Abstract
Coccidiosis is an economically significant disease in the global poultry industry, but little is known about the mechanisms of bone defects caused by coccidiosis; thus, the study focused on effects of coccidiosis on the bone homeostasis of young broiler chickens. A total of 480 male Cobb500 broilers were randomly allocated into four treatment groups, including an uninfected control consuming diet ad libitum, two infected groups were orally gavaged with two different concentrations of sporulated Eimeria oocysts, and an uninfected pair-fed group fed the same amount of feed as the high Eimeria-infected group consumed. Growth performance and feed intake were recorded, and samples were collected on 6 days post infection. Results indicated that coccidiosis increased systemic oxidative status and elevated immune response in bone marrow, suppressing bone growth rate (P < 0.05) and increasing bone resorption (P < 0.05) which led to lower bone mineral density (P < 0.05) and mineral content (P < 0.05) under Eimeria infection. With the same amount of feed intake, the uninfected pair-fed group showed a distinguished bone formation rate and bone resorption level compared with the Eimeria infected groups. In conclusion, inflammatory immune response and oxidative stress in broilers after Eimeria infection were closely associated with altered bone homeostasis, highlighting the role of inflammation and oxidative stress in broiler bone homeostasis during coccidiosis.
Subject terms: Animal biotechnology, Biotechnology
Introduction
Coccidiosis induced by protozoan parasites, Eimeria spp., causes a sizeable economic impact in the poultry industry worldwide1. Eimeria spp. infect enterocytes and cause severe digestive tract damage, leading to inflammation and malabsorption of nutrients2–4. Coccidiosis affects chickens of all ages, although the negative impact of coccidiosis was more severe at younger ages owing to the immature immune system5. Furthermore, because the modern broiler chicken is characterized by relatively higher porosity and lower mineral content in long bones6,7, broiler chickens’ leg bones might be more frangible under the infection with Eimeria spp.8–10.
Coccidiosis significantly reduced tibia bone ash content and adversely affected femur breaking strength11. Sporozoites of some species, especially E. acervulina and E. maxima, also decreased bone mineral content (BMC) and showed lower bone mineral density (BMD) in infected broilers12. Bone mineral loss caused by Eimeria spp. in broiler chickens has been linked to nutrition imbalance and malabsorption with significantly reduced absorption of essential minerals and vitamins13–17. Other than the nutritional factors, emerging evidence suggests that bone homeostasis is mediated by oxidative stress and immune response under disease conditions18,19. It has been shown that oxidative stress is a negative impact factor of osteoblast activity in broilers during Eimeria infection, and there is a potential link between oxidative stress and lower bone quality20. Moreover, the activity of osteoclasts can be another factor to mediate skeletal homeostasis during pathogen infection21,22. Osteoclasts, unique multinuclear cells, originate from hematopoietic stem cells in bone marrow23 and are located on the surface of the bone24. Osteoclasts highly express tartrate-resistant acid phosphatase (TRAP, TRAPase)25. The expression and activity of the TRAP enzyme was not only considered as a histochemical biomarker of osteoclasts activity but also considered as an important messenger between skeleton homeostasis and the immune system26. Bone marrow also serves as the cradle of hematopoiesis and as a reservoir of growth factors and cytokines27. Many inflammatory cytokines trigger osteoclastic bone resorption by mediating osteoclast formation, cell activity, and lifespan, which ultimately leads to bone mineral loss28–31. The crosstalk between bone homeostasis and immunity was referred as osteoimmunology32. The receptor activator of nuclear factor kappa B (RANK) is essential in activating the nuclear factor kappa B (NFKB) pathway and the c-Fos (FOS, Fos proto-oncogene) pathway by mediating the expression of intracellular promoter nuclear factor of activated T cell (NFATC1) that stimulates the differentiation of osteoclasts33–41. NFKB ligand (RANKL) is a critical cytokine produced mainly in osteoblasts and regulates osteoclast formation32,42. The binding of RANKL to its receptor RANK on the surface of osteoclast progenitor cells triggers the differentiation of osteoclast precursors into osteoclasts, which increases the number of osteoclasts on their bone surfaces43. Besides, pro-osteoclastogenic cytokines produced by macrophages include tumor necrosis factor-α (TNF-α; tumor necrosis factor-α-like in chicken), interleukin-1β (IL1B), and interleukin-6 (IL6) are essential cytokines in mediating osteoclast activity43–47. Meanwhile, those cytokines are excessively released in response to Eimeria infection48–50. Moreover, several transcript factors have multiple roles in inflammatory response, osteogenesis, and osteoclastogenesis. For example, osteoprotegerin (OPG) has been shown to be an inhibitor of TNF-related apoptosis51. It is produced by osteoblast lineage cells and acts as a natural decoy receptor for RANKL, negatively regulating RANK-RANKL signaling and reducing bone resorption. The ratio of RANKL/OPG was used as a biomarker that indicates the occurrence of bone remodeling52. Modulating the expression of OPG/RANKL can affect the activity of osteoblasts, osteoclastogenesis, and osteoclast activity. SMAD1 is a key element that intermediates transforming growth factor-beta signaling and the bone morphogenetic protein (BMP) signaling pathways that are essential in osteoblast activity, bone mineralization, and osteoclast differentiation53–55.
In poultry studies, Kakhki et al.56 have reported Eimeria spp. had adverse effects on long bone homeostasis, which is attributed to bone remodeling status. However, the biomechanical properties of specific bone homeostasis and its precise etiology remain to be fully elucidated. Based on the aforementioned information, we hypothesized that broiler bone health during coccidiosis is not simply caused by nutritional deficiency but is also associated with inflammatory immune responses and oxidative stress in the broiler chicken. Thus, the study was conducted to better understand bone homeostasis in broiler chickens and the relationship between inflammation/oxidative stress and skeletal development during acute Eimeria infection.
Results
Performance variables and intestinal permeability
On post-infection day 5 (5 dpi), the Control and the pair-fed (PF) groups showed no sign of intestinal damage, whereas a higher concentration of FITC-d in the serum was detected in the Eimeria challenged groups, which revealed damage to the gut epithelium owing to the Eimeria infection. Feed restriction in the PF group did not cause intestinal lesions. The high challenge group (High) had higher gastrointestinal permeability than the Control or PF (P < 0.05; Fig. 1). The average daily feed intake (ADFI) was recorded during the trial (Table 1). Because the same amount feed was provided to the PF to match with the High groups, there were no differences in ADFI between the PF and the High (P > 0.05). From 4 to 6 dpi, Eimeria infection significantly decreased ADFI in the Low (P < 0.05) and High (P < 0.05) groups when compared to the uninfected Control group. The result of the cumulative feed consumed (FI), body weight gain (BWG), and feed conversion ratio (FCR) clearly showed that Eimeria infection adversely affected all performance parameters (P < 0.05; Table 1). The low dose of Eimeria infection significantly reduced BWG and increased FCR (P < 0.05) in broilers compared with the Control during the whole acute challenge period (0–6 dpi); however, it did not significantly alter the FI during the infection. The high dose of Eimeria infection significantly reduced BWG (P < 0.05) and FI (P < 0.001), and increased FCR (P < 0.001) compared with the Control or Low group. Significantly lower BWG (P < 0.05), lower cumulative FI (P < 0.05), and higher FCR (P < 0.05) were observed in the PF group compared with the Control. Besides, the PF group showed a 16.21% higher BWG (P > 0.05) and significantly lower FCR (P < 0.05) when compared with the High.
Figure 1.
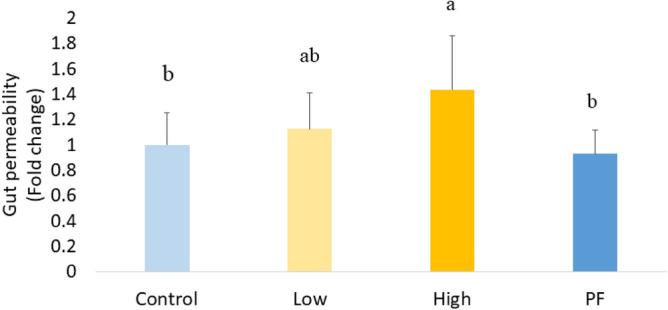
Gut permeability at 5 dpi. Higher concentration of FITC-d in the serum revealed more severe damage to the gut epithelium owing to the Eimeria challenge.a,ab,b Treatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P < 0.05, N = 10. The relatively level of FITC-d in serum was presented as fold change.
Table 1.
Average daily feed intake (ADFI), cumulative feed intake (FI), body weight gain (BWG, kg) and feed conversion ratio (FCR) from 0 to 6 dpi.
| Treatments1 | ADFI (g) | |||||
|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 3 dpi | 4 dpi | 5 dpi | 6 dpi | |
| Control | 126.01 | 95.42 | 104.23 | 108.19a | 86.38a | 96.68a |
| Low | 134.75 | 103.97 | 107.01 | 86.89b | 68.09b | 64.51b |
| High | 133.40 | 101.93 | 100.40 | 75.22b | 42.18c | 44.89c |
| PF | 127.27 | 93.33 | 93.33 | 76.36b | 42.42c | 46.82c |
| SEM | 1.66 | 2.32 | 2.16 | 2.92 | 3.56 | 3.86 |
| P-value | 0.158 | 0.316 | 0.128 | < 0.001 | < 0.001 | < 0.001 |
| Treatments | 0–6 dpi | |||||
|---|---|---|---|---|---|---|
| FI | BWG | FCR | ||||
| Control | 617.75a | 352.59a | 1.77c | |||
| Low | 566.53a | 250.43b | 2.32b | |||
| High | 499.47b | 178.06c | 2.93a | |||
| PF | 480.38b | 206.94bc | 2.35b | |||
| SEM | 12.098 | 12.86 | 0.09 | |||
| P-value | < 0.001 | < 0.001 | < 0.001 | |||
1Control: uninfected controls fed diet ad libitum and gavaged with water; Low: low Eimeria-infected group fed diet ad libitum and gavaged with 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina; High: severely Eimeria-infected group fed diet ad libitum diet and gavaged with 12,500 oocysts of E. maxima; 12,500 oocysts of E. tenella; 62,500 oocysts of E. acervulina; PF: an uninfected pair-fed group that fed the same amount of feed as the High group, gavaged with water.
a,ab,b,cTreatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P < 0.05, N = 10.
Bone mineral density was reduced in the Eimeria-infected groups
Bone morphology parameters were analyzed in diaphysis and metaphysis sections using micro-computed tomography (micro-CT) (Table 2). Tibia diaphyseal BMC was significantly reduced in the High and PF groups (P < 0.05), and diaphyseal BMD was reduced in the Low (P < 0.05), High (P < 0.05) and PF groups (P < 0.05) compared with the Control. Tibia metaphyseal BMD (total) was significantly reduced in the Low (P < 0.05) and the High (P < 0.05), whereas there was no significant difference between the PF group (P > 0.05) and the Control. Trabecular BMD and the number of trabecular bone (Tb. N) at metaphyseal regions were significantly reduced in the Low, High, and PF groups (P < 0.05) compared to the Control. However, metaphyseal total BMC, metaphyseal trabecular BMC, metaphyseal cortical BMD, and metaphyseal cortical BMC were not affected by Eimeria infection or feed restriction.
Table 2.
Femur bone metaphysis and diaphysis structure in broiler chickens at 6 dpi.
| Section | Unit | Control1 | Low | High | PF | SEM | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Metaphysis | Total | BMC2 | g | 149.403 | 137.218 | 117.639 | 132.312 | 6.984 | 0.469 |
| BMD | g/mm2 | 0.288a | 0.233b | 0.239b | 0.243ab | 0.009 | 0.017 | ||
| TV | mm3 | 579.742 | 606.012 | 534.340 | 559.222 | 22.068 | 0.704 | ||
| BV | mm3 | 315.092 | 359.677 | 301.793 | 335.881 | 20.723 | 0.783 | ||
| Cortical | BMD | g/mm2 | 0.556 | 0.511 | 0.522 | 0.492 | 0.0136 | 0.449 | |
| BMC | g | 84.366 | 97.495 | 86.034 | 95.740 | 4.9120 | 0.325 | ||
| Trabecular | BMD | g/mm2 | 0.519a | 0.398b | 0.392b | 0.411b | 0.0135 | < 0.001 | |
| BMC | g | 4.339 | 4.286 | 3.304 | 5.483 | 0.4082 | 0.294 | ||
| BS | mm2 | 403.078 | 410.676 | 317.433 | 443.159 | 37.1790 | 0.660 | ||
| Tb.N | - | 11.325a | 9.1304b | 9.236b | 8.2574b | 0.2855 | 0.004 | ||
| Diaphysis | Total | BMC | g | 136.838a | 120.375ab | 104.044b | 96.318b | 4.8538 | 0.013 |
| BMD | g/mm2 | 1.028a | 0.902b | 0.912b | 0.898b | 0.0130 | < 0.001 | ||
| BV | Mm3 | 133.672 | 133.649 | 114.335 | 107.172 | 4.8897 | 0.126 |
1Control: uninfected controls fed diet ad libitum and gavaged with water; Low: low Eimeria-infected group fed diet ad libitum and gavaged with 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina; High: severely Eimeria-infected group fed diet ad libitum diet and gavaged with 12,500 oocysts of E. maxima; 12,500 oocysts of E. tenella; 62,500 oocysts of E. acervulina; PF: an uninfected pair-fed group that fed the same amount of feed as the High group, gavaged with water.
2BMC, bone mineral content; BMD, bone mineral density; TV, total bone volume; BV, bone volume (TV minus bone marrow volume); BS, bone surface area; Tb. N, trabecular number.
a,ab,bTreatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P < 0.05, N = 10.
Eimeria-infected broilers exhibited suppression in bone formation
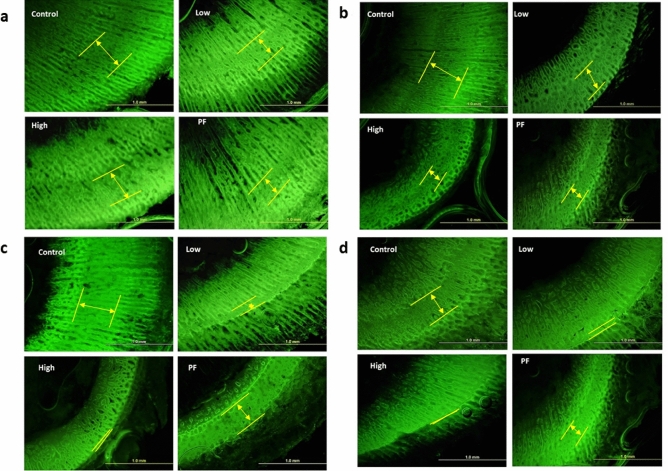
The distance between double layers of calcein bands was measured at the diaphysis of the tibia and femurs to evaluate bone growth rate (Fig. 2; Table 3). From 0 to 4 dpi (mild infection period), the femoral growth rate was significantly decreased in the High and PF groups compared to the Control, whereas the tibial growth rate was not significantly changed during 0–4 dpi. From 4 to 8 dpi (severe infection period), tibial and femoral growth was significantly reduced in the Low group (P < 0.05) and the High group (P < 0.05), however, it was not changed in the PF group (P > 0.05) when compared with the Control.
Figure 2.
Fluorescence image of the femoral or tibial cross-section of broilers (measurement data shown as Table 3). Bone formation was visualized by double calcein labeling in the femoral bone and tibia bone, respectively. Representative picture (a): femoral bone at 0–4 dpi; (b): tibial bone at 0–4; (c): femoral bone at 4–8 dpi; (d): tibial bone at 4–8 dpi; Photos were taken at 4 × objective. The yellow scale bar is 1 mm in length. The yellow lines marked the distance between two calcein injections.
Table 3.
Bone growth rate measured by calcein injection method.
| Femur: unit (mm) | |||
|---|---|---|---|
| Treatment1 | 0–4 dpi | 4–8 dpi | 0–8 dpi |
| Control | 0.253a | 0.212a | 0.479a |
| Low | 0.175ab | 0.081b | 0.219bc |
| High | 0.157b | 0.032b | 0.190c |
| PF | 0.145b | 0.157a | 0.300b |
| SEM | 0.0132 | 0.0143 | 0.0241 |
| P-value | 0.014 | < 0.001 | < 0.001 |
| Tibia: unit (mm) | |||
|---|---|---|---|
| Treatment1 | 0–4 dpi | 4–8 dpi | 0–8 dpi |
| Control | 0.208 | 0.186a | 0.387a |
| Low | 0.128 | 0.030b | 0.147b |
| High | 0.115 | 0.036b | 0.152b |
| PF | 0.125 | 0.140a | 0.270ab |
| SEM | 0.0177 | 0.0158 | 0.0285 |
| P-value | 0.197 | < 0.001 | 0.002 |
1Control, control group; Low, the lower challenge dose; High, the high challenge dose; PF, pair-feeding group, paired with the High group.
2Unit: mm.
a,ab,b,bc,cTreatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P < 0.05, N = 10.
By adding data together from both injection stages, during 0–8 dpi, the Low, High, and PF treatment groups showed a significant decrease in bone formation (only femur), whereas the High group had the lowest bone growth rate (P < 0.05), followed by the Low (P < 0.05) and PF (P < 0.05) when compared with the Control. Compared with the Control, tibial growth in the Low and High groups was significantly reduced (P < 0.05) by the Eimeria challenge. However, the tibial growth of the PF group was not statistically different compared to the Control or infected groups (P > 0.05).
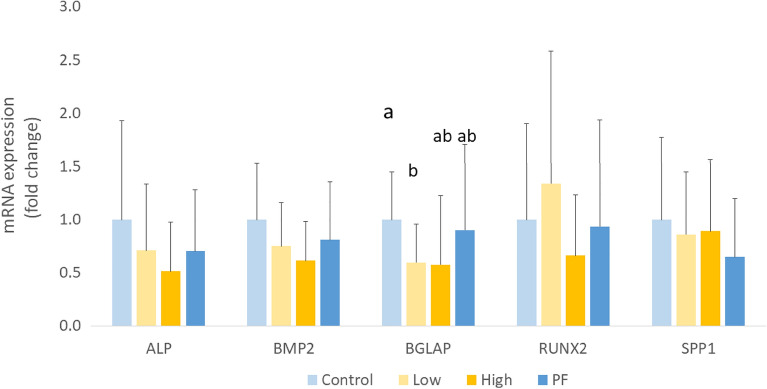
Relative mRNA expression of BGLAP was significantly reduced in the High group when compared with the Control (P < 0.05; Fig. 3), confirming that the severe infection of Eimeria adversely affected long bone growth. However, the expression of other bone formation markers was not significantly changed among the treatments. Moreover, higher cumulative FI was linearly correlated with higher femoral bone formation rate (R2 = 0.4655, P = 0.010), but not in tibia (P > 0.05). The tibial bone growth rate was highly correlated with metaphyseal trabecular BMD (R2 = 0.710, P < 0.001). Tibial bone growth rate (by calcein injection method) was positively correlated with tibial diaphysis BMD (R2 = 0.4308, P = 0.032).
Figure 3.
Osteogenesis-related gene expression in broilers bone marrow of different treatment groups. Control, non-challenge control group; Low, the low challenge dose of Eimeria; High, the high challenge dosage of Eimeria; PF, pair-feeding group that paired with High group.a,ab,b Treatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P = 0.002, N = 10.
Eimeria infected broilers exhibited an increased bone resorption
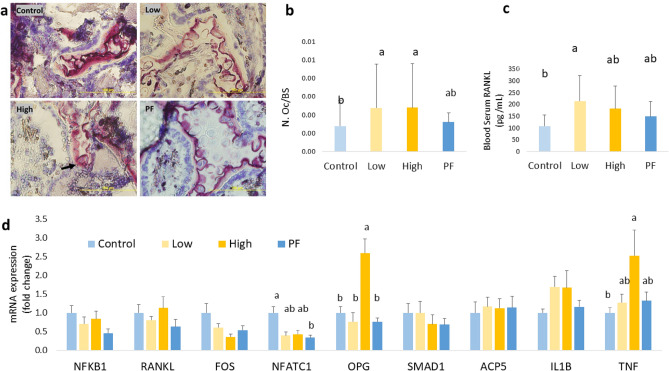
The number of osteoclasts per bone surface (N. Ocl/BS) in the Low and High groups was significantly higher than in the Control (P < 0.05; Fig. 4a and b). Eimeria challenge increased the formation of TRAP-positive cells and bone resorption activity on the surface of tibia metaphysis trabecular bone. Meanwhile, an increased serum level of RANKL was observed in the Low (P < 0.05; Fig. 4c), whereas the High and PF serum levels of RANKL had numeric increasing compared to the Control (P > 0.05). Moreover, the mRNA expression of NFATC1 was significantly decreased in the PF (P < 0.05; Fig. 4d), and expression of TNFRSF11B (OPG) and TNF (tumor necrosis factor-like) was significantly increased in the High (P < 0.001) when compared with the Control. However, there were no significant changes in the expression of NFKB1, RANKL, FOS, ACP5(TRAP), IL1B, and SMAD1 among the treatments. A higher ratio of RANKL/OPG was observed in the Low (P < 0.05) and the PF (P < 0.05) when compared with the High (Fig. 5). Together, results suggest that Eimeria infection resulted in bone remodeling along with higher osteoclast number and activity in broilers. There was a negative correlation between TNF and metaphyseal BMD (R2 = 0.419, P = 0.030).
Figure 4.
Eimeria infection increases osteoclast formation. (a): Illustration of TRAP staining at tibial metaphysis section. Osteoclasts were defined in this study as TRAP-positive cells with three or more nuclei. Osteoclast was indicated with arrow. Photos were taken at 10 × objective. The yellow scale bar is 100 µm in length. (b): Number of osteoclasts (N. Oc) were counted and bone circumference (BS) were measured. The increased ratio of N. Oc/BS indicated an increased formation of osteoclast activity at metaphysis section in Eimeria infected groups (P = 0.004). (c): Serum level of RANKL in broilers at 6 dpi. The low group has higher level of RANKL than the Control group (P = 0.047). (d): Osteoclastogenesis-related gene expression in broilers bone marrow of different treatment groups.a,ab,b Treatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P < 0.050, N = 10. Control, non-challenge control group; Low, the low challenge dose of Eimeria; High, the high challenge dosage of Eimeria; PF, pair-feeding group that paired with High group.
Figure 5.
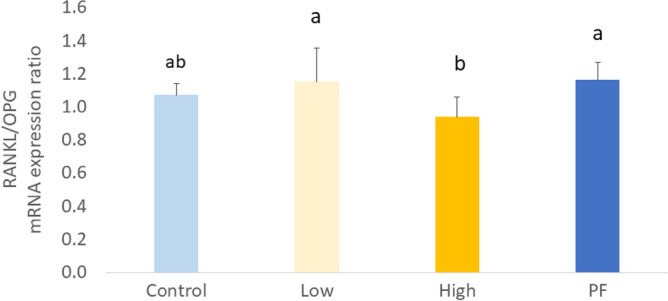
mRNA expression ratio of RANKL/OPG in bone marrow. Higher rate of RANKL/OPG indicated bone remodeling status.a,ab,b Treatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P = 0.005, N = 10. Control, non-challenge control group; Low, the low challenge dose of Eimeria; High, the high challenge dosage of Eimeria; PF, pair-feeding group that paired with High group.
Eimeria infection increased lipid peroxidation and decreased antioxidant capacity, and the correlation between redox status and bone parameters
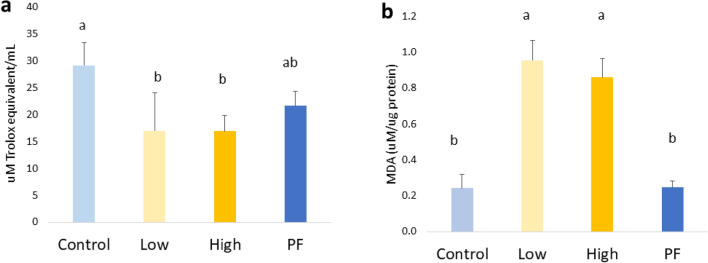
The total antioxidant capacity of serum in the Eimeria spp. infected broilers (the Low and High groups) decreased significantly at 6 dpi compared with the non-infected Control (P < 0.05; Fig. 6a). Meanwhile, the total antioxidant capacity of serum in the PF group showed a numeric decrease compared with the Control, but the change was not statistically significant. In contrast to the antioxidant parameters in serum, the level of MDA in the liver was significantly increased by Eimeria infection compared with the Control and PF groups (P < 0.01; Fig. 6b) at 6 dpi.
Figure 6.
Oxidative status at 6 dpi. (a): serum total antioxidant capacity was measured by antioxidant assay kit, that coccidial infection significantly decreased the antioxidant capacity (P = 0.001). (b): liver lipid peroxidation was measured by TBAR assay kit, that coccidial infection significantly elevated lipid peroxidation in liver (P < 0.001). Control, non-challenge control group; Low, the low challenge dose of Eimeria; High, the high challenge dosage of Eimeria; PF, pair-feeding group that paired with High group.a,ab,b Treatments with different letters means a significantly difference between treatments by using Tukey’s HSD test, P < 0.05, N = 10.
Pearson correlation analyses revealed a negative correlation of the liver TBAR level with femur growth rate (R2 = 0.249, P = 0.005) and between the liver TBAR level and tibia growth rate (R2 = 0.330, P = 0.001). There was a positive correlation between serum total antioxidant capacity and metaphysis bone mineral density (R2 = 0.219, P = 0.0182); and a negative correlation between the liver TBAR level and metaphysis bone mineral density (R2 = 0.391, P < 0.001). Meanwhile, bone marrow BGLAP mRNA level was negatively correlated with the liver TBAR level (R2 = 0.130, P = 0.031) and positively correlated with serum total antioxidant capacity (R2 = 0.227, P = 0.004).
Discussion
With increasing concerns around farm animal welfare, poultry bone abnormalities have become one of the significant challenges for the poultry industry. Bone is an essential multifunctional organ that not only provides static functions such as structural support and internal organ protection but also acts as a dynamic endocrine organ that releases hormones for mineral homeostasis, acid–base balance, and reservoir of energy and minerals57,58. However, for a long time, bone health in broiler production yet gained enough attention until recently because more research suggests that current commercial broiler chicken breeds grow fast to heavyweight that predisposes the chickens to leg weakness and skeletal abnormalities59,60. With coccidiosis so widespread, the Eimeria-challenge model was chosen as a disease model to understand the possible link between intestinal parasite infection and bone health in the current study. With a mild infection, broilers was able to compensate for growth loss partially at later stage after recover from coccidiosis, even so, the growth potential remains severely compromised48. Uncoupling bone remodeling may not be apparent at early growth but may show up later in the market age, resulting in clinically leg bone abnormality, eventually decreasing the market value61. Thus, the early stage of bone health is vital in broiler growth performance and critical in product profitability.
The etiology of leg abnormalities under intestinal infection is generally complex. The factors that affect bone metabolism during infection include but are not limited to nutrition, immunity and physical stress. Eimeria infection could affect epithelium cells directly by mediating nutrition transporter activity or indirectly by causing apoptosis of cells and damaging the integrity of the intestine3,62,63. The damage to the digestive tract can cause malabsorption of nutrients, the deficiency of macronutrients including carbohydrates, crude protein, lipid, and minerals, and is associated with a bone remodeling imbalance that increases markers of bone resorption and decreases markers of bone formation64–66. Bone also serves as a mineral and energy reservoir, playing a role in maintaining glucose and phosphorus level within a narrow range in blood67. Long-term calcium deficiency is a potential risk factor for osteoporosis and bone fracture68,69. Depleting dietary calcium and phosphate could increase bone resorption and decrease bone mineral density70,71, but increased calcium intake alone is insufficient to compensate for the severe bone mineral loss under acute disease conditions72. Besides, protein deficiency could significantly decrease BMD and cancellous bone mass, reducing bone strength13,14,73. FI is positively correlated with the bone formation rate74. Lower energy, essential amino acid or mineral level can subsequently decrease osteoblastic activity suppressing bone formation. Moreover, nutrition deficiency could modify the plasma levels of certain essential hormones, such as growth hormone and insulin-like growth factor75,76, and elevated plasma stress hormones such as corticosterone77–81. Endocrine changes can directly mediate bone remodeling by regulating the activity of osteoblasts or osteoclasts, and those variables should also be considered in the experimental design. Monitoring the daily feed intake amount is very important for evaluating the growth performance especially with the impact of Eimeria infection on broiler chicken growth. In order to permit a clear interpretation of the results and limit the variables of nutrient and endocrine factors, the pair-feeding method was incorporated in this study. Pair-feeding is a technique to determine the effect of treatment on growth that is independent of nutritional factors. It has been widely used in the animal in vivo study model and was particularly well adapted to a study of energy, protein, and mineral intake deficiency82,83. In the current study, the amount of feed provided to the uninfected PF group was matched with the High group. However, the growth performance in the PF group was numerically higher than in the High group but not significant. The growth difference between the two groups reflected the metabolic cost of immune activation, oxidative stress, and other factors, rather than feed intake difference. Comparing the High group and the PF group provided more direct evidence to find the possible link between immune response/oxidative stress and bone metabolism.
Moreover, the micro-CT analyses revealed more specific details of bone structural changes. A significant drop in BMD was observed in bone metaphysis and diaphysis in both Eimeria-infected groups. Furthermore, the PF group and the infected Low and High groups showed different responses in the metaphysis and diaphysis sections. For example, the diaphysis is the mid-section (shaft) of a long bone and is the primary site of radial growth in young animals84. The diaphysis is made up of cortical bone, which has higher mineral content and density, and less water content when compared with trabecular bone, providing structural strength85. According to the current results, a significant decrease in BMD, BMC, and bone growth rate was observed in the diaphysis section. Both infected groups and the PF groups showed suppression of bone formation over this site. The PF group had the lowest diaphyseal BMC compared with the other groups. Lowest metaphyseal BMD was observed in the High group. It showed that bone metabolic activity appears site-specific during Eimeria infection, and bone mineral loss occurs distinctly in the proximal tibia metaphysis and diaphysis. Moreover, trabecular bones are metabolically more active than cortical bone, which not only contributes to the strength of the bone but also serves as a source of calcium for the body because trabecular bones are remodeled more rapidly during physiological processes86–88. Cortical bone loss occurs slower than trabecular loss due to the fact that less surface per unit of bone matrix volume is available for bone remodeling89. Nutrient deficiency, stress, and infectious skeletal disorders could cause bone mass loss and decrease bone quality by altering the trabecular bone microarchitecture90,91. In human and mouse research, the rate of bone turnover is more rapid in trabecular bone with a larger surface area than in cortical bone92. The current results showed that the bone mineral loss at the metaphysis section mainly occurred in the trabecular bone structure. Larger bone endo-surface could provide broader space for osteoblast or osteoclast attachment in trabecular bone. We hypothesized that bone resorption happens more frequently around the trabecular bone. Also, the tibial growth rate was highly correlated with tibial trabecular BMD instead of BMC, which indicates metaphysis trabecular bone BMD is more suited to evaluate early biochemical changes of bone during pathogen infection in broilers. Total mineral content (BMC) or bone ash weight may not accurately reflect the metabolic change of bone during infection.
Metabolic changes can be further assessed by different methods focusing on bone formation and resorption individually. Calcein labeling was used in the current study to visualize the newly formatted bone, and the RT-qPCR method was used to examine the bone formation-related marker genes in the bone marrow. The calcein labeling method is commonly used to assess bone growth, which directly visualizes bone growth in vivo. According to the results, by correlating FI with bone formation in each group, the Low group had significantly higher FI compared with the PF group; however, the bone formation rate was significantly lower than the PF (P < 0.05), which is in agreement with the findings of the microstructure analysis. With the same amount of FI, the PF group has a 4.71% higher BMD than the High group (P < 0.05). With a similar amount of FI (P > 0.05) between the Control and Low groups, diaphyseal BMD in the Low group was significantly lower than in the Control group. Based on the current results, even though provided with the same amount of feed, the Eimeria-challenged chickens had worse bone health status than the PF group, suggesting that apart from nutrition deficiency, other factors may be involved in bone homeostasis in both direct and indirect manners. In our previous studies, we have reported the changed redox status in broilers on 6 dpi after Eimeria spp. challenge20. Oxidative stress has been acknowledged as a major contributor to the immune response. The increased production of ROS is an inflammatory response that functions for the recruitment and activation of immune cells that lead to pathogen killing93. ROS production is involved in mineral homeostasis and contributes to bone remodeling by promoting bone resorption and suppressing bone formation. Human and mice studies have found a tight association between oxidative stress and pathogenesis of the bone disorder, that the redox state changes are related to the bone modeling and remodeling processes94,95. The redox state can directly impact osteoblast activity that regulates bone formation rate96. Oxidative stress suppressed the osteoblastic differentiation process of primary bone marrow stem cells95. In the current study, the oxidative stress (increased TBAR level and decreased total antioxidant capacity) was negatively correlated with bone growth rate and mRNA expression of BGLAP. This result was consistent with our previous finding20, that decreased bone quality was associated with systemic oxidative stress in broiler during Eimeria infection. Oxidative stress can be a co-factor involved in loss of osteoblastic activity that ultimately led to poor bone quality.
Eimeria infection can cause a complex host immune responses, encompassing both cellular and humoral mechanisms during infection48. Studies indicated that humoral immunity and antibodies produced by B cells were increased during severe Eimeria infection97. Different from other species, the bursa of Fabricius, a unique central immune organ of birds located near the cloaca, is the location of B lymphocyte differentiation and maturation instead of bone marrow98. Fully differentiated B lymphocytes migrate to peripheral lymphoid organs to participate in immune responses, such as producing antibodies and participating in humoral immunity48,99. B cell-produced proteins such as RANKL and OPG are critical for bone metabolism100–104. B lineage cells produce more than 60% of total OPG in bone marrow102. Mice that were injected with RANKL inhibitor resulted in a larger bone mass105. According to our study, an increased mRNA expression of OPG in bone marrow indicated that the system was actively producing more OPG, but the source of OPG remains unknown. The drastically higher expression of OPG in bone marrow may be related to a negative feedback loop, that increased OPG subsequently affects osteoclastogenesis106. We hypothesize that the bone marrow could actively reduce or inhibit highly-elevated osteoclast activity by increased expression of OPG, then preserving minerals for bone homeostasis during Eimeria infection. A similar pattern of expression was observed in gene expression of tight junction protein during acute Eimeria infection, that Eimeria infection damaged intestine integrity, but tight junction protein gene expression was significantly elevated to repair the damage4.
Bone marrow not only contains different cell types that perform bone formation and resorption but also serves as the cradle of hematopoiesis and a reservoir of growth factors and cytokines, providing an ideal environment for communicating between bone metabolism and the immune system. Essentially all the units that participate in cellular immunity can influence bone cells, particularly impacting the activity and formation of osteoclasts2,97,107. The cytokines IL-1β, IL-6, and TNF-α are known to increase bone resorption by stimulating both osteoclast activity and differentiation in mammals45. The number of osteoclast precursors increases under inflammatory conditions, characterized by high levels of the potent inflammatory cytokine TNF-α108,109. The current study found significantly higher mRNA expression of TNF (tumor necrosis factor-like) in the High group than in the other groups. Meanwhile, activation of osteoclast formation was detected in the High group over the metaphysis trabecular bone. It is important to mention that, with the same amount of FI, the expression of TNF was relatively low in the PF group compared with the High, as well as the number of osteoclast and enzyme activity of RANKL was relatively higher in the High group than the PF group. Based on the comparison between the High group and the PF group, we concluded that the increased osteoclastic bone resorption is associated with the activation of immune response in broiler chickens during Eimeria infection. Moreover, NFKB is involved in many signaling pathways and plays an important role in osteoclast formation and survival rate35,110,111. NFKB ligand (RANKL), one of the most critical molecules that regulate osteoclast formation, provides the crosstalk between bone and immune systems32,42. The binding of RANKL to its receptor RANK triggers osteoclast precursors to differentiate into osteoclasts, which increases the number of osteoclasts on their bone surfaces43,112,113. In the present study, the High group showed the lowest level of RANKL/OPG, indicating that the negative feedback loop was turned on because less osteoclastic activity was in need to preserve the minerals for bone structure and support114. The different expressions of the RANKL/OPG ratio between the Low and the High indicated the bone homeostasis is infection-dose-dependent during coccidiosis. However, with the difference in B-cell development in avian species, immunity, particularly humoral immunity, might interact with bone metabolism differently from mammals. How osteoimmunology plays a role in avian bone homeostasis needs more profound studies. Taken together, delayed bone development in the parasite-challenged groups was attributable to an uncoupling of osteoblast and osteoclast activity, whereby increased bone resorption and decreased bone formation were closely associated with immune response/oxidative stress during Eimeria infection. With the long-held notion that the central pathophysiology of bone disorder was nutrition deficiency and physical stress during Eimeria infection, we demonstrated that bone disorder is also closely connected with bone modeling and remodeling which are associated with immune response/oxidative stress. Both nutrition and concurrent diseases will influence the occurrence of leg disorders. Further study on osteoimmunology needs to address bone disorder issues and will further lead to a more precise understanding of the mechanism underlying the pathogenesis of bone mineral loss and bone disease in broilers, eventually improving animal production and welfare in the future.
Materials and methods
Ethics statement
The experiment followed the guideline of the Institutional Animal Care and Use Committee and was conducted at the Poultry Research Farm, University of Georgia, Athens, GA. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Georgia (ethical approval code: A2021 12-012).
Experimental design
The study was carried out in compliance with the ARRIVE guidelines. A total of 480 one-day-old male broiler chickens (Cobb 500) were randomly distributed into four treatment groups with ten replicates and twelve birds per cage. All broiler chicks were fed the same starter basal diet during day 1 to 14, and the starter diet were formulated following Cobb500 broiler management guide115. On day 14, all experimental groups received either water or Eimeria spp. challenge. Experimental groups included uninfected controls (Control) fed diet ad libitum (gavaged with water), a low Eimeria-infected group (Low) fed diet ad libitum (gavaged with 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina), a severely Eimeria-infected group (High) fed diet ad libitum diet (gavaged with 12,500 oocysts of E. maxima; 12,500 oocysts of E. tenella; 62,500 oocysts of E. acervulina), and an uninfected pair-fed group (PF; gavaged with water) that fed the same amount of feed as the High group consumed. To ensure that the pair-fed group (PF) had the same intake as the high challenge group, the amount of feed provided to each group was carefully monitored and matched. Preliminary data was used to estimate the daily feed intake of each group, and the average feed intake of the high challenge group was calculated. The same amount of feed was then provided to the pair-fed group. The feed was weighed and distributed evenly to the pair-fed group broilers at three or four intervals throughout the day (7:30 am, 3:30 pm, and 9:30 pm), with the 9:30 pm feeding time serving as an opportunity to adjust the intake of the pair-fed group to match that of the high challenge group. The grower (15–20 days of age) basal diets were formulated following Cobb500 broiler management guide115. Diet information is shown in Supplementary Table S1 online. All chicks were raised under the same house, feeding, and environmental management conditions based on the broiler management guide115. Chicks were allowed to consume water on an ad libitum basis, and daily feed intake was measured during the study. On 6 days post infection (dpi), one bird per replicate was randomly selected to collect tissue samples. The experimental design flow chart shown in Supplementary Fig. S1 online. The tissue samples were snap-frozen in liquid nitrogen and kept in − 80 °C until future processing.
Gut permeability
The gut permeability was measured on 5 dpi by the method used in our previous study3,116. Briefly, fluorescein isothiocyanate dextran (FITC-d; MW 4000; Sigma-Aldrich, Ontario, Canada) was dissolved in distilled water and made into 2.2 mg/mL solution. One bird per cage was randomly selected and gavaged with 1 mL of FITC-d solution. Two hours after inoculation, the blood was collected from birds and kept in the dark at room temperature for clotting. The clotted blood was centrifuged at 1500 g for 15 min to serum collection. The standard curve solution was made from a serial dilution of FITC-d stock (2.2 mg/mL). Dilution buffer was made from the pooled serum of non-infection birds with the basal diet. Sample and standard solutions were loaded into black 96-well plates, and FITC-d concentrations were measured by a spectrophotometer (SpectraMax M5; Molecular Devices, San Jose, CA). The excitation wavelength was set at 485 nm, and the emission wavelength was set at 528 nm.
Micro-computed tomography (micro-CT)
A total of 40 samples (one bird per cage) were randomly collected to evaluate 3-D bone morphologic changes in the broiler. The proximal and shaft of the tibia were assessed by micro-computed tomography (micro-CT). The scanning process was performed according to our previous publications20,117, with setting as 83 kV, 121 µA, and a 0.5 mm aluminum filter, the pixel size as 26 µm with 360° complete rotation, and 42 min of acquisition time. Scanning was performed with SkyScan 1172 (SkyScan, Kontich, Belgium). 2-D images were transferred to CTAn software (CTAn, SkyScan) for structure construction and quantification. The metaphyseal region of interest (ROI) was post-operated to automatically separate trabecular bone from cortical bone and preserve its morphology using a threshold of 800. Average bone mineral density (BMD), bone mineral content (BMC), and bone micro-architectural parameters of each group were taken from the same ROI. Cortical and trabecular bone parameters were quantified and analyzed separately. The following parameters were quantified: total volume (TV), bone volume (BV), bone surface (BS), bone volume per tissue volume (BV/ TV), and trabecular number (Tb. N)20.
Calcein labeling
For dynamic histomorphometry measures of bone formation, calcein (Cat no. C0875, Sigma Aldrich, St. Louis, MO) was dissolved in a 1 M sodium hydroxide solution and then mixed with sterilized distilled water to make the 2.0% working solution. The birds were injected with the calcein solution intraperitoneally at 20 mg/kg of body weight. On day 4 after the first injection of calcein, the birds were injected again as previously described. Bone samples were collected on 4 days after the second injection. The muscle was removed immediately, and bones were preserved in 70% ethanol. Upon analysis, a thin slice of bone was taken from mid-diaphysis by a circular saw (Ryobi, Anderson, SC, USA), sanded each bone slice down by using sandpaper then mounted on a glass slide. Calcein has a high calcium affinity and translates into a relatively broad fluorescent band. A fluorescence microscope (Leica DC500 camera, Leica Microsystems Inc., Buffalo Groove, IL) was used to visualize new bone formation and determine the distance between the two calcein labels on the bones. Eight measurements at different angles were performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The average values were calculated for data analysis.
Serum receptor activator of nuclear factor kappa B ligand enzyme-linked immunosorbent assay (RANKL ELISA)
Chicken RANKL concentrations were measured by commercially available kits (MyBioSource, San Diego, CA, USA). All procedures were performed according to the manufacturer’s protocol. The method was two-site sandwich ELISA, the pre-coated antibody was Chicken PRM1 monoclonal antibody and the detecting antibody was polyclonal antibody with biotin labeled. A standard curve was created, and the RANKL concentration of the examined samples was calculated and expressed in pg/ml. Background OD values were subtracted from the calculation, and the color depth was directly proportional to the amount of RANKL in the sample.
Tartrate-resistant acid phosphatase staining (TRAP staining)
All tibia bones were collected at 6 dpi. After removing the muscle tissue, tibias were fixed in 4% PBS-buffered formaldehyde at 4 °C for three days and then moved into 70% ethanol for preservation. Tibial tuberosity was used as a landmark to cut the bone slides by the circular saw. The bone slides were demineralized with 10% ethylenediaminetetraacetic acid (EDTA) at 4 °C for 13 days. Each bone slide was equally cut into four pieces perpendicularly. The samples were then embedded in paraffin and cut into 4 µm sections using Leitz 1512 rotary microtome (Leica Microsystems, Wetzlar, Germany). The paraffin sections were stained with tartrate-resistant acid phosphatase (TRAP) solution prepared by mixing acetate buffer (pH 5.0), naphthol AS-MX phosphate (Sigma Chemical, St. Louis, MO, USA), Fast Red Violet LB Salt (Sigma), and 50 mM sodium tartrate (Sigma). The sections were counterstained with hematoxylin (Sigma). Osteoclasts were defined in this study as TRAP-positive cells with three or more nuclei. Bone circumference was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Lipid peroxidation and antioxidant status assay
Chicken total antioxidant capacity in serum was analyzed using a QuantiChrom antioxidant assay kit (BioAssay Systems, Hayward, CA, USA), and the level of liver lipid peroxidation was determinated by using QuantiChro TBARS Assay Kit (BioAssay Systems, Hayward, CA, USA). Serum was collected, centrifuged, and then kept at − 80 °C. A liver sample from one bird per cage was collected, snap-frozen in liquid nitrogen, and then kept at − 80 °C. Liver samples were homogenized and centrifuged in the assay buffer, and all assay procedures were performed according to the manufacturer’s protocols. The protein concentration was measured by protein quantification assay (Pierce™ BCA Protein Assay Kit, Thermo Scientific, Rockford, IL, United States) following the procedure indicated in our previous publication20.
Real-time quantitative PCR analysis of gene expression in bone marrow
Bone marrow from femur bones was extracted, snap-frozen in liquid nitrogen, and stored immediately at -80 °C until RNA isolation (N = 10). Bone marrow total RNA was extracted using Qiazol reagent (Qiagen, USA) according to the manufacturer’s instruction. A Nano-Drop 1000 Spectrophotometer (ThermoFisher Scientific, Pittsburgh, PA) was used to determine the quantity of RNA. The cDNA was synthesized from total RNA (2000 ng) using high-capacity cDNA reverse transcription kits (Thermo Fisher Scientific, Waltham, MA).
Real-time reverse transcription polymerase chain reaction (RT-qPCR) was performed to measure mRNA expression. Primers were designed using the Primer-BLAST program (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The specificity of primers was validated by melting curve analysis and gel electrophoresis. RT-qPCR was performed on an Applied Biosystems StepOnePlus™ (Thermo Fisher Scientific, Waltham, MA) with iTaq™ Universal SYBR Green Supermix (BioRad, Hercules, CA) using the following conditions for all genes: 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s, annealing temperature for 20 s, and extending at 72 °C for 1 min.
The geometric mean of 18S, HBMS and GAPDH were used for normalization118. The stability of housekeeping genes were confirmed by their consistent Ct values among the treatments (P > 0.1)119. BGLAP, RUNX2, SPP1, BMP2, and ALP were used as genetic markers of bone formation in the bone marrow120. NFKB, RANKL, FOS, ACP5, NFATC1, IL1B, TNF, SMAD1, and TNFRSF11B (OPG)121 were used as the genetic markers for osteoclastic activity in the bone marrow. Details of primer sequences used for the experiment are presented in Table 4. Moreover, the ratio of OPG/RANKL in bone marrow was calculated. Samples were run in triplicate, and relative gene expression data were analyzed using the 2−ΔΔCt. The mean ΔCt of each marker gene from the control group was used to calculate the ΔΔCt value, and 2−ΔΔCt expression levels were normalized to 1 for the control group. Expression levels of the treatment groups were presented as fold change.
Table 4.
Nucleotide sequences of the primers used for real-time RT-PCR.
| Primer sequence (5′–3′) | Product length (bp) | Annealing temperature (°C) | Accession # | |
|---|---|---|---|---|
| GAPDH |
F-GCTAAGGCTGTGGGGAAAGT R-TCAGCAGCAGCCTTCACTAC |
161 | 55 | NM_204305.1 |
| RNA18S1 |
F-AGCCTGCGGCTTAATTTGAC R-CAACTAAGAACGGCCATGCA |
121 | 56.5 | AF_173612.1 |
| HMBS |
F- GGCTGGGAGAATCGCATAGG R- TCCTGCAGGGCAGATACCAT |
131 | 59 | XM_004947916.3 |
| SPP1 |
F-GCCCAACATCAGAGCGTAGA R-ACGGGTGACCTCGTTGTTTT |
204 | 57 | NM_204535.4 |
| BMP2 |
F-TCAGCTCAGGCCGTTGTTAG R-GTCATTCCACCCCACGTCAT |
163 | 57 | XM_025148488.1 |
| RUNX2 |
F-ACTTTGACAATAACTGTCCT R-GACCCCTACTCTCATACTGG |
192 | 60 | XM_015285081.2 |
| ALP |
F-CGACCACTCACACGTCTTCA R-CGATCTTATAGCCAGGGCCG |
140 | 58 | NM_205360.1 |
| BGLAP |
F-GGATGCTCGCAGTGCTAAAG R-CTCACACACCTCTCGTTGGG |
142 | 57 | NM_205387.3 |
| NFKB1 |
F-GAAGGAATCGTACCGGGAACA R-CTCAGAGGGCCTTGTGACAGTAA |
131 | 59 | XM_015285418.2 |
| FOS |
F-CTTCGACGAGCTGCTTTTCT R-TGGAGGTGTAGGTGCTAGGG |
191 | 60 | NM_205508.1 |
| TNFRSF11B |
F-ACGCTTGTGCTCTTGGACAT R-CAGCGTAGTACTGGTCTGGG |
193 | 60 | NM_001033641.1 |
| TNFSF11 |
F-ACACGCCCTTTGAAAATCAG R-GCAAAAGGTTGCTTCTCTGG |
196 | 60 | XM_015275777.2 |
| ACP5 |
F-GCTTCCAGGAGACCTTCGAG R-CAGGCGGAGGCTGTAGTAGT |
170 | 61 | XM_040693093.1 |
| NFATC1 |
F-CAGTCCTGCAGTCCAACTCA R-TCCTCAGGTTCTCGCTTGAT |
173 | 60 | XM_040663226.1 |
| SMAD1 |
F-GTTTTGTAAAGGGTTGGGGAGC R-AATGCAGGAGCTTGGGACCTTA |
174 | 61 | XM_040698719.1 |
| IL1B |
F-AGATGAAGCGGGTCAGCTC R-GCATCAAGGGCTACAAGCTC |
120 | 59 | XM_015297469.2 |
| TNF |
F-CGTGGTTCGAGTCGCTGTAT R-CCGTGCAGGTCGAGGTAC |
100 | 60 | XM_040694846.2 |
1GAPDH: glyceraldehyde-3-phosphate dehydrogenase; HMBS: hydroxymethylbilane synthase; RNA18S1: RNA, 18S ribosomal 1; ACP5: TRAP, acid phosphatase 5, tartrate resistant; TNFSF11: RANKL,TNF superfamily member 11; TNF: tumor necrosis factor-like; NFATC1:nuclear factor of activated T cells 1; TNFRSF11B: OPG, TNF receptor superfamily member 11b; IL1B: interleukin 1 beta; BGLAP: bone gamma-carboxyglutamate protein; RUNX2: runt-related transcription factor 2; ALP: alkaline phosphatase; SPP1: secreted phosphoprotein 1; BMP2: bone morphogenetic protein 2; FOS: Fos proto-oncogene, AP-1 transcription factor subunit; NFKB1: nuclear factor kappa B subunit 1; SMAD1: SMAD family member 1.
Statistical analysis
All experimental data were expressed as mean with standard error of the means (SEM). Data were tested for homogeneity of variances and normality of studentized residuals. The differences among the treatment groups were analyzed by one-way ANOVA, whereas the means were analyzed statistically by Tukey’s test using JMP Pro14 (SAS Institute, Inc., Cary, NC). Statistical significance was set at P < 0.05, and 0.05 ≤ P ≤ 0.1 were also presented to show the trending toward statistical significance122. Pair wise correlations (JMP Pro14) were evaluated for all bone and growth variables.
Supplementary Information
Acknowledgements
This study was financed in part by a cooperative agreement 58-6040-8-034 from United States Department of Agriculture-Agricultural Research Service.
Author contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. Y.T., P.T., T.S., and W.K. conceived and designed this study. Y.T., P.T., and J.C. contributed to broilers husbandry and sample collection. Y.T. and M.Y. contributed to data analyses. The paper was written through contribution and critical review of the manuscript by all authors (Y.T., J.C., P.T., M.Y., T.S. and W.K.).
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-27585-5.
References
- 1.Blake DP, et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:115. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yun C. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000;24:303–324. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]
- 3.Teng PY, et al. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020;99:4203–4216. doi: 10.1016/j.psj.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teng PY, Choi J, Tompkins Y, Lillehoj H, Kim W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021;52:81. doi: 10.1186/s13567-021-00949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fatoba AJ, Adeleke MA. Diagnosis and control of chicken coccidiosis: A recent update. J. Parasit. Dis. 2018;42:483–493. doi: 10.1007/s12639-018-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook ME. Skeletal deformities and their causes: Introduction. Poult. Sci. 2000;79:982–984. doi: 10.1093/ps/79.7.982. [DOI] [PubMed] [Google Scholar]
- 7.Thorp BH. Skeletal disorders in the fowl: A review. Avian Pathol. 1994;23:203–236. doi: 10.1080/03079459408418991. [DOI] [PubMed] [Google Scholar]
- 8.Kestin SC, Knowles TG, Tinch AE, Gregory NG. Prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 1992;131:190–194. doi: 10.1136/vr.131.9.190. [DOI] [PubMed] [Google Scholar]
- 9.Oikeh I, Sakkas P, Blake DP, Kyriazakis I. Interactions between dietary calcium and phosphorus level, and vitamin D source on bone mineralization, performance, and intestinal morphology of coccidia-infected broilers1. Poult. Sci. 2019;98:5679–5690. doi: 10.3382/ps/pez350. [DOI] [PubMed] [Google Scholar]
- 10.Sakkas P, et al. Does selection for growth rate in broilers affect their resistance and tolerance to Eimeria maxima? Vet. Parasitol. 2018;258:88–98. doi: 10.1016/j.vetpar.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakkas P, Oikeh I, Blake DP, Smith S, Kyriazakis I. Dietary vitamin D improves performance and bone mineralisation, but increases parasite replication and compromises gut health in Eimeria-infected broilers. Br. J. Nutr. 2019;122:676–688. doi: 10.1017/S0007114519001375. [DOI] [PubMed] [Google Scholar]
- 12.Fetterer RH, Miska KB, Mitchell AD, Jenkins MC. The use of dual-energy X-ray absorptiometry to assess the impact of Eimeria infections in broiler chicks. Avian Dis. 2013;57:199–204. doi: 10.1637/10392-092812-Reg.1. [DOI] [PubMed] [Google Scholar]
- 13.Southern LL, Baker DH. Zinc toxicity, zinc deficiency and zinc-copper interrelationship in Eimeria acervulina-infected chicks. J. Nutr. 1983;113:688–696. doi: 10.1093/jn/113.3.688. [DOI] [PubMed] [Google Scholar]
- 14.Turk DE. Calcium absorption during coccidial infections in chicks. Poult. Sci. 1973;52:854–857. doi: 10.3382/ps.0520854. [DOI] [PubMed] [Google Scholar]
- 15.Gautier AE, Latorre JD, Matsler PL, Rochell SJ. Longitudinal characterization of coccidiosis control methods on live performance and nutrient utilization in broilers. Front. Vet. Sci. 2019;6:468. doi: 10.3389/fvets.2019.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turk DE, Stephens JF. Coccidial infections of the ileum, colon, and ceca of the chick and nutrient absorption. Poult. Sci. 1969;48:586–589. doi: 10.3382/ps.0480586. [DOI] [PubMed] [Google Scholar]
- 17.Joyner LP, et al. Amino-acid malabsorption and intestinal leakage of plasma-proteins in young chicks infected with Eimeria acervulina. Avian Pathol. 1975;4:17–33. doi: 10.1080/03079457509353847. [DOI] [PubMed] [Google Scholar]
- 18.Allen, M. R. & Burr, D. B. In Basic and Applied Bone Biology 75–90 (2014).
- 19.Frost HM. Wolff's Law and bone's structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994;64:175–188. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Tompkins YH, Teng P, Pazdro R, Kim WK. Long bone mineral loss, bone microstructural changes and oxidative stress after Eimeria challenge in broilers. Front. Physiol. 2022;13:945740. doi: 10.3389/fphys.2022.945740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hambli R. Connecting mechanics and bone cell activities in the bone remodeling process: An integrated finite element modeling. Front. Bioeng. Biotechnol. 2014;2:6. doi: 10.3389/fbioe.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prisby R, et al. Kinetic examination of femoral bone modeling in broilers. Poult. Sci. 2014;93:1122–1129. doi: 10.3382/ps.2013-03778. [DOI] [PubMed] [Google Scholar]
- 23.Holtrop ME, King GJ. The ultrastructure of the osteoclast and its functional implications. Clin. Orthopaed. Relat. Res. 1977 doi: 10.1097/00003086-197703000-00062. [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum SL. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukushima O, Bekker PJ, Gay CV. Ultrastructural localization of tartrate-resistant acid phosphatase (purple acid phosphatase) activity in chicken cartilage and bone. Am. J. Anat. 1991;191:228–236. doi: 10.1002/aja.1001910303. [DOI] [PubMed] [Google Scholar]
- 26.Hayman AR. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 2008;41:218–223. doi: 10.1080/08916930701694667. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, et al. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology. 2010;129:133–145. doi: 10.1111/j.1365-2567.2009.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon LB, et al. Time dependent loss of trabecular bone in human tibial plateau fractures. J. Orthop. Res. 2018;36:2865–2875. doi: 10.1002/jor.24057. [DOI] [PubMed] [Google Scholar]
- 29.Kamibayashi L, Wyss UP, Cooke TDV, Zee B. Trabecular microstructure in the medial condyle of the proximal tibia of patients with knee osteoarthritis. Bone. 1995;17:27–35. doi: 10.1016/8756-3282(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 30.Lee MSJ, et al. Plasmodium products persist in the bone marrow and promote chronic bone loss. Sci. Immunol. 2017 doi: 10.1126/sciimmunol.aam8093. [DOI] [PubMed] [Google Scholar]
- 31.Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: Interactions of the bone and immune system. Endocr. Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakashima T, Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2011;1240:E13–18. doi: 10.1111/j.1749-6632.2011.06373.x. [DOI] [PubMed] [Google Scholar]
- 34.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 35.Jimi E, et al. Activation of NF-kappaB is involved in the survival of osteoclasts promoted by interleukin-1. J. Biol. Chem. 1998;273:8799–8805. doi: 10.1074/jbc.273.15.8799. [DOI] [PubMed] [Google Scholar]
- 36.Yao Z, Xing L, Qin C, Schwarz EM, Boyce BF. Osteoclast precursor interaction with bone matrix induces osteoclast formation directly by an interleukin-1-mediated autocrine mechanism. J. Biol. Chem. 2008;283:9917–9924. doi: 10.1074/jbc.M706415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beedles KE, Sharpe PT, Wagner EF, Grigoriadis AE. A putative role for c-Fos in the pathophysiology of Paget's disease. J. Bone Miner. Res. 1999;14(Suppl 2):21–28. doi: 10.1002/jbmr.5650140206. [DOI] [PubMed] [Google Scholar]
- 38.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 39.Scott EW, et al. PU.1 functions in a cell-autonomous manner to control the differentiation of multipotential lymphoid-myeloid progenitors. Immunity. 1997;6:437–447. doi: 10.1016/s1074-7613(00)80287-3. [DOI] [PubMed] [Google Scholar]
- 40.Huang H, et al. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem. Biophys. Res. Commun. 2006;351:99–105. doi: 10.1016/j.bbrc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. Clin. Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taichman RS. Blood and bone: Two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 43.Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells. 2017;40:706–713. doi: 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rifas L. Bone and cytokines: beyond IL-1, IL-6 and TNF-alpha. Calcif. Tissue Int. 1999;64:1–7. doi: 10.1007/s002239900570. [DOI] [PubMed] [Google Scholar]
- 45.Kwan-Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Schett G. Review: Immune cells and mediators of inflammatory arthritis. Autoimmunity. 2008;41:224–229. doi: 10.1080/08916930701694717. [DOI] [PubMed] [Google Scholar]
- 47.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005;115:282–290. doi: 10.1172/jci200523394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lillehoj HS. Role of T lymphocytes and cytokines in coccidiosis. Int. J. Parasitol. 1998;28:1071–1081. doi: 10.1016/s0020-7519(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 49.Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 2006;114:209–223. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Byrnes S, Eaton R, Kogut M. In vitro interleukin-1 and tumor necrosis factor-alpha production by macrophages from chickens infected with either Eimeria maxima or Eimeria tenella. Int. J. Parasitol. 1993;23:639–645. doi: 10.1016/0020-7519(93)90170-4. [DOI] [PubMed] [Google Scholar]
- 51.Neville-Webbe HL, et al. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res. Treat. 2004;86:269–279. doi: 10.1023/b:brea.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- 52.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou ML, et al. The smad dependent TGF-beta and BMP signaling pathway in bone remodeling and therapies. Front. Mol. Biosci. 2021;8:593310. doi: 10.3389/fmolb.2021.593310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tasca A, et al. SMAD1/5 signaling in osteoclasts regulates bone formation via coupling factors. PLoS ONE. 2018;13:e0203404. doi: 10.1371/journal.pone.0203404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tasca A, et al. Smad1/5 and Smad4 expression are important for osteoclast differentiation. J. Cell Biochem. 2015;116:1350–1360. doi: 10.1002/jcb.25092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kakhki RAM, et al. Eimeria challenge adversely affected long bone attributes linked to increased resorption in 14-day-old broiler chickens. Poult. Sci. 2019;98:1615–1621. doi: 10.3382/ps/pey527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guntur AR, Rosen CJ. Bone as an endocrine organ. Endocr. Pract. 2012;18:758–762. doi: 10.4158/EP12141.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suchacki KJ, et al. Skeletal energy homeostasis: A paradigm of endocrine discovery. J. Endocrinol. 2017;234:R67–R79. doi: 10.1530/JOE-17-0147. [DOI] [PubMed] [Google Scholar]
- 59.Bradshaw RH, Kirkden RD, Broom DM. A review of the aetiology and pathology of leg weakness in broilers in relation to welfare. Avian Poult. Biol. Rev. 2002;13:45–103. doi: 10.3184/147020602783698421. [DOI] [Google Scholar]
- 60.Hartcher KM, Lum HK. Genetic selection of broilers and welfare consequences: A review. Worlds Poult. Sci. J. 2019;76:154–167. doi: 10.1080/00439339.2019.1680025. [DOI] [Google Scholar]
- 61.Waldenstedt L. Nutritional factors of importance for optimal leg health in broilers: A review. Anim. Feed Sci. Technol. 2006;126:291–307. doi: 10.1016/j.anifeedsci.2005.08.008. [DOI] [Google Scholar]
- 62.Kong S, Zhang YH, Zhang W. Regulation of intestinal epithelial cells properties and functions by amino acids. Biomed. Res. Int. 2018;2018:2819154. doi: 10.1155/2018/2819154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yadav S, et al. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020;99:5936–5945. doi: 10.1016/j.psj.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papageorgiou M, Dolan E, Elliott-Sale KJ, Sale C. Reduced energy availability: Implications for bone health in physically active populations. Eur. J. Nutr. 2018;57:847–859. doi: 10.1007/s00394-017-1498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nattiv A, et al. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007;39:1867–1882. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 66.MacDonell R, Hamrick MW, Isales CM. Protein/amino-acid modulation of bone cell function. Bonekey Rep. 2016;5:827. doi: 10.1038/bonekey.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou R, et al. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021;9:25. doi: 10.1038/s41413-021-00142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nordin BE, Morris HA. The calcium deficiency model for osteoporosis. Nutr. Rev. 1989;47:65–72. doi: 10.1111/j.1753-4887.1989.tb02794.x. [DOI] [PubMed] [Google Scholar]
- 69.Imari ZK, Hassanabadi A, Nassiri-Moghaddam H. Response of broiler chickens to calcium and phosphorus restriction: Effects on growth performance, carcase traits, tibia characteristics and total tract retention of nutrients. Ital. J. Anim. Sci. 2020;19:929–939. doi: 10.1080/1828051x.2020.1808101. [DOI] [Google Scholar]
- 70.Akesson K, Lau KH, Johnston P, Imperio E, Baylink DJ. Effects of short-term calcium depletion and repletion on biochemical markers of bone turnover in young adult women. J. Clin. Endocrinol. Metab. 1998;83:1921–1927. doi: 10.1210/jcem.83.6.4891. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, He T, Li M, Hu J, Piao X. Effects of dietary calcium and phosphorus levels and supplementation of 25-hydroxycholecalciferol on performance and bone properties of broiler starters. Arch. Anim. Nutr. 2019;73:445–456. doi: 10.1080/1745039X.2019.1667192. [DOI] [PubMed] [Google Scholar]
- 72.Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal women: A randomized controlled trial. Am. J. Med. 1995;98:331–335. doi: 10.1016/s0002-9343(99)80310-6. [DOI] [PubMed] [Google Scholar]
- 73.Bourrin S, Toromanoff A, Ammann P, Bonjour JP, Rizzoli R. Dietary protein deficiency induces osteoporosis in aged male rats. J. Bone Miner. Res. 2000;15:1555–1563. doi: 10.1359/jbmr.2000.15.8.1555. [DOI] [PubMed] [Google Scholar]
- 74.de Paula FJ, Rosen CJ. Bone remodeling and energy metabolism: New perspectives. Bone Res. 2013;1:72–84. doi: 10.4248/BR201301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruggeman V, Vanmontfort D, Renaville R, Portetelle D, Decuypere E. The effect of food intake from two weeks of age to sexual maturity on plasma growth hormone, insulin-like growth factor-I, insulin-like growth factor-binding proteins, and thyroid hormones in female broiler breeder chickens. Gen. Comp. Endocrinol. 1997;107:212–220. doi: 10.1006/gcen.1997.6917. [DOI] [PubMed] [Google Scholar]
- 76.Buyse J, et al. Food deprivation and feeding of broiler chickens is associated with rapid and interdependent changes in the somatotrophic and thyrotrophic axes. Br. Poult. Sci. 2000;41:107–116. doi: 10.1080/00071660086493. [DOI] [PubMed] [Google Scholar]
- 77.Iyasere OS, Beard AP, Guy JH, Bateson M. Elevated levels of the stress hormone, corticosterone, cause 'pessimistic' judgment bias in broiler chickens. Sci. Rep. 2017;7:6860. doi: 10.1038/s41598-017-07040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Jong IC, van Voorst S, Ehlhardt DA, Blokhuis HJ. Effects of restricted feeding on physiological stress parameters in growing broiler breeders. Br. Poult. Sci. 2002;43:157–168. doi: 10.1080/00071660120121355. [DOI] [PubMed] [Google Scholar]
- 79.Bowling M, Forder R, Hughes RJ, Weaver S, Hynd PI. Effect of restricted feed intake in broiler breeder hens on their stress levels and the growth and immunology of their offspring. Transl. Anim. Sci. 2018;2:263–271. doi: 10.1093/tas/txy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shini S, Kaiser P. Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress. 2009;12:388–399. doi: 10.1080/10253890802526894. [DOI] [PubMed] [Google Scholar]
- 81.Almeida M, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 2017;97:135–187. doi: 10.1152/physrev.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitchell HH, Beadles JR. The paired-feeding method in nutrition experiments and its application to the problem of cystine deficiencies in food proteins. J. Nutr. 1930;2:225–243. doi: 10.1093/jn/2.3.225. [DOI] [Google Scholar]
- 83.Walker BR. Dietary regulation of cortisol production and metabolism in humans. Endocr. Abstr. 2015 doi: 10.1530/endoabs.37.S1.1. [DOI] [Google Scholar]
- 84.Sanchez-Rodriguez E, et al. Changes with age (from 0 to 37 D) in tibiae bone mineralization, chemical composition and structural organization in broiler chickens. Poult. Sci. 2019;98:5215–5225. doi: 10.3382/ps/pez363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gong JK, Arnold JS, Cohn SH. Composition of Trabecular and cortical bone. Anat. Rec. 1964;149:325–331. doi: 10.1002/ar.1091490303. [DOI] [PubMed] [Google Scholar]
- 86.Passi N, Gefen A. Trabecular bone contributes to strength of the proximal femur under mediolateral impact in the avian. J. Biomech. Eng. 2005;127:198–203. doi: 10.1115/1.1835366. [DOI] [PubMed] [Google Scholar]
- 87.Whitehead CC, Fleming RH. Osteoporosis in Cage Layers. Poult. Sci. 2000;79:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
- 88.Kim C, Park D. The effect of restriction of dietary calcium on trabecular and cortical bone mineral density in the rats. J. Exerc. Nutr. Biochem. 2013;17:123–131. doi: 10.5717/jenb.2013.17.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 90.Aguado E, Pascaretti-Grizon F, Goyenvalle E, Audran M, Chappard D. Bone mass and bone quality are altered by hypoactivity in the chicken. PLoS ONE. 2015;10:e0116763. doi: 10.1371/journal.pone.0116763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kierończyk B, Rawski M, Józefiak D, Świątkiewicz S. Infectious and non-infectious factors associated with leg disorders in poultry—a review. Ann. Anim. Sci. 2017;17:645–669. doi: 10.1515/aoas-2016-0098. [DOI] [Google Scholar]
- 92.Kent GN, et al. Human lactation: Forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J. Bone Miner. Res. 1990;5:361–369. doi: 10.1002/jbmr.5650050409. [DOI] [PubMed] [Google Scholar]
- 93.Georgieva NV, Koinarski V, Gadjeva V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 2006;172:488–492. doi: 10.1016/j.tvjl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 94.Mody N. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radical Biol. Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 95.Bai XC, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 96.Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017;14:209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim WH, Chaudhari AA, Lillehoj HS. Involvement of T cell immunity in avian coccidiosis. Front. Immunol. 2019;10:2732. doi: 10.3389/fimmu.2019.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ifrah ME, Perelman B, Finger A, Uni Z. The role of the bursa of Fabricius in the immune response to vaccinal antigens and the development of immune tolerance in chicks (Gallus domesticus) vaccinated at a very young age. Poult. Sci. 2017;96:51–57. doi: 10.3382/ps/pew232. [DOI] [PubMed] [Google Scholar]
- 99.Masteller EL, Thompson CB. B cell development in the chicken. Poult. Sci. 1994;73:998–1011. doi: 10.3382/ps.0730998. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Titanji K, et al. Dysregulated B cell expression of RANKL and OPG correlates with loss of bone mineral density in HIV infection. PLoS Pathog. 2014;10:e1004497. doi: 10.1371/journal.ppat.1004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, Terauchi M, Vikulina T, Roser-Page S, Weitzmann MN. B Cell Production of Both OPG and RANKL is Significantly Increased in Aged Mice. Open Bone J. 2014;6:8–17. doi: 10.2174/1876525401406010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Titanji K. Beyond antibodies: B cells and the OPG/RANK-RANKL pathway in health, non-HIV disease and HIV-induced bone loss. Front. Immunol. 2017;8:1851. doi: 10.3389/fimmu.2017.01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Onal M, et al. Receptor activator of nuclear factor kappaB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J. Biol. Chem. 2012;287:29851–29860. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Furuya Y, et al. Increased bone mass in mice after single injection of anti-receptor activator of nuclear factor-kappaB ligand-neutralizing antibody: evidence for bone anabolic effect of parathyroid hormone in mice with few osteoclasts. J. Biol. Chem. 2011;286:37023–37031. doi: 10.1074/jbc.M111.246280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brandstrom H, et al. Tumor necrosis factor-alpha and -beta upregulate the levels of osteoprotegerin mRNA in human osteosarcoma MG-63 cells. Biochem Biophys Res Commun. 1998;248:454–457. doi: 10.1006/bbrc.1998.8993. [DOI] [PubMed] [Google Scholar]
- 107.Zaiss MM, et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J. Immunol. 2010;184:7238–7246. doi: 10.4049/jimmunol.0903841. [DOI] [PubMed] [Google Scholar]
- 108.Yao Z, et al. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J. Biol. Chem. 2006;281:11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 109.Li P, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50:265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 110.Xing L, et al. NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis. J. Bone Miner. Res. 2002;17:1200–1210. doi: 10.1359/jbmr.2002.17.7.1200. [DOI] [PubMed] [Google Scholar]
- 111.Miyazaki T, et al. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J. Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rucci N, Rufo A, Alamanou M, Teti A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J. Cell Biochem. 2007;100:464–473. doi: 10.1002/jcb.21059. [DOI] [PubMed] [Google Scholar]
- 113.Pivonka P, et al. Theoretical investigation of the role of the RANK-RANKL-OPG system in bone remodeling. J. Theor. Biol. 2010;262:306–316. doi: 10.1016/j.jtbi.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 114.Logar DB, et al. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J. Bone Miner. Metab. 2007;25:219–225. doi: 10.1007/s00774-007-0753-0. [DOI] [PubMed] [Google Scholar]
- 115.Cobb. Cobb 500 Broiler Performance and Nutrient Supplement Guide. Siloam Springs (AR): Cobb-Vantress (2019).
- 116.Teng PY, Yadav S, Dos Santos TS, Fuller AL, Kim WK. 2-Nitro-1-propanol improved nutrient digestibility and oocyst shedding but not growth performance of Eimeria-challenged broilers. Poult. Sci. 2020;99:4314–4322. doi: 10.1016/j.psj.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tompkins YH, et al. The effects of maternal fish oil supplementation rich in n-3 PUFA on offspring-broiler growth performance, body composition and bone microstructure. PLoS ONE. 2022;17:e0273025. doi: 10.1371/journal.pone.0273025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stephens AS, Stephens SR, Morrison NA. Internal control genes for quantitative RT-PCR expression analysis in mouse osteoblasts, osteoclasts and macrophages. BMC Res. Notes. 2011;4:410. doi: 10.1186/1756-0500-4-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen C, White DL, Marshall B, Kim WK. Role of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in chicken embryo osteogenesis, adipogenesis, myogenesis, and vitamin D3 metabolism. Front. Physiol. 2021;12:637629. doi: 10.3389/fphys.2021.637629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paludo E, et al. The involvement of RUNX2 and SPARC genes in the bacterial chondronecrosis with osteomyelitis in broilers. Animal. 2017;11:1063–1070. doi: 10.1017/S1751731116002433. [DOI] [PubMed] [Google Scholar]
- 122.Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J. Thorac. Dis. 2016;8:E928–E931. doi: 10.21037/jtd.2016.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.