Abstract
Objective:
To compare rates of severe maternal morbidity (SMM) for pregnant patients with a cardiac diagnosis classified by the modified World Health Organization (mWHO) classification to those without a cardiac diagnosis.
Study design:
This retrospective study using the 2015–2019 Nationwide Readmissions Database identified hospitalizations, comorbidities, and outcomes using diagnosis and procedure codes. The primary exposure was cardiac diagnosis, classified into low-risk (mWHO class I and II) and moderate-to-high-risk (mWHO class II/III, III, or IV). The primary outcome was SMM or death during the delivery hospitalization; secondary outcomes included cardiac-specific SMM during delivery hospitalizations and readmissions after the delivery hospitalization.
Results:
A weighted national estimate of 14,995,122 delivery admissions was identified, including 46,541 (0.31%) with mWHO I-II diagnoses and 37,330 (0.25%) with mWHO II/III-IV diagnoses. Patients with mWHO II/III-IV diagnoses experienced SMM at the highest rates (22.8% vs 1.6% for no diagnosis; with adjusted relative risk (aRR) of 5.67 [95% CI: 5.36 to 6.00]). The risk of death was also highest for patients with mWHO II/III-IV diagnoses (0.3% vs. <0.1% for no diagnosis; aRR 18.07 [95% CI: 12.25–26.66]). Elevated risk of SMM and death persisted to 11 months postpartum for those patients with mWHO II/III-IV diagnoses.
Conclusions:
In this nationwide database, SMM is highest among individuals with moderate-to-severe cardiac disease based on mWHO classification. This risk persists in the year postpartum. These results can be used to enhance pregnancy counseling.
Keywords: Modified World Health Organization Classification, cardiac disease in pregnancy, severe maternal morbidity, maternal mortality, maternal adverse cardiac events
INTRODUCTION
Cardiovascular disease is the leading cause of maternal mortality in the United States. Data extracted from the Centers for Disease Control and Prevention’s Pregnancy Mortality Surveillance System from 2011 to 2013 suggest that cardiovascular conditions and cardiomyopathy together account for 26% of pregnancy-related deaths.1 The rising impact of cardiovascular disease in pregnancy is attributed to rising maternal age and obesity, increasing prevalence of acquired cardiovascular co-morbidities among women of childbearing age, improved pediatric congenital heart disease outcomes permitting survival into adulthood, and persistent racial/socioeconomic disparities.2–7 Pregnant people with cardiac disease are at higher risk for adverse cardiovascular, neonatal, and obstetric complications, although the degree and breadth of risk can vary widely depending on the underlying diagnosis and is often not well-quantified in the literature.8, 9
There are currently three primary risk or classification models used to predict adverse cardiovascular events in pregnancy for women with pre-existing cardiac disease. These are the CARPREG II risk score, the ZAHARA risk score, and the modified World Health Organization classification of maternal cardiovascular risk (mWHO).10–12 The ZAHARA risk score is specifically designed for women with congenital heart disease, while CARPREG II and the mWHO classification provide estimates of the risk of adverse cardiac events for women with both congenital and acquired heart disease. None of these models are comprehensive in their inclusion of cardiac diagnoses. The mWHO classification outperforms CARPREG I (the first iteration of CARPREG) and ZAHARA in terms of predicting adverse cardiac events in pregnancy among women with congenital heart disease13, 14 and acquired heart disease.15, 16 The performance of the mWHO classification has not been directly compared to CARPREG II. The outcome predicted for all the models is adverse cardiac events, typically including arrhythmia, heart failure or pulmonary edema, transient ischemic attack, stroke, dissection, myocardial infarction, cardiac arrest, or maternal death. The models do not capture the risk of other adverse maternal outcomes, such as mechanical ventilation, sepsis, preeclampsia, or transfusion. However, the mWHO classification does divide the risk of maternal morbidity and mortality into no, small, intermediate, significantly and extremely high risk, providing a general guide to the overall risks of pregnancy for individuals with cardiac disease.
The mWHO classification divides cardiac diagnoses into five classes based on risk of pregnancy and provides predicted maternal adverse cardiac event rates.12, 17, 18 Women in mWHO class I are considered at no detectable increased risk of maternal morbidity and mortality, mWHO II are at small increased risk, mWHO II/III are at intermediate increased risk, mWHO III are at significantly increased risk, and mWHO IV are at such high risk of maternal morbidity and mortality that pregnancy is considered contraindicated. Validation of the predicted maternal adverse cardiac event rates and risk of morbidity and mortality associated with the mWHO classes in a large US population has yet to be completed.
The primary aim of this study was to quantify and compare rates of severe maternal morbidity (SMM) and mortality during delivery hospitalization in a large, nationwide database among pregnant women with no, low-, and high-risk cardiac disease using the mWHO classification. Secondly, we aimed to examine SMM and mortality up to 11 months postpartum and to compare outcomes between those who did and did not experience cardiac morbidity during their delivery hospitalization.
METHODS
This was a retrospective cohort study utilizing the Nationwide Readmissions Database (NRD), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality.19 The NRD dataset is a large administrative dataset that, in 2019, contained hospitalizations for 30 states, representing 61.8% of the U.S. population and 60.4% of all hospitalizations. Data available in each record include demographic information (e.g. age, gender, primary payer), diagnosis and procedure codes, hospital characteristics, including location and academic affiliation, hospital length of stay, inpatient charges, and discharge disposition. Methods used for the purposes of this analysis are further described in detail elsewhere.20 Delivery hospitalizations from October 1, 2015 to December 31, 2019 were included. The final quarter of 2015 was selected as the start date for the study as this was the date of adoption of International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) for billing in the United States, , which provided a significant increase in the granularity of disease coding. At the time of this analysis, December 2019 was the most recent NRD data available for review.
The exposure of interest was a diagnosis code during the delivery hospitalization consistent with cardiac disease in pregnancy. ICD-10 codes were used to identify women with a cardiac disease diagnosis (Supplemental Table 1). Women were divided into three comparator groups. The first included all women with a delivery hospitalization within the given timeframe who did not have one of the cardiac diagnosis codes of interest. The remaining two groups included women with a diagnosis of cardiac disease, which were subsequently classified by mWHO classification and divided into groups by mild or moderate-to-severe cardiac disease.12 The use of billing codes limited the degree of precision of the cardiac diagnoses and ability to divide into the five separate mWHO classes. For example, repaired congenital heart disease (CHD) lesions could not be differentiated from unrepaired CHD lesions, as they are in the mWHO classification, and moderate left ventricular impairment cannot be differentiated from severe. However, when mWHO class I and mWHO class II diagnoses were combined, they were mutually exclusive of the diagnoses included in mWHO classes II-III, III, and IV (Supplementary Table 1). This allowed a comparison of the outcomes between diagnoses included in mWHO classes I and II, which are associated with a lower risk of adverse cardiac events, and those diagnoses included in mWHO classes II/III, III, and IV, which are associated with a moderate-to-high risk of adverse cardiac events. Repaired aortic coarctation was only included in the mWHO I-II group. To summarize, the cohorts included women without cardiac disease, women with mWHO I-II cardiac disease (mWHO I-II), and women with mWHO II-III, III, or IV cardiac disease (mWHO II/III-IV). For individuals who might have more than one cardiac diagnosis in different mWHO categories, the individual was assigned a cohort according to the most severe diagnosis. Aortic root dilation was excluded from the exposures of interest for two reasons. First, the degree of aortic root dilation was unable to be specified by ICD-10 codes. Second, the ICD-10 code for aortic aneurysm was included in the SMM primary outcome, as noted below. Underlying aortopathies, such as Marfan syndrome and bicuspid aortic valve, were included.
The primary outcome was a composite of severe maternal morbidity, as defined by the Centers for Disease Control and Prevention (CDC), and maternal mortality occurring during the delivery hospitalization. Table 1 includes the specific CDC Severe Maternal Morbidity Indicators included in the primary outcome, as well as secondary outcomes.21 Secondary outcomes during the delivery hospitalization included cardiac-specific severe maternal morbidity (as outlined in Table 1) and mortality, non-transfusion severe maternal morbidity or mortality, preterm birth (defined by presence of ICD-10 code Z3A .xx, with xx the digits coding gestational age < 37 weeks), and cesarean delivery rather than vaginal delivery.
Table 1.
Primary and secondary outcomes.
| Primary outcome | Secondary outcomes | |
|---|---|---|
| Severe maternal morbidity (SMM) or mortality | Non-transfusion SMM or mortality | |
| Acute myocardial infarction Aortic aneurysm Acute renal failure Adult respiratory distress syndrome Amniotic fluid embolism Cardiac arrest/ventricular fibrillation Conversion of cardiac rhythm Disseminated intravascular coagulation Eclampsia Heart failure/arrest during surgery or procedure |
Puerperal cerebrovascular disorders Pulmonary edema/acute heart failure Severe anesthesia complications Sepsis Shock Sickle cell disease with crisis Air and thrombotic embolism Blood products transfusion Hysterectomy Temporary tracheostomy Mechanical ventilation |
SMM minus blood product transfusion |
| Cardiac SMM or mortality | ||
| Acute myocardial infarction Aortic aneurysm Cardiac arrest/ventricular fibrillation Conversion of cardiac rhythm Heart failure/arrest during surgery or procedure Pulmonary edema/acute heart failure | ||
|
Preterm birth
Cesarean delivery | ||
In addition to analyzing events during the delivery hospitalization, we also evaluated readmissions for SMM and cardiac-specific SMM, as well as readmissions ending in death following delivery. The NRD is a calendar year dataset (i.e., there is no crossover between years of the dataset) and reports discharge date at the level of month; making the conservative assumption that discharges occur at the end of the month, the longest follow-up period available is thus 11 months (for patients discharged in January), and we accordingly treated patients as being censored (lost to follow-up) based on their month of discharge (e.g., 1 month for November, 2 months of October, etc.). In evaluating readmissions, patients were stratified based on whether they had experienced a cardiac-specific SMM event during their delivery hospitalization in order to examine rates of SMM occurring after the delivery admission both for those who had an initial adverse cardiac event during their delivery hospitalization and those that did not.
Covariates of interest that were available in the NRD dataset included age in years at admission, calendar year and quarter, primary expected payer, hospital characteristics, delivery type, and medical comorbidities, using a previously validated obstetric-specific comorbidity index.22 The comorbidity index includes preexisting heart disease and pulmonary hypertension as comorbid conditions, and since these were incorporated into the mWHO categories, we removed these variables from our comorbidity adjustment. Univariate statistical methods, incorporating the weighted, stratified, and clustered nature of the NRD, were used to assess the univariate associations between demographics, cardiac disease, and outcomes. Relative risk regression (incorporating the NRD’s weighting, stratification, and clustering) using a Poisson model was used to assess for associations between cardiac disease and delivery hospitalization outcomes, adjusting for patient age, comorbidities, delivery mode (except for the cesarean outcome), and calendar year and quarter. Similarly, a weighted Cox proportional hazards model was used to analyze readmissions data, controlling for the same covariates and again accounting for the complex survey methods of the NRD. Kaplan-Meier failure estimates were calculated for readmissions for SMM or mortality, cardiac-specific SMM or mortality, death, or readmission for any reason or death occurring after discharge from the delivery hospitalization for each mWHO classification group and by whether any cardiac-specific SMM had occurred during the delivery stay. Stratification by whether subjects had experienced cardiac SMM during their delivery hospitalization was performed to further examine the risks of cardiac disease in the postpartum period based on the outcome of their delivery hospitalization. Statistical analysis was completed in Stata Statistical Software, Version 16.1 (Statacorp, College Station, Texas). A two-sided alpha value of 0.05 was considered statistically significant. Given the retrospective nature of this analysis using an existing, limited dataset, the Duke University School of Medicine Institutional Review Board deemed it exempt from review.
RESULTS
A total of 8,004,184 patients experienced a delivery hospitalization between October 2015 and December 2019 (Figure 1). When weighted to produce estimates of the national population, rather than only the sample included in the NRD dataset, this sample equated to 14,995,122 patients. For the remainder of this manuscript, numbers are reported as a weighted statistic. Of the total weighted delivery hospitalizations, 14,911,251 (99.5%) women did not have cardiac disease, 46,541 (0.3%) had mWHO I-II cardiac disease, 37,330 (0.2%) had mWHO II/III-IV cardiac disease. The sample sizes for individual cardiac diagnoses, when available, are provided in Supplementary Table 1. Women with cardiac disease differed in age (28.8 vs. 30.2 vs. 29.8 years for no cardiac disease, mWHO I-II, and mWHO II/III-IV, respectively; p<0.001), delivery at a metropolitan teaching hospital (69.5%, 76.6% and 81.2% for no cardiac disease, mWHO I-II, II/III-IV, and unclassified, respectively; p<0.001), and comorbid conditions compared to women without cardiac disease (Table 1).
Figure 1:
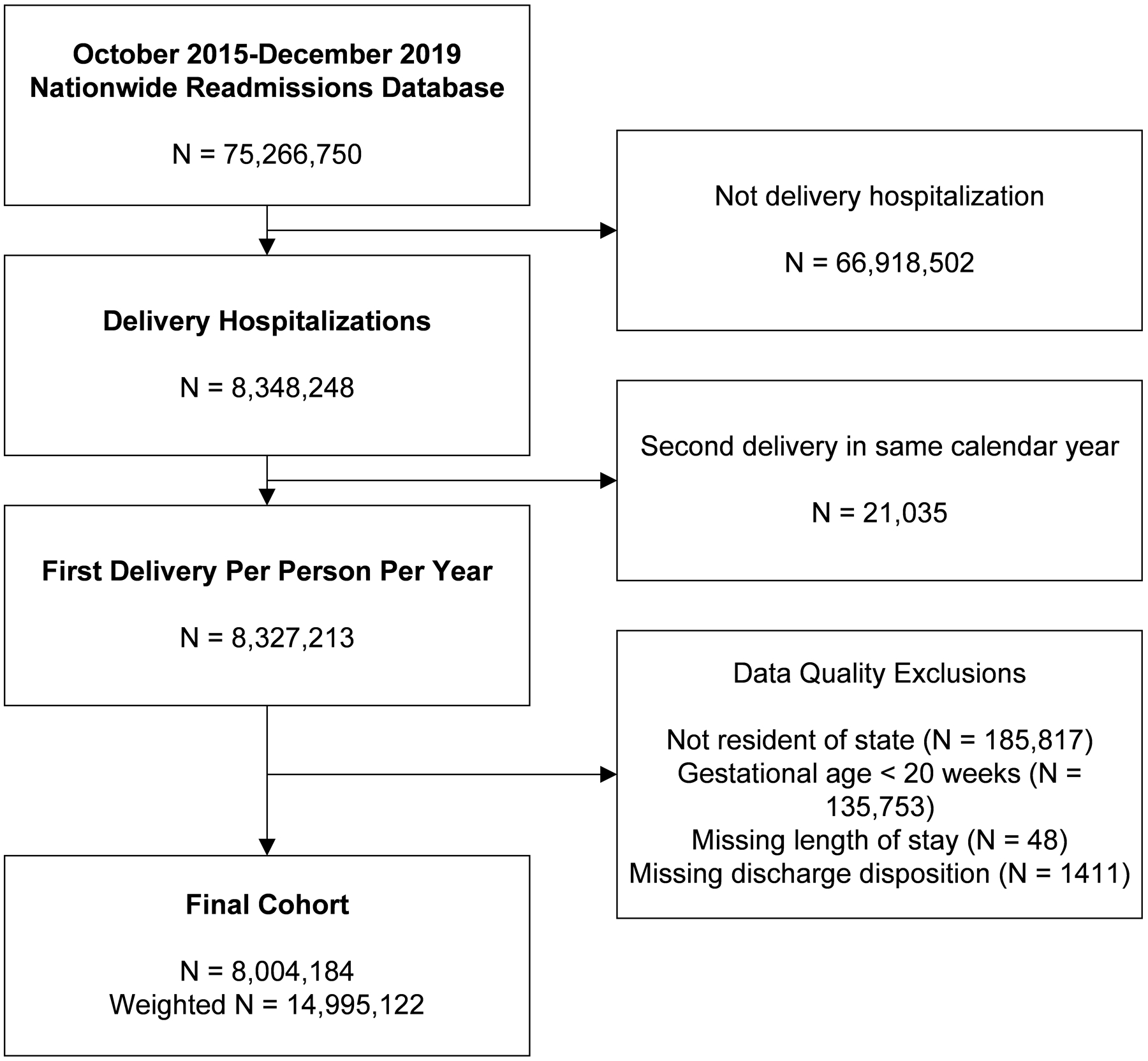
Derivation of Study Cohort
Detailed SMM event rates are provided in Supplementary Table 2. All delivery hospitalization outcomes demonstrated increased probability in unadjusted analyses with increasing severity of mWHO class (Figure 2). Specifically, mWHO I-II was associated with higher rates of SMM, including non-transfusion SMM and cardiac-specific SMM, death, preterm birth, and cesarean delivery when compared to patients without cardiac disease; mWHO II-III/IV was associated with even higher rates of all outcomes. These findings were confirmed in adjusted analyses (Figure 3). Patients with mWHO II/III-IV disease had a 5.67-times higher adjusted probability of any SMM or mortality during their delivery hospitalization (95% confidence interval: 5.36, 6.00) compared with no cardiac disease, with similar notably higher rates of non-transfusion and cardiac-specific SMM. Death, preterm birth, and cesarean delivery rates were similarly elevated with one-quarter of those with mWHO II/III-IV disease delivering preterm and one-half delivering by cesarean. Results from unweighted regression models (not incorporating the complex design of the NRD) were similar (Supplementary Figure 1).
Figure 2:
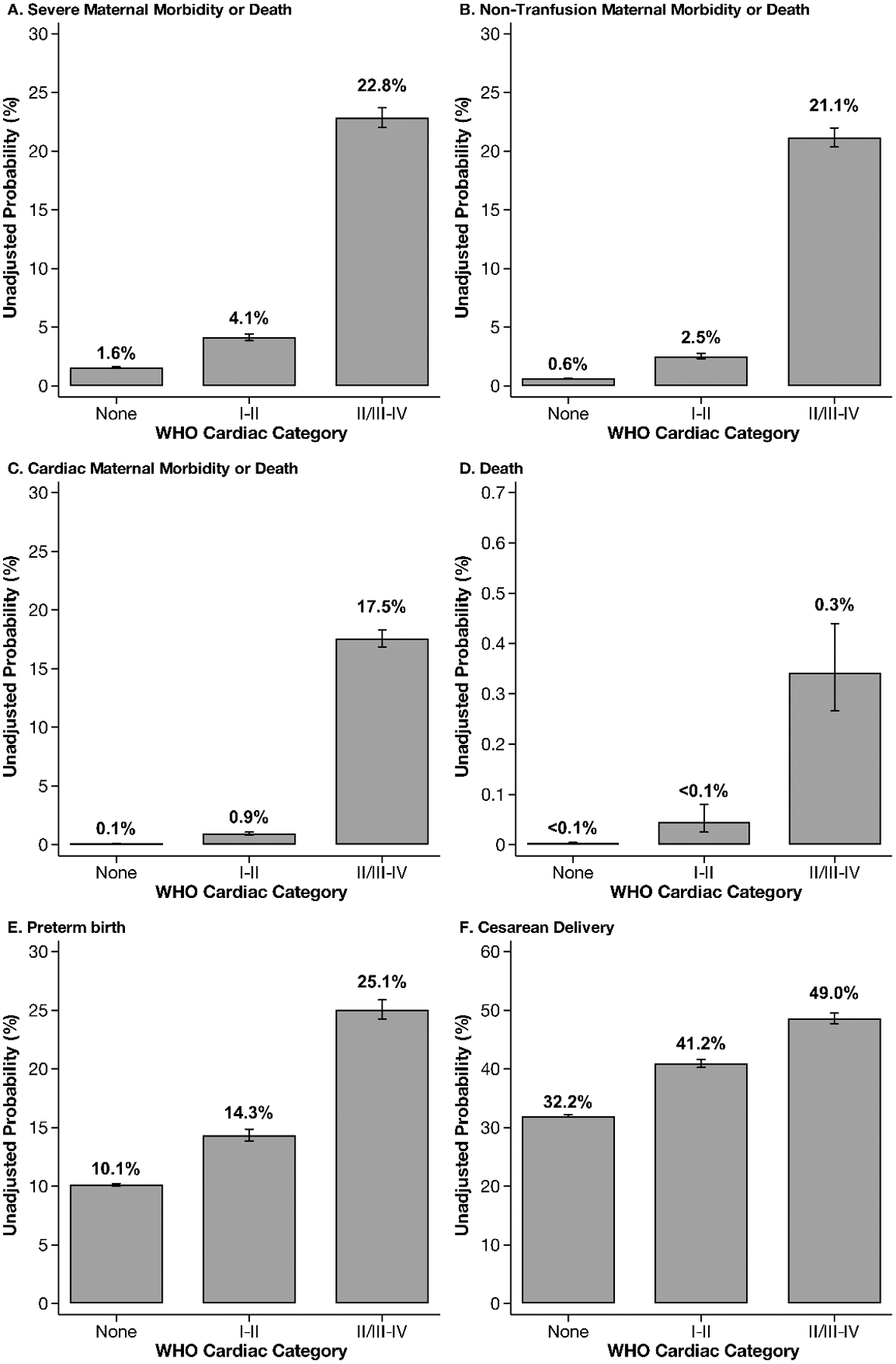
Unadjusted results from delivery hospitalizations, stratified by modified World Health Organization cardiac category
Figure 3:
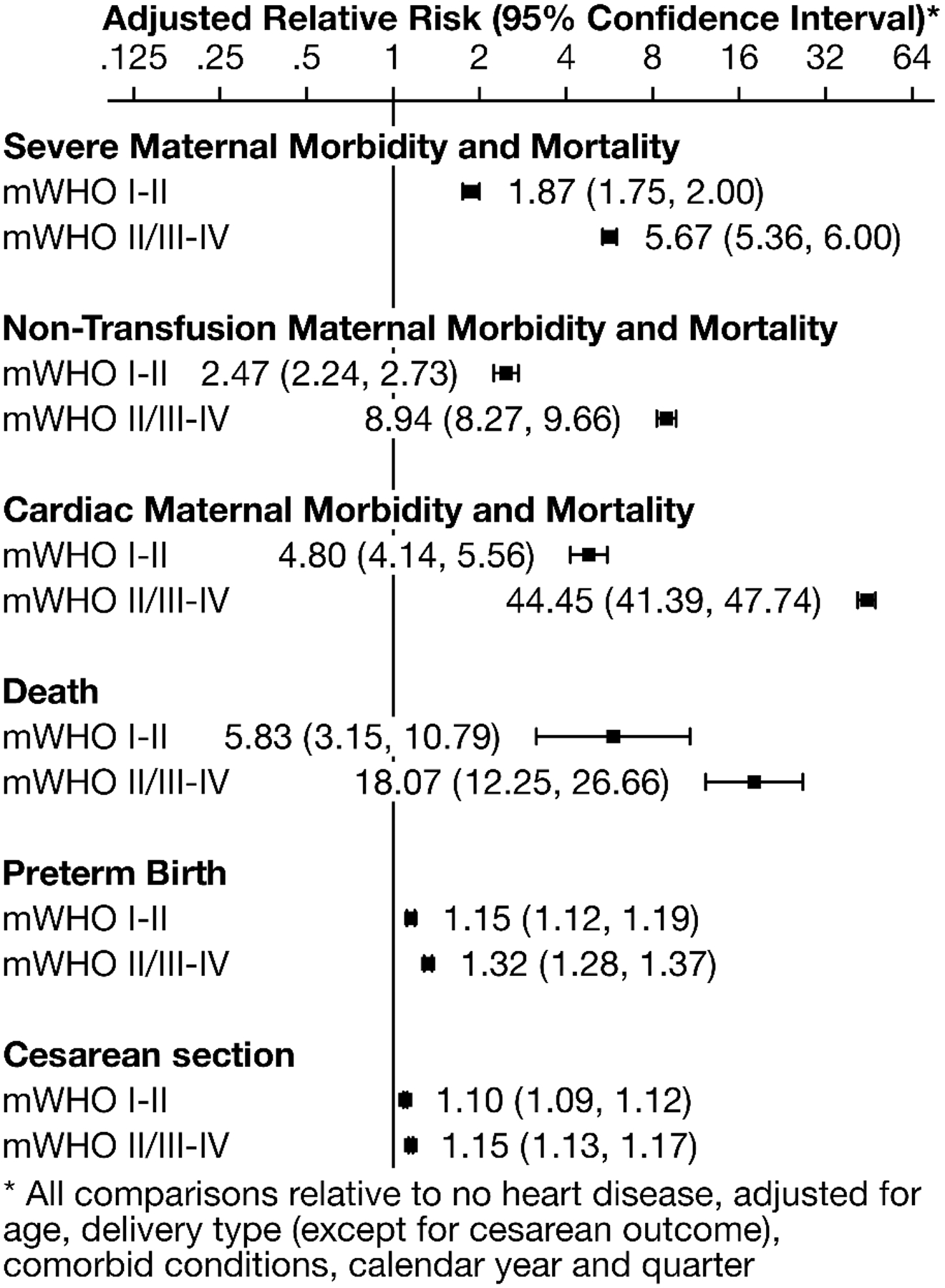
Adjusted in-hospital outcomes, stratified by modified World Health Organization cardiac category, compared to no heart disease
Among patients surviving their delivery hospitalization, we examined outcomes for those with cardiac disease compared to no cardiac disease up to 11 months postpartum following the delivery hospitalization (Figure 4). Occurrence of a cardiac SMM event during the delivery hospitalization was observed to be a powerful predictor of subsequent adverse outcomes, and for this reason, analyses were performed stratified on this variable. In unadjusted analyses, with the exception of death for those with mWHO I-II cardiac disease, rates of adverse outcomes were higher for patients with cardiac disease than those without cardiac disease regardless of whether they had experienced cardiac SMM during their delivery stay. These differences in postpartum outcomes were less pronounced for those with mWHO class I-II disease.
Figure 4:
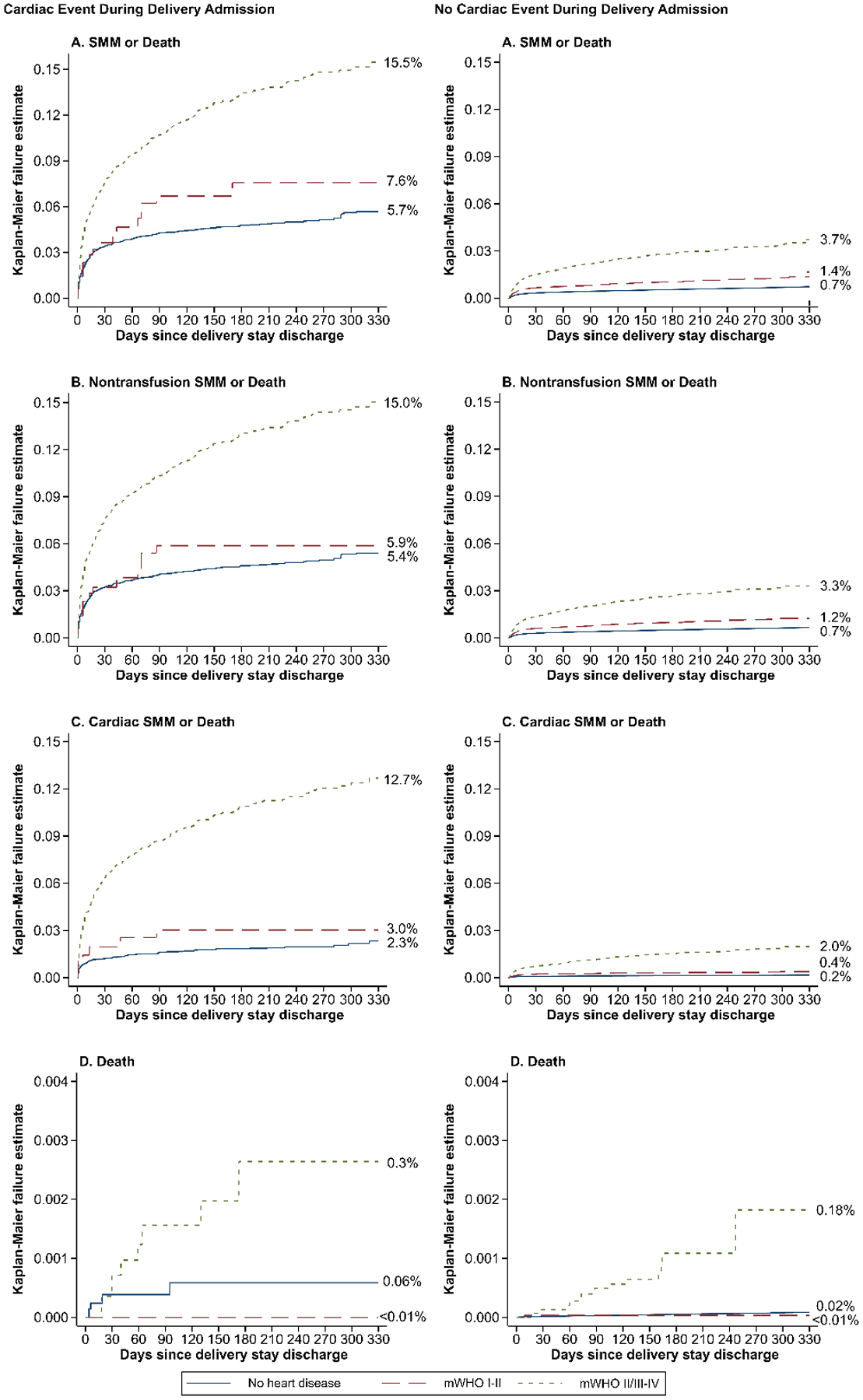
Unadjusted Kaplan-Maier curves comparing rates of readmission, stratified by modified World Health Organization cardiac category, compared to no heart disease
Finally, comparisons were adjusted for potential confounding factors in Cox proportional hazard models (Table 3). For patients with cardiac SMM during their delivery stay, associations between mWHO I-II disease versus no cardiac disease and rates of adverse outcome were not statistically significant. In contrast, except for death, there were significant positive associations noted between mWHO II/III-IV disease and adverse outcomes. Among patients without cardiac SMM during the delivery stay, both mWHO I-II and mWHO II/III-IV cardiac disease compared to no cardiac disease was associated with increased risk of SMM-related rehospitalizations. However, death was only positively associated with mWHO II/III-IV cardiac disease, but not mWHO I-II cardiac disease, compared to no cardiac disease in this group that did not experience cardiac SMM during the delivery stay. Results from unweighted regression models (not incorporating the complex design of the NRD were similar (Supplementary Table 3).
Table 3:
Covariate-adjusted outcomes for events following delivery hospitalization
| Cardiac SMM during Delivery Hospitalization | No Cardiac SMM during Delivery Hospitalization | |
|---|---|---|
| Adjusted Hazard Ratio (95% Confidence Interval)* | ||
| Any SMM or Death | ||
| mWHO I-II | 1.72 (0.996, 2.98) | 1.53 (1.34, 1.76) |
| mWHO II/III-IV | 1.70 (1.42, 2.03) | 3.17 (2.84, 3.54) |
| Nontransfusion SMM or Death | ||
| mWHO I-II | 1.46 (0.79, 2.69) | 1.57 (1.36, 1.81) |
| mWHO II/III-IV | 1.69 (1.42, 2.02) | 3.20 (2.85, 3.59) |
| Cardiac SMM or Death | ||
| mWHO I-II | 1.75 (0.73, 4.17) | 1.89 (1.48, 2.40) |
| mWHO II/III-IV | 3.03 (2.41, 3.81) | 6.97 (5.99, 8.01) |
| Death | ||
| mWHO I-II | † | 0.48 (0.07, 3.29) |
| mWHO II/III-IV | 2.16 (0.59, 7.95) | 9.61 (5.13, 18.02) |
All comparisons versus patients without cardiac disease. Adjusted for age, delivery type, comorbid conditions, calendar year/quarter, and patient age
Unable to estimate due to low event rate
DISCUSSION
In this nationwide cohort, pregnant people with mWHO II/III, III, or IV cardiac lesions in pregnancy, as defined by the modified World Health Organization classification of maternal cardiovascular risk, were more likely to experience severe maternal morbidity or mortality both during their delivery hospitalization and during readmissions up to 11 months postpartum compared to pregnant people without cardiac disease. In fact, nearly one-quarter of pregnant people with mWHO II/III-IV cardiac lesions experienced severe maternal morbidity or death, 18% experienced cardiac-specific morbidity or mortality, and the risk of death alone was 0.3% during the delivery hospitalization.
Our results also provide important insight into the temporal relationship between cardiac disease and SMM and mortality. Notably, regardless of whether they had experienced cardiac SMM during their delivery hospitalization, those with mWHO II/III-IV cardiac disease experienced a significantly increased risk of SMM, non-transfusion SMM, and cardiac SMM throughout the 11 months postpartum compared to those without a cardiac diagnosis. In contrast, only those with a mWHO I-II cardiac diagnosis who did not experience cardiac SMM during their delivery hospitalization remained at increased risk for SMM and death in the 11 months postpartum. It is possible that this group with lower risk cardiac disease who do not experience cardiac SMM during the delivery hospitalization are followed less closely in the postpartum period. These findings suggest that vigilance must be maintained throughout the postpartum period in people with mWHO II/III-IV and mWHO I-II cardiac disease, even if they do not experience significant adverse cardiac events during their delivery hospitalization. Such vigilance could be accomplished by continued close follow-up by the pregnancy heart team during the year postpartum.12, 18
Our findings corroborate the mWHO classification of maternal cardiovascular risk. The mWHO classification was initially devised based on expert opinion and theoretical risk, with the risk of adverse cardiac events ranging from 2–5% in mWHO class I, to 11–19% in mWHO class II/III, to >27% in mWHO class IV.18 The unadjusted probability of cardiac SMM or mortality in our study for the higher-risk mWHO classes was 18%, which is consistent with the predicted adverse cardiac event rates. Albeit based on expert opinion, this classification system often performs superiorly in the prediction of adverse cardiac events in pregnancy to other risk prediction models, such as CARPREG and ZAHARA.13–16 The data from our large, nationwide study provides one of the most comprehensive assessments, based on standardized definitions, of severe maternal morbidity and mortality in individuals with cardiac disease to date. While representing an average of three of the mWHO classes (mWHO classes II/III, III, and IV), this study confirms the significantly elevated risk of severe complications among pregnant people with mWHO class II/III-IV heart disease compared to no heart disease and compared to mWHO class I-II disease. Not to be trivialized, however, our results also highlight the increased risk of severe complications in those with cardiac diagnoses considered to be mild (mWHO class I and II). “Low risk” is not the equivalent of “no risk”, with this group experiencing a nearly 2-fold increase in the risk of severe maternal morbidity and mortality compared to those with no cardiac diagnosis. These findings can be used to strengthen the mWHO classification. Furthermore, the results may also aid clinicians in counseling patients with cardiac disease about the risks of pregnancy, delivery, and the postpartum period.
Finally, our results shed light on some of the increased obstetric risks for patients with cardiac disease in pregnancy. Both those with mWHO I-II and mWHO II/III-IV cardiac disease were at higher risk for preterm birth and cesarean delivery. While we were unable to determine what proportions of these differences were due to iatrogenic causes, these differences are notable. Preterm birth carries long-term risks to the neonate,23 while cesarean delivery carries increased risks of maternal complications.24, 25 Most guidelines regarding management of cardiovascular disease in pregnancy recommend vaginal delivery over cesarean in the absence of obstetric indications for cesarean delivery.12, 26 In fact, data from the ROPAC registry suggests that planned cesarean delivery in patients with cardiac disease results in earlier delivery and lower neonatal birth weight.27 There are only a select few cardiac diagnoses, mostly in mWHO class IV, that warrant a planned cesarean birth. The discrepancy in cesarean birth rates seen in our data between those without cardiac disease and those with mWHO class I-II and class II/III-IV cardiac disease highlights the need for continued education of obstetric and cardiology providers regarding the safe and appropriate intrapartum management of cardiac disease in pregnancy.
The strengths of this study include the use of a large, nationwide database providing one of the largest samples available to analyze outcomes of cardiac disease in pregnancy. Additional strengths include the use of the CDC’s standardized definition of severe maternal morbidity, as well as the use of validated ICD-10 comorbidity indices.21, 22 While assessment of outcomes was limited to a maximum of 11-months postpartum given lack of linkage between years in the NRD database, the analysis of outcomes for such a long postpartum period provides insight into the potential delayed risks of pregnancy for individuals with cardiac disease and is consistent with the definition of pregnancy-related death.
We recognize several limitations. The NRD dataset only provides data on hospitalizations and readmissions. Rather than only including pregnant patients with antepartum admissions, which could have overestimated rates of adverse events, we included delivery hospitalizations (which would capture most pregnancies ending in delivery) and subsequent readmissions. However, this limitation may have resulted in an underestimation of the true event rate in this population. For example, events (e.g. ICU admission) occurring prior to a delivery hospitalization that did not culminate in delivery were not included in the estimation. These limitations further highlight that the true risk of any severe maternal morbidity or death related to cardiac disease in pregnancy is likely higher than what is presented here. As is true for all retrospective analyses utilizing administrative datasets, this study was further limited by reliance on ICD-10 diagnosis codes, which may not have captured all exposures and outcomes. Under-coding may have led to an underestimation cardiac diagnoses, comorbidities, and outcomes. For example, the 3.3% of subjects identified as having a BMI ≥ 40 is lower than the expected rate of 9% in the US population reported by the CDC in 2017–2018.28 The reliance on ICD-10 diagnosis codes prevented more granular division of the mWHO classes into classes I, II, II/III, III, and IV.
In conclusion, the results of this study provide valuable supporting data that can be used when counseling people with cardiac disease in pregnancy. Beyond insights into the absolute risk of severe maternal morbidity and mortality from a large, nationwide database, this study highlights that the risks of pregnancy complicated by cardiac disease extend beyond delivery into the year postpartum. While pregnant people with mWHO II/III-IV disease are at highest risk for SMM and mortality, those with lower risk lesions classified as mWHO I-II also have a significantly elevated risk compared to those without cardiac disease that should not be minimized.
Supplementary Material
Table 2:
Baseline characteristics, stratified by Modified World Health Organization Classification
| Modified World Health Organization classification | |||||
|---|---|---|---|---|---|
| Overall (N=8,004,184) (Weighted N = 14,995,122) |
No heart disease (N=7,959,609) (Weighted N = 14,911,251) |
WHO I-II (N=24,947) (Weighted N = 46,541) |
WHO II/III-IV (N=19,628) (Weighted N = 37,330) |
P* | |
| Mean (Standard Deviation) or % | |||||
| Patient Demographics | |||||
| Age in years at admission | 28.8 (5.8) | 28.8 (5.8) | 30.2 (5.9) | 29.8 (6.0) | <0.001 |
| Primary expected payer† | <0.001 | ||||
| Medicare | 119,392 (0.8) | 117,712 (0.8) | 635 (1.4) | 1,045 (2.8) | |
| Medicaid | 6,300,543 (42.1) | 6,268,908 (42.1) | 15,512 (33.4) | 16,124 (43.2) | |
| Private | 7,902,339 (52.8) | 7,855,343 (52.7) | 28,396 (61.1) | 18,600 (49.9) | |
| Self-pay | 220,180 (1.5) | 219,297 (1.5) | 446 (1.0) | 437 (1.2) | |
| No charge | 7,558 (0.1) | 7,528 (0.1) | 11 (0.0) | 19 (0.1) | |
| Other | 427,594 (2.9) | 425,058 (2.9) | 1,478 (3.2) | 1,057 (2.8) | |
| Zip Code Median Household Income† | <0.001 | ||||
| Quartile 1 (Lowest) | 4,107,300 (27.6) | 4,085,289 (27.6) | 11,134 (24.1) | 10,877 (29.3) | |
| Quartile 2 | 3,894,478 (26.1) | 3,873,575 (26.1) | 11,221 (24.3) | 9,681 (26.1) | |
| Quartile 3 | 3,780,479 (25.4) | 3,758,884 (25.4) | 12,423 (26.9) | 9,172 (24.7) | |
| Quartile 4 (Highest) | 3,116,885 (20.9) | 3,098,057 (20.9) | 11,467 (24.8) | 7,361 (19.8) | |
| Hospital Characteristics | |||||
| Hospital Bed Size | <0.001 | ||||
| Small | 2,514,262 (16.8) | 2,503,663 (16.8) | 6,294 (13.5) | 4,305 (11.5) | |
| Medium | 4,295,153 (28.6) | 4,275,238 (28.7) | 11,865 (25.5) | 8,050 (21.6) | |
| Large | 8,185,708 (54.6) | 8,132,350 (54.5) | 28,382 (61.0) | 24,976 (66.9) | |
| Hospital location / teaching status | <0.001 | ||||
| Metropolitan non-teaching | 3,178,062 (21.2) | 3,165,133 (21.2) | 7,804 (16.8) | 5,125 (13.7) | |
| Metropolitan teaching | 10424392 (69.5) | 10358423 (69.5) | 35,668 (76.6) | 30,302 (81.2) | |
| Non-metropolitan hospital | 1,392,668 (9.3) | 1,387,695 (9.3) | 3,070 (6.6) | 1,904 (5.1) | |
| Comorbid Conditions | |||||
| Advanced maternal age | 2,624,113 (17.5) | 2,604,334 (17.5) | 11,210 (24.1) | 8,570 (23.0) | <0.001 |
| Asthma | 785,843 (5.2) | 774,202 (5.2) | 5,189 (11.1) | 6,453 (17.3) | <0.001 |
| Bariatric surgery | 41,532 (0.3) | 41,056 (0.3) | 245 (0.5) | 231 (0.6) | <0.001 |
| Bleeding disorder | 327,773 (2.2) | 323,751 (2.2) | 2,009 (4.3) | 2,013 (5.4) | <0.001 |
| BMI greater or equal to 40 | 501,552 (3.3) | 497,045 (3.3) | 2,088 (4.5) | 2,419 (6.5) | <0.001 |
| Chronic hypertension | 499,773 (3.3) | 491,341 (3.3) | 3,324 (7.1) | 5,109 (13.7) | <0.001 |
| Chronic renal disease | 38,633 (0.3) | 37,375 (0.3) | 270 (0.6) | 988 (2.6) | <0.001 |
| Connective tissue or autoimmune disease | 30,824 (0.2) | 29,970 (0.2) | 352 (0.8) | 502 (1.3) | <0.001 |
| Gastrointestinal disease | 847,655 (5.7) | 837,319 (5.6) | 5,587 (12.0) | 4,749 (12.7) | <0.001 |
| Gestational Diabetes Mellitus | 1,143,423 (7.6) | 1,136,040 (7.6) | 3,997 (8.6) | 3,386 (9.1) | <0.001 |
| Gestational hypertension / preeclampsia without severe features | 1,240,779 (8.3) | 1,232,357 (8.3) | 4,541 (9.8) | 3,881 (10.4) | <0.001 |
| HIV/AIDS | 13,531 (0.1) | 13,406 (0.1) | 50 (0.1) | 75 (0.2) | <0.001 |
| Mental health disorder | 1,149,200 (7.7) | 1,135,363 (7.6) | 7,801 (16.8) | 6,036 (16.2) | <0.001 |
| Multiples gestation | 269,703 (1.8) | 267,374 (1.8) | 1,187 (2.6) | 1,142 (3.1) | <0.001 |
| Neuromuscular disease | 74,543 (0.5) | 73,461 (0.5) | 547 (1.2) | 535 (1.4) | <0.001 |
| Placenta accreta spectrum | 16,928 (0.1) | 16,729 (0.1) | 102 (0.2) | 98 (0.3) | <0.001 |
| Placenta previa | 69,523 (0.5) | 68,954 (0.5) | 338 (0.7) | 232 (0.6) | <0.001 |
| Placental abruption | 161,879 (1.1) | 160,470 (1.1) | 642 (1.4) | 767 (2.1) | <0.001 |
| Preeclampsia with severe features | 496,658 (3.3) | 489,464 (3.3) | 2,437 (5.2) | 4,756 (12.7) | <0.001 |
| Preexisting anemia | 1,971,304 (13.1) | 1,955,613 (13.1) | 8,131 (17.5) | 7,560 (20.3) | <0.001 |
| Preexisting diabetes mellitus | 203,955 (1.4) | 201,432 (1.4) | 969 (2.1) | 1,555 (4.2) | <0.001 |
| Preterm birth | 1,497,610 (10.0) | 1,481,719 (9.9) | 6,586 (14.2) | 9,304 (24.9) | <0.001 |
| Prior cesarean birth | 2,630,037 (17.5) | 2,611,406 (17.5) | 9,855 (21.2) | 8,775 (23.5) | <0.001 |
| Substance use disorder | 978,538 (6.5) | 970,828 (6.5) | 3,311 (7.1) | 4,399 (11.8) | <0.001 |
| Thyrotoxicosis | 39,656 (0.3) | 38,979 (0.3) | 392 (0.8) | 284 (0.8) | <0.001 |
| Delivery type | <0.001 | ||||
| Spontaneous vaginal | 9,494,266 (63.7) | 9,451,868 (63.8) | 25,389 (54.9) | 17,009 (46.0) | |
| Operative vaginal | 608,019 (4.1) | 604,398 (4.1) | 1,778 (3.8) | 1,844 (5.0) | |
| Cesarean | 4,802,249 (32.2) | 4,765,067 (32.2) | 19,046 (41.2) | 18,136 (49.0) | |
P-values by weighted linear regression for continuous variables and weighted chi2 test for binary/categorical variables
Missing values in Payer (8,744 observations) and ZIP Code Income Quartile (54,174 observations)
ACKNOWLEDGEMENTS
The authors appreciate the HCUP Data Partners who contribute data to the NRD. A complete list of partners can be found at (www.hcup-us.ahrq.gov/hcupdatapartners.jsp).
FUNDING
Work contained in this manuscript were made possible by the following grants from the National Institutes of Health (TL1-TR002555 [JJF]). Data acquisition was also supported by funding from the Foundation for Women and Girls with Blood Disorders to JJF. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstetrics & Gynecology 2017;130(2):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangel V, White RS, Nachamie AS, Pick JS. Racial and Ethnic Disparities in Maternal Outcomes and the Disadvantage of Peripartum Black Women: A Multistate Analysis, 2007–2014. American Journal of Perinatology 2018;36(08):835–848. [DOI] [PubMed] [Google Scholar]
- 3.Briller J, Koch AR, Geller SE. Maternal Cardiovascular Mortality in Illinois, 2002–2011. Obstetrics & Gynecology 2017;129(5):819–826. [DOI] [PubMed] [Google Scholar]
- 4.Ntiloudi D, Giannakoulas G, Parcharidou D, Panagiotidis T, Gatzoulis MA, Karvounis H. Adult congenital heart disease: A paradigm of epidemiological change. International Journal of Cardiology 2016;218:269–274. [DOI] [PubMed] [Google Scholar]
- 5.Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS data brief 2009(21):1–8. [PubMed] [Google Scholar]
- 6.Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and Obstetric Outcomes Among Pregnant Women With Congenital Heart Disease. Obstetrics & Gynecology 2015;126(2):346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis JM, Menard MK, Gee RE. Racial and Ethnic Disparities in Maternal Morbidity and Mortality. Obstetrics & Gynecology 2015;125(3):690–694. [DOI] [PubMed] [Google Scholar]
- 8.Koutrolou-Sotiropoulou P, Parikh PB, Miller C, Lima FV, Butler J, Stergiopoulos K. Impact of Heart Disease on Maternal and Fetal Outcomes in Pregnant Women. The American Journal of Cardiology 2015;116(3):474–480. [DOI] [PubMed] [Google Scholar]
- 9.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier L-A, Morton BC, et al. Prospective Multicenter Study of Pregnancy Outcomes in Women With Heart Disease. Circulation 2001;104(5):515–521. [DOI] [PubMed] [Google Scholar]
- 10.Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, et al. Pregnancy Outcomes in Women With Heart Disease The CARPREG II Study. Journal of the American College of Cardiology 2018;71(21):2419–2430. [DOI] [PubMed] [Google Scholar]
- 11.Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJM, et al. Predictors of pregnancy complications in women with congenital heart disease. European Heart Journal 2010;31(17):2124–2132. [DOI] [PubMed] [Google Scholar]
- 12.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39(34):3165–3241. [DOI] [PubMed] [Google Scholar]
- 13.Balci A, Sollie-Szarynska KM, Bijl AGLvd, Ruys TPE, Mulder BJM, Roos-Hesselink JW, et al. Prospective validation and assessment of cardiovascular and offspring risk models for pregnant women with congenital heart disease. Heart 2014;100(17):1373. [DOI] [PubMed] [Google Scholar]
- 14.Kim YY, Goldberg LA, Awh K, Bhamare T, Drajpuch D, Hirshberg A, et al. Accuracy of risk prediction scores in pregnant women with congenital heart disease. Congenit Heart Dis 2019;14(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu C-W, Shih J-C, Chen S-Y, Chiu H-H, Wang J-K, Chen C-A, et al. Comparison of 3 Risk Estimation Methods for Predicting Cardiac Outcomes in Pregnant Women With Congenital Heart Disease. Circ J 2015;79(7):1609–1617. [DOI] [PubMed] [Google Scholar]
- 16.Pijuan-Domènech A, Galian L, Goya M, Casellas M, Merced C, Ferreira-Gonzalez I, et al. Cardiac complications during pregnancy are better predicted with the modified WHO risk score. International Journal of Cardiology 2015;195:149–154. [DOI] [PubMed] [Google Scholar]
- 17.Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart 2006;92(10):1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ACOG Practice Bulletin No. 212. Obstetrics & Gynecology 2019;133(5):e320–e356. [DOI] [PubMed] [Google Scholar]
- 19.[dataset] HCUP Nationwide Readmissions Database (NRD). Healthcare Cost and Utilization Project (HCUP). 2014, 2016, and 2017. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nrdoverview.jsp [Google Scholar]
- 20.Federspiel J, Suresh S, Darwin K, Szymanski L. Hospitalization Duration Following Uncomplicated Cesarean Delivery: Predictors, Facility Variation, and Outcomes. Am J Perinatology Reports 2020;10(02):e187–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Division of Reproductive Health National Center for Chronic Disease Prevention and Health Promotion. How Does CDC Identify Severe Maternal Morbidity? [Google Scholar]
- 22.Leonard SA, Kennedy CJ, Carmichael SL, Lyell DJ, Main EK. An Expanded Obstetric Comorbidity Scoring System for Predicting Severe Maternal Morbidity. Obstet Gynecol 2020;136(3):440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371(9608):261–9. [DOI] [PubMed] [Google Scholar]
- 24.Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018;392(10155):1349–1357. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS, et al. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ 2007;176(4):455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, et al. Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association. Circulation 2020;141(23):e884–e903. [DOI] [PubMed] [Google Scholar]
- 27.Ruys TP, Roos-Hesselink JW, Pijuan-Domenech A, Vasario E, Gaisin IR, Iung B, et al. Is a planned caesarean section in women with cardiac disease beneficial? Heart 2015;101(7):530–6. [DOI] [PubMed] [Google Scholar]
- 28.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. In: NCHS Data Brief. Hyattsville, MD: National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


