Abstract
The clinical approval of immune checkpoint inhibitors is an important advancement in the field of cancer immunotherapy. However, the percentage of beneficiaries is still limited and it is becoming clear that combination therapies are required to further enhance the treatment efficacy. The potential of strategies targeting the immunoregulatory network by “hitting the gas pedal” as opposed to “blocking the brakes” is being recognized and intensively investigated. Hence, next to immune checkpoint inhibitors, agonists of co-stimulatory receptors of the tumor necrosis factor superfamily (TNF-SF) are emerging as promising options to expand the immunomodulatory toolbox. In this review the development of different categories of recombinant antibody and ligand-based agonists of 4-1BB, OX40, and GITR is summarized and discussed in the context of the challenges presented by the structural and mechanistical features of the TNFR-SF. An overview of current formats, trends, and clinical studies is provided.
Key Points
| Targeting the co-stimulatory receptors 4-1BB, OX40, and GITR of the TNF superfamily holds potential for cancer immunotherapy. |
| Current developments of agonists focus on effective receptor clustering, site-specific activity, and reduced toxicity. |
| A variety of mono- and bispecific antibodies as well as antibody-ligand fusion proteins has been generated, which are now being evaluated in clinical trials. |
Introduction
Interfering with the regulatory network of the immune system holds great potential for cancer immunotherapy. This has been impressively demonstrated by the successful clinical development of many immune checkpoint inhibitors that act by enhancing an antitumor immune response blocking coinhibitory receptors (e.g. CTLA-4, PD-1). However, treatment responses are still limited to a small percentage of patients [1]. Thus, current efforts focus on also exploring the opposite regulatory approach, i.e. enhancing an antitumor immune response by activating co-stimulatory receptors. Members of the tumor necrosis factor receptor superfamily (TNFR-SF), in particular 4-1BB, OX40, and GITR, have emerged here as promising targets [2]. However, the translation of the concept has been challenged by their particular structural and mechanistic features. Their influence and impact on the development of therapeutic reagents are discussed in this review.
Costimulatory Receptors of the Tumor Necrosis Factor Superfamily (TNF-SF)
4-1BB (CD137/TNFRSF9), OX40 (CD134/TNFRSF4), and GITR (CD357, TNFRSF18) are amongst the most intensively investigated co-stimulatory members of the TNFR-SF for cancer therapy so far. They are mainly expressed on activated T cells and NK cells, enhancing the processes of proliferation, differentiation, survival, and effector functions (for reviews, see [3, 4]). Accordingly, treatment effects of 4-1BB agonists in several preclinical mouse models were demonstrated to impact and depend on CD8+ T cells and NK cells [5, 6]. Importantly, upregulation of 4-1BB on antigen-primed T cells in the tumor allowed to identify and target tumor-specific T cells on site [7]. The expression of 4-1BB was shown to be enhanced by the hypoxic conditions of the tumor microenvironment [8]. Treatment with 4-1BB agonists induced expansion of tumor infiltrating CD8+ T cells [9, 10], prevention of activation-induced cell death (AICD) [11], and restoration of exhausted tumor infiltrating lymphocyte (TIL) function [12]. Furthermore, intratumoral persistence [13], reversion of anergy [14], and an increase in effector memory CD8+ T cells [15] was reported. In addition, the expression of 4-1BB on tumor micro-vessels was shown to be involved in enhancing the recruitment of activated T cells [16]. Early co-stimulatory studies indicated a more prominent effect of 4-1BB on CD8+ T cells and OX40 on CD4+ T cells, respectively [17, 18]. Indeed, OX40 signaling was shown to enhance the cooperation between CD4+ T cells and CD8+ T cells for antitumor activity [19], and both subpopulations were shown to participate in agonist-mediated tumor regression in preclinical mouse models [20]. Also, enhanced infiltration and function of tumor-specific CD8+ T cells and the generation of tumor-specific memory was reported [21, 22]. In the case of GITR, co-stimulation by agonistic antibodies was shown to promote antitumor response by enhancing both CD8+ and CD4+ effector T-cell activity and in particular reducing the number and activity of tumor-infiltrating Tregs [23, 24]. Although GITR, OX40, and 4-1BB appear to have the potential to drive the proliferation of Tregs, they also seem to be implicated in antagonizing Treg generation and Treg-mediated suppression (for review, see [25, 26]). Thus, the co-stimulatory impact on Tregs and the implication for cancer treatment still remain unclear. Importantly, agonists of 4-1BB, OX40, and GITR have shown great potential for combination therapies, for example, with each other, immune checkpoint inhibitors, and conventional strategies (for review, see [26, 27]).
Mechanism of Activation
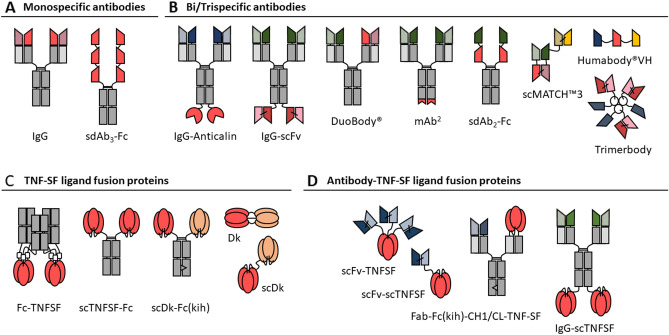
In general, co-stimulatory TNFRs are expressed on immune cells and interact with their respective ligands expressed on antigen presenting cells (APC). Consequently, under physiological conditions, co-stimulation takes place in a local manner via cell-cell interaction. From a structural point of view, TNF-SF receptors are characterized by repeats of a cysteine-rich domain (CRD) in their ectodomain that can promote diverse degree of receptor self-assembly. Thus, prior to their activation the receptors present in monomeric, dimeric or trimeric state. TNF-SF ligands on the other side are characterized by an external TNF homology domain (THD) that usually leads to stable homotrimeric ligand assemblies [28]. X-ray crystal structures showed that receptor-ligand binding takes place in a symmetric ligand trimer-receptor trimer configuration, involving the typical THD and CRD domains [29]. According to the prevalent two-step model, further clustering of this trimeric receptor-ligand complex is required to achieve efficient signaling pathway activation [30]. This step is supported by the given alignment, restricted mobility, and high local density of the ligand in its transmembrane form. In fact, many TNF-SF ligands can bind in soluble form with high affinity to their receptors, but fail to activate them efficiently, unless additional oligomerization is induced. Most of the co-stimulatory members of the TNFR-SF, including 4-1BB, OX40, and GITR, fall into this category. Thus, the induction of receptor clustering is considered essential for the efficacy of agonistic reagents. This insight has ultimately guided the development of agonists, leading to diverse antibody and ligand-based formats (Fig. 1), many of them now entering clinical trials (Tables 1, 2 and 3).
Fig. 1.
Schematic overview of agonists for co-stimulatory tumor necrosis factor superfamily (TNF-SF) receptors. (A) monospecific antibodies, (B) bispecific antibodies, (C) TNF-SF ligand fusion proteins, (D) antibody-TNF-SF ligand fusion proteins. Target specificity: red/orange, costimulatory receptor; blue, TAA; green, PD-1/PD-L1; yellow, human serum albumin. sdAb single-domain antibody, TNF-SF extracellular domain of costimulatory TNF super family ligand, kih knob-into-hole, Dk duokine, sc single-chain
Table 1.
Tumor necrosis factor super family (TNF-SF) agonistic monospecific antibodies in clinical studies (www.clinicaltrials.gov)
| Target | Name | Format | Tumor type | Combination | Phase | Status | Clinical ID | Information by |
|---|---|---|---|---|---|---|---|---|
| 4-1BB |
Urelumab (BMS-663513) |
huIgG4 | Melanoma | – | II |
Completed (10/2009) |
NCT00612664 | Bristol-Myers Squibb |
| Urothelial carcinoma/bladder cancer | Nivolumab (αPD-1) | II | Recruiting | NCT02845323 | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | |||
|
Utomilumab (PF-05082566) |
huIgG2 | Adv. solid tumors | Pembrolizumab (αPD-1) | I |
Completed (02/217) |
NCT02179918 | Pfizer | |
| Solid tumor/B-cell lymphoma | Rituximab (αCD20) | I |
Completed (02/2019) |
NCT01307267 | Pfizer | |||
| Her2-positive breast cancer | Trastuzumab (αHer-2)/Trastuzumab Emtansine (αHer2-ADC) | I | Active | NCT03364348 | George W. Sledge Jr., Stanford University | |||
| Adv. cancers | Avelumab (αPD-L1)/CMP-001 (TLR9 agonist)/PF-04518600 (αOX40) | II | Active | NCT02554812 | Pfizer | |||
| YH004 | huIgG1 | Cancer | – | I | Recruiting | NCT05040932 | Eucure (Beijing) Biopharma Co., Ltd. | |
| ATOR-1017 | huIgG4 | Solid tumor | – | I | Recruiting | NCT04144842 | Alligator Bioscience AB | |
| EU101 | n.i.a. | Solid tumor | – | I/II | Recruiting | NCT04903873 | Eutilex | |
| ADG106 | huIgG4 | Metastatic NSCLC | Nivolumab (αPD-1) | I/II | Recruiting | NCT05236608 | National University Hospital, Singapore | |
| AGEN2372 | huIgG4 | Adv. solid tumor | AGEN1181 (αCTLA-4) | I | Recruiting | NCT04121676 | Agenus Inc. | |
| LVGN6051 | huIgG (huFcγRIIB selective) | Soft tissue sarcoma | Anlotinib | I/II | Not yet recruiting | NCT05301764 | Lyvgen Biopharma Holdings Limited | |
| OX40 | MOXR0916 | huIgG1 | Adv. or metastatic solid tumors | Atezolizumab (αPD-L1) | I |
Completed (11/2019) |
NCT02410512 | Genentech, Inc. |
| PF-04518600 | huIgG2 | Adv. or metastatic carcinoma | PF-05082566 (α4-1BB) | I |
Completed (11/2020) |
NCT02315066 | Pfizer | |
| Adv. malignancies | Avelumab (αPD-L1)/Utomilumab (α4-1BB)/radiation therapy | I/II | Active | NCT03217747 | M.D. Anderson Cancer Center | |||
| Adv. malignancies | Avelumab (αPD-L1)/Utomilumab(α4-1BB)/CMP-001 (TLR9 agonist)/PD 0360324 (Anti-M-CSF) | II | Active | NCT02554812 | Pfizer | |||
| Recurrent/refractory acute myeloid leukemia | Avelumab (αPD-L1)/Azacitidine | I/II | Active | NCT03390296 | M.D. Anderson Cancer Center | |||
| Follicular Lymphoma | Rituximab (αCD20)/Utomilumab (α4-1BB) | I | Active | NCT03636503 | Caron A. Jacobson, Dana-Farber Cancer Institute | |||
| Triple negative breast cancer | Avelumab (αPD-L1) | II | Recruiting | NCT03971409 | Hope Rugo, MD, University of California, San Francisco | |||
| Metastatic kidney cancer | Axitinib | II | Recruiting | NCT03092856 | University of Southern California | |||
| MEDI6469 | mIgG1 | Metastatic breast cancer | – | I |
Completed (08/2018) |
NCT01862900 | Providence Health & Services | |
| Head and neck cancer | – | I | Active | NCT02274155 | Providence Health & Services | |||
| MEDI0562 | huIgG | Adv. solid tumors | – | I |
Completed (01/2018) |
NCT02318394 | MedImmune LLC | |
| Ovarian cancer | Durvalumab (αPD-1), Tremelilumab (αCTLA-4), MEDI 9447 (αCD73) | II |
Completed (09/2021) |
NCT03267589 | Nordic Society of Gynaecological Oncology - Clinical Trials Unit | |||
| Adv. solid tumors | Durvalumab (αPD-1)/Tremelimumab (αCTLA-4) | I |
Completed (08/2019) |
NCT02705482 | MedImmune LLC | |||
| Head and neck squamous cell carcinoma or melanoma | – | I | Active | NCT03336606 | Providence Health & Services | |||
| BMS-986178 | huIgG1 | Adv. solid tumors | Nivolumab (αPD-1)/Ipilimumab (αCTLA-4) | I/II |
Completed (11/2020) |
NCT02737475 | Bristol-Myers Squibb | |
| Adv. or metastatic solid tumors | SD-101 (TLR9 agonist) | I | Active | NCT03831295 | Ronald Levy, Stanford University | |||
| Low-grade B-cell non-Hodgkin lymphomas | Radiation/SD-101 (TLR9 agonist) | I | Active | NCT03410901 | Ronald Levy, Stanford University | |||
| INCAGN01949 | huIgG1 | Adv. or metastatic solid tumors | – | I/II |
Completed (03/2019) |
NCT02923349 | Incyte Corporation | |
| Adv. malignancies | Nivolumab (αPD-1)/Ipilimumab (αCTLA-4) | I/II |
Completed (09/2019) |
NCT03241173 | Incyte Corporation | |||
| Pancreatic cancer and others | CMP-001 (TLR9 agonist) | I/II | Recruiting | NCT04387071 | University of Southern California | |||
| IBI101 | huIgG1 | Adv. solid tumors | Sintilimab (αPD-1) | I | Active | NCT03758001 | Innovent Biologics (Suzhou) Co. Ltd. | |
| HFB301001 | huIgG1 | Adv. solid tumors | – | I | Recruiting | NCT05229601 | HiFiBiO Therapeutics | |
| ES102/INBRX-106 | sdAb3Fc | Adv. solid tumors | – | I | Recruiting | NCT04730843 | Elpiscience Biopharma. Ltd. | |
| Adv. solid tumors | Toripalimab (αPD-1) | I | Recruiting | NCT04991506 | Elpiscience Biopharma. Ltd. | |||
| Adv. or metastatic solid tumors | Pembrolizumab (αPD-1) | I | Recruiting | NCT04198766 | Inhibrx, Inc. | |||
| BGB-A445 | n.i.a. | Adv. solid tumors | Tislelizumab (αPD-1) | I | Recruiting | NCT04215978 | BeiGene | |
| BAT6026 | n.i.a. | Adv. solid tumors | - | I | Recruiting | NCT05105971 | Bio-Thera Solutions | |
| Adv. solid tumors | BAT1308 (αPD-1) | I | Not yet recruiting | NCT05109650 | Bio-Thera Solutions | |||
| GITR | MK-4166 | huIgG1 | Solid tumors | Pembrolizumab (αPD-1) | I |
Completed (07/2019) |
NCT02132754 | Merck Sharp & Dohme LLC |
| TRX518 | huIgG1 | Melanoma/solid tumors | – | I |
Completed (09/2018) |
NCT01239134 | Leap Therapeutics, Inc. | |
| GWN323 | IgG1 | Solid tumors/lymphomas | PDR001 (αPD-1) | I |
Completed (03/2020) |
NCT02740270 | Novartis | |
| INCAGN01876 | huIgG1 | Adv. or metastatic solid tumors | – | I/II |
Completed (12/2019) |
NCT02697591 | Incyte Corporation | |
| Adv. or metastatic malignancies | Ipilimumab (αCTLA-4)/Nivolumab (αPD-1) | I/II |
Completed (11/2021) |
NCT03126110 | Incyte Corporation | |||
| Glioblastoma | INCMGA00012 (αPD-1)/Stereotactic Radiosurgery | II | Active | NCT04225039 | University of Pennsylvania | |||
| ASP1951 | tetravalent huIgG4 | Adv. solid tumors | Pembrolizumab (αPD-1) | I | Active | NCT03799003 | Astellas Pharma Inc | |
| REGN6569 | n.i.a. | Squamous cell carcinoma of head and neck | Cemiplimab (αPD-1) | I | Recruiting | NCT04465487 | Regeneron Pharmaceuticals | |
| BMS-986156 | huIgG1 | Metastatic lung/chest/liver tumors | Ipilimumab (αCTLA-4)/Nivolumab (αPD-1)/radiation | I/II | Recruiting | NCT04021043 | M.D. Anderson Cancer Center |
n.i.a. no information available, adv. advanced
Table 2.
Tumor necrosis factor super family (TNF-SF) agonistic bi- and trispecific antibodies in clinical studies (www.clinicaltrials.gov)
| Targets | Name | Format | Tumor type | Combination | Phase | Status | Clinical ID | Information by |
|---|---|---|---|---|---|---|---|---|
| Her2 x 4-1BB | PRS-343 | (αHer2) IgG4mut-(α4-1BB) Anticalin | Her2-positive adv. or metastatic solid tumors | – | I |
Completed (10/2021) |
NCT03330561 | Pieris Pharmaceuticals, Inc. |
| Adv. or metastatic Her2-positive solid tumors | Atezolizumab (αPD-L1) | I | Active | NCT03650348 | Pieris Pharmaceuticals, Inc. | |||
| Her2-positive gastric cancer | Ramucirumab (αVEGFR2)/Paclitaxel/Tucatinib | II | Active | NCT05190445 | Pieris Pharmaceuticals, Inc. | |||
| PD-L1 x 4-1BB |
PRS-344/ S095012 |
(αPD-L1) IgG4mut-(α4-1BB) Anticalin | Solid tumors | – | I/II | Recruiting | NCT05159388 | Pieris Pharmaceuticals, Inc. |
| GEN1046 | DuoBody® | Solid tumors | – | I | Recruiting | NCT04937153 | Genmab | |
| Metastatic NSCLC | Pembrolizumab(αPD-1) | II | Recruiting | NCT05117242 | Genmab | |||
| ABL503 | (αPD-L1) IgG1mut-(α4-1BB) scFv | Adv. solid tumors | – | I | Recruiting | NCT04762641 | ABL Bio, Inc. | |
| INBRX-105 | (αPD-L1) sdAb-(α4-1BB) sdAb-Fcmut | Solid tumors | Pembrolizumab (αPD-1) | I | Recruiting | NCT03809624 | Inhibrx, Inc. | |
| FS222 | mAb2 | Advanced cancers | – | I | Recruiting | NCT04740424 | F-star Therapeutics Limited | |
| OX40 x 4-1BB | FS120 | mAb2 | Adv./metastatic cancer | – | I | Recruiting | NCT04648202 | F-star Therapeutics Limited |
| PD-L1 x OX40 | EMB-09 | FIT-Ig® | Adv. solid tumors | – | I | Not yet recruiting | NCT05263180 | Shanghai EpimAb Biotherapeutics Co., Ltd. |
| 4-1BB x PD-L1 x HSA | NM21-1480 | scMATCH™3 | Adv. solid tumors/NSCLC | – | I/II | Recruiting | NCT04442126 | Numab Therapeutics AG |
| PSMA x 4-1BB x HSA | CB307 | Humabody® | Adv. and/or metastatic PSMA-positive solid tumors | – | I | Recruiting | NCT04839991 | Crescendo Biologics Ltd. |
Adv advanced, NSCLC non-small cell lung cancer
Table 3.
Agonistic tumor necrosis factor super family (TNF-SF) ligand-fusion proteins in clinical studies (www.clinicaltrials.gov)
| Targets | Name | Format | Tumor type | Combination | Phase | Status | Clinical ID | Information by |
|---|---|---|---|---|---|---|---|---|
| OX40 | MEDI6383 | Fcγ4(S228P)-TD-OX40L | Adv. solid tumors | +/– Durvalumab (αPD-L1) | I | Completed (07/2017) | NCT02221960 | MedImmune LLC |
| GITR | MEDI1873 | Fcγ1-TD-GITRL(N161D) | Adv. solid tumors | – | I | Completed (12/2018) | NCT02583165 | MedImmune LLC |
| FAP x 4-1BB | RO7122290 | (αFAP) Fab-Fcγ1mut-CH1/CL-4-1BBL | Metastatic colorectal cancer | Cibisatamab (αCEAxCD3) /Obinutuzumab (αCD20) | I/II | Recruiting | NCT04826003 | Hoffmann-La Roche |
| CD19 x 4-1BB | RO7227166 | (αCD19) Fab-Fcγ1mut-CH1/CL-4-1BBL | Lymphoma, non-Hodgkin | Obinutuzumab (αCD20)/Glofitamab (αCD20xCD3)/Tozilizumab (αIL-6R) | I | Recruiting | NCT04077723 | Hoffmann-La Roche |
| PDL-1 x OX40 | SL-279252 | PD1-Fcγ4-OX40L | Adv. solid tumors or lymphomas | – | I | Recruiting | NCT03894618 | Shattuck Labs, Inc. |
TD trimerization domain, adv. advanced
Agonistic Monospecific Antibodies
In principle, the bivalency of a classical monoclonal IgG antibody entails the potential for cross-linking and agonistic cluster induction, whereupon the position of the epitope rather than high affinity is critical [31, 32]. However, it is actually the Fc region that plays a dominant role in modulating this process. It was shown that FcγR-mediated cell surface binding of the targeted antibody can become crucial for the efficacy of co-stimulatory TNFR-SF clustering and activation [33]. Unfortunately, this makes the approach dependent on the presence of FcγR-expressing immune cells and prone to unreliable factors like FcγR expression levels and competition with serum IgG. Furthermore, isotype-dependent binding to particular FcγR types impacts the therapeutic outcome. For instance, in preclinical studies with 4-1BB agonistic antibodies of different isotypes it was shown, that binding to inhibitory FcγRIIB was required for anti-tumor efficacy, while binding to the activating FcγRIII reduced tumor effects due to T cell depletion by antibody-dependent cellular cytotoxicity (ADCC). However, an isotype with low activating/inhibitory FcγR binding ratio (reduced ADCC) was only combinable with a weak intrinsic agonist. In combination with a strong intrinsic agonist liver toxicity was observed [34]. Thus, intrinsic cross-linking capacity, isotype and availability and distribution of FcγR types determine not only the treatment efficacy, but also the side effect profile. Consequently, the development of monospecific antibodies has been challenged by these factors.
By now several 4-1BB-directed agonistic monoclonal antibodies have entered clinical trials (Table 1). Initial leading molecules were Urelumab (BMS-663513) [35] and Utolimumab (PF-05082566) [36]. Urelumab is a non-ligand-blocking fully human IgG4 antibody with a hinge mutation (S228P) for improved stability that showed clinical activity, but also dose-limiting hepatotoxicity (doses ≥ 1mg/kg) [37, 38]. Utolimumab on the other hand is a ligand-blocking humanized IgG2 antibody that has shown a favorable safety profile, but was less effective relative to Urelumab [31, 39]. Structural analysis revealed that the epitope position of Urelumab in comparison to Utolimumab enabled stronger 4-1BB cross-linking through the bivalent binding of the IgG, enhancing its intrinsic agonistic strength. In addition, both isotypes presented reduced ADCC capacity and enabled FcγRIIB-mediated cross-linking [31], whereupon affinity for FcγRIIB is 10-fold higher for IgG4 than for IgG2 [40]. Looking into optimizing the cross-linking balance of agonistic strength and FcγR affinity led to the development of LVGN6051 that combines weak intrinsic 4-1BB agonism, i.e. FcγR cross-linking requirement, with engineered FcγRIIB selectivity. Preclinical mouse studies showed effective antitumor activity without signs of concomitant liver toxicity [34]. Mutations in isotypes of immunostimulatory antibodies are reviewed in detail by Boulard et al. [41]. Most clinical studies with co-stimulatory agonists include the evaluation of combinatory treatments with immune checkpoint inhibitors (Table 1).
Further developments focus on enhancing the intrinsic agonistic efficacy. Here, a consistent strategy to improve the cross-linking property of an antibody is the generation of recombinant antibody formats with increased multivalency. This included fusing small binding units, e.g. three OX40-directed single-domain antibodies (sdAb), in a row to an Fc part, leading to hexavalent antibodies with enhanced avidity and therefore cross-linking capacity (ES102/INBRX-106). Also, a tetravalent hinge-stabilized IgG4 molecule targeting GITR (ASP1951), has been reported [42]. Both formats are currently listed in clinical trials (Table 1). Moreover, in preclinical studies the design of tetravalent and in addition biepitopic antibodies was shown to retrieve robust OX40 agonists, independent of extrinsic crosslinking [43].
Other approaches address in particular the reduction of immune-related adverse events. Thus, to avoid systemic toxicity, local treatment and local activation of monoclonal antibodies is being investigated. Local treatment by low-dose intratumoral injections with a 4-1BB agonistic antibody in mice was shown to result in antitumor effects without liver inflammation [8]. A clinical phase I/II study with intratumoral urelumab treatment in combination with systemic applied nivolumab in patients with solid tumors has been announced (NCT03792724). Local activation is the strategy of the Probody-approach with a 4-1BB agonist antibody prodrug. Here, a peptide fused via a protease-cleavable linker to the N-terminus of the light chain, masks the antigen-binding site in solution. Once arrived at the tumor microenvironment (TME) the peptide is cleaved by tumor-associated proteases, enabling co-stimulatory receptor binding, i.e. agonistic activity at the tumor site. Thus, in syngeneic mouse models the antitumor efficacy of the original antibody was preserved while liver inflammation was reduced [44]. Next to the efforts to improve monospecific antibodies, the introduction of bispecific antibodies is on the rise in the field.
Bispecific Antibodies
Bispecific antibodies targeting a co-stimulatory receptor and a tumor-associated antigen (TAA) have the potential to localize the agonistic activity at the tumor site. Binding to the co-stimulatory receptor on the immune cell is in general not sufficient for an effective activation, but tethering the antibody by its TAA-specificity to the tumor cell surface, i.e., adopting a transmembrane-like form, enables the dynamic of efficient receptor clustering and therefore target-dependent activation. Thus, the strategy seeks for high local co-stimulatory efficacy and reduced peripheric toxicity. Furthermore, the tumor-directed antibody unit can also contribute to diversify the mode of action, for example, by blocking a target receptor (TAA). Most advanced developments include a variety of monovalent/bivalent bispecific antibodies with a silenced Fc part or a human serum albumin (HSA) binding unit (Fig.1B, Table 2). PRS-343 was the first bispecific 4-1BB agonist to enter clinical trials. It is a Her-2 specific IgG4 variant of trastuzumab, fused at the C-terminus to a 4-1BB-directed, non-ligand-blocking, anticalin molecule. The Fc region is engineered (S228P, F234A, L235A) to avoid half-antibody exchange and FcγR-binding (i.e., excluding ADCC and targeting-independent cross-linking), without interfering with FcRn-binding (i.e., prolonged plasma half-life). It was shown that the co-stimulatory activity of PRS-343 was related to the Her-2 expression levels in vitro and induced localized immune effects and antitumor efficacy in preclinical in vivo studies [45]. The first clinical phase I trial as monotherapy was recently completed (NCT03330561). PRS-343 was well tolerated and showed clinical benefit, associated with increased CD8+T cell numbers and proliferation index [46]. A second phase I trial of PRS-343 in combination with atezolizumab (NCT03650348) and a phase II trial of PRS-343 in combination with ramucirumab and paclitaxel or tucatinib (NCT05190445) is ongoing. Another important target on the rise is PD-L1. The ligand forms part of the PD-L1/PD-1 checkpoint inhibitor axis and is overexpressed in many solid tumors [47]. Bispecific antibodies targeting PD-L1 and a co-stimulatory receptor seem a particular promising strategy, because combination of localized checkpoint inhibition and co-stimulation is expected to synergize in enhancing T-cell and NK-cell function, increasing treatment response rate and durability. Bispecific antibodies in development seek to translate this concept mostly by targeting PD-L1 and 4-1BB. 4-1BB is prominently expressed on PD-1 high positive CD8+ TILs, and PD-1 blockade can further upregulate the 4-1BB expression [48], thus supporting a combined action. Formats entering clinical trials include IgGs fused at the C-terminus to an Anticalin [46] or scFv [49], DuoBody® [50], mAb2 [51], and sdAbs fused to either a Fc region or an HSA-specific sdAb [52] (Fig.1B, Table 2). Preclinical studies confirmed blocking of checkpoint inhibition and targeting-dependent co-stimulatory activity. Furthermore, bispecific antibodies were able to outperform the combination of respective monoclonal antibodies in vitro and showed superior antitumor effects in comparison with the treatment with immune checkpoint inhibitor only in divers tumor mouse models [46, 49–51]. Mechanistic studies with NM21-1480, a monovalent trispecific antibody (single-chain of three λcap™-stabilized Fvs) targeting PD-L1, 4-1BB, and HSA, respectively, addressed the issues of target density, epitope position and antibody affinities. Targeting-mediated co-stimulation was demonstrated at a broad range of PD-L1 expression levels, whereupon the co-stimulatory strength correlated with the target density. For this antibody format, 4-1BB clustering resulted more effectively from binding a membrane distal epitope than a proximal one. Furthermore, increasing the affinity to PD-L1 significantly over 4-1BB converged the dosing for maximal dual activity [53]. Hence, target density, epitope position, and affinity need to be concerted adequately to deliver the strategy. PD-L1x4-1BB bispecifics were in general well tolerated in toxicity studies in cynomolgus monkeys without signs of liver inflammation [49–51, 53]. First results of a phase I trial of the PD-L1x4-1BB DuoBody GEN1046 in heavily pretreated patients with advanced refractory solid tumors (NCT03917381) showed a manageable safety profile and disease control in 65.6% of the patients mostly in the form of stable disease [50].
Other developments focus on the combination of small antibody or antibody-like units without including an Fc-part. In the trimer body format, a 4-1BB-directed scFv is connected to an EGFR-directed VHH by a linker with the murine collagen XVIII homotrimerization domain. Consequently, the molecule assembled into a homotrimer with the binding units presented in a hexagonal conformation. Targeting-enhanced co-stimulation was confirmed in vitro and antitumor effects demonstrated in CDX and PDX humanized mouse models [54]. No signs of systemic or liver toxicity were observed for respective surrogates in corresponding syngeneic mouse models [55, 56]. The principle of targeting-mediated co-stimulation is also pursued by bispecific antibody-mimetics composed of designed ankyrin repeat proteins (DARPins). MP0310, a bispecific DARPin® drug candidate directed against the fibroblast activation protein (FAP) and 4-1BB, is currently being evaluated in a clinical phase I trial in patients with advanced solid tumors (NCT04049903).
Costimulatory TNF-SF Ligands
Naturally, co-stimulatory receptors of the TNFR-SF can also be activated by recombinant forms of their respective ligands (Fig. 1C, Table 3). The basic functional unit is usually a self-assembling, non-covalently linked homotrimer of the extracellular domain (ECD) of the ligand, which requires further oligomerization to induce effective receptor clustering. This can be facilitated for example by fusing the ECD of the ligand to an Fc region and enforcing ligand trimerization by introducing an isoleucine zipper coiled coil domain in the linker. Thus, a Fc-mediated covalently linked hexameric ligand form was generated that showed co-stimulatory properties for Fc-GITRL and Fc-OX40L in preclinical in vitro and in vivo studies [57, 58]. Similar to the situation observed with agonistic monoclonal antibodies, cross-linking via Fc/FcγR interactions were shown to play an important role in the activity of these molecules. Both fusion proteins entered clinical phase I studies with patients with advanced solid tumors (NCT02221960, NCT02583165) (Table 3). Fc-GITRL (MEDI1873) was reported to show an overall acceptable safety profile and prolonged stable disease in some patients. However, the lack of tumor response discouraged the company from further clinical development [59].
Other developments include the generation of recombinant ligands in the single-chain format, i.e. connecting three ECDs with short linkers, thus enforcing intramolecular trimerization rather than intermolecular trimerization. Concomitant fusion to the N-terminus of a silenced Fcγ1 region retrieves a covalently linked homodimer with a hexavalent ligand configuration. scGITRL-Fc showed co-stimulatory activity and antitumor effects that were independent of FcγR-mediated cross-linking [60]. This property was also confirmed for scCD40L-Fc and scCD27L-Fc [61, 62].
Another approach conceives the generation of Duokines (Dk), i.e. fusion proteins composed of two different co-stimulatory TNF-SF ligands (e.g., combinations of 4-1BBL, OX40L, CD27L, and CD40L). Here, the respective ECDs are connected by a 15-20 amino acid linker, leading to a bifunctional homotrimer formation. Alternatively, ligand units in the single-chain format are fused, generating scDuokines (scDk) [63]. Receptor clustering is here facilitated by simultaneous receptor binding in cis or trans. Thus, dual-targeting translates into combined co-stimulatory activity. Following the same principle, further developments to increase the plasma half-life included scDk-Fc fusion proteins utilizing a silenced, heterodimeric (knob-into-hole) Fc design [64]. Both formats, scDk and scDk-Fc, showed similar co-stimulatory properties and the potential to enhance the antitumor effect of a T-cell bispecific antibody (TAA × CD3) in a syngeneic tumor mouse model [63, 64].
Antibody-Fusion Proteins with Co-Stimulatory TNF-SF Ligands
Antibody-fusion proteins composed of a tumor-directed antibody and the ECD of a co-stimulatory TNF-SF ligand constitute another approach to achieve tumor-localized co-stimulation. Antibody-mediated binding to a tumor-associated antigen leads to the cell surface presentation of the co-stimulatory ligand, mimicking its physiological active membrane-bound form. Targeting-dependent activity was demonstrated for antibody fusion proteins with different TNF-SF members (e.g., 4-1BBL, OX40L, GITRL, LIGHT), target specificities (e.g., FAP, EGFR, Endoglin, EDA, CD19), and formats [65–70] (Fig. 1D, Table 3). To translate this concept, initially scFv-TNF-SF were created by fusing a scFv antibody to the N-terminus of the TNF-SF ligand (ECD). Due to the trimerization property of the ligand, homotrimeric molecules with three antibody units and a trimeric ligand unit were generated [65–68]. Advanced design introduced the ligand in the single-chain format, creating a monomeric scFv-scTNF-SF variant with only one antibody unit and one trimeric ligand unit, showing improved activity and stability. Importantly, targeting a ligand trimer to the cell surface was shown to be sufficient for the induction of an effective receptor stimulation in vitro and to enhance antitumor effects in mice [71]. Furthermore, the single-chain design of the TNF-SF ligand enabled single-site fusion of the ligand trimer and consequently the incorporation into fusion protein formats of higher complexity [72]. Currently, the most advanced and in clinical studies is an IgG-like format composed of a FAP or CD19-directed Fab fragment, a heterodimeric Fcγ1 region, and a 4-1BBL trimer (RO7122290/RO7227166). The ligand trimer assembles from two ECDs fused as single-chain to the CL(RK) domain connected to the CH2-CH3 of the Fc region and a single ECD fused to a CH1(EE) domain forming a complementary light chain-like arm. The heterodimeric (knob-into-hole) Fc region is modified to inhibit FcγR binding without interfering with FcRn binding. Thus, tumor target-dependent, but FcγR-cross-linking independent, co-stimulatory activity was combined with a prolonged serum half-life [70]. Preclinical studies in xenograft-humanized mouse models showed FAP- and CD19-directed antibody-4-1BBL fusion proteins to increase the accumulation and activation of intratumoral CD8+ T cell and enhance the antitumor effects of T-cell bispecific CEA × CD3 and CD20 × CD3 antibodies, respectively. No accumulation of immune cells in the liver was observed [70]. Clinical phase I studies with patients with metastatic colorectal cancer (NCT04826003) and non-Hodgkin lymphoma (NCT04077723) have been initiated (Table 3).
Recently, a format for the blockade of PD-1 checkpoint inhibition in combination with GITR agonism has been proposed. The corresponding antibody-fusion protein is composed of an anti-PD-1 IgG1 antibody fused at the C-terminus of the silenced Fc to scGITRL. Taking advantage of the co-expression and cross-regulation of PD-1 and GITR on activated T cells, PD-1 targeting-mediated GITR-clustering in cis was shown to induce effective tumor growth inhibition in diverse syngeneic, genetically engineered, and xenograft-humanized mouse tumor models [73]. Instead of using an antibody, targeting and blocking of PD-L1 can also be achieved by introducing the ECD of PD-1. In the design of PD-1-Fc-OX40L, the ECD of PD-1 and OX40L were fused to the N- and C-terminus of a silenced Fc region, respectively. Indeed, the stimulatory activity on activated T cells and the antitumor responses in mice appeared to be superior to the treatment effect obtained by the combination of corresponding monoclonal antibodies [74]. Currently, a clinical phase I study is recruiting participants (NCT03894618).
In the current treatment strategies co-stimulatory agonists and immune checkpoint inhibitors are usually combined simultaneously, either in the form of a single molecule or as co-applied separate molecules. Preclinical studies in mice showed for the combination of an OX40 agonist and a PD-1 checkpoint inhibitor that the sequential administration and the order of application were crucial to improve the antitumor efficacy and obtain effects superior to the concurrent combination therapy [75]. Thus, accounting the dynamic of a natural immune response, exploring the potential of different timing should be of interest to further improve dosing and treatment efficacy of co-stimulatory agonists in combinatory approaches. Considering their mode of action as enhancer molecules, their therapeutic efficacy will always be intrinsically dependent on the presence of a natural underlying or an artificially induced antitumor immune response. Thus, in order to tune the antitumor immune response adequately and minimize immune-related adverse events, their application will have to be carefully adjusted for each particular combination strategy.
Conclusions
Agonists of co-stimulatory TNF-SF receptors are required to induce effective receptor clustering. The application of conventional monoclonal antibodies has been shown to be complicated by the dependence on FcγR-mediated cross-linking. Thus, current drug developments focus mainly on enhancing the cross-linking capacity of antibodies and ligands in an FcγR-independent manner. Next to the generation of multivalent antibody and oligomeric ligand molecules, the design of bispecific antibodies and bifunctional antibody-ligand fusion proteins driving receptor complex clustering by cell-cell interactions is emerging as a promising option to enhance and localize the co-stimulatory activity in the tumor. Site-directed activity in combination with immune checkpoint inhibition is expected to further increase the therapeutic efficacy. Currently, multiple co-stimulatory TNF-SF agonists have entered clinical trials. In the near future upcoming results of toxicity and treatment efficacy will define the potential of the optimized formats and concepts. It will be interesting to see which candidates will come out on top and take the lead.
Declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. D.M. is supported by the German Cancer Aid (Grant 70114233).
Conflicts of interest/competing interests
D.M. is named inventor on a patent application covering the Duokine and scDuokine technology.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
No datasets were generated for this article. References are indicated.
Code availability
Not applicable.
Author contributions
Conception, literature search and writing of the manuscript was done by D.M.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerson DA, Redmond WL. Overcoming Tumor-Induced Immune Suppression: From Relieving Inhibition to Providing Costimulation with T Cell Agonists. BioDrugs. 2018;32(3):221–231. doi: 10.1007/s40259-018-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croft M. The TNF family in T cell differentiation and function–unanswered questions and future directions. Semin Immunol. 2014;26(3):183–190. doi: 10.1016/j.smim.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4–1BB T- cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 6.Houot R, Goldstein MJ, Kohrt HE, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114(16):3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Q, Song D-G, Poussin M, et al. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014;20:44–55. doi: 10.1158/1078-0432.CCR-13-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palazón A, Martínez- Forero I, Teijeira A, et al. The HIF-1α hypoxia response in tumor- infiltrating T lymphocytes induces functional CD137 (4–1BB) for immunotherapy. Cancer Discov. 2012;2:608–623. doi: 10.1158/2159-8290.CD-11-0314. [DOI] [PubMed] [Google Scholar]
- 9.Harao M, Forget MA, Roszik J, et al. 4–1BB-enhanced expansion of CD8+ TIL from triple-negative breast cancer unveils mutation-specific CD8+ T cells. Cancer Immunol Res. 2017;5(6):439–445. doi: 10.1158/2326-6066.CIR-16-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innamarato P, Asby S, Morse J, et al. Intratumoral activation of 41BB costimulatory signals enhances CD8 T Cell expansion and modulates tumor-infiltrating myeloid cells. Immunol. 2020;205(10):2893–2904. doi: 10.4049/jimmunol.2000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Chacon JA, Li Y, Wu RC, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. Immunother. 2011;34(3):236–250. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JB, Horton BL, Zheng Y, et al. The EGR2 targets LAG-3 and 4–1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J Exp Med. 2017;214:381–400. doi: 10.1084/jem.20160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigelin B, Bolanos E, Teijeira A, et al. Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137mAb. Proc Natl Acad Sci U S A. 2015;112:7551–7556. doi: 10.1073/pnas.1506357112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox RA, Tamada K, Flies DB, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD81 cytolytic T lymphocytes in vivo. Blood. 2004;103(1):177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 15.Muth ST, Saung MT, Blair AB, et al. CD137 agonist-based combination immunotherapy enhances activated, effector memory T cells and prolongs survival in pancreatic adenocarcinoma. Cancer Lett. 2021;499:99–108. doi: 10.1016/j.canlet.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palazón A, Teijeira A, Martínez-Forero I, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71(3):801–811. doi: 10.1158/0008-5472.CAN-10-1733. [DOI] [PubMed] [Google Scholar]
- 17.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4–1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173(10):5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 18.Habib-Agahi M, Phan TT, Searle PF. Co-stimulation with 4–1BB ligand allows extended T-cell proliferation, synergizes with CD80/CD86 and can reactivate anergic T cells. Int Immunol. 2007;19(12):1383–1394. doi: 10.1093/intimm/dxm106. [DOI] [PubMed] [Google Scholar]
- 19.Song A, Song J, Tang X, Croft M. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37(5):1224–1232. doi: 10.1002/eji.200636957. [DOI] [PubMed] [Google Scholar]
- 20.Kjaergaard J, Tanaka J, Kim JA, et al. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60(19):5514–5521. [PubMed] [Google Scholar]
- 21.Gough MJ, Ruby CE, Redmond WL, et al. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68(13):5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 22.Peng W, Williams LJ, Xu C, et al. Anti-OX40 antibody directly enhances the function of tumor-reactive CD8+ T cells and synergizes with PI3Kβ inhibition in PTEN loss melanoma. Clin Cancer Res. 2019;25(21):6406–6416. doi: 10.1158/1078-0432.CCR-19-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riccardi C, Ronchetti S, Nocentini G. Glucocorticoid-induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin Ther Targets. 2018;22(9):783–797. doi: 10.1080/14728222.2018.1512588. [DOI] [PubMed] [Google Scholar]
- 24.Buzzatti G, Dellepiane C, Del Mastro L. New emerging targets in cancer immunotherapy: the role of GITR. ESMO Open. 2020;4(Suppl 3):e000738. doi: 10.1136/esmoopen-2020-000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 2018;95:77–99. doi: 10.1016/j.jaut.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y, Lin Q, Zhang Z, Zhang L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm Sin B. 2020;10(3):414–433. doi: 10.1016/j.apsb.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav R, Redmond WL. Current clinical trial landscape of OX40 agonists. Curr Oncol Rep. 2022;24(7):951–960. doi: 10.1007/s11912-022-01265-5. [DOI] [PubMed] [Google Scholar]
- 28.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27(1):19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 29.Wajant H. Principles of antibody-mediated TNF receptor activation. Cell Death Differ. 2015;22(11):1727–1741. doi: 10.1038/cdd.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucka K, Wajant H. Receptor oligomerization and its relevance for signaling by receptors of the tumor necrosis factor receptor superfamily. Front Cell Dev Biol. 2021;8:615141. doi: 10.3389/fcell.2020.615141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin SM, Kimberlin CR, Roe-Zurz Z, et al. Structure of the 4–1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab. Nat Commun. 2018;9(1):4679. doi: 10.1038/s41467-018-07136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho SK, Xu Z, Thakur A, et al. Epitope and Fc-mediated cross-linking, but not high affinity, are critical for antitumor activity of CD137 agonist antibody with reduced liver toxicity. Mol Cancer Ther. 2020;19(4):1040–1051. doi: 10.1158/1535-7163.MCT-19-0608. [DOI] [PubMed] [Google Scholar]
- 33.Medler J, Nelke J, Weisenberger D, et al. TNFRSF receptor-specific antibody fusion proteins with targeting controlled FcγR-independent agonistic activity. Cell Death Dis. 2019;10(3):224. doi: 10.1038/s41419-019-1456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi X, Li F, Wu Y, et al. Optimization of 4–1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat Commun. 2019;10(1):2141. doi: 10.1038/s41467-019-10088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jure-Kunkel, M. et al. Polynucleotides encoding fully human antibodies against human 4-1BB. US patent 7659384B2 (2010).
- 36.Fisher TS, Kamperschroer C, Oliphant T, et al. Targeting of 4–1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother. 2012;61(10):1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal NH, Logan TF, Hodi FS, et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res. 2017;23(8):1929–1936. doi: 10.1158/1078-0432.CCR-16-1272. [DOI] [PubMed] [Google Scholar]
- 38.Ascierto PA, Simeone E, Sznol M, et al. Clinical experiences with anti - CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4–1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131(1):49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Wu Y, Ye K, et al. Antibody-targeted TNFRSF activation for cancer immunotherapy: the role of FcγRIIB cross-linking. Front Pharmacol. 2022;13:924197. doi: 10.3389/fphar.2022.924197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boulard P, Gouilleux-Gruart V, Watier H. Finding the right heavy chains for immunostimulatory antibodies. Int J Mol Sci. 2022;23(18):10367. doi: 10.3390/ijms231810367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seidel-Dugan, C. et al 2018 => 33rd annual meeting & pre-conference programs of the society for immunotherapy of cancer (SITC 2018): Washington, D.C., USA. 7-11 November 2018. J Immunother Cancer 2018; 6(Suppl 1):114. doi: 10.1186/s40425-018-0422-y [DOI] [PMC free article] [PubMed]
- 43.Yang Y, Yeh SH, Madireddi S, et al. Tetravalent biepitopic targeting enables intrinsic antibody agonism of tumor necrosis factor receptor superfamily members. MAbs. 2019;11(6):996–1011. doi: 10.1080/19420862.2019.1625662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etxeberria I, Bolaños E, Teijeira A, et al. Antitumor efficacy and reduced toxicity using an anti-CD137 Probody therapeutic. Proc Natl Acad Sci U S A. 2021;118(26):e2025930118. doi: 10.1073/pnas.2025930118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinner MJ, Aiba RSB, Jaquin TJ, et al. Tumor-Localized Costimulatory T-Cell Engagement by the 4–1BB/HER2 Bispecific Antibody-Anticalin Fusion PRS-343. Clin Cancer Res. 2019;25(19):5878–5889. doi: 10.1158/1078-0432.CCR-18-3654. [DOI] [PubMed] [Google Scholar]
- 46.Peper-Gabriel JK, Pavlidou M, Pattarini L, et al. The PD-L1/4-1BB bispecific antibody-anticalin fusion protein PRS-344/S095012 elicits strong T-cell stimulation in a tumor-localized manner. Clin Cancer Res. 2022;28(15):3387–3399. doi: 10.1158/1078-0432.CCR-21-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi M, Niu M, Xu L, et al. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14(1):10. doi: 10.1186/s13045-020-01027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyung-Don K, Park S, Jeong S, et al. 4–1BB delineates distinct activation status of exhausted tumor-infiltrating CD8+ T cells in hepatocellular carcinoma. Hepatology. 2020;71:955–971. doi: 10.1002/hep.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeong S, Park E, Kim HD, et al. Novel anti-4-1BB×PD-L1 bispecific antibody augments anti-tumor immunity through tumor-directed T-cell activation and checkpoint blockade. J Immunother Cancer. 2021;9(7):e002428. doi: 10.1136/jitc-2021-002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muik A, Garralda E, Altintas I, et al. Preclinical characterization and phase i trial results of a bispecific antibody targeting PD-L1 and 4–1BB (GEN1046) in patients with advanced refractory solid tumors. Cancer Discov. 2022;12(5):1248–1265. doi: 10.1158/2159-8290.CD-21-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lakins MA, Koers A, Giambalvo R, et al. FS222, a CD137/PD-L1 tetravalent bispecific antibody, exhibits low toxicity and antitumor activity in colorectal cancer models. Clin Cancer Res. 2020;26(15):4154–4167. doi: 10.1158/1078-0432.CCR-19-2958. [DOI] [PubMed] [Google Scholar]
- 52.Homepage of Inhibrx, https://inhibrx.com/inbrx-105/; homepage of Crescendo Biologics, https://www.crescendobiologics.com/. Accessed 10 Oct 2022.
- 53.Snell et al., AACR Annual Meeting 2020, Poster #2276 ; AACR Annual Meeting 2022, Poster #2870
- 54.Compte M, Harwood SL, Erce-Llamazares A, et al. An Fc-free EGFR-specific 4–1BB-agonistic trimerbody displays broad antitumor activity in humanized murine cancer models without toxicity. Clin Cancer Res. 2021;27(11):3167–3177. doi: 10.1158/1078-0432.CCR-20-4625. [DOI] [PubMed] [Google Scholar]
- 55.Compte M, Harwood SL, Muñoz IG, et al. A tumor-targeted trimeric 4–1BB-agonistic antibody induces potent anti-tumor immunity without systemic toxicity. Nat Commun. 2018;9(1):4809. doi: 10.1038/s41467-018-07195-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Compte M, Harwood SL, Martínez-Torrecuadrada J, et al. Case report: an EGFR-targeted 4–1BB-agonistic Trimerbody does not induce hepatotoxicity in transgenic mice with liver expression of human EGFR. Front Immunol. 2021;11:614363. doi: 10.3389/fimmu.2020.614363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oberst MD, Augé C, Morris C, et al. Potent immune modulation by MEDI6383, an engineered human OX40 ligand IgG4P Fc fusion protein. Mol Cancer Ther. 2018;17(5):1024–1038. doi: 10.1158/1535-7163.MCT-17-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tigue NJ, Bamber L, Andrews J, et al. MEDI1873, a potent, stabilized hexameric agonist of human GITR with regulatory T-cell targeting potential. Oncoimmunology. 2017;6(3):e1280645. doi: 10.1080/2162402X.2017.1280645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balmanoukian AS, Infante JR, Aljumaily R, et al. Safety and clinical activity of MEDI1873, a novel GITR agonist, in advanced solid tumors. Clin Cancer Res. 2020;26(23):6196–6203. doi: 10.1158/1078-0432.CCR-20-0452. [DOI] [PubMed] [Google Scholar]
- 60.Richards DM, Marschall V, Billian-Frey K, et al. HERA-GITRL activates T cells and promotes anti-tumor efficacy independent of FcγR-binding functionality. J Immunother Cancer. 2019;7(1):191. doi: 10.1186/s40425-019-0671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merz C, Sykora J, Marschall V, et al. The hexavalent CD40 agonist HERA-CD40L induces T-cell-mediated antitumor immune response through activation of antigen-presenting cells. J Immunother. 2018;41(9):385–398. doi: 10.1097/CJI.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 62.Thiemann M, Richards DM, Heinonen K, et al. A single-chain-based hexavalent CD27 agonist enhances T cell activation and induces anti-tumor immunity. Front Oncol. 2018;8:387. doi: 10.3389/fonc.2018.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fellermeier-Kopf S, Gieseke F, Sahin U, et al. Duokines: a novel class of dual-acting co-stimulatory molecules acting in cis or trans. Oncoimmunology. 2018;7(9):e1471442. doi: 10.1080/2162402X.2018.1471442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aschmoneit N, Kocher K, Siegemund M, et al. Fc-based Duokines: dual-acting costimulatory molecules comprising TNFSF ligands in the single-chain format fused to a heterodimerizing Fc (scDk-Fc) Oncoimmunology. 2022;11(1):2028961. doi: 10.1080/2162402X.2022.2028961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Müller D, Frey K, Kontermann RE. A novel antibody-4-1BBL fusion protein for targeted costimulation in cancer immunotherapy. J Immunother. 2008;31(8):714–722. doi: 10.1097/CJI.0b013e31818353e9. [DOI] [PubMed] [Google Scholar]
- 66.Hornig N, Kermer V, Frey K, et al. Combination of a bispecific antibody and costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. J Immunother. 2012;35(5):418–429. doi: 10.1097/CJI.0b013e3182594387. [DOI] [PubMed] [Google Scholar]
- 67.Hornig N, Reinhardt K, Kermer V, et al. Evaluating combinations of costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. Cancer Immunol Immunother. 2013;62(8):1369–1380. doi: 10.1007/s00262-013-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sapski S, Beha N, Kontermann RE, Müller D. Influence of antigen density and immunosuppressive factors on tumor-targeted costimulation with antibody-fusion proteins and bispecific antibody-mediated T cell response. Cancer Immunol Immunother. 2020;69(11):2291–2303. doi: 10.1007/s00262-020-02624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mock J, Stringhini M, Villa A, et al. An engineered 4–1BBL fusion protein with "activity on demand". Proc Natl Acad Sci U S A. 2020;117(50):31780–31788. doi: 10.1073/pnas.2013615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Claus C, Ferrara C, Xu W, et al. Tumor-targeted 4–1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019;11(496):eaav5989. doi: 10.1126/scitranslmed.aav5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fellermeier S, Beha N, Meyer JE, et al. Advancing targeted co-stimulation with antibody-fusion proteins by introducing TNF superfamily members in a single-chain format. Oncoimmunology. 2016;5(11):e1238540. doi: 10.1080/2162402X.2016.1238540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beha N, Harder M, Ring S, et al. IL15-based trifunctional antibody-fusion proteins with costimulatory TNF-superfamily ligands in the single-chain format for cancer immunotherapy. Mol Cancer Ther. 2019;18(7):1278–1288. doi: 10.1158/1535-7163.MCT-18-1204. [DOI] [PubMed] [Google Scholar]
- 73.Chan S, Belmar N, Ho S, et al. An anti-PD-1-GITR-L bispecific agonist induces GITR clustering-mediated T cell activation for cancer immunotherapy. Nat Cancer. 2020;3(3):337–354. doi: 10.1038/s43018-022-00334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fromm G, de Silva S, Johannes K, et al. Agonist redirected checkpoint, PD1-Fc-OX40L, for cancer immunotherapy. J Immunother Cancer. 2018;6(1):149. doi: 10.1186/s40425-018-0454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Messenheimer DJ, Jensen SM, Afentoulis ME, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res. 2017;23(20):6165–6177. doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]