Abstract
Background:
Hepatic epithelioid hemangioendothelioma (HEH) is a rare vascular tumor of unknown etiology and unpredictable natural history. To date, no large-scale studies have been published evaluating this disease due to its rare occurrence.
Methods:
The National Cancer Database was reviewed between 2004 and 2016 to identify patients with HEH. Univariate analysis with overall survival (OS) was performed by Cox proportional hazards model. Kaplan–Meier method was used to create OS curves and compared using the log-rank test.
Results:
We identified 229 patients with HEH. The majority of patients were female (61.1%), white (84.3%), and had a Charlson–Deyo score of 0 (75%). Chemotherapeutic intervention was seen in 26% of the patients while 33% received surgical intervention in the form of wedge/segmental liver resection (n = 27), hepatectomy lobectomy/extended lobectomy (n = 18), and liver transplant (n = 22). Five-year survival in surgical patients was 90.5%, 66.5% and 81%, respectively (p = 0.485). Age greater than 55 years (hazard ratio [HR], 2.78; p < 0.001), Asian ethnicity compared to white (HR, 2.84; p = 0.012), and a higher Charlson–Deyo score (score 1: HR, 2.28; p < 0.001 and score ≥2: HR, 2.76; p = 0.011) were associated with worse OS.
Conclusion:
Treatment for HEH remains variable with only a third of the patients undergoing surgery. International collaboration is necessary to determine the optimal treatment for this rare disease.
Keywords: epithelioid hemangioendothelioma, HEH, HEHE
1 |. INTRODUCTION
Epithelioid hemangioendothelioma is a rare tumor of vascular origin characterized by epithelioid and histiocytoid vascular endothelial cells, known to occur in the liver and other parts of the body such as spleen, heart, head and neck, bone, and lungs.1–4 The term epithelioid hemangioendothelioma was first proposed by Weiss and Enzinger in 1982,5 and is used to name those vascular neoplasms that show a borderline biological behavior, between benign hemangiomas and highly malignant sarcomas.6 It has an estimated incidence of 1–2 cases in every 1 million people and occurs more frequently in women with a male to female ratio of 2:3.7,8
The most common clinical manifestations of hepatic epithelioid hemangioendothelioma (HEH) are right upper quadrant pain, followed by hepatomegaly and weight loss.8 HEH lesions are hypodense on computed tomography, and on magnetic resonance imaging showed low signal intensity on T1 and high heterogeneous signal intensity on T2,9 with some cases showing the characteristic “lollipop sign” on imaging.10 Diagnosis is aided by the presence of at least one endothelial marker including CD31, CD34, or factor VIII-related antigen.11
The differential diagnosis includes hepatocellular carcinoma, cholangiocarcinoma, angiosarcoma, and metastatic carcinoma among others.11 The treatment options are broad and inconsistent due to lack of sufficient data promulgating one type of treatment over another, mostly due to the paucity of this disease.
With multiple management strategies and treatment options ranging from drugs to liver transplant, added to the lack of established guidelines, there emerges a dire need for an improved understanding of this disease. The purpose of this study is to review the National Cancer Database (NCDB) experience with HEH and identify all treatment approaches and outcomes to consider optimal management for this rare disease.
2 |. MATERIALS AND METHODS
2.1 |. Data source
The NCDB was queried from 2004 to 2016 to conduct a retrospective study in patients diagnosed with HEH. NCDB is jointly sponsored by the American College of Surgeons and the American Cancer Society. It is a clinical oncology database sourced from hospital registry data that are collected in more than 1500 Commission on Cancer (CoC) accredited facilities across the United States and Puerto Rico, representing more than 70% of newly diagnosed cancer cases nationwide. It is used to explore trends in cancer care and to serve as a basis for quality improvement.12
2.2 |. Patient selection
Following exemption from Institutional Review Board review, all patients with liver cancer were identified from 2004 to 2016. Only patients with a diagnosis of HEH (specified histology code: 9133)— according to the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3), were included in this set. Subjects who received treatment with radiofrequency ablation and palliative care patients were further excluded.
2.3 |. Demographics and treatment variables
Data of interest included patient characteristics (age, sex, ethnicity, and Charlson–Deyo score), facility location (Northeast, Midwest, West, and South), and type (Community Cancer Program, Comprehensive Community Cancer Program, Academic/Research Program, and Integrated Network Cancer Program), oncological variables (tumor size, metastasis, lymph node involvement, lymphovascular invasion, and margin status), treatment types (surgery, chemotherapy, and radiation), and sequence of treatment. Surgery of the primary site (liver) was categorized into wedge/segmental resection, lobectomy/extended lobectomy, and transplantation.
2.4 |. Statistics
Descriptive statistics were used to summarize patient characteristics. The Kaplan–Meier method was used to estimate overall survival (OS) and survival curves were compared between three surgical intervention groups using the log-rank test. Univariate analysis was performed to assess factors associated with OS using Cox proportional hazards model. Firth’s penalized likelihood bias-reduction approach was used to account for small sample and low event rates. A p value of <0.05 was considered statistically significant for all analyses. All analyses were conducted using SAS Version 9.4 (SAS Institute Inc.).
3 |. RESULTS
3.1 |. Demographics
Among 192,418 patients with liver cancer between 2004 and 2016, 229 patients met inclusion criteria (Figure 1). The majority of patients were young (median age = 55), female (61.1%), with a male-to-female ratio of 2:3 (Table 1). Univariate association with OS for patients above median age (≥55, n = 102) was independently associated with worse OS (hazard ratio [HR], 2.78; p <0.001) compared to patients below median (≤55 years; Table 2). The majority of the cases were seen in white population (84.3%). The Asian population (3.75%) was associated with worse OS (HR, 2.84; p = 0.012) compared to the white population. Patients with a Charlson–Deyo score of 2+ predicted a worse OS (HR, 2.76; p = 0.011), followed by a score of 1+ (HR, 2.28; p < 0.001) when compared to a Charlson–Deyo score of 0.
FIGURE 1.
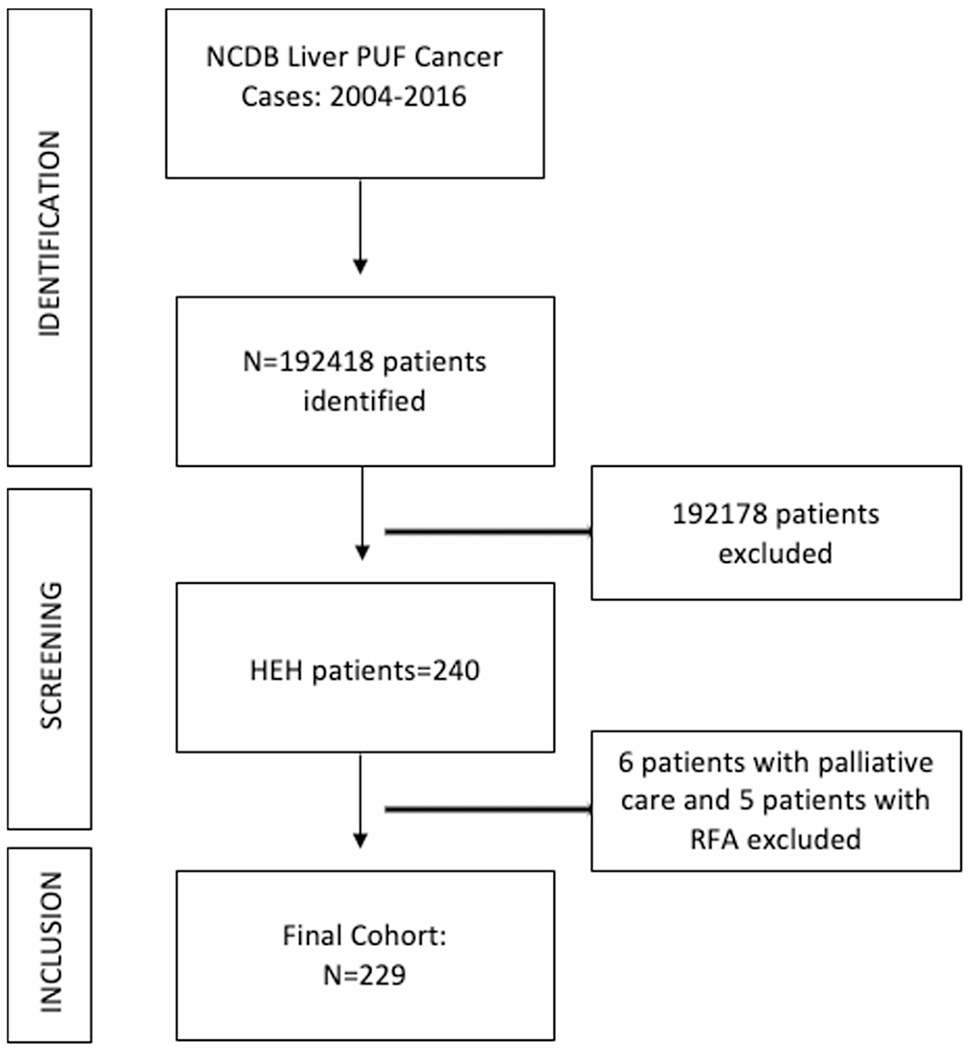
Schematic depicting patients inclusion and exclusion criteria. HEH, hepatic epithelioid hemangioendothelioma; N, number; NCDB, National Cancer Database; PUF, participant user data file; RFA, radiofrequency ablation
TABLE 1.
Descriptive statistics including demographics, tumor characteristics, and surgical treatment methods used for intervention in patients with hepatic epithelioid hemangioendothelioma
| Variable | Level | N (%) = 229 |
|---|---|---|
| Cohort | Wedge/segmental resection | 27 (40.3) |
| Lobectomy/extended lobectomy | 18 (26.9) | |
| Transplant | 22 (32.8) | |
| Missing | 162 | |
|
| ||
| Facility location | Northeast | 35 (20.5) |
| Midwest | 56 (32.7) | |
| West | 29 (17.0) | |
| South | 51 (29.8) | |
| Missing | 58 | |
|
| ||
| Age (categorial) | Below median (≤55) | 118 (51.5) |
| Above median (55) | 111 (48.5) | |
|
| ||
| Sex | Male | 89 (38.9) |
| Female | 140 (61.1) | |
|
| ||
| Race | White | 193 (84.3) |
| Black | 20 (8.7) | |
| Other | 8 (3.5) | |
| Asian | 8 (3.5) | |
|
| ||
| Charlson–Deyo score | 0 | 172 (75.1) |
| 1 | 44 (19.2) | |
| 2+ | 13 (5.7) | |
|
| ||
| Primary Payor | Medicaid/other government/not insured/unknown | 30 (13.1) |
| Private | 131 (57.2) | |
| Medicare | 68 (29.7) | |
|
| ||
| Grade | Well differentiated | 8 (36.4) |
| Moderately differentiated | 11 (50.0) | |
| Poorly differentiated/undifferentiated | 3 (13.6) | |
| Missing | 207 | |
|
| ||
| Lymph Vascular Invasion, 2010 | Not present | 17 (63.0) |
| Present | 10 (37.0) | |
| Missing | 202 | |
|
| ||
| Surgical margins status | Negative | 52 (82.5) |
| Positive | 11 (17.5) | |
| Missing | 166 | |
TABLE 2.
Univariate association with overall survival analyzing demographics, treatment modalities, and tumor-related characteristics in patients with hepatic epithelioid hemangioendothelioma
| OS |
|||||
|---|---|---|---|---|---|
| Covariate | Level | N | Hazard ratio (95% CI) | HR p value | Log-rank p value |
| Cohort | Wedge/segmental resection | 25 | 0.91 (0.23–3.52) | 0.893 | 0.485 |
| Lobectomy/extended lobectomy | 15 | 1.96 (0.50–7.61) | 0.345 | ||
| Transplant | 21 | – | – | ||
|
| |||||
| Facility type | Community Cancer Program | 11 | 0.48 (0.16–1.29) | 0.164 | 0.074 |
| Comprehensive Community Cancer Program | 45 | 0.45 (0.22–0.98) | 0.037 | ||
| Academic/Research Program | 91 | 0.39 (0.21–0.81) | 0.008 | ||
| Integrated Network Cancer Program | 12 | – | – | ||
|
| |||||
| Age (category) | Above median | 102 | 2.78 (1.77–4.48) | <0.001 | <0.001 |
| Below median | 111 | – | – | ||
|
| |||||
| Race | Black | 19 | 1.01 (0.43–2.01) | 0.983 | 0.127 |
| Other | 7 | 1.28 (0.35–3.25) | 0.661 | ||
| Asian | 8 | 2.84 (1.14–5.89) | 0.012 | ||
| White | 179 | – | – | ||
|
| |||||
| Charlson–Deyo score | 1 | 41 | 2.28 (1.39–3.66) | <0.001 | <0.001 |
| 2+ | 11 | 2.76 (1.17–5.59) | 0.011 | ||
| 0 | 161 | – | – | ||
|
| |||||
| Primary Payor | Medicaid/other government/not insured/unknown | 27 | 0.36 (0.16–0.72) | 0.007 | <0.001 |
| Private | 124 | 0.34 (0.21–0.53) | <0.001 | ||
| Medicare | 62 | – | – | ||
|
| |||||
| Grade | Moderately differentiated | 10 | 1.30 (0.30–7.33) | 0.758 | 0.676 |
| Poorly differentiated/undifferentiated | 3 | 2.44 (0.36–16.26) | 0.368 | ||
| Well differentiated | 7 | – | – | ||
|
| |||||
| Lymph Vascular Invasion, 2010 | Not present | 16 | 1.67 (0.52–6.72) | 0.435 | 0.355 |
| Present | 8 | – | – | ||
|
| |||||
| Surgical margins status | Positive | 9 | 1.51 (0.29–5.26) | 0.575 | 0.757 |
| Negative | 49 | – | – | ||
|
| |||||
| Radiation therapy | None | 196 | 0.53 (0.08–66.93) | 0.661 | 0.607 |
| Beam radiation | 9 | 1.01 (0.11–132.46) | 0.996 | ||
| Radioactive implants | 4 | 0.84 (0.07–116.39) | 0.914 | ||
| Radiation therapy, NOS | 1 | – | – | ||
|
| |||||
| Metastatic Lung Involvement, 2010–2015 | None | 73 | 1.30 (0.62–3.02) | 0.509 | 0.450 |
| Yes | 31 | – | – | ||
|
| |||||
| Chemotherapy | None | 117 | 1.07 (0.36–5.18) | 0.923 | 0.298 |
| Chemotherapy administered, type and number of agents not documented | 5 | 1.65 (0.32–9.96) | 0.557 | ||
| Single-agent chemotherapy | 33 | 1.63 (0.51–8.20) | 0.483 | ||
| Multiagent chemotherapy | 16 | 1.19 (0.30–6.47) | 0.822 | ||
| Chemotherapy recommended, unknown if administered | 7 | – | – | ||
|
| |||||
| Firth’s penalized maximum likelihood estimation was used. | |||||
Abbreviations: CI, confidence interval; HR, hazard ratio; NOS, not otherwise specified; OS, overall survival.
3.2 |. Facility providing care and type of intervention
Patients treated at Academic/Research Programs predicted an improved OS (HR, 0.39; p = 0.008) followed by Comprehensive Community Cancer Programs (HR, 0.45; p = 0.037), and Community Cancer Programs (HR, 0.48; p = 0.164) compared to Integrated Network Cancer Program. Surgery of the primary site was performed in 67 out of 201 patients, of which 25 patients underwent wedge/segmental resection (HR, 0.91; p = 0.893), 15 received lobectomy/extended lobectomy (HR, 1.96; p = 0.345) compared to 21 patients who received a liver transplant. There was no difference in OS between types of surgical resection (p = 0.485). Of these 67 patients, 63 were alive 30 and 90 days after surgery, 2 patients died in less than 30 days and 2 were lost to follow-up. A moderately differentiated grade of tumor was noted in 10 patients (HR, 1.30; p = 0.758), while 3 patients had poorly differentiated/undifferentiated tumors (HR, 2.44; p = 0.368) when compared to 7 patients with well-differentiated tumors. Regional lymph nodes were positive in 17 patients (HR, 1.61; p = 0.497) and negative in 12.
The majority of patients (196 out of 210) did not receive radiation therapy. Among the 14 patients with radiation therapy, 9 had beam radiation (HR, 1.01; p = 0.99) and 4 had radioactive implants (HR, 0.84; p = 0.914) compared to 1 patient with radiation therapy not otherwise specified. Radiation therapy was administered before surgery in one patient and as adjuvant therapy in two patients, with no difference is survival between the groups (p = 0.670). The majority of patients did not receive chemotherapy (145 out of 206). Single-agent chemotherapy was documented in 33 patients and multiagent therapy in 16 patients. Chemotherapy was administered before surgery in three patients, and eight patients received adjuvant chemotherapy. No noticeable survival benefit was noted with respect to the type and sequence of chemotherapy in any group (p = 0.298 and p = 0.383, respectively).
3.3 |. Kaplan–Meier survival analysis of surgical patients
Patients who underwent wedge/segmental resections had the highest 5-year OS of 90.5% (95% CI: 67%–97.5%). This dropped down to 74.6% (95% CI: 44%–90.1%) for the 10-year OS rate. For lobectomy/extended lobectomy, the 5- and 10-year OS rates were the same at 66.5% (95% CI: 31.8%–86.4%) and for liver transplant recipients, the rates were same at 81.0% (95% CI: 56.9%–92.4%). While transplant patients demonstrated an improved 10-year OS, the results did not prove to be statistically significant (p = 0.4851; Figure 2).
FIGURE 2.
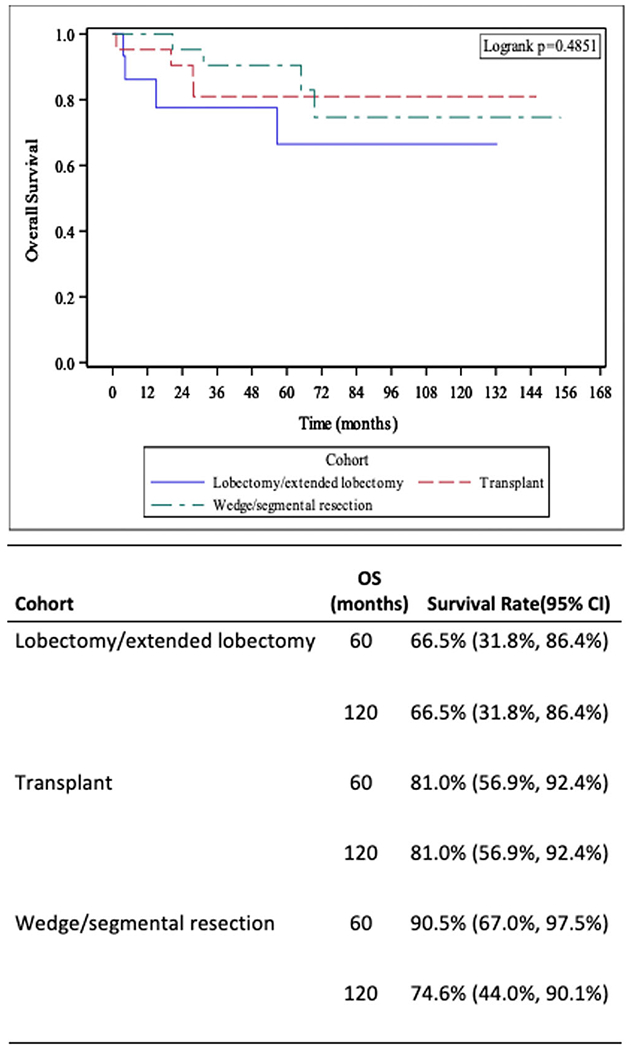
Kaplan–Meier plots demonstrating survival curves by type of surgical resection of primary site (liver)—wedge/segmental resection, hepatectomy lobectomy/extended lobectomy, and liver transplantation. Five- and 10-year overall survival statistics included. CI, confidence interval; OS, overall survival.
4 |. DISCUSSION
The purpose of this study was to examine the NCDB for all cases of HEH to describe patient characteristics and current management trends in the United States and analyze outcomes in a large cohort of patients with this rare disease. Through our analysis, we found that HEH occurred more commonly in women than men, with a male to female ratio of 2:3, consistent with existing data.8 Patients above the age of 55 and Asian ethnicity had an association with worse OS. None of the treatment results elucidated a strategic method to improve survival in these patients.
In our analysis, surgery of the primary site was the most common form of treatment seen in 33% of patients in this study. Wedge or segmental resection was seen in 40% of this population, followed by transplant (33%) and lobectomy/extended lobectomy (27%). There was no significant difference in OS between these cohorts. A literature review by Mehrabi et al.,8 between 1984 and 2005, revealed liver transplantation to be the most common surgical treatment modality (83%) followed by liver resection (17%). This demonstrates a substantial change in trend with current strategies favoring wedge or segmental resection, compared to transplantation. This may reflect the negative impact of prolonged immuno-suppressive therapy following transplantation or scarce availability of donors. However, multifocal presentation seen in the majority of patients with HEH may be a limiting factor for liver resection, and in such situations transplantation has proven to have favorable long-term outcomes,13–15 despite extrahepatic disease as well as lymph node involvement.14 Both wedge/segmental resection and liver transplantation had acceptable 5-year survival rates (90% and 81%, respectively) in our study.
Nonsurgical treatment options like chemotherapy were administered in 26% of patients in our analysis. Single and multiagent chemotherapeutic strategies were tried but no significant difference in survival was noted between the groups. Two case reports by Lakkis et al.,16 showed the benefits of cyclophosphamide-based metronomic chemotherapy, reporting a partial radiological response. Multifactorial modes of action, including immunological and antiangiogenic functions, were attributed to this response. HEH is a vascular tumor and antiangiogenic agents like thalidomide have been tested to show mixed results.17–20 A phase 2 study by Chevreau et al.21 on sorafenib, showed a 9-month progression-free rate of only 30.7%. A study on immunotherapy, highlighting the use of interferon-alpha 2b (IFN-a 2b) on 42 patients, showed a partial and complete response in 47.6% and 4.8% of the patients, respectively with encouraging 1-, 3-, and 5-year progression-free survival rates of 81.0%, 69.2%, and 62.3%, respectively.22 The use of mammalian target of rapamycin inhibitor, sirolimus, proved to be beneficial in the treatment of HEH in children and adults.23,24 Drugs such as propranolol/prednisolone have been tried in infantile HEH with 56% of the patients doing well while 44% needing salvage therapy.25 Only 7% of the patients in this NCDB review received radiation therapy with no significant impact on OS. A case report of palliative radiation therapy in a patient with multiple HEH lesions resulted in complete metabolic response of treated nodules.26 The limited literature restricts our understanding of the impact of radiation in the management of these tumors. While radiofrequency ablation has shown promise,27 the lack of proper management guidelines has made surgery a more viable option along with liver transplantation for multilobar and metastatic disease.28
Our study has several limitations. First, the retrospective nature of this study can lead to a selection bias. Additionally, the NCDB is limited in longitudinal data like disease-free survival. Clinically relevant information regarding patient comorbidities, exact tumor location, details of systemic therapy (name, dose, frequency, and duration), drug toxicity, complications during treatment, and cause of death is not captured in the NCDB. Missing data is another drawback of the NCDB. Despite these limitations, our study is the first large descriptive analysis of HEH patients, analyzing patient characteristics, available treatment strategies, and outcomes.
5 |. CONCLUSION
HEH has a better prognosis compared to other hepatic malignancies. Our descriptive analysis identifies patient characteristics and treatment strategies that may help improve understanding of this rare disease, and potentially lead to collaboration between institutions. Both wedge/segmental resection and liver transplantation have shown reasonable 5-year survival rates. New treatment strategies with antiangiogenic agents and immunotherapy have demonstrated promise but require more clinical investigation.
ACKNOWLEDGMENTS
Research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. This study was supported in part by the Contardi Research Fellowship.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Ellis GL, Kratochvil FJ 3rd. Epithelioid hemangioendothelioma of the head and neck: a clinicopathologic report of twelve cases. Oral Surg Oral Med Oral Pathol. 1986;61:61–68. [DOI] [PubMed] [Google Scholar]
- 2.Marchiano D, Fisher F, Hofstetter S. Epithelioid hemangioendothelioma of the heart with distant metastases. A case report and literature review. J Cardiovasc Surg. 1993;34:529–533. [PubMed] [Google Scholar]
- 3.Tiu CM, Chou YH, Wang HT, Chang T. Epithelioid hemangioendothelioma of spleen with intrasplenic metastasis: ultrasound and computed-tomography appearance. Comput Med Imaging Graph. 1992;16:287–290. [DOI] [PubMed] [Google Scholar]
- 4.Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol. 1986;3:259–287. [PubMed] [Google Scholar]
- 5.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. [DOI] [PubMed] [Google Scholar]
- 6.Requena L, Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30:29–44. [DOI] [PubMed] [Google Scholar]
- 7.Kou K, Chen YG, Zhou JP, et al. Hepatic epithelioid hemangioendothelioma: update on diagnosis and therapy. World J Clin Cases. 2020;8:3978–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma. Cancer. 2006;107:2108–2121. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Cui M-Y, Xiong J, et al. Spectrum of appearances on CT and MRI of hepatic epithelioid hemangioendothelioma. BMC Gastroenterol. 2015;15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alomari AI The lollipop sign: a new cross-sectional sign of hepatic epithelioid hemangioendothelioma. Eur J Radiol. 2006;59:460–464. [DOI] [PubMed] [Google Scholar]
- 11.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562–582. [DOI] [PubMed] [Google Scholar]
- 12.American College of Surgeons, National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb
- 13.Agrawal N, Parajuli S, Zhao P, et al. Liver transplantation in the management of hepatic epithelioid hemangioendothelioma: a single-center experience and review of the literature. Transplant Proc. 2011;43:2647–2650. [DOI] [PubMed] [Google Scholar]
- 14.Lerut JP, Orlando G, Adam R, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949–957. Discussion 957. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez JA, Becker NS, O’Mahony CA, Goss JA, Aloia TA. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg. 2008;12:110–116. [DOI] [PubMed] [Google Scholar]
- 16.Lakkis Z, Kim S, Delabrousse E, et al. Metronomic cyclophosphamide: an alternative treatment for hepatic epithelioid hemangioendothelioma. J Hepatol. 2013;58:1254–1257. [DOI] [PubMed] [Google Scholar]
- 17.Bölke E, Gripp S, Peiper M, et al. Multifocal epithelioid hemangioendothelioma: case report of a clinical chamaeleon. Eur J Med Res. 2006;11:462–466. [PubMed] [Google Scholar]
- 18.Mascarenhas RC, Sanghvi AN, Friedlander L, Geyer SJ, Beasley HS, Van Thiel DH. Thalidomide inhibits the growth and progression of hepatic epithelioid hemangioendothelioma. Oncology. 2004;67:471–475. [DOI] [PubMed] [Google Scholar]
- 19.Raphael C, Hudson E, Williams L, Lester JF, Savage PM. Successful treatment of metastatic hepatic epithelioid hemangioendothelioma with thalidomide: a case report. J Med Case Rep. 2010;4:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soape MP, Verma R, Payne JD, Wachtel M, Hardwicke F, Cobos E. Treatment of hepatic epithelioid hemangioendothelioma: finding uses for thalidomide in a new era of medicine. Case Rep Gastrointest Med. 2015;2015:326795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevreau C, Le Cesne A, Ray-Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO). Cancer. 2013;119:2639–2644. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Zhang Z, Huang J, Tan H, Yang Z. Efficacy and safety of interferon-alpha 2b for patients with hepatic epithelioid hemangioendothelioma: outcomes of a case-series analysis. Cancer Manag Res. 2021;13:8273–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel ER, Cournoyer E, Adams DM, Stapleton S. A retrospective review of the use of sirolimus for pediatric patients with epithelioid hemangioendothelioma. J Pediatr Hematol Oncol. 2020;42:e826–e829. [DOI] [PubMed] [Google Scholar]
- 24.Stacchiotti S, Provenzano S, Dagrada G, et al. Sirolimus in advanced epithelioid hemangioendothelioma: a retrospective case-series analysis from the Italian Rare Cancer Network Database. Ann Surg Oncol. 2016;23:2735–2744. [DOI] [PubMed] [Google Scholar]
- 25.Emad A, Fadel S, El M, et al. Outcome of children treated for infantile hepatic hemangioendothelioma. J Pediatr Hematol Oncol. 2020;42:126–130. [DOI] [PubMed] [Google Scholar]
- 26.Suga K, Kawakami Y, Hiyama A, Hori K. F-18 FDG PET/CT monitoring of radiation therapeutic effect in hepatic epithelioid hemangioendothelioma. Clin Nucl Med. 2009;34:199–202. [DOI] [PubMed] [Google Scholar]
- 27.Kamarajah SK, Robinson D, Littler P, White SA. Small, incidental hepatic epithelioid haemangioendothelioma the role of ablative therapy in borderline patients. J Surg Case Rep. 2018;2018:rjy223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emamaullee JA, Edgar R, Toso C, et al. Vascular endothelial growth factor expression in hepatic epithelioid hemangioendothelioma: implications for treatment and surgical management. Liver Transpl. 2010;16:191–197. [DOI] [PubMed] [Google Scholar]


