Abstract
The most characteristic features of the Lyme disease pathogens, the Borrelia burgdorferi sensu lato (s.l.) group, are their ability to invade tissues and to circumvent the immune defenses of the host for extended periods of time, despite elevated levels of borrelia-specific antibodies in serum and other body fluids. Our aim in the present study was to determine whether B. burgdorferi is able to interfere with complement (C) at the level of C3 by accelerating C3b inactivation and thus to inhibit the amplification of the C cascade. Strains belonging to different genospecies (Borrelia garinii, B. burgdorferi sensu stricto, and Borrelia afzelii) were compared for their sensitivities to normal human serum and abilities to promote factor I-mediated C3b degradation. B. burgdorferi sensu stricto and B. afzelii strains were found to be serum resistant. When the spirochetes were incubated with radiolabeled C3b, factor I-mediated degradation of C3b was observed in the presence of C-resistant B. afzelii (n = 3) and B. burgdorferi sensu stricto (n = 1) strains but not in the presence of C-sensitive B. garinii (n = 7) strains or control bacteria (Escherichia coli, Staphylococcus aureus, and Enterococcus faecalis). Immunoblotting and radioligand binding analyses showed that the C-resistant strains had the capacity to acquire the C inhibitors factor H and factor H-like protein 1 (FHL-1) from growth medium and human serum. A novel surface protein with an apparent molecular mass of 35 kDa was found to preferentially bind to the N terminus region of factor H. Thus, the serum-resistant B. burgdorferi s.l. strains can circumvent C attack by binding the C inhibitors factor H and FHL-1 to their surfaces and promoting factor I-mediated C3b degradation.
The Borrelia burgdorferi sensu lato (s.l.) group of spirochetes are the causative agents of Lyme disease, a vector-borne illness with a widespread distribution in the Northern hemisphere. Lyme disease occurs at least in Europe, North America, and Asia and is transmitted by locally occurring ticks of the Ixodes ricinus complex (29). Progression of the infection can be divided into localized (I), disseminated (II), and persistent (III) stages (28), where patients at the localized stage usually present with erythema migrans and fever. The second stage of infection has more-variable clinical manifestations, with specific organs being infected. Disorders of the central nervous system, heart, liver, spleen, and eye have all been described in conjunction with disseminated Lyme borreliosis. In the third stage, nonpurulent arthritis is the most common manifestation but other immunological phenomena and acrodermatitis chronica atrophicans are occasionally observed. Three genospecies of the B. burgdorferi s.l. group have been identified (3). They seem to prefer different organs and, thus, cause different clinical manifestations in the disseminated and persistent stages. Borrelia garinii is often associated with neuroborreliosis, and B. burgdorferi sensu stricto (s.s.) is often associated with arthritis, whereas Borrelia afzelii seems to cause acrodermatitis chronica atrophicans more often than the other genospecies (1, 2, 32). Overall, the Lyme disease spirochetes are characterized by their ability to invade tissues and evade host immune defenses for extended periods of time despite the appearance of antibodies in serum and other body fluids.
The complement system is one of the most versatile parts of the immune system. In general, most microbes activate the complement system, which eventually leads to phagocytosis of the target and/or formation of the membrane attack complexes (MAC). The MAC can usually be effectively assembled only on the surfaces of gram-negative bacteria that have an outer cell membrane. In both the classical and the alternative pathways the most central step in complement activation is generation of C3b. C3b is a ligand for immune adhesion and immune complex clearance as well as a subunit in the alternative-pathway C3/C5 convertase, C3bBb, and in the classical-pathway C5 convertase, C4b2a3b. Considerable amplification of the complement cascade occurs at the C3 level by cleavage of C3 to C3b by C3bBb. The newly formed C3b binds factor B. C3bBb generated after cleavage of B to Bb makes this step a self-amplifying process that leads to efficient C3b opsonization. In addition, by converting C5 into C5b the C5 convertases start the downstream cascade that leads to MAC formation and lysis of susceptible cells.
Considering the characteristic features of B. burgdorferi infection it is obvious that the B. burgdorferi s.l. spirochetes must somehow avoid complement attack. In the tick gut the spirochetes may be protected by complement-inhibiting factors in tick saliva, which prevent C3b deposition on complement-activating surfaces (25). However, upon invasion of subcutaneous tissues and in disseminated infection Borrelia spirochetes must have other means of avoiding the complement system. Several microbes are known to interfere with complement at the C3b level, for example, by accelerating decay of the C3 convertase enzymes (Trypanosoma cruzi) (31), by promoting C3b inactivation (vaccinia virus complement control protein, VCP, or herpes simplex virus type 1) (14, 15, 17), or by binding host complement regulators onto their own cell surfaces (e.g., Streptococcus pyogenes binding host factor H [8]). Host cells protect themselves from complement-mediated destruction with the help of membrane regulators complement receptor type 1 (CR1; CD35), membrane cofactor protein (MCP; CD46), and decay accelerating factor (DAF; CD55) (19) and by binding plasma protein factor H or factor H-like protein 1 (FHL-1) to their membranes (18). Factor H is the most important soluble regulator of the alternative pathway. It can (i) act as a cofactor for serine protease factor I in C3b inactivation, (ii) replace Bb from the C3bBb convertase, and (iii) compete with factor B for binding to C3b. In performing these functions factor H uses as many as three distinct binding sites for C3b (10, 27) and several binding sites for glycosaminoglycans and/or sialic acids. Multiple interactions between surface-bound C3b and factor H are needed for discrimination between alternative-pathway activators (e.g., microbes) and nonactivators (host cells).
In an earlier study it was observed that B. garinii strains are sensitive to the bactericidal activity of serum (i.e., complement sensitive), whereas the B. burgdorferi s.s. strains are only partially sensitive and the B. afzelii strains are resistant (4). These findings are correlated with the susceptibility of these strains to complement-dependent phagocytosis in human serum (30). Our aim in this study was to determine whether bacterial control of the alternative complement pathway might contribute to the pathogenicity of B. burgdorferi s.l. To this end we analyzed the complement sensitivities of different genospecies of B. burgdorferi s.l. and their effects on C3b degradation in vitro. We observed that B. afzelii and B. burgdorferi s.s. strains, which were complement resistant in a serum immobilization assay, promoted factor I-mediated inactivation of C3b while the serum-sensitive B. garinii strains did not. The underlying mechanism was revealed when we observed that the complement-resistant Borrelia strains had acquired complement inhibitors factor H and FHL-1 from serum containing growth media. B. burgdorferi s.s. and B. afzelii bound these soluble complement inhibitors from serum by a surface protein with a molecular mass of 35 kDa. Acceleration of C3b inactivation by acquisition of soluble complement regulators factor H and FHL-1 is thus probably an important immune evasion mechanism of serum-resistant strains of B. burgdorferi.
MATERIALS AND METHODS
Spirochetal strains.
Eleven low-passage (n < 15) B. burgdorferi s.l. strains were used in this study. All strains were obtained from The National Public Health Institute, Department in Turku (NPHI). B. burgdorferi s.s. strain ia was isolated by the NPHI from one of the first culture-confirmed Lyme disease patients in Finland (M. K. Viljanen, J. Oksi, P. Salomaa, M. Skurnik, R. Peltonen, and H. Kalimo, Letter, J. Infect. Dis. 165:596–597, 1992). Three B. afzelii strains (A91, 570, and 1082) were used. A91 and 1082 were patient isolates from Turku, Finland, and 570 was isolated from a tick caught in Helsinki in 1996. The B. garinii strains (3/96, 5/96, 13/96, 28/97, 40/97, 46/97, and 50/97) were isolated in 1996 and 1997 from erythema migrans lesions of patients in the archipelago of Turunmaa close to Turku.
Spirochetes were cultured in BSK-H medium (Sigma Chemicals, St. Louis, Mo.) at 33°C in a 5% CO2 atmosphere. The medium contained 6% heat-inactivated rabbit serum. Growth was maintained for 2 weeks or until the culture had reached a density of at least 107 bacteria/ml, as calculated by dark-field microscopy. The bacteria were centrifuged (8,000 × g) and washed extensively with Veronal-buffered saline (VBS), pH 7.4. The final concentration was adjusted to 109 bacteria/ml.
Serum sensitivity test.
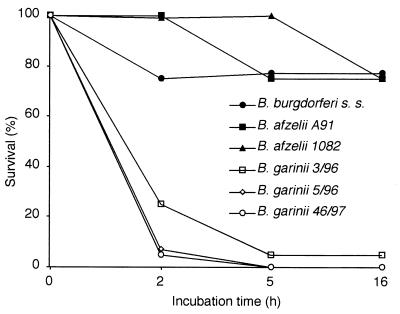
Borrelia bacteria were cultured until a density of at least 107/ml was reached. The concentration of bacteria was estimated by counting 10 microscope fields at ×40 magnification. Serum from a healthy laboratory worker without B. burgdorferi antibodies was extracted by drawing blood and letting it stand for 30 min at room temperature. Subsequently the blood was centrifuged and the serum was aliquoted and frozen (−70°C). For the serum sensitivity test the reaction mixtures were pipetted to a volume of 100 μl. Bacteria (50 μl/reaction mixture) were centrifuged (6,000 × g) and washed three times with BSK-II buffer. Four different reaction mixtures were set up for each strain: 10, 20, and 40% normal human serum (NHS) and heat-inactivated serum. Serum was diluted with the BSK-II medium. The mixtures were incubated at 37°C for a total of 16 h, and 10-μl aliquots were removed for analysis at 2, 5, and 16 h. The aliquots were pipetted onto microscope slides and analyzed using an Olympus microscope with dark field. The number of bacteria and the percentages of live bacteria were estimated and categorized into one of the following groups: 100, 75, 25, 5, or 0% live bacteria at each time point. The strains were classified as either sensitive or resistant based on the end point category at 16 h (Fig. 1).
FIG. 1.
Analysis of serum resistance of B. burgdorferi s.l. strains. The indicated strains were incubated in nonimmune human serum at 37°C for 16 h. The survival of bacteria at specified time points was calculated as the percentage of motile, live bacteria by dark-field microscopy. The different B. burgdorferi strains fell into two distinct categories. While the B. burgdorferi s.s. and B. afzelii strains were resistant to serum killing, all the tested B. garinii strains were classified as serum sensitive.
Complement components.
Factor I used in the experiments was either supplied by Calbiochem-Novabiochem (La Jolla, Calif.) or purified from human plasma as described previously (13). Factor H and C3 were purified from human plasma (18), and C3b was generated from C3 with factors B and D in the presence of Ni2+ ions as described previously (12, 13). Recombinant FHL-1 was kindly provided by Jens Hellwage and Peter Zipfel (6). C4b was purchased from Quidel Corp. (La Jolla, Calif.), and C4bp was provided by Anna Blom (University of Lund, Lund, Sweden). C3b, C4b, factor H, and FHL-1 were radiolabeled with 125I using the Iodogen method (26) (Pierce Chemical Group, Rockford, Ill.).
Factor I cofactor assay.
In the optimized factor I cofactor assay the bacteria (approximately 4 × 107) were incubated with factor I (500 ng) and radiolabeled C3b (50,000 cpm) at 37°C for 1.5 h in 40 μl of VBS containing 0.1% gelatin. Gelatin was present in the reaction mixture to prevent adhesion of the reactants to surfaces of the reaction tube. After incubation the samples were run on sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis (SDS–8% PAGE) gels under reducing conditions. The gel was fixed in 5% acetic acid for 30 min, dried, and autoradiographed using the Fujifilm BAS 2500 instrument (Fuji Photo Film, Tokyo, Japan).
In controls, the C3b degradation assay was carried out in the absence of Borrelia bacteria or in the presence of selected strains of Escherichia coli, Staphylococcus aureus, and Enterococcus faecalis instead of B. burgdorferi. The control bacteria were isolated at the Helsinki University Central Hospital diagnostics laboratory and were selected randomly for the assay. The positive control mixture consisted of factor H (5 or 0.5 μg/ml), factor I, and 125I-C3b. In addition, a sample of 125I-C3b or 125I-C4b without factors I and H was used to monitor for any spontaneous degradation under the experimental conditions.
Immunoblotting analysis of factor H binding.
Factor H binding by the strains used in the cofactor assay was analyzed by an immunoblotting assay. Two different preparations of B. afzelii A91, two B. garinii strains (3/96 and 46/97), and B. burgdorferi s.s. ia that had been grown in the BSK-H medium and washed three times with VBS were mixed with a reducing SDS-PAGE sample buffer. The samples containing 107 bacteria/ml were incubated at 37°C for 20 min on a shaker, and aliquots were run on an SDS–8% PAGE gel. The samples were electrotransferred onto a nitrocellulose membrane, and the membrane was blocked with 5% fat-free milk in phosphate-buffered saline (PBS). Polyclonal goat anti-factor H antibody (1:5,000 dilution) was added, and the membrane was incubated for 12 h at 4°C. The membrane was washed five times, and a secondary peroxidase-conjugated donkey anti-goat antibody (diluted 1:5,000 in 1% bovine serum albumin-PBS) was added and incubated with the membrane for 60 min at room temperature on a shaker. The membrane was washed with PBS, and positive reactions were visualized by the electrochemiluminescence method (Amersham, Little Chalfont, United Kingdom).
Analysis of factor H and FHL-1 binding by ligand blotting.
Outer membranes (OM) and protoplasmic cylinders (PC) of one strain of each genospecies of Borrelia bacteria were isolated using a method described previously (21). The OM and PC specimens were run on an SDS–8% PAGE gel under nonreducing conditions and transferred onto a nitrocellulose membrane. The membranes were blocked with 5% fat-free milk in PBS for 60 min at room temperature. 125I-labeled factor H and FHL-1 (106 cpm/membrane) were incubated on the membranes for 24 h at 4°C on a shaker. The membranes were washed five times with PBS and analyzed by autoradiography.
RESULTS
Serum sensitivity testing of Borrelia species.
The serum sensitivities of various strains of B. burgdorferi were tested by incubating (37°C) the bacteria in 10, 20, and 40% NHS for 16 h. Samples from the reaction mixtures were taken at 2, 5, and 16 h for microscopy, and the percentages of live bacteria were estimated. B. burgdorferi s.s. ia, B. afzelii 1082, and B. afzelii A91 were classified as resistant, and B. garinii 3/96, 5/96, and 46/97 were classified as sensitive, at the 16-h endpoint (Fig. 1).
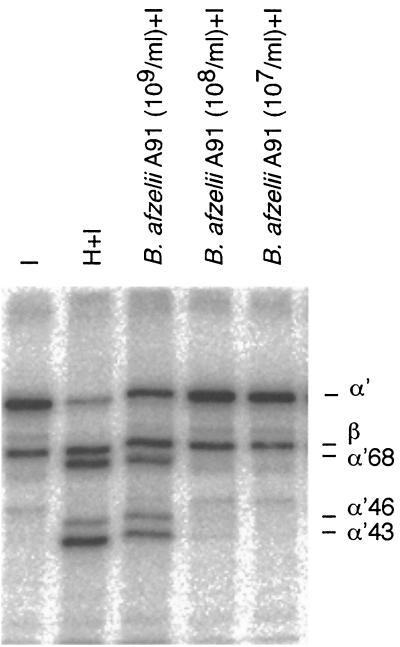
With regard to the differences in serum sensitivity we next compared the promotion of C3b cleavage by the various Borrelia genospecies. When 125I-C3b was incubated for 1.5 h at 37°C with B. afzelii (A91) and factor I, a distinct C3b degradation pattern was observed (Fig. 2). The α′ chain of C3b became cleaved into 68-, 46-, and 43-kDa fragments. The amount of cleavage product increased with an increasing concentration of bacteria (Fig. 2), and the pattern of cleavage resembled that obtained with factors H and I. In the absence of factor I or bacteria, no cleavage of C3b took place. As controls we tested other bacteria that had been isolated at our diagnostics laboratory. Neither E. faecalis (n = 1), S. aureus (n = 1), nor E. coli (n = 1) exhibited C3b-degrading activity in the presence or absence of factor I (not shown).
FIG. 2.
SDS-PAGE and autoradiography analysis of C3b cleavage by B. afzelii strain A91. Various amounts of bacteria (lanes 3 to 5 [numbering from the left]: 109, 108, and 107/ml, respectively) grown in BSK-H medium were incubated in a final volume of 40 μl for 1.5 h at 37°C with radiolabeled C3b (1.25 μg/ml; 106 cpm/μg) in the presence of factor I (12.5 μg/ml). Lane 1, negative control with 125I-C3b plus I; lane 2, positive control (125I-C3b, factor I, and 0.5 μg of factor H/ml). Note the cleavage of the α′ chain of C3b into fragments representing those of iC3B (lane 3). The image was produced using the Fujifilm BAS 2500 instrumentation and MacBAS, version 2.5, and Adobe Photoshop, version 5.5, software.
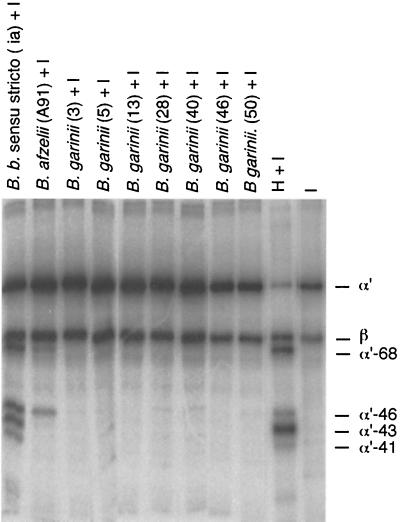
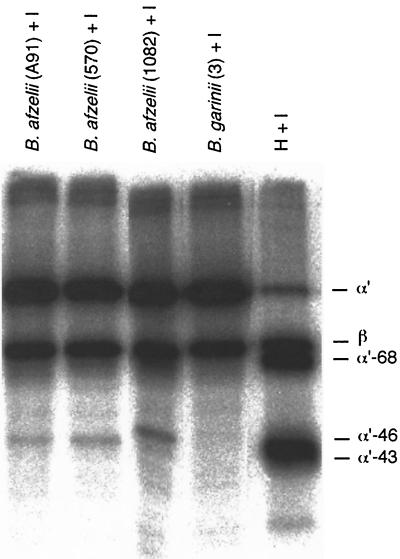
When a larger panel of Borrelia strains was tested, it was observed that B. burgdorferi s.s. (ia) and B. afzelii strains (A91, 570, and 1082) promoted cleavage of C3b in the cofactor assay, whereas the seven strains of B. garinii (strains 3/96, 5/96, 13/96, 28/97, 40/97, 46/97, and 50/97) did not (Fig. 3 and 4). A similar cleavage pattern was observed with all B. afzelii strains (Fig. 4). The cleavage pattern obtained with B. burgdorferi s.s. was somewhat different from that obtained with the B. afzelii strains. Incubation of the B. burgdorferi s.s. ia strain with 125I-C3b and factor I produced a fourth α′-chain 41-kDa band in addition to the 68-, 46-, and 43-kDa bands. This cleavage pattern possibly indicated a more efficient C3b inactivation by B. burgdorferi s.s. than by B. afzelii. No cleavage occurred with either B. burgdorferi s.s. or B. afzelii in the absence of factor I, indicating that the cleavage was not due to a protease on the borrelial surface. The concentration of bacteria that induced C3b cleavage was about 108 bacteria/ml, and the concentration of factor H used in controls was approximately 5 μg/ml. At the latter concentration factor H with I produced almost complete cleavage of 125I-C3b (50,000 cpm) during the 1.5-h incubation period.
FIG. 3.
Comparison of levels of C3b cleavage by factor I (12.5 μg/ml) in the presence of the three genospecies of B. burgdorferi s.l. grown in BSK-H medium. The C3b inactivation-promoting activity for factor I was tested with approximately 109 bacteria/ml. Lanes 1 and 2 (numbering from the left), cleavage of C3b produced by B. burgdorferi (B. b.) s.s. ia and B. afzelii A91, respectively. Under the conditions used, the cleavage of 125I-C3b progressed to a different degree in the presence of factor H (lane 10; 5 μg/ml) and the two strains. Unlike the other strains tested, none of the B. garinii strains (3/96, 5/96, 13/96, 28/97, 40/97, 46/97, and 50/97) promoted 125I-C3b cleavage. The image was produced as in Fig. 2.
FIG. 4.
Analysis of 125I-C3b cleavage by three B. afzelii strains grown in BSK-H. All three strains (A91, 570, and 1082) yielded weak but similar C3b cleavage patterns with factor I (12.5 μg/ml). No cleavage of C3b with B. garinii was observed. The positive control shows 125I-C3b cleavage with factors H (5 μg/ml) and I. The image was produced as in Fig. 2.
Acquisition of factor H and FHL-1 from growth medium by Borrelia.
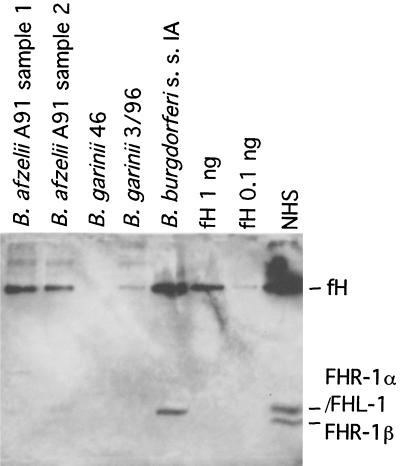
In order to determine the factor responsible for the observed cofactor activity, we tested for the possibility that the bacteria had acquired factor H from their growth media. The presence of factor H was analyzed by immunoblotting using a goat antibody that recognizes both human and rabbit factor H. Two different preparations of B. afzelii A91, two B. garinii strains (3/96 and 46/97), and B. burgdorferi s.s. ia that had been grown in the BSK-H medium were washed and incubated in SDS-PAGE sample buffer and run on an SDS-PAGE gel. Immunoblotting with the polyclonal anti-factor H antibody revealed factor H on the surfaces of B. afzelii A91 and B. burgdorferi s.s. ia strains (Fig. 5). In addition, B. burgdorferi s.s. ia appeared to have bound a lower-molecular-weight protein that corresponded to FHL-1, an alternatively spliced 43-kDa product of the factor H gene. No bound factor H or the putative FHL-1 on either of the B. garinii strains was observed.
FIG. 5.
Uptake of complement factor H and FHL-1 from the growth medium by the different B. burgdorferi strains. Two batches of B. afzelii A91, two strains of B. garinii, and B. burgdorferi s.s. strain ia were grown in BSK-H medium. After being washed three times with VBS the bacterial preparations were run on SDS-PAGE gel and immunoblotted on nitrocellulose with a polyclonal anti-factor H antibody. Purified factor H (1.0 and 0.1 ng on lanes 6 and 7 [lanes are numbered from the left], respectively) and NHS (lane 8) were run as positive controls. Uptake of 150-kDa factor H occurred with the B. afzelii and B. burgdorferi s.s. strains but not with the B. garinii strains. In addition, B. burgdorferi s.s. bound a protein that corresponds to FHL-1. FHL-1 can be separated from the comigrating FHR-1α because FHL-1 runs as a single band at 43 kDa (lane 5) and FHR-1 runs as a doublet at 43 (FHR-1α) and 37 kDa (FHR-1β; lane 8).
Binding of factor H and FHL-1 to borrelial surface proteins.
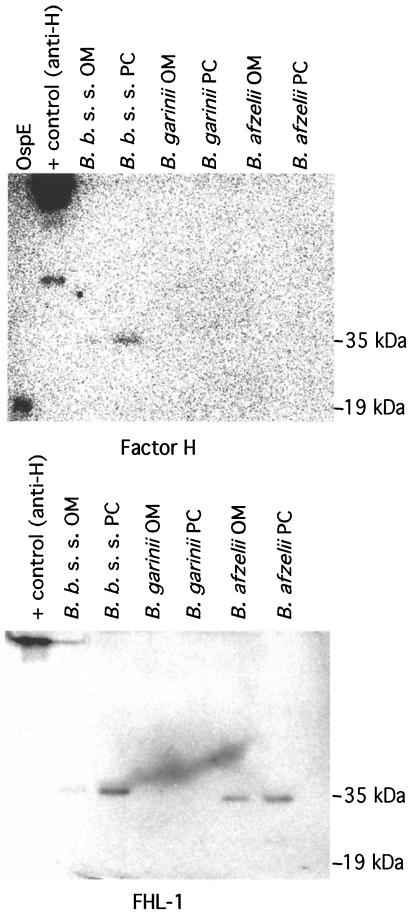
B. burgdorferi s.l. has a two-layer OM with very few proteins on the outermost membrane. In order to localize the factor H binding molecule(s), OM of strains of all three genospecies were isolated by ultracentrifugation through sucrose density gradient columns. OM and PC were collected, run on SDS-PAGE gels, and transferred onto nitrocellulose membranes. Factor H and FHL-1 binding to the OM and PC of the strains representing all three genospecies was analyzed by incubating the membranes with radiolabeled factor H (Fig. 6, top) and FHL-1 (Fig. 6, bottom). Factor H binding to B. burgdorferi s.s. ia OM and PC fractions was observed, suggesting that a factor H binding molecule is present on the OM. The factor H binding protein had an approximate molecular mass of 35 kDa. This protein was distinct from 19-kDa outer surface protein OspE, which in a separate study (7) was identified as a factor H ligand. FHL-1 binding to the 35-kDa protein on B. afzelii A91 OM and PC fractions as well as on the B. burgdorferi s.s. PC fraction was observed. This suggests that a molecule binding FHL-1 can be found on the OM of at least the B. afzelii strains. No binding of either factor H or FHL-1 to B. garinii proteins was observed. Also, no binding of 125I-labeled C4bp to Borrelia bacteria was observed. The results therefore suggest that factor H and FHL-1 selectively bind to the OM of both B. burgdorferi s.s. and B. afzelii strains. This tentatively accounts for the observed differences in serum sensitivity and C3b degradation-promoting activity.
FIG. 6.
Ligand blotting analysis of 125I-factor H and 125I-FHL-1 binding to B. burgdorferi s.l. strains. OM and PC of three strains representing all three genospecies were isolated using ultracentrifugation through sucrose gradients. OM and PC were run on a nonreducing SDS-PAGE gel and electrotransferred onto a nitrocellulose membrane. Factor H binding protein OspE (19 kDa) and a factor H antibody were run as positive controls. The samples were probed with radiolabeled factor H (top) and FHL-1 (bottom). B. burgdorferi (B. b.) s.s. was observed to bind both factor H and FHL-1, whereas B. afzelii A91 bound preferentially FHL-1 and B. garinii bound neither. Factor H and FHL-1 bound to a protein with an approximate molecular mass of 35 kDa which is distinct from the 19-kDa OspE protein.
DISCUSSION
In this study we observed that the complement-resistant B. burgdorferi s.s. and B. afzelii strains can promote C3b inactivation and thereby escape complement attack, whereas the B. garinii strains did not have this activity. The C3b cleavage-promoting activity of the B. burgdorferi s.s. and B. afzelii genospecies was associated with the ability of these strains to bind the soluble complement inhibitors factor H and FHL-1. B. garinii strains did not bind either factor. This suggests that the ability to bind factor H or FHL-1 is protective for the bacteria in serum in vitro and presumably also in the mammalian host and possibly in the tick. The acquisition of factor H from the rabbit serum containing growth medium explains why in vitro-cultivated bacteria had C3b cleavage-promoting activity.
To confirm the localization of the factor H and FHL-1 binding molecule, we isolated OM of three strains representing all three genospecies of B. burgdorferi s.l. Factor H binding to B. burgdorferi s.s. OM could be confirmed as well as the binding of FHL-1 to the OM of B. afzelii. The target molecule for factor H and FHL-1 had an approximate molecular mass of 35 kDa. In a separate study (7) factor H was observed to bind to the 19-kDa OspE outer surface protein of B. burgdorferi s.s. via its C terminus. This, together with the fact that FHL-1, an N-terminally truncated form of factor H, preferentially bound to B. afzelii and recognized a 35-kDa protein, suggests that there are two separate mechanisms for binding factor H and FHL-1 to complement-resistant strains of Borrelia. Apparently, these mechanisms protect the OM of the bacteria against complement attack in the mammalian host. As such the binding of factor H and FHL-1 represents a novel virulence mechanism of B. burgdorferi s.s. and B. afzelii strains.
The binding of factor H and FHL-1 as a virulence mechanism has been reported previously for other bacteria. For example, the M protein of group A streptococci (8) and Hic of pneumococci (9) have been shown to function as factor H binding proteins. By binding factor H or FHL-1 to their surfaces the bacteria can protect themselves by promoting factor I-mediated degradation of C3b. In Neisseria gonorrhoeae a somewhat similar protection mechanism has been described; the sialylated surface lipooligosaccharide or the porin 1A protein binds factor H thus protecting the bacteria against complement attack (22, 23). The binding of factor H and FHL-1 thus represents an important complement resistance mechanism, which is shared by a growing number of microbes.
Bacteria and viruses use different kinds of mechanisms of complement evasion (24). While bacteria mostly seem to bind host complement regulators, viruses utilize endogenous promoters for C3b cleavage. The VCP protein of vaccinia virus acts as a cofactor in C3b degradation (15). This protein contains short consensus repeat (SCR) domains, which are typical structural subunits of mammalian complement regulators, such as factor H, CD46, and CD35. Genes for similar factors have tentatively been identified in the highly pathogenic monkeypox and smallpox viruses (14; S. N. Shchelkunov, A. V. Totmenin, P. F. Safronov, O. I. Ryazankina, V. V. Gutorov, and N. A. Petrov, Abstract, Immunologist 6:412, 1998). Herpes simplex virus type 1 has a protein (gC-1) that lacks SCR domains but that binds to C3b and exhibits decay-accelerating activity for the C3bBb convertase (16). T. cruzi possesses a DAF-like protein, T-DAF, which seems to be crucial in protecting this protozoan parasite against complement attack (31). The facts that the protein synthesis machinery of bacteria is not very good in making proteins with disulfide bonds and that no proteins with significant homology to factor H or MCP/CD46 are present in the borrelial genome (5) suggest that the Borrelia bacteria do not have intrinsic cofactors with an SCR structure.
In our serum sensitivity test, we observed that the B. burgdorferi s.s. and B. afzelii strains were resistant to serum immobilization and vacuolization, whereas all the B. garinii strains were sensitive to these effects of serum. Earlier, Breitner-Ruddock and colleagues observed in their study (4) that B. garinii strains activated the alternative complement pathway and became lysed in nonimmune human serum, whereas the B. afzelii strains did not. However, this was not due to differences in C3 deposition, that is, both strains activated the complement system. Kochi et al. also observed that B. burgdorferi s.s. strain 297 was killed by the classical complement pathway in the presence of antibody but not via the alternative pathway (11). Our results indicate that the preferential activity to bind factor H and FHL-1 and promote C3b degradation by B. afzelii and s.s. strains explains why they are not killed by the alternative pathway. So far, our studies have not shown any evidence for direct control of the classical pathway by borreliae. No binding of C4bp was observed (data not shown). Patarakul et al. suggested in their work that effective MAC-mediated lysis of B. burgdorferi was not achieved because of different C3 acceptor proteins on serum-resistant WT297 and MUT297 strains (20). As pathogens, borreliae are likely to have multiple mechanisms to evade complement attack, including multiple acceptors for factor H and FHL-1 and inhibition of the terminal complement cascade.
On the basis of the present results one could offer an attractive explanation for some of the basic aspects of B. burgdorferi infection. Antibody production is typically marked in Lyme disease but is also markedly ineffective, as evidenced by the fact that the immune system is in many cases incapable of eradicating the bacterium. With reduced or ineffective MAC formation the efficiency of antibody-mediated killing of spirochetes would be decreased. The observed differences in the abilities of the different genospecies to cleave C3b are consistent with the previously reported (4) differences in serum sensitivities, suggesting that the ability to bind factor H and FHL-1 and to promote C3b inactivation is protective in serum and presumably also in the mammalian host. According to Suhonen et al. phagocytosis of B. burgdorferi s.l. is mostly complement mediated (30). The efficacy of phagocytosis of different borrelia genospecies correlated inversely, we observed, with the abilities of different genospecies to cleave C3b. The fact that B. garinii strains are more susceptible to complement than the other strains may be related to their preference for causing borreliosis in the central nervous system, where the amount of complement activity is much smaller than in other compartments of the human body.
In summary, our observations suggest that B. burgdorferi s.s. and B. afzelii strains can evade complement activation by promoting factor I-mediated inactivation of C3b and controlling the amplification of the complement cascade. Complement resistance associates with an ability to bind soluble complement regulators factor H and FHL-1. Neutralization of this resistance may prove useful in the prevention and/or treatment of borreliosis.
ACKNOWLEDGMENTS
We thank The Helsinki Biomedical Graduate School, The Academy of Finland, The Sigrid Jusélius Foundation, The University of Helsinki, and The Helsinki University Central Hospital Research Funds for funding.
We thank Miikka Peltomaa for coordinating the collection of ticks in the Helsinki area, Jens Hellwage and Peter Zipfel for FHL-1, and Anna Blom for C4bp.
REFERENCES
- 1.Anthonissen F M, De Kesel M, Hoet P P, Bigaignon G H. Evidence for the involvement of different genospecies of Borrelia in the clinical outcome of Lyme disease in Belgium. Res Microbiol. 1994;145:327–331. doi: 10.1016/0923-2508(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 2.Assous V M, Postic D, Paul G, Nevot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 3.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Breitner-Ruddock S, Würzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol. 1997;185:253–260. doi: 10.1007/s004300050038. [DOI] [PubMed] [Google Scholar]
- 5.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 6.Hellwage J, Kuhn S, Zipfel P F. The human complement regulatory factor-H-like protein 1, which represents a truncated form of factor H, displays cell-attachment activity. Biochem J. 1997;326:321–327. doi: 10.1042/bj3260321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, Seppala I J, Meri S. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276:8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- 8.Horstmann R D, Sievertsen H J, Knobloch J, Fischetti V A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci USA. 1988;85:1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janulczyk R, Iannelli F, Sjoholm A G, Pozzi G, Bjorck L. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J Biol Chem. 2000;275:37257–37263. doi: 10.1074/jbc.M004572200. [DOI] [PubMed] [Google Scholar]
- 10.Jokiranta S, Hellwage J, Koistinen V, Zipfel P F, Meri S. Each of the three binding sites on complement factor H interacts with a distinct site on C3b. J Biol Chem. 2000;275:27657–27662. doi: 10.1074/jbc.M002903200. [DOI] [PubMed] [Google Scholar]
- 11.Kochi S K, Johnson R C, Dalmasso A P. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi. Role of antibody in formation of an effective membrane attack complex. J Immunol. 1991;146:3964–3970. [PubMed] [Google Scholar]
- 12.Koistinen V. Effect of complement-protein-C3b density on the binding of complement factor H to surface-bound C3b. Biochem J. 1991;280:255–259. doi: 10.1042/bj2800255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koistinen V, Wessberg S, Leikola J. Common binding region of complement factors B, H and CR1 on C3b revealed by monoclonal anti-C3d. Complement Inflamm. 1989;6:270–280. doi: 10.1159/000463102. [DOI] [PubMed] [Google Scholar]
- 14.Kotwal G J. Poxviral mimicry of complement and chemokine system components: what's the end game? Immunol Today. 2000;21:242–248. doi: 10.1016/s0167-5699(00)01606-6. [DOI] [PubMed] [Google Scholar]
- 15.Kotwal G J, Isaacs S N, McKenzie R, Frank M M, Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990;250:827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- 16.Lubinski J, Wang L, Mastellos D, Sahu A, Lambris J D, Friedman H M. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J Exp Med. 1999;190:1637–1646. doi: 10.1084/jem.190.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenzie R, Kotwal G J, Moss B, Hammer C H, Frank M M. Regulation of complement activity by vaccinia virus complement-control protein. J Infect Dis. 1992;166:1245–1250. doi: 10.1093/infdis/166.6.1245. [DOI] [PubMed] [Google Scholar]
- 18.Meri S, Pangburn M K. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci USA. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan B P, Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15:369–396. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- 20.Patarakul K, Cole M F, Hughes C A. Complement resistance in Borrelia burgdorferi strain 297: outer membrane proteins prevent MAC formation at lysis susceptible sites. Microb Pathog. 1999;27:25–41. doi: 10.1006/mpat.1999.0280. [DOI] [PubMed] [Google Scholar]
- 21.Radolf J D, Goldberg M S, Bourell K, Baker S I, Jones J D, Norgard M V. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun. 1995;63:2154–2163. doi: 10.1128/iai.63.6.2154-2163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ram S, McQuillen D P, Gulati S, Elkins C, Pangburn M K, Rice P A. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ram S, Sharma A K, Simpson S D, Gulati S, McQuillen D P, Pangburn M K, Rice P A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rautemaa R, Meri S. Complement-resistance mechanisms of bacteria. Microbes Infect. 1999;1:785–794. doi: 10.1016/s1286-4579(99)80081-1. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro J M. Ixodes dammini: salivary anti-complement activity. Exp Parasitol. 1987;64:347–353. doi: 10.1016/0014-4894(87)90046-4. [DOI] [PubMed] [Google Scholar]
- 26.Salacinski P R, McLean C, Sykes J E, Clement-Jones V V, Lowry P J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha, 6 alpha-diphenyl glycoluril (Iodogen) Anal Biochem. 1981;117:136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A K, Pangburn M K. Identification of three physically and functionally distinct binding sites for C3b in human complement factor H by deletion mutagenesis. Proc Natl Acad Sci USA. 1996;93:10996–11001. doi: 10.1073/pnas.93.20.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 29.Steere A C. Lyme disease: a growing threat to urban populations. Proc Natl Acad Sci USA. 1994;91:2378–2383. doi: 10.1073/pnas.91.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suhonen J, Hartiala K, Tuominen-Gustafsson H, Viljanen M K. Borrelia burgdorferi induced oxidative burst, calcium mobilization, and phagocytosis of human neutrophils are complement dependent. J Infect Dis. 2000;181:195–202. doi: 10.1086/315195. [DOI] [PubMed] [Google Scholar]
- 31.Tambourgi D V, Kipnis T L, da Silva W D, Joiner K A, Sher A, Heath S, Hall B F, Ogden G B. A partial cDNA clone of trypomastigote decay-accelerating factor (T-DAF), a developmentally regulated complement inhibitor of Trypanosoma cruzi, has genetic and functional similarities to the human complement inhibitor DAF. Infect Immun. 1993;61:3656–3663. doi: 10.1128/iai.61.9.3656-3663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]