases a variety of proinflammatory and anti-inflammatory factors, including the adipokines leptin (LEP) and adiponectin (ADIPOQ), as well as cytokines and chemokines such as TNF-α, IL-6, and MCP-1 [92].
ASCs: Their Role in the Physiological Wound
Adipose tissue has been established as a viable source of stromal/stem cells that exhibit both multipotency and immunomodulatory characteristics [93–95]. The regenerative capacity of ASCs is harnessed through their secretome. ASCs secrete a wide variety of growth factors (PDGF, FGF, VEGF, and HGF) and cytokines/adipokines [SDF-1, IL-6, IL-8, TGF-α, angiopoietin (ANGPT)] that act as mediators of angiogenesis, immune modulation, and stromal remodeling [96–98]. In vivo, ASCs reside in the stem cell niche, where they are surrounded by the ECM and other supporting cells [99]. The stem cell niche, more commonly referred to as the microenvironment, modulates the ability of ASCs to differentiate, proliferate, and migrate as they aid in the restoration of cellular age defects and tissue repair [100].
In response to injury, ASCs shift the inflammatory phenotype of local immune cells to a more anti-inflammatory phenotype via soluble factors [101]. This shift is done through the modulation of inflammatory profiles of macrophages, T cells, B cells, and dendritic cells, which in turn furthers the proliferative and remodeling phases of the wound healing process [102,103]. Many of the soluble factors described above are also associated with modulating the expression and/or secretion of multiple growth factors, cytokines, chemokines, and inflammatory markers that are linked to both wound healing and cancer development and progression [82,104–111]. Here, we will first evaluate these secreted factors as they pertain to wound healing.
ASCs promote vascularization of the wound via the secretion of angiogenic factors. VEGF is a key angiogenic growth factor because of its ability to promote endothelial progenitor cell mobilization, recruitment, and migration, which accelerates angiogenesis in the wound [112]. Many angiogenic factors, including VEGF, PDGF, and ANGPT, are secreted throughout several phases of the wound healing process to facilitate angiogenesis [113]. Heo et al. determined that TNF-α-activated ASCs produce proinflammatory cytokines IL-6 and IL-8 that further aid in angiogenesis and epithelium regeneration in wound repair [114].
The proinflammatory cytokine TNF-α is not only secreted by ASCs but is also upregulated in the inflammatory phase of wound healing. ASCs stimulate macrophages to secrete TNF-α, which further aids in activation, proliferation, apoptosis, and differentiation of other macrophages. In physiological wound healing, ASC-secreted IL-6 aids in macrophage recruitment and macrophage pro- and anti-inflammatory-like phenotypes [115].
Macrophages consist of two subtypes, the classically active proinflammatory M1 and the alternatively activated anti-inflammatory M2 [116]. ASC secretion of specific cytokines and factors such as IL-6 stimulates M2 polarization, which promotes healing and inhibits inflammation in tissues [117–119]. MCP-1, another proinflammatory cytokine produced by ASCs, promotes macrophage recruitment during wound healing. Studies have shown that LEP, an adipokine secreted by ASCs, stimulates angiogenesis, cell proliferation, and differentiation, and migration of keratinocytes to enhance wound healing [80].
Circulating levels and protein content of growth differentiation factor 11 (GDF11), a member of the TGF-β superfamily, is affected by pathological conditions and age [120]. It has a role in multiple phases of the wound healing process by increasing cell proliferation and migration, angiogenesis, and ECM production [100]. TGF-β1 is active in multiple steps of the wound healing process. It plays the role of a paracrine mediator that activates fibroblasts, macrophages, and ASC secretions [100]. Increased expression of the chemokine SDF-1 by ASCs amplifies ASC migration to the wound site [100]. SDF-1 has also been noted as an important factor in the spread of breast cancer cells [81]. Although these specific soluble mediators have roles in wound healing, the question arises concerning how their roles are altered by aging.
Many of the regenerative qualities of ASCs, including proliferation and differentiation capacity, change with age. The ASC secretome has been harnessed as a viable treatment option for chronic nonhealing wounds; however, studies have yet to characterize the ASC secretome as a function of age [121]. Several of the aforementioned mediators, including PDGF, VEGF, IL-6, TNF-α, SDF-1, TGF-β1, and LEP are affected by aging [1,122]. Although no studies have shown that ASC-secreted PDGF specifically is impacted by age, studies indicate that aging results in the enhanced release of PDGF, TGF-β, and TGF-α from α-granules [1]. Increased age also correlates with a decline in phagocytic activity of wound macrophages, leading to an accumulation in the proinflammatory cytokines IL-6 and TNF-α [1].
In contrast to the tissue environment as a whole, Pandey et al. revealed that aged ASCs exhibit lower expression of TNF-α relative to young ASCs [123]. This suggests that the impact of aging on each cell type is distinct. Aging may have different effects on immune cells compared to tissue-derived cells such as ASCs. The expression of VEGF, an essential regulator of angiogenesis, is also dependent on cell type. In cutaneous wound healing, VEGF expression declines with aging [1]. In contrast, studies have noted that ASC-secreted VEGF levels is unaffected by age when active in bone healing [124]. Aging also results in a decrease in SDF-1 expression in wound healing.
Similarly, a recent study demonstrated that age affects ASC production of SDF-1 [1], suggesting that ASC homing could be impacted by poor SDF-1 at the wound site [1,122]. In addition, age-associated changes in response to hypoxic conditions play a significant role in the physiologic wound healing process [125].
With aging, wound response to hypoxia diminishes; thus, responsiveness to TGF-β1 also diminishes. In normal tissues, TGF-β1 increases the deposition of collagen by stimulating its synthesis and minimizing its degradation. In aged wound tissue, this ratio of synthesis to degradation is imbalanced [1]. Although many of the aforementioned ASC-secreted factors have been examined as a function of aging, little is known about the role of gender on ASC paracrine function in tumorigenesis and wound healing [126]. More specifically, there are no studies to date that have focused on gender specific changes as a function of aging in ASCs.
ASCs: Their Role in the TME
As stated above, PDGF, VEGF, IL-6, TNF-α, SDF-1, TGF-β1, and LEP are all repressed with age and may result in decreased wound healing. Although beneficial during wound repair, both VEGF and PDGF are important for endothelial cell proliferation and vascularization of tumors [113]. Other proangiogenic factors and proinflammatory cytokines are elevated in tumors, for example, high serum levels of both IL-6 and IL-8 are correlated with strong tumor invasion and poor prognosis in breast cancer [127].
Of note, TNF-α is secreted by ASCs and is associated with the development of the tissue architecture necessary for tumor growth and metastasis [80]. Notably, long-term exposure of hormone receptor-positive (HR+) breast cancer cells to TNF-α results in epithelial to mesenchymal transition (EMT), endocrine resistance, and a more aggressive phenotype [128]. Studies have exhibited that chronic inflammation associated with excessive IL-6 secretion facilitates tumor development [129].
Similar to previously mentioned mediators, MCP-1 is also involved in breast cancer tumor progression and metastasis [130]. Some studies have demonstrated that GDF11 induces tumor-suppressive properties, while others indicate GDF11 promotes tumorigenesis [120]. LEP also plays a crucial role in tumorigenesis. In HR+ breast cancer, LEP is correlated with higher recurrence rates and increased invasiveness [81].
In breast cancer, TGF-β1 enhances the progression of breast malignancies into more malignant phenotypes [81]. SDF-1 and its receptor, chemokine receptor type 4 (CXCR4), play an important role in HR+ breast cancer [131]. The expression of SDF-1-CXCR4 is associated with the EMT phenotype. Furthermore, crosstalk between estrogen receptor-α (ER-α), SDF-1, and CXCR4 contributes to hormone independence, making it increasingly difficult to treat with endocrine therapy [131].
LEP also increases TNF-α expression, ROS production, MCP-1 expression, and endothelial cell proliferation and migration, all of which increase cancer cell growth and mobility [80]. As previously mentioned in terms of keloid formation, PAI-1 contributes to proper wound healing through the breakdown of the fibrin clot. Studies reveal that there is an association between ASC-secreted PAI-1 and tumor cell invasion and metastasis, as well as a poor prognostic indicator in breast and colon cancers [132,133].
Due to parallels between wound healing and tumor progression, it may be of value to determine if a tumor's ability to induce wound healing factors such as TNFα, IL-6, LEP, GDF11, TGFβ, and MCP-1 is heightened in tumors derived from young patients. As stated above, PDGF, VEGF, IL-6, TNF-α, SDF-1, TGF-β1, and LEP are altered by aging [1]. Identifying differences in expression of these factors in a tumor due to patient age may provide insight on novel ways to combat the aggressiveness of tumors from younger individuals. For example, there are elevated levels of LEP in young ASCs and links to both wound healing and tumorigenesis, LEP could be a potential target underlying amplified tumor aggressiveness observed in young patients. LEP not only promotes wound healing, but it also plays an oncogenic role in breast cancer [91,134,135].
In a study by Pandey et al., LEP expression in aged ASCs was significantly lower compared to young ASCs [123]. With elevated levels in young ASCs and links to both wound healing and tumorigenesis, LEP could be a potential target underlying amplified tumor aggressiveness observed in young patients.
Once recruited to the TME, ASCs also undergo phenotypic changes into a more aggressive CAF cell type. CAFs, an α-SMA, tenascin-C, nestin, neural/NG2, and PDGFR-α-positive cell population, have been shown to increase tumor aggressiveness through cytokine-mediated activities [80]. There are no studies to date validating the role of age in ASC-CAF transition and tumor stroma integration.
Comparison of ASCs in Breast Cancer
Breast tissue is composed of 90% adipose tissue, generating permanent interactions between epithelial cells and adipose cells [136]. Evidence has demonstrated that adipocytes and MSCs may maintain tumor phenotypes by either behaving as energy reservoirs for neighboring cancer cells or secreting molecules and vesicles [137–139]. Many studies have focused on the interactions of ASCs and breast cancer, but few have emphasized the role of age as a factor in tissue response to breast cancer [81,140]. Although the relationship between the wound healing response and breast cancer has yet to be fully explored, Agresti et al. and Troester et al. provide compelling research that solidifies the necessity to define this relationship more fully.
After investigating the wound healing fluid (WHF) composition of breast carcinoma patients after surgery, results revealed that fluid obtained from the surgical sites mirrored molecular features of the removed tumor [141]. Multiple factors, including IL-6, G-CSF, and MCP-1, were upregulated in WHF from patients with invasive breast cancer. Elevated expression of these factors is also associated with more aggressive tumors [142,143]. It is also important to note that the WHF composition varied based on primary tumor histology, intrinsic subtype, size, grade, and lymph node status, further reiterating the need to also focus on patient-specific characteristics such as age when identifying the role of the wound-healing response in breast tumorigenesis.
The study by Troester et al. not only illustrates an association between the wound healing gene expression signature and breast cancer survival but also suggests that tumor heterogeneity contributes to the differential expression of wound response signatures. The future development of additional microenvironment-targeted therapies would require a more personalized approach in the investigation of tumor ECM-heterogeneity [144,145].
Conclusions
The link between wound healing and tumorigenesis is undeniable based on the evidence presented in this review. Tumor heterogeneity has been presented as an ongoing challenge for the development of cancer therapeutics. Heterogeneity is observed between cancers from different patients (intratumor heterogeneity) and within a single tumor (intertumor heterogeneity) [146].
Data are suggestive that ASCs may play a critical role in the observed wound healing response to cancer. Without recognition of tumor heterogeneity, the comparison of wound healing and cancer takes on a more generalized approach. Here, we suggest that tumor heterogeneity and the tumor-induced wound healing response may be regulated by patient stem cell age. The body of evidence investigating ASCs from this perspective is sparse. Many of the cytokines and growth factors presented in this review are potential links between ASCs and the tumor wound.
Specifically, these cytokines have been previously cited in epithelial stem cell lines [147]; however, there has been little focus on the effect of ASC age and paracrine activity. With this said, more short-term experiments focused on the differences in aged and young ASC secretome and tumorigenesis would be beneficial to bridge this gap in knowledge. Further, we suggest that, with respect to breast cancer, studies need to focus on the specific cancer subtype when investigating the effects of ASC age in tumor progression. As highlighted with keloid scars, a semblance exists between the body's response to benign tumors and chronic wounds.
Keloids and TNBC are observed predominantly in both younger individuals and African Americans. The relationship between keloids and TNBC offers compelling evidence that the wound healing response mirrors the tumor response and suggests an age dependent mechanism. To date, an underlying commonality between the wound healing response in tumors and patient age has yet to be clearly defined.
Author Disclosure Statement
Jeffrey M. Gimble is a cofounder, co-owner, and executive at Obatala Sciences, Inc.
Funding Information
This publication was supported by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center.
References
- 1. Sgonc R and Gruber J. (2013). Age-related aspects of cutaneous wound healing: a mini-review. Gerontology 59:159–164. [DOI] [PubMed] [Google Scholar]
- 2. Tricoli JV, Blair DG, Anders CK, Bleyer WA, Boardman LA, Khan J, Kummar S, Hayes-Lattin B, Hunger SP, et al. (2016). Biologic and clinical characteristics of adolescent and young adult cancers: acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma, and sarcoma. Cancer 122:1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N and Perou CM. (2011). Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 29:e18–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, et al. (2008). Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324–3330. [DOI] [PubMed] [Google Scholar]
- 5. Palmer ML, Herrera L and Petrelli NJ. (1991). Colorectal adenocarcinoma in patients less than 40 years of age. Dis Colon Rectum 34:343–346. [DOI] [PubMed] [Google Scholar]
- 6. Marble K, Banerjee S and Greenwald L. (1992). Colorectal carcinoma in young patients. J Surg Oncol 51:179–182. [DOI] [PubMed] [Google Scholar]
- 7. Schäfer M and Werner S. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 9:628–638. [DOI] [PubMed] [Google Scholar]
- 8. Dvorak HF. (2015). Tumors: wounds that do not heal-redux. Cancer Immunol Res 3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacCarthy-Morrogh L and Martin P. (2020). The hallmarks of cancer are also the hallmarks of wound healing. Sci Signal 13:eaay8690. [DOI] [PubMed] [Google Scholar]
- 10. Hanahan D and Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell 144:646–674. [DOI] [PubMed] [Google Scholar]
- 11. Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D and Brown PO. (2004). Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol 2:E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, et al. (1999). The transcriptional program in the response of human fibroblasts to serum. Science 283:83–87. [DOI] [PubMed] [Google Scholar]
- 13. Troester MA, Lee MH, Carter M, Fan C, Cowan DW, Perez ER, Pirone JR, Perou CM, Jerry DJ and Schneider SS. (2009). Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res 15:7020–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riss J, Khanna C, Koo S, Chandramouli GVR, Yang HH, Hu Y, Kleiner DE, Rosenwald A, Schaefer CF, et al. (2006). Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res 66:7216–7224. [DOI] [PubMed] [Google Scholar]
- 15. Groessl M, Slany A, Bileck A, Gloessmann K, Kreutz D, Jaeger W, Pfeiler G and Gerner C. (2014). Proteome profiling of breast cancer biopsies reveals a wound healing signature of cancer-associated fibroblasts. J Proteome Res 13:4773–4782. [DOI] [PubMed] [Google Scholar]
- 16. Balkwill F and Mantovani A. (2001). Inflammation and cancer: back to Virchow? Lancet 357:539–545. [DOI] [PubMed] [Google Scholar]
- 17. Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y and Martin P. (2015). The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J 34:2219–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eming SA, Krieg T and Davidson JM. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127:514–525. [DOI] [PubMed] [Google Scholar]
- 19. de Visser KE, Eichten A and Coussens LM. (2006). Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6:24–37. [DOI] [PubMed] [Google Scholar]
- 20. Balkwill F, Charles KA and Mantovani A. (2005). Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217. [DOI] [PubMed] [Google Scholar]
- 21. Chamberlain G, Fox J, Ashton B and Middleton J. (2007). Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 22. Jackson WM, Nesti LJ and Tuan RS. (2012). Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 1:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fitzsimmons REB, Mazurek MS, Soos A and Simmons CA. (2018). Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int 2018:8031718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garg RK, Rennert RC, Duscher D, Sorkin M, Kosaraju R, Auerbach LJ, Lennon J, Chung MT, Paik K, et al. (2014). Capillary force seeding of hydrogels for adipose-derived stem cell delivery in wounds. Stem Cells Transl Med 3:1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badiavas EV and Falanga V. (2003). Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol 139:510–516. [DOI] [PubMed] [Google Scholar]
- 26. Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, Longaker MT and Gurtner GC. (2012). Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 33:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, et al. (2007). Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol 212:702–709. [DOI] [PubMed] [Google Scholar]
- 28. Aggarwal S and Pittenger MF. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 29. Tsyb AF, Petrov VN, Konoplyannikov AG, Saypina EV, Lepechina LA, Kalsina S, Semenkova IV and Agaeva EV. (2008). In vitro inhibitory effect of mesenchymal stem cells on zymosan-induced production of reactive oxygen species. Bull Exp Biol Med 146:158–164. [DOI] [PubMed] [Google Scholar]
- 30. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V and Uccelli A. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372. [DOI] [PubMed] [Google Scholar]
- 31. Li P, Gong Z, Shultz LD and Ren G. (2019). Mesenchymal stem cells: from regeneration to cancer. Pharmacol Ther 200:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ren G, Liu Y, Zhao X, Zhang J, Zheng B, Yuan ZR, Zhang L, Qu X, Tischfield JA, Shao C and Shi Y. (2014). Tumor resident mesenchymal stromal cells endow naïve stromal cells with tumor-promoting properties. Oncogene 33:4016–4020. [DOI] [PubMed] [Google Scholar]
- 33. Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, et al. (2012). CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell 11:812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Escobar P, Bouclier C, Serret J, Bièche I, Brigitte M, Caicedo A, Sanchez E, Vacher S, Vignais ML, et al. (2015). IL-1β produced by aggressive breast cancer cells is one of the factors that dictate their interactions with mesenchymal stem cells through chemokine production. Oncotarget 6:29034–29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, Wang Y and Shi YF. (2017). TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2(+) neutrophils. Oncogene 36:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu MS, Borrelli MR, Lorenz HP, Longaker MT and Wan DC. (2018). Mesenchymal stromal cells and cutaneous wound healing: a comprehensive review of the background, role, and therapeutic potential. Stem Cells Int 2018:6901983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang Y, Yu P, Li W, Ren G, Roberts AI, Cao W, Zhang X, Su J, Chen X, et al. (2014). p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene 33:3830–3838. [DOI] [PubMed] [Google Scholar]
- 38. Gazdic M, Simovic Markovic B, Jovicic N, Misirkic-Marjanovic M, Djonov V, Jakovljevic V, Arsenijevic N, Lukic ML and Volarevic V. (2017). Mesenchymal stem cells promote metastasis of lung cancer cells by downregulating systemic antitumor immune response. Stem Cells Int 2017:6294717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han Z, Tian Z, Lv G, Zhang L, Jiang G, Sun K, Wang C, Bu X, Li R, et al. (2011). Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J Cell Mol Med 15:2343–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, Rabson AB, Roberts AI, Wang Y and Shi Y. (2014). Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res 74:1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liotta F, Querci V, Mannelli G, Santarlasci V, Maggi L, Capone M, Rossi MC, Mazzoni A, Cosmi L, et al. (2015). Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br J Cancer 112:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shaw TJ and Martin P. (2009). Wound repair at a glance. J Cell Sci 122:3209–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ridiandries A, Tan JTM and Bursill CA. (2018). The role of chemokines in wound healing. Int J Mol Sci 19:3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leibovich SJ and Ross R. (1975). The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 45. Demidova-Rice TN, Hamblin MR and Herman IM. (2012). Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 25:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schröter D and Höhn A. (2018). Role of advanced glycation end products in carcinogenesis and their therapeutic implications. Curr Pharm Des 24:5245–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim CS, Park S and Kim J. (2017). The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J Exerc Nutrition Biochem 21:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turner NJ and Badylak SF. (2015). The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv Wound Care (New Rochelle) 4:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown LF, Van de Water L, Harvey VS and Dvorak HF. (1988). Fibrinogen influx and accumulation of cross-linked fibrin in healing wounds and in tumor stroma. Am J Pathol 130:455–465. [PMC free article] [PubMed] [Google Scholar]
- 50. Dvorak HF. (2002). Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20:4368–4380. [DOI] [PubMed] [Google Scholar]
- 51. Dvorak HF. (2003). Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am J Pathol 162:1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF and van de Water L. (1992). Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 176:1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J and Dvorak AM. (1987). Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest 57:673–686. [PubMed] [Google Scholar]
- 54. Martin P. (1997). Wound healing—aiming for perfect skin regeneration. Science 276:75–81. [DOI] [PubMed] [Google Scholar]
- 55. Coussens LM and Werb Z. (2002). Inflammation and cancer. Nature 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Homey B, Müller A and Zlotnik A. (2002). Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol 2:175–184. [DOI] [PubMed] [Google Scholar]
- 57. Rossi D and Zlotnik A. (2000). The biology of chemokines and their receptors. Annu Rev Immunol 18:217–242. [DOI] [PubMed] [Google Scholar]
- 58. Dong X, Mao S and Wen H. (2013). Upregulation of proinflammatory genes in skin lesions may be the cause of keloid formation (Review). Biomed Rep 1:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tuan TL, Wu H, Huang EY, Chong SS, Laug W, Messadi D, Kelly P and Le A. (2003). Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol 162:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tang W, Zhou M, Dorsey TH, Prieto DA, Wang XW, Ruppin E, Veenstra TD and Ambs S. (2018). Integrated proteotranscriptomics of breast cancer reveals globally increased protein-mRNA concordance associated with subtypes and survival. Genome Med 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fuentes-Duculan J, Bonifacio KM, Suárez-Fariñas M, Kunjravia N, Garcet S, Cruz T, Wang CQF, Xu H, Gilleadeau P, et al. (2017). Aberrant connective tissue differentiation towards cartilage and bone underlies human keloids in African Americans. Exp Dermatol 26:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T and Jeschke MG. (2011). Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 17:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li S, Wei X, He J, Tian X, Yuan S and Sun L. (2018). Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother 105:83–94. [DOI] [PubMed] [Google Scholar]
- 64. Chalmers RL. (2011). The evidence for the role of transforming growth factor-beta in the formation of abnormal scarring. Int Wound J 8:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barcellos-Hoff MH and Akhurst RJ. (2009). Transforming growth factor-beta in breast cancer: too much, too late. Breast Cancer Res 11:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zarzynska JM. (2014). Two faces of TGF-beta1 in breast cancer. Mediat Inflamm 2014:141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. LeFlore IC. (1980). Misconceptions regarding elective plastic surgery in the black patient. J Natl Med Assoc 72:947–948. [PMC free article] [PubMed] [Google Scholar]
- 68. Marneros AG, Norris JE, Olsen BR and Reichenberger E. (2001). Clinical genetics of familial keloids. Arch Dermatol 137:1429–1434. [DOI] [PubMed] [Google Scholar]
- 69. Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH and El Saghir NS. (2013). Epidemiology and prognosis of breast cancer in young women. J Thorac Dis 5(Suppl 1):S2–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Narod SA. (2012). Breast cancer in young women. Nat Rev Clin Oncol 9:460–470. [DOI] [PubMed] [Google Scholar]
- 71. Newman LA. (2014). Breast cancer disparities: high-risk breast cancer and African ancestry. Surg Oncol Clin N Am 23:579–592. [DOI] [PubMed] [Google Scholar]
- 72. Dunn BK, Agurs-Collins T, Browne D, Lubet R and Johnson KA. (2010). Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat 121:281–292. [DOI] [PubMed] [Google Scholar]
- 73. Paget S. (1989). The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98–101. [PubMed] [Google Scholar]
- 74. Dvorak HF. (2019). Tumors: Wounds That Do Not Heal-A Historical Perspective with a Focus on the Fundamental Roles of Increased Vascular Permeability and Clotting. Semin Thromb Hemost 45:576–592. [DOI] [PubMed] [Google Scholar]
- 75. Hanahan D and Coussens LM. (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322. [DOI] [PubMed] [Google Scholar]
- 76. Bertolini F, Lohsiriwat V, Petit JY and Kolonin MG. (2012). Adipose tissue cells, lipotransfer and cancer: a challenge for scientists, oncologists and surgeons. Biochim Biophys Acta 1826:209–214. [DOI] [PubMed] [Google Scholar]
- 77. Sadlonova A, Mukherjee S, Bowe DB, Gault SR, Dumas NA, Van Tine BA, Frolova N, Page GP, Welch DR, Novak L and Frost AR. (2007). Human breast fibroblasts inhibit growth of the MCF10AT xenograft model of proliferative breast disease. Am J Pathol 170:1064–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Erler JT and Weaver VM. (2009). Three-dimensional context regulation of metastasis. Clin Exp Metastasis 26:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Whiteside TL. (2008). The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Strong AL, Burow ME, Gimble JM and Bunnell BA. (2015). Concise review: the obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells 33:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schweizer R, Tsuji W, Gorantla VS, Marra KG, Rubin JP and Plock JA. (2015). The role of adipose-derived stem cells in breast cancer progression and metastasis. Stem Cells Int 2015:120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sabol RA, Giacomelli P, Beighley A and Bunnell BA. (2019). Adipose stem cells and cancer: concise review. Stem Cells 37:1261–1266. [DOI] [PubMed] [Google Scholar]
- 83. Preisner F, Leimer U, Sandmann S, Zoernig I, Germann G and Koellensperger E. (2018). Impact of human adipose tissue-derived stem cells on malignant melanoma cells in an in vitro co-culture model. Stem Cell Rev Rep 14:125–140. [DOI] [PubMed] [Google Scholar]
- 84. Scioli MG, Storti G, D'Amico F, Gentile P, Kim BS, Cervelli V and Orlandi A. (2019). Adipose-derived stem cells in cancer progression: new perspectives and opportunities. Int J Mol Sci 20:3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sun L-Y, Pang C-Y, Li D-K, Liao C-H, Huang W-C, Wu C-C, Chou Y-Y, Li WW, Chen S-Y, et al. (2013). Antioxidants cause rapid expansion of human adipose-derived mesenchymal stem cells via CDK and CDK inhibitor regulation. J Biomed Sci 20:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li T-S and Marbán E. (2010). Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 28:1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thomson JA and Odorico JS. (2000). Human embryonic stem cell and embryonic germ cell lines. Trends Biotechnol 18:53–57. [DOI] [PubMed] [Google Scholar]
- 88. Balkwill F and Coussens LM. (2004). Cancer: an inflammatory link. Nature 431:405–406. [DOI] [PubMed] [Google Scholar]
- 89. Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF and Karin M. (2004). IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296. [DOI] [PubMed] [Google Scholar]
- 90. Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and Ben-Neriah Y. (2004). NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431:461–466. [DOI] [PubMed] [Google Scholar]
- 91. Brock CK, Hebert KL, Artiles M, Wright MK, Cheng T, Windsor GO, Nguyen K, Alzoubi MS, Collins-Burow BM, et al. (2021). A role for adipocytes and adipose stem cells in the breast tumor microenvironment and regenerative medicine. Front Physiol 12:751239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fantuzzi G. (2005). Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115:911–919; quiz 920. [DOI] [PubMed] [Google Scholar]
- 93. Gimble JM, Katz AJ and Bunnell BA. (2007). Adipose-derived stem cells for regenerative medicine. Circ Res 100:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K and Gimble JM. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McIntosh KR, Frazier T, Rowan BG and Gimble JM. (2013). Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol 9:175–184. [DOI] [PubMed] [Google Scholar]
- 96. Dubey NK, Mishra VK, Dubey R, Deng YH, Tsai FC and Deng WP. (2018). Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells. Int J Mol Sci 19:2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, Bosia A, Marchisio PC and Mantovani A. (1991). In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest 87:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV and March KL. (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298. [DOI] [PubMed] [Google Scholar]
- 99. Voog J and Jones DL. (2010). Stem cells and the niche: a dynamic duo. Cell Stem Cell 6:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mazini L, Rochette L, Admou B, Amal S and Malka G. (2020). Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci 21:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M and Raeisi Dehkordi S. (2019). Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther 10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hyldig K, Riis S, Pennisi CP, Zachar V and Fink T. (2017). Implications of extracellular matrix production by adipose tissue-derived stem cells for development of wound healing therapies. Int J Mol Sci 18:1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Baharlou R, Ahmadi-Vasmehjani A, Faraji F, Atashzar MR, Khoubyari M, Ahi S, Erfanian S and Navabi SS. (2017). Human adipose tissue-derived mesenchymal stem cells in rheumatoid arthritis: regulatory effects on peripheral blood mononuclear cells activation. Int Immunopharmacol 47:59–69. [DOI] [PubMed] [Google Scholar]
- 104. Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, Broaddus R, Kolonin M, Mok SC, et al. (2013). Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One 8:e81859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR and Ghaderi A. (2011). Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol 266:116–122. [DOI] [PubMed] [Google Scholar]
- 106. Ning H, Lin G, Fandel T, Banie L, Lue TF and Lin CS. (2008). Insulin growth factor signaling mediates neuron-like differentiation of adipose-tissue-derived stem cells. Differentiation 76:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T and Kaneda Y. (2006). Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb 13:77–81. [DOI] [PubMed] [Google Scholar]
- 108. Song YH, Gehmert S, Sadat S, Pinkernell K, Bai X, Matthias N and Alt E. (2007). VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem Biophys Res Commun 354:999–1003. [DOI] [PubMed] [Google Scholar]
- 109. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ and Park JS. (2007). Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48:15–24. [DOI] [PubMed] [Google Scholar]
- 110. Xiong L, Sun J, Hirche C, Yang J, Yang Y, Xia Y, Lehnhardt M, Wang R and Fu X. (2012). In vitro N-acetyl-L-cysteine promotes proliferation and suppresses interleukin-8 expression in adipose-derived stem cells. Aesthetic Plast Surg 36:1260–1265. [DOI] [PubMed] [Google Scholar]
- 111. Lin XH, Liu N, Xiao YC, Chen RH, Du HW, Wang JH, Zhang YX and Liu DS. (2011). [Effects of adipose-derived stem cells transplantation on the neuronal apoptosis and the expression of Bcl-2 and caspase-12 in the brain post focal cerebral ischemia in rats]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 27:40–43. [PubMed] [Google Scholar]
- 112. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ and Holash J. (2000). Vascular-specific growth factors and blood vessel formation. Nature 407:242–248. [DOI] [PubMed] [Google Scholar]
- 113. Gentile P and Garcovich S. (2019). Concise review: adipose-derived stem cells (ASCs) and adipocyte-secreted exosomal microRNA (A-SE-miR) modulate cancer growth and proMote wound repair. J Clin Med 8:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB and Kim JH. (2011). Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol 131:1559–1567. [DOI] [PubMed] [Google Scholar]
- 115. Yang CY, Chang PY, Chen JY, Wu BS, Yang AH and Lee OK. (2021). Adipose-derived mesenchymal stem cells attenuate dialysis-induced peritoneal fibrosis by modulating macrophage polarization via interleukin-6. Stem Cell Res Ther 12:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Heo JS, Choi Y and Kim HO. (2019). Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int 2019:7921760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhu D, Johnson TK, Wang Y, Thomas M, Huynh K, Yang Q, Bond VC, Chen YE and Liu D. (2020). Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther 11:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Manferdini C, Paolella F, Gabusi E, Gambari L, Piacentini A, Filardo G, Fleury-Cappellesso S, Barbero A, Murphy M and Lisignoli G. (2017). Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis Cartilage 25:1161–1171. [DOI] [PubMed] [Google Scholar]
- 119. Heo JS, Choi Y and Kim HO. (2019). Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int 2019:7921760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Simoni-Nieves A, Gerardo-Ramírez M, Pedraza-Vázquez G, Chávez-Rodríguez L, Bucio L, Souza V, Miranda-Labra RU, Gomez-Quiroz LE and Gutiérrez-Ruiz MC. (2019). GDF11 implications in cancer biology and metabolism. Facts and Controversies. Front Oncol 9:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lombardi F, Palumbo P, Augello FR, Cifone MG, Cinque B and Giuliani M. (2019). Secretome of adipose tissue-derived stem cells (ASCs) as a novel trend in chronic non-healing wounds: an overview of experimental in vitro and in vivo studies and methodological variables. Int J Mol Sci 20:3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Park JS, Park G and Hong HS. (2021). Age affects the paracrine activity and differentiation potential of human adipose-derived stem cells. Mol Med Rep 23:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pandey AC, Semon JA, Kaushal D, O'Sullivan RP, Glowacki J, Gimble JM and Bunnell BA. (2011). MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res Ther 2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dufrane D. (2017). Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant 26:1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ruthenborg RJ, Ban JJ, Wazir A, Takeda N and Kim JW. (2014). Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells 37:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Frazier T, Lee S, Bowles A, Semon J, Bunnell B, Wu X and Gimble J. (2018). Gender and age-related cell compositional differences in C57BL/6 murine adipose tissue stromal vascular fraction. Adipocyte 7:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ma Y, Ren Y, Dai ZJ, Wu CJ, Ji YH and Xu J. (2017). IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv Clin Exp Med 26:421–426. [DOI] [PubMed] [Google Scholar]
- 128. Antoon JW, Lai R, Struckhoff AP, Nitschke AM, Elliott S, Martin EC, Rhodes LV, Yoon NS, Salvo VA, et al. (2012). Altered death receptor signaling promotes epithelial-to-mesenchymal transition and acquired chemoresistance. Sci Rep 2:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hodge DR, Hurt EM and Farrar WL. (2005). The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41:2502–2512. [DOI] [PubMed] [Google Scholar]
- 130. Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M and Ochiai A. (2009). Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer 125:1276–1284. [DOI] [PubMed] [Google Scholar]
- 131. Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, et al. (2010). Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat 121:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hogan NM, Joyce MR, Murphy JM, Barry FP, O'Brien T, Kerin MJ and Dwyer RM. (2013). Impact of mesenchymal stem cell secreted PAI-1 on colon cancer cell migration and proliferation. Biochem Biophys Res Commun 435:574–579. [DOI] [PubMed] [Google Scholar]
- 133. Sternlicht MD, Dunning AM, Moore DH, Pharoah PD, Ginzinger DG, Chin K, Gray JW, Waldman FM, Ponder BA and Werb Z. (2006). Prognostic value of PAI1 in invasive breast cancer: evidence that tumor-specific factors are more important than genetic variation in regulating PAI1 expression. Cancer Epidemiol Biomarkers Prev 15:2107–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L and Sánchez-Margalet V. (2019). Obesity and breast cancer: role of leptin. Front Oncol 9:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tadokoro S, Ide S, Tokuyama R, Umeki H, Tatehara S, Kataoka S and Satomura K. (2015). Leptin promotes wound healing in the skin. PLoS One 10:e0121242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pallegar NK and Christian SL. (2020). Adipocytes in the tumour microenvironment. Adv Exp Med Biol 1234:1–13. [DOI] [PubMed] [Google Scholar]
- 137. D'Esposito V, Ambrosio MR, Giuliano M, Cabaro S, Miele C, Beguinot F and Formisano P. (2020). Mammary adipose tissue control of breast cancer progression: impact of obesity and diabetes. Front Oncol 10:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Park J, Morley TS, Kim M, Clegg DJ and Scherer PE. (2014). Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bandini E, Rossi T, Gallerani G and Fabbri F. (2019). Adipocytes and microRNAs crosstalk: a key tile in the mosaic of breast cancer microenvironment. Cancers (Basel) 11:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Freese KE, Kokai L, Edwards RP, Philips BJ, Sheikh MA, Kelley J, Comerci J, Marra KG, Rubin JP and Linkov F. (2015). Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis: a systematic review. Cancer Res 75:1161–1168. [DOI] [PubMed] [Google Scholar]
- 141. Agresti R, Triulzi T, Sasso M, Ghirelli C, Aiello P, Rybinska I, Campiglio M, Sfondrini L, Tagliabue E and Bianchi F. (2019). Wound healing fluid reflects the inflammatory nature and aggressiveness of breast tumors. Cells 8:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lippitz BE and Harris RA. (2016). Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis. OncoImmunology 5:e1093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ocana A, Nieto-Jiménez C, Pandiella A and Templeton AJ. (2017). Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer 16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bergamaschi A, Tagliabue E, Sørlie T, Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, et al. (2008). Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol 214:357–367. [DOI] [PubMed] [Google Scholar]
- 145. Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G and Palacios J. (2008). Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68:989–997. [DOI] [PubMed] [Google Scholar]
- 146. Prasetyanti PR and Medema JP. (2017). Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer 16:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Arwert EN, Hoste E and Watt FM. (2012). Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 12:170–180. [DOI] [PubMed] [Google Scholar]