Abstract
Objective
The nanohydroxyapatite/polyamide‐66 (n‐HA/PA66) cage is a novel bioactive nonmetal cage that is now used in some medical centers, while the polyetheretherketone (PEEK) cage is a typical device that has been widely used for decades with excellent clinical outcomes. This study was performed to compare the long‐term radiographic and clinical outcomes of these two different cages used in transforaminal lumbar interbody fusion (TLIF).
Methods
In this retrospective and matched‐pair case control study, we included 200 patients who underwent TLIF from January 2010 to December 2014 with a minimum 7‐year follow‐up. One hundred patients who used n‐HA/PA66 cages were matched with 100 patients who used PEEK cages for age, sex, diagnosis, and fusion level. The independent student's t‐test and Pearson's chi‐square test were used to compare the two groups regarding radiographic (fusion status, cage subsidence rate, segmental angle [SA], and interbody space height [IH]) and clinical (Oswestry Disability Index [ODI], and Visual Analog Scale [VAS] for back and leg) parameters preoperatively, postoperatively, and at the final follow‐up.
Results
The n‐HA/PA66 and PEEK groups had similar fusion rates of bone inside and outside the cage at the final follow‐up (95.3% vs 91.8%, p = 0.181, 92.4% vs 90.1%, p = 0.435). The cage union ratios exposed to the upper and lower endplates of the n‐HA/PA66 group were significantly larger than those of the PEEK group (p < 0.05). The respective cage subsidence rates in the n‐HA/PA66 and PEEK groups were 10.5% and 17.5% (p = 0.059). There were no significant differences between the two groups in the SA, IH, ODI scores, or VAS scores at any time point. The n‐HA/PA66 group showed high fusion and low subsidence rates during long‐term follow‐up.
Conclusion
Both n‐HA/PA66 and PEEK cages can achieve satisfactory long‐term clinical and radiographic outcomes in TLIF. However, the n‐HA/PA66 group showed significantly larger cage union ratios than the PEEK group. Therefore, the results indicated that the n‐HA/PA66 cage is an ideal alternative material comparable to the PEEK cage in TLIF.
Keywords: Lumbar degenerative disease, Lumbar fusion, Long‐term follow‐up, Cage union ratio, Nanohydroxyapatite/polyamide‐66 cage, Polyetheretherketone cage
Both n‐HA/PA66 and PEEK cages can achieve satisfactory long‐term clinical and radiographic outcomes in TLIF, while the n‐HA/PA66 group showed significantly larger cage union ratios than the PEEK group. And n‐HA/PA66 cage is an ideal alternative material comparable to the PEEK cage in TLIF.

Introduction
Transforaminal lumbar interbody fusion (TLIF), which was described by Harms and Rolinger, has become a commonly used and effective surgical treatment for lumbar degenerative disease (LDD) with favorable outcomes. 1 , 2 The materials inserted into the disc space after discectomy should provide good mechanical support in the anterior column as well as facilitate bone growth between the two vertebral bodies. Autogenous iliac crest was once considered the “gold standard” for TLIF due to its excellent biocompatibility and high fusion rate without immunogenicity. 3 However, it requires a second surgical site, which increases the operative time and blood loss, and there are some donor‐site complications, including hematoma, persistent pain, infection, and fracture. 4
To avoid the complications of autografts, various interbody implant devices, including polyetheretherketone (PEEK) cages, carbon fiber cages, titanium mesh cages, and bioactive glass ceramic spacers, have been designed, and their comparison has been widely reported. 5 , 6 , 7 , 8 , 9 Among them, PEEK cages could reduce stress shielding due to their lower elastic modulus, similar to natural bone, and their radiolucency helps surgeons observe postoperative bone healing around the implants. 10 , 11 , 12 The low elastic modulus and radiolucency of PEEK cages make them extremely popular with spine surgeons. However, the osseointegration capacity of PEEK cages is relatively poor due to their bioinert property. 13
The hollow bullet nanohydroxyapatite/polyamide‐66 (n‐HA/PA66) cage is a bioactive nonmetal cage with good radiolucency made by a composite of n‐HA and PA66, mimicking the structure of natural bone. 14 It has a stiffness and elastic modulus similar to those of natural bone, which could provide satisfactory mechanical support, high fusion rates, and low subsidence rates in anterior reconstruction. 15 To date, the n‐HA/PA66 cage has been reported to treat cervical spondylosis and thoraco‐lumbar fractures with satisfactory long‐term clinical outcomes. 15 , 16 , 17 , 18
However, only one study compared the short‐term clinical results of the n‐HA/PA66 cage and the PEEK cage in the treatment of LDD. 19 Since there is still a lack of comparative studies between the two kinds of cages with long‐term follow‐up, whether they could achieve similar long‐term radiographic and clinical outcomes is still questionable. Therefore, the purposes of this study were (i) to compare the long‐term radiographic outcomes, such as the subsidence rate, fusion rate, and state, of the n‐HA/PA66 and PEEK cages used in TLIF, and (ii) to identify whether the two different cages could achieve satisfactory clinical outcomes and were maintained well during the long‐term follow‐up.
Methods
This was a matched‐pair study of patients with retrospectively collected data from our hospital, and it was approved by the local ethics committee (No. 2019–654). The inclusion criteria were (1) patients underwent TLIF as the primary surgery between January 2010 to December 2014, and (2) no previous surgical intervention at lumbar. The exclusion criteria were as follows: (1) more than three‐level; (2) lumbar degenerative scoliosis, spinal tumors, spinal tuberculosis, or spinal infection; (3) severe osteoporosis (T < –2.5); and (4) postoperative clinical and radiographic follow‐up period less than 7 years. Overall, 1470 patients underwent TLIF within this time period were identified, while 1174 patients among them had to be excluded due to the exclusion criteria. In the remaining 296 patients, there were 149 patients who underwent TLIF using the n‐HA/PA66 cage (the Institution of Materials Science and Technology, Sichuan University) and 147 patients using the PEEK cage (Medtronic, Memphis, TN, USA). To eliminate the selective bias, we performed a 1:1 matching pair according to their age, sex, diagnosis, and fusion level. Finally, there was a total of 200 patients enrolled in this study, 100 patients in n‐HA/PA66 group and 100 in PEEK group (Fig. 1).
Fig. 1.
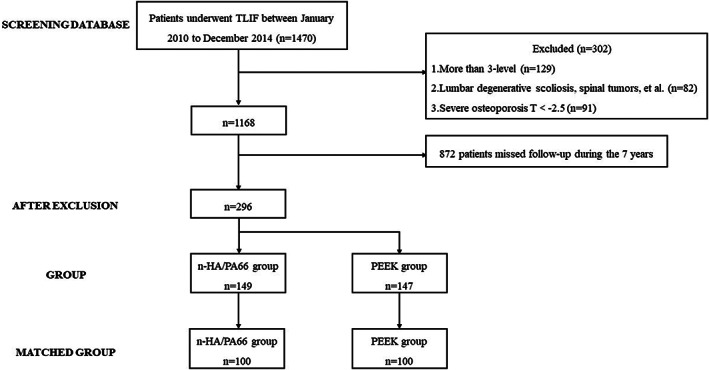
The flow diagram of patients in this study
Surgical Procedure
All patients were treated by one of four senior spine surgeons after general anesthesia was administered by a skilled anesthesiologist. The patients were not randomized to the type of cage, and the decision to use n‐HA/PA66 or PEEK cages was made by the surgeon. After pedicle screw positioning, TLIF was conducted as described by Meyer et al. 20 Harvested local bone was acquired by removing cartilage and fibrous tissue from excised bone, which was then morselized. After filling the cage with morselized bone, residual bone was inserted into the anterior and contralateral disc space, and then the cage was inserted into the interbody space. Finally, the spinal screw rod system was compressed longitudinally, and the cage was confirmed to be located nicely under fluoroscopy.
Clinical and Radiographic Evaluation
Information regarding operative time, intraoperative blood loss, and complications was collected. Clinical outcome assessments in terms of the Oswestry Disability Index (ODI) and Visual Analog Scale (VAS) for the back and leg were evaluated in all patients before the operation, 3 months after the operation, and at the last follow‐up. X‐ray and three‐dimensional CT of the lumbar spine were taken preoperatively, postoperatively, and at the final follow‐up. The radiographic assessment on X‐ray included (Fig. 2): segmental angle (SA), the angle between the superior endplate of the upper vertebra and the inferior endplate of the lower vertebra; intervertebral space height (IH), the average value of the anterior, middle, and posterior intervertebral space height. The loss of SA and IH was defined as the d‐value between postoperative and final follow‐up. Subsidence was defined as any loss of IH more than 3 mm. 21 The fusion status was evaluated on three‐dimensional CT at the final follow‐up. The success of fusion was defined when there was continuous contact of trabecular bone between the upper and lower end plates of the fusion segments, mature bony trabeculae bridging the intervertebral space (sentinel sign), 22 cortication at the peripheral edges of the fusion masses, and an absence of identifiable radiographic clefts. 23 , 24 The radiographic assessment on three‐dimensional CT included the cage union ratio, 25 percentage of fusion bone for the end plate of the vertebra that comes in contact with the local bone inserted within the cage (Fig. 3), fusion rate of local bone within the cage, and fusion rate of local bone outside the cage. A total of 60 patients were randomly selected to undergo an evaluation to determine the reliability of imaging measurements. One week after one orthopaedic surgeon (Z.Z.) measured the radiographic parameters for every patient, he repeated the measurements in the 60 randomly selected patients to evaluate intra‐observer reliability. Another orthopaedic surgeon (B.H.) also measured these parameters in the 60 patients to evaluate inter‐observer reliability. The intraclass coefficients (ICCs) of the intra‐observer and inter‐observer reliability were 0.877 and 0.892, respectively.
Fig. 2.
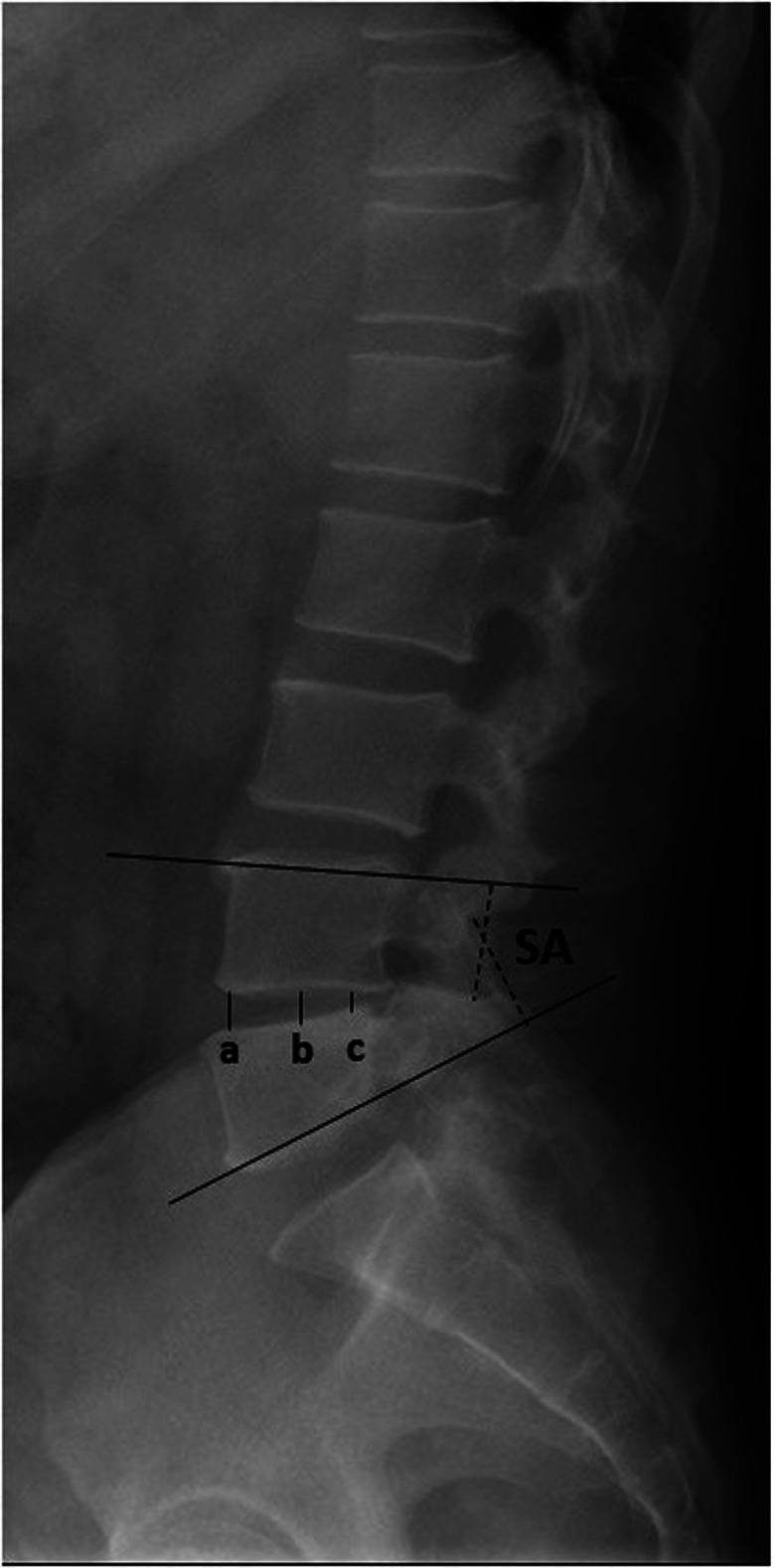
Measurement methods: SA (segmental angle), the angle between the superior endplate of the upper vertebra and the inferior endplate of the lower vertebra; IH (intervertebral space height), the average value of the anterior (a), middle (b), and posterior (c) intervertebral space heights
Fig. 3.
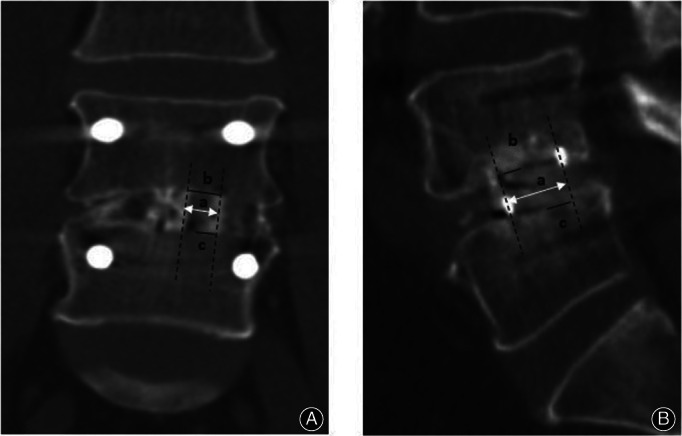
(A) Coronal cage union ratio, percentage of coronal union cage length. Upper, b/a × 100; lower, c/a × 100. (B) Sagittal cage union ratio, percentage of sagittal union cage length. Upper, b/a × 100; lower, c/a × 100
Statistical Analysis
All data were analyzed using SPSS 23.0 software (SPSS, Inc., Chicago, Illinois). Continuous data are presented as the mean ± standard deviation and were analyzed by using independent Student's t‐test. Categorical data were compared by using Pearson's chi‐square test or Fisher's exact test. A p‐value less than 0.05 indicated a significant difference.
Results
The present study enrolled 200 patients (76 males, 124 females) with an average age of 56.2 years (range 30–78 years). The mean duration of follow‐up was 95.4 months (range 86–114 months). The n‐HA/PA66 cage was used in 100 patients (n‐HA/PA66 group), whereas the PEEK cage was used in 100 patients (PEEK group) (Figs. 4 and 5). The two groups were well matched in terms of age, sex, BMI, diagnosis, and fusion level. The demographic data and preoperative clinical characteristics are summarized in Table 1, with no significant differences between the two groups.
Fig. 4.
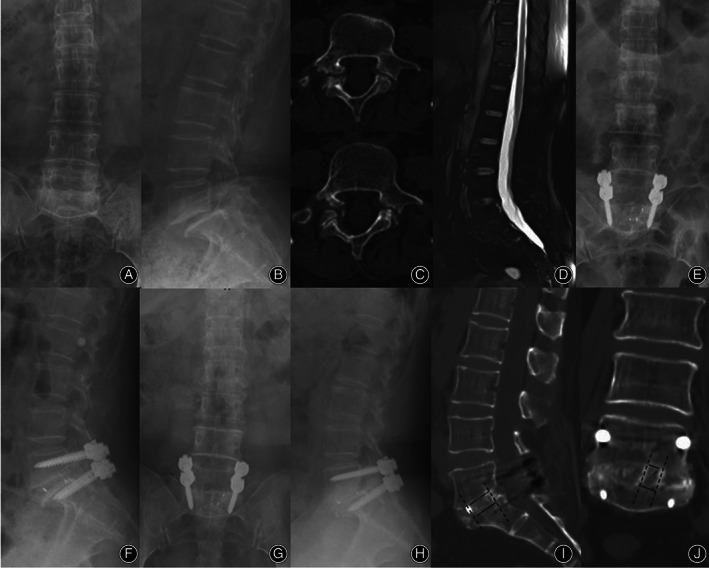
A 58‐year‐old female patient underwent TLIF with an n‐HA/PA66 cage due to L5/S1 isthmic spondylolisthesis. Preoperative lumbar radiographs (A‐D). The postoperative radiographs (E‐F). The final follow‐up radiographs at 92 months after surgery showed that both the autogenous bone granules within and outside the cage achieved satisfactory bony fusion (G‐J). Of note, the surface of the n‐HA/PA66 cage fits closely with the bone tissue and is well integrated with both the upper and lower endplates. (Cage union rate at the upper endplate: coronal = 66.7%, sagittal = 76.9%; at the lower endplate: coronal = 95.8%, sagittal = 92.5%)
Fig. 5.
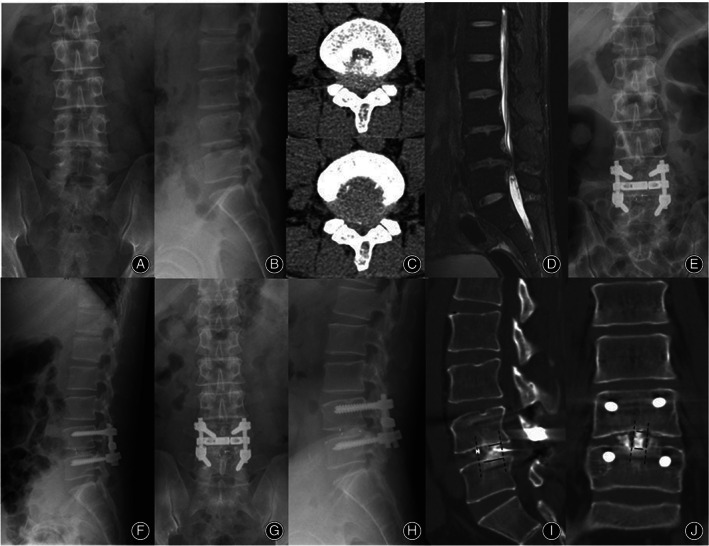
A 43‐year‐old male patient underwent TLIF with a PEEK cage due to spinal stenosis in L4‐5. Preoperative radiographs (A‐D). The postoperative radiographs (E‐F). The X‐rays at 87 months after surgery showed bone union at the L4‐5 level without internal fixation loosening, breakage, or cage subsidence (G, H). The three‐dimensional CT at the final follow‐up indicated that there was still a gap between the autogenous bone granules and the surface of the PEEK cage, and the bone integrated between the material and both the upper and lower endplates was relatively poorer than that in the n‐HA/PA66 cage (I, J). (Cage union rate at the upper endplate: coronal = 49.2%, sagittal = 51.2%; at the lower endplate: coronal = 68.2%, sagittal = 74.3%)
TABLE 1.
Patient demographics and surgical details between the two groups>
| Parameters | n‐HA/PA66 cage (n = 100) | PEEK cage (n = 100) | t / X 2 | p value |
|---|---|---|---|---|
| Age (years) | 56.5 ± 11.2 | 56.0 ± 11.9 | −0.432 | 0.811 |
| Sex (n) | 0.285 | 0.771 | ||
| Male | 37 | 39 | ||
| Female | 63 | 61 | ||
| BMI (kg/m2) | 24.3 ± 3.0 | 24.3 ± 3.3 | 0.032 | 0.975 |
| Diabetes (n) | 9 | 11 | 0.222 | 0.637 |
| Smoking (n) | 12 | 14 | 0.177 | 0.674 |
| T‐score for BMD | −0.5 ± 1.2 | −0.3 ± 1.5 | −0.345 | 0.618 |
| Diagnosis (n) | 0.870 | 0.886 | ||
| Spinal stenosis | 38 | 40 | ||
| Recurrent lumbar disc herniation | 4 | 2 | ||
| Degenerative spondylolisthesis | 33 | 35 | ||
| Isthmic spondylolisthesis | 25 | 23 | ||
| Surgical level (n) | 0.025 | 0.876 | ||
| One‐level | 28 | 29 | ||
| Two‐level | 72 | 71 | ||
| Operative time (min) | 198.5 ± 37.2 | 187.0 ± 34.6 | 0.457 | 0.562 |
| Intraoperative blood loss (ml) | 385.2 ± 110.5 | 381.7 ± 126.3 | −0.311 | 0.650 |
| Follow‐up time (moths) | 96.3 ± 11.8 | 94.5 ± 8.7 | 0.829 | 0.315 |
Abbreviations: BMD, bone mineral density; BMI, body mass index.
Radiographic Outcomes
The IH significantly increased after surgery in both groups. In the n‐HA/PA 66 group, the average IH increased from 9.1 ± 2.7 mm preoperatively to 12.7 ± 2.2 mm postoperatively and slightly decreased at the final follow‐up. In the PEEK group, it increased from 8.7 ± 2.8 mm before surgery to 12.3 ± 2.5 mm postoperatively and slightly decreased at the final follow‐up. The mean corrections of IH were 3.7 ± 2.2 mm and 3.5 ± 1.8 mm in the n‐HA/PA 66 group and the PEEK group, respectively, and the mean losses of IH were 1.3 ± 1.3 mm and 1.5 ± 1.4 mm, respectively. There were no significant differences between the two groups for these parameters at any time point (Table 2). The SA also did not differ significantly between the two groups. At the final follow‐up, the fusion rates of local bone inside and outside the cage were 95.3% and 92.4% in the n‐HA/PA66 group and 91.8% and 90.1% in the PEEK group, respectively. The cage subsidence rate at the final follow‐up was 10.5% (18/172 cages) in the n‐HA/PA66 group and 17.5% (30/171 cages) in the PEEK group. No significant difference was observed in the fusion rate or subsidence rate between the two groups (Table 2). The mean cage union ratios exposed to the upper and lower endplates of the n‐HA/PA66 group were significantly larger than those of the PEEK group (79.2 ± 8.7% vs 65.9 ± 12.6%, p = 0.000; 88.0 ± 10.6% vs 72.6 ± 11.7%, p = 0.000) by sagittal three‐dimensional CT. A similar situation was also observed by coronal three‐dimensional CT (Table 3).
TABLE 2.
Radiographic and fusion state between the two groups
| Parameters | n‐HA/PA66 group | PEEK group | t / X 2 | p value |
|---|---|---|---|---|
| Fusion rate (%) | ||||
| Local bone inside the cage | 95.3 (164/172) | 91.8 (157/171) | 1.786 | 0.181 |
| Local bone outside the cage | 92.4 (159/172) | 90.1 (154/171) | 0.610 | 0.435 |
| IH (mm) | ||||
| Preoperative | 9.1 ± 2.7 | 8.7 ± 2.8 | 0.591 | 0.556 |
| Postoperative | 12.7 ± 2.2* | 12.3 ± 2.5* | 1.016 | 0.312 |
| Final follow‐up | 11.4 ± 2.1*, # | 10.8 ± 2.3* , # | 1.584 | 0.116 |
| Correction | 3.7 ± 2.2 | 3.5 ± 1.8 | 0.358 | 0.721 |
| Loss | 1.3 ± 1.3 | 1.5 ± 1.4 | −0.791 | 0.431 |
| Final subsidence rate (%) | 10.5 (18/172) | 17.5 (30/171) | 3.570 | 0.059 |
| SA (°) | ||||
| Preoperative | 15.3 ± 8.0 | 15.4 ± 8.0 | −0.070 | 0.944 |
| Postoperative | 17.9 ± 8.5 | 17.7 ± 7.4 | 0.158 | 0.875 |
| Final follow‐up | 17.6 ± 8.2 | 17.3 ± 7.3 | 0.224 | 0.823 |
| Correction | 2.6 ± 3.6 | 2.3 ± 4.3 | 0.470 | 0.639 |
| Loss | −0.3 ± 1.5 | −0.4 ± 3.1 | 0.205 | 0.838 |
Abbreviations: IH, intervertebral space height; SA, segmental angle
p < 0.05 compared with post‐operation
p < 0.05 compared with pre‐operation.
TABLE 3.
Comparison of cage union rate at the final follow‐up between the two groups
| Parameters | n‐HA/PA66 group | PEEK group | t / X 2 | p value |
|---|---|---|---|---|
| Coronal | ||||
| Upper endplate | 74.6 ± 12.2 | 66.2 ± 10.8 | 4.282 | 0.000* |
| Lower endplate | 75.6 ± 14.0 | 71.6 ± 8.8 | 2.518 | 0.027* |
| Sagittal | ||||
| Upper endplate | 79.2 ± 8.7 | 65.9 ± 12.6 | 6.024 | 0.000* |
| Lower endplate | 88.0 ± 10.6 | 72.6 ± 11.7 | 5.112 | 0.000* |
Statistical significance between two groups (p < 0.05).
Clinical Outcomes
The ODI and VAS scores significantly improved in both groups after surgery, and the effect was maintained well until the final follow‐up. There was no significant difference in ODI or VAS score between the n‐HA/PA66 group and the PEEK group at any time point (Table 4).
TABLE 4.
Clinical outcomes between the two groups
| Parameters | n‐HA/PA66 group | PEEK group | t / X 2 | p value |
|---|---|---|---|---|
| VAS back | ||||
| Preoperative | 6.7 ± 1.5 | 6.2 ± 1.8 | −0.648 | 0.485 |
| 3‐month postoperative | 3.4 ± 1.1* | 3.1 ± 1.6* | 0.311 | 0.672 |
| Final follow‐up | 1.9 ± 0.8* , # | 2.2 ± 0.6* , # | 0.215 | 0.836 |
| VAS leg | ||||
| Preoperative | 6.3 ± 1.3 | 6.8 ± 1.7 | 0.215 | 0.611 |
| 3‐month postoperative | 2.1 ± 0.8* | 2.4 ± 1.1* | −0.871 | 0.357 |
| Final follow‐up | 2.0 ± 0.9* | 1.8 ± 1.0* | 0.457 | 0.581 |
| ODI scores | ||||
| Preoperative | 53.5 ± 7.8 | 50.8 ± 6.5 | −0.225 | 0.637 |
| 3‐month postoperative | 32.7 ± 10.2* | 35.1 ± 13.8* | 0.715 | 0.471 |
| Final follow‐up | 14.6 ± 5.1* , # | 15.2 ± 4.8* , # | 0.302 | 0.632 |
Abbreviations: ODI, Oswestry Disability Index; VAS, Visual Analog Scale
p < 0.05 compared with 3‐month post‐operation
p < 0.05 compared with pre‐operation.
Complications
No cage migration or breakage occurred in either group at the final follow‐up. For the n‐HA/PA66 group, one patient presented with leakage of cerebrospinal fluid (CSF) and recovered after prolonged application of pressured drainage postoperatively. There was one patient with nerve root injury who completely recovered with proper exercise and oral neurotrophic drugs within 1 year after surgery. For the PEEK group, there was one patient with CSF leakage, one patient with wound infection, and one patient with nerve root injury (Table 5). All cases resolved completely by conservative treatment.
TABLE 5.
Complications and re‐admissions between the two groups
| Parameters | n‐HA/PA66 group | PEEK group | t/X 2 | p value |
|---|---|---|---|---|
| Perioperatively | ||||
| CSF leakage | 1 | 1 | ||
| Wound infection | 0 | 1 | ||
| Nerve root injury | 1 | 1 | ||
| Epidural hematoma | 0 | 0 | ||
| Implant‐related | ||||
| Screw loosening | 0 | 0 | ||
| Screw broken | 1 | 2 | ||
| Cage retropulsion/migration | 0 | 0 | ||
| Re‐admission | 0 | 0 | ||
| Total | 3 | 5 | 0.521 | 0.721 |
Abbreviation: CSF, cerebrospinal fluid.
Discussion
PEEK has become one of the most common materials used as interbody cages in lumbar fusion and has shown excellent clinical outcomes. 10 , 11 , 12 Nevertheless, one main shortcoming of the PEEK cage is its relatively low osseointegration capacity due to its surface bioinert property, which might lead to some complications, such as nonunion, subsidence, and migration of the cage. 5 , 7 , 13 As a bioactive material, the n‐HA/PA66 cage showed better biocompatibility and osteoconductive qualities. 26 , 27 It has been widely applied in the treatment of LDD during the past decade. In the present study, we retrospectively reviewed 100 patients who underwent TLIF using n‐HA/PA66 cage and matched with 100 patients using PEEK cage. Through the 7‐year follow‐up, we found that the two cages all could achieve good long‐term radiographic and clinical outcomes in TLIF. Moreover, as a bioactive material, n‐HA/PA66 cage indeed showed some advantages in lumbar fusion.
Better Fusion State in the n‐HA/PA66 Cage
In this study, both groups showed satisfactory fusion rates at the final follow‐up without any significant difference, which was similar to a previous study. 15 , 16 , 17 , 19 However, the traditional method for determining the success of fusion was observing mature bony trabecula bridging the interbody space (sentinel sign) and an absence of identifiable radiographic clefts. 22 , 23 , 24 Of note, the union ratio between the cage and endplate is important for evaluating the solidity of fusion, as well as for evaluating fusion itself. 25 In addition, the fusion segment becomes more stable with increasing fusion area, which is mechanically beneficial to load transmission, even with the segment determined to have fused. 28 The traditional method can only qualitatively analyze whether fusion is achieved but cannot quantitatively evaluate the above index. To assess the fusion state of n‐HA/PA66 and PEEK more precisely, we adopted the qualitative analysis method described by Lee et al. 25 At the final follow‐up, we found that the cage union ratios in both the upper and lower endplates of the n‐HA/PA66 group were significantly larger than those of the PEEK group, indicating that the former exhibited a better fusion state. Previous animal experiments have demonstrated that the n‐HA/PA66 cage can release Ca2+ and PO4 3− from its surface, which gradually forms a crystal layer on the cage surface that bridges the graft and implant bed for osteogenesis. 29 , 30 , 31 In addition, it is also an ideal three‐dimensional microstructure material for sufficient proliferation of rat bone marrow mesenchymal stem cells, and the dynamic perfusion culture condition can significantly improve osteogenic effectiveness. 30 In contrast, the PEEK cage is a bioinert cylinder without osteo conduction or osteoinduction. This might explain why the n‐HA/PA66 group showed a significantly larger ratio directly fused to the endplate than the PEEK group at the final follow‐up.
Subsidence Rates of the Two Different Cages
Cage subsidence is a common complication after TLIF and is associated with fusion failures and poor clinical outcomes. 4 It is influenced by the lower fused segment, amount of morselized bone, number of fused segments, endplate manipulation, cage size, cage position, and the material characteristics of the cage. 5 , 6 , 32 Both n‐HA/PA66 and PEEK cages have a low Young's modulus that is similar to natural bone, resulting in lower stress shielding. 33 , 34 , 35 Furthermore, the n‐HA/PA66 cage shape is characterized by a wide rim with several shallow recesses, and the PEEK cage also has a high and regular jag. These unique shapes could significantly increase the friction and footprint area, which dispersed the pressure on the cage surface. Thus, diverse previous studies have reported that these two kinds of cages are superior to titanium mesh cages in reducing the subsidence rate after cervical reconstruction. 15 , 33 , 34 , 36 , 37 In this study, satisfactory subsidence rates were achieved in both groups through long‐term follow‐up. Of note, although there was no significant difference, a slightly lower cage subsidence rate was observed in the n‐HA/PA66 group (10.5% vs 17.5%, p = 0.059). Kumar et al. 38 reported that a larger area of contact between the cage surface and endplate could produce a lower stress distribution. Although the footprint area is an important factor of subsidence in the early stage of fusion, successful bony fusion and the fused area inside cages are more important in terms of the final subsidence rate. 25 , 38 Therefore, the relatively larger cage union ratio in the n‐HA/PA66 group might be the reason for the slight difference in the cage subsidence rates between the two groups at the final follow‐up.
Long‐Term Outcomes of the Two Different Cages
The long‐term radiographic and clinical outcomes were satisfactory in both the n‐HA/PA66 and PEEK groups. There were no significant differences in IH or SA at preoperative, postoperative, or final follow‐up between the two groups. The mean magnitudes of loss in IH of the operative segment were also similar (n‐HA/PA66 group vs PEEK group = 1.3 mm vs 1.5 mm, p = 0.431). Although both groups showed slight loss of IH during follow‐up, the VAS and ODI scores were obviously improved after surgery, and this effect was maintained well at the final follow‐up. Thus, the above long‐term follow‐up data suggest that n‐HA/PA66 cages are comparable with PEEK cages as ideal implants for application in TLIF.
Strengths and Limitations
The present study firstly reported the long‐term follow‐up outcomes of the two cages used in TLIF through a relatively large sample size. Furthermore, we used cage union ratio to evaluate the fusion state on three‐dimensional CT, and found that bioactive n‐HA/PA66 cage had better fusion effect than the PEEK cage. However, several limitations still exist in our study. First, it was a retrospective study from a single center. Prospective studies are necessary to confirm the present findings. Second, the choice of the two different cages was not randomized, and the final results might be influenced by surgeon‐related factors to a certain degree.
Conclusion
This retrospective and matched‐pair study demonstrated that both n‐HA/PA66 and PEEK cages can achieve high fusion and low subsidence rates in TLIF, with an average 7‐year follow‐up. As a bioactive material, the n‐HA/PA66 group showed significantly larger cage union ratios than the PEEK group. And the use of both cages can obtain satisfactory long‐term clinical outcomes. Therefore, our results indicated that the n‐HA/PA66 cage is an ideal alternative material comparable to the PEEK cage in TLIF.
Authorship declaration
All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript.
Author's Contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Z.Z. and S.Y. Acquisition of data: Z.Z., B.H., L.W., and H.Y. Analysis and interpretation of the data: Z.Z. and B.H. Drafting of the manuscript: Z.Z., T.L., and L.W. Critical revision of the manuscript for important intellectual content: L.L., Y.S., and X.Y. Statistical analysis: Z.Z. and H.Y. Obtained funding: B.H., H.Y., and Y.S. Study supervision: Y.S., and X.Y.
Conflicts of Interest
The authors have no relevant financial or non‐financial interests to disclose.
Ethical Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the West China hospital (No. 2019–654). Written informed consent was obtained from the parents.
Acknowledgments
We wish to thank all patients who generously agreed to be interviewed for this study. This study was supported by China Postdoctoral Science Foundation General Program (2021M702337); the 1‐3‐5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD21001); the National Natural Science Foundation of China (82072386 and 82102521); the Science and Technology Project of the Health Planning Committee of Sichuan (21PJYY0810); and Chengdu Science and Technology Project (2021‐YF05‐00743‐SN).
First Author: Zhuang Zhang and Bo‐wen Hu contributed equally to this study.
Yue‐ming Song and Xi Yang contributed equally to this study and should be considered co‐corresponding authors.
References
- 1. Harms J, Rolinger H. A one‐stager procedure in operative treatment of spondylolistheses: dorsal traction‐reposition and anterior fusion (author's transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343–7. [DOI] [PubMed] [Google Scholar]
- 2. Kurra S, Lavelle WF, Silverstein MP, Savage JW, Orr RD. Long‐term outcomes of transforaminal lumbar interbody fusion in patients with spinal stenosis and degenerative scoliosis. Spine J. 2018;18(6):1014–21. [DOI] [PubMed] [Google Scholar]
- 3. McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81(6):859–80. [DOI] [PubMed] [Google Scholar]
- 4. Rihn JA, Patel R, Makda J, Hong J, Anderson DG, Vaccaro AR, et al. Complications associated with single‐level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623–9. [DOI] [PubMed] [Google Scholar]
- 5. Le TV, Baaj AA, Dakwar E, Burkett CJ, Murray G, Smith DA, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976). 2012;37(14):1268–73. [DOI] [PubMed] [Google Scholar]
- 6. Kim MC, Chung HT, Cho JL, Kim DJ, Chung NS. Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2013;26(2):87–92. [DOI] [PubMed] [Google Scholar]
- 7. Nemoto O, Asazuma T, Yato Y, Imabayashi H, Yasuoka H, Fujikawa A. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation. Eur Spine J. 2014;23(10):2150–5. [DOI] [PubMed] [Google Scholar]
- 8. Bartels RH, Donk RD, Feuth T. Subsidence of stand‐alone cervical carbon fiber cages. Neurosurgery. 2006;58(3):502–8. [DOI] [PubMed] [Google Scholar]
- 9. Lee JH, Kim SK, Kang SS, Han SJ, Lee CK, Chang BS. A long‐term follow‐up, multicenter, comparative study of the radiologic, and clinical results between a CaO‐SiO2‐P2O5‐B2O3 bioactive glass ceramics (BGS‐7) intervertebral spacer and titanium cage in 1‐level posterior lumbar Interbody fusion. Clin Spine Surg. 2020;33(7):E322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Massaad E, Fatima N, Kiapour A, Hadzipasic M, Shankar GM, Shin JH. Polyetheretherketone versus titanium cages for posterior lumbar Interbody fusion: meta‐analysis and review of the literature. Neurospine. 2020;17(2):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seaman S, Kerezoudis P, Bydon M, Torner JC, Hitchon PW. Titanium vs. polyetheretherketone (PEEK) interbody fusion: meta‐analysis and review of the literature. J Clin Neurosci. 2017;44:23–9. [DOI] [PubMed] [Google Scholar]
- 12. Rousseau MA, Lazennec JY, Saillant G. Circumferential arthrodesis using PEEK cages at the lumbar spine. J Spinal Disord Tech. 2007;20(4):278–81. [DOI] [PubMed] [Google Scholar]
- 13. Phan K, Hogan JA, Assem Y, Mobbs RJ. PEEK‐halo effect in interbody fusion. J Clin Neurosci. 2016;24:138–40. [DOI] [PubMed] [Google Scholar]
- 14. Huang M, Feng J, Wang J, Zhang X, Li Y, Yan Y. Synthesis and characterization of nano‐HA/PA66 composites. J Mater Sci Mater Med. 2003;14(7):655–60. [DOI] [PubMed] [Google Scholar]
- 15. Hu B, Wang L, Song Y, Hu Y, Lyu Q, Liu L, et al. A comparison of long‐term outcomes of nanohydroxyapatite/polyamide‐66 cage and titanium mesh cage in anterior cervical corpectomy and fusion: a clinical follow‐up study of least 8 years. Clin Neurol Neurosurg. 2019;176:25–9. [DOI] [PubMed] [Google Scholar]
- 16. Hu B, Yang X, Hu Y, Lyu Q, Liu L, Zhu C, et al. The n‐HA/PA66 cage versus the PEEK cage in anterior cervical fusion with single‐level discectomy during 7 years of follow‐up. World Neurosurg. 2019;123:e678–84. [DOI] [PubMed] [Google Scholar]
- 17. Zhong W, Liang X, Luo X, Quan Z, Jiang D. Imaging evaluation of nano‐hydroxyapatite/polyamide 66 strut in cervical construction after 1‐level corpectomy: a retrospective study of 520 patients. Eur J Med Res. 2020;25(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang X, Song Y, Liu L, Liu H, Zeng J, Pei F. Anterior reconstruction with nano‐hydroxyapatite/polyamide‐66 cage after thoracic and lumbar corpectomy. Orthopedics. 2012;35(1):e66–73. [DOI] [PubMed] [Google Scholar]
- 19. Deng QX, Ou YS, Zhu Y, Zhao ZH, Liu B, Huang Q, et al. Clinical outcomes of two types of cages used in transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases: n‐HA/PA66 cages versus PEEK cages. J Mater Sci Mater Med. 2016;27(6):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garg B, Mehta N. Minimally invasive transforaminal lumbar interbody fusion (MI‐TLIF): a review of indications, technique, results and complications. J Clin Orthop Trauma. 2019;10(Suppl 1):S156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu C, He M, Mao L, Yang H, Hu B, Zhang L, et al. Titanium interlayer‐mediated hydroxyapatite‐coated polyetheretherketone cage in transforaminal lumbar interbody fusion surgery. BMC Musculoskelet Disord. 2021;22(1):918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McAfee PC, Regan JJ, Geis WP, Fedder IL. Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine (Phila Pa 1976). 1998;23(13):1476–84. [DOI] [PubMed] [Google Scholar]
- 23. Lee JH, Lee JH, Park JW, Lee HS. Fusion rates of a morselized local bone graft in polyetheretherketone cages in posterior lumbar interbody fusion by quantitative analysis using consecutive three‐dimensional computed tomography scans. Spine J. 2011;11(7):647–53. [DOI] [PubMed] [Google Scholar]
- 24. Boden SD, Grob D, Damien C. Ne‐Osteo bone growth factor for posterolateral lumbar spine fusion: results from a nonhuman primate study and a prospective human clinical pilot study. Spine (Phila Pa 1976). 2004;29(5):504–14. [DOI] [PubMed] [Google Scholar]
- 25. Lee JH, Jeon DW, Lee SJ, Chang BS, Lee CK. Fusion rates and subsidence of morselized local bone grafted in titanium cages in posterior lumbar interbody fusion using quantitative three‐dimensional computed tomography scans. Spine (Phila Pa 1976). 2010;35(15):1460–5. [DOI] [PubMed] [Google Scholar]
- 26. Huang D, Zuo Y, Li J, Zou Q, Zhang L, Gong M, et al. Bioactive composite gradient coatings of nano‐hydroxyapatite/polyamide66 fabricated on polyamide66 substrates. J R Soc Interface. 2012;9(72):1450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano‐hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28(22):3338–48. [DOI] [PubMed] [Google Scholar]
- 28. Closkey RF, Parsons JR, Lee CK, Blacksin MF, Zimmerman MC. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine (Phila Pa 1976). 1993;18(8):1011–5. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Li Y, Wei J, de Groot K. Development of biomimetic nano‐hydroxyapatite/poly(hexamethylene adipamide) composites. Biomaterials. 2002;23(24):4787–91. [DOI] [PubMed] [Google Scholar]
- 30. Qian X, Yuan F, Zhimin Z, Anchun M. Dynamic perfusion bioreactor system for 3D culture of rat bone marrow mesenchymal stem cells on nanohydroxyapatite/polyamide 66 scaffold in vitro. J Biomed Mater Res B Appl Biomater. 2013;101(6):893–901. [DOI] [PubMed] [Google Scholar]
- 31. Xu Q, Lu H, Zhang J, Lu G, Deng Z, Mo A. Tissue engineering scaffold material of porous nanohydroxyapatite/polyamide 66. Int J Nanomedicine. 2010;5:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stein IC, Than KD, Chen KS, Wang AC, Park P. Failure of a polyether‐ether‐ketone expandable interbody cage following transforaminal lumbar interbody fusion. Eur Spine J. 2015;24(Suppl 4):S555–9. [DOI] [PubMed] [Google Scholar]
- 33. Yang X, Chen Q, Liu L, Song Y, Kong Q, Zeng J, et al. Comparison of anterior cervical fusion by titanium mesh cage versus nano‐hydroxyapatite/polyamide cage following single‐level corpectomy. Int Orthop. 2013;37(12):2421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Quan Z, Zhao Z, Luo X, Tang K, Li J, et al. Evaluation of anterior cervical reconstruction with titanium mesh cages versus nano‐hydroxyapatite/polyamide66 cages after 1‐ or 2‐level corpectomy for multilevel cervical spondylotic myelopathy: a retrospective study of 117 patients. PLoS One. 2014;9(5):e96265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kersten RF, van Gaalen SM, de Gast A, Oner FC. Polyetheretherketone (PEEK) cages in cervical applications: a systematic review. Spine J. 2015;15(6):1446–60. [DOI] [PubMed] [Google Scholar]
- 36. Cabraja M, Oezdemir S, Koeppen D, Kroppenstedt S. Anterior cervical discectomy and fusion: comparison of titanium and polyetheretherketone cages. BMC Musculoskelet Disord. 2012;13:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Y, Wang X, Lu X, Yang L, Yang H, Yuan W, et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7‐year follow‐up. Eur Spine J. 2013;22(7):1539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar N, Judith MR, Kumar A, Mishra V, Robert MC. Analysis of stress distribution in lumbar interbody fusion. Spine (Phila Pa 1976). 2005;30(15):1731–5. [DOI] [PubMed] [Google Scholar]


