Abstract
Introduction: In many low and middle-income countries (LMIC), drug promotional literature (DPL) remains one of the main sources of drug information for physicians. Studies conducted in many LMICs showed poor compliance to the WHO guidelines for ethical drug promotion especially in the area of information about excipients, adverse drug reactions, drug-drug interactions and contra-indications. These inadequacies in the information provided may mislead the prescriber with potential adverse consequences among patients using the medicines. Nigeria has a big pharmaceutical sector which is poorly regulated and we hypothesize that such unethical drug promotional practices may exist. This study therefore set out to assess compliance to the WHO ethical drug promotion (using DPL) at the Ekiti State University Teaching Hospital (EKSUTH), Ado-Ekiti, South-West Nigeria. Methodology: This was a descriptive cross-sectional study conducted in several specialist clinics of EKSUTH, Ado-Ekiti. Printed DPLs (brochures and leaflets) were collected from these clinics, collated using a pre-designed data collection form and analyzed using the WHO ethical criteria for medicinal drug promotion. Results:Two hundred seventy-five DPLs were screened A total of out of which 234 DPLs were selected after screening to after removal of duplications. Only 5 (2.1%) DPLs met all the WHO criteria. DPLs promoting antibiotics, cardiovascular drugs, and vitamins/nutritional supplements were in the majority (22.2%, 17.1%, and 11.5% respectively. Most of the DPLs had the generic (223; 95.3%) and brand (234; 100%) names, active ingredients (209; 89.3%), excipients (149; 63.7%), and indications (232; 99.1%). Information about adverse drug reactions (76; 32.5%), contra-indications (73; 31.2%), and drug interactions (46; 19.7%) was less represented. Only 59 (25.2%) DPLs had references. Fixed-dose combination drugs made up 34.6% of drugs being promoted. Conclusion:The DPLs assessed in this study had low adherence to WHO ethical drug promotion criteria especially those related to adverse drug reaction, drug interactions, and contra-indications.
Keywords: drug advertisement, drug brochure, compliance, prescribers, rational use of medicine, criteria, Nigeria
Introduction
Physicians and prescribers typically have a considerable task in keeping up with details of medicines especially new and re-formulated ones. Apart from journals, online sources and textbooks, direct marketing of medicines using pharmaceutical representatives serves as a major source of information about new medicines, and subsequently influencing prescribing, in a number of countries especially low- and middle-income countries (LMICs).1 -6 In many instances, the pharmaceutical sales representatives (PSR) leave drug promotional literature in the form of posters, leaflets, and brochures in physicians’ offices following their visit. In LMICs, the pressure on pharmaceutical companies to make a profit, coupled with often a lack of regulation of physician- pharmaceutical sales representative activities typically seen in higher income countries, exposes physicians to numerous drug promotion literature (DPL).3,7 This relationship between pharmaceutical sales representatives and physicians is associated with considerable ethical issues and concerns across countries.8 -10 A number of studies have shown that the interaction with PSRs influences the prescribing pattern of physicians, which can lead to irrational prescribing of medicines and higher costs.2,5,11 -14 However, others have shown that the influence of PSRs on the prescribing pattern of physicians can be minor.15,16 A concern is that pharmaceutical companies can omit vital information about adverse effects of the medicines they market to make them more attractive to prescribers.17 -19 In addition, many physicians are not adequately trained to critically appraise information about new medicines, which can leave them exposed to potentially biased information from pharmaceutical companies unless educational input is provided by health authorities and others. 18 As a result of this, the WHO’s Ethical Criteria for Medicinal Drug Promotion was developed and released in 1988 to establish typical information that should be made available by pharmaceutical companies when marketing their medicines. 20 According to their suggestions, contents of DPLs should include key information sets. This includes their brand name, the name of the active ingredient (International non-proprietary name—INN), content of the active ingredients per dosage form or regimen, other ingredients that may cause concerns, therapeutic indications, dosage form, adverse effects, contra- indications, drug interactions, name and address of manufacturers or distributor, and pertinent references to the scientific literature. 20 The regulation of drug promotion varies from country to country with countries including Brazil, France, and the USA having strict regulations.21,22 The same cannot be said for many LMICs including India, Pakistan, and Nepal.16,23 In Nigeria, regulation of advertisement of medicines is under the purvey of the National Agency for Drug and Food Administration and Control (NAFDAC). A recent update on the law was released in 2021; however, the regulation is not typically enforced reflecting the general regulatory environment of pharmaceuticals in the country. 24 Studies conducted in many LMIC have shown poor compliance to the WHO guidelines for ethical drug promotion especially in the area of information about excipients, adverse drug reactions, drug-drug interactions, and contra-indications.25,26 These inadequacies may mislead the prescriber with potential adverse consequences among patients prescribed these medicines. Whilst there have been studies in Nigeria discussing the promotional activities of pharmaceutical companies, 3 there is currently a paucity of information regarding the contents of DPLs in Nigeria. A study conducted by Adibe et al 27 in 2015 did not strictly analyze the information provided using the components of the WHO Ethical Criteria for Medicinal Drug Promotion. This is important because Nigeria has an appreciable pharmaceutical sector, which is currently poorly regulated. We hypothesized that sub-optimal drug promotional practices may exist with potentially adverse implications for the health of its citizens.
Consequently, this study set out to assess compliance to the WHO ethical drug promotion (using DPL) at the Ekiti State University Teaching Hospital (EKSUTH), Ado-Ekiti, South-West Nigeria. The findings can be used to provide guidance to national authorities in Nigeria to help improve the future care of patients especially given concerns with the extent and functioning of Drugs and Therapeutic Committees (DTCs) even among tertiary hospitals in Nigeria. 28
Methodology
This was a descriptive cross-sectional study conducted between April 1 and 30, 2020 in several specialist clinics of EKSUTH, Ado-Ekiti, Nigeria.
We chose this setting for this initial study as this hospital is a government-owned public tertiary healthcare facility located in Ekiti State, South-West Nigeria, and caters for the healthcare needs of the 3.2 million inhabitants of Ekiti State as well as patients from neighboring states. The healthcare facility offers primary, secondary, and tertiary level care to patients through its team of medical personnel comprising of consultants, resident doctors, pharmacists, nurses, and other healthcare professionals. Primary care is accessed by patients at the Family Medicine clinics and those requiring the services of other specialists are referred appropriately. Consequently, these clinics should be able to provide examples of DPL across all sectors of care, and be representational of other similar facilities in Nigeria.
Study Procedure
Printed DPLs (brochures and leaflets) were collected from the Internal Medicine, Pediatrics, Family Medicine and Gynecology specialist clinics of the hospital. When multiple DPLs of same brand of medicine were found, this was counted as one. A content analysis was undertaken using the checklist of the various components of the WHO’s Ethical Criteria for Medicinal Drug Promotion. These include their international non-proprietary name (INN), brand name, excipients, indication of use, dosage form, adverse drug reactions, contra-indications, and drug interactions. Other required information included precautions, name and address of company, references and nature of the components, for example, whether single or in a fixed dose combination (FDC). 20 The latter because there have been concerns with the role and value of FDCs such as irrational drug combinations, drug-interactions, and high costs especially among LMICs. 29
Information was extracted from the DPLs by the PI (JOF) and other co-authors in the hospital (IB, AOB, PA). The same set of DPLs were screened by 2 reviewers (JOF and IB). In case of divergent outcomes, a consensus was agreed by the 2 reviewers. Extracted information was collated using a pre-designed data collection form built on the WHO ethical criteria for medicinal drug promotion (Appendix A).
Data Management
Data was entered into Excel spreadsheet and analyzed using IBM SPSS Statistics for Windows, Version 25.0. (Armonk, NY: IBM Corp). The collected DPLs were categorized according to their therapeutic class (WHO ATC classification) and analyzed descriptively by JOF and BAO. 30 The various components of the WHO’s Ethical Criteria for Medicinal Drug Promotion were summarized using proportions/percentages.
Ethical Considerations
This study made use of documents that were in the public domain. Consequently, ethical approval was not necessary.
Results
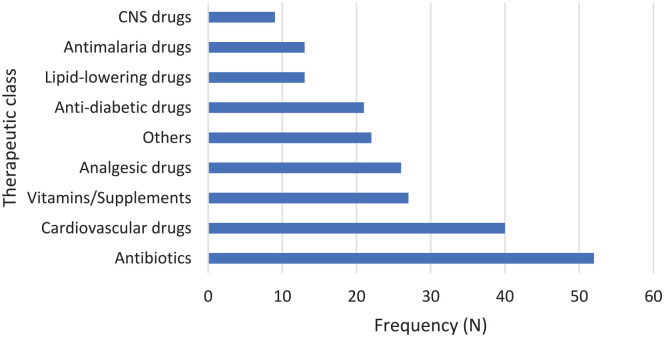
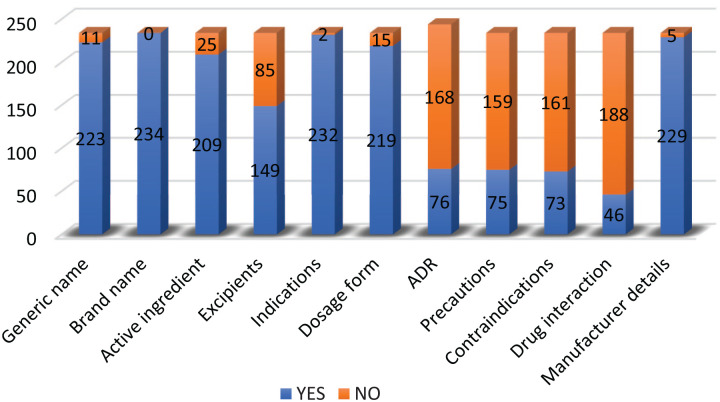
Two hundred seventy-five DPLs were screened out of which 234 DPLs were selected after removal of duplications. Only 5 (2.1%) DPLs met all the WHO criteria. DPLs promoting antibiotics, cardiovascular drugs, and vitamins/nutritional supplements were the majority (22.2%, 17.1%, and 11.5% respectively). Details of the distribution of selected DPLs according to their therapeutic class is shown in Figure 1. Most of the DPLs had the generic (INN) (223; 95.3%) and brand (234; 100%) names of the drug being marketed. Information about adverse drug reactions (76; 32.5%), contra-indications (73; 31.2%), and drug interactions (46; 19.7%) was less represented in the DPLs. Figure 2 highlights the availability of components of the WHO’s Ethical Criteria for Medicinal Drug Promotion in the DPLs. Fifty-nine (25.2%) DPLs had no references while 81 (34.6%) were FDCs. The median number of references was 2 (range 0-25).
Figure 1.
Drug promotional literature by therapeutic classes.
Figure 2.
Components of WHO criteria for ethical drug promotion.
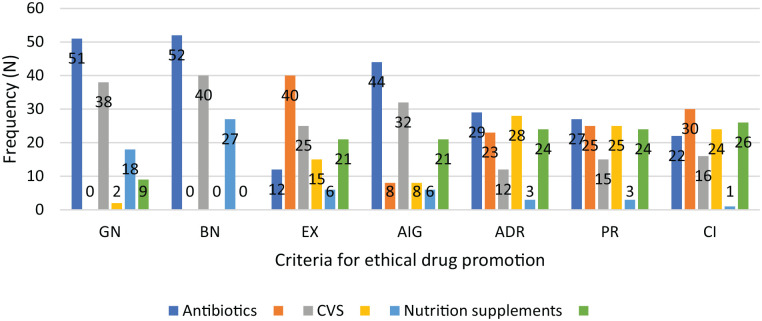
A sub-analysis comparing a number of the therapeutic classes, that is, antibiotics, cardiovascular drugs, and nutritional supplements/vitamins, by compliance to criteria for ethical drug promotion is shown in Figure 3. This chart shows that non-compliance to the criteria was greater with nutritional supplements/vitamins.
Figure 3.
Compliance of some therapeutic classes to WHO criteria on drug promotion.
GN = generic name; BN = brand name; EX = excipients; AIG = active ingredients; ADR = adverse drug reactions; PR = precautions; CI = contraindications.
Discussion
We believe this is one of the first studies conducted in Nigeria to fully evaluate adherence to WHO Ethical Drug Promotion criteria among selected DPLs from several outpatient clinics of the Ekiti State University Teaching Hospital, Ado-Ekiti. This is important given high levels of patient co-payments in Nigeria and the fact that physicians should be looking to minimize costs where possible to maximize patient care within available resources.31,32 Encouragingly, there was high levels of compliance to information on INN and brand names, active ingredients, therapeutic indications, and dosage forms. Overall compliance was 95.3% to 100% regarding information on INN and brand names and therapeutic indications for the advertised drug in this study, similar to studies conducted in India, Saudi Arabia, Ethiopia, and Nepal.33 -36 Proper identification of a medicine being marketed is essential. Consequently, it is not surprising that these items were also found in majority of the DPLs screened in these studies. However, the level of compliance to safety-related components was very low in this study. Only, 32.5%, 32.1%, 31.2%, and 19.7% respectively of the DPLs had information on the potential adverse effects, precautions, contra-indications, and drug interactions. Similar results have been reported by Kamath and Hoovinaliole, 37 who found that over 80% of screened DPLs in their study did not have any information on adverse drug reactions, contra-indications and precautions while only less than 10% of DPLs in Nepalese studies had information on potential adverse drug reactions.25,36 Similarly, only 11% and 13.5% of screened DPLs contained information about potential adverse drug reactions and drug interactions in a study conducted by Jhanwar. 38 It was also interesting to note that DPLs on nutritional supplements/vitamins performed worse in compliance to safety-related criteria than those on antibiotics and cardiovascular drugs. This may be due to the general belief that nutritional supplements are not harmful. In addition, typically less regulations and data requirements among the authorities for nutritional supplements/vitamins than seen with licensed medicines. The lack of adequate information about adverse drug reactions, contra-indications, and precautions was also highlighted in other studies conducted in Saudi Arabia and Iraq.34,39 In addition, a study on the quality of information provided in drug promotion to physicians by PSRs in Canada, France, and the United States found that serious adverse effects were rarely mentioned. 40 This is a concern as the primary goal of the prescriber should be to ensure safe use of medicines. Consequently, the lack of information about safety-related components including potential adverse drug reactions, contra-indications, precautions, and drug interactions is a cause for concern which needs to be addressed going forward. Prescribers and healthcare institutions may need to challenge PSRs and their companies in the future to improve on the situation perhaps with the aid of Drug and Therapeutic Committees (DTCs) in hospitals. The regulatory authorities may also need to intervene in the future to ensure inclusion of safety-related information in drug advertisements including DPLs especially in LMICs where there is typically less control over pharmaceutical company promotional activities.
Antimicrobials (22.2%), cardiovascular drugs (17.1%), and nutritional supplements (11.5%) were the most commonly promoted medicines in the DPLs. Antimicrobials, analgesics and nutritional supplements were also the most common therapeutic classes promoted in DPLs in the earlier Nigerian study. 40 This is not surprising as infections and cardiovascular diseases are highly prevalent in Nigeria.32,41 -43 Nutritional supplements/vitamins are also widely used in Nigeria especially together with antimicrobials and antimalarials. This is because of the anecdotal belief of the negative effect of these anti-infective medications on the general well-being and the need to beef up one’s immunity when taking them. Antimicrobials were also the most common medicines promoted in studies conducted in Ethiopia and India35,44, while vitamins/nutritional supplements made up 27.9% of all DPLs screened in another Indian study. 45 Cardiovascular (26.4%), endocrine drugs (18.9%), and nutritional supplements (18.9%) were also the most advertised among screened DPLs in a similar study by NAikwadi et al 46 in India.
As mentioned, the use of FDCs can be problematic because of issues of rationality, potential interactions, and adverse drug reactions as well as potentially increased costs when compared to each component separately.29,47 Consequently, a concern is that approximately a third (34.6%) of advertised medicines in this study were FDCs, similar to a study by Ganashree et al 33 and co-workers in India. This compares to FDCs accounting for 45.2%, 49%, and 65% of DPLs in other studies conducted mainly in South-East Asia.44,45,48 There is a need therefore for stakeholders in the healthcare sectors, especially the regulatory agencies, to critically assess and regulate the FDCs component of the pharmaceutical sector. In addition, DTCs in hospitals to fully evaluate their value compared with prescribing components separately given high patient co-payment levels in Nigeria.
The provision of references to substantiate claims made about the efficacy and safety of promoted medicines is an ethical obligation in drug promotion. However, in this study, only 25.2% of screened DPLs had references included, which is less than 48.6% and 42.8% reported in studies conducted in Ethiopia and India.35,49 An appreciably higher proportion of references was also reported in studies from Saudi Arabia (64%) and Iraq (72%),34,39 which needs to be a minimum in Nigeria going forward.
Since DPLs remain a principal source of information for many prescribers in Nigeria and other LMICs, training physicians to be able to critically appraise information supplied by pharmaceutical companies either in form of promotional literature or scientific publications becomes a priority, which is supported by conclusions from other similar studies.50,51 We have seen the catastrophic consequences of misinformation regarding potential medicines to treat COVID-19 in Nigeria, which needs to be avoided going forward.52,53 This should be via DTCs and their activities in hospitals to promote the rational use of medicines. However, as mentioned, there can be concerns regarding their existence and activities even among tertiary hospitals in Nigeria. 32 Drug information centers (DICs) within healthcare facilities can play a significant role in ensuring that prescribers have the correct and up to date information about medicines. Unfortunately, DICs are currently not widely available in Nigeria, and not fully functional where established.54,55 There is a need therefore for the establishment of functional DICs in healthcare facilities to bridge this gap as the availability and role of DTCs.
We are aware of a number of limitations with this study. The key limitation of this study was the non-verification of accuracy of the cited references in the DPLs and their accessibility. This can be the focus of future research. Whilst this was a single-center study, we believe that the contents of DPLs distributed to healthcare facilities across the country are the same. There is also the possibility of bias by co-authors who screened the DPLs. We believe though that this possibility was appreciably reduced by ensuring that same set of DPLs were screened by 2 different reviewers and concurrence established when there were differences. Consequently, we believe our findings are robust providing guidance to key stakeholder groups across Nigeria interested in enhancing the rational use of medicines.
Conclusion
The adherence to WHO ethical drug promotion among sourced DPLs was sub-optimal in Nigeria especially in the areas of adverse drug reactions, drug interactions, and contra-indications. This needs to be urgently addressed by regulatory authorities in Nigeria. In addition, there is a need for building the capacity of prescribers for the critical assessment of DPLs; DTCs can play a major role here going forward. DTCs and other educational activities among physicians should equip them in making the right choices when confronted with multiple choices of medicines for a variety of conditions advertised in DPLs.
Acknowledgments
The authors are grateful to the medical interns who collected the drug brochures and leaflets from the outpatients’ clinics.
Appendix A.
| S/N | GN | BN | AID | TC | EX | IN | DF | ADRs | PR | CI | DI | Refs (N) | MN | MA |
GN: Generic name; BN: Brand name; AID: Active ingredient/dose; TC: Therapeutic class; EX: Excipients; IN: Indications; DF: Dosage form
ADR: Adverse drug reactions; PR: Precaution; CI: Contra-indications; DI: Drug interactions; MN: Manufacturer/Marketer name; MA Manufacturer/Marketer address.
Footnotes
Authrors’ Contributions: JOF and FA conceptualized the study. AB, OOS, IB and OA assisted in the acquisition of data and its extraction. JOF, IB, BG interpreted and analyzed the data. Initial draft of manuscript had inputs from JOF, BG, IB, FA and AB. BG and OOS revised the manuscript for critical intellectual content. All authors read and approved the final draft of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Joseph O Fadare  https://orcid.org/0000-0002-5641-1402
https://orcid.org/0000-0002-5641-1402
Oladele Simeon Olatunya  https://orcid.org/0000-0003-2564-3064
https://orcid.org/0000-0003-2564-3064
References
- 1. Riaz H, Godman B, Hussain S, et al. Prescribing of bisphosphonates and antibiotics in Pakistan: challenges and opportunities for the future. J Pharm Health Serv Res. 2015;6(2):111-121. [Google Scholar]
- 2. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7:e016408. doi: 10.1136/bmjopen-2017-016408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fadare JO, Oshikoya KA, Ogunleye OO, et al. Drug promotional activities in Nigeria: impact on the prescribing patterns and practices of medical practitioners and the implications. Hosp Pract. 2018;46:77-87. doi: 10.1080/21548331.2018.1437319 [DOI] [PubMed] [Google Scholar]
- 4. Fadlallah R, Alkhaled L, Brax H, et al. Extent of physician-pharmaceutical industry interactions in low- and middle-income countries: a systematic review. Eur J Public Health. 2018;28:224-230. doi: 10.1093/eurpub/ckx204 [DOI] [PubMed] [Google Scholar]
- 5. Datta A, Dave D. Effects of physician-directed pharmaceutical promotion on prescription behaviors: longitudinal evidence. Health Econ. 2017;26:450-468. doi: 10.1002/hec.3323 [DOI] [PubMed] [Google Scholar]
- 6. Khazzaka M. Pharmaceutical marketing strategies’ influence on physicians’ prescribing pattern in Lebanon: ethics, gifts, and samples. BMC Health Serv Res. 2019;19:80. doi: 10.1186/s12913-019-3887-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamal S, Holmberg C, Russell J, et al. Perceptions and attitudes of Egyptian health professionals and policy-makers towards pharmaceutical sales representatives and other promotional activities. PLoS One. 2015;10:e0140457. doi: 10.1371/journal.pone.0140457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373-380. doi: 10.1001/jama.283.3.373 [DOI] [PubMed] [Google Scholar]
- 9. Workneh BD, Gebrehiwot MG, Bayo TA, et al. Influence of medical representatives on prescribing practices in Mekelle, northern Ethiopia. PLoS One. 2016;11:e0156795. doi: 10.1371/journal.pone.0156795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz D, Caplan AL, Merz JF. All gifts large and small: toward an understanding of the ethics of pharmaceutical industry gift-giving. Am J Bioeth. 2010;10:11-17. doi: 10.1080/15265161.2010.519226 [DOI] [PubMed] [Google Scholar]
- 11. Mitchell AP, Trivedi NU, Gennarelli RL, et al. Are financial payments from the pharmaceutical industry associated with physician prescribing? A systematic review. Ann Intern Med. 2021;174:353-361. doi: 10.7326/M20-5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogunleye OO, Fadare JO, Eriksen J, et al. Reported needs of information resources, research tools, connectivity and infrastructure among African pharmacological scientists to improve future patient care and health. Expert Rev Clin Pharmacol. 2019;12:481-489. doi: 10.1080/17512433.2019.1605903 [DOI] [PubMed] [Google Scholar]
- 13. Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7:e1000352. doi: 10.1371/journal.pmed.1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brax H, Fadlallah R, Al-Khaled L, et al. Association between physicians’ interaction with pharmaceutical companies and their clinical practices: a systematic review and meta-analysis. PLoS One. 2017;12:e0175493. doi: 10.1371/journal.pone.0175493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lotfi T, Morsi RZ, Rajabbik MH, et al. Knowledge, beliefs and attitudes of physicians in low and middle-income countries regarding interacting with pharmaceutical companies: a systematic review. BMC Health Serv Res. 2016;16:57. doi: 10.1186/s12913-016-1299-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gereffi G. The Pharmaceutical Industry and Dependency in the Third World. The Pharmaceutical Industry and Dependency in the Third World. Princeton University Press; 2017. [Google Scholar]
- 17. Altawalbeh SM, Ibrahim IA, Al-Shatnawi SF. Influence of pharmaceutical promotion on prescribers in Jordan. Int J Clin Pharm. 2020;42:744-755. doi: 10.1007/s11096-020-01006-3 [DOI] [PubMed] [Google Scholar]
- 18. Malmström RE, Godman BB, Diogene E, et al. Dabigatran – a case history demonstrating the need for comprehensive approaches to optimize the use of new drugs. Front Pharmacol. 2013;4:39. doi: 10.3389/fphar.2013.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen D. Dabigatran: how the drug company withheld important analyses. BMJ. 2014;349:g4670. doi: 10.1136/bmj.g4670 [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization. Ethical criteria for medicinal drug promotion. 1988. Accessed on May 24, 2022. https://apps.who.int/iris/handle/10665/38125 [DOI] [PubMed]
- 21. Francer J, Izquierdo JZ, Music T, et al. Ethical pharmaceutical promotion and communications worldwide: codes and regulations. Philos Ethics Humanit Med. 2014;9:7. doi: 10.1186/1747-5341-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vianna JL, Figueiredo L, Ribeiro A. Distribution and marketing of drugs in Brazil: overview. ACC (Association of corporate counsel); 2018:1-8. [Google Scholar]
- 23. Ahmed RR, Saeed A. Pharmaceutical drug promotion practices in Pakistan: Issues in ethical and non-ethical pharmaceutical practices. Middle East J Sci Res. 2014;20(11):1630-1640. [Google Scholar]
- 24. Federal Government of Nigeria. Drugs and related product advertisement regulations. 2021. Accessed July 28, 2022. https://www.nafdac.gov.ng
- 25. Alam K, Shah AK, Ojha P, Palaian S, Shankar PR. Evaluation of drug promotional materials in a hospital setting in Nepal. South Med Rev. 2009;2:2-6. [PMC free article] [PubMed] [Google Scholar]
- 26. Vachhani PM, Solanki MN, Desai MK. An evaluation of drug promotional literatures published in scientific medical journals. J Pharm Bioallied Sci. 2016;8(3):248-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adibe M, Igboeli N, Ubaka C, Udeogaranya P, Onwudiwe N, Ita O. Evaluation of information contained in drug advertisement and promotion materials in Nigeria. Trop J Pharm Res. 2015;14(3):539-544. [Google Scholar]
- 28. Fadare JO, Ogunleye O, Obiako R, et al. Drug and therapeutics committees in Nigeria: evaluation of scope and functionality. Expert Rev Clin Pharmacol. 2018;11:1255-1262. doi: 10.1080/17512433.2018.1549488 [DOI] [PubMed] [Google Scholar]
- 29. Godman B, McCabe H, D Leong T, et al. Fixed dose drug combinations – are they pharmacoeconomically sound? Findings and implications especially for lower- and middle-income countries. Expert Rev Pharmacoecon Outcomes Res. 2020;20(1):1-26. doi: 10.1080/14737167.2020.1734456 [DOI] [PubMed] [Google Scholar]
- 30. World Health Organisation. Anatomical therapeutic chemical (ATC) classification. 2021. Accessed May 2, 2022. https://www.who.int/tools/atc-ddd-toolkit/atc-classification
- 31. Aregbeshola BS, Khan SM. Out-of-Pocket payments, catastrophic health expenditure and poverty among households in Nigeria 2010. Int J Health Policy Manag. 2018;7:798-806. doi: 10.15171/ijhpm.2018.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fadare JO, Enwere OO, Adeoti AO, Desalu OO, Godman B. Knowledge and attitude of physicians towards the cost of commonly prescribed medicines: a case study in three Nigerian healthcare facilities. Value Health Reg Issues. 2020;22:68-74. doi: 10.1016/j.vhri.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 33. Ganashree P, Bhuvana K, Sarala N. Critical review of drug promotional literature using the World Health Organization guidelines. J Res Pharm Pract. 2016;5:162-165. doi: 10.4103/2279-042X.185711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al-Aqeel SA, Al-Sabhan JF, Sultan NY. Analysis of written advertising material distributed through community pharmacies in Riyadh, Saudi Arabia. Pharm Pract. 2013;11:138-143. doi: 10.4321/s1886-36552013000300003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hailu HG, Gobezie MY, Yesuf TA, Workneh BD. Critical evaluation of the validity of drug promotion materials in Ethiopia. Drug Healthc Patient Saf. 2019;11:47-54. doi: 10.2147/DHPS.S200487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jha N, Sapkota Y, Shankar PR. Critical Evaluation of drug advertisements in a medical college in Lalitpur, Nepal. J Multidiscip Healthc. 2020;13:717-725. doi: 10.2147/jmdh.S259708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamath A, Hoovinahole S. A study of adherence of drug promotional literatures from various clinical specialties to the World Health Organization ethical criteria for drug promotion. J Pharm Negat Results. 2016;7(1):37. [Google Scholar]
- 38. Jhanwar A. Analysis of drug promotional literature and its adherence to WHO guidelines. J Pharm Sci Res. 2022;14(1):686-688. [Google Scholar]
- 39. Mikhael EM. Evaluating the reliability and accuracy of the promotional brochures for the generic pharmaceutical companies in Iraq using World Health Organization guidelines. J Pharm Bioallied Sci. 2015;7:65-68. doi: 10.4103/0975-7406.148781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mintzes B, Lexchin J, Sutherland JM, et al. Pharmaceutical sales representatives and patient safety: a comparative prospective study of information quality in Canada, France and the United States. J Gen Intern Med. 2013;28:1368-1375. doi: 10.1007/s11606-013-2411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Godman B, Egwuenu A, Haque M, et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life. 2021;11:528. doi: 10.3390/life11060528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akunne OO, Godman B, Adedapo AD, Truter I, Fadare J. Statin prescribing among hypertensive patients in southwest Nigeria: findings and implications for the future. J Comp Eff Res. 2016;5:281-288. doi: 10.2217/cer.15.65 [DOI] [PubMed] [Google Scholar]
- 43. Ogunleye OO, Fadare JO, Yinka-Ogunleye AF, Anand Paramadhas BD, Godman B. Determinants of antibiotic prescribing among doctors in a Nigerian urban tertiary hospital. Hosp Pract. 2019;47:53-58. doi: 10.1080/21548331.2018.1475997 [DOI] [PubMed] [Google Scholar]
- 44. Khakhkhar T, Mehta M, Shah R, Sharma D. Evaluation of drug promotional literatures using WHO guidelines. J Pharm Negat Results. 2013;4(1):33-38. [Google Scholar]
- 45. Gautam SR, Chugh PK, Sah RK, Tripathi CD. Critical appraisal of drug promotional literature using World Health Organisation guidelines. Int J Basic Clin Pharmacol. 2017;6:2014. [Google Scholar]
- 46. Naikwadi SA, Jadhav RB, Patil AP. Critical analysis of Indian drug promotional literature (DPL) using World Health Organization criteria for ethical medicinal drug promotion. IOSR J Dent Med Sci. 2017;16:49-54. doi:10.9790/0853-1609084954 [Google Scholar]
- 47. Gautam CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol. 2008;65:795-796. doi: 10.1111/j.1365-2125.2007.03089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mangla N, Gupta M. Evaluation of rationality of drug promotional literature using who ethical criteria for medicinal drug promotion. Int J Health Sci Res. 2018;8(4):55-62. [Google Scholar]
- 49. Parli K, Reema R, Devang R, Supriya M. Evaluation of promotional drug literature provided by medical representative at a tertiary care hospital. Int J Pharm Sci Res. 2017;8(4):1744. 1. [Google Scholar]
- 50. Sharma S, Akhoon N, Moe HW, Nair DR, Shashidhar V. A study of perceptions and exposure of drug promotional literature among clinicians in a teaching hospital. Perspect Clin Res. 2021;12:140-145. doi: 10.4103/picr.PICR_36_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shetty VV, Karve AV. Promotional literature: how do we critically appraise? J Postgrad Med. 2008;54(3):217-221. 1. [DOI] [PubMed] [Google Scholar]
- 52. Abena PM, Decloedt EH, Bottieau E, et al. Chloroquine and hydroxychloroquine for the prevention or treatment of COVID-19 in Africa: caution for inappropriate off-label use in healthcare settings. Am J Trop Med Hyg. 2020;102:1184-1188. doi: 10.4269/ajtmh.20-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haque M, Abubakar AR, Ogunleye OO, et al. Changes in availability, utilization, and prices of medicines and protection equipment for COVID-19 in an urban population of Northern Nigeria. J Res Pharm Pract. 2021;10:17-22. doi: 10.4103/jrpp.JRPP_20_92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olowofela A, Ayinbuomwan SA, Isah AO. Sources of information on the use of medicines utilized by resident doctors in a tertiary health care facility in Nigeria. Highland Med Res J. 2017;17(2):81-85. [Google Scholar]
- 55. Okoye I, Onyebuchi O. Utilization of drug information services in selected tertiary hospitals in Enugu State, Nigeria. J Curr Biomed Res. 2021;1(2):17-26. [Google Scholar]