Abstract
Purpose
Preeclampsia occurs in up to 15% of pregnancies and constitutes a major risk factor for cardiovascular disease. This observational cohort study aimed to examine the association between preeclamptic pregnancies and cardiovascular outcomes as well as primary and specialized care utilization after delivery.
Methods
Using statutory claims data we identified women with singleton live births between 2010 and 2017. Main outcomes included the occurrence of either hypertension or cardiovascular disease after one or more preeclamptic pregnancies, number of contacts to a general practitioner or cardiologist after delivery and prescribed antihypertensive medication. Data were analyzed using Cox proportional hazard regression models adjusted for maternal age, diabetes, dyslipidemia, and obesity.
Results
The study cohort consisted of 181,574 women with 240,698 births. Women who experienced preeclampsia once had an increased risk for cardiovascular (hazard ratio, HR = 1.29) or hypertensive (HR = 4.13) events. In women affected by recurrent preeclampsia, risks were even higher to develop cardiovascular disease (HR = 1.53) or hypertension (HR = 6.01). In the following years after delivery, general practitioners were seen frequently, whereas cardiologists were consulted rarely (0.3 and 2.4%).
Conclusion
Women affected by preeclampsia experience an increased risk of developing chronic hypertension and cardiovascular disease, especially those with recurrent preeclampsia. Future medical guidelines should take this potential risk into account.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00404-022-06561-w.
Keywords: Preeclampsia, Cardiovascular disease, Hypertension, Pregnancy, Primary care
Background
Pregnancy-related hypertensive disorders, including preeclampsia (PE) and gestational hypertension (GH), rank among the most common causes of maternal death and develop in up to 10% of pregnancies [1], with even higher rates of recurrence in up to 15% [2]. PE is defined either by new-onset hypertension and proteinuria after the 20th week of gestation (GW) or, alternatively, by the onset of PE-associated signs encompassing maternal organ dysfunction, even organ failure, or placental dysfunction [3, 4].
PE is presumably caused by placental hypoperfusion and hypoxia owing to abnormal development of the uterine placental spiral arteries. Hence, the emerging inflammatory response can cause endothelial dysfunction and vasoconstriction [5–7].
The pathophysiology of PE in its entirety is not fully understood and findings in the current literature diverge regarding mechanisms underlying future cardiovascular disease (CVD) [8, 9]. Widely discussed is the influence of factors such as obesity, diabetes, dyslipidemia, hypertension, a positive family history, and the occurrence of a metabolic syndrome [10–12]. Better known, however, are the diverse complications associated with PE, which, in turn, are due to the underlying vascular dysfunction [13, 14]. These include in particular placental insufficiency or abruption, intrauterine growth restriction, or intrauterine fetal death [15].
Most of the underlying pathological conditions and symptoms of pregnancy-related hypertensive disorders seem to resolve on their own shortly after birth [8]. Usually, spontaneous resolution of maternal hypertension and proteinuria is observed within the first week postpartum [8, 16].
Nevertheless, previous long-term studies have shown that hypertensive disorders during pregnancy are among the most important risk factors for cardiovascular diseases (CVD) [13, 17, 18]. Long-term cardiovascular adverse outcomes identified in women with a history of hypertensive disorders include the manifestation of chronic hypertension, ischemic heart disease, and stroke [19–21]. Consequently, both the American Heart Association (AHA) and the European Society of Cardiology (ESC) included hypertensive disorders during pregnancy as an official risk factor for CVDs [22, 23]. CVDs, in turn, constitute the leading causes of mortality in women and are responsible for 32–34% of deaths among women [24]. Thus, precautions and preventive measures in terms of lifestyle changes and minimizing underlying risk factors are of major importance [25, 26].
Despite the well-established risk association between a history of PE or GH and long-term complications, which present a major global health burden, current recommendations and guidelines regarding cardiovascular risk assessment, screening for high-risk patients, integrated care concepts for long-term follow-up or preventative intervention programs during the postpartum period are scarce [27]. The German Association of Gynecology and Obstetrics (DGGG) recommends a medical check-up for blood pressure and renal function parameters at three months postpartum and, in particular, a comprehensive explanation of potential risks for women shortly after giving birth [28]. However, the clinical implementation of these guidelines is still insufficient [29]. According to a nationwide study, half of the participating physicians were not even aware of the existing guidelines and did not offer structured follow-up advice for women at risk [8, 30]. Nevertheless, a recently published study has proven evidence for quality improvement in terms of postpartum compliance regarding clinical follow-ups and blood pressure regulation due to a bundled care concept for women with a history of PE [31].
The purpose of this large-scale cohort study is to investigate the risk for long-term cardiovascular maternal outcomes after both, first-time and recurrent PE using claims data from German statutory health insurance. Secondary goal of this study is to analyze the current utilization of primary and specialized care following a PE pregnancy. We hypothesize that postpartum care settings in Germany are insufficient regarding the probable considering impact on cardiovascular lifetime risk caused by preeclampsia.
Materials and methods
Data and study design
Data sets were obtained by searching claims data from the AOK Baden-Wuerttemberg, a major regional German statutory health insurance company, presently covering around 4.5 million people. Regularly insured women who delivered a live infant between January 1, 2010, and December 31, 2017, were included. We analyzed a subsample of mothers whose data could be matched with the data of their child/children (222,779 women with 291,091 births). Pregnancies with a history of hypertensive disorder were identified using ICD-10 codes (International Classification of Diseases). Women who developed CVD (including the entities listed in Table 1 below) or chronic hypertension after delivery were identified. Follow-up data were collected until September 30, 2019.
Table 1.
List of ICD, DRG and OPS codes for disease definitions
| ICD-10 and ATC-codes | DRG codes | |
|---|---|---|
| Delivery outcome | O36.4, O60.1, O60.2, O60.3, O75.7, O80, O81, O82, Z37.-, Z38.- | O01, O02, O60 |
| Preeclampsia (exposure) | O14.- | None |
| Hypertension | I10.-, I11.-, I12.-, I13.-, I15.- | None |
| CVD | I20.-;I121; I22.-; I23.-; I24.-, I25.-, I51.6, I51.8; I51.9 | None |
| Adiposity, overnutrition | E66.-, E67.8, E68, E78.- | None |
| Dyslipidemia | E78.- | None |
| Diabetes | E10.-, E11.-, E12.-, E13.-, E14.-, | None |
| Gestational diabetes | O24 | None |
|
Medication Diuretics Beta-blockers Calcium-channel blocker ACE inhibitors AT-II-receptor antagonist |
C.- C03.- C07.- C08.- C09A.-, C09B C09C.-, C09D |
None |
Additionally, (Official Classification for Operations and Procedures) and DRG (Diagnosis Related Groups) OPS codes were used. A full list of ICD, OPS, and DRG codes is summarized in Table S1. Implausible delivery data were excluded (n = 964) from all analyses due to the miscoding of the quarter of birth in the claims data. We also excluded births of multiples (n = 5872) since they are generally associated with higher peripartum risks regarding fetal and maternal morbidity [32]. Moreover, births with a maternal age < 15 years (n = 23) were excluded as were subjects who were insured with the AOK for less than 40% of the observation period (n = 26,365). Births with preexisting maternal diagnoses of CVD (n = 6611) or hypertension (n = 12,747) (between January 1, 2007 and September 30, 2019) were excluded at baseline. Overall, n = 50,393 births were excluded (in some births more than one exclusion criterion applied) and the final sample consisted of 181,574 women with 240,698 births.
All study data were anonymized and cannot be traced to the study team. Patient identification numbers that were originally used for linking files within the insurance databases were encrypted. The study fulfills the eligibility criteria according to the STROBE guidelines for observational cohort studies. Ethical approval was obtained from the Ethics Committee of Tuebingen University Hospital and the University Medical Faculty.
Study variables
Exposure variables
The main exposure of interest was a history of PE, as defined by the International Classification of Diseases (ICD-10 German modification see Table S1). PE presents one entity of pregnancy-related hypertensive disorders and should be distinguished from chronic and gestational hypertension, defined as the sole presence of hypertension after the 20th GW, as well as from isolated gestational proteinuria, defined as a protein-creatinine ratio > 30 mg/mmol in the absence of hypertension.
For the main analysis, we considered PE as a time-dependent variable so that a woman could contribute pregnancies and person-time to both unexposed and exposed groups during follow-up. Women were considered “unexposed” (1) if they never had a PE pregnancy, or (2) from the date of their first delivery without prior PE until the date of their first PE pregnancy, irrespective of subsequent pregnancy outcomes.
Thus, we finally stratified the births into three strata according to the status of the PE prior risk exposure: (1) no exposure to PE, (2) exposure to one PE pregnancy, and (3) exposure to at least two PE pregnancies.
Outcome variables
The main outcome variables of interest encompass cardiovascular health outcomes following PE pregnancies that were identified using ICD-10 codes. The set of hospitalization endpoints regarding cardiovascular outcomes, including chronic hypertension as well as all clinically relevant diagnoses summarized under the term “cardiovascular disease”, can be found in Table 2. Chronic hypertension is defined as a persistent mean arterial blood pressure over 140/90 mmHg or higher.
Table 2.
Outcome variables: ICD codes
| Category | ICD codes |
|---|---|
| Hypertension | I10.-, I11.-, I12.-, I13.-, I15.- |
|
CVD Angina pectoris Myocardial infarction Recurrent myocardial infarction Acute complications after myocardial infarction Acute and chronic ischemic heart disease Unspecified cardiovascular disease Other heart diseases |
I20.-; I121; I22.-; I23.-; I24.-, I25.-, I51.6, I51.8; I51.9 |
Covariates
Potential confounders included: maternal age, diabetes (preexisting as well as gestational diabetes), dyslipidemia, and obesity. Maternal exposure to obesity encompassed adiposity, overnutrition, and dyslipidemia. Possible confounders were selected according to findings of previous research and expertise [33–35].
Statistical analysis
The statistical analyses were performed using R version 4.0.2 and R-Studio v. 1.3.1056 for Windows (32/64 bit) [36]. The observation period started with the date of delivery and, hence, women were followed until they received one or more diagnoses of the respective ICD codes for CVD or hypertension. Other possible endpoints of the study period included death, migration, change to another insurance company, or end of the study period. Thus, in these survival data (i.e., data regarding the occurrence of time-dependent events and censored data), we aimed to compare risk groups (at least one risk exposure to PE) with a reference group (no risk exposure to PE), adjusted for defined binary and parametric covariates.
Cox proportional hazard regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). In our proportional hazard model, the risk groups (ii & iii) were compared to the reference group of individuals who were not exposed to PE (i). The respective time scale was defined as a number of years postpartum.
The HRs are interpreted as relative risks: an HR = 1 meant that there was no difference in the event rate between a risk group and the reference. An HR > 1 or < 1 meant that the event-rate of a risk group was > 1 or < 1 times the event rate of the reference group, respectively. The model was adjusted for confounders, such as maternal age, diabetes or obesity, and dyslipidemia. We set the critical α-error to α ≤ 0.01. However, as the sample size was extensive, and thus the statistical power to detect even small effects (HRs ≈ 1) amounted near to 1 − β = 1.00, we cannot conclude that an effect had any substantial meaning only because of its statistical significance. Thus, in this study, we focused on the interpretation of the effect sizes, i.e., the HR’s.
Moreover, the distribution of visits to a general practitioner and/or a cardiologist in the outpatient setting, stratified by the occurrence of PE throughout the observation time after birth at the descriptive level.
Results
Sample characteristics
The study population consisted of 181,574 women with 240,698 singleton live births. Mean maternal age at delivery was 30.62 years (standard deviation, SD 5.26 years). The average inter-pregnancy-intervals amounted to 2.75 years (SD 1.24 years). Mean observation time was 4.74 years (SD = 2.27 years). Further characteristics of the study population are listed in Table 3.
Table 3.
Sample characteristics
| f | % | f | % | ||
|---|---|---|---|---|---|
| Number of births | Birth mode | ||||
| 1 | 125,930 | 52.32 | C-Section | 74,231 | 30.84 |
| 2 | 95,024 | 39.48 | Vaginal | 165,363 | 68.70 |
| ≥ 3 | 19,744 | 8.20 | Undefined | 78 | 0.03 |
| Missing | 0 | 0.00 | Missing | 1026 | 0.43 |
| Obesity/dyslipidemia | (Gestational) diabetes | ||||
| False | 196,170 | 81.50 | False | 199,119 | 82.73 |
| True | 44,528 | 18.50 | True | 41,579 | 17.27 |
| Missing | 0 | 0.00 | Missing | 0 | 0.00 |
| Preterms | Preeclampsia | ||||
| False | 225,035 | 93.49 | False | 217,954 | 90.55 |
| True | 15,663 | 6.51 | True | 22,744 | 9.45 |
| Missing | 0 | 0.00 | Missing | 0 | 0.00 |
| Cardiovascular disease | Hypertension | ||||
| False | 235,586 | 97.88 | False | 220,760 | 91.72 |
| True | 5112 | 2.12 | True | 19,938 | 8.28 |
| Missing | 0 | 0.00 | Missing | 0 | 0.00 |
We observed a proportion of 8.40% of women (n = 20,233) with a history of one PE pregnancy and 1.04% of women (n = 2511) with a recurrent diagnosis.
Regarding outcome variables in the timeframe between 2010 and 2019, at least one diagnosis (inpatient and outpatient setting) from the CVD group was detected in 5,112 (2.12%), and hypertension in 19,938 (8.28%) women (Table 4).
Table 4.
Outcome case numbers stratified by occurrence of preeclampsia
| No PE | One PE pregnancy | Two or more PE pregnancies | |
|---|---|---|---|
| Cardiovascular disease | |||
| f | 4418 | 597 | 97 |
| % | 2.03 | 2.95 | 3.86 |
| Hypertension | |||
| f | 12,684 | 6009 | 1245 |
| % | 5.82 | 29.70 | 49.58 |
f = frequency; % = percentage of strata "No PE" n = 217,954; "One PE pregnancy" n = 20,233; "Two or more PE pregnancies" n = 2511
Mean age at initial diagnosis of a CVD was 30.47 years (SD = 5.59 years) and hypertension 30.45 years (SD = 5.59 years).
Regarding our three strata according to the status of the risk exposure, there were
217,954 cases with no exposure to PE.
20,233 cases with one PE pregnancy.
2,511 cases with at least two PE pregnancies.
Main analyses
Compared to women with no risk exposure, women with a history of one PE pregnancy had a 28.8% increased risk of a subsequent CVD (see Table 5). For women, who had two or more PE pregnancies, the increased risk was 52.8%. The effect of obesity was comparable to the effects of the single PE exposures (HR = 1.384, see Table 3). The effects of maternal age and diabetes seemed low (maternal age: HR = 1.035; diabetes: HR = 1.151). The cumulative hazard plot regarding the risk of any subsequent CVD is depicted in Fig. 1.
Table 5.
Cox regressions onto cardiovascular disease and chronic hypertension by risk exposures
| Outcome | Exposure | HR | 95% CI HR | z | p( >|z|) |
|---|---|---|---|---|---|
| Cardiovascular disease | One preeclampsiad | 1.288 | [1.181; 1.405] | 5.727 | < 0.001 |
| Two or more PE pregnanciesd | 1.528 | [1.248; 1.873] | 4.096 | < 0.001 | |
| Maternal age | 1.035 | [1.030; 1.040] | 13.362 | < 0.001 | |
| Diabetes | 1.151 | [1.073; 1.236] | 3.924 | < 0.001 | |
| Obesity | 1.384 | [1.298; 1.476] | 9.866 | < 0.001 | |
| Hypertension | One preeclampsiad | 4.128 | [4.000; 4.261] | 88.080 | < 0.001 |
| Two or more PE pregnanciesd | 6.007 | [5.659; 6.376] | 58.890 | < 0.001 | |
| Maternal age | 1.060 | [1.057; 1.063] | 45.190 | < 0.001 | |
| Diabetes | 1.353 | [1.310; 1.397] | 18.400 | < 0.001 | |
| Obesity | 2.086 | [2.025; 2.150] | 48.110 | < 0.001 |
HR hazard ratio, 95% CI HR 95% confidence interval of hazard ratio, z z-value, p( >|z|) empirical significance level
aN = 240,698
bNumber of events = 5112
cNumber of events = 19,938
dReference group = no exposure to preeclampsia
Fig. 1.
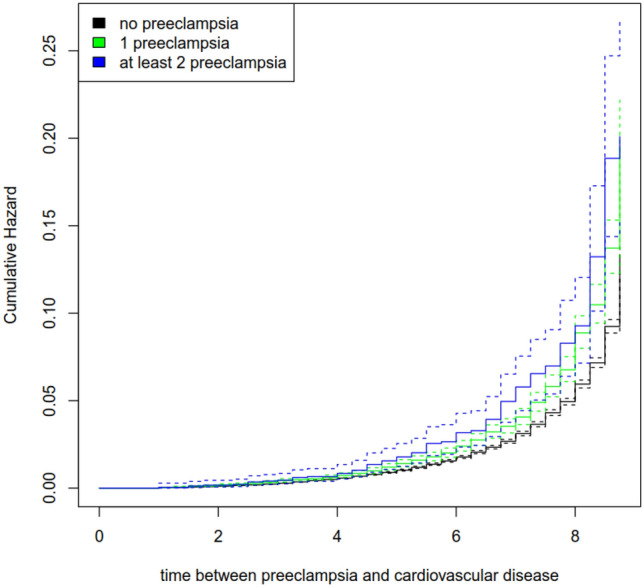
Cumulative hazard plot on the risk of any subsequent CVD
The effects of PE exceeded the respective effects from the prior model for the outcome of hypertension: compared to women with no risk exposure, women with a history of one PE pregnancy had a more than fourfold risk (i.e., an increase in risk by 312.8%) for subsequent, hypertension (see Table 3). For a history of at least two PE pregnancies, the risk was increased by 500.7%, which is more than a sixfold risk compared to women without any risk exposure. Maternal age, diabetes, and obesity had weaker effects, although the effects of diabetes and obesity seemed non-negligible (maternal age: HR = 1.060, diabetes: HR = 1.353, obesity: HR = 2.086) (Fig. 2).
Fig. 2.
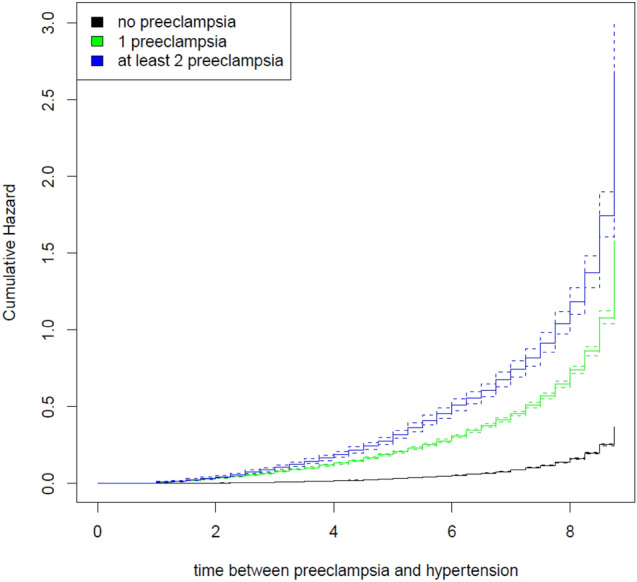
Cumulative hazard plot on the risk of subsequent hypertension
Additional analyses
Medical follow-up in women with a history of one or more PE pregnancies
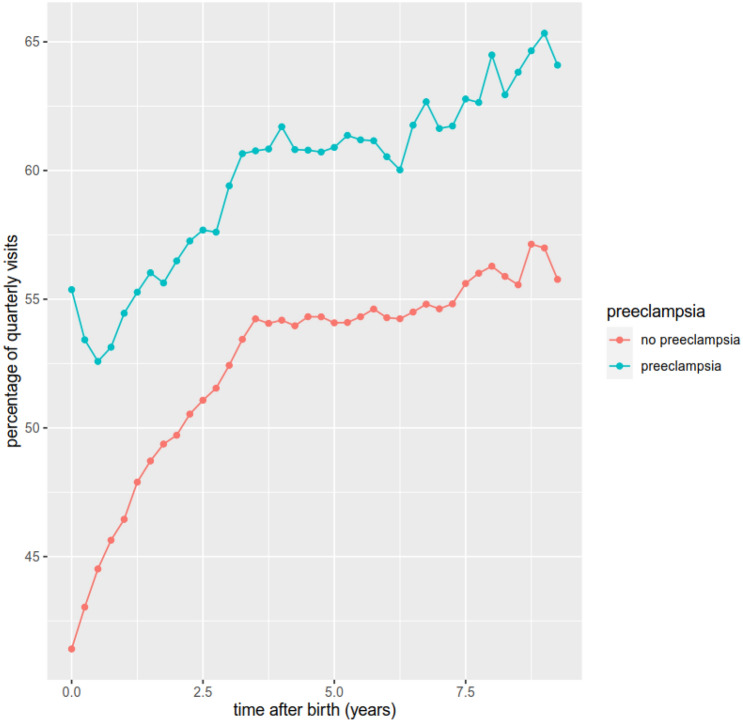
As shown in Fig. 3, the quarterly visits to a general practitioner mainly increase over time after birth. Between 41.4 and 65.3% of patients visit a general practitioner every quarter. The number of visits per quarter are increased in women who experienced a PE pregnancy by between 5.8 and 14.0%, with the highest gap immediately after birth.
Fig. 3.
Visits to general practitioner stratified for the occurrence of PE
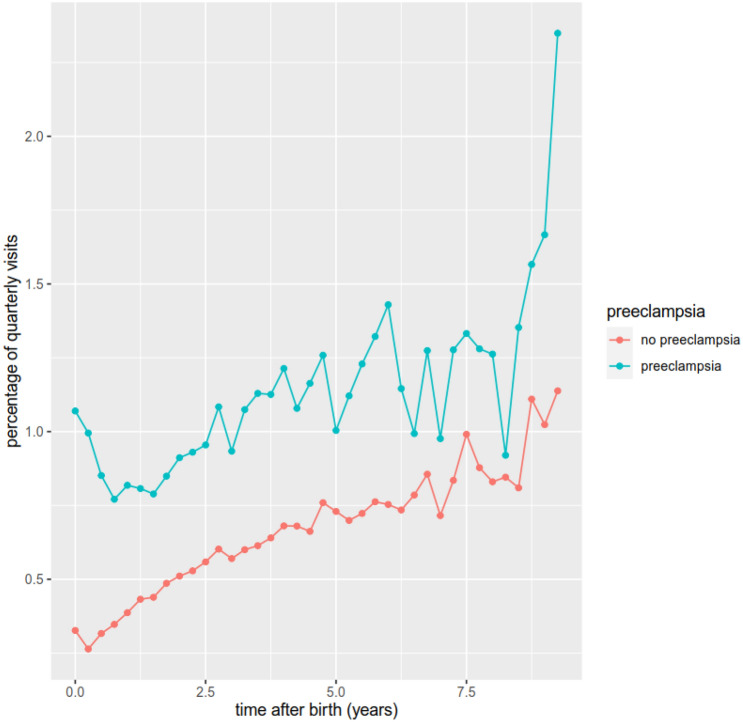
Accordingly, the number of quarterly visits to the cardiologist mainly increased by the time after the birth. However, the quarterly rates of visits to the specialist vary between 0.3 and 2.4% and are disproportionately lower than the rates of visits to the general practitioner. The rate of visits per quarter is generally increased in the group of women who experienced a PE pregnancy, varying between 0.1 and 1.2% with the highest gap at the end of the observation period at 9.25 years. Only 1% of patients who experienced a PE pregnancy consulted an internal specialist as recommended in the guidelines (Fig. 4) [28].
Fig. 4.
Visits to cardiologist stratified for the occurrence of PE
Discussion
Principal findings
To our knowledge, this observational, retrospective cohort study is the first to provide information on current postpartum disease incidence, care utilization, and medication intake in affected women. Our main analyses have shown that the occurrence of at least one PE pregnancy was significantly associated with a greater risk for cardiovascular adverse events and hypertension. The associated detrimental risk was even more pronounced when PE occurred in the second rather than in the first pregnancy. The adjusted risks ranged from 1.32 in one PE pregnancy to 2.03 in two or more for the outcome CVD. Regarding postpartum hypertension, we found HRs of 4.03 and up to 5.06 (two or more PE pregnancies). Thus, our findings are in accordance with previous research confirming PE to be a relevant risk factor for cardiovascular health in the long term and showing similar risk increases [25]. A large epidemiological review by Melchiorre et al. encompassing 87 publications emphasizes the elevated cardiovascular mortality as well and especially the urge for postpartum screening programs that should be initiated as early as possible [13]. In accordance with our findings, Magnussen et al. also observed a risk elevation according to the number of PE pregnancies [11]. Our study results also confirm those of Auger et al., who focused on recurrent PE and the risk of cardiovascular outcomes [37]. The authors also showed a decrease in the time period until the manifestation of the first cardiovascular event compared to women with no history of PE [38, 39].
Regarding postpartum follow-up, our data show that the relative number of medical check-ups decreases to a minimum shortly after giving birth for both general practitioner and cardiologist visits. The difference between affected and non-affected women remains negligible, especially in terms of specialized medical health care, which is rather unfavorable, as the postpartum period represents an important time frame in which an adequate risk management could significantly influence the risk for CVD in the long term [40]. In addition, previous research has shown a relatively low risk perception among women with a history of PE regarding cardiovascular outcomes in the long term [41]. Similar results have been published within the framework of the CVD prevention initiative in North America, showing that only 50% of referred women were seen by a cardiologist postpartum [42]. However, a scoping review published in 2019 reported that not only affected women but also health care providers lack in knowledge regarding long-term cardiovascular outcomes and the need for postpartum follow-up visits [30].
Perspectives
Our results suggest that women with a history of PE should be offered preventive counseling and lifestyle education in the postpartum period to improve access to early interventions and specialized care to optimize long-term outcomes.
Strengths and limitations
As we used claims data in these analyses, the main limitation of our study is the missing adjustment regarding socioeconomic status and smoking. However, previous studies have shown that lifestyle factors do not constitute relevant risk factors for PE as compared to several genetic factors [43].
We could not assess the effect of timing regarding the onset of PE and the potentially different impacts of early- and late-onset PE. Moreover, further research could distinguish between different entities of pregnancy-related hypertensive disorders, including gestational hypertension, proteinuria, or preexisting hypertension, as they may lead to different long-term outcomes according to the current literature. Riise et al., however, found that long-term cardiovascular risks are similar for underlying PE and gestational hypertension, supporting the focus solely on PE in our work [44].
One of the strengths of our study, however, is that preexisting and known risk factors were excluded to examine PE as an independent risk factor insofar as possible. Furthermore, data were collected by insurance claims and not by self-report, which tends to over- or underrate results as diagnoses are stated in the context of billing purposes. The accuracy of diagnosis is guaranteed as far as possible by the use of medical records from a German insurance company. Hence, data could be collected in both the clinical and the outpatient setting, rendering our method of data collection far more reliable than that of other large studies based on self-report or merely on clinical data obtained from the hospital [45, 46]. The large sample size and the volume of observational claims data also render our findings highly precise and reduce potential sources of bias to a minimum.
Furthermore, we also focused on the current postpartum follow-up situation regarding the outpatient care setting in Germany to enhance the need for implementing feasible and cost-effective postpartum screening and developing prevention strategies for women at risk.
Our results are in line with the study of Heidrich et al., who showed that a large proportion of physicians are not aware of current recommendations regarding follow-up after giving birth. Less than 40% of the participating physicians even provided advice to reduce cardiovascular risk[47]. Thus, there is not only a need to establish an effective preventive program in high-risk populations but also to raise awareness among pregnant women and physicians alike regarding the burden of potential CVDs [48].
Conclusion
Women with a history of PE pregnancies have a substantially higher risk of CVD and chronic hypertension in the long term. So far, there is still little knowledge and awareness among affected women and physicians regarding cardiovascular outcomes and existing recommendations for screening women at risk in Germany are scarce. Our findings emphasize the necessity for a structured postpartum disease and prevention management taking the potential adverse outcomes into account. Further research should focus on detecting women at high risk and evaluating the effectiveness of optimized primary care programs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The AOK Baden-Wuerttemberg kindly provided data for the analyses.
Abbreviations
- AHA
American heart association
- ACE
Angiotensin-converting enzyme
- AOK
Allgemeine Ortskrankenkasse (general local health insurance)
- AT
Angiotensin
- ATC
Anatomic therapeutic chemical classification system
- CKD
Chronic kidney disease
- CI
Confidence interval
- CVC
Cardiovascular disease
- DGGG
German association of gynecology and obstetrics
- DRG
Diagnosis related groups
- ESC
European society of cardiology
- ESRD
End-stage renal disease
- GH
Gestational hypertension
- GW
Gestational week
- HR
Hazard ratio
- ICD
International classification of diseases
- OPS
Operation and procedure classification system
- PE
Preeclampsia
- SD
Standard deviation
Author contributions
KH: study concept, data analysis, manuscript writing and editing; MM: data analysis, manuscript writing and editing; RG: data analysis, manuscript writing and editing; MG: data analysis; manuscript editing; AB: study concept, study management; MW: data analysis, manuscript editing; SYB: study concept, manuscript editing; SJ: manuscript editing; MGC: manuscript editing; SHK: manuscript editing; AC: manuscript editing; GK: manuscript editing; FS: data management, manuscript editing; SW: study concept, data analysis, manuscript writing and editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the AOK Baden-Wuerttemberg. The funders had no role in study design and analysis, decision to publish or drafting of the manuscript.
Availability of data and materials
The data that support the findings of this study are available from AOK Baden-Wuerttemberg but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of AOK Baden-Wuerttemberg.
Declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
Ethical approval was obtained from the Ethics Committee of Tuebingen University Medical Faculty and University Hospital.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Hernández-Díaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACOG ACoOaG (2013) Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol 122(5):1122–1131. 10.1097/01.Aog.0000437382.03963.88. [DOI] [PubMed]
- 4.Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Chaiworapongsa T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Investig. 2013;123(7):2775–2777. doi: 10.1172/JCI70431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 7.Melchiorre K, Giorgione V, Thilaganathan B (2021) The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol [DOI] [PubMed]
- 8.Heidrich MB, Wenzel D, von Kaisenberg CS, Schippert C, von Versen-Höynck FM. Preeclampsia and long-term risk of cardiovascular disease: what do obstetrician-gynecologists know? BMC Pregn Childbirth. 2013;13:61. doi: 10.1186/1471-2393-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130(8):703–714. doi: 10.1161/circulationaha.113.003664. [DOI] [PubMed] [Google Scholar]
- 10.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365–1370. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 11.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114(5):961–970. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 12.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335(7627):978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melchiorre K, Thilaganathan B, Giorgione V, Ridder A, Memmo A, Khalil A. Hypertensive disorders of pregnancy and future cardiovascular health. Front Cardiovasc Med. 2020;7:59. doi: 10.3389/fcvm.2020.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–715. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 15.Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29(7–8):518–522. doi: 10.1016/s1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- 16.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42(1):39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- 17.Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol. 2009;114(6):1307–1314. doi: 10.1097/AOG.0b013e3181c14e3e. [DOI] [PubMed] [Google Scholar]
- 18.Clark SL, Belfort MA, Dildy GA, Englebright J, Meints L, Meyers JA, et al. Emergency department use during the postpartum period: implications for current management of the puerperium. Am J Obstet Gynecol. 2010;203(1):38. doi: 10.1016/j.ajog.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Engeland A, Bjørge T, Klungsøyr K, Skjærven R, Skurtveit S, Furu K. Preeclampsia in pregnancy and later use of antihypertensive drugs. Eur J Epidemiol. 2015;30(6):501–508. doi: 10.1007/s10654-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Collaborating Centre for Ws, Children's H. National Institute for Health and Clinical Excellence: Guidance. Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press Copyright © 2011 (2010) Royal college of obstetricians and gynaecologists [PubMed]
- 21.Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:3078. doi: 10.1136/bmj.j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoet GA, Koster MP, Velthuis BK, de Groot CJ, Maas AH, Fauser BC, et al. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas. 2015;82(2):153–161. doi: 10.1016/j.maturitas.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Staff AC, Redman CW, Williams D, Leeson P, Moe K, Thilaganathan B, et al. Pregnancy and long-term maternal cardiovascular health: progress through harmonization of research cohorts and biobanks. Hypertension. 2016;67(2):251–260. doi: 10.1161/hypertensionaha.115.06357. [DOI] [PubMed] [Google Scholar]
- 24.Mathers CD, Boerma T, Ma FD. Global and regional causes of death. Br Med Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 25.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28(1):1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 26.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132(17):1667–1678. doi: 10.1161/circulationaha.114.008720. [DOI] [PubMed] [Google Scholar]
- 27.Kamravamanesh M, Kohan S, Rezavand N, Farajzadegan Z. A comprehensive postpartum follow-up health care program for women with history of preeclampsia: protocol for a mixed methods research. Reprod Health. 2018;15(1):1–8. doi: 10.1186/s12978-018-0521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(DGGG) DGfGuG. AWMF-Leitlinie 015/018. Diagnostik und Therapie hypertensiver Schwangerschaftserkrankungen. 2008. Internet: http://www.awmf.org/uploads/tx_szleitlinien/015-018_S1_Diagnostik_und_Therapie_hypertensiver_Schwangerschaftserkrankungen_05_2008_12-2011.pdf.
- 29.Bro Schmidt G, Christensen M, Breth KU. Preeclampsia and later cardiovascular disease—What do national guidelines recommend? Pregnancy Hypertens. 2017;10:14–17. doi: 10.1016/j.preghy.2017.07.139. [DOI] [PubMed] [Google Scholar]
- 30.Roth H, LeMarquand G, Henry A, Homer C. Assessing knowledge gaps of women and healthcare providers concerning cardiovascular risk after hypertensive disorders of Pregnancy—A scoping review. Front Cardiovasc Med. 2019;6:178. doi: 10.3389/fcvm.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suresh SC, Duncan C, Kaur H, Mueller A, Tung A, Perdigao JL, et al. Postpartum outcomes with systematic treatment and management of postpartum hypertension. Obstet Gynecol. 2021;138(5):777–787. doi: 10.1097/AOG.0000000000004574. [DOI] [PubMed] [Google Scholar]
- 32.Su R-N, Zhu W-W, Wei Y-M, Wang C, Feng H, Lin L, et al. Maternal and neonatal outcomes in multiple pregnancy: A multicentre study in the Beijing population. Chronic Dis Transl Med. 2015;1(4):197–202. doi: 10.1016/j.cdtm.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawlor DA, Hart CL, Hole DJ, Smith GD. Reverse causality and confounding and the associations of overweight and obesity with mortality. Obesity. 2006;14(12):2294–2304. doi: 10.1038/oby.2006.269. [DOI] [PubMed] [Google Scholar]
- 34.Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169(4):224–232. doi: 10.7326/M17-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart. 2013;99(15):1118–1121. doi: 10.1136/heartjnl-2013-303945. [DOI] [PubMed] [Google Scholar]
- 36.Team RC (2013) R: A language and environment for statistical computing
- 37.Auger N, Fraser WD, Schnitzer M, Leduc L, Healy-Profitós J, Paradis G. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart. 2017;103(3):235–243. doi: 10.1136/heartjnl-2016-309671. [DOI] [PubMed] [Google Scholar]
- 38.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9(3):147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 39.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–2869. doi: 10.1161/circulationaha.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine L, Nkonde-Price C, Limaye M, Srinivas S. Factors associated with postpartum follow-up and persistent hypertension among women with severe preeclampsia. J Perinatol. 2016;36(12):1079–1082. doi: 10.1038/jp.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traylor J, Chandrasekaran S, Limaye M, Srinivas S, Durnwald CP. Risk perception of future cardiovascular disease in women diagnosed with a hypertensive disorder of pregnancy. J Matern Fetal Neonatal Med. 2016;29(13):2067–2072. doi: 10.3109/14767058.2015.1081591. [DOI] [PubMed] [Google Scholar]
- 42.Gladstone RA, Pudwell J, Pal RS, Smith GN. Referral to cardiology following postpartum cardiovascular risk screening at the maternal health clinic in Kingston. Ontario Can J Cardiol. 2019;35(6):761–769. doi: 10.1016/j.cjca.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Lindström L, Skjaerven R, Bergman E, Lundgren M, Klungsøyr K, Cnattingius S, et al. Chronic hypertension in women after perinatal exposure to preeclampsia, being born small for gestational age or preterm. Paediatr Perinatal Epidemiol. 2017;31(2):89–98. doi: 10.1111/ppe.12346. [DOI] [PubMed] [Google Scholar]
- 44.Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Iversen AC, et al. Association between gestational hypertension and risk of cardiovascular disease among 617,589 Norwegian women. J Am Heart Assoc. 2018;7(10):1. doi: 10.1161/jaha.117.008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. doi: 10.1161/hypertensionaha.109.130765. [DOI] [PubMed] [Google Scholar]
- 46.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seely EW, Rich-Edwards J, Lui J, Nicklas JM, Saxena A, Tsigas E, et al. Risk of future cardiovascular disease in women with prior preeclampsia: a focus group study. BMC Pregn Childbirth. 2013;13:240. doi: 10.1186/1471-2393-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutherland L, Neale D, Henderson J, Clark J, Levine D, Bennett WL. Provider counseling about and risk perception for future chronic disease among women with gestational diabetes and preeclampsia. J Womens Health (Larchmt) 2020;29(9):1168–1175. doi: 10.1089/jwh.2019.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from AOK Baden-Wuerttemberg but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of AOK Baden-Wuerttemberg.