Abstract
Squamous mucosal epithelial cells constitutively express calprotectin in the cytoplasm. To study how this antimicrobial protein complex confers epithelial resistance to invading bacteria, an epithelial cell line was stably transfected to express the calprotectin complex. Cells expressing calprotectin resist invasion by Listeria monocytogenes and Salmonella enterica serovar Typhimurium. Calprotectin expression was accompanied by altered actin organization, increased α3 integrin expression, and spreading cell morphology. In this study, we assessed whether calprotectin expression affects bacterial binding and uptake. Threefold-fewer Listeria organisms bound to the surfaces of calprotectin-expressing cells, and 10-fold fewer were localized intracellularly by immunofluorescence. Similarly, fewer Salmonella organisms bound to cells expressing calprotectin. Calprotectin-expressing and sham-transfected cells showed similar levels of expression of surface E-cadherin and intracellular adhesion molecule 1 (ICAM-1) by flow cytometry. Calprotectin-expressing transfectants expressed calprotectin on the cell surface as well as in the cytosol. In conclusion, two bacterial pathogens showed reduced binding to calprotectin-expressing epithelial cells. Calprotectin-expressing cells appeared to have internalized disproportionately fewer Listeria organisms, suggesting that reduced binding and translocation supplemented direct antimicrobial effects in calprotectin-expressing cells.
Among their functions, epithelia serve as a physical barrier to protect the connective tissue from infiltration and infection by microbes from the surface. In response to certain microbes, epithelial cells express several inflammatory cytokines and potent antimicrobial proteins, suggesting an additional role in the innate defense of the host (3, 30). A broad-spectrum antimicrobial protein (19, 28, 29), calprotectin is constitutively expressed in neutrophils, monocytes (8), activated macrophages (22), and keratinocytes (33). Squamous mucosal epithelia constitutively express calprotectin (33). In contrast, normal skin and other mucosal epithelia do not express calprotectin (33). Expression of calprotectin is induced, however, in response to inflammation such as in psoriasis, lichen planus (15), and ulcerative colitis (17).
Calprotectin is a heterodimer of two small anionic proteins, MRP8 and MRP14, which belong to the S100 calcium-binding protein family (16). Also described as L1 antigen, cystic fibrosis antigen, calgranulin A-calgranulin B, MRP8-MRP14, and S100A8-S100A9 (13), several functions of calprotectin are speculated. Calprotectin in serum and body fluids is significantly increased during inflammation such as in rheumatoid arthritis and cystic fibrosis, suggesting a regulatory role in inflammatory reactions (2, 12). Purified calprotectin inhibits cell growth and induces apoptosis in mouse and human cell lines (20, 34). Calprotectin expression is also associated with differentiation stages of myelomonocytic and keratinocyte cell lineages (16, 23). Finally, calprotectin may protect epithelia against infection and contribute to innate immunity, given its broad-spectrum antimicrobial effects (5).
We developed stable mucosal epithelial cell lines expressing calprotectin which were less susceptible to invasion by pathogens, including Listeria monocytogenes and Salmonella enterica serovar Typhimurium, than sham-control transfectants (K. Nisapukultorn, K. F. Ross, and M. C. Herzberg, submitted for publication). The finding supports the role of calprotectin in innate host defense. In addition, calprotectin expression was associated with increased long actin filament formation, increased α3 integrin expression, and spreading cell morphology. These changes may potentially affect bacterial binding and internalization. Therefore, in this study, we determined whether calprotection expression affects bacterial binding and uptake.
MATERIALS AND METHODS
Stable epithelial cell lines expressing calprotectin.
Stable KB epithelial cells expressing calprotectin (KB-MRP8/14) and KB sham-control transfectants (KB-EGFP) were generated previously (Nisapakultorn et al., submitted). Briefly, KB cells (American Type Culture Collection, ATCC CCL-17), a calprotectin-negative oral carcinoma cell line, were cotransfected with the mammalian expression vector pIRES-EGFP (Clontech, Palo Alto, Calif.), containing MRP8 and MRP14 genes and the selectable marker pSV2-neo, to generate the KB-MRP8/14 cell line. A sham-control transfectant cell line, KB-EGFP, was generated by cotransfection of insertless pIRES-EGFP and pSV2-neo. As previously determined (Nisapakultorn et al., submitted), calprotectin expression in the cytosols of transfected cells was verified both by sandwich enzyme-linked immunosorbent assay and by indirect immunofluorescence using monoclonal antibody (MAb) 27E10 (Bachem, King of Prussia, Pa.), which is specific for the MRP8-MRP14 heterodimer (4).
Cell culture.
KB-EGFP and KB-MRP8/14 cells were maintained in modified Eagle medium supplemented with 10% fetal bovine serum and 700 μg of Geneticin (G418 sulfate; Mediatech, Herndon, Va.) per ml. To avoid an antimicrobial effect from Geneticin, KB transfectants were grown in medium without Geneticin for 4 days before experiments.
Bacteria.
L. monocytogenes ATCC 43249 and Salmonella serovar Typhimurium ATCC 14028 were grown in brain heart infusion medium and on tryptic soy agar (Difco). Listeria cells were harvested from log-phase cultures cells were (optical density of 0.4 to 0.6 at 620 nm), and Salmonella harvested from stationary-phase cultures. Freshly harvested bacteria at the multiplicity of infection (MOI) noted below were used to infect KB transfectants.
Immunofluorescence analysis of intracellular and extracellular Listeria.
Fluorescence labeling to differentiate intracellular and extracellular bacteria was performed as described previously (31). KB-EGFP and KB-MRP8/14 (1.2 × 105 cells) were grown overnight on gelatin-coated coverslips. The KB transfectants were infected with L. monocytogenes at an MOI of 100 for 2 h, washed twice with Dulbecco's phosphate-buffered saline (PBS), and incubated with gentamicin (100 μg/ml)-containing medium for 1.5 h. The monolayers were washed again to remove nonadherent bacteria and fixed for 10 min with 4% paraformaldehyde. Extracellular Listeria cells were stained by incubation with rabbit anti-Listeria serum (dilution, 1:3,000; Biodesign, Kennebunk, Maine) for 2 h and then with goat anti-rabbit Alexa 350 (dilution, 1:100; Molecular Probes, Eugene, Oreg.) for 1 h. All antibodies were diluted in PBS containing 3% (wt/vol) bovine serum albumin. Cell monolayers were then permeabilized with 0.2% Triton X-100 for 2 min to allow staining of both intracellular and extracellular Listeria cells (total Listeria). The monolayers were washed and incubated with rabbit anti-Listeria serum (dilution, 1:3,000) for 2 h and then with goat anti-rabbit-Alexa 568 (dilution, 1:500; Molecular Probes) for 1 h. Coverslips were mounted with Fluoromount G (Southern Biotechnologies, Birmingham, Ala.) and observed with a Nikon Eclipse fluorescence microscope. Images from 20 random microscopic fields at a ×400 magnification were captured with a Spot camera (Diagnostic Instruments Inc., Sterling Heights, Mich.). The numbers of total Listeria organisms (Alexa 568) and extracellular Listeria organisms (Alexa 350) from the same field were counted. The number of intracellular Listeria organisms was obtained by subtracting the number of extracellular Listeria organisms (Alexa 350) from the number of total Listeria organisms (Alexa 568).
Bacterial-adhesion assay.
KB-EGFP and KB-MRP8/14 cells were grown to confluence on gelatin-coated coverslips. KB transfectants were incubated with Listeria (1.2 × 107 CFU) or Salmonella (1.2 × 106 CFU) for 15, 30, 45, and 60 min at 37°C. Salmonella cells bound in such high numbers that a smaller input was used to permit counting with more confidence. The monolayers were washed twice with Dulbecco's PBS and fixed for 10 min with 4% paraformaldehyde. Adherent bacteria were stained with rabbit anti-Listeria (dilution, 1:3,000) or rabbit anti-Salmonella (dilution, 1:3,000; Biodesign) and then with goat anti-rabbit Alexa 568 (dilution, 1:500). At each time point, images from 10 random microscopic fields at a ×200 magnification were captured with a Spot camera (Diagnostic Instruments Inc.) and the number of adherent bacteria in each field was counted.
Flow cytometry.
To obtain cells in suspension, the KB-EGFP and KB-MRP8/14 cells were removed from flasks by incubating them with 0.02% (wt/vol) EDTA for 2 min and then with 0.17% trypsin–0.02% EDTA for 1 min. The KB transfectants were washed once with wash buffer (PBS with 2% fetal bovine serum) and fixed (5 × 105 cells in 100 μl) for 10 min with 4% paraformaldehyde. Cells were then washed twice with wash buffer and incubated with 1 μg of a primary antibody (mouse immunoglobulin G1 isotype control [Pharmingen, San Diego, Calif.], 27E10 MAb, anti-E-cadherin antibody [Santa Cruz Biotechnology, Santa Cruz, Calif.], or anti-intracellular adhesion molecule 1 (ICAM-1) antibody [Sigma, St. Louis, Mo.]) for 30 min at 4°C. Cells were washed and incubated with donkey anti-mouse Fab fragment conjugated with phycoerythrin (1:50 dilution; Jackson ImmunoResearch, West Grove, Pa.) for 30 min at 4°C. Cells were washed again and resuspended in 500 μl of wash buffer. The labeled KB transfectants (10,000 events for each sample) were analyzed by flow cytometric analysis with a FACscan (Becton Dickinson, San Jose, Calif.).
Statistical analysis.
Data are presented as means ± standard deviations (SD). Statistically significant differences between KB-EGFP and KB-MRP8/14 results were determined by using a two-sample t test for intracellular and extracellular Listeria data and a two-way analysis of variance for bacterial-adhesion assay data.
RESULTS
Analysis of intracellular and extracellular Listeria associated with KB transfectants.
To understand how calprotectin might promote resistance to invasion, extracellular and total Listeria cells associated with KB transfectants were visualized and enumerated. Fivefold fewer total Listeria cells were associated with KB-MRP8/14 monolayers than with KB-EGFP monolayers (Fig. 1). About threefold fewer Listeria cells bound extracellularly to the plasma membranes of KB-MRP8/14 than to those of KB-EGFP cells. By contrast, approximately 10-fold fewer intracellular bacteria were observed in KB-MRP8/14 than in KB-EGFP cells.
FIG. 1.
Number of L. monocytogenes bacteria associated with KB-EGFP and KB-MRP8/14 cells. The monolayers of KB-EGFP and KB-MRP8/14 cells were incubated with L. monocytogenes at an MOI of 100 for 2 h and washed, followed by treatment for 1.5 h with gentamicin. Intracellular and extracellular bacteria were differentiated by double fluorescence staining as described in Materials and Methods. Results are means ± SD of bacterial counts from 20 random microscopic fields at a magnification of ×400. Significantly fewer total bacteria, intracellular bacteria, and extracellular bacteria associated with KB-MRP8/14 than with KB-EGFP cells. ∗, P < 0.01.
Binding of Listeria and Salmonella to KB transfectants.
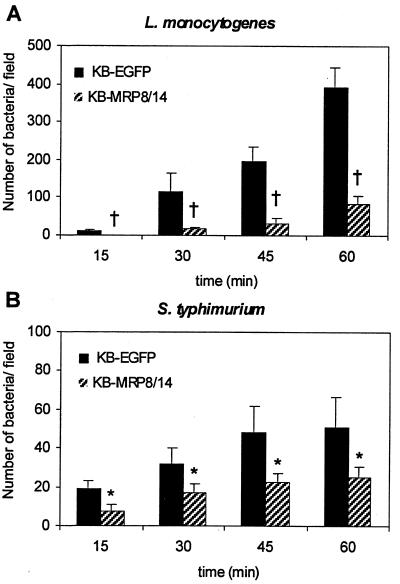
Listeria and Salmonella were incubated with KB transfectants for 15, 30, 45, and 60 min. After each incubation period, bacteria that bound extracellularly to KB transfectants were stained and counted. The number of Listeria and Salmonella organisms that bound to KB transfectants increased over time (Fig. 2). At all time points, fewer Listeria and Salmonella organisms bound to KB-MRP8/14 than to KB-EGFP cells.
FIG. 2.
Binding of L. monocytogenes and Salmonella serovar Typhimurium organisms to KB transfectants. The confluent monolayers of KB-EGFP and KB-MRP8/14 cells were incubated with L. monocytogenes (1.2 × 107 CFU) or Salmonella serovar Typhimurium (1.2 × 106 CFU) for 15, 30, 45, and 60 min. After each time point, the nonadherent bacteria were removed by washing. Bacteria that remained bound to the monolayers were fluorescently labeled and counted. Results are means ± SD of bacterial counts from 10 random microscopic fields at a magnification of ×200. Significantly fewer Listeria and Salmonella organisms bound to KB-MRP8/14 than to KB-EGFP cells. †, P < 0.01; ∗, P < 0.05.
Expression of cell surface proteins on KB transfectants.
MRP8-MRP14 complexes are expressed in the cytoplasms of KB-MRP8/14 but not KB-EGFP cells (unpublished data). We analyzed KB-MRP8/14 and KB-EGFP cells for surface expression by flow cytometry. MRP8-MRP14 complexes were expressed on the cell surfaces of about 66% of KB-MRP8/14 cells (Fig. 3A); no MRP8-MRP14 complexes were detected on KB-EGFP cells (Fig. 3B).
FIG. 3.
Cell surface expression of calprotectin by KB-MRP8/14 (A) and KB-EGFP (B) cells. A total of 10,000 events were counted for each sample. The M1 bars indicate the range of immunofluorescence intensity attributed to the isotype control. The M2 bars represented fluorescence intensity by positively stained cells.
L. monocytogenes expresses internalin, encoded by inlA, which is required for invasion into epithelial cells (10). On epithelial cells, E-cadherin serves as an internalin receptor to facilitate uptake of Listeria (18). By flow cytometric analysis, levels of E-cadherin expression were similar on KB-EGFP and KB-MRP8/14 cells (Fig. 4). Approximately 44% of KB-EGFP and KB-MRP8/14 cells expressed E-cadherin. ICAM-1, a prominent cell surface adhesion molecule, served as a positive control. Levels of ICAM-1 expression did not appear to differ on KB-EGFP and KB-MRP8/14 cells (Fig. 4).
FIG. 4.
Cell surface expression of E-cadherin and ICAM-1 by KB-EGFP and KB-MRP8/14 cells. A total of 10,000 events were counted for each sample. The M1 bars indicate the fluorescence intensity attributed to the isotype control. The M2 bars represent fluorescence intensity by positively stained cells.
DISCUSSION
Calprotectin is a protein complex with broad-spectrum antimicrobial activity. By transfection of genes for MRP8 and MRP14 into a calprotectin-negative oral epithelial cell line, we showed that epithelial calprotectin expression reduced invasion by L. monocytogenes and Salmonella serovar Typhimurium (Nisapakultorn et al., submitted). In the present study, we further explored how calprotectin expression affected the invasion process. We showed that calprotectin expression reduced binding of L. monocytogenes and Salmonella serovar Typhimurium and that it may affect internalization of these bacteria into epithelial cells.
Listeria and Salmonella exploit very different mechanisms to facilitate entry into epithelial cells (11, 18), yet calprotectin expression reduces the binding of both species over time. While the binding proteins on epithelial cells for Salmonella are presently unknown, E-cadherin serves as a receptor for Listeria and is required for uptake into epithelial cells (10, 18). As determined by fluorescence-activated cell sorter analysis, we showed that levels of cell surface expression of E-cadherin in KB-EGFP and KB-MRP8/14 cells were similar. ICAM-1 is an epithelial cell surface adhesion molecule and pathogen receptor for species as diverse as Plasmodium falciparum and Coxsackie viruses (1). Expression of ICAM-1 is also unaltered. Therefore, decreased bacterial binding to KB-MRP8/14 cells is not due to changes in expression of E-cadherin or ICAM-1. Reduced bacterial binding, however, may be a result of increased expression of α3 integrin in cells expressing calprotectin (Nisapakultorn et al., submitted). A calprotectin-associated increase in α3 integrin expression may enhance intercellular junctions and attachment to the substratum (6, 7), limiting access to bacterial receptors on epithelial cells.
Calprotectin is expressed on the plasma membranes of 15 to 20% of freshly isolated neutrophils and monocytes as well as on inflamed epithelial cells in vivo (8, 27, 35). Expressed primarily in cell cytosol, calprotectin lacks a signal peptide, N-linked glycosylation, and membrane anchor sequences (16). In the absence of these structural features common to exported or plasma membrane proteins, calprotectin, like other S100 proteins, can be expressed on the cell surface and released or secreted from cells (9). In the present study, we found that approximately 66% of KB-MRP8/14 cells express calprotectin on cell surfaces. Cells in culture also release calprotectin into the medium (our unpublished observation). How calprotectin gets to the cell surface and is released is unclear. In monocytes, the release of calprotectin requires an intact microtubule network (25). The calprotectin heterocomplex, but not the single subunits, has a high calcium-dependent affinity for arachidonic acid (14, 26). In neutrophils, calprotectin is released in heterotypic complex with arachidonic acid (14). If similar mechanisms operate in KB-MRP8/14 cells, it remains to be determined if membrane-associated or released calprotectin is directly antimicrobial and how the binding of bacteria would be affected.
Although the invasion process can be artificially divided into steps involving binding of bacteria to the cell surface and uptake of the bacteria into host cells, there is no clear distinction between each step. Some surface-bound bacteria may actually be in the process of translocating into the host cells. Hence, the reduction in the number of Listeria and Salmonella cells bound to KB-MRP8/14 cells may also reflect an effect of calprotectin on bacterial uptake. In fact, using double immunofluorescence to differentiate intracellular and extracellular bacteria, we found that almost 10-fold fewer intracellular Listeria organisms but only 3-fold fewer Listeria organism bound to KB-MRP8/14 than to KB-EGFP cells. This discrepancy suggests that (i) calprotectin expression may interfere with bacterial internalization and/or (ii) calprotectin kills or inhibits growth of intracellular bacteria. Thus, not only may calprotectin have an intracellular antimicrobial affect, as suggested by in vitro data (21, 30), calprotectin may also interfere with internalization of bacteria into epithelial cells.
Reduced bacterial internalization may be associated with altered actin organization in calprotectin-expressing cells and interference with signal transduction by calprotection. We previously observed increased long actin filament formation in cells expressing calprotectin (Nisapakultorn et al., submitted) compared to that in sham-transfected and untransfected parental cells. During invasion by Listeria and Salmonella, host cells rapidly respond through an elevation of intracellular calcium (24, 32). Calcium is a key secondary messenger during signal transduction. As a calcium-binding protein, calprotectin may bind free intracellular calcium and interfere with signal transduction. Hence, calprotectin expression may contribute to reduced bacterial internalization by altering actin organization and calcium-dependent signaling.
In conclusion, these data provide supporting evidence that calprotectin expression in mucosal epithelial cells may play a protective role in innate host defense. Calprotectin expression reduces epithelial invasion by pathogens, including Listeria and Salmonella. Calprotectin mediates protection against invasion in vitro by several novel mechanisms. Associated with expression, bacterial binding is reduced. It remains unclear if the effect of calprotectin is associated directly or indirectly with cell surface calprotectin. Cell surface α3 integrin is upregulated with calprotectin and is likely to reduce access to key receptors. Calprotectin also interferes with internalization, perhaps by modifying calcium signaling and actin organization. These mechanisms may complement antimicrobial activity of calprotectin within the cytoplasm. Based on in vitro experiments, calprotectin is a multifunctional protein employing several modes of action to contribute to innate epithelial immunity against infection.
ACKNOWLEDGMENTS
This project was supported by NIH NIDCR grants R01DE11831 and P30DE09737. K. Nisapakultorn also was supported by a scholarship from the Royal Thai Government.
We appreciate the assistance with flow cytometry provided by Massimo Costalonga and with statistical analysis provided by James Hodges.
REFERENCES
- 1.Bella J, Rossmann M G. Review: rhinoviruses and their ICAM receptors. J Struct Biol. 1999;128:69–74. doi: 10.1006/jsbi.1999.4143. [DOI] [PubMed] [Google Scholar]
- 2.Berntzen H B, Munthe E, Fagerhol M K. A longitudinal study of the leukocyte protein L1 as an indicator of disease activity in patients with rheumatoid arthritis. J Rheumatol. 1989;16:1416–1420. [PubMed] [Google Scholar]
- 3.Bevins C L. Antimicrobial peptides as agents of mucosal immunity. Ciba Found Symp. 1994;186:250–269. doi: 10.1002/9780470514658.ch15. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj R S, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, Mahnke K, Falk M, Meinardus-Hager G, Sorg C. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol. 1992;22:1891–1897. doi: 10.1002/eji.1830220732. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Gabrielsen T O, Dale I, Muller F, Steinbakk M, Fagerhol M K. The leucocyte protein L1 (calprotectin): a putative nonspecific defence factor at epithelial surfaces. Adv Exp Med Biol. 1995;371A:201–206. doi: 10.1007/978-1-4615-1941-6_41. [DOI] [PubMed] [Google Scholar]
- 6.Carter W G, Kaur P, Gil S G, Gahr P J, Wayner E A. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter W G, Wayner E A, Bouchard T S, Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale I, Brandtzaeg P, Fagerhol M K, Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985;84:24–34. doi: 10.1093/ajcp/84.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 10.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 11.Ginocchio C C, Olmsted S B, Wells C L, Galan J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 12.Golden B E, Clohessy P A, Russell G, Fagerhol M K. Calprotectin as a marker of inflammation in cystic fibrosis. Arch Dis Child. 1996;74:136–139. doi: 10.1136/adc.74.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johne B, Fagerhol M K, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen C F, Dale I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113–123. doi: 10.1136/mp.50.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 15.Kunz M, Roth J, Sorg C, Kolde G. Epidermal expression of the calcium binding surface antigen 27E10 in inflammatory skin diseases. Arch Dermatol Res. 1992;284:386–390. doi: 10.1007/BF00372067. [DOI] [PubMed] [Google Scholar]
- 16.Lagasse E, Clerc R G. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol. 1988;8:2402–2410. doi: 10.1128/mcb.8.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugering N, Stoll R, Kucharzik T, Schmid K W, Rohlmann G, Burmeister G, Sorg C, Domschke W. Immunohistochemical distribution and serum levels of the Ca(2+)-binding proteins MRP8, MRP14 and their heterodimeric form MRP8/14 in Crohn's disease. Digestion. 1995;56:406–414. doi: 10.1159/000201267. [DOI] [PubMed] [Google Scholar]
- 18.Mengaud J, Ohayon H, Gounon P, Mege R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 19.Miyasaki K T, Bodeau A L, Murthy A R, Lehrer R I. In vitro antimicrobial activity of the human neutrophil cytosolic S-100 protein complex, calprotectin, against Capnocytophaga sputigena. J Dent Res. 1993;72:517–523. doi: 10.1177/00220345930720020801. [DOI] [PubMed] [Google Scholar]
- 20.Murao S, Collart F, Huberman E. A protein complex expressed during terminal differentiation of monomyelocytic cells is an inhibitor of cell growth. Cell Growth Differ. 1990;1:447–454. [PubMed] [Google Scholar]
- 21.Murthy A R, Lehrer R I, Harwig S S, Miyasaki K T. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–6301. [PubMed] [Google Scholar]
- 22.Odink K, Cerletti N, Bruggen J, Clerc R G, Taresay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 23.Olsen E, Rasmussen H H, Celis J E. Identification of proteins that are abnormally regulated in differentiated cultured human keratinocytes. Electrophoresis. 1995;16:2241–2248. doi: 10.1002/elps.11501601356. [DOI] [PubMed] [Google Scholar]
- 24.Pace J, Hayman M J, Galan J E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 25.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Bio Chem. 1997;272:9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 26.Siegenthaler G, Roulin K, Chatellard-Gruaz D, Hotz R, Saurat J H, Hellman U, Hagens G. A heterocomplex formed by the calcium-binding proteins MRP8 (S100A8) and MRP14 (S100A9) binds unsaturated fatty acids with high affinity. J Biol Chem. 1997;272:9371–9377. doi: 10.1074/jbc.272.14.9371. [DOI] [PubMed] [Google Scholar]
- 27.Simon M, Jr, Hunyadi J, Gomez R S. Macrophage markers 25F9 and 27E10 on human keratinocytes in normal and diseased skin. J Dermatol. 1993;20:618–622. doi: 10.1111/j.1346-8138.1993.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 28.Sohnle P G, Collins-Lech C, Wiessner J H. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991;163:187–192. doi: 10.1093/infdis/163.1.187. [DOI] [PubMed] [Google Scholar]
- 29.Steinbakk M, Naess-Andresen C F, Lingaas E, Dale I, Brandtzaeg P, Fagerhol M K. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 30.Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defense. Curr Opin Microbiol. 1999;2:99–105. doi: 10.1016/s1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- 31.Tang P, Foubister V, Pucciarelli G, Finlay B B. Methods to study bacterial invasion. J Microbiol Methods. 1993;18:227–240. [Google Scholar]
- 32.Wadsworth S J, Goldfine H. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect Immun. 1999;67:1770–1778. doi: 10.1128/iai.67.4.1770-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson M M, Busuttil A, Hayward C, Brock D J, Dorin J R, Van Heyningen V. Expression pattern of two related cystic fibrosis-associated calcium-binding proteins in normal and abnormal tissues. J Cell Sci. 1988;91:221–230. doi: 10.1242/jcs.91.2.221. [DOI] [PubMed] [Google Scholar]
- 34.Yui S, Mikami M, Yamazaki M. Purification and characterization of the cytotoxic factor in rat peritoneal exudate cells: its identification as the calcium binding protein complex, calprotectin. J Leukoc Biol. 1995;58:307–316. doi: 10.1002/jlb.58.3.307. [DOI] [PubMed] [Google Scholar]
- 35.Zwadlo G, Schlegel R, Sorg C. A monoclonal antibody to a subset of human monocytes found only in the peripheral blood and inflammatory tissues. J Immunol. 1986;137:512–518. [PubMed] [Google Scholar]