Abstract
Psoriasis is a systemic immune-mediated disease associated with an increased risk of comorbidities, such as psoriatic arthritis, cardiovascular disease, metabolic syndrome, inflammatory bowel disease, psychiatric disorders, and malignancy. In recent years, with the advent of biological agents, the efficacy and safety of psoriasis treatments have dramatically improved. Presently, tumor necrosis factor-α inhibitors, interleukin-17 inhibitors, interleukin-12/23 inhibitors, and interleukin-23 inhibitors are approved to treat moderate-to-severe psoriasis. Small-molecule inhibitors, such as apremilast and deucravacitinib, are also approved for the treatment of psoriasis. Although it is still unclear, systemic agents used to treat psoriasis also have a significant impact on its comorbidities by altering the systemic inflammatory state. Data from clinical trials and studies on the safety and efficacy of biologics and small-molecule inhibitors provide important information for the personalized care and treatment for patients with psoriasis. Notably, treatment with interleukin-17 inhibitors is associated with new-onset or exacerbations of inflammatory bowel disease. In addition, great caution needs to be taken when using tumor necrosis factor-α inhibitors in patients with psoriasis with concomitant congestive heart failure, multiple sclerosis, and malignancy. Apremilast may induce weight loss as an adverse effect, presenting also with some beneficial metabolic actions. A better understanding of the characteristics of biologics and small-molecule inhibitors in the treatment of psoriasis comorbidities can provide more definitive guidance for patients with distinct comorbidities.
Key Points
| Treatment regimens for patients with psoriasis should be tailored to meet the specific needs based on disease severity, the impact on quality of life, the response to previous therapies, and the presence of comorbidities. |
| Evidence from clinical trials provides pertinent information regarding the safety and efficacy of biologic agents and small-molecule drugs for psoriasis, which should be integrated into clinical decision making. Furthermore, practical recommendations are necessary regarding first-line systemic therapy options for patients with psoriasis and distinct comorbid conditions. |
| We provide evidence-based recommendations for the use of psoriasis treatments in patients with distinct comorbidities. |
Introduction
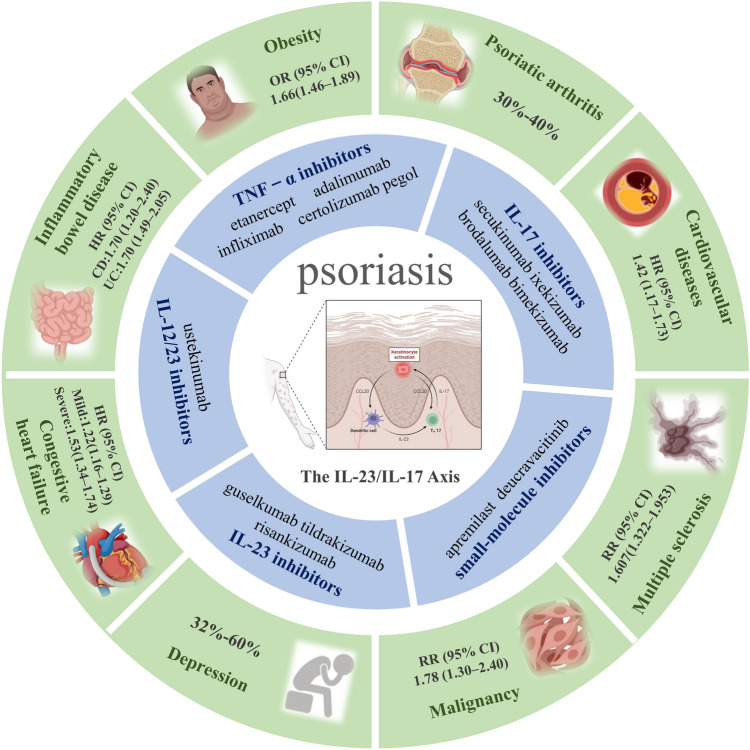
Psoriasis is a chronic, inflammatory, immune-mediated, systemic disease affecting approximately 2–3% of the population worldwide [1, 2]. An improved understanding of psoriasis pathogenesis has led to the identification of multiple new therapeutic targets, including anti-tumor necrosis factor (TNF)-α, anti-interleukin (IL)-17, anti-IL-12/23, and anti-IL-23 therapies, which have revolutionized the treatment options for psoriasis [3, 4]. However, no single therapy options work for all patients. Psoriasis is associated with certain comorbid conditions, such as psoriatic arthritis (PsA), cardiovascular disease, metabolic syndrome, inflammatory bowel disease (IBD), psychiatric disorders, multiple sclerosis (MS), and malignancy (Fig. 1) [5]. The shared immunologic and genetic features may in part help explain the epidemiological association between psoriasis and psoriasis-related comorbidities [6, 7]. These comorbid disease conditions often impact the clinical decision to select one therapy over another, and it is important to consider that certain treatments may improve or exacerbate comorbidities of psoriasis [8]. Indeed, biological therapies targeting pro-inflammatory cytokines in the pathogenesis of psoriasis might not only improve skin symptoms but also alter systemic inflammation, hence influencing long-term outcomes of psoriasis comorbidities [9, 10]. Currently, there are four classes of biologic agents approved by the US Food and Drug Administration (FDA) for use in moderate-to-severe psoriasis, including TNF-α inhibitors (etanercept, adalimumab, infliximab, certolizumab pegol), anti-IL-12/23 agents (ustekinumab), anti-IL-17 agents (secukinumab, ixekizumab, brodalumab), and anti-IL-23 agents (guselkumab, tildrakizumab, risankizumab) (Fig. 1) [11]. Although not currently approved by FDA, we also discussed the treatment recommendation of bimekizumab, which is a monoclonal antibody that selectively neutralises IL-17A and IL-17F [12].
Fig. 1.
Summary of systemic treatment options and comorbidities in psoriasis. The inner ring depicts current biologic and small-molecule inhibitors for psoriasis, the outer ring depicts the strength of the association between psoriasis and comorbidities. CI confidence interval, HR hazard ratio, IL interleukin, OR odds ratio, RR relative risk, TNF tumor necrosis factor, UC ulcerative colitis
In addition, our review included data on small-molecule inhibitors, including oral phosphodiesterase 4 inhibitor (apremilast) and tyrosine kinase 2 inhibitor (deucravacitinib). Importantly, each biologic agent and small-molecule inhibitor has its own advantages and limitations for the treatment of psoriasis [13]. As the available therapeutic targets for psoriasis increased, psoriasis-related comorbidities may complicate the choice of psoriasis treatment. Dermatologists should be aware of the presence of these comorbidities when formulating an individualized treatment plan for a patient with psoriasis [14]. The review focuses on the possible effects of biologic agents and small-molecule inhibitors used to treat psoriasis on psoriasis comorbidities and provides insight into selecting appropriate biologic therapy options for patients with psoriasis with distinct comorbidities.
PsA
Psoriatic arthritis, which is characterized by dactylitis and enthesis, is closely associated with psoriasis. Up to 30–40% of patients with psoriasis will develop PsA, with an average of 10 years between psoriasis and PsA [15]. The overlapping of genetic variations and inflammatory mediators involved in psoriasis and PsA implies a common pathogenic mechanism between the two diseases [16].
TNF-α Inhibitors
The therapeutic effect of TNF-α inhibitors in PsA has been the subject of many clinical trials. In a randomized controlled trial (RCT), patients with psoriasis and PsA receiving etanercept experienced significant clinical improvement at 12 weeks, including Psoriatic Arthritis Response Criteria, American College of Rheumatology 20% response criteria (ACR20), and the Psoriasis Area and Severity Index (PASI) [17]. Mease et al. compared the efficacy of etanercept versus methotrexate in PsA at week 24, and found that etanercept monotherapy sustained superior efficacy than methotrexate monotherapy for ACR20 response at week 24 (etanercept: 60.9%, methotrexate: 50.7%; p = 0.029) [18]. Similarly, other anti-TNF-α agents (adalimumab, infliximab, and certolizumab) also demonstrated a significant impact on the signs and symptoms in patients with PsA and have been approved for clinical use [19–21]. In a network meta-analysis of 46 RCTs comparing the efficacy of various systemic therapies for active PsA, anti-TNF therapies ranked highest in the ACR response [22]. Therefore, we recommend anti-TNF-α agents as the first-line option (Table 1).
Table 1.
Treatment recommendations for patients with psoriasis with distinct comorbidities
| Patients with psoriasis with psoriatic arthritis |
First line: TNF inhibitors, ixekizumab, secukinumab, guselkumab, risankizumab Second line: brodalumab, bimekizumab, apremilast Third line: tildrakizumab, ustekinumab, deucravacitinib |
| Patients with psoriasis with obesity |
First line: IL-17 inhibitors, ustekinumab Second line: IL-23 inhibitors, apremilast Third line: TNF inhibitors |
| Patients with psoriasis with inflammatory bowel disease |
First line: adalimumab, infliximab Second line: certolizumab pegol (for CD), ustekinumab, IL-23 inhibitors (for CD) Third line: apremilast (for UC) Avoid: IL-17 inhibitors |
| Patients with psoriasis with cardiac diseases |
First line: TNF-α inhibitors Second line: IL-17 inhibitors, IL-23 inhibitors, apremilast Third line: ustekinumab |
| Patients with psoriasis with congestive heart failure |
First line: IL-17 inhibitors, IL-23 inhibitors, ustekinumab TNF inhibitors: Avoid in NYHA class III and IV Avoid in patients with ejection fraction <50% |
| Patients with psoriasis with depression |
First line: IL-23 inhibitors Second line: secukinumab, ixekizumab, ustekinumab Third line: TNF inhibitors |
| Patients with psoriasis with multiple sclerosis |
First line: IL-17 inhibitors Second line: ustekinumab Third line: IL-23 inhibitors Avoid: TNF inhibitors |
| Patients with psoriasis with malignancy |
First line: IL-17 inhibitors, IL-23 inhibitors Second line: ustekinumab Avoid: TNF inhibitors |
CD Crohn’s disease, IL interleukin, NYHA New York Heart Association, TNF tumor necrosis factor, UC ulcerative colitis
IL-17 Inhibitors
A phase III RCT for secukinumab reported that secukinumab 150 or 300 mg remarkably improved the signs and symptoms of axial PsA versus placebo [23]. Additionally, secukinumab was tested in two long-term clinical trials for patients with active PsA and demonstrated sustained inhibition of radiographic progression through 104 weeks of therapy [24, 25]. A phase III RCT evaluating secukinumab versus adalimumab in patients with psoriasis with concomitant PsA reported that secukinumab has a higher PASI 100 response (secukinumab: 39.1%, adalimumab: 23.8%; p = 0.0136); responses to ACR20 were similar (secukinumab: 76.4%, adalimumab: 68.3%; p = 0.1752) [26]. In a phase III RCT to assess the safety and efficacy of ixekizumab in biologic-naïve patients with active PsA, ixekizumab led to a significant improvement in disease activity and physical function at week 24 [27]. Another phase III RCT for ixekizumab demonstrated an improvement in health-related quality of life and work productivity through 52 weeks of treatment [28]. An open-label head-to-head study compared the efficacy of ixekizumab versus adalimumab in patients with psoriasis with comorbid PsA for 24 weeks as measured by ACR50 and PASI 100. Data suggested that ixekizumab was superior for PASI 100 (ixekizumab: 60%, adalimumab: 47%; p = 0.001) but non-inferior for ACR50 (ixekizumab: 51%, adalimumab: 47%), with fewer serious adverse events (adalimumab: 8.5%; ixekizumab: 3.5%) [29]. Similarly, in a 52-week multicenter study (SPIRIT), a significantly higher proportion of patients treated with ixekizumab versus adalimumab achieved PASI 100 (64% vs 41%), but there was no difference in ACR50 (49.8% vs 49.8%) at week 52 [30]. Therefore, secukinumab and ixekizumab are also recommended as first-line drugs for patients with PsA.
Brodalumab, although not currently approved for PsA, has shown good results in several studies. A integrative analysis of two phase III RCTs studying brodalumab in patients with PsA indicated that brodalumab treatment led to improvements in signs and symptoms of PsA assessed by ACR20 as compared with placebo (45.8% and 47.9% for 140 mg and 210 mg, respectively, and 20.9% for placebo) [31]. Similarly, a higher proportion of patients achieved patient-reported improvements in the brodalumab group versus placebo at weeks 24 in a phase II study [32]. In a subgroup analysis (n = 928) comparing brodalumab and ustekinumab in patients with psoriasis with comorbid PsA status, brodalumab was more effective in treating PsA than ustekinumab, including quality-of-life improvement and complete skin clearance [33]. Thus, brodalumab was considered to be second-line treatment.
With regard to bimekizumab, in a phase IIb RCT (BE ACTIVE; n = 206) in patients with active PsA for 48 weeks, significantly higher proportions of patients experienced ACR50 response in all groups receiving bimekizumab (24–46%) compared with the placebo group (7%) at week 12, with fewer adverse events (bimekizumab: 41%; placebo: 57%) [34]. The treatment efficacy in skin and joint outcomes was sustained for up to 48 weeks [34]. Merola et al. compared the efficacy of bimekizumab versus placebo in patients with PsA who had previously responded inadequately to TNF inhibitors, and reported significant improvements in the primary endpoint (ACR50; 43.4% vs 6.8%; p < 0.001) at week 12 [35]. The open-label extension study of BE ACTIVE assessed the long-term efficacy and tolerability of bimekizumab in patients with PsA up to a total of 152 weeks. Patients in the open-label extension maintained treatment response and improvements in pain, fatigue, physical function, and health-related quality of life over 3 years [36]. Ongoing phase III studies of bimekizumab in PsA will help to better understand its therapeutic effects. At this time, we recommend bimekizumab as a second-line option for patients with PsA.
IL-23 Inhibitors
Guselkumab was tested in two phase III clinical trials for patients with PsA, and did demonstrate robust efficacy in the signs and symptoms of PsA [37, 38]. Sweet et al. found that the serum protein levels of Th17 effector cytokines (IL-17A and IL-17F) in patients with active PsA were decreased after guselkumab or ustekinumab treatment, but the reductions in the guselkumab group were greater than that in ustekinumab-treated patients [39]. In two phase III RCTs (KEEPsAKE 1 AND 2; n = 964/467), risankizumab has been shown to significantly improve key disease outcomes (ACR20/50/70, PASI 90) versus placebo, and was well tolerated in patients with PsA [40, 41]. Thus, we recommend guselkumab and risankizumab as first-line treatments.
In a phase IIb RCT (n =391), obviously higher ACR20 response rates were identified in patients receiving tildrakizumab versus placebo (tildrakizumab: 71.4–79.5%, placebo: 50.6%; all p < 0.01). However, tildrakizumab treatment did not demonstrate a benefit in dactylitis and enthesitis, and adverse events occurred in 64.5% of patients [42]. Phase III trials of tildrakizumab in patients with PsA are still underway. At this time, we recommend tildrakizumab as a third-line option for patients with PsA.
IL-12/23 Inhibitors
In two phase III RCTs, more patients receiving ustekinumab achieved ACR20 response versus placebo at week 24, and the treatment effect was sustained through week 52 [43, 44]. Another phase III RCT explored the treatment benefits of ustekinumab in PsA at 12 weeks and reported a significant improvement in all study measures [45]. In particular, the efficacy of ustekinumab on skin symptoms appears to be better than that on joint inflammation. Siebert et al. found that the C-reactive protein levels in serum samples, but not the levels of IL-17A or IL-17F, were significantly decreased after ustekinumab treatment in patients with PsA [46]. In a case series, seven patients with psoriasis experienced new-onset or worsening PsA after conversion from a TNF-α inhibitor to ustekinumab [47]. Bonifati and Graceffa reported that five patients who discontinued ustekinumab experienced a remission of joint inflammation after reinstituting TNF-α inhibitor treatment, suggesting TNF-α inhibitors had a higher degree of effectiveness than ustekinumab [48]. As ustekinumab is more effective in treating skin diseases than arthritis, it is considered a third-line treatment in patients with PsA.
Small-Molecule Inhibitors
Apremilast, an oral small-molecule inhibitor of phosphodiesterase 4, is approved by the FDA to treat patients with active PsA [49]. A phase IIIb RCT compared apremilast monotherapy (n = 110) and placebo (n = 109) in biological-naïve patients with PsA for 52 weeks [50]. Significantly higher proportions of patients receiving apremilast achieved the ACR20 response versus placebo (apremilast: 38.2%, placebo: 20.2%; p = 0.004) at week 16, and clinical improvements were sustained through 52 weeks with continued apremilast treatment [50]. Two other phase III RCTs for apremilast in active PsA also demonstrated clinically meaningful improvements in signs, symptoms, physical function, and multiplicity-controlled secondary endpoints [51, 52]. Apremilast is effective in treating PsA, and we recommend apremilast as a second-line treatment.
Deucravacitinib is an oral selective tyrosine kinase 2 inhibitor. In a phase II RCT (n = 203), a significantly higher ACR20 was observed with deucravacitinib compared with placebo (52.9% and 62.7% for 6 mg and 12 mg, respectively, and 31.8% for placebo) [53]. These results suggest that deucravacitinib is a promising treatment option for PsA. Further investigations are required to confirm the efficacy of deucravacitinib for PsA. At this time, we consider deucravacitinib to be a third-line option.
Obesity
The bidirectional association between obesity and psoriasis has been confirmed in multiple studies. First of all, a higher prevalence of obesity has been observed in patients with psoriasis compared with the general population. Armstrong et al. performed a random-effects meta-analysis of 16 observational studies and found that patients with psoriasis have a higher risk of being obese compared with patients without psoriasis (odds ratio [OR] 1.66; 95% confidence interval [CI] 1.46–1.89) [54]. In particular, severe psoriasis showed an increased odds of obesity compared with patients with mild psoriasis (mild, OR 1.46; 95% CI 1.17–1.82; severe, OR 2.23; 95% CI 1.63–3.05). In addition, the impact of weight gain is associated with an increased incidence of developing psoriasis. In a large longitudinal study enrolling 33,734 individuals, obese people have 1.87 times the odds of having psoriasis compared with normal-weight individuals. Moreover, there is a substantially increased risk of psoriasis for people with a long-term weight gain > 10 kg compared with being weight stable (relative risk [RR] 1.72; 95% CI 1.15–2.58) [55].
TNF-α Inhibitors
Infliximab in the therapy of psoriasis is dosed based on weight (5 mg/kg). Petridis et al. explored the impact of body mass index (BMI) on the treatment response and quality-of-life improvement in patients with psoriasis treated with infliximab. Through 1 year of treatment, infliximab significantly improved PASI 75 response and Dermatology Life Quality Index scores in patients with psoriasis, independent of baseline BMI [56]. In the diet-induced obesity animal model, infliximab led to a reduction in the levels of blood glucose and insulin, and restored glucose homeostasis [57]. Surprisingly, patients with psoriasis with concomitant overweight or obesity are more likely to sustain the long-term response to infliximab than patients with normal weight for at least 5 years in a real-world study [58]. The dose of etanercept, adalimumab, and certolizumab pegol in the therapy of psoriasis is fixed, and their efficacy and safety are impaired by obesity. A retrospective study investigated the influence of obesity on the long-term efficacy of etanercept for psoriasis. Data suggested that obesity (BMI ≥ 30 kg/m2) led to a reduction in etanercept efficacy compared with healthy or overweight patients [59]. According to the data of the APHRODITE trial, significantly greater proportions of patients with psoriasis after adalimumab treatment achieved a PASI 50 response in healthy or overweight patient versus obese groups (79% vs 58%, p = 0.02) [60]. Similarly, Prussick et al. reported that normal-weight or overweight patients had higher PASI 75 response rates with adalimumab versus obese patients (normal: 85.0%; overweight: 85.7%; obese: 61.3%) [61]. A pooled analysis of 33 clinical trials explored the role of BMI on the risk of adverse events in 8747 patients following certolizumab pegol therapy. The serious infectious events were observed more frequently in people with a BMI >35 kg/m2 as compared with patients with normal weight [62].
It is also noteworthy that some studies have shown that TNF-α therapies were significantly associated with weight gain. In a cross-sectional study (n = 191), patients with psoriasis gained an average weight of 1.6 kg (2.1%) after 1 year of infliximab treatment compared with baseline weight, mainly in patients with severe psoriasis [63]. As well, in a cross-sectional study of 191 patients with psoriasis, infliximab treatment was associated with severe weight increments [63]. Saraceno et al. performed a retrospective analysis and reported that patients with psoriasis using TNF-α inhibitors experienced an obvious increase in their body weight and BMI at week 24 compared with the control [64]. Similarly, in a meta-analysis comparing the impact of various biologics for psoriasis on the change in body weight and BMI, patients taking TNF-α inhibitors had increases in body weight and BMI more than those on conventional systemic treatments [65]. Tumor necrosis factor-α inhibitors are considered third line for obese patients with psoriasis owing to the potential risk of weight gain with this class of drugs.
IL-12/23 Inhibitors
In contrast to anti-TNF-α agents, patients with psoriasis initiated on ustekinumab did not experience an increase in their body weight or BMI in a prospective cohort study [66]. In particular, ustekinumab in the treatment of psoriasis is also weight dosed. The patients with body weight <100 kg were administered with the 45-mg dose whereas patients with body weight >100 kg were administered with the 90-mg dose [67]. Overall, because ustekinumab is weight adjusted, we recommend this drug as the first-line treatment.
IL-17 Inhibitors
Interleukin-17 inhibitors are highly effective agents independent from body weight. In a summary analysis of three RCTs evaluating the impact of body weight on the treatment efficacy of ixekizumab in patients with psoriasis, ixekizumab showed similar degrees of effectiveness in various weight groups [66]. However, in a phase II dose-ranging study, higher PASI 75 responses rates were sustained in patients with psoriasis weighed < 90 kg than those weighing ≥ 90 kg [68]. Based on the pooled analysis of three phase III studies (FIXTURE, ERASURE, and SCULPTURE), secukinumab treatment led to a decreasing trend in body weight at a follow-up of 52 weeks [69]. Babino et al. presented a case of patient with psoriasis affected by morbid obesity who was treated with secukinumab and, after 48 weeks of the treatment, experienced significant improvement in skin symptoms and metabolic parameters [70]. Likewise, compared with obese patients with psoriasis, normal-weight patients treated with brodalumab had higher PASI 75 and PASI 90 responses at week 52 in a phase III RCT [71]. Here, we recommend anti-IL-17 agents as the first-line treatment choice.
IL-23 Inhibitors
The weight-related data regarding the efficacious anti-IL-23 agents are still sparse, but they are promising therapeutic approaches for obese patients with psoriasis nonetheless. A post hoc analysis of two clinical trials (n = 837/992) evaluated the efficacy of guselkumab in patients with psoriasis grouped by different body weights. Guselkumab was highly efficacious for psoriasis regardless of patient weight [72]. In a real-life study, after guselkumab treatment, the levels of PASI scores in obese and non-obese patients with psoriasis decreased to a similar extent, though the baseline PASI score was higher in obese patients than in non-obese patients [73]. Ruiz-Villaverde et al. reported a case of an obese patient with psoriasis who achieved a successful and sustained response through 52 weeks with risankizumab [74]. Taken together, we also recommend anti-IL-23 agents as second-line therapy.
Small-Molecule Inhibitors
In several clinical trials for psoriasis (ESTEEM1/2 and PALACE3), apremilast has been shown to reduce body weight and increase insulin sensitivity, suggesting some beneficial metabolic actions [51, 75]. Malara et al. evaluated the real-world effectiveness of apremilast in patients with psoriasis and reported a significant improvement in PASI and Dermatology Life Quality Index scores, irrespective of their weight [76]. Considering the potentially advantageous effect on weight loss, we recommend apremilast as the second-line option for patients with obesity and psoriasis.
IBD
Both IBD and psoriasis are chronic inflammatory diseases, and there is a significant association between IBD and psoriasis. The prevalence of psoriasis in patients with Crohn’s disease (CD) was 9.6%, whereas the prevalence was 2.2% in the general population. Likewise, the prevalence of CD was 0.5% in patients with psoriasis but only 0.2% in control groups. [77]. Similar trends occur with ulcerative colitis (UC) [78]. In patients with psoriasis, the OR of CD and UC were 1.70 (CI 1.20–2.40) and 1.75 (CI 1.49–2.05), respectively [79]. The shared immunologic and genetic features have been observed in psoriasis and active CD and UC, and treatments used for psoriasis were also used for IBD [80].
TNF-α Inhibitors
Adalimumab and infliximab have been approved by the FDA for patients with UC and CD, and certolizumab pegol have been approved for the treatment of CD [81]. In a large real-life study, patients with CD receiving long-term therapy of infliximab experienced a sustained clinical benefit through almost 5 years of follow-up, with a low incidence of hospitalization or surgery [82]. In two RCTs, infliximab maintenance therapy after resective intestinal surgery was effective for preventing CD recurrence for more than 1 year [83, 84]. For patients with moderate-to-severe active UC, significantly higher proportions receiving infliximab 5 mg or 10 mg achieved a clinical response at week 54, defined as a Mayo score reduction of at least 3 points, compared with placebo (5 mg: 45%, 10 mg: 44%, placebo: 20%; p < 0.001) [85].
In the CHARM trial (n = 854), adalimumab is more effective in maintaining clinical remission in patients with CD than placebo through 56 weeks (adalimumab 40 mg weekly: 41%, placebo: 12%; p < 0.001) [86]. In several long-term studies assessing the treatment benefits of adalimumab, adalimumab was effective for maintaining clinical response and remission in patients with CD for up to 4 years, with no new safety signals reported [87, 88]. The treatment effect of adalimumab was also observed in UC. In an RCT, adalimumab induced clinical remission in 18% of patients with active UC at week 8, compared with 9.2% in the patients receiving placebo (p = 0.031) [89]. In particular, adalimumab treatment significantly reduced the incidence of hospitalization and surgery and indirect costs in patients with CD [90, 91] and patients with UC [92]. Furthermore, adalimumab appears to be a safe and clinically beneficial alternative for patients with CD and UC who have not responded to infliximab treatment or cannot tolerate infliximab [93, 94].
In an RCT (n = 930), certolizumab pegol maintenance therapy led to a maintained response and remission in patients with active CD who had a response to induction therapy of certolizumab pegol compared with patients switching to placebo (certolizumab pegol: 63%, placebo: 36%; p < 0.001) [95]. Another RCT evaluating the efficacy outcomes and safety of certolizumab pegol in patients with CD up to 7 years demonstrated certolizumab pegol to be well tolerated for CD, with sustained remission in some patients as long as 7 years [96]. However, in two RCTs, certolizumab pegol did not meet statistical significance in maintaining remission compared with placebo, although the response rate was improved [97, 98]. In regard to UC, a small and noncontrolled study suggested limited or no efficacy for certolizumab pegol in patients with active UC [99].
Roughly speaking, adalimumab and infliximab showed similar degrees of treatment effectiveness in CD or UC, whereas certolizumab pegol appeared to be less effective in patients with CD. Therefore, we recommend adalimumab and infliximab as the first-line treatments for patients with CD or UC and certolizumab pegol as the second-line choice for CD.
Although safe, etanercept does not show the same efficacy as other TNF-α inhibitors in the therapy of CD. An RCT determining the efficacy and safety of etanercept in patients with active CD indicated that etanercept 25 mg was safe, but not effective for the treatment of patients with CD in the primary study endpoint, Crohn’s Disease Activity Index score (etanercept: 39%, placebo: 45%; p = 0.763) [100]. It should be noted that etanercept treatment might induce exacerbation of CD. In a nationwide cohort study (n = 17,018), etanercept treatment led to a potential risk of being diagnosed with CD and UC compared with an unexposed cohort (adjusted hazard ratio [HR] 2.0 [CI 1.4–2.8] and 2.0 [CI 1.5–2.8], respectively) [101]. Several studies also presented the cases of CD occurring in patients with psoriasis following a course of etanercept treatment [102, 103].
IL-12/23 Inhibitors
Ustekinumab was approved by the FDA to treat patients with CD and UC in 2016 and 2019, respectively, after phase III RCTs demonstrated an effective clinical response and remission [104]. Patients with active CD receiving ustekinumab achieved a higher induction response compared with placebo at 6 weeks. Subcutaneous ustekinumab was efficacious for maintaining remission in patients with active CD who had a response to induction therapy through 5 years [104]. Ustekinumab therapy also provided patients with CD with a significant improvement in quality of life and histologic disease activity [105, 106]. Similarly, a pivotal phase III UNIFI trial studying ustekinumab in patients with UC indicated that ustekinumab maintained a superior efficacy versus placebo in clinical response and endoscopic outcomes [107]. However, a real-world study comparing ustekinumab and adalimumab reported that higher rates of induction response and remission were noted in the adalimumab-treated patients than the ustekinumab groups (clinical response: 73.2% vs 50%; remission: 44.3% vs 27.7%) [108]. In a network meta-analysis comparing the efficacy of different first-line agents for patients with active UC, infliximab showed a superiority over ustekinumab in the induction of clinical remission and endoscopic improvement [109]. Overall, ustekinumab should be recommended as a second-line agent for the co-occurrence of psoriasis and IBD.
IL-17 Inhibitors
A phase IIA RCT explored the efficacy and safety of secukinumab in patients with active CD. Secukinumab was ineffective in treating CD, and higher rates of adverse outcomes were noted in patients taking secukinumab compared with placebo [110]. Based on the safety data of ten phase II/III clinical studies, a pooled analysis reported nine cases of new-onset or exacerbations of IBD in patients with psoriasis taking secukinumab, compared with one in patients taking etanercept [111]. Two phase III RCTs (UNCOVER-2 and UNCOVER-3) of ixekizumab reported 11 cases of IBD (seven UC and four CD) compared with zero in the placebo-treated patients [112]. Brodalumab 210 mg, 350 mg, or 700 mg were tested in a phase II RCT (n = 212) for moderate-to-severe CD and did not demonstrate any benefit compared with placebo, and led to several cases of worsening CD [113]. The association between IL-17 inhibitors and the potential risk of IBD exacerbation was also documented in several case reports. Haidari et al. reported a case of asymptomatic CD in patients with psoriasis following secukinumab treatment [114]. Johnston and Veettil presented a case of a patient with psoriasis who developed UC during treatment initiation with secukinumab [115]. Mu et al. described a patient with chronic plaque psoriasis who manifested CD after treatment with ixekizumab, with no recurrence of clinical disease after stopping ixekizumab treatment [116]. Another case report also presented a patient with psoriasis who was treated with ixekizumab and after 12 weeks of the induction period developed diarrheal illness and rectal bleeding [117]. Marin et al. reported a case of acute self-limited UC occurring in a patient treated with ixekizumab for psoriasis, and the colitis was quickly resolved after treatment withdrawal [118]. For patients with psoriasis and IBD, anti-IL-17 agents should be avoided.
IL-23 Inhibitors
Preliminary studies investigating anti-IL-23 agents for the therapy of CD demonstrated encouraging data. In a phase II RCT (n = 309), guselkumab led to significant improvements in clinical and endoscopic response in patients with moderately to severely active CD at week 12 versus placebo [119]. In two case reports of concomitant psoriasis and CD, patients were successfully treated with guselkumab after not responding to infliximab or ixekinumab therapy [120, 121]. After 12 weeks, patients with active CD treated with intravenous risankizumab showed a higher rate of clinical remission than did those receiving placebo in a phase II RCT (risankizumab: 31%, placebo: 15%, p = 0.0489) [122]. In an open-label extension study, risankizumab maintenance therapy was well tolerated and sustained clinical remission (> 71%) and endoscopic response (> 42%) up to 184 weeks [123]. More recently, phase III RCTs (ADVANCE and MOTIVATE) evaluated the efficacy and safety of intravenous risankizumab as induction therapy in patients with moderately to severely active CD. In both trials, risankizumab treatment (600 and 1200 mg) resulted in early symptom control at week 4, and endoscopic evidence of improvement of the mucosa at week 12, with no new safety risks identified [124]. Subsequently, the efficacy of continuing subcutaneous risankizumab as maintenance therapy was assessed in the phase III FORTIFY trial. Significantly greater clinical remission and endoscopic response rates were identified in patients treated with risankizumab compared with placebo [125]. Thus, IL-23 inhibitors are considered second-line treatments for CD. There are no published studies of IL-23 inhibitors for UC.
Small-Molecule Inhibitors
Danese and Peyrin-Biroulet conducted a phase II RCT for apremilast in patients with active UC. A higher proportion of patients who achieved clinical remission were identified in patients treated with apremilast 30 mg versus placebo (apremilast: 31.6%, placebo: 12.1%; p = 0.01) at week 12 [126]. Furthermore, apremilast treatment significantly reduced C-reactive protein and fecal calprotectin compared with the placebo group [126]. At this time, we recommend apremilast as a third-line option for patients with psoriasis with concomitant with UC. Animal models of colitis have shown that phosphodiesterase 4 inhibitors are beneficial, but clinical data of apremilast on CD are not available at present [76].
A number of studies have shown that tyrosine kinase 2 regulates signaling and functional responses downstream of IL-12, IL-23, and type I interferon receptors that are involved in IBD pathophysiology [127, 128]. In the anti-CD40 antibody-induced colitis model, treatment with deucravacitinib dose-dependently protected mice from histologically evident colitis [129]. Clinical trials of deucravacitinib are ongoing in UC (NCT03934216) and CD (NCT03599622).
Cardiovascular Diseases
There is growing evidence to support an association between having psoriasis and an increased incidence of major adverse cardiovascular events (MACE), a composite endpoint including myocardial infarction, stroke, and cardiovascular mortality [130, 131]. In a population-based cohort study, the risk of MACE was higher in patients with severe psoriasis compared with unexposed controls (HR 1.42; CI 1.17–1.73) [132].
TNF-α Inhibitors
A number of studies have shown that treatment of psoriasis with TNF-α inhibitors may decrease the risk of cardiovascular comorbidities. Wu et al. assessed the major cardiovascular event risk in patients with psoriasis receiving TNF-α inhibitors (n = 9148) compared with methotrexate (n = 8581), and reported that TNF-α inhibitors users had a reduced frequency of cardiovascular events compared with methotrexate (1.45% vs 4.09%; p < 0.01) [133]. Moreover, treatment with TNF-α inhibitors leads to an 11% reduction in the risk of cardiovascular event for every 6 months of cumulative exposure [133]. In a retrospective cohort study of patients with psoriasis, the HR for MACE for patients with psoriasis initiated on TNF-α inhibitors compared with those receiving topical agents was 0.80 (95% CI 0.66–0.98) [134]. Similarly, in a secondary analysis determining the myocardial infarction risk in patients with psoriasis, the use of TNF-α inhibitors for patients with psoriasis was associated with a significant lower risk of myocardial infarction compared with topical therapy (HR 0.27; CI 0.11–0.67) [135]. In a nationwide cohort study investigating the risk of MACE in patients with PsA receiving therapy with different classes of biologics, the risk of MACE was significantly greater with IL-12/23 and IL-17 inhibitors than TNF inhibitors [136]. As TNF-α inhibitors exert a cardioprotective effect, these drugs are considered first-line systemic agents in patients with psoriasis with cardiovascular risk factors.
IL-12/23 Inhibitors
In the case of IL-12/23 inhibitors, briakinumab reported safety concerns of an increased risk of MACE during the initial analyses, which led to trial discontinuation in 2011 [137]. Briakinumab is no longer under development in any indication. Regarding ustekinumab, a case-time-control study for patients with psoriasis with a high cardiovascular risk found a significantly increased risk of MACE with the use of ustekinumab (OR 4.17; CI 1.19–14.59) [138]. A meta-analysis by Tzellos et al. of nine RCTs (five ustekinumab and four briakinumab) compared the risk of MACE in patients with psoriasis receiving IL-12/23 inhibitors versus those receiving placebo using the Peto one-step method, and found a statistically significant increased OR of 4.23 (CI 1.07–16.75) [139]. However, in another meta-analysis by Ryan et al. of the same data using a different method (Mantel–Haenszel fixed-effects method), there was no significant statistical difference in the rate of MACEs observed in patients with psoriasis receiving IL-12/23 inhibitors compared with placebo [140]. Of note, a large 5-year post-marketing study for psoriasis indicated that ustekinumab treatment does not increase the risk of MACE [141]. Similarly, several long-term studies support the safety of ustekinumab with regard to MACE risk [142, 143]. Further investigations are needed to confirm whether ustekinumab may have beneficial or detrimental effects on cardiovascular comorbidities in patients with psoriasis. Overall, we consider ustekinumab to be a third-line option.
IL-17 Inhibitors
Interleukin-17 may exert both pro-atherogenic and anti-atherogenic roles according to the specific context and microenvironment [144]. Despite these contradictory findings, the current clinical data at least indicate that IL-17 inhibitors did not increase the risk of cardiovascular disease [144]. In a pooled analysis of ten RCTs reviewing the safety data of secukinumab in 3993 patients with psoriasis, the exposure-adjusted incidence of MACE over 52 weeks was comparable across secukinumab 300 mg, secukinumab 150 mg, and etanercept (0.42, 0.35, and 0.34, respectively) [111]. Secukinumab treatment has been shown to improve the endothelial function of patients with psoriasis at 52 weeks assessed by flow-mediated dilation [145]. With regard to ixekizumab, data reported a low incidence of MACE with continued exposure for ixekizumab through 60 weeks, and the rates of MACE were similar between ixekizumab and etanercept [146]. Based on the data from phase III RCTs (UNCOVER-1, UNCOVER-2, and UNCOVER-3), ixekizumab exhibited a neutral impact on cardiovascular-related parameters in patients with psoriasis at week 60 [147]. As for brodalumab, a phase III RCT investigating the efficacy and safety of brodalumab in patients with psoriasis (n = 661) reported no cases of MACE during the 12-week induction phase and five cases of MACE over 52 weeks, suggesting a low rate of MACE for brodalumab [71]. Further investigations are required to confirm whether IL-17 inhibitors may have beneficial effects on the cardiovascular system in patients with psoriasis. Given the low risk of MACE associated with IL-17 inhibitors, we recommend these biologics as a second-line treatment.
IL-23 Inhibitors
From the present published RCT evidence, there is no safety signal for MACE with IL-23 inhibitors in patients with psoriasis [137]. Crowley et al. reviewed the safety data of IL-23 inhibitors (tildrakizumab, guselkumab, and risankizumab) from phase III RCTs in patients with psoriasis and reported no significant increase in MACE rates [148]. In a pooled analysis of three RCTs assessing the incidence of cardiovascular events among tildrakizumab-treated patients with psoriasis, there were no statistically significant difference in the risk of MACE with initiation of tildrakizumab as compared with placebo [149]. For guselkumab, a 5-year pooled analysis of two phase III RCTs (VOYAGE 1 and VOYAGE 2) showed a low incidence of MACE for guselkumab (0.29/100 patient-years) [150]. A recent analysis of adverse event reports in the FDA Adverse Event Reporting System revealed a potential safety signal for a cerebrovascular accident with the use of risankizumab in comparison to other alternative psoriasis therapeutics [151]. In order to better characterize this unconfirmed potential safety signal, further long-term observational data are necessary. Thus, we consider IL-23 inhibitors as a second-line treatment for patients with psoriasis and cardiovascular disease.
Small-Molecule Inhibitors
In a cohort study of 68,678 patients with PsA treated with apremilast, TNF-α inhibitors, IL-12/23 inhibitors, IL-17 inhibitors, and conventional treatments, the incidence rates of MACE were lowest for users of TNF-α inhibitors, and similar for all other treatments, including apremilast [152]. In another nationwide cohort study assessing the risk of MACE for patients with PsA, there were no statistically difference between apremilast new users and TNF inhibitors new users [136]. A 156-week long-term safety study of apremilast for patients with psoriasis reported a low incidence of MACE (0.5/100 patient-years) [153]. Therefore, we also recommend apremilast as a second-line treatment.
CHF
A nationwide cohort study has reported a psoriasis severity-dependent increased risk of congestive heart failure (CHF). The HRs for CHF in mild and severe psoriasis were 1.22 (95% CI 1.16–1.29) and 1.53 (95% CI 1.34–1.74) respectively, although the precise mechanism of this correlation is unclear [154].
TNF-α Inhibitors
The benefits of anti-TNF-α in patients with CHF is controversial. One RCT assessed the treatment safety and outcomes of infliximab in patients with the New York Heart Association class III and IV and a left ventricular ejection fraction less than 35%. Patients receiving high doses of infliximab (10 mg/kg) had an increased risk of hospitalization or death for CHF as compared with placebo (HR 2.84, CI 1.01–7.97) through 28 weeks [155]. Similar findings were supported in several case reports, with patients with psoriasis treated with TNF-α inhibitors experiencing a new onset or exacerbation of CHF. Lazarewicz et al. published a case of CHF that developed in a patient with rheumatoid arthritis receiving certolizumab pegol therapy, and the heart failure almost fully resolved after stopping the medication [156]. Furthermore, a case of sudden death after receiving a single infusion of infliximab 200 mg in a patient without CHF was reported [157]. Another case report described a patient with CD who developed CHF after infliximab treatment [158]. Recently, a case report presents a patient with relapsing hidradenitis suppurativa who was diagnosed with decompensated CHF after receiving adalimumab during hospitalization [159].
In contrast, some clinical data have suggested that etanercept treatment is beneficial for patients with CHF. An RCT (n = 47) of etanercept for patients with heart failure demonstrated a significant improvement in left ventricular ejection fraction and left ventricular remodeling in a dose-dependent manner [160]. Patients receiving etanercept 4 or 10 mg/m2 experienced significant functional status improvements in another phase I study [161]. Although controversial, TNF inhibitors should be avoided in patients with CHF with New York Heart Association class III and IV, as well as patients with a left ventricular ejection fraction less than 50% [162]. Furthermore, echocardiogram and cardiology consultations are recommended before TNF inhibitor treatment in patients with CHF with the New York Heart Association class I or II [8].
IL-12/23 Inhibitors, IL-17 Inhibitors, and IL-23 Inhibitors
Clinical data on ustekinumab, anti-IL-17 agents, and anti-IL-23 agents are limited. A meta-analysis assessing the short-term risk of CHF in patients with psoriasis receiving ustekinumab, anti-IL-17 agents, and anti-IL-23 agents did not demonstrate any significant change in CHF incidence [163]. Han et al. compared the risk of CHF in patients with psoriasis receiving ustekinumab and TNF-α inhibitors. Patients initiated on ustekinumab exhibited a decreased risk of CHF compared with TNF-α inhibitors (HR 0.641; CI 0.415–0.985, p = 0.0426) [164]. In a phase III RCT (n = 1346) for patients with psoriasis, ixekizumab treatment reported no case of CHF [165]. A real-world study of secukinumab in patients with psoriasis with a 6-month follow-up reported no new onset or exacerbation of CHF [166]. Similarly, no CHF exacerbation or new onset was reported with brodalumab in a phase II open-label study with 5 years of long-term follow-up [167]. Preliminary clinical data have suggested that IL-12/23 inhibitors, IL-17 inhibitors, or IL-23 inhibitors appear to be safe for patients with CHF and concomitant psoriasis.
Depression
Many patients with psoriasis develop erythematous and scaly plaques on the visible areas of the body, resulting in a sense of stigma, lowered self-confidence, psycho-social burden, and depression [168]. The incidence of depression among patients with psoriasis is estimated to range from 32 to 60%, higher than that in the general population and other dermatology patients [169]. Although the psychological burden of psoriasis may be the source of depression, the strong association between psoriasis and depression is possible because of the overlapping inflammatory cytokines and shared physiological mechanisms [170].
TNF-α Inhibitors
A phase III RCT evaluated the effect of etanercept on the symptoms of depression in patients with psoriasis, as verified using the Beck Depression Inventory and Hamilton Rating Scale for Depression [171]. As a result, patients taking etanercept for 12 weeks had a meaningful improvement in Hamilton Rating Scale for Depression and Beck Depression Inventory scores compared with those receiving the placebo. Similarly, etanercept treatment significantly decreased both the Hospital Anxiety and Depression Scale-Anxiety (HADS-A) score and the HADS-Depression (HADS-D) score in patients with psoriasis in a prospective cohort study [172]. However, sustained depression (HADS-D ≥ 8 at month 0 and month 1) was found to be associated with decreased PASI 75/90 responses to etanercept at month 6 [172]. Jin et al. also reported that patients with psoriasis with concomitant depression at baseline exhibited lower PASI 75/90 responses after 6 months of etanercept treatment compared with control patients [173]. According to the real-world study data, adalimumab therapy was associated with a decreased risk of depression compared with conventional systemic agents (HR 0.63, CI 0.46–0.86) [174]. Furthermore, adalimumab treatment led to a significant improvement in depressive symptoms in patients with psoriasis [175].
IL-12/23 Inhibitors
In a phase III RCT (n = 1230), patients with psoriasis receiving ustekinumab experienced a large improvement in HADS and Dermatology Life Quality Index scores [176]. Ustekinumab was tested in an open-label trial and also has been shown to significantly relieve the depressive symptoms in patients with psoriasis, as assessed by Beck Depression Inventory scores and psychiatric interviews [177].
IL-17 Inhibitors
In a pooled analysis of three phase III RCTs for patients with psoriasis affected by moderately severe depressive symptoms (Quick Inventory of Depressive Symptomatology-Self-Report 16 score ≥ 11), about 40% of comorbid patients initiated on ixekizumab exhibited depression remission (Quick Inventory of Depressive Symptomatology-Self-Report 16 score ≤ 5), while only 17.8% of patients receiving placebo showed depression remission [178]. A post hoc analysis of a phase IIIb RCT (SUPREME; n = 434) reported that secukinumab treatment led to improvement in symptoms of anxiety/depression for 48 weeks, as verified using HADS [179]. Furthermore, 80–87% of patients with psoriasis treated with secukinumab experienced a PASI 90 response, regardless of baseline depression/anxiety status. Strober et al. performed a pooled analysis of ten clinical trials in patients with psoriasis and reported that secukinumab treatment did not increase the risk of depression, anxiety, or suicidality [180]. However, Komori et al. presented a case of depression exacerbation in a patient with PsA initiated on secukinumab [181]. The safety of brodalumab on depression remains controversial because of the enhanced number of suicidal ideation and behavior cases observed in the patients treated with brodalumab [181]. However, Chiricozzi et al. demonstrated that there is an accidental but not causal association between suicidal behavior events and brodalumab use [182]. Furthermore, brodalumab treatment resulted in an improvement in depression/anxiety scores versus placebo [182].
IL-23 Inhibitors
In a phase IIII RCT (VOYAGE2; n = 992), more patients with psoriasis with baseline Hospital Anxiety and Depression Scale-Anxiety or HADS-D ≥8 reported Hospital Anxiety and Depression Scale-Anxiety or HADS-D <8 after receiving guselkumab for 24 weeks compared with adalimumab (anxiety: 58.4% vs 42.9%, p = 0.028; depression: 59.8% vs 46.4%, p = 0.079) [183]. Reich et al. also analyzed the data from VOYAGE2 and reported that guselkumab therapy was associated with greater improvements in work productivity compared with adalimumab in patients with psoriasis with or without depression/anxiety status [184]. Augustin et al. combined data from UltIMMa-1 and 2 of risankizumab (n = 997) and demonstrated that risankizumab treatment had a superior effect in reducing psychological distress compared with ustekinumab (anxiety: 69.1% vs 57.1%, p = 0.004; depression: 71.1% vs 60.4%, p = 0.01) [185].
Interleukin-23 inhibitors are the first-line choice as they seem to be more effective in improving symptoms of anxiety and depression than TNF inhibitors and ustekinumab. Because brodalumab may increase the risk of suicide ideation cases, we recommended carefully assessing the use of brodalumab in patients with psoriasis with a history of psychiatric comorbidities. Other IL-17 inhibitors and ustekinumab are the second-line options as several clinical trials have shown good tolerance and efficacy for depression. While depression symptoms in patients with psoriasis decreased the treatment response to TNF inhibitors, this class should be considered a third-line treatment for patients with psoriasis with depression.
MS
Psoriasis and MS are both T-cell-mediated autoimmune diseases. In a meta-analysis of nine observational studies, psoriasis has been associated with an increased risk of developing MS (RR 1.607; CI 1.322–1.953, p < 0.0001) [186]. According to data from a national cohort study in Denmark, the association between psoriasis and MS became stronger as psoriasis became more severe (adjusted incidence rate ratios 1.84, and 2.61 for mild and severe psoriasis, respectively) [187]. Although the mechanism is unclear, accumulating evidence suggests that pathophysiological factors may be responsible for the relationship between psoriasis and MS. Both diseases have an aberrant immune infiltration of Th17 cells with the production of IL-17 [187]. Additionally, the two diseases share genetic links such as polymorphisms within the IL-23 receptor gene [188].
TNF-α Inhibitors
Although TNF-α inhibitors have been commonly used in immune-mediated disorders, they were ineffective in patients with MS and even exacerbated MS. A phase II RCT (n = 168) evaluated the effects of lenercept, a TNF receptor fusion protein-like etanercept (did not progress to market), on the number of new lesions in MS up to 48 weeks. As a result, obviously greater proportions of patients treated with lenercept experienced MS exacerbations compared with placebo-treated patients [189]. In a phase I safety trial of infliximab, leukocyte counts and immunoglobulin in cerebrospinal fluid significantly increased in two patients with rapidly progressive MS after treatment with infliximab, suggesting TNF neutralization increased the disease activity of MC and caused immune activation [190]. Several case reports supported similar results, with TNF-α inhibitor treatment worsening the signs and symptoms of MS. Sukal et al. described a patient with psoriasis treated with etanercept who developed neuroradiological and clinical signs of MS after 1 year of the therapy [191]. Another patient with psoriasis who received infliximab developed MS during treatment, and the neurological symptoms resolved after discontinuation of infliximab [192]. Furthermore, another case report presented a patient with PsA and uveitis who manifested MS after treatment with adalimumab [193]. Therefore, it is best to avoid this class of drugs in patients with psoriaswith established demyelinating disease.
IL-17 Inhibitors
Secukinumab was tested in a phase II RCT for patients with MS and has been shown to dramatically reduce the number of new active brain lesions in patients with MS compared with placebo [194]. A case report described a patient with PsA with concomitant MS status who responded unsatisfactory to ustekinumab, secukinumab treatment reduced the psoriasis activity and halted the MS deterioration [195]. In the cuprizone-induced MS experimental model, secukinumab treatment played a neuroprotective effect by modulating oxidative and inflammatory signaling [196]. In a case study of the co-occurrence of psoriasis and MS, treatment with ixekizumab resulted in complete skin clearance without MS progression [197]. Based on the evidence provided by clinical trials and case reports, the use of anti-IL-17 agents could be a safe and promising option for patients with psoriasis with concomitant MS.
IL-12/23 Inhibitors
Chang et al. presented two patients with psoriasis with comorbid MS who were treated with ustekinumab and achieved clinical remission without MS progression after treatment [198]. However, patients receiving ustekinumab did not demonstrate superior efficacy in reducing the MS lesions compared with placebo in another phase II clinical trial, though ustekinumab was well tolerated for patients with MS [199]. Thus, in patients with psoriasis and MS, ustekinumab is a second-line treatment option.
IL-23 Inhibitors
Data on anti-IL-23 agents are limited, but there are no reports that these drugs lead to disease worsening of MS. Based on the data from three phase III RCTs (n = 2081), Blauvelt et al. assessed the long-term safety of tildrakizumab in psoriasis and reported no cases of MS after 64 weeks of therapy [200]. A phase III RCT exploring the safety of guselkumab versus placebo and adalimumab in psoriasis did not report any MS cases or MS exacerbations [201]. Similarly, there was no adverse event of MS during risankizumab treatment in a phase III RCT [202]. Given the limited evidence, anti-IL-23 agents are considered a third-line treatment option.
Malignancy
Increasing evidence suggests that patients with psoriasis might have a higher risk of malignancy, especially non-melanoma skin cancer (NMSC) and lymphoma, compared with psoriasis-free patients [203, 204]. A cohort study conducted by Margolis et al. revealed that patients with psoriasis had a higher risk of overall malignancy (RR 1.78; CI 1.30–2.40) [205]. Although the reasons for this increased risk are still inconclusive, it seems evident that chronic inflammation conditions and immunosuppressive therapies play important roles in the pathogenesis of tumors [206].
TNF-α Inhibitors
Considering the role of TNF in mediating tumor growth, concerns remain about the risk of malignancy with anti-TNF therapy. In a meta-analysis assessing the short-term risk of NMSC in patients receiving anti-TNF inhibitors (including RA, psoriasis, PsA, ankylosing spondylitis, and CD), the relative risks associated with anti-TNF inhibitors were 2.02 (95% CI 1.11–3.95) [207]. Another study supported the similar findings, with patients with psoriasis receiving TNF inhibitors showing an increased incidence of NMSC, especially cutaneous squamous cell carcinoma (adjusted HR 1.81; CI 1.23–2.67) [208]. An increase of developing all cancers has been found in patients with psoriasis treated with adalimumab and infliximab compared with the general population in a pooled analysis of 71 controlled trials (OR 3.3; CI 1.2–9.1) [209]. The association between tumorigenesis and anti-TNF therapy was also documented in multiple case reports [210–212]. In contrast, based on the data of two meta-analyses and one observational study, TNF inhibitors did not meet the statistical significance in increasing the risk of malignancy [213–215]. Overall, it is best to avoid this class of drugs in patients with psoriasis with concomitant malignancy.
IL-17 Inhibitors
Gottlieb et al. summarized the incidence of malignancy in patients with psoriasis with brodalumab treatment (n = 4019) based on the data of one phase II study and three phase III RCTs, reporting two cases of lymphomas and three cases of NMSC. In particular, significantly lower rates of malignancy at 52 weeks were identified in patients receiving brodalumab, as compared with ustekinumab-treated patients [216]. In a integrative analysis of ten clinical trials (n = 3430), three NMSC cases were reported in patients with psoriasis during secukinumab treatment, suggesting that secukinumab did not lead to an increased risk in malignancy [111]. Likewise, the safety data from seven clinical trials of ixekizumab in patients with psoriasis (n = 4209) demonstrate a low incidence of malignancy with long-term exposure to ixekizumab [146].
IL-23 Inhibitors
Two RCTs assessing the safety of guselkumab in psoriasis (n = 825) reported three cases of NMSC and no lymphoma cases. In a pooled analysis of two RCTs, Reich et al. evaluated the long-term treatment outcomes and safety of tildrakizumab in psoriasis for up to 148 weeks and reported low exposure-adjusted rates of malignancies [217]. Based on the safety data from two phase III RCTs (n = 997), NMSC was reported in two patients with psoriasis after 18 weeks of exposure to risankizumab [202]. As anti-IL-17 and anti-IL-23 therapies have low rates of malignancy, they are considered first-line options in patients with psoriasis with malignancy.
IL-12/23 Inhibitors
Papp et al. assessed the long-term safety of ustekinumab in patients with psoriasis (n = 3117) for up to 5 years and reported 47 cases of NMSC and 54 cases of other malignancies, the rates of which were comparable with rates expected in the general population [218]. The impact of ustekinumab treatment on malignancy risk was also evaluated in another study and did not demonstrate an obvious increase in the risk of malignancies compared with no exposure [219]. Similarly, the safety data from pooled phase II and III RCTs of ustekinumab in patients with psoriasis (n = 3117) did not suggest higher rates of malignancy with ustekinumab exposure [220]. However, in animal studies, prolonged treatment with ustekinumab was associated with an increased risk of tumor development [221]. Overall, ustekinumab may be considered a second-line agent.
Conclusions
The development of multiple powerful biologic agents and small-molecule inhibitors has broadened the treatment regimens and transformed patient outcomes for psoriasis. However, comorbid disease conditions often complicate the clinical therapy decision, and it is important to consider the strengths and limitations of each biologic agent when formulating a personalized treatment protocol for patients with psoriasis. We hope our evidence-based recommendations can help guide dermatologists in selecting appropriate biologic therapy for individuals with comorbid PsA, obesity, depression, IBD, CHF, MS, and malignancy. Accordingly, the therapeutic guidelines should be modified as more strong evidence emerges.
Declarations
Funding
This work was sponsored by grants from National Natural Science Foundation of China (No. 81872522, 82073429), Innovation Program of Shanghai Municipal Education Commission (No. 2019-01-07-00-07-E00046), Clinical Research Plan of SHDC (No. SHDC2020CR1014B), Program of Shanghai Academic Research Leader (No. 20XD1403300), National Natural Science Foundation of China (No. 81903205), and Shanghai Pujiang Program (No. 22PJD058).
Conflicts of interest/competing interests
Yuxiong Jiang, Youdong Chen, Qian Yu, and Yuling Shi have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data obtained from public domain resources.
Code availability
Not applicable.
Author contributions
Initial concept by YJ and YS. YJ and DY characterized and wrote the manuscript with oversight by YS and QY. All authors discussed the results, read, and approved the final manuscript.
Contributor Information
Qian Yu, Email: yuervictory@163.com.
Yuling Shi, Email: shiyuling1973@tongji.edu.cn.
References
- 1.Boehncke W-H, Schoen MP. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/s0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 2.Hjuler KF, Gormsen LC, Vendelbo MH, Egeberg A, Nielsen J, Iversen L. Increased global arterial and subcutaneous adipose tissue inflammation in patients with moderate-to-severe psoriasis. Br J Dermatol. 2017;176:732–740. doi: 10.1111/bjd.15149. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 4.Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci. 2020 doi: 10.3390/ijms21051690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182:840–848. doi: 10.1111/bjd.18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisondi P, Bellinato F, Girolomoni G, Albanesi C. Pathogenesis of chronic plaque psoriasis and its intersection with cardio-metabolic comorbidities. Front Pharmacol. 2020 doi: 10.3389/fphar.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paroutoglou K, Papadavid E, Christodoulatos GS, Dalamaga M. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obesity Rep. 2020;9:165–178. doi: 10.1007/s13679-020-00380-3. [DOI] [PubMed] [Google Scholar]
- 8.Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27–40. doi: 10.1016/j.jaad.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 9.Girolomoni G, Griffiths CEM, Krueger J, et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: a hypothesis paper. J Dermatol Treat. 2015;6:103–112. doi: 10.3109/09546634.2014.880396. [DOI] [PubMed] [Google Scholar]
- 10.Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:2552–2564. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahil SK, Ezejimofor MC, Exton LS, et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol. 2020;183:638–649. doi: 10.1111/bjd.19325. [DOI] [PubMed] [Google Scholar]
- 12.Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis. Drugs. 2021;1:1751–1762. doi: 10.1007/s40265-021-01612-z. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Chen Z, Gong Y, Shi Y. A review of switching biologic agents in the treatment of moderate-to-severe plaque psoriasis. Clin Drug Invest. 2018;38:191–199. doi: 10.1007/s40261-017-0603-3. [DOI] [PubMed] [Google Scholar]
- 14.Monks G, Rivera-Oyola R, Lebwohl M. The psoriasis decision tree. J Clin Aesthet Dermatol. 2021;14:14–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;91:2273–2284. doi: 10.1016/s0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 16.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;4:423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–390. doi: 10.1016/s0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- 18.Mease PJ, Gladman DD, Collier DH, Ritchlin CT, Helliwell PS, Liu L, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol. 2019;71:111–124. doi: 10.1002/art.40851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 20.Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann Rheumatic Dis. 2014;73:48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syversen SW, Jorgensen KK, Goll GL, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance infliximab therapy on disease control in patients with immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;326:2375–2384. doi: 10.1001/jama.2021.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McInnes IB, Sawyer LM, Markus K, LeReun C, Sabry-Grant C, Helliwell PS. Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes. RMD Open. 2022 doi: 10.1136/rmdopen-2021-002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheumc Dis. 2021;80:582–590. doi: 10.1136/annrheumdis-2020-218808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McInnes IB, Mease PJ, Ritchlin CT, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology. 2017;56:1993–2003. doi: 10.1093/rheumatology/kex301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mease PJ, Landewe R, Rahman P, et al. Secukinumab provides sustained improvement in signs and symptoms and low radiographic progression in patients with psoriatic arthritis: 2-year (end-of-study) results from the FUTURE 5 study. RMD Open. 2021 doi: 10.1136/rmdopen-2021-001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottlieb AB, Merola JF, Reich K, et al. Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study. Br J Dermatol. 2021;185:1124–1134. doi: 10.1111/bjd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb AB, Strand V, Kishimoto M, et al. Ixekizumab improves patient-reported outcomes up to 52 weeks in bDMARD-naive patients with active psoriatic arthritis (SPIRIT-P1) Rheumatology. 2018;57:1777–1788. doi: 10.1093/rheumatology/key161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79:123–131. doi: 10.1136/annrheumdis-2019-215386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naive to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis. 2020;79:1310–1319. doi: 10.1136/annrheumdis-2020-217372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mease PJ, Helliwell PS, Hjuler KF, Raymond K, McInnes I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185–193. doi: 10.1136/annrheumdis-2019-216835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mease PJ, Genovese MC, Mutebi A, Viswanathan HN, Chau D, Feng J, et al. Improvement in psoriasis signs and symptoms assessed by the Psoriasis Symptom Inventory with brodalumab treatment in patients with psoriatic arthritis. J Rheumatol. 2016;43:343–349. doi: 10.3899/jrheum.150182. [DOI] [PubMed] [Google Scholar]
- 33.Kokolakis G, Vadstrup K, Hansen JB, Carrascosa JM. Brodalumab is associated with high rates of complete clearance and quality of life improvement: a subgroup analysis of patients with psoriasis and concomitant psoriatic arthritis. Dermatology. 2021 doi: 10.1159/000520290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395:427–440. doi: 10.1016/S0140-6736(19)33161-7. [DOI] [PubMed] [Google Scholar]
- 35.Merola JF, McInnes I, Ritchlin CT, et al. OP0255 bimekizumab in patients with active psoriatic arthritis and an inadequate response to tumor necrosis factor inhibitors: 16-week efficacy; SAFETY FROM BE COMPLETE, a phase 3, multicentre, randomised placebo controlled study. Ann Rheum Dis. 2022;81:167–169. doi: 10.1136/annrheumdis-2022-eular.2265. [DOI] [Google Scholar]
- 36.Mease PJ, Asahina A, Gladman DD, et al. Effect of bimekizumab on symptoms and impact of disease in patients with psoriatic arthritis over 3 years: results from BE ACTIVE. Rheumatology. 2022 doi: 10.1093/rheumatology/keac353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNF alpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–1125. doi: 10.1016/s0140-6736(20)30265-8. [DOI] [PubMed] [Google Scholar]
- 38.Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1126–1136. doi: 10.1016/s0140-6736(20)30263-4. [DOI] [PubMed] [Google Scholar]
- 39.Sweet K, Song Q, Loza MJ, et al. Guselkumab induces robust reduction in acute phase proteins and type 17 effector cytokines in active psoriatic arthritis: results from phase 3 trials. RMD Open. 2021 doi: 10.1136/rmdopen-2021-001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81:225v31. doi: 10.1136/annrheumdis-2021-221019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostor A, Van den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis. 2022;81:351–358. doi: 10.1136/annrheumdis-2021-221048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mease PJ, Chohan S, Fructuoso FJG, et al. Efficacy and safety of tildrakizumab in patients with active psoriatic arthritis: results of a randomised, double-blind, placebo-controlled, multiple-dose, 52-week phase IIb study. Ann Rheum Dis. 2021;80:1147–1157. doi: 10.1136/annrheumdis-2020-219014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi: 10.1016/s0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 44.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–999. doi: 10.1136/annrheumdis-2013-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman P, Puig L, Gottlieb AB, et al. Ustekinumab treatment and improvement of physical function and health-related quality of life in patients with psoriatic arthritis. Arthritis Care Res. 2016;68:812–822. doi: 10.1002/acr.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siebert S, Sweet K, Dasgupta B, Campbell K, McInnes IB, Loza MJ. Responsiveness of serum C-reactive protein, interleukin-17A, and interleukin-17F levels to ustekinumab in psoriatic arthritis: lessons from two phase III, multicenter, double-blind, placebo-controlled trials. Arthritis Rheumatol. 2019;71:1660–1669. doi: 10.1002/art.40921. [DOI] [PubMed] [Google Scholar]
- 47.Jones BB, Millsop JW, Walsh JA, Krueger GG, Duffin KC. Onset of psoriatic arthritis during ustekinumab treatment for psoriasis: a case series of seven patients. Br J Dermatol. 2015;173:272–274. doi: 10.1111/bjd.13645. [DOI] [PubMed] [Google Scholar]
- 48.Bonifati C, Graceffa D. How effective is ustekinumab in controlling psoriatic arthritis? Dermatol Ther. 2016;9:155–159. doi: 10.1111/dth.12322. [DOI] [PubMed] [Google Scholar]
- 49.Crocetti L, Floresta G, Cilibrizzi A, Giovannoni MP. An overview of PDE4 inhibitors in clinical trials: 2010 to early 2022. Molecules. 2022 doi: 10.3390/molecules27154964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nash P, Ohson K, Walsh J, Delev N, Nguyen D, Teng L, et al. Active Investigators. Early and sustained efficacy with apremilast monotherapy in biological-naive patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE) Ann Rheum Dis. 2018;77:690–698. doi: 10.1136/annrheumdis-2017-211568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3) Ann Rheum Dis. 2016;75:1065–1073. doi: 10.1136/annrheumdis-2015-207963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020–1026. doi: 10.1136/annrheumdis-2013-205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mease PJ, Deodhar AA, van der Heijde D, et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81:815–822. doi: 10.1136/annrheumdis-2021-221664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012 doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snekvik I, Smith CH, Nilsen TIL, Langan SM, Modalsli EH, Romundstad PR, et al. Obesity, waist circumference, weight change, and risk of incident psoriasis: prospective data from the HUNT Study. J Invest Dermatol. 2017;137:2484–2490. doi: 10.1016/j.jid.2017.07.822. [DOI] [PubMed] [Google Scholar]
- 56.Petridis A, Panagakis P, Moustou E, et al. A multicenter, prospective, observational study examining the impact of risk factors, such as BMI and waist circumference, on quality of life improvement and clinical response in moderate-to-severe plaque-type psoriasis patients treated with infliximab in routine care settings of Greece. J Eur Acad Dermatol Venereol. 2018;2:768–775. doi: 10.1111/jdv.14802. [DOI] [PubMed] [Google Scholar]
- 57.Araujo EP, De Souza CT, Ueno M, Cintra DE, Bertolo MB, Carvalheira JB, et al. Infliximab restores glucose homeostasis in an animal model of diet-induced obesity and diabetes. Endocrinology. 2007;148:5991–5997. doi: 10.1210/en.2007-0132. [DOI] [PubMed] [Google Scholar]
- 58.Haque EK, Azhar A, Corbett J, Frieder J, Wang X, Menter A. A real-world evaluation of the long-term safety and efficacy of infliximab in the treatment of moderate-to-severe psoriasis. Dermatol Ther. 2020;10:1121–1135. doi: 10.1007/s13555-020-00436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giunta A, Babino G, Ruzzetti M, Manetta S, Chimenti S, Esposito M. Influence of body mass index and weight on etanercept efficacy in patients with psoriasis: a retrospective study. J Int Med Res. 2016;44:72–75. doi: 10.1177/0300060515593254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cassano N, Galluccio A, De Simone C, et al. Influence of body mass index, comorbidities and prior systemic therapies on the response of psoriasis to adalimumab: an exploratory analysis from the Aphrodite data. J Biol Regul Homeost Agents. 2008;22:233–237. [PubMed] [Google Scholar]
- 61.Prussick R, Unnebrink K, Valdecantos WC. Efficacy of adalimumab compared with methotrexate or placebo stratified by baseline BMI in a randomized placebo-controlled trial in patients with psoriasis. J Drugs Dermatol. 2015;14:864–868. [PubMed] [Google Scholar]
- 62.Bykerk VP, Blauvelt A, Curtis JR, et al. Associations between safety of certolizumab pegol, disease activity, and patient characteristics, including corticosteroid use and body mass index. ACR Open Rheumatol. 2021;3:501–511. doi: 10.1002/acr2.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahe E, Reguiai Z, Barthelemy H, et al. Evaluation of risk factors for body weight increment in psoriatic patients on infliximab: a multicentre, cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:151–159. doi: 10.1111/jdv.12066. [DOI] [PubMed] [Google Scholar]
- 64.Saraceno R, Schipani C, Mazzotta A, Esposito M, Di Renzo L, De Lorenzo A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57:290–295. doi: 10.1016/j.phrs.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Wu M-Y, Yu C-L, Yang S-J, Chi C-C. Change in body weight and body mass index in psoriasis patients receiving biologics: a systematic review and network meta-analysis. J Am Acad Dermatol. 2020;82:101–109. doi: 10.1016/j.jaad.2019.07.103. [DOI] [PubMed] [Google Scholar]
- 66.Gisondi P, Conti A, Galdo G, Piaserico S, De Simone C, Girolomoni G. Ustekinumab does not increase body mass index in patients with chronic plaque psoriasis: a prospective cohort study. Br J Dermatol. 2013;168:1124–1127. doi: 10.1111/bjd.12235. [DOI] [PubMed] [Google Scholar]
- 67.Dalal RS, Allegretti JR. Ustekinumab dose optimization in Crohn disease: one size does not fit all. Inflamm Bowel Dis. 2021;27:e70. doi: 10.1093/ibd/izab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412–421. doi: 10.1111/bjd.12110. [DOI] [PubMed] [Google Scholar]
- 69.Gerdes S, Pinter A, Papavassilis C, Reinhardt M. Effects of secukinumab on metabolic and liver parameters in plaque psoriasis patients. J Eur Acad Dermatol Venereol. 2020;34:533–541. doi: 10.1111/jdv.16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Babino G, Fulgione E, Giorgio CM, Agozzino M, Alfano R, Argenziano G. Efficacy and safety of secukinumab in a psoriatic patient affected by comorbid metabolic disorders. Dermatol Ther. 2019 doi: 10.1111/dth.12858. [DOI] [PubMed] [Google Scholar]
- 71.Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- 72.Papp K, Crowley J, Rubel D, Landells I, Song M, Wasfi Y, et al. Consistency of response by weight across subgroups of patients with psoriasis treated with guselkumabl: results from the VOYAGE 1 and 2 Trials. Acta Dermato Venereol. 2018;98:20. [Google Scholar]
- 73.Galluzzo M, Tofani L, Lombardo P, Petruzzellis A, Silvaggio D, Egan CG, et al. Use of guselkumab for the treatment of moderate-to-severe plaque psoriasis: a 1 year real-life study. J Clin Med. 2020 doi: 10.3390/jcm9072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruiz-Villaverde R, Ayen-Rodriguez A, Llamas-Molina JM, Ruiz-Carrascosa JC. Risankizumab as a promising therapeutic approach in obese patients. Dermatol Ther. 2020 doi: 10.1111/dth.13323. [DOI] [PubMed] [Google Scholar]
- 75.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173:1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 76.Malara G, Politi C, Trifiro C, Verduci C, D'Arrigo G, Testa A, et al. Effectiveness of apremilast in real life in patients with psoriasis: a longitudinal study. Acta Dermato Venereol. 2021 doi: 10.2340/00015555-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee FI, Bellary SV, Francis C. Increased occurrence of psoriasis in patients with Crohns disease and their relatives. Am J Gastroenterol. 1990;85:962–963. [PubMed] [Google Scholar]
- 78.Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn's disease. J Eur Acad Dermatol Venereol. 2009;23:561–565. doi: 10.1111/j.1468-3083.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 79.Fu Y, Lee C-H, Chi C-C. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417–1423. doi: 10.1001/jamadermatol.2018.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitlock SM, Enos CW, Armstrong AW, et al. Management of psoriasis in patients with inflammatory bowel disease: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2018;78:383–394. doi: 10.1016/j.jaad.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 81.Bruner M, Dige A, Loft AG, et al. Spondylitis-psoriasis-enthesitis-enterocolitis-dactylitis-uveitis-peripheral synovitis (SPEED-UP) treatment. Autoimmun Rev. 2021 doi: 10.1016/j.autrev.2020.102731. [DOI] [PubMed] [Google Scholar]
- 82.Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 83.Regueiro M, Kip KE, Baidoo L, Swoger JM, Schraut W. Postoperative therapy with infliximab prevents long-term Crohn's disease recurrence. Clin Gastroenterol Hepatol. 2014;12:1494–502.e1. doi: 10.1016/j.cgh.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 84.Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology. 2009;136:441–450. doi: 10.1053/j.gastro.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 85.Moss AC, Farrell RJ. Infliximab for induction and maintenance therapy for ulcerative colitis. Gastroenterology. 2006;131:1649–1651. doi: 10.1053/j.gastro.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 86.Colombel J-F, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 87.Panaccione R, Colombel JF, Sandborn WJ, D'Haens G, Zhou Q, Pollack PF, Thakkar RB, Robinson AM. Adalimumab maintains remission of Crohn's disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38:1236–1247. doi: 10.1111/apt.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watanabe M, Hibi T, Mostafa NM, Chao J, Arora V, Camez A, Petersson J, Thakkar R. Long-term safety and efficacy of adalimumab in Japanese patients with moderate to severe Crohn's disease. J Crohns Colitis. 2014;8:1407–1416. doi: 10.1016/j.crohns.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 89.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis. Gastroenterology. 2010;138:S114–S115. doi: 10.1016/S0016-5085(10)60526-4. [DOI] [PubMed] [Google Scholar]
- 90.Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn's disease: results from the CHARM Study. Gastroenterology. 2008;135:1493–1499. doi: 10.1053/j.gastro.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 91.Louis E, Lofberg R, Reinisch W, Camez A, Yang M, Pollack PF, et al. Adalimumab improves patient-reported outcomes and reduces indirect costs in patients with moderate to severe Crohn's disease: results from the CARE trial. J Crohns Colitis. 2013;7:34–43. doi: 10.1016/j.crohns.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 92.Feagan BG, Sandborn WJ, Lazar A, et al. Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology. 2014;146:110–18.e3. doi: 10.1053/j.gastro.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 93.Lichtiger S, Binion DG, Wolf DC, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–1239. doi: 10.1111/j.1365-2036.2010.04466.x. [DOI] [PubMed] [Google Scholar]
- 94.Sandborn WJ, Hanauer S, Loftus EV, et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn's disease. Am J Gastroenterol. 2004;99:1984–1989. doi: 10.1111/j.1572-0241.2004.40462.x. [DOI] [PubMed] [Google Scholar]
- 95.Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OO, Hanauer SB, McColm J, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 96.Sandborn WJ, Lee SD, Randall C, et al. Long-term safety and efficacy of certolizumab pegol in the treatment of Crohn's disease: 7-year results from the PRECiSE 3 study. Aliment Pharmacol Ther. 2014;40:903–916. doi: 10.1111/apt.12930. [DOI] [PubMed] [Google Scholar]
- 97.Baker DE. Certolizumab pegol for the treatment of Crohn's disease. Expert Rev Clin Immunol. 2009;5:683–691. doi: 10.1586/eci.09.48. [DOI] [PubMed] [Google Scholar]
- 98.Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, et al. Certolizumab pegol for active Crohn's disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011;9:670–U84. doi: 10.1016/j.cgh.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 99.Lee SD, Osterman MT, Parrott SC, Wheat CL. Assessment of the efficacy and safety of certolizumab pegol for the treatment of moderate to severe ulcerative colitis. Gastroenterology. 2012;142:S75-S. doi: 10.1016/S0016-5085(12)60287-X. [DOI] [Google Scholar]
- 100.Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088–94. doi: 10.1053/gast.2001.28674. [DOI] [PubMed] [Google Scholar]
- 101.Korzenik J, Larsen MD, Nielsen J, Kjeldsen J, Norgard BM. Increased risk of developing Crohn's disease or ulcerative colitis in 17 018 patients while under treatment with anti-TNF alpha agents, particularly etanercept, for autoimmune diseases other than inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:289–94. doi: 10.1111/apt.15370. [DOI] [PubMed] [Google Scholar]
- 102.Ahmad K, Rogers S. Development of Crohn disease in a patient on etanercept for psoriasis. Br J Dermatol. 2007;157:396. doi: 10.1111/j.1365-2133.2007.08009.x. [DOI] [PubMed] [Google Scholar]
- 103.Tichy M, Hercogova J. Manifestation of Crohn's disease in a young woman during the course of treatment for severe form of chronic plaque psoriasis with etanercept. Dermatol Ther. 2014;27:211–4. doi: 10.1111/dth.12119. [DOI] [PubMed] [Google Scholar]
- 104.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375:1946–60. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 105.Li K, Friedman JR, Chan D, et al. Effects of ustekinumab on histologic disease activity in patients with Crohn's disease. Gastroenterology. 2019;157:1019. doi: 10.1053/j.gastro.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 106.Sands BE, Han C, Gasink C, et al. The effects of ustekinumab on health-related quality of life in patients with moderate to severe Crohn's disease. J Crohns Colitis. 2018;12:883–95. doi: 10.1093/ecco-jcc/jjy055. [DOI] [PubMed] [Google Scholar]
- 107.Rowan CR, Boland K, Harewood GC. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2020;382:91. doi: 10.1056/NEJMc1915042. [DOI] [PubMed] [Google Scholar]
- 108.Ahmed Z, Venkata K, Zhang N, Malik TA. Comparative effectiveness of ustekinumab versus adalimumab in induction of clinical response and remission in Crohn's disease: experience of a real-world cohort at a tertiary care inflammatory bowel disease referral center. Gastroenterol Res. 2019;12:245–51. doi: 10.14740/gr1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2179. doi: 10.1016/j.cgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van de Kerkhof PCM, Griffiths CEM, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83. doi: 10.1016/j.jaad.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 112.Gordon KB, Colombel J-F, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis reply. N Engl J Med. 2016;375:2102. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 113.Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn's disease. Am J Gastroenterol. 2016;111:1599–607. doi: 10.1038/ajg.2016.298. [DOI] [PubMed] [Google Scholar]
- 114.Haidari W, Al-Naqshabandi S, Ahn CS, Bloomfeld RS, Feldman SR. Asymptomatic Crohn's disease identified in a patient being treated with secukinumab: a case report. Sage Open Med Case Rep. 2019 doi: 10.1177/2050313x19893580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnston DN, Veettil R. A case of new onset ulcerative colitis following secukinumab treatment. Br J Hosp Med. 2019;80:544–5. doi: 10.12968/hmed.2019.80.9.544. [DOI] [PubMed] [Google Scholar]
- 116.Mu X, Fardy J, Reid S, Trahey J. Severe drug-associated colitis with Crohn's features in setting of ixekizumab therapy for chronic plaque psoriasis. BMC Gastroenterol. 2021 doi: 10.1186/s12876-021-01936-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith MK, Pai J, Panaccione R, Beck P, Ferraz JG, Jijon H. Crohn's-like disease in a patient exposed to anti-Interleukin-17 blockade (ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. 2019 doi: 10.1186/s12876-019-1067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marin M, Alzueta N, Pio M, Gascon A, Castresana M. Ulcerative colitis induced by ixekizumab: a case report. Eur J Hosp Pharm. 2021;28:50–2. doi: 10.1136/ejhpharm-2019-002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sandborn WJ, D'Haens GR, Reinisch W, et al. Guselkumab for the treatment of Crohn's disease: induction results from the phase 2 GALAXI-1 study. Gastroenterology. 2022 doi: 10.1053/j.gastro.2022.01.047. [DOI] [PubMed] [Google Scholar]
- 120.Berman HS, Villa NM, Shi VY, Hsiao JL. Guselkumab in the treatment of concomitant hidradenitis suppurativa, psoriasis, and Crohn's disease. J Dermatol Treat. 2021;2:261–3. doi: 10.1080/09546634.2019.1654067. [DOI] [PubMed] [Google Scholar]
- 121.Grossberg LB. A case report of successful treatment of Crohn's disease and psoriasis with guselkumab. Inflamm Bowel Dis. 2019;25:E84-E. doi: 10.1093/ibd/izz033. [DOI] [PubMed] [Google Scholar]
- 122.Feagan BG, Sandborn WJ, D'Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn's disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–709. doi: 10.1016/s0140-6736(17)30570-6. [DOI] [PubMed] [Google Scholar]
- 123.Feagan BG, Panes J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn's disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3:671–80. doi: 10.1016/s2468-1253(18)30233-4. [DOI] [PubMed] [Google Scholar]
- 124.D'Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn's disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015–30. doi: 10.1016/s0140-6736(22)00467-6. [DOI] [PubMed] [Google Scholar]
- 125.Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn's disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399:2031–46. doi: 10.1016/s0140-6736(22)00466-4. [DOI] [PubMed] [Google Scholar]
- 126.Nittala R, Singh A. Effects of apremilast, an oral inhibitor of phosphodiesterase 4, in a randomized trial of patients with active ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:3057. doi: 10.1016/j.cgh.2020.02.038. [DOI] [PubMed] [Google Scholar]
- 127.Danese S, Peyrin-Biroulet L. Selective tyrosine kinase 2 inhibition for treatment of inflammatory bowel disease: new hope on the rise. Inflamm Bowel Dis. 2021;27:2023–30. doi: 10.1093/ibd/izab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nash P. Janus kinase inhibitors: safety in patients with psoriatic arthritis. J Rheumatol. 2022;49:44–7. doi: 10.3899/jrheum.21132.9. [DOI] [PubMed] [Google Scholar]
- 129.Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aaw1736. [DOI] [PubMed] [Google Scholar]
- 130.Caiazzo G, Fabbrocini G, Di Caprio R, Raimondo A, Scala E, Balato N, et al. Psoriasis, cardiovascular events, and biologics: lights and shadows. Frontiers in Immunol. 2018 doi: 10.3389/fimmu.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu SC-S, Lan C-CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017 doi: 10.3390/ijms18102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hugh J, Van Voorhees AS, Nijhawan RI, Bagel J, Lebwohl M, Blauvelt A, et al. From the Medical Board of the National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168–77. doi: 10.1016/j.jaad.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 133.Wu JJ, Guerin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81–90. doi: 10.1016/j.jaad.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 134.Wu JJ, Joshi AA, Reddy SP, Batech M, Egeberg A, Ahlehoff O, et al. Anti-inflammatory therapy with tumour necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1320–6. doi: 10.1111/jdv.14951. [DOI] [PubMed] [Google Scholar]
- 135.Wu JJ, Poon K-YT. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167–8. doi: 10.1016/j.jaad.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 136.Vegas LP, Le Corvoisier P, Penso L, Paul M, Sbidian E, Claudepierre P. Risk of major adverse cardiovascular events in patients initiating biologics/apremilast for psoriatic arthritis: a nationwide cohort study. Rheumatology. 2022;61:1589–99. doi: 10.1093/rheumatology/keab522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Brito M, Yiu ZZN. Cardiovascular Safety of biologics targeting interleukin (IL)-12 and/or IL-23: what does the evidence say? Am J Clin Dermatol. 2021;22:587–601. doi: 10.1007/s40257-021-00612-9. [DOI] [PubMed] [Google Scholar]
- 138.Poizeau F, Nowak E, Kerbrat S, et al. Association between early severe cardiovascular events and the initiation of treatment with the anti-interleukin 12/23p40 antibody ustekinumab. JAMA Dermatol. 2020;156:1208–15. doi: 10.1001/jamadermatol.2020.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2013;27:622–7. doi: 10.1111/j.1468-3083.2012.04500.x. [DOI] [PubMed] [Google Scholar]
- 140.Ryan C, Leonardi CL, Krueger JG, et al. Association between biologic therapies for chronic Plaque Psoriasis and Cardiovascular Events: a meta-analysis of randomized controlled trials. JAMA. 2011;306:864–71. doi: 10.1001/jama.2011.1211. [DOI] [PubMed] [Google Scholar]
- 141.Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535–45. doi: 10.1111/jdv.12046. [DOI] [PubMed] [Google Scholar]
- 142.Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Drugs Dermatology. 2015;14:58–66. [PubMed] [Google Scholar]
- 143.Reich K, Langley RG, Lebwohl M, Szapary P, Guzzo C, Yeilding N, et al. Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studies. Br J Dermatol. 2011;164:862–72. doi: 10.1111/j.1365-2133.2011.10257.x. [DOI] [PubMed] [Google Scholar]
- 144.Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J Am Acad Dermatol. 2018;79:345–52. doi: 10.1016/j.jaad.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 145.von Stebut E, Reich K, Thaci D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139:1054–62. doi: 10.1016/j.jid.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 146.Strober B, Leonardi C, Papp KA, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol. 2017;76:432. doi: 10.1016/j.jaad.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 147.Egeberg A, Wu JJ, Korman N, Solomon JA, Goldblum O, Zhao F, et al. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J Am Acad Dermatol. 2018;79:104. doi: 10.1016/j.jaad.2018.02.074. [DOI] [PubMed] [Google Scholar]
- 148.Crowley JJ, Warren RB, Cather JC. Safety of selective IL-23p19 inhibitors for the treatment of psoriasis. J Eur Acad Dermatol Venereol. 2019;33:1676–84. doi: 10.1111/jdv.15653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bissonnette R, Fernandez-Penas P, Puig L, Mendelsohn AM, Rozzo SJ, Menter A. Incidence of cardiovascular events among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: pooled data from three large randomised clinical trials. J Eur Acad Dermatol Venereol. 2020;34:E21–4. doi: 10.1111/jdv.15866. [DOI] [PubMed] [Google Scholar]
- 150.Blauvelt A, Tsai T-F, Langley RG, Miller M, Shen Y-K, You Y, et al. Consistent safety profile with up to 5 years of continuous treatment with guselkumab: pooled analyses from the phase 3 VOYAGE 1 and VOYAGE 2 trials of patients with moderate-to-severe psoriasis. J Am Acad Dermatol. 2022;86:827–34. doi: 10.1016/j.jaad.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 151.Woods RH. Potential cerebrovascular accident signal for risankizumab: a disproportionality analysis of the FDA Adverse Event Reporting System (FAERS) Br J Clin Pharmacol. 2022 doi: 10.1111/bcp.15581. [DOI] [PubMed] [Google Scholar]
- 152.Persson R, Hagberg KW, Qian Y, Vasilakis-Scaramozza C, Jick S. The risks of major cardiac events among patients with psoriatic arthritis treated with apremilast, biologics, DMARDs or corticosteroids. Rheumatology. 2021;60:1926–31. doi: 10.1093/rheumatology/keaa683. [DOI] [PubMed] [Google Scholar]
- 153.Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2) J Am Acad Dermatol. 2017;77:310. doi: 10.1016/j.jaad.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 154.Khalid U, Ahlehoff O, Gislason GH, Kristensen SL, Skov L, Torp-Pedersen C, et al. Psoriasis and risk of heart failure: a nationwide cohort study. Eur J Heart Fail. 2014;16:743–8. doi: 10.1002/ejhf.113. [DOI] [PubMed] [Google Scholar]
- 155.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti-TNF Therapy Against Congestive Heart Failure Investigators Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the Anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.cir.0000077913.60364.d2. [DOI] [PubMed] [Google Scholar]
- 156.Lazarewicz K, Shaw S, Haque S. Certolizumab pegol-induced heart failure. J Clin Rheumatol. 2018;24:55–6. doi: 10.1097/rhu.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 157.de'Clari F, Salani I, Safwan E, Giannacco A. Sudden death in a patient without heart failure after a single infusion of 200 mg infliximab: does TNF-alpha have protective effects on the failing heart, or does infliximab have direct harmful cardiovascular effects? Circulation. 2002;105:E183. doi: 10.1161/01.cir.0000017216.41471.df. [DOI] [PubMed] [Google Scholar]
- 158.Grillo TG, Almeida LR, Beraldo RF, et al. Heart failure as an adverse effect of infliximab for Crohn's disease: a case report and review of the literature. World J Clin Cases. 2021;9:10382–91. doi: 10.12998/wjcc.v9.i33.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lopez CM, Laboy PT, Bou MO, Noriega AQ, Rivera VC. Fatal new-onset congestive heart failure related to adalimumab use in apPatient with relapsing hidradenitissSuppurativa: a case report. Am J Case Rep. 2021 doi: 10.12659/ajcr.929148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103:1044–7. doi: 10.1161/01.cir.103.8.1044. [DOI] [PubMed] [Google Scholar]
- 161.Deswal A, Bozkurt B, Seta Y, Parilti-Eiswirth S, Hayes FA, Blosch C, et al. Safety and efficacy of a soluble P75 tumor necrosis factor receptor (enbrel, etanercept) in patients with advanced heart failure. Circulation. 1999;99:3224–6. doi: 10.1161/01.cir.99.25.3224. [DOI] [PubMed] [Google Scholar]
- 162.Amin M, No DJ, Egeberg A, Wu JJ. Choosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say? Am J Clin Dermatol. 2018;19:1–13. doi: 10.1007/s40257-017-0328-3. [DOI] [PubMed] [Google Scholar]
- 163.Champs B, Degboe Y, Barnetche T, Cantagrel A, Ruyssen-Witrand A, Constantin A. Short-term risk of major adverse cardiovascular events or congestive heart failure in patients with psoriatic arthritis or psoriasis initiating a biological therapy: a meta-analysis of randomised controlled trials. RMD Open. 2019 doi: 10.1136/rmdopen-2018-000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Han JH, Park HE, Kim YH, Jung J-H, Lee JH, Park YM, et al. Comparison of the risk of heart failure in psoriasis patients using anti-TNF alpha inhibitors and ustekinumab. Esc Heart Failure. 2022;9:1502–4. doi: 10.1002/ehf2.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blauvelt A, Gooderham M, Iversen L, Ball S, Zhang L, Agada NO, et al. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: rsults through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3) J Am Acad Dermatol. 2017;77:855–62. doi: 10.1016/j.jaad.2017.06.153. [DOI] [PubMed] [Google Scholar]
- 166.Strober BE, Germino R, Guana A, Greenberg JD, Litman HJ, Guo N, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatol Treat. 2020;31:333–41. doi: 10.1080/09546634.2019.1603361. [DOI] [PubMed] [Google Scholar]
- 167.Lebwohl MG, Blauvelt A, Menter A, Papp KA, Guenthner S, Pillai R, et al. Efficacy, safety, and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis treated with brodalumab for 5 years in a long-term, open-label, phase II study. Am J Clin Dermatol. 2019;20:863–71. doi: 10.1007/s40257-019-00466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Holsken S, Krefting F, Schedlowsk MI, Sondermann W. Common fundamentals of psoriasis and depression. Acta Derm Venereol. 2021 doi: 10.2340/actadv.v101.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kovitwanichkanont T, Chong AH, Foley P. Beyond skin deep: addressing comorbidities in psoriasis. Med J Aust. 2020;212:528–34. doi: 10.5694/mja2.50591. [DOI] [PubMed] [Google Scholar]
- 170.Wang X, Li Y, Wu L, Xiao S, Ji Y, Tan Y, et al. Dysregulation of the gut-brain-skin axis and key overlapping inflammatory and immune mechanisms of psoriasis and depression. Biomed Pharmacother. 2021 doi: 10.1016/j.biopha.2020.111065. [DOI] [PubMed] [Google Scholar]
- 171.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;67:29–35. doi: 10.1016/s0140-6736(05)67763-x. [DOI] [PubMed] [Google Scholar]
- 172.Yang A, Xin X, Yang W, Li M, Yang W, Li L, et al. Etanercept reduces anxiety and depression in psoriasis patients, and sustained depression correlates with reduced therapeutic response to etanercept. Ann Dermatol Venereol. 2019;146:363–71. doi: 10.1016/j.annder.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 173.Jin W, Zhang S, Duan Y. Depression symptoms predict worse clinical response to etanercept treatment in psoriasis patients. Dermatology. 2019;235:55–64. doi: 10.1159/000492784. [DOI] [PubMed] [Google Scholar]
- 174.Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Am Acad Dermatol. 2018;78:70–80. doi: 10.1016/j.jaad.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 175.Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812–8. doi: 10.1016/j.jaad.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 176.Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu M-C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63:457–65. doi: 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 177.Kim S-J, Park M-Y, Pak K, Han J, Kim G-W, Kim H-S, et al. Improvement of depressive symptoms in patients with moderate-to-severe psoriasis treated with ustekinumab: an open label trial validated using beck depression inventory, Hamilton Depression Rating Scale measures and (18)fluorodeoxyglucose (FDG) positron emission tomography (PET) J Dermatol Treat. 2018;29:761–8. doi: 10.1080/09546634.2018.1466021. [DOI] [PubMed] [Google Scholar]
- 178.Griffiths CEM, Fava M, Miller AH, Russell J, Ball SG, Xu W, et al. Impact of ixekizumab treatment on depressive symptoms and systemic inflammation in patients with moderate-to-severe psoriasis: an integrated analysis of three phase 3 clinical studies. Psychother Psychosom. 2017;86:260–7. doi: 10.1159/000479163. [DOI] [PubMed] [Google Scholar]
- 179.Talamonti M, Malara G, Natalini Y, et al. Secukinumab improves patient perception of anxiety and depression in patients with moderate to severe psoriasis: a post hoc analysis of the SUPREME study. Acta Derm Venereol. 2021 doi: 10.2340/00015555-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Strober BE, Langley RGB, Menter A, Magid M, Porter B, Fox T, et al. No elevated risk for depression, anxiety or suicidality with secukinumab in a pooled analysis of data from 10 clinical studies in moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:E105–7. doi: 10.1111/bjd.16051. [DOI] [PubMed] [Google Scholar]
- 181.Komori T, Otsuka A, Honda Y, Kanameishi S, Honda T, Kabashima K. Exacerbation of depression in a psoriatic arthritis patient possibly induced by secukinumab. Eur J Dermatol. 2016;26:506–7. doi: 10.1684/ejd.2016.2832. [DOI] [PubMed] [Google Scholar]
- 182.Chiricozzi A, Romanelli M, Saraceno R, Torres T. No meaningful association between suicidal behavior and the use of. Expert Opin Drug Saf. 2016;15:1653–9. doi: 10.1080/14740338.2016.1228872. [DOI] [PubMed] [Google Scholar]
- 183.Gordon KB, Armstrong AW, Han C, Foley P, Song M, Wasfi Y, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the Phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol. 2018;32:1940–9. doi: 10.1111/jdv.15012. [DOI] [PubMed] [Google Scholar]
- 184.Reich K, Foley P, Han C, McElligott S, Muser E, Li N, et al. Guselkumab improves work productivity in patients with moderate-to-severe psoriasis with or without depression and anxiety: results from the VOYAGE 2 comparator study versus adalimumab. J Dermatol Treat. 2020;31:617–23. doi: 10.1080/09546634.2019.1628172. [DOI] [PubMed] [Google Scholar]
- 185.Augustin M, Lambert J, Zema C, et al. Effect of risankizumab on patient-reported outcomes in moderate to severe psoriasis: the UltIMMa-1 and UltIMMa-2 randomized clinical trials. JAMA Dermatol. 2020;156:1344–53. doi: 10.1001/jamadermatol.2020.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Islam MM, Poly TN, Yang H-C, Wu C-C, Li Y-C. Increase risk of multiple sclerosis in patients with psoriasis disease: an evidence of observational studies. Neuroepidemiology. 2019;2:152–60. doi: 10.1159/000495112. [DOI] [PubMed] [Google Scholar]
- 187.Egeberg A, Mallbris L, Gislason GH, Skov L, Hansen PR. Risk of multiple sclerosis in patients with psoriasis: a Danish nationwide cohort study. J Invest Dermatol. 2016;136:93–8. doi: 10.1038/jid.2015.350. [DOI] [PubMed] [Google Scholar]
- 188.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Human Genet. 2007;80:273–90. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Arnason BGW, Jacobs G, Hanlon M, et al. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457–65. doi: 10.1212/wnl.53.3.457. [DOI] [PubMed] [Google Scholar]
- 190.vanOosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, vonBlomberg BME, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531–4. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- 191.Sukal SA, Nadiminti L, Granstein RD. Etanercept and demyelinating disease in a patient with psoriasis. J Am Acad Dermatol. 2006;54:160–4. doi: 10.1016/j.jaad.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 192.Kalinowska-Lyszczarz A, Fereidan-Esfahani M, Guo Y, Lucchinetti CF, Tobin WO. Pathological findings in central nervous system demyelination associated with infliximab. Mult Scler J. 2020;26:1124–9. doi: 10.1177/1352458519894710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baresic M, Crnogaj MR, Zadro I, Anic B. Demyelinating disease (multiple sclerosis) in a patient with psoriatic arthritis treated with adalimumab: a case-based review. Rheumatol Int. 2021;41:2233–9. doi: 10.1007/s00296-021-04995-0. [DOI] [PubMed] [Google Scholar]
- 194.Havrdova E, Belova A, Goloborodko A, Tisserant A, Wright A, Wallstroem E, et al. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J Neurol. 2016;263:1287–95. doi: 10.1007/s00415-016-8128-x. [DOI] [PubMed] [Google Scholar]
- 195.Assefa GT, Kaneko S, Oguro H, Morita E. Treatment of psoriasis and psoriatic arthritis with secukinumab after unsatisfactory response to ustekinumab in multiple sclerosis patient. J Dermatol. 2019;46:E112–3. doi: 10.1111/1346-8138.14619. [DOI] [PubMed] [Google Scholar]
- 196.Abdel-Maged AE-S, Gad AM, Rashed LA, Azab SS, Mohamed EA, Awad AS. Repurposing of secukinumab as neuroprotective in cuprizone-induced multiple sclerosis experimental model via inhibition of oxidative, inflammatory, and neurodegenerative signaling. Mol Neurobiol. 2020;57:3291–306. doi: 10.1007/s12035-020-01972-9. [DOI] [PubMed] [Google Scholar]
- 197.Herrera-Acosta E, Garriga-Martina GG, Suarez-Perez JA, Martinez-Garcia EA, Herrera-Ceballos E. Ixekizumab for patients with plaque psoriasis affected by multiple sclerosis: case report. Sultan Qaboos Univ Med J. 2021;21:488–90. doi: 10.18295/squmj.4.2021.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chang S, Chambers CJ, Liu F-T, Armstrong AW. Successful treatment of psoriasis with ustekinumab in patients with multiple sclerosis. Dermatol Online J. 2015;21(7). 10.5070/D3217028112. [PubMed]
- 199.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH, Ustekinumab MS Investigators Repeated subcutaneous injections of IL12/23 P40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/s1474-4422(08)70173-x. [DOI] [PubMed] [Google Scholar]
- 200.Blauvelt A, Reich K, Papp KA, et al. Safety of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br J Dermatol. 2018;179:615–22. doi: 10.1111/bjd.16724. [DOI] [PubMed] [Google Scholar]
- 201.Blauvelt A, Papp KA, Griffiths CEM, Randazzo B, Wasfi Y, Shen Y-K, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparatore-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–17. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 202.Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–61. doi: 10.1016/s0140-6736(18)31713-6. [DOI] [PubMed] [Google Scholar]
- 203.Butron-Bris B, Dauden E, Rodriguez-Jimenez P. Psoriasis therapy and skin cancer: a review. Life (Basel). 2021 doi: 10.3390/life11101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Geller S, Xu H, Lebwohl M, Nardone B, Lacouture ME, Kheterpal M. Malignancy risk and recurrence with psoriasis and its treatments: a concise update. Am J Clin Dermatol. 2018;19:363–75. doi: 10.1007/s40257-017-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Margolis D, Bilker W, Hennessy S, Vittorio C, Santanna J, Strom BL. The risk of malignancy associated with psoriasis. Arch Dermatol. 2001;137:778–83. [PubMed] [Google Scholar]
- 206.Patel S, Patel T, Kerdel FA. The risk of malignancy or progression of existing malignancy in patients with psoriasis treated with biologics: case report and review of the literature. Int J Dermatol. 2016;55:487–93. doi: 10.1111/ijd.13129. [DOI] [PubMed] [Google Scholar]
- 207.Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119–30. doi: 10.1002/pds.2046. [DOI] [PubMed] [Google Scholar]
- 208.Asgari MM, Ray GT, Geier JL, Quesenberry CP. Malignancy rates in a large cohort of patients with systemically treated psoriasis in a managed care population. J Am Acad Dermatol. 2017;76:632–8. doi: 10.1016/j.jaad.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 209.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies—systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 210.Farah RA, Alduaij A, Ugas C, Navarro R. Primary central nervous system lymphoma in a patient on adalimumab therapy for chronic plaquepPsoriasis. World Neurosurg. 2020;139:260–3. doi: 10.1016/j.wneu.2020.03.155. [DOI] [PubMed] [Google Scholar]
- 211.Girard C, Guillot B, Bessis D. Gastric MALT lymphoma in a patient receiving infliximab for psoriasis. Br J Dermatol. 2008;159:497–8. doi: 10.1111/j.1365-2133.2008.08664.x. [DOI] [PubMed] [Google Scholar]
- 212.Miranda LQ, Bressan AL, Rehfeldt FVdS, Vasconcelos BN, Gripp AC. Psoriasis, lymphoma and etanercept: is there a correlation? An Bras Dermatol. 2012;87:139–41. doi: 10.1590/S0365-05962012000100020. [DOI] [PubMed] [Google Scholar]
- 213.Dommasch ED, Abuabara K, Shin DB, Josephine N, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035–50. doi: 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, Pollono EN, Polo Cueto J, Rosa Gonzales-Crespo M, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA. 2012;308:898–908. doi: 10.1001/2012.jama.10857. [DOI] [PubMed] [Google Scholar]
- 215.Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895–904. doi: 10.1136/ard.2010.149419. [DOI] [PubMed] [Google Scholar]
- 216.Gottlieb A, Lebwohl M, Liu C, Israel RJ, Jacobson A. Malignancy rates in brodalumab clinical studies for psoriasis. Am J Clinl Dermatol. 2020;21:421–30. doi: 10.1007/s40257-020-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Reich K, Warren RB, Iversen L, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol. 2020;182:605–17. doi: 10.1111/bjd.18232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Papp KA, Griffiths CEM, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. B J Dermatol. 2013;168:844–54. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- 219.Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845. doi: 10.1016/j.jaad.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 220.Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742–51. doi: 10.1016/j.jaad.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 221.Ustekinumab: new drug. Suspicion of carcinogenicity: too great a risk for psoriasis patients. Prescrire Int. 2009.18:202–4. [PubMed]