Abstract
Spinal cord injury (SCI) remains a life-altering event that devastates those injured and the families that support them. Numerous laboratories are engaged in preclinical and clinical trials to repair the injured spinal cord with stem cell–derived therapeutics. A new developmental paradigm reveals early bifurcation of brain or trunk neurons in mammals via neuromesodermal progenitors (NMPs) relevant to therapies requiring homotypic spinal cord neural populations. Human-induced pluripotent stem cell (hiPSC) NMP-derived spinal motor neurons generated ex vivo following this natural developmental route demonstrate robust survival in vivo when delivered as suspension grafts or as in vitro preformed encapsulated neuronal circuitry when transplanted into a rat C4-C5 hemicontusion injury site. Use of in vitro matured neurons avoids in vivo differentiation challenges of using pluripotent hiPSC or multipotent neural stem cell (NSC) or mesenchymal stem cell therapeutics. In this review, we provide an injury to therapeutics overview focusing on how stem cell and developmental fields are merging to generate exquisitely matched spinal motor neurons for SCI therapeutic studies. The complexity of the SCI microenvironment generated by trauma to neurons and vasculature, along with infiltrating inflammatory cells and scarring, underlies the challenging cytokine microenvironment that therapeutic cells encounter. An overview of evolving but limited stem cell–based SCI therapies that have progressed from preclinical to clinical trials illustrates the challenges and need for additional stem cell–based therapeutic approaches. The focus here on neurons describes how NMP-based neurotechnologies are advancing parallel strategies such as transplantation of preformed neuronal circuitry as well as human in vitro gastruloid multicellular models of trunk central and peripheral nervous system integration with organs. NMP-derived neurons are expected to be powerful drivers of the next generation of SCI therapeutics and integrate well with combination therapies that may utilize alternate biomimetic scaffolds for bridging injuries or flexible biodegradable electronics for electrostimulation.
Keywords: Neuromesodermal progenitor, human-induced pluripotent stem cell spinal motor neuron subtype, gastruloid, clinical trial, bioengineering, microenvironment
Impact Statement
The ability to generate stem cell–derived neurons for spinal cord injury (SCI) therapeutics, which survive and functionally integrate when transplanted, brings neuronal circuitry restoration strategies to the forefront. Challenges persist with the current use of multipotent progenitor cells for complete and sufficient differentiation to neurons, exacerbated by a cytokine complex SCI microenvironment. A new developmental paradigm bifurcates distinct brain or spinal neuron populations and is being implemented with hiPSCs to generate spinal motor neurons as well as gastruloid models of human central nervous system (CNS)–peripheral nervous system (PNS) development in the trunk and spine. These insights are coupled with detailed molecular signatures of neurons to ensure anatomical and regional matching of therapeutic cells for SCI. The next generation of SCI cell therapies will apply the right spinal motor neuron subtype and advanced delivery in innovative platforms, including preformed transplantable neuronal circuitry, that can be combined with electrostimulation to accelerate functional recovery outcomes for SCI patients.
Introduction
Spinal cord injury (SCI) is one of the most debilitating unresolved neurological pathologies that destroys the quality of life by temporal or permanent motor, sensory, and autonomic dysfunction and resulting in a two-to-five-fold increase in premature death. 1 The World Health Organization estimates between 250,000 and 500,000 people worldwide are living with SCI, impacting primarily two age groups, 15–29 years old or 50 years and above. 2 SCIs are complex in pathology and frequent injuries seen in the civilian population versus military may differ, with the latter including high-energy blast mechanisms that generate a broader range of injuries and poorer neurologic recovery. 3 Broadly stated, SCIs are either complete, in which no significant spared motor or sensory function remains below the injury site or incomplete, in which partial severing retains some level of signaling through the injury SCI. 4 The heterogeneous nature of SCI, inability of intrinsic neural regeneration alone to provide recovery, and rapid progression from an acute to a chronic and more refractive injury microenvironment are driving a range of innovative-targeted therapeutic strategies. Of significant interest are efforts to engineer replacement cell therapies using stem cell neurotechnology. Here, we describe advances with the generation and transplantation of anatomical region–specific spinal motor neurons and support cells as preformed neuronal circuitry, currently being done in animal models of SCI. We highlight the next generation–associated technologies that will broaden the ability to treat a larger variety of SCIs, including more complex injuries, and discuss the importance of standardization of treatments to allow consensus.
Complexity of the SCI microenvironment in defining treatments
Insight into the pathophysiology of SCI
Along with initial physical trauma that results in the primary mechanical damage to the spine are compounding secondary changes, including vascular injury and biochemical changes that can lead to scarring in chronic stages. Changes to anatomical integrity and continuity of the spinal cord drive the biphasic response that results from the primary injury as well as force type and direction. This includes (1) impacts with persistent compression seen with burst fractures, fracture-dislocations, and acute disk ruptures; (2) transient compression injuries, such as hyperextension; (3) distraction by forcible stretching of spinal column in the axial plane; and (4) laceration/transection injuries such as due to missiles, sharp bone fragment dislocation, or severe distraction. 5 Loss of function occurs upon direct damage to ascending and descending neurons in neural circuitry pathways as well as neuronal death accelerated by microvascular disruption of blood vessels and the blood–brain barrier (BBB) generating edema, ionic imbalance, and changes to energy metabolism mechanisms for neurons. The narrowing of spared blood vessels, as vasospasm, along with local swelling, edema, both lead to ischemia. These events contribute to initial loss of sensation and motor paralysis in spinal shock as well as systemic pendulum effects in blood pressure, including systemic hypotension. 6 Demyelination of neuronal axons during mechanical compression, neuronal apoptosis, and the continued breakdown of myelin, and astrocytic responses further engage inflammatory responses during SCI. 7
Loss of neurons and support cells in acute stages of cell death triggers the release of cell- and blood-derived damage-associated molecular pattern, DAMP, molecules that include at least 35 factors related to extracellular matrix (ECM) and membrane proteins as well as intracellular molecules of nucleic acids, histones, mitochondrial DNA and reactive oxygen species, heat shock proteins, and more. DAMPs are part of the innate immune response and activate purinergic receptors to induce microglial chemotaxis to the damage site. Additional contributing roles of oxidative stress, derangements in ionic homeostasis, neurotransmitter accumulation, plasma membrane compromise (increased permeability), lipid peroxidation, and necrotic cell death exacerbate the injury. Progression from acute into the subacute phase is evident by the Wallerian degeneration that triggers gliosis and neuroinflammation, along with apoptosis, further demyelination of surviving axons, axonal dieback, and ECM remodeling. A reactive response to increasing levels of adenosine triphosphate (ATP) also occurs and results in increased levels of oligodendrocyte precursor cells (OPCs), oligodendrocytes, microglia, and astrocytes. The proliferation of reactive astrocytes during gliosis generates increased secretion of matrix chondroitin-sulfate proteoglycans, CSPGs, which remodel the injury microenvironment and interfere with neuronal plasticity.7,8 These temporal cascades of events during secondary stages of injury drive the pathophysiological response that is acute, immediate over seconds to minutes, subacute that occurs over minutes to weeks, or chronic that evolves over months to years. 9 The complexity of SCIs suggests that a balance of critical factors may be needed to switch reactionary responses away from neuroprotective barriers toward one primed to favor neural circuitry regeneration.
The SCI microenvironment and roles of astrocytes, inflammation, and scarring
Astrocytes play a critical role in neuronal metabolism 10 and synaptic function as part of a “tripartite synapse” 11 in a non-injury environment, but within 1 h of focal mechanical trauma, reactive astrogliosis occurs as an immediate response to injury. 12 During this acute phase of SCI, astrocyte cell processes elongate, evident by an increase in glial fibrillary acidic protein (GFAP) intermediate filament protein and surround clusters of fibrotic and infiltrating inflammatory cells to allow tissue repair and functional improvement. As reactive astrocytes entangle with pericytes and tightly interweave, an inhibitory mesh-like scar array is created. Astrocytic glial scarring, while neuroprotective, interferes with axonal regeneration and contributes to a chronic state of SCI. 13
In SCI, inflammation provides phagocytic clearance of cellular debris but may also contribute to peripheral damage that spreads to the surrounding tissue and interferes with neural regeneration. The inflammatory response is initiated by peripherally derived immune cells, that includes macrophages, neutrophils, and T-cells along with activated glia, both astrocytes undergoing astrogliosis and microglia. The infiltration of leukocytes and activation of microglia and astrocytes can further contribute to tissue damage through released proteases, reactive oxygen intermediates, lysosomal enzymes, proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, IL-6 and IL-10, and chemokines.14,15 This detrimental–beneficial duality is the result of the capacity of immune cells to be polarized with pro- or anti-inflammatory functions at different stages and distances from the injury. 16 Microglia are highly dynamic and proliferate during the first week following SCI to form a dense cellular interface between reactive astrocytes and monocyte-derived macrophages. 17 Upon SCI, inflammatory microglia transform into a reactive M1 type, that exacerbates neuroinflammation, and M2 type that promotes tissue repair and exerts anti-inflammatory effects. The ratio of M1 or M2 types directs SCI response and shortly after injury, M1 microglial predominates and M2 microglia does not persist as long. 18 The microglial scar component regulates inflammation versus functional recovery, but M1-type microglia are associated with chronic neuroinflammation following SCI. The M1 microglia polarization induces astrocytes to increase CSPG secretion and local deposition. 19 These growth-inhibitory ECM glycoproteins include neurocan, versican, brevican, phosphacan, and NG2. CSPGs become a chemo-physical barrier and contribute to the failure of SCI regeneration by attenuating axon growth cone activity and preventing oligodendrocyte progenitor cell maturation into functionally mature oligodendrocytes capable of remyelination. 20 The CSPG inhibition of neurite outgrowth acts via the Rho/Rho-associated protein kinase (ROCK) signaling pathway. 21 The microglia enhancement of glial scars is hypothesized to be related to a failed switch from M1 back to M2 type. 22 Resolving scar tissue barriers requires understanding the intricate mixture of cell types that include astrocytic, fibrotic, and microglial cells.
Scarring separates healthy tissue from necrotic tissue and prevents non-CNS cells from invading the CNS parenchyma and includes astrocytic and microglia cell types along with changes to ECM as well as fibrotic scarring. 23 The fibrotic scar provides the necessary initial support structure to allow the injured area to maintain tissue integrity and forms adjacent to the medial side of a forming astrocytic scar. However, the persistence of fibrotic cells interferes with a return to normal tissue structure and hinders axonal regeneration and functional recovery, including excess deposition of ECM molecules with inhibitive effects. The dense ECM that constitutes the fibrotic scar is composed of fibronectin, collagen, and laminin. Microvascular CD13 positive endothelial cells, which aid in myelin debris engulfment, and platelet-derived growth factor receptor beta (PDGFRβ)-positive pericytes have been suggested to promote fibrotic scar formation.23,24 The gradual proliferation and migration of fibroblasts also contributes to encapsulation of macrophages in the injured core. 25
Therapeutic approaches to improve SCI recovery must target a diverse set of conditions and cell types over a temporal cascade (Figure 1) and will require combined strategies to bring flexibility needed to treat the complex range of SCIs 26 including providing neural cells to replace damaged or lost cells, targeting the injury microenvironment to remove barriers to regeneration and encourage intrinsic regeneration, and integration with additional connectivity assisting strategies such as electrostimulation and exercise.
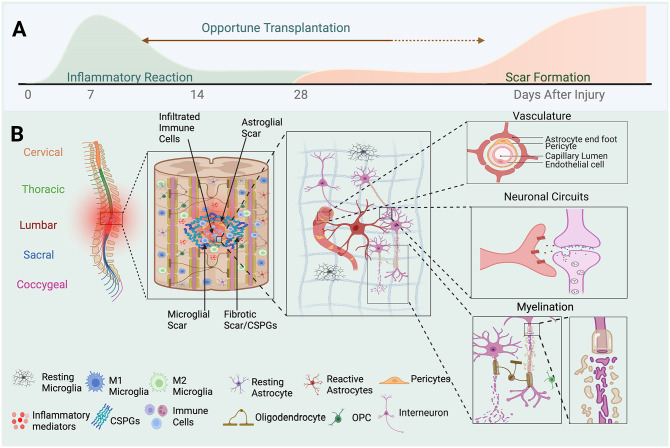
Figure 1.
Complexity of cell types in the SCI microenvironment and injury impact. (A) Timeline of microenvironment pathological changes following SCI. A wave of inflammatory response is followed by decreased inflammation and long-term glial scar formation. Immediate therapeutic intervention into the post-traumatic inflammatory microenvironment is challenging regarding survival of grafted cells and the presence of complex cytokine regulators. Human clinical trials have shown transplantation of cell therapy as early as 10 days. (B) Diagram of the spinal column. Shown in increasing detail are the complexity of the SCI microenvironment, cell types involved, and functional impacts. Perturbations to vascular, neural circuitry and metabolism, and myelination functions are critical to survival and regeneration of neuronal circuits following SCI.
Historical perspective on stem cell–based SCI therapeutics
Transitioning stem cell technologies from models into clinical trials
Rodent models of neuropathologies and SCI have been at the forefront of innovative stem cell therapeutic strategies due to their ability to provide embryonic, developmental, and physiological functional studies that co-inform and set the stage for studies in larger animal models and human clinical trials. Although vastly different in scale, the anatomical, physiological, and genetic similarities of rodents to humans, along with abundant genetic resources, have proven to be invaluable. 27 Studies with mouse embryonic stem cells (mESCs) historically began in 1981, with in vitro cultures from inner cell mass blastocysts.28,29 Human embryonic stem cell (hESC) isolation and characterization came near two decades later 30 following advances in in vitro fertilization and the first human test tube baby in 1978. The breakthrough development of human-induced pluripotent stem cell (hiPSC) technology 31 further extends the capabilities of human stem cell technologies, including greater ethnic population diversity 32 along with the ability to study neurological disorders across developmental and adult stages.
The first clinical trial with pluripotent stem cell–based therapeutics, GRNOPC1 done by Geron, Inc. (Menlo Park, CA, USA) in 2010 evaluated hESC WA09 oligodendrocytes in five patients with SCI; however, financial challenges with frontier therapies halted the trial, revealing challenges to companies and patients.33,34 Previously, mesenchymal stem cells (MSCs) had been used, based on evidence of transdifferentiation into neurons. 35 MSCs for SCI derived from autologous bone marrow met safety considerations in SCI observational studies36 –39 as did additional sourcing of MSCs from adipose tissue40,41 and umbilical cord. 42 Although the results of these studies support the safe use of MSCs without complications over the periods studied, no significant clinical therapeutic potential has yet been demonstrated. Numerous rodent studies with hiPSC-derived NSCs indicate the potential for therapeutic benefits, but concerns remain for reproducibility and incomplete differentiation in preclinical trial studies. 43 Meanwhile hiPSC-based therapeutics were first used in clinical trials in 2013 for macular degeneration, transplanting autologous retinal pigment epithelial cell sheets with improvement to vision. 44 Over 81 clinical trial studies with hiPSC technology, mostly observational, have been conducted worldwide, but only a handful for therapeutic treatment of SCI. The first clinical trial using hiPSC-derived NSC neural progenitor cells for SCI initiated in 2018 to evaluate hiPSC-derived NSC neural progenitor cells in the treatment of subacute injury between the third/fourth vertebrae and the tenth thoracic vertebrae, with an American Spinal Cord Injury Association Injury Scale (AIS) classification of A. 45 The intent of the study is to evaluate post-transplant graft cell mortality, adverse events such as hyperproliferation of transplanted graft cells, efficacy of treatment on restoration of motor function, sensory function, reduced spasticity, and improved quality of life. The use of multipotent NSCs as a more directed approach to neurons, without transdifferentiation such as with MSCs, has advanced but still faces challenges complicated by their multipotency, primarily complete differentiation and maturation of cells to avoid neurotomas, as well as the need for sufficient graft differentiation in vivo of spinal motor neurons, myelinating oligodendrocytes, and support cells to promote functional neural circuitry recovery and regeneration. This study that applies neural progenitors represents a new breakthrough in fine-tuning SCI treatments with more appropriate cell types.
Restoring neural circuitry – neuromesodermal progenitors
The importance of making the right neuron
The use of neurons as therapeutics was first demonstrated with fetal embryonic tissue.46,47 Until recently, success in using stem cell technologies to generate neurons has been historically unsuccessful and has remained understudied. Recently, induced reprogramming to generate autologous motor neurons in a bypass of developmental steps promoted recovery in a rat T9 compression injury. 48 New studies in Parkinson’s disease demonstrate that pluripotent stem cell in vitro derived and matured CNS dopaminergic neurons survive in rodent and monkey models of Parkinson’s disease and generate significant therapeutic improvement when the appropriate neuron subtype is grafted.49,50 Pioneering studies in treating Parkinson’s disease advanced initially through allogeneic transplantation of human fetal dopamine cells for Parkinson’s disease.51 –54 Analysis of several tissues and stem cell–derived cells based on anatomical classification of midbrain dopaminergic neurons, along with single-cell gene expression profiling in the mouse brain, revealed the existence of several molecularly distinct dopaminergic neuron subtypes. 55 The shift in therapeutic cell focus to attention around the molecular diversity of substantia nigra dopaminergic neurons and relevance for Parkinson’s disease has been instrumental in effecting advanced therapeutics. In addition, use of hiPSC-derived dopaminergic progenitors56,57 enables a pipeline for therapeutic cell generation, with near unlimited availability of cells, increased purity, standardized manufacturing, and the ability to better withstand cryopreservation before neuronal differentiation. 58 Such significant findings with Parkinson’s disease have not escaped notice by the SCI field in regard to developmentally appropriate derivation of neurons including subtype specification to generate optimal therapeutic outcomes.
NMPs and homotypic spinal motor neurons
The earliest models of nervous system embryology arose from elegant studies on amphibian development,59,60 in which a single founding pool of NSC neural progenitors in the early epiblast gives rise to both brain and trunk lineages. Transformation along the anterior–posterior body length then continues by caudalizing, or posteriorizing signals. A parallel strategy for vertebrate CNS development awaited verification but meanwhile a similar paradigm was assumed and has influenced SCI therapeutic studies with pluripotent stem cells and generation of NSCs. An important study in mice 61 followed by detailed molecular analysis of transcriptomics by RNA-Seq in both mouse and human pluripotent stem cells 62 demonstrated that developmental lineages of the vertebrate brain and spine arise from distinct populations of neural progenitors in vivo and in vitro. 63 In an immediate bifurcation event during embryonic neurogenesis in vertebrates, NSCs in the anterior neural plate region generate cranial neurons in the brain and descending white matter tracts, while a separate pool of caudal axial progenitor cells, termed neuromesodermal progenitors (NMPs), present in the posterior plate generate and pattern the spinal cord as the common origin of the CNS spine and trunk musculoskeletal system, first described as axial stem cells.64,65 NMPs, therefore, represent the most natural route for in vivo and in vitro generation of therapeutic cells and recapitulation of cell phenotypes in vivo along the neuraxis to benefit SCI. Additional detail is also available now through transcriptional RNA-Seq profiling of spinal interneurons 66 and spinal motor neurons 67 that provides a fine-grained map of the cellular heterogeneity, individual subclasses of neurons, and circuit and physiological specializations. The ground-breaking revelations around vertebrate NMPs in development of the nervous system and transcriptional profiling of spinal neurons in rodent studies are expected to be synergistic to accelerate and transform SCI therapeutics in the next decade, allowing a renewed focus on neuronal circuitry restoration.
Transplantable preformed neural circuitry for SCI therapeutics
The leap in stem cell neurotechnologies and use of neurons as SCI cell therapeutics will accelerate progress in understanding, treatment, and recovery of CNS and PNS neural circuitry following SCI. Preformed transplantable neuronal circuitry incorporating in vitro NMP-derived and functionally matured human caudal spinal motor neurons, along with interneurons and OPCs encapsulated in alginate 68 (Figure 2), have been demonstrated to functionally integrate in a rat hemicontusion model of SCI. 69 These synaptically connected neuronal networks, termed neural ribbons (Figure 3), demonstrated retention of high synaptic density, integration into the host parenchyma, and long-term viability. Preliminary eight-week duration functional recovery studies in the rat model (unpublished studies) indicate improvement in limb reaching function, consistent with host–graft integration seen at earlier timepoints. 69 Next steps in optimization of neural ribbon technology include focus on spinal cell types and ratios, inclusion of ECM modulators such as chondroitinase to counter refractory injury site CSPGs, and scaling to supply sufficient neural therapeutic cells. Worth noting in initial in vivo studies is the substantial reduction in number of neurons needed to achieve minimal recovery (~5000) and the robust survival and host integration of the transplanted matured neurons without ECM matrix modifiers, such as chondroitinase for modulation of refractive CSPGs. Spinal motor neuron networks delivered in neural ribbons also contained OPCs. To what degree co-transplantation with matured oligodendrocytes, capable of axonal myelination, would impact functional integration of grafted neurons and intrinsic host neuron regeneration also needs further exploration. Both immature OPCs and mature oligodendrocytes may be important regarding providing critical signaling factors for transplanted neurons in addition to myelination and stabilization of neuronal axons. In comparison to a parallel study using the same strategy for generating regionally matched NMP-derived human spinal motor neurons and OPCs but delivered as a suspension and in logarithmically larger numbers, 200,000 cells, 70 also survive but appear to penetrate less deeply into the host parenchyma. Alginate neural ribbons appear to preferentially align along the host parenchyma rather than being retained in the cavity, which may promote integration and host–graft synaptic connectivity. Both studies are consistent with homotypic matching as a dominant factor in graft survival. Whether neural ribbons can be directed to be more productive in terms of generating circuitry recovery with the host in lieu of suspension cells that may also generate independent circuitry that will need to be reorganized is an important question in moving ahead with NMP-derived neuronal cell therapeutics. Neural ribbon technology constitutes an exciting next generation strategy for reproducible delivery, placement, and retention of transplantable neuronal circuitry in vivo. In addition, neural ribbons have been tested as shippable neurotechnology, allowing them to be generated in one location and shipped overnight for performing transplantation studies elsewhere. Ex vivo differentiation of spinal neural cells for transplantation also critically allows comprehensive analysis by RNA-Seq and single-cell RNA-Seq transcriptomic profiling, in addition to analysis of biomarkers with cell phenotypes, functional assays, and electrophysiology analysis that will help to create greatest uniformity in treatments and enable rapid advancements in technology.
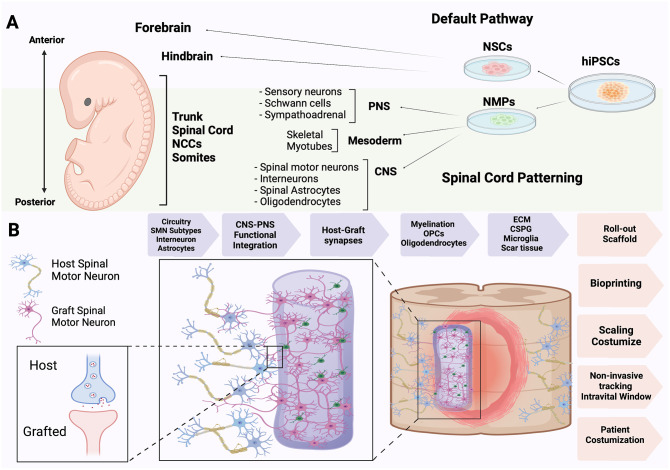
Figure 2.
hiPSC NMP-trunk specification and neural ribbons. (A) The anterior–posterior neuraxis in the human embryo is specified from NSCs that generate brain neurons or NMP-derived cells that form the trunk and spine, directing somites and neural crest cells (NCCs) for CNS–PNS integration. (B) Application of NMPs to achieve trunk regionally matched CNS, PNS, and mesodermal lineage cells is showing promise in transplantation circuitry. Grafted NMP-derived regionally matched spinal motor neurons and support cells in neural ribbons as preformed synaptically connected networks facilitates host–graft interactions and neural circuitry integration, as alginate-based encapsulation provides temporary support for networks before dissolution. Advantages of neural ribbon circuitry and prospective assisting technologies for improved clinical translation.
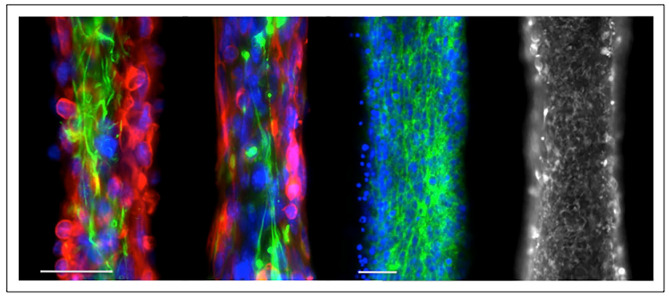
Figure 3.
Preformed neural circuitry ribbons for SCI. Encapsulated hiPSC NMP-derived spinal motor neurons and oligodendrocytes in a 1:5 ratio in RGD-alginate-type I collagen hydrogel as 150-micron diameter neural ribbons. Images left to right: images 1 and 2, DAPI, nuclei (blue), antibodies: SMI312, pan-axonal neurofilament (red), β-III tubulin, Tuj1 (green); image 3, DAPI, nuclei (blue), pan-cadherin (green); image 4, mitochondria (mitotracker). Technology is published in Olmsted et al.67,68 Scale bars are 50 microns.
Scaffolds and electrostimulation in corticospinal connectivity
Alginate neural ribbons provide degradable short-term support for transplanted preformed neuronal circuitry to integrate with the host parenchyma and drive host–graft interactions and are being explored along with other research efforts to test additional platforms that biomimic the spine or integrate electrostimulation (Figure 4). Printable three-dimensional (3D) biomimetic scaffolds may offer an alternate reproducible platform to bridge the injury site and provide a suitable scaffold and microenvironment for retaining grafted cells and promoting repair.71,72 The use of a fabricated collagen/silk fibroin scaffold designed to simulate the spinal cord anatomy 73 also reduced scarring in vivo. In another study, a bioink scaffold designed to biomimic the white matter of the native spinal cord, composed of hydroxypropyl chitosan, thiolated hyaluronic acid, vinyl sulfonated hyaluronic acid, and matrigel, resulted in an increase in axon regeneration, decreased scarring, and significant locomotor recovery in a rat model of SCI. 74 Combination of hydrogels with other techniques that show promise, such as electrical stimulation, are also being developed to improve functional circuitry regeneration. 75 In this study, stem cell–derived neural cells encapsulated in a 3D gelatin scaffold and transplanted into the transected rat spinal cord with electroacupuncture exhibited increased graft cell survival and neuronal differentiation along with synapse formation. An alternate strategy is use of a macroporous flexible mesh as a combined roll-out electronics and scaffold that is deliverable by syringe injection 76 Improved ability to monitor grafting cells in the acute injury site in real-time by integrating advanced imaging approaches with intravital windows 77 will allow observation and quantitation of integration and regeneration over a timespan of days to months to advance and compare innovative SCI therapeutic strategies more quickly.
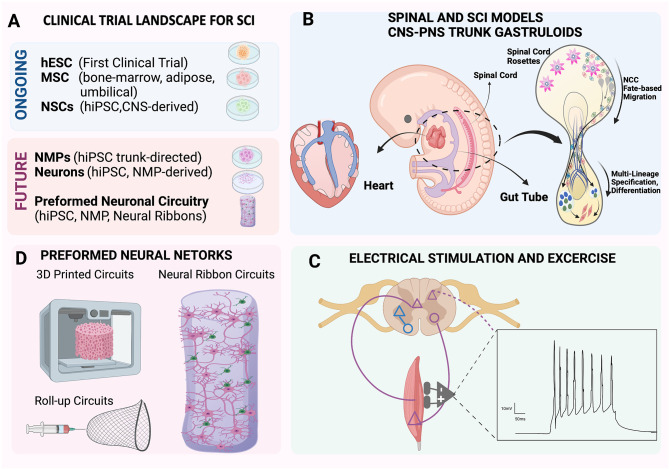
Figure 4.
Current clinical trial landscape and forefront of SCI therapeutic approaches. (A) Brief overview of current stem cell therapeutic sources for SCI and future landscape for the application of hiPSC NMP neurotechnology and preformed neuronal circuitry in clinical trials. (B to D) Technologies at the forefront of SCI therapeutic approaches. (B) Gastruloid models based on NMP protocols can mimic human multilineage development of the trunk and spine regions in a dish and are ideal models to understand integration of CNS and PNS neurons. Shown center in the diagram is a human embryo near 28 days. Gastruloid EMLO and EMLOC technologies model neurons of the multichambered embryonic heart (left) or enteric gut (right). (C) Implementation of electrical stimulus and exercise as a synergistic and complementing therapeutic approach for improved results is expected to be a required step to optimize clinical translational recovery of SCI therapy. (D) The future of SCI therapeutic approaches is expected to advance transplantable neural circuitry considerations applying novel platforms with hiPSC NMP-derived subtype-specific networks.
SCI therapeutics in the context of human development
A powerful capability of pluripotent stem cells is their ability to generate all cell lineages relevant to early embryonic gastrulation that is the formation of endoderm, mesoderm, and ectoderm, as well as the downstream lineages relevant to human development. 78 The important discovery of NMPs beyond their use to generate anatomically matched neurons for SCI repair 79 also allows embryo-like gastruloids to be developed to study the complex integration of CNS–PNS neural circuitry. Two recently developed NMP gastruloid strategies are human elongating multilineage organized (EMLO) gastruloids, 80 in which trunk CNS and PNS neurons form around the enteric gut, as well as cardiac directed EMLOCs, 81 that recapitulate formation of the human embryonic multichambered heart along with its intrinsic neural circuitry. 81 These living human gastruloid EMLO and EMLOC platforms allow the study of previously unobtainable stages of human neurodevelopment for CNS–PNS integration, including key developmental events in neural crest cell (NCC) migration. The advancement of human gastruloid technology along with existing organoid technologies now allows multitissue insights into human trunk development and organ integration with the spinal cord.
Conclusions and future directions
The spinal cord critically links elements of the CNS and PNS for conduction of sensory and motor signals. The complex neuronal network of the spinal cord coordinates multiple actions including motor function, voluntary, and involuntary movements. In SCI, spinal neurons may suffer abrupt discontinuation of axonal projections generating gaps in CNS connections harming ascending sensory and descending CNS–PNS motor and autonomic pathways. Depending on the location and severity of the injury, the effects can range from loss of motor function and sensation below the injury site to loss of bowel control, loss of bladder control, and sexual dysfunction. 82 To target repair of SCI and stimulate reformation of new synaptic connections via therapeutic cells and intrinsic regeneration, strategies that focus primarily on neuronal circuitry and combined strategies will bring considerable advancements as alternate approaches. With refined, developmentally appropriate, robust protocols for spinal motor neuron differentiation, neuroanatomical, and cellular diversity along the rostral–caudal and dorsal–ventral axes can be achieved and may be the key missing element in functional recovery with transplanted mature neuron survival and host integration.69,70 No comparative functional studies in animal models have yet been published to evaluate SCI cell therapies that use non-NMP-generated NSCs preprogrammed for brain neural cells versus NMP-trunk–derived NSCs. As new analysis approaches emerge and are integrated into SCI studies, such as spatial single-cell RNA-Seq of tissues along with proteomics and cell–cell interaction data, more rapid and detailed quantitative comparisons at the molecular level will be possible. These detailed data can be integrated together with results on functional recovery after treatment to improve outcomes. The next decade of stem cell–based SCI therapeutics is expected to deliver rapid next level advances and NMP-based spinal motor neuron differentiation strategies now bring neurons as cell therapeutics back to the forefront.
Acknowledgments
The authors acknowledge unpublished neural ribbon data provided by Zachary T. Olmsted in Figure 3, generated as part of the New York State Spinal Cord Injury Research Board (NYSCIRB) grant awarded to Dr Paluh.
Footnotes
Authors’ Contributions: JLP and MBP-E contributed equally to the writing of the manuscript. MBP-E was primarily responsible for generating Figures 1, 2, and 4 using BioRender and researching references. JLP generated Figure 3.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The EMLOC technology referenced in the text is covered by a provisional patent 63/311,498 via RFSUNY by Drs J.L. Paluh and Z.T. Olmsted.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The SCI research was funded by the New York State Department of Health (NYS DOH) Spinal Cord Injury Research Board (NYSCIRB), Projects to Accelerate Research Translation (PART) award C33278GG and SUNY Polytechnic SEED award 917035-21, and used a published ethnically diverse hiPSC line developed in the Paluh laboratory through previous New York State Stem Cell Research (NYSTEM) funding (C026184).
ORCID iDs: Maria Belen Paredes-Espinosa  https://orcid.org/0000-0002-7249-5140
https://orcid.org/0000-0002-7249-5140
Janet L Paluh  https://orcid.org/0000-0002-5988-6075
https://orcid.org/0000-0002-5988-6075
References
- 1. Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Prim 2017;3:1–21 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. International perspectives on spinal cord injury. https://www.who.int/publications/i/item/international-perspectives-on-spinal-cord-injury (2013, accessed 3 March 2022).
- 3. Bernstock JD, Caples CM, Wagner SC, Kang DG, Lehman RA., Jr. Characteristics of combat-related spine injuries: a review of recent literature. Mil Med 2015;180:503–12 [DOI] [PubMed] [Google Scholar]
- 4. Roberts TT, Leonard GR, Cepela DJ. Classifications in brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin Orthop Relat Res 2017;475:1499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 2001;24:254–64 [DOI] [PubMed] [Google Scholar]
- 6. Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res 2006; 152:223–9 [DOI] [PubMed] [Google Scholar]
- 7. Anjum A, Yazid MD, Daud MF, Idris J, Hwei Ng AM, Naicker AS, Rashidah Ismail OH, Kumar RKA, Lokanathan Y. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 2020;21:1–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun 2019;10:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–99 [DOI] [PubMed] [Google Scholar]
- 10. Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism 2011;14: 724–38 [DOI] [PubMed] [Google Scholar]
- 11. Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 2009;32:421–31 [DOI] [PubMed] [Google Scholar]
- 12. Mucke L, Oldstone MB, Morris JC, Nerenberg MI. Rapid activation of astrocyte-specific expression of GFAP-lacZ transgene by focal injury. New Biol 1991;3:465–74 [PubMed] [Google Scholar]
- 13. Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew M, v. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 2013;33:12870–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian J Med Res 2012;135:287–96 [PMC free article] [PubMed] [Google Scholar]
- 15. Bethea JR. Spinal cord injury-induced inflammation: a dual-edged sword. Prog Brain Res 2000;128:33–42 [DOI] [PubMed] [Google Scholar]
- 16. Couillard-Despres S, Bieler L, Vogl M. Pathophysiology of traumatic spinal cord injury. Neurol Asp Spinal Cord Inj 2017;20:503–28 [Google Scholar]
- 17. Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle ME, Vernoux N, Tremblay MÈ, Fuehrmann T, Shoichet MS, Lacroix S. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun 2019;10:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 2009;29:13435–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu SS, Li ZY, Xu XZ, Yao F, Luo Y, Liu YC, Cheng L, Zheng MG, Jing JH. M1-type microglia can induce astrocytes to deposit chondroitin sulfate proteoglycan after spinal cord injury. Neur Regener Res 2022;17:1072–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karus M, Ulc A, Ehrlich M, Czopka T, Hennen E, Fischer J, Mizhorova M, Qamar N, Brüstle O, Faissner A. Regulation of oligodendrocyte precursor maintenance by chondroitin sulphate glycosaminoglycans. Glia 2016;64:270–86 [DOI] [PubMed] [Google Scholar]
- 21. Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 2003;22:319–30 [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Yang S, Liu C, Han X, Gu X, Zhou S. Deciphering glial scar after spinal cord injury. Burns Trauma 2021;9:tkab035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 2013;33:13882–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou T, Zheng Y, Sun L, Badea SR, Jin Y, Liu Y, Rolfe AJ, Sun H, Wang X, Cheng Z, Huang Z, Zhao N, Sun X, Li J, Fan J, Lee C, Megraw TL, Wu W, Wang G, Ren Y. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat Neurosci 2019;22:421–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Z, Yu S, Hu X, Li Y, You X, Tian D, Cheng L, Zheng M, Jing J. Fibrotic scar after spinal cord injury: crosstalk with other cells, cellular origin, function, and mechanism. Front Cell Neurosci 2021;15:720938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buzoianu-Anguiano V, Rivera-Osorio J, Orozco-Suárez S, Vega-García A, García-Vences E, Sánchez-Torres S, Jiménez-Estrada I, Guizar-Sahagún G, Mondragon-Caso J, Fernández-Valverde F, Madrazo I, Grijalva I., Single vs. combined therapeutic approaches in rats with chronic spinal cord injury. Front Neurol 2020;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bryda EC. The mighty mouse: the impact of rodents on advances in biomedical research. Mo Med 2013;110:207–11 [PMC free article] [PubMed] [Google Scholar]
- 28. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154–6 [DOI] [PubMed] [Google Scholar]
- 29. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 1981;78:7634–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, Jones J. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–7 [DOI] [PubMed] [Google Scholar]
- 31. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76 [DOI] [PubMed] [Google Scholar]
- 32. Chang EA, Tomov ML, Suhr ST, Luo J, Olmsted ZT, Paluh JL, Cibelli J. Derivation of ethnically diverse human induced pluripotent stem cell lines. Sci Rep 2015;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scott CT, Magnus D. Wrongful termination: lessons from the Geron clinical trial. Stem Cells Transl Med 2014;3:1398–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scott C, Huggett B. Geron’s quixotic fate. Nat Biotechnol 2012;30:497. [DOI] [PubMed] [Google Scholar]
- 35. Scuteri A, Miloso M, Foudah D, Orciani M, Cavaletti G, Tredici G. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther 2011;6:82–92 [DOI] [PubMed] [Google Scholar]
- 36. Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, Dixit A, Rauthan A, Murgod U, Totey S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy 2009;11:897–911 [DOI] [PubMed] [Google Scholar]
- 37. Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, Choi BH, Park H, Ha Y. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: phase I/II clinical trial. Stem Cells 2007;25:2066–73 [DOI] [PubMed] [Google Scholar]
- 38. Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, Montenegro X, Gonzalez R, Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant 2008;17:1277–93 [DOI] [PubMed] [Google Scholar]
- 39. Chernykh ER, Stupak VV, Muradov GM, Sizikov MY, Shevela EY, Leplina OY, Tikhonova MA, Kulagin AD, Lisukov IA, Ostanin AA, Kozlov VA. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull Exp Biol Med 2007;143:543–7 [DOI] [PubMed] [Google Scholar]
- 40. Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med 2016;39:655–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011;20:1297–308 [DOI] [PubMed] [Google Scholar]
- 42. Xiao Z, Tang F, Zhao Y, Han G, Yin N, Li X, Chen B, Han S, Jiang X, Yun C, Zhao C, Cheng S, Zhang S, Dai J. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with neuroregen scaffolds and mesenchymal stem cells. Cell Transplant 2018;27:907–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang L, Fu C, Xiong F, He C, Wei Q. Stem cell therapy for spinal cord injury. Cell Transplant 2021;30:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata K-I, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M. Autologous induced stem-cell–derived retinal cells for macular degeneration. N Engl J Med 2017;376:1038–46 [DOI] [PubMed] [Google Scholar]
- 45. Sugai K, Sumida M, Shofuda T, Yamaguchi R, Tamura T, Kohzuki T, Abe T, Shibata R, Kamata Y, Ito S, Okubo T, Tsuji O, Nori S, Nagoshi N, Yamanaka S, Kawamata S, Kanemura Y, Nakamura M, Okano H. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen Ther 2021;18:321–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embyronic spinal cord tissue in neonatal and adult rats. J Comp Neurol 1986; 247:275–96 [DOI] [PubMed] [Google Scholar]
- 47. Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol 1993;123:3–16 [DOI] [PubMed] [Google Scholar]
- 48. Lee H, Lee HY, Lee BE, Gerovska D, Park SY, Zaehres H, Araúzo-Bravo MJ, Kim JI, Ha Y, Schöler HR, Kim JB. Sequentially induced motor neurons from human fibroblasts facilitate locomotor recovery in a rodent spinal cord injury model. Elife 2020;9:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takahashi J. Strategies for bringing stem cell-derived dopamine neurons to the clinic: the Kyoto trial. Prog Brain Res 2017;230:213–26 [DOI] [PubMed] [Google Scholar]
- 50. Takahashi J. iPS cell-based therapy for Parkinson’s disease: a Kyoto trial. Regen Ther 2020;13:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, Lindholm T, Björklund A, Leenders KL, Rothwell JC, Frackowiak R, Marsden D, Johnels B, Steg G, Freedman R, Hoffer BJ, Seiger A, Bygdeman M, Strömberg I, Olson L. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol 1989;46:615–31 [DOI] [PubMed] [Google Scholar]
- 52. Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Marsden CD, Björklund A. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 1990;247:574–7 [DOI] [PubMed] [Google Scholar]
- 53. Lindvall O, Widner H, Rehncrona S, Brundin P, Odin P, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC. Transplantation of fetal dopamine neurons in Parkinson’s disease: one-year clinical and neurophysiological observations in two patients with putaminal implants. Ann Neurol 1992;31:155–65 [DOI] [PubMed] [Google Scholar]
- 54. Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Wells TH, Barrett JN, Grafton ST, Huang SC, Eidelberg D, Rottenberg DA. Transplantation of human fetal dopamine cells for Parkinson’s disease. Arch Neurol 1990;47:505–12 [DOI] [PubMed] [Google Scholar]
- 55. Poulin J-F, Zou J, Cicchetti F, Awatramani RB. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling correspondence. Cell Rep 2014;9:930–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, Jung JH, Kim J, Hwang D, Cohen BM, Teicher MH, Leblanc P, Carter BS, Kordower JH, Bolshakov VY, Kong SW, Schweitzer JS, Kim KS. Human autologous iPSC–derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest 2020;130:904–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schweitzer JS, Song B, Herrington TM, Park T-Y, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, Henchcliffe C, Kaplitt M, Neff C, Rapalino O, Seo H, Lee I-H, Kim J, Kim T, Petsko GA, Ritz J, Cohen BM, Kong S-W, Leblanc P, Carter BS, Kim K-S. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N Engl J Med 2020;382:1926–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci 2020;21:103–15 [DOI] [PubMed] [Google Scholar]
- 59. Nieuwkoop PD. Activation and organization of the central nervous system in amphibians. Part III. Synthesis of a new working hypothesis. J Exp Zool 1952;120:83–108 [Google Scholar]
- 60. Nieuwkoop PD, Nigtevecht G, v. Neural activation and transformation in explants of competent ectoderm under the influence of fragments of anterior notochord in urodeles. Development 1954;2:175–93 [Google Scholar]
- 61. Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell 2009;17:365–76 [DOI] [PubMed] [Google Scholar]
- 62. Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol 2014;12:e1001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henrique D, Abranches E, Verrier L, Storey KG. Neuromesodermal progenitors and the making of the spinal cord. Development 2015;142: 2864–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development 2002;129:4855–66 [DOI] [PubMed] [Google Scholar]
- 65. Wilson V, Olivera-Martinez I, Storey KG. Stem cells, signals and vertebrate body axis extension. Development 2009;136:1591–604 [DOI] [PubMed] [Google Scholar]
- 66. Lu DC, Niu T, Alaynick WA. Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci 2015;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Osseward PJ, 2nd, Pfaff SL. Cell type and circuit modules in the spinal cord. Curr Opin Neurobiol 2019;56:175–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Olmsted ZT, Stigliano C, Badri A, Zhang F, Williams A, Koffas MAG, Xie Y, Linhardt RJ, Cibelli J, Horner PJ, Paluh JL. Fabrication of homotypic neural ribbons as a multiplex platform optimized for spinal cord delivery. Sci Rep 2020;10:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Olmsted ZT, Stigliano C, Scimemi A, Wolfe T, Cibelli J, Horner PJ, Paluh JL. Transplantable human motor networks as a neuron-directed strategy for spinal cord injury. iScience 2021;24:e102827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Olmsted ZT, Stigliano C, Marzullo B, Cibelli J, Horner PJ, Paluh JL. Fully characterized mature human iPS- and NMP-derived motor neurons thrive without neuroprotection in the spinal contusion cavity. Front Cell Neurosci 2022;15:e725195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Joung D, Lavoie NS, Guo SZ, Park SH, Parr AM, McAlpine MC. 3D printed neural regeneration devices. Adv Funct Mater 2020;30:e906237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med 2019;25:263–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jiang JP, Liu XY, Zhao F, Zhu X, Li XY, Niu XG, Yao ZT, Dai C, Xu HY, Ma K, Chen XY, Zhang S. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neur Reg Res 2020;15:959–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu X, Hao M, Chen Z, Zhang T, Huang J, Dai J, Zhang Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 2021; 272:120771. [DOI] [PubMed] [Google Scholar]
- 75. Jin H, Zhang YT, Yang Y, Wen LY, Wang JH, Xu HY, Lai BQ, Feng B, Che MT, Qiu XC, Li ZL, Wang LJ, Ruan JW, Jiang B, Zeng X, Deng QW, Li G, Ding Y, Zeng YS. Electroacupuncture facilitates the integration of neural stem cell-derived neural network with transected rat spinal cord. Stem Cell Rep 2019;12:274–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu J, Fu TM, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM. Syringe-injectable electronics. Nat Nanotechnol 2015;10:629–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Alieva M, Ritsma L, Giedt RJ, Weissleder R, van Rheenen J. Imaging windows for long-term intravital imaging. Intravital 2014;3:e29917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, Lutolf MP, Duboule D, Arias AM. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 2018;562:272–6 [DOI] [PubMed] [Google Scholar]
- 79. Olmsted ZT, Paluh JL. Stem cell neurodevelopmental solutions for restorative treatments of the human trunk and spine. Front Cell Neurosci 2021;15:667590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Olmsted ZT, Paluh JL. Co-development of central and peripheral neurons with trunk mesendoderm in human elongating multi-lineage organized gastruloids. Nat Commun 2021;12:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Olmsted ZT, Paluh JL. A combined human gastruloid model of cardiogenesis and neurogenesis. iScience 2022;25:104486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park SE, Elliott S, Noonan VK, Thorogood NP, Fallah N, Aludino A, Dvorak MF. Impact of bladder, bowel and sexual dysfunction on health status of people with thoracolumbar spinal cord injuries living in the community. J Spinal Cord Med 2017;40:548–59 [DOI] [PMC free article] [PubMed] [Google Scholar]