Abstract
Depression, or major depressive disorder, is a common mental disorder that affects individuals’ behavior, mood, and physical health, and its prevalence has increased during the lockdowns implemented to curb the COVID-19 pandemic. There is an urgent need to update the treatment recommendations for mental disorders during such crises. Conventional interventions to treat depression include long-term pharmacotherapy and cognitive behavioral therapy. Electroencephalogram-neurofeedback (EEG-NF) training has been suggested as a non-invasive option to treat depression with minimal side effects. In this systematic review, we summarize the recent literature on EEG-NF training for treating depression. The 12 studies included in our final sample reported that despite several issues related to EEG-NF practices, patients with depression showed significant cognitive, clinical, and neural improvements following EEG-NF training. Given its low cost and the low risk of side effects due to its non-invasive nature, we suggest that EEG-NF is worth exploring as an augmented tool for patients who already receive standard medications but remain symptomatic, and that EEG-NF training may be an effective intervention tool that can be utilized as a supplementary treatment for depression. We conclude by providing some suggestions related to experimental designs and standards to improve current EEG-NF training practices for treating depression.
Keywords: Depression, EEG, Neurofeedback, Intervention tool, Therapy
1. Introduction
The current catastrophic global situation caused by the coronavirus disease 2019 (COVID-19) pandemic has led to great disruptions in people's daily lives. The pandemic has affected many people's socioeconomic and mental statuses, leading to various psychological issues such as post-traumatic stress disorder, suicidal tendencies, anxiety, and depression. Depression, or major depressive disorder (MDD), is a common yet serious psychological illness that negatively affects the way a person feels, thinks, and acts. Depression affects over 120 million people worldwide, and epidemiological studies have estimated the prevalence of depression to be around 10% to 15% in the general population. In 2001, the World Health Organization (WHO) predicted that by 2020, depression would rank second in the global disease burden and would be one of the priority mental diseases covered by the WHO's Mental Health Gap Action Programme (Sayers, 2001). Recent meta-analytic studies have demonstrated the prevalence of depression worldwide. Its prevalence in Europe and the US has been reported to range between 11.9% and 17%, with a similarly high rate in Asia (16.7%) and the lowest rates in Australia and Africa (7.3% to 11.5%), from 1994 to 2004 (Lim et al., 2018). Another meta-analysis showed the prevalence of depression to be higher in high-income countries (5.5% to 14.6%) than in low-income countries (5.9% to 11.1%) (Bromet et al., 2011).
Depression symptoms include sadness, loss of interest, guilt, sleep problems, tiredness, poor concentration, and a loss of interest in day-to-day activities (Lin et al., 2021; Lu et al., 2014). More severe cases of depression can lead to an increased risk of death (Cuijpers and Smit, 2002) and suicide (Choo et al., 2014; Large, 2016; Lin et al., 2021). Depression is a chronic disorder that affects an individual's quality of life (Daly et al., 2010; Mayor, 2015), social and emotional functioning (Huang et al., 2019; Huang and Huang, 2019; Wong et al., 2016), and ability to work (Hirschfeld et al., 2000; Lerner and Henke, 2008; Stewart et al., 2003). Depression also affects people's social lives. People with depression show a bias during social processing and interactions, with a tendency to pay more attention to negative aspects of social information. For example, in laboratory studies, people with depression tend to show preferential attention to sad words, sad faces, and emotional words ((Gotlib et al., 2004a, Gotlib et al., 2004b); Mogg and Bradley, 2005). People with depression are more likely to perceive the negative attributes of social interactions or to attribute negative outcomes to themselves, and less likely to feel a sense of belonging (Joiner et al., 1999). Furthermore, depressed people are likely to exert negative effects on others and to experience a sense of rejection and the loss of a sense of reward (Joiner Jr. and Katz, 1999).
Depression can be reliably diagnosed and treated with the help of physicians (Wahlbeck, 2015). Current clinical recommendations for the treatment of depression are pharmacotherapy, psychotherapy, and a combination of the two (American Psychiatric Association, 2009; National Collaborating Centre for Mental Health (UK),2010). Pharmacology-based therapies for depression include medications such as anti-depressants and selective serotonin reuptake inhibitors. Numerous psychological therapies are considered effective treatment options, including cognitive behavioral therapy and interpersonal therapy (Churchill et al., 2001; National Collaborating Centre for Mental Health (UK) 2010). The use of neurofeedback (NF)-based interventions have been reported recently for patients with depression and/or treatment-resistant depression (Lee et al., 2019) even after they have received conventional pharmacotherapeutic methods.
In this article, we provide a brief overview of current state-of-the-art neurofeedback systems, focusing on electroencephalogram-based neurofeedback (EEG-NF), and elaborate on the various protocols used in NF studies involving patients with depressive symptoms. We then discuss how EEG-NF can be used as a psychotherapeutic intervention to treat depression. Using an example of a generalized EEG-NF system, we first describe the fundamentals of EEG and EEG-NF systems and how they have been used to date. We then explore published studies conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021) that explore EEG-NF protocols for the treatment of depression, such as alpha symmetry and high-beta down-training. To understand the role of the EEG-NF protocols in use, we examine studies that used EEG-NF to improve cognition and other conditions in depressed people. We conclude with a discussion of methodological considerations and protocols for EEG-NF and how EEG-NF can be used as an interventional tool to treat depression.
NF-based training is used to control psycho-physiological processes in the brain through the use of external feedback. NF was developed to assist brain–machine interfaces to help individuals overcome injuries, disabilities, and/or mental illnesses (Kamiya, 1971; Lubar et al., 1995; Lubar and Bahler, 1976; Mann et al., 1992; Nowlis and Kamiya, 1970; Ordikhani-Seyedlar et al., 2016; Rozelle and Budzynski, 1995; Seifert and Lubar, 1975). NF can help an individual control the function of a brain region by training a specific set of brain waves using EEGs. EEG signals are categorized into five basic frequency components that represent specific functions of the brain: (1) delta, the person is asleep (0.5–4 Hz); (2) theta, the person is in a drowsy, meditative state (4–8 Hz); (3) alpha, the person is attentive (8–12 Hz); (4) beta, the person is alert (14–30 Hz); and (5) gamma, the person is engaged in higher cognitive function (30–100 Hz). NF protocols are based on these frequency components and defined as delta, theta, alpha, beta, and gamma protocols or as ratios of the frequency components, such as the alpha/theta and beta/theta ratios (Dempster, 2012). The most important component of an NF technique is the feedback provided by an audio, video, or audio-video stimulus, which regulates the participant's brain waves.
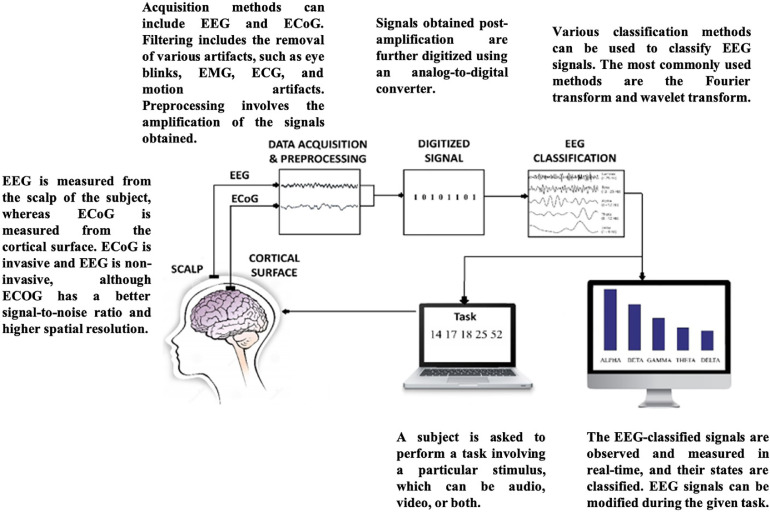
The generalized EEG-NF system depicted in Fig. 1 is the simplest and most widely used system. EEG is primarily used in NF because it is non-invasive, cost-effective, and simple to use (Gruzelier, 2014). Recent studies have shown improvements in the use of EEG-NF for various clinical and non-clinical applications (Jeunet et al., 2019; Marzbani et al., 2016; Omejc et al., 2019). EEG-NF has been applied in the treatment of diseases such as epilepsy (Morales-Quezada et al., 2019; Nigro, 2019; Sterman et al., 1974; Tan et al., 2009; Walker and Kozlowski, 2005), ADHD (Chabot et al., 2001), autism (Karimi et al., 2011), schizophrenia (Gandara et al., 2020; McCarthy-Jones, 2012; Nan et al., 2012; Pazooki et al., 2019; Singh et al., 2020; Surmeli et al., 2012), insomnia (Hammer et al., 2011), anxiety (Blaskovits et al., 2017; Gadea et al., 2020; Patel et al., 2020; Tolin et al., 2020; Wang et al., 2019), and drug and alcohol addiction (Dalkner et al., 2017; Ko and Park, 2018; Lackner et al., 2016), and of learning disorders such as dyslexia, dyscalculia, and dysgraphia (Bearden et al., 2003; Breteler et al., 2010; Patil et al., 2022; Nazari et al., 2012; Wang and Sourina, 2013). Studies have also shown post-NF improvements in executive function and depression symptoms (Choi, 2011; Dias and van Deusen, 2011). DeRubeis et al. (2008) concluded that the NF system showed promising treatment effects in patients with depression in recent clinical trials.
Fig. 1.
Summary of components of a generalized neurofeedback system.
The COVID-19 pandemic has highlighted the urgent need to develop more effective mental health treatments. Mitigation strategies are needed to promote mental health awareness and treatments for mental health disorders. Additional non-pharmaceutical treatments for depression and anxiety disorders are required to avoid the side effects of pharmacotherapeutic interventions. EEG-NF is one such promising technique for treating depression. In this systematic review, we first review the literature on psychotherapeutic interventions with a focus on EEG-NF for people with depression. Then, we elaborate on the protocols used to assist or treat depression in NF studies. Lastly, we discuss how current state-of-the-art EEG-NF protocols could facilitate future research and improve global health amid the COVID-19 pandemic and future public health emergencies.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards were followed for this systematic review (Page et al., 2021).
2.1. Search strategy
EEG-NF studies were selected using a systematic search process. A thorough search for peer-reviewed articles published in English between 2010 and August 2020 that included the treatment of depression symptoms was conducted using the PubMed database, using the following keyword combinations: (“EEG” or “Electroencephalography” or “Neurofeedback”) and (“Depression” or “Major Depressive Disorder” or Treatment-Resistant Depression”).
2.2. Inclusion criteria
Independent searches and screening were conducted to ensure that the inclusion criteria were met. Studies were only included if they utilized neurofeedback (NF) based on electroencephalogram (EEG) studies as an intervention to treat participants with depression symptoms. We did not review or include studies using other modalities, such as functional magnetic resonance imaging (fMRI) or magneto-electrography (MEG. Furthermore, the studies were screened to ensure that they met the following criteria: (1) were clinical or randomized empirical trials; (2) involved depression, MDD, or treatment-resistant depression; (3) included participants older than 18 years; (4) involved EEG-NF; and (5) were published between 2010 and 2020 in English. After conducting literature searches, we eliminated duplicate records and excluded theoretical papers and reviews.
3. Results
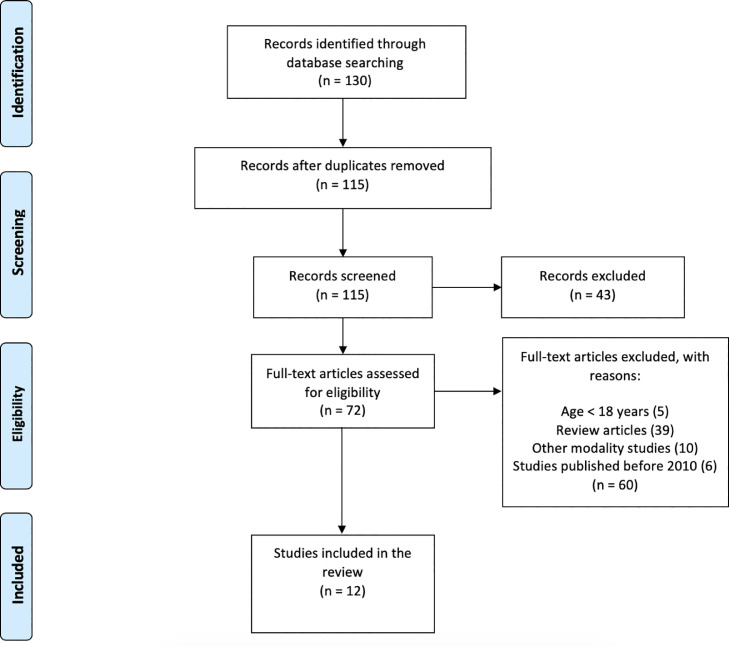
Among the 130 original reports discovered through the database searches, we evaluated 72 full texts for eligibility. Studies were excluded if the subjects did not fit our predetermined inclusion criteria or the researchers did not provide adequate EEG-specific findings. Finally, 12 published studies were included in the current systematic review (Fig. 2 ).
Fig. 2.
The preferred reporting items for systematic review and meta-analysis (PRISMA) flow diagram for selected studies involving EEG-NF to treat depression.
The 12 studies were divided into two groups based on the two NF protocols most commonly used to treat depression symptoms: (1) alpha-asymmetry (ALAY) and (2) high-beta down-training NF protocols. Other protocols used in the research to treat depression symptoms are discussed in following sections.
3.1. Alpha-asymmetry (ALAY) NF protocol for treating depression
The theory of frontal alpha asymmetry was proposed by Richard Davidson (1992) as a useful neurophysiological method to treat the symptoms of depression (Davidson, 1992). The frontal ALAY method involves the identification of differences in alpha activity between the left and right frontal hemispheres during an EEG session. As indicated by Stewart et al. (2011), the left frontal hemisphere involves the behavioral activation system, which is associated with positive emotion and motivation, and the right hemisphere involves the behavioral inhibition activation system, which is associated with negative emotions, anxiety, and depression (Mathersul et al., 2008; Stewart et al., 2011; Thibodeau et al., 2006). Alpha asymmetry is indicated by a score and is abbreviated as A1. It is a potential marker of frontal ALAY (Baehr et al., 1997) and is usually calculated by the formula,
| (1) |
where log F4 and log F3 represent the powers of the left and right frontal alpha components, respectively. The A1 score is an important indicator in resting and task-based studies involving participants with depression (Kaiser et al., 2018; Smith et al., 2017; Stewart et al., 2011). Baehr et al. (1997) defined an ALAY-NF protocol that was used to elevate the A1 score and reduce depression symptoms. A number of ALAY-based NF studies have shown reductions in symptoms related to depression over both short-term (Baehr et al., 1997; Choi, 2011; Dias and van Deusen, 2011; Mennella et al., 2017; Rosenfeld, 2000; Wang et al., 2019) and long-term (Baehr et al., 2001) follow-ups.
A pilot study by Choi et al. (2011) showed improvements in a variety of neurocognitive functions and reductions in depression-related symptoms. The improvements in the depressive symptoms of participants using the ALAY-NF protocol were assessed in clinical interviews. The 23 participants were randomly assigned to either an NF training group (n = 23; age = 28.46 ± 9.69 years; 10 females) or a placebo group (N = 11; age = 28.54 ± 6.84 years; 7 females). The NF group received the ALAY-NF protocol for about 30 min per session over a 5-week period. The placebo group participants received placebo psychotherapy training consisting of psychological assessments, interpretation of and information about test results, and treatment of mood disorders. The NF group showed significantly improved scores on the Beck Depression Inventory (BDI-II), Hamilton Rating Scale for Depression (HAM-D), and Automatic Thoughts Questionnaire-Negative (ATQ-N) compared with the placebo group. The A1 score also improved significantly in the NF group compared with the placebo group in both the eyes-open and eyes-closed conditions. The study showed that EEG-NF based on ALAY theory was associated with a reduction in depressive symptoms.
Mennella et al. (2017) reported results similar to those of Choi et al. (2011) in a sample of 32 healthy women. The participants were randomly allocated to either an ALAY-NF (N = 16; age = 22.9 ± 1.2 years) or an active control (N = 16; age = 23.3 ± 1.2 years) group. Both groups received seven mid-frontal alpha activity NF sessions with a duration of 45 min over 2 weeks. The Beck Anxiety Inventory (BAI) and Positive and Negative Affect Scales (PANAS) were applied to both groups before and after the EEG-NF training. The participants in the ALAY-NF group scored significantly lower on the behavioral scales of the BAI and the PANAS Negative effect scores compared with the active control group from before to after NF training. The study also examined the alpha power in both hemispheres and found increased left alpha power in the ALAY-NF group compared with the controls, which increased the A1 score in the ALAY-NF group. The study further provided a rationale for using the EEG-NF-based frontal alpha asymmetry protocol as a clinical application to treat or improve symptoms related to depression. Table 1
Table 1.
Summary of studies based on EEG-NF using the ALAY protocol for treating depression.
| Authors (year) | Protocol | Sample Characteristics (% female) | Groups (if any) | Electrodes Used for NF | Session Details | Depression Assessments | Behavioral Results | EEG-wave Results |
|---|---|---|---|---|---|---|---|---|
| Choi (2011) | ↑ Frontal alpha asymmetry |
N = 23 Depressive mood (74%) |
NF group (N = 12; age = 28.46 ± 9.69 years) Placebo group (N = 11; age = 28.54 ± 6.84 years) |
Alpha training: F3, F4 referenced at Cz | 10 sessions over 5 weeks; each session = 30 mins |
|
BDI-II, ATQ-N, and HAM-D; a trend toward a significant reduction in scores was observed | Alpha power difference between pre- and post-tests found in the NF group |
| Peeters et al. (2014) | ↓ Alpha asymmetry (AA) |
N = 9 MDD age = 46.55 ± 11.71 years (44%) |
N/A | F3, F4 | Maximum 30 sessions over 10 weeks; each session = 33 mins |
|
During NF, QIDS-SR decreased significantly | Mean baseline AA decreased significantly over the sessions |
| Mennella et al. (2017) | Frontal alpha symmetry ; ↑ Mid-frontal alpha |
N = 32 Healthy (100%) |
Asymmetry group (N = 16; age = 22.9 ± 1.2 years) Control group (N = 16; age = 23.3 ± 1.2 years) |
Alpha asymmetry training: F3-F4; Mid-frontal training: Fz |
7 sessions over 2 weeks; each session = 45 mins |
|
Significant decreases in PANAS negative affect and BAI scores | Significant increase in alpha power at F4 (right) in alpha asymmetry group; no significant increase in control group |
| Cheon et al. (2016) | ↑ Beta; ↑ theta/ ↓ alpha |
N = 20 MDD age = 43.25 ± 14.29 years (80%) |
N/A | Beta training: F3; Alpha/theta training: Pz |
16–24 sessions over 8 weeks; each session = 30 mins |
|
Mean HAM-D, HAM-A and BDI-II scores improved significantly | No significant differences in A1 scores post-NF sessions |
Note: MDD: major depressive disorder; NF: neurofeedback training; F3: left frontal electrode; Fz: middle frontal electrode; F4: right frontal electrode; Cz: middle central electrode; Pz: middle parietal electrode; A1: alpha asymmetry score; HAM-D: Hamilton Depression Rating Scale; HAM-A: Hamilton Anxiety Rating Scale; BDI-II: Beck Depression Inventory; BAI: Beck Anxiety Inventory; ATQ-P: Positive Automatic Thoughts Questionnaire; ATQ-N: Negative Automatic Thoughts Questionnaire; DSS: Depression Stigma Scale; QIDS-SR: Quick Inventory of Depressive Symptomatology–Self-report; PANAS: Positive and Negative Affect Schedule; AA: alpha asymmetry.
A number of contrasting studies based on ALAY-NF have shown a reduction in the asymmetry score, with an overall reduction in depressive symptoms after the EEG-NF sessions (Cheon et al., 2016; Peeters et al., 2014). Peeters et al. (2014) administered 30 sessions of EEG-NF over 10 weeks to reduce the alpha asymmetry in participants with depression symptoms (N = 9; age = 46.55 ± 11.71 years; 4 females). They used the Quick Inventory of Depressive Symptomatology (Self-Report) to assess depression symptoms and found a significant and feasible improvement in the post-EEG-NF session scores. The study further reported a reduction in alpha symmetry after the EEG-NF sessions compared with the baseline, although there was no significant improvement in the A1 score. The study empirically validated the ability of EEG-NF to reduce relative frontal asymmetry. Cheon et al. (2016) used an 8-week ALAY-NF protocol to increase the beta power in the left frontal electrode region (F3) and reduce the alpha power in the central electrode location (Pz) in participants with depression (n = 20; age = 43.25 ± 14.29 years; 16 females). The HAM-D, BDI-II, and Hamilton Anxiety Rating Scale (HAM-A) were used to assess depression-related symptoms. The scores on the HAM-D, HAM-A, and BDI-II were significantly reduced after the ALAY-NF sessions. However, there was no significant improvement in the A1 score. This was an ideal study for understanding the clinical application of EEG-NF in alleviating the symptoms of depression and anxiety in a small sample.
3.2. High-beta down-training NF protocol for treating depression
The EEG beta band (14 Hz–30 Hz) can be divided into two types of beta activity: (1) low/slow beta (12 Hz–15 Hz) activity, indicating a state of concentration and alertness, and (2) high/fast beta (22 Hz–38 Hz) activity, indicating a state of anxiety and stress (Collura, 2014; Hoffmann, 2005). The high-beta reduction protocol, also called the high-beta down-training EEG-NF protocol, was developed to reduce arousal and manage anxiety, stress, and rumination (Collura, 2014). Various applications of this protocol have been used to improve stress management in athletes (Dupee and Werthner, 2011), people with post-traumatic stress disorder (Walker, 2009), and children with autism (Thompson et al., 2010).
Wang et al. (2019) compared the effects of ALAY-NF and high-beta down-training NF in 70 participants with depression. The participants were divided into three groups: an ALAY-NF group (N = 24; age = 33 ± 14.741 years; 19 females), a high-beta down-training NF group (N = 23; age = 42.83 ± 15.816 years; 12 females), and a control group (N = 23; age = 42.61 ± 13.937 years; 16 females). The ALAY-NF and high-beta NF groups received 10 sessions of EEG-NF training over 5 weeks, while the control group received no training. The ALAY and high-beta NF groups showed significant reductions in depression-related symptoms and a slight deviation in the A1 score. Compared with the ALAY-NF group, the high-beta NF group showed greater reductions in beta power and in depression and anxiety symptoms. A recent study by Chen and Lin (2020) examined the effect of high-beta down-training on participants with depression or depression symptoms (N = 23; age = 43.17 ± 15.82 years; 12 females). The participants received 10 sessions of high-beta down-training over 5 weeks. Depression and symptoms related to depression were assessed using the BDI-II and BAI, and the participants’ scores were significantly reduced after the training. The study also reported significant reductions in low- and high-beta activity over both hemispheres. Table 2 lists the studies based on EEG-NF using the high-beta down-training protocol for treating depression.
Table 2.
Summary of studies based on EEG-NF using the high-beta down-training protocol for treating depression.
| Authors (year) | Protocol | Sample Characteristics (% female) | Groups (if any) | Electrodes Used for NFT | Session Details | Depression Assessments | Behavioral Results | EEG-wave Results |
|---|---|---|---|---|---|---|---|---|
| Wang et al. (2019) | Alpha asymmetry (↑ A1 score); ↓ High-beta |
N = 70 MDD (59%) |
ALAY group (N = 24; age = 33 ± 14.741 years) High-beta group (N = 23; age = 42.83 ± 15.816 years) Control group (N = 23; age = 42.61 ± 13.937 years) |
ALAY training: F3, F4 High-beta down-training: P3, P4 |
10 sessions over 5 weeks |
|
Improvements in BDI-II and BAI found in the ALAY and high-beta group | Significant decreases in P3 and P4 electrode high-beta in beta group, whereas control showed increased beta at P3 and P4 |
| Chen & Lin (2020) | ↓ High beta |
N = 23 MDD and anxiety age = 43.17 ± 15.82 years (52%) |
N/A | P3, P4 | 10 sessions over 5 weeks; each session = 60 mins |
|
Significant decrease in BDI-II and BAI scores post-NF sessions | Significant decrease in low and high beta at P3 and P4 post-NF sessions |
Note: F3: left frontal electrode; NF: neurofeedback training; MDD: major depressive disorder; ALAY: Alpha-asymmetry neurofeedback protocol; Fz: middle frontal electrode; F4: right frontal electrode; Cz: middle central electrode; P3: left parietal electrode; P4: right parietal electrode; A1: alpha asymmetry score; BDI-II: Beck Depression Inventory; BAI: Beck Anxiety Inventory.
3.3. Other EEG-NF protocols used in depression-based studies
Studies using EEG-NF to alleviate depression and its symptoms have also shown improvements in other areas of cognition. A number of studies have also shown the alleviation of various conditions that are directly or indirectly related to depression. These studies are discussed in the following section.
3.4. EEG-NF to improve cognition
Lee et al. (2019) used two EEG-NF protocols and assessed the serum concentrations of brain-derived neurotrophic factor (BDNF) in patients with treatment-resistant depression (TRD) and healthy controls. The TRD patients were divided into two groups. Over a 12-week period, the EEG-NF augmentation group (N = 12; age = 48.25 ± 14.44 years; 9 females) received 12–24 sessions of training using the elevation of the sensorimotor rhythm (SMR)/beta for 30 min, followed by training using the theta/alpha protocol for 30 min. This group received combined EEG-NF training and medication treatment. The treatment as usual (TAU) group (N = 12; age = 54.33 ± 12.67 years; 8 females) received medication and psychotherapy placebo treatment sessions instead of EEG-NF training. Patients in the TRD groups and healthy controls provided blood samples for BDNF assessment at baseline. HAM-D and BDI-II depression scores were assessed in the TRD groups. The results showed significant decreases in the HAM-D and BDI-II scores in the EEG-NF group compared with the TAU group. Compared with the TAU group, the EEG-NF augmentation group also saw significant reductions in the quality of life and functional recovery scores measured using the Sheehan Disability Scale (SDS) and the 5-level version of the European Quality of Life Questionnaire 5-Dimensional Classification (EQ-5D-5 L) scores. However, the study did not find a significant difference in the baseline serum BDNF concentrations between the three groups.
Several studies have incorporated EEG-NF protocols such as the upper-alpha protocol and peak-alpha protocol to examine cognitive performance in participants with depression symptoms. Escolano et al. (2014) used EEG-NF and compared improvements in working memory performance in 74 participants with depression, divided into the NF group (N = 40; age = 53.7 ± 10.87 years; 25 females) and the control group (N = 20; age = 49.5 ± 10.18 years; 16 females). Both groups received the increasing upper alpha protocol over the parieto-occipital region over a 5-week period. The participants with depression showed improvements in working memory performance, especially processing speed and performance, compared with the control participants. The Patient Health Questionnaire (PHQ-9) was used to assess depression and its severity, and there was a significant decrease in scores after the EEG-NF sessions. The study further observed a significant increase in the upper peak alpha power across participants with depression, both during rest and during task performance. This study thus indicates that the upper-alpha EEG-NF protocol can be used to improve working memory and alleviate depression symptoms.
3.5. EEG-NF improves other conditions associated with depression
A number of studies have used EEG-NF-based protocols, including SMR/beta and alpha/theta, to improve not only symptoms of depression but also associated conditions (Table 3 ). Kayiran et al. (2010) used an EEG-NF-based increasing SMR/theta protocol to alleviate anxiety and depression symptoms in 36 participants with fibromyalgia syndrome. The participants were randomly assigned to two groups: a medication plus NF group (N = 18; age = 31.78 ± 6.17 years) and a control group (N = 18; 32.39 ± 6.72 years). The NF group received the EEG-NF treatment and medication, whereas the control group received medication only. The NF group was trained to elevate SMR and reduce theta for about 30 min per session, with 5 sessions per week for 4 weeks, whereas the patients in the control group received medication for 8 weeks. The NF group showed significantly reduced depression scores compared with the control group. There was also a significant decrease in the theta/SMR ratio in the fourth week of training, indicating the potential clinical use of the protocol.
Table 3.
Summary of studies based on EEG-NF using other protocols for treating cognitive functions and depression in various conditions/diseases.
| Authors (year) | Protocol | Sample characteristics (% female) | Groups (if any) | Electrodes Used for NF | Session Details | Depression Assessments | Behavioral Results | EEG-wave Results |
|---|---|---|---|---|---|---|---|---|
| Kayiran et al., (2010) | ↑SMR; ↓theta |
N = 36 FMS (N/A) |
NF group (N = 18; age = 31.78 ± 6.17 years) Control group (N = 18; age = 32.39 ± 6.72 years) |
SMR training: C4 | 20 sessions over 8 weeks; each session = 30 mins |
|
The NF group showed significantly lower mean HDS, BDS, HAS, and BAS scores than the control group | Theta/SMR ratio showed a significant decrease at the 4th week in the NF group |
| Escolano et al. (2014) | ↑ Upper alpha (UA) |
N = 60 Healthy (68%) |
NF group (N = 40; age = 53.7 ± 10.87 years) Control group (N = 20; age = 49.5 ± 10.18 years) |
P3, P4, Pz, O1, and O2 | 8 NF sessions over 5 weeks; each session = 32 mins |
|
No significant change in BDI-II; the mean PHQ-9 score was slightly lower in the NF group | Significant increase in UA power in rest and task-related activity in the NF group |
| Choobforoushzadeh et al. (2015) | ↓ Theta and ↓ alpha; ↑ beta |
N = 24 Relapsing-remitting multiple sclerosis (50%) |
NF group (N = 12; age = 34.28 ± 8.17 years) TAU group (N = 12; age = 33.39 ± 7.72 years) |
F3 for NF group | 16 sessions over 8 weeks; each session = 30 mins |
|
Depression scores improved between the pre- and post-tests | N/A |
| Lackner et al. (2016) | ↑ Alpha and theta (simultaneously) |
N = 25 AUD (0%) |
NF group (N = 13; age = 38.9 ± 9.1 years) Control group (N = 12; age = 40.5 ± 8.8 years) |
Fz (Alpha NF) Pz (Theta NF) Cz (Control) |
12 sessions over 6 weeks; each session = 20 mins |
|
Significant improvements in the BDI-V, BSI, and PPR scores compared with control group | Increased absolute alpha and theta amplitudes post-NF compared with the control group |
| Lee et al. (2019) | Two NF:
|
N = 24 (TRD) & N = 12 (Healthy) (67%) |
NF augmentation group (N = 12; age = 48.25 ± 14.44 years) TAU group (N = 12; age = 54.33 ± 12.67 years) Healthy control group (N = 12; age = 43.50 ± 13.80 years) |
SMR training: T4 Beta training: F3/T3 Alpha/theta training: Pz |
12–24 sessions over 12 weeks; each session = 30 mins |
|
HAM-D and BDI-II in the NF augmentation group significantly decreased over 12 weeks compared with the TAU group | N/A |
| Yu et al. (2020) | ↑ Peak alpha |
N = 26 Dysphoric (54%) |
NF group (N = 14; age = 22.33 ± 2.43 years) Sham-control group (N = 12; age = 23.05 ± 3.25 years) |
Peak alpha training: Fp1, Fp2 | 20 sessions over 10 weeks; each session = 30 mins |
|
Depression and rumination significantly decreased after NF sessions | Increased peak alpha indicated enhanced executive function and reduced rumination and depression |
Note: NF: neurofeedback training; FMS: fibromyalgia syndrome; SMR: sensorimotor rhythms; AUD: alcohol use disorder; TRD: treatment-resistant depression; TAU: treatment as usual; C4: right central electrode; P3: left parietal electrode; Pz: middle parietal electrode; O1: left occipital electrode; O2: right occipital electrode; F3: left frontal electrode; Fz: middle frontal electrode; Cz: middle central electrode; T4: right temporal electrode; T3: left temporal electrode; Fp1: left frontal pole; Fp2: right frontal pole; HDS/HAM-D: Hamilton Depression Rating Scale; BDS: Beck Depression Scale; HAS: Hamilton Anxiety Scale; BAS: Beck Anxiety Scale; BDI-II: Beck Depression Inventory; PHQ-9: Patient Health Questionnaire; FSS: Fatigue Severity Scale; HADS: Hospital Anxiety and Depression Scale; BSI: Brief Symptom Inventory; FKV-lis: Freiburg Questionnaire on Coping with Illness; FPTM-23: Therapy Motivation Questionnaire; PPR: Posttraumatic Growth Inventory; SOC-13: Sense of Coherence Scale.
Choobforoushzadeh et al. (2015) used EEG-NF to alleviate depression and fatigue symptoms in 24 participants with multiple sclerosis. The participants were randomly assigned to two groups: an EEG-NF group (N = 12; age = 34.28 ± 8.17 years; 6 females) and a TAU group (N = 12; age = 33.39 ± 7.72 years; 6 females). The study protocol aimed to elevate beta and reduce alpha and theta during 16 30-minute sessions over 8 weeks. Scores on the Hospital Anxiety and Depression Scale and Fatigue Severity Scale showed significant decreases in the NF group compared with the TAU group. The effects were maintained at a 2-month follow-up, indicating that EEG-NF may have long-term effects and be successful in clinical applications.
A similar EEG-NF study by Lackner et al. (2016) used a simultaneous theta and alpha increase protocol in 25 participants with alcohol use disorder and symptoms related to depression. The participants were randomly assigned to two groups: an EEG-NF group (N = 13; age = 38.9 ± 9.1 years) and a control group (N = 12; age = 40.5 ± 8.8 years). The NF group received the EEG-NF training sessions, whereas the control group received TAU. The NF group was trained to elevate alpha and theta simultaneously for 20 min per session over 6 weeks. The study reported significant improvements in depression symptoms and anxiety measured by the BDI-V, Posttraumatic Growth Inventory, and Brief Symptom Inventory (BSI) in the EEG-NF group compared with the control group. The EEG-NF group also showed significant improvements in theta and alpha amplitudes compared with the control group.
Yu et al. (2020) compared the effect of increasing peak alpha in participants with dysphoria who were randomly assigned to an EEG-NF group (N = 12; age = 22.33 ± 2.43 years; 8 females) and a sham-control group (N = 12; age = 23.05 ± 3.25 years; 6 females). The NF group received EEG-NF peak alpha training during 20 30-minute sessions over 10 weeks, and the sham-control group received an audio signal–detecting task. The NF group showed significant reductions in rumination and depression, as measured by the BDI-II, compared with the sham-control group. The peak alpha also showed a significant increase in the NF group compared with the sham-control group, indicating improvements in working memory capacity and executive functions, as well as a reduction in depressive symptoms.
4. Discussion
This systematic review of studies on EEG-NF for the treatment of depression shows that the included studies produced statistically significant clinical improvements. The most important element in the design of an EEG-NF experiment is the EEG protocol. To compare the between-group clinical effects across various EEG-NF studies, we grouped the studies in this review according to the EEG protocols used.
One of the most commonly used protocols is the ALAY protocol; this is based on Davidson's model of emotion, which indicates the degree of anger associated with approach behavior and links it to the corresponding brain region (i.e., activity in the left prefrontal region) (Davidson et al., 1990). This model characterizes the potential association of emotional processing and emotional regulation with unbalanced neural resources in the left and right frontal cortex (Harmon-Jones et al., 2010). The model further indicates that hypoactivation in the left frontal regions of the brain is associated with depression (Henriques and Davidson, 1990, Henriques and Davidson, 1991) and that the alpha power is higher in the right than in the left frontal region because of hypoactivation in the left region. In the ALAY protocol, participants are trained to decrease left frontal alpha activity and increase right frontal activity, thus increasing the A1 score. However, EEG-NF studies using the ALAY protocol have received criticism (Esmail and Linden, 2011). Although the ALAY protocol has received support from a few studies, other evidence suggests that there is no specific association between increased left frontal alpha activity and depression (Bhogal et al., 2004). A few studies have been able to modulate the A1 score, whereas others have shown no modulation in participants with depression, again indicating an inconsistency in the findings of studies using the ALAY protocol.
ALAY-based EEG-NF studies have also shown smaller group differences in control conditions compared with NF groups. Although these studies have reported substantial clinical effects of EEG-NF, their sample sizes have been rather small. A recent EEG-NF study by Wang et al. (2019) compared two protocols, ALAY and high-beta down-training, in participants with depression and concluded that the high-beta down-training protocol was more effective. The efficacy of high-beta down-training was further supported by Chen and Lin (2020), who reported superior behavioral and clinical outcomes compared with ALAY in participants with depression.
Another limitation of studies on EEG-NF for treating depression is that many of them include only one group. Very few studies have compared participants with depression to a healthy control group. Hammond (2005) observed some improvement in participants with depression after three to six 30-minute NF sessions, a significant improvement after 10 to 12 sessions, and complete recovery after 20 to 22 sessions. Further, the treatment relieved not only depression but also other conditions, including anxiety and rumination, introversion, and withdrawal. Most of the studies in this review used >8 sessions of neurofeedback training, although there was some variance in the number and duration of sessions. Despite the encouraging results, controlled trials of EEG-NF for treating depression are needed.
EEG-NF training to alleviate depression symptoms is an encouraging development because it is non-invasive and has minimal side effects compared with methods such as pharmacotherapy, intense transcranial magnetic stimulation, and electroconvulsive therapy. Recent studies have suggested that EEG-NF could be a major therapeutic tool for various disorders. Despite this potential, more high-quality research is needed. It would be beneficial to develop more protocols based on bimodal or multimodal NF to improve the efficacy of the training and its role in clinical applications. It would also be helpful to incorporate EEG-NF and fMRI-NF, as the overlap between time and spatial resolution would provide insight into the effects of NF on participants with depression and facilitate the identification of suitable and predictive biomarkers of depression. Currently, the use of EEG-NF as a treatment for depression is limited, but its potential seems to be enormous.
The findings of this review support the use of EEG-based NF as an evidence-based treatment for depression. Nevertheless, the evidence is limited because NF is a complex intervention that involves various independent factors in the experimental design. These include control designs featuring variations such as the comparison of a group receiving standard medication plus EEG-NF with a control group receiving standard medication only (also referred to as a passive control design), or of a group receiving continued standard medication with EEG-NF with a group receiving continued standard medication with targeted EEG-NF (also referred to as an active control design). It was difficult to select an acceptable design for EEG-NF research (Lubianiker et al., 2019; Sorger et al., 2019), but some studies have provided compelling evidence for its efficacy (Thibault et al., 2016; Sorger et al., 2019; Ros et al., 2020). In addition to the control design, EEG-NF systems offer several benefits to the user, including individualized treatment, ease of use, and detailed user-specific EEG analysis. Future studies aiming to understand the neural mechanisms of EEG-NF and its effects on depression should use larger sample sizes, as well as longer follow-up periods to understand the efficacy and clinical effects of EEG-NF (Becerra et al., 2006; Gevensleben et al., 2010; Lubianiker et al., 2019; Mehler et al., 2018; Rance et al., 2018). The current systematic review sheds light on how EEG-NF can be utilized as a treatment for depressive symptoms. The effectiveness of EEG-NF protocols in treating depression symptoms in the general population is limited. For a deeper knowledge of the benefits of EEG-NF, further EEG-NF research that compares participants without pharmacological therapies, participants with depression, and healthy control participants are needed. Future studies also should concentrate on EEG-based biomarkers in individuals with depression to examine how they change or remain constant with age and how they respond to EEG-NF training. EEG and fMRI treatments can be used simultaneously to better understand the impact of the neurobiology of depression throughout a lifespan. Overall, the evidence from the 12 published studies included in this systematic review suggests that more research is needed to understand the efficacy of EEG-NF research and its use in treating depression symptoms. This is the first systematic review to examine the efficacy of EEG-NF as an intervention to treat depression symptoms, and the findings indicate that EEG-NF training may be an effective intervention tool that can be utilized as a supplementary treatment for depression
The global pandemic caused by COVID-19 has raised many concerns and disrupted the everyday lives of people around the globe (Kokou-Kpolou et al., 2020; Voitsidis et al., 2020; Wu, 2020). For most of the population, this global public health crisis has been an extremely stressful period (Qiu et al., 2020; Zhang et al., 2020) and has affected many people's mental and psychological health both directly and indirectly. Research has reported the increased prevalence of psychological problems such as anxiety disorders (Huang and Zhao, 2020), depression symptoms (Elbay et al., 2020), insomnia (Kokou-Kpolou et al., 2020; Voitsidis et al., 2020), and post-traumatic stress symptoms (Lin et al., 2020) in both developing and developed countries. Hence, there is a need to update the treatment recommendations for mental disorders during such crises. The development of EEG-NF to alleviate depression symptoms is particularly encouraging amid the current COVID-19 pandemic because it is a complex yet relatively non-invasive intervention compared with other pharmacotherapeutic interventions. EEG-NF targets both cognitive and affective processes in patients with depression. As it has minimal or no side effects, the approach has good face validity for treating depression. Patients with depression have shown significant clinical and neural improvements following EEG-NF training. Moreover, given its low cost and the low risk of side effects due to its non-invasive nature, we consider EEG-NF worth exploring as an augmented tool for patients who receive standard medications but remain symptomatic.
However, this systematic review reveals that previous studies have not always followed best practices in EEG-NF designs, and it highlights several issues that should be considered when developing such designs. Some of the major issues in many of these studies are small and unbalanced sample sizes, blinding in random control designs, and a lack of follow-up protocols. These issues increase the difficulty of evaluating the clinical effects of EEG-NF and should be addressed in future studies. We recommend that future studies include sufficient investigations, improved techniques, and controlled research trials to better understand the efficacy of EEG-NF in treating depression.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgement
This work was supported by the Ministry of Science and Technology (105-2420-H-009-001-MY2; 107-2410-H-009-028-MY3; 110-2410-H-A49-059; 111-2410-H-A49 -071-MY3) and the Center for Intelligent Drug Systems and Smart Biodevices (IDS2B) from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. This work was also supported by the Hong Kong Institute for Advanced Studies, City University of Hong Kong (9360157) in Hong Kong.
References
- American Psychiatric Association, 2009. Practice guideline for the treatment of patients with major depressive disorder (3rd). http://psychiatryonline.org/guidelines.aspx. [PubMed]
- Baehr E., Rosenfeld J.P., Baehr R. The clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression. J. Neurother. 1997;2:10–23. doi: 10.1300/J184v02n03_02. [DOI] [Google Scholar]
- Baehr E., Rosenfeld J.P., Baehr R. Clinical use of an alpha asymmetry neurofeedback protocol in the treatment of mood disorders. J. Neurother. 2001;4:11–18. doi: 10.1300/J184v04n04_03. [DOI] [Google Scholar]
- Bearden T.S., Cassisi J.E., Pineda M. Neurofeedback training for a patient with thalamic and cortical infarctions. Appl. Psychophysiol. Biofeedback. 2003;28:241–253. doi: 10.1023/a:1024689315563. [DOI] [PubMed] [Google Scholar]
- Becerra J., Fernández T., Harmony T., Caballero M.I., García F., Fernández-Bouzas A., Santiago-Rodríguez E., Prado-Alcalá R.A. Follow-up study of learning-disabled children treated with neurofeedback or placebo. Clin. EEG Neurosci. 2006;37:198–203. doi: 10.1177/155005940603700307. [DOI] [PubMed] [Google Scholar]
- Bhogal S.K., Teasell R., Foley N., Speechley M. Lesion location and poststroke depression: systematic review of the methodological limitations in the literature. Stroke. 2004;35:794–802. doi: 10.1161/01.STR.0000117237.98749.26. [DOI] [PubMed] [Google Scholar]
- Blaskovits F., Tyerman J., Luctkar-Flude M. Effectiveness of neurofeedback therapy for anxiety and stress in adults living with a chronic illness: a systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2017;15:1765–1769. doi: 10.11124/JBISRIR-2016-003118. [DOI] [PubMed] [Google Scholar]
- Breteler M.H.M., Arns M., Peters S., Giepmans I., Verhoeven L. Improvements in spelling after QEEG-based neurofeedback in dyslexia: a randomized controlled treatment study. Appl. Psychophysiol. Biofeedback. 2010;35:5–11. doi: 10.1007/s10484-009-9105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet E., Andrade L.H., Hwang I., Sampson N.A., Alonso J., de Girolamo G., de Graaf R., Demyttenaere K., Hu C., Iwata N., Karam A.N., Kaur J., Kostyuchenko S., Lépine J.-.P., Levinson D., Matschinger H., Mora M.E.M., Browne M.O., Posada-Villa J., Viana M.C., Williams D.R., Kessler R.C. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot R.J., Michele F., Prichep L., John E.R. The clinical role of computerized EEG in the evaluation and treatment of learning and attention disorders in children and adolescents. J. Neuropsychiatr. 2001;13:171–186. doi: 10.1176/jnp.13.2.171. [DOI] [PubMed] [Google Scholar]
- Chen T.-.C., Lin I.-.M. The learning effects and curves during high beta down-training neurofeedback for patients with major depressive disorder. J. Affect. Disord. 2020;266:235–242. doi: 10.1016/j.jad.2020.01.175. [DOI] [PubMed] [Google Scholar]
- Cheon E.-.J., Koo B.-.H., Choi J.-.H. The efficacy of neurofeedback in patients with major depressive disorder: an open labeled prospective study. Appl. Psychophysiol. Biofeedback. 2016;41:103–110. doi: 10.1007/s10484-015-9315-8. [DOI] [PubMed] [Google Scholar]
- Choi S.W. Is alpha wave neurofeedback effective with randomized clinical trials in depression? A pilot study. Neuropsychology. 2011;63:43–51. doi: 10.1159/000322290. [DOI] [PubMed] [Google Scholar]
- Choo C., Diederich J., Song I., Ho R. Cluster analysis reveals risk factors for repeated suicide attempts in a multi-ethnic Asian population. Asian J. Psychiatr. 2014;8:38–42. doi: 10.1016/j.ajp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Choobforoushzadeh A., Neshat-Doost H.T., Molavi H., Abedi M.R. Effect of neurofeedback training on depression and fatigue in patients with multiple sclerosis. Appl. Psychophysiol. Biofeedback. 2015;40:1–8. doi: 10.1007/s10484-014-9267-4. [DOI] [PubMed] [Google Scholar]
- Churchill R., Hunot V., Corney R., Knapp M., McGuire H., Tylee A., Wessely S. A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression. Health Technol. Assess. 2001;5:1–173. doi: 10.3310/hta5350. [DOI] [PubMed] [Google Scholar]
- Collura T.F. Taylor & Francis; 2014. Technical Foundations of Neurofeedback. [DOI] [Google Scholar]
- Cuijpers P., Smit F. Excess mortality in depression: a meta-analysis of community studies. J. Affect. Disord. 2002;72:227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- Dalkner N., Unterrainer H.F., Wood G., Skliris D., Holasek S.J., Gruzelier J.H., Neuper C. Short-term beneficial effects of 12 sessions of neurofeedback on avoidant personality accentuation in the treatment of alcohol use disorder. Front. Psychol. 2017;8:1688. doi: 10.3389/fpsyg.2017.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly E.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Gaynes B.N., Warden D., Morris D.W., Luther J.F., Farabaugh A., Cook I., Rush A.J. Health-related quality of life in depression: a STAR*D report. Ann. Clin. Psychiatr. 2010;22:43–55. [PubMed] [Google Scholar]
- Davidson R.J. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Ekman P., Saron C.D., Senulis J.A., Friesen W.V. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. J. Pers. Soc. Psychol. 1990;58:330–341. [PubMed] [Google Scholar]
- Dempster, T.J., 2012. An investigation into the optimum training paradigm for alpha electroencephalographic biofeedback.
- DeRubeis R.J., Siegle G.J., Hollon S.D. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat. Rev. Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A.M., van Deusen A. A new neurofeedback protocol for depression. Span. J. Psychol. 2011;14:374–384. doi: 10.5209/rev_sjop.2011.v14.n1.34. [DOI] [PubMed] [Google Scholar]
- Dupee M., Werthner P. Managing the stress response: the use of biofeedback and neurofeedback with Olympic athletes. Biofeedback. 2011;39:92–94. doi: 10.5298/1081-5937-39.3.02. [DOI] [Google Scholar]
- Elbay R.Y., Kurtulmuş A., Arpacıoğlu S., Karadere E. Depression, anxiety, stress levels of physicians and associated factors in Covid-19 pandemics. Psychiatry Res. 2020;290 doi: 10.1016/j.psychres.2020.113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolano C., Navarro-Gil M., Garcia-Campayo J., Congedo M., De Ridder D., Minguez J. A controlled study on the cognitive effect of alpha neurofeedback training in patients with major depressive disorder. Front. Behav. Neurosci. 2014;8:296. doi: 10.3389/fnbeh.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmail S., Linden D.E.J. Cognitive Sciences. Nova Science Publishers; 2011. Emotion regulation networks and neurofeedback in depression; pp. 101–128. [Google Scholar]
- Gadea M., Aliño M., Hidalgo V., Espert R., Salvador A. Effects of a single session of SMR neurofeedback training on anxiety and cortisol levels. Neurophysiol. Clin. 2020;50:167–173. doi: 10.1016/j.neucli.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Gandara V., Pineda J.A., Shu I.-.W., Singh F. A systematic review of the potential use of neurofeedback in patients with schizophrenia. Schizophr. Bull. Open. 2020;1 doi: 10.1093/schizbullopen/sgaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevensleben H., Holl B., Albrecht B., Schlamp D., Kratz O., Studer P., Rothenberger A., Moll G.H., Heinrich H. Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. Eur. Child Adolesc. Psychiatry. 2010;19:715–724. doi: 10.1007/s00787-010-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Kasch K.L., Traill S., Joormann J., Arnow B.A., Johnson S.L. Coherence and specificity of information-processing biases in depression and social phobia. J. Abnorm. Psychol. 2004;113:386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Krasnoperova E., Yue D.N., Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J. Abnorm. Psychol. 2004 doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gruzelier J.H. EEG-neurofeedback for optimising performance. I: a review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014;44:124–141. doi: 10.1016/j.neubiorev.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Hammer B.U., Colbert A.P., Brown K.A., Ilioi E.C. Neurofeedback for insomnia: a pilot study of Z-score SMR and individualized protocols. Appl. Psychophysiol. Biofeedback. 2011;36:251–264. doi: 10.1007/s10484-011-9165-y. [DOI] [PubMed] [Google Scholar]
- Hammond D.C. Neurofeedback treatment of depression and anxiety. J. Adult Dev. 2005;12:131–137. [Google Scholar]
- Harmon-Jones E., Gable P.A., Peterson C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol. Psychol. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Henriques J.B., Davidson R.J. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J. Abnorm. Psychol. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques J.B., Davidson R.J. Left frontal hypoactivation in depression. J. Abnorm. Psychol. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Hirschfeld R.M., Montgomery S.A., Keller M.B., Kasper S., Schatzberg A.F., Möller H.J., Healy D., Baldwin D., Humble M., Versiani M., Montenegro R., Bourgeois M. Social functioning in depression: a review. J. Clin. Psychiatry. 2000;61:268–275. doi: 10.4088/jcp.v61n0405. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. Brain training against stress: theory, methods and results from an outcome study. Stress Rep. 2005;4:1–24. [Google Scholar]
- Huang C.-.M., Fan Y.-.T., Lee S.-.H., Liu H.-.L., Chen Y.-.L., Lin C., Lee T.M.C. Cognitive reserve-mediated neural modulation of emotional control and regulation in people with late-life depression. Soc. Cogn. Affect. Neurosci. 2019;14:849–860. doi: 10.1093/scan/nsz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-.M., Huang H.-.W. Chapter six - Aging, neurocognitive reserve, and the healthy brain. Psychol. Learn. Motiv. 2019;71:175–213. doi: 10.1016/bs.plm.2019.07.006. [DOI] [Google Scholar]
- Huang Y., Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288 doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeunet C., Glize B., McGonigal A., Batail J.-.M., Micoulaud-Franchi J.-.A. Using EEG-based brain computer interface and neurofeedback targeting sensorimotor rhythms to improve motor skills: theoretical background, applications and prospects. Neurophysiol. Clin. 2019;49:125–136. doi: 10.1016/j.neucli.2018.10.068. [DOI] [PubMed] [Google Scholar]
- Joiner T., Coyne J.C., Blalock J. American Psychological Association; Washington, DC: 1999. On the Interpersonal Nature of depression: Overview and synthesis, in: The Interactional Nature of Depression: Advances in Interpersonal Approaches; pp. 3–19. [DOI] [Google Scholar]
- Kaiser A.K., Doppelmayr M., Iglseder B. Electroencephalogram alpha asymmetry in geriatric depression. Z. Gerontol. Geriatr. 2018;51:200–205. doi: 10.1007/s00391-016-1108-z. [DOI] [PubMed] [Google Scholar]
- Kamiya J. Biofeedback training in voluntary control of EEG alpha rhythms. Calif. Med. 1971;115:44. [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Haghshenas S., Rostami R. Neurofeedback and autism spectrum: a case study. Procedia Soc. Behav. Sci. 2011;30:1472–1475. doi: 10.1016/j.sbspro.2011.10.285. [DOI] [Google Scholar]
- Kayiran S., Dursun E., Dursun N., Ermutlu N., Karamürsel S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Appl. Psychophysiol. Biofeedback. 2010;35:293–302. doi: 10.1007/s10484-010-9135-9. [DOI] [PubMed] [Google Scholar]
- Ko S., Park W. Effects of quantitative electroencephalography based neurofeedback training on autonomous regulations in patients with alcohol use disorder. Asian Nurs. Res. (Korean Soc Nurs Sci) 2018;12:136–144. doi: 10.1016/j.anr.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Kokou-Kpolou C.K., Megalakaki O., Laimou D., Kousouri M. Insomnia during COVID-19 pandemic and lockdown: prevalence, severity, and associated risk factors in French population. Psychiatry Res. 2020 doi: 10.1016/j.psychres.2020.113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner N., Unterrainer H.F., Skliris D., Wood G., Wallner-Liebmann S.J., Neuper C., Gruzelier J.H. The effectiveness of visual short-time neurofeedback on brain activity and clinical characteristics in alcohol use disorders: practical issues and results. Clin. EEG Neurosci. 2016;47:188–195. doi: 10.1177/1550059415605686. [DOI] [PubMed] [Google Scholar]
- Large M. Study on suicide risk assessment in mental illness underestimates inpatient suicide risk. BMJ. 2016 doi: 10.1136/bmj.i267. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Lee G.W., Seo W.S., Koo B.H., Kim H.G., Cheon E.J. Neurofeedback treatment on depressive symptoms and functional recovery in treatment-resistant patients with major depressive disorder: an open-label pilot study. J. Korean Med. Sci. 2019;34:e287. doi: 10.3346/jkms.2019.34.e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner D., Henke R.M. What does research tell us about depression, job performance, and work productivity? J. Occup. Environ. Med. 2008;50:401–410. doi: 10.1097/JOM.0b013e31816bae50. [DOI] [PubMed] [Google Scholar]
- Lim G.Y., Tam W.W., Lu Y., Ho C.S., Zhang M.W., Ho R.C. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018;8:2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Huang C.-.M., Fan Y.-.T., Liu H.-.L., Chen Y.-.L., Aizenstein H.J., Lee T.M.-C., Lee S.-.H. Cognitive reserve moderates effects of white matter hyperintensity on depressive symptoms and cognitive function in late-life depression. Front. Psychiatry. 2020;11:249. doi: 10.3389/fpsyt.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Huang C.-.M., Karim H.T., Liu H.-.L., Lee T.M.-C., Wu C.W., Toh C.H., Tsai Y.-.F., Yen T.-.H., Lee S.-.H. Greater white matter hyperintensities and the association with executive function in suicide attempters with late-life depression. Neurobiol. Aging. 2021;103:60–67. doi: 10.1016/j.neurobiolaging.2020.12.016. [DOI] [PubMed] [Google Scholar]
- Lu Y., Tang C., Liow C.S., Ng W.W.N., Ho C.S.H., Ho R.C.M. A regressional analysis of maladaptive rumination, illness perception and negative emotional outcomes in Asian patients suffering from depressive disorder. Asian J. Psychiatr. 2014;12:69–76. doi: 10.1016/j.ajp.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Lubar J.F., Bahler W.W. Behavioral management of epileptic seizures following EEG biofeedback training of the sensorimotor rhythm. Biofeedback Self Regul. 1976;1:77–104. doi: 10.1007/BF00998692. [DOI] [PubMed] [Google Scholar]
- Lubar J.F., Swartwood M.O., Swartwood J.N., O'Donnell P.H. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 1995;20:83–99. doi: 10.1007/BF01712768. [DOI] [PubMed] [Google Scholar]
- Lubianiker N., Goldway N., Fruchtman-Steinbok T., Paret C., Keynan J.N., Singer N., Cohen A., Kadosh K.C., Linden D.E.J., Hendler T. Process-based framework for precise neuromodulation. Nat. Hum. Behav. 2019;3:436–445. doi: 10.1038/s41562-019-0573-y. [DOI] [PubMed] [Google Scholar]
- Mann C.A., Lubar J.F., Zimmerman A.W., Miller C.A., Muenchen R.A. Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: controlled study with clinical implications. Pediatr. Neurol. 1992;8:30–36. doi: 10.1016/0887-8994(92)90049-5. [DOI] [PubMed] [Google Scholar]
- Marzbani H., Marateb H.R., Mansourian M. Neurofeedback: a comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 2016;7:143–158. doi: 10.15412/J.BCN.03070208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathersul D., Williams L.M., Hopkinson P.J., Kemp A.H. Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8:560–572. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- Mayor S. Persistent depression doubles stroke risk despite treatment, study finds. BMJ. 2015;350 doi: 10.1136/bmj.h2611. [DOI] [PubMed] [Google Scholar]
- McCarthy-Jones S. Taking back the brain: could neurofeedback training be effective for relieving distressing auditory verbal hallucinations in patients with schizophrenia? Schizophr. Bull. 2012;38:678–682. doi: 10.1093/schbul/sbs006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler D.M.A., Sokunbi M.O., Habes I., Barawi K., Subramanian L., Range M., Evans J., Hood K., Lührs M., Keedwell P., Goebel R., Linden D.E.J. Targeting the affective brain-a randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology. 2018;43:2578–2585. doi: 10.1038/s41386-018-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella R., Patron E., Palomba D. Frontal alpha asymmetry neurofeedback for the reduction of negative affect and anxiety. Behav. Res. Ther. 2017;92:32–40. doi: 10.1016/j.brat.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognit. Ther. Res. 2005;29:29–45. doi: 10.1007/s10608-005-1646-y. [DOI] [Google Scholar]
- Morales-Quezada L., Martinez D., El-Hagrassy M.M., Kaptchuk T.J., Sterman M.B., Yeh G.Y. Neurofeedback impacts cognition and quality of life in pediatric focal epilepsy: an exploratory randomized double-blinded sham-controlled trial. Epilepsy Behav. 2019;101 doi: 10.1016/j.yebeh.2019.106570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan W., Rodrigues J.P., Ma J., Qu X., Wan F., Mak P.-.I., Mak P.U., Vai M.I., Rosa A. Individual alpha neurofeedback training effect on short term memory. Int. J. Psychophysiol. 2012;86:83–87. doi: 10.1016/j.ijpsycho.2012.07.182. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health (UK), 2010. Depression: The Treatment and Management of Depression in Adults. Leicester (UK).
- Nazari M.A., Mosanezhad E., Hashemi T., Jahan A. The effectiveness of neurofeedback training on EEG coherence and neuropsychological functions in children with reading disability. Clin. EEG Neurosci. 2012;43:315–322. doi: 10.1177/1550059412451880. [DOI] [PubMed] [Google Scholar]
- Nigro S.E. The efficacy of neurofeedback for pediatric epilepsy. Appl. Psychophysiol. Biofeedback. 2019;44:285–290. doi: 10.1007/s10484-019-09446-y. [DOI] [PubMed] [Google Scholar]
- Nowlis D.P., Kamiya J. The control of electroencephalographic alpha rhythms through auditory feedback and the associated mental activity. Psychophysiology. 1970;6:476–484. doi: 10.1111/j.1469-8986.1970.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Omejc N., Rojc B., Battaglini P.P., Marusic U. Review of the therapeutic neurofeedback method using electroencephalography: EEG Neurofeedback. Bosn. J. Basic Med. Sci. 2019;19:213–220. doi: 10.17305/bjbms.2018.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordikhani-Seyedlar M., Lebedev M.A., Sorensen H.B.D., Puthusserypady S. Neurofeedback therapy for enhancing visual attention: state-of-the-art and challenges. Front. Neurosci. 2016;10:352. doi: 10.3389/fnins.2016.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K., Sutherland H., Henshaw J., Taylor J.R., Brown C.A., Casson A.J., Trujillo-Barreton N.J., Jones A.K.P., Sivan M. Effects of neurofeedback in the management of chronic pain: a systematic review and meta analysis of clinical trials. Eur. J. Pain. 2020;24:1440–1457. doi: 10.1002/ejp.1612. [DOI] [PubMed] [Google Scholar]
- Patil A.U., Madathil D., Fan Y.T., Tzeng Ovid J.L., Huang C.M., Huang H.W. Neurofeedback for the education of children with ADHD and specific learning disorders: a review. Brain Sci. 2022 doi: 10.3390/brainsci12091238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazooki K., Leibetseder M., Renner W., Gougleris G., Kapsali E. Neurofeedback treatment of negative symptoms in schizophrenia: two case reports. Appl. Psychophysiol. Biofeedback. 2019;44:31–39. doi: 10.1007/s10484-018-9417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters F., Oehlen M., Ronner J., van Os J., Lousberg R. Neurofeedback as a treatment for major depressive disorder – a pilot study. PLoS ONE. 2014;9:e91837. doi: 10.1371/journal.pone.0091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Shen B., Zhao M., Wang Z., Xie B., Xu Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen. Psychiatr. 2020 doi: 10.1136/gpsych-2020-100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance M., Walsh C., Sukhodolsky D.G., Pittman B., Qiu M., Kichuk S.A., Wasylink S., Koller W.N., Bloch M., Gruner P., Scheinost D., Pittenger C., Hampson M. Time course of clinical change following neurofeedback. Neuroimage. 2018;181:807–813. doi: 10.1016/j.neuroimage.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J.P., Dr. An EEG biofeedback protocol for affective disorders. Clin. Electroencephalogr. 2000;31:7–12. doi: 10.1177/155005940003100106. [DOI] [PubMed] [Google Scholar]
- Rozelle G.R., Budzynski T.H. Neurotherapy for stroke rehabilitation: a single case study. Biofeedback Self Regul. 1995;20:211–228. doi: 10.1007/BF01474514. [DOI] [PubMed] [Google Scholar]
- Sayers J. Bull World Health Organ; 2001. The World Health Report 2001-Mental health: New understanding, New Hope. [Google Scholar]
- Seifert A.R., Lubar J.F. Reduction of epileptic seizures through EEG biofeedback training. Biol. Psychol. 1975;3:157–184. doi: 10.1016/0301-0511(75)90033-2. [DOI] [PubMed] [Google Scholar]
- Singh F., Shu I.-.W., Granholm E., Pineda J.A. Revisiting the potential of EEG neurofeedback for patients with schizophrenia. Schizophr. Bull. 2020;46:741–742. doi: 10.1093/schbul/sbaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E., Reznik S.J., Stewart J.L., Allen J.J.B. Assessing and conceptualizing frontal EEG asymmetry: an updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 2017;111:98–114. doi: 10.1016/j.ijpsycho.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterman M., MacDonald L., Stone R.K. Biofeedback training of the sensorimotor electroencephalogram rhythm in man: effects on epilepsy. Epilepsia. 1974;15:395–416. doi: 10.1111/j.1528-1157.1974.tb04016.x. [DOI] [PubMed] [Google Scholar]
- Stewart J.L., Coan J.A., Towers D.N., Allen J.J.B. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J. Affect. Disord. 2011;129:167–174. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W.F., Ricci J.A., Chee E., Hahn S.R., Morganstein D. Cost of lost productive work time among US workers with depression. J. Am. Med. Assoc. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Surmeli T., Ertem A., Eralp E., Kos I.H. Schizophrenia and the efficacy of qEEG-guided neurofeedback treatment: a clinical case series. Clin. EEG Neurosci. 2012;43:133–144. doi: 10.1177/1550059411429531. [DOI] [PubMed] [Google Scholar]
- Tan G., Thornby J., Hammond D.C., Strehl U., Canady B., Arnemann K., Kaiser D.A. Meta-Analysis of EEG biofeedback in treating epilepsy. Clin. EEG Neurosci. 2009;40:173–179. doi: 10.1177/155005940904000310. [DOI] [PubMed] [Google Scholar]
- Thibodeau R., Jorgensen R.S., Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J. Abnorm. Psychol. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Thompson L., Thompson M., Reid A. Neurofeedback outcomes in clients with Asperger's syndrome. Appl. Psychophysiol. Biofeedback. 2010;35:63–81. doi: 10.1007/s10484-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Tolin D.F., Davies C.D., Moskow D.M., Hofmann S.G. Biofeedback and neurofeedback for anxiety disorders: a quantitative and qualitative systematic review. Adv. Exp. Med. Biol. 2020;1191:265–289. doi: 10.1007/978-981-32-9705-0_16. [DOI] [PubMed] [Google Scholar]
- Voitsidis P., Gliatas I., Bairachtari V., Papadopoulou K., Papageorgiou G., Parlapani E., Syngelakis M., Holeva V., Diakogiannis I. Insomnia during the COVID-19 pandemic in a Greek population. Psychiatry Res. 2020 doi: 10.1016/j.psychres.2020.113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlbeck K. Public mental health: the time is ripe for translation of evidence into practice. World Psychiatry. 2015;14:36–42. doi: 10.1002/wps.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.E. Anxiety associated with post traumatic stress disorder-The role of quantitative electro-encephalograph in diagnosis and in guiding neurofeedback training to remediate the anxiety. Biofeedback. 2009;37:67–70. doi: 10.5298/1081-5937-37.2.67. [DOI] [Google Scholar]
- Walker J.E., Kozlowski G.P. Neurofeedback treatment of epilepsy. Child Adolesc. Psychiatr. Clin. N. Am. 2005;14:163–176. doi: 10.1016/j.chc.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Wang Q., Sourina O. Real-time mental arithmetic task recognition from EEG signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2013;21:225–232. doi: 10.1109/TNSRE.2012.2236576. [DOI] [PubMed] [Google Scholar]
- Wang S.-.Y., Lin I.-.M., Fan S.-.Y., Tsai Y.-.C., Yen C.-.F., Yeh Y.-.C., Huang M.-.F., Lee Y., Chiu N.-.M., Hung C.-.F., Wang P.-.W., Liu T.-.L., Lin H.-.C. The effects of alpha asymmetry and high-beta down-training neurofeedback for patients with the major depressive disorder and anxiety symptoms. J. Affect. Disord. 2019;257:287–296. doi: 10.1016/j.jad.2019.07.026. [DOI] [PubMed] [Google Scholar]
- Wong N.M.L., Liu H.-.L., Lin C., Huang C.-.M., Wai Y.-.Y., Lee S.-.H., Lee T.M.C. Loneliness in late-life depression: structural and functional connectivity during affective processing. Psychol. Med. 2016;46:2485–2499. doi: 10.1017/S0033291716001033. [DOI] [PubMed] [Google Scholar]
- Wu B. Social isolation and loneliness among older adults in the context of COVID-19: a global challenge. Glob. Health Res. Policy. 2020;5:27. doi: 10.1186/s41256-020-00154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.-.H., Tseng C.-.Y., Lin W.-.L. A neurofeedback protocol for executive function to reduce depression and rumination: a controlled study. Clin. Psychopharmacol. Neurosci. 2020;18:375–385. doi: 10.9758/cpn.2020.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.X., Wang Y., Rauch A., Wei F. Unprecedented disruption of lives and work: health, distress and life satisfaction of working adults in China one month into the COVID-19 outbreak. Psychiatry Res. 2020;288 doi: 10.1016/j.psychres.2020.112958. [DOI] [PMC free article] [PubMed] [Google Scholar]