Abstract
Introduction
S1P1 receptor modulators (S1P1-RM) are oral disease-modifying therapies (DMTs) for multiple sclerosis (MS). Several authorities have raised doubts that S1P1-RM are responsible for an increased risk of melanoma in patients with MS. We studied the in vitro effects of S1P1-RM on different melanoma cell lines to compare the effect of available S1P1-RM on the proliferation of human melanoma cells.
Methods
Four S1P1-RM were studied which are currently approved for managing MS, namely fingolimod (Gilenya®), siponimod (Mayzent®), ozanimod (Zeposia®), and ponesimod (Ponvory®). We tested these four drugs at different concentrations, including therapeutic doses (0.5, 1.6, 5.5, 18, and 60 µM), on human melanoma cell lines (501Mel cells, 1205LU cells, and M249R cells) to analyze in vitro cell proliferation monitored with the IncuCyte ZOOM live cell microscope (Essen Bioscience).
Results
At therapeutic doses, median confluence increased overall for all lineages: + 122% for ozanimod (p < 0.001), + 71% for ponesimod (p < 0.001), + 67% for siponimod (NS), and + 41% for fingolimod (p = 0.094). Ozanimod- and ponesimod-treated cells increased confluency in 501Mel, 1205LU, and M249R cell lines (p < 0.001).
Conclusion
These data suggest an increased proliferation of various melanoma cell lines with S1P1-RM treatments used at therapeutic concentrations for patients with MS and should raise the question of increased dermatologic surveillance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00429-6.
Keywords: Sphingosine-1 phosphate, Multiple sclerosis, Inflammation, Siponimod, Ozanimod, Ponesimod, Fingolimod, Melanoma cell lines
Key Summary Points
| There is some (but not sufficiently supported) evidence that drugs for MS such as fingolimod, siponimod, ozanimod, and ponesimod can increase the risk of malignancies, including skin cancers. |
| This problem is not well defined by data from randomized controlled trials, but several case reports have confirmed the occurrence of melanoma in patients treated with these new medications called S1P1 receptor modulators (S1P1-RM). |
| This study assessed the in vitro effects of S1P1-RMs on different melanoma cell lines to compare their effect on the proliferation of human melanoma cells. |
| An increased proliferation of various melanoma cell lines was observed suggesting (and confirming) the advisability of a careful dermatologic surveillance of patients treated with this new family of drugs. |
Introduction
Multiple sclerosis (MS) is the most common inflammatory immune-mediated demyelinating disease of the central nervous system (CNS) and represents the most common cause of neurological disability in young adults [1]. An early diagnosis of MS is essential to receiving timely treatment aimed at reducing permanent neurological disability. Many disease-modifying therapies (DMTs) with different immunomodulatory mechanisms have become available with a marked effect on MS inflammatory activity [2].
Early immunosuppression and immunomodulation have been the mainstay of therapeutic strategies in relapsing–remitting forms of MS (RRMS). Sphingosine 1-phosphate receptor modulators (S1P1-RM) are a class of treatment that enables the sequestration of lymphocytes within lymphatic tissue [3]. The primary mechanism of action of S1P1-RM in MS is through receptor binding on lymphocytes, resulting in the internalization of the receptor–drug complex resulting in the loss of responsiveness to the S1P gradient that drives lymphocyte egress from lymph nodes [4]. The reduction in circulating lymphocytes presumably limits inflammatory cell migration into the CNS [4]. Even if clinical trials or follow-up studies involving fingolimod, such as TRANSFORMS [5], FREEDOMS [6], and INFORMS [7], have not revealed any significant differences in the incidence of cancers, many pharmacovigilance cases have been reported recently [8], including lymphoma, HPV-related cancers, and cutaneous malignancies (Table 1). Several serious adverse events have been described due to the non-selective interactions with other receptors, specifically S1P3–5 [9]. The development of new-generation S1P1-RM with more enhanced selectivity, such as siponimod, ozanimod, and recently marketed ponesimod (March 2021) [10, 11], may result in improved safety and efficacy. Nevertheless, skin cancers such as melanoma remain a safety concern. Indeed, some publications have described new cases of skin cancer in patients treated with fingolimod [8, 12, 13] and ozanimod [14]. However, until now, no study has been performed to evaluate the safety of different doses of fingolimod, siponimod, ozanimod, and ponesimod and their effect on human melanoma cell line proliferation. This study analyzed the in vitro effects of S1P1-RM through established melanoma cell lines.
Table 1.
Prevalence of malignancies under S1P1-RM treatment
| Drug | Trials/duration | Malignancies prevalence |
|---|---|---|
| Fingolimod |
[3] FREEDOMS 24 months [5] TRANSFORM 12 months |
• 0.5 mg group (n = 429) o Basal cell carcinoma, n = 4 (0.9%) • 1.25 mg group (n = 425) o Basal cell carcinoma, n = 1 (0.2%) o Breast cancer, n = 1 (0.2%) o Malignant melanoma, n = 1 (0.2%) o Bowen’s disease, n = 1 (0.2%) • Placebo group (n = 418) o Basal cell carcinoma, n = 3 (0.7%) o Breast cancer, n = 3 (0.7%) o Malignant melanoma, n = 1 (0.2%) o Cervical carcinoma stage 0, endometrial cancer, n = 1 (0.2%) o Prostate cancer, n = 1 (0.2%) • 0.5 mg group (n = 429) o Melanocytic nevus, n = 28 (6.5%) o Basal cell carcinoma, n = 3 (0.7%) o Melanoma (including in situ), n = 3 (0.7%) o Breast cancer, n = 2 (0.5%) • 1.25 mg group (n = 420) o Melanocytic nevus, n = 42 (10.0) o Basal cell carcinoma, n = 2 (0.5%) o Melanoma (including in situ), n = 0 o Breast cancer, n = 2 (0.5%) • Interferon beta-1a n = 431 o Basal cell carcinoma, n = 1 (0.2%) o Melanoma (including in situ), n = 0 o Breast cancer, n = 0 |
| Fingolimod |
[7] INFORMS 36 months |
• 0.5 mg group (n = 336) o Basal cell carcinoma, n = 14 (4%) o Squamous cell carcinoma of skin, n = 6 (2%) o Malignant melanoma (including in situ), n = 1 (< 1%) o Breast cancer, n = 1 (< 1%) o Invasive lobular breast carcinoma, 0 o Non-Hodgkin lymphoma, 1 (< 1%) o Lung neoplasm, malignant, 1 (< 1%) o Ovarian cancer, 1 (< 1%) o Prostate cancer, 1 (< 1%) • Placebo group (n = 487) o Basal cell carcinoma, n = 9 (2%) o Squamous cell carcinoma of skin, n = 1 (< 1%) o Malignant melanoma (including in situ), n = 0 o Breast cancer, n = 0 o Invasive lobular breast carcinoma, n = 1 (< 1%) o Non-Hodgkin lymphoma, n = 0 o Lung neoplasm, malignant, n = 0 o Ovarian cancer, n = 0 o Prostate cancer, n = 1 (< 1%) |
| Siponimod |
[15] BOLD 24 months [16] EXPAND 36 months |
• 0.25 mg group (n = 50) o None • 0.5 mg group (n = 29) o Cervix neoplasm n = 1, (3.4%) • 1.25 mg (n = 43) o Basal cell carcinoma, n = 1 (2.3%) • 2 mg (n = 29) o None • 10 mg (n = 33) o None • 0.5 to 2 mg (n = 1099) o Skin neoplasms, malignant and unspecified, n = 14 (1%) o Basal cell carcinoma, n = 11 (1%) • Placebo (n = 546) o Skin neoplasms, malignant and unspecified, n = 8 (1%) o Basal cell carcinoma, n = 6 (1%) |
| Ozanimod |
[17] RADIANCE 24 months [18] SUNBEAM 12 months |
• 0.5 mg (n = 439) o Malignant melanoma in situ, n = 1 (0.2%) o Medulloblastoma, n = 1 (0.2%) o Basal cell carcinoma, n = 1 (0.2) • 1.0 mg (n = 434) o Invasive breast carcinoma, n = 1 (0.2%) o Keratoacanthoma, n = 1 (0.2%) o Basal cell carcinoma, n = 1 (0.2%) o Brest cancer, n = 1(0.2%) • Interferon beta-1a (n = 440) o Chronic lymphocytic leukemia, n = 1 (0.2%) o Basal cell carcinoma, n = 1 (0.2%) • 0.5 mg (n = 453) o Invasive breast carcinoma and basal cell carcinoma, n = 2 (0.4%) • 1.0 mg (n = 448) o Testicular seminar, n = 1 (0.2%) • Interferon beta-1a (n = 445) o None |
| Ponesimod |
[11] OPTIMUM 48 months |
• Ponesimod 20 mg (n = 565) o Skin malignant condition, n = 5 (0.9%) o Non skin malignant condition, n = 1 (0.2%) • Teriflunomide 14 mg (n = 566) o Skin malignant condition, n = 1 (0.2%) o Non skin malignant condition, n = 1 (0.2%) |
Methods
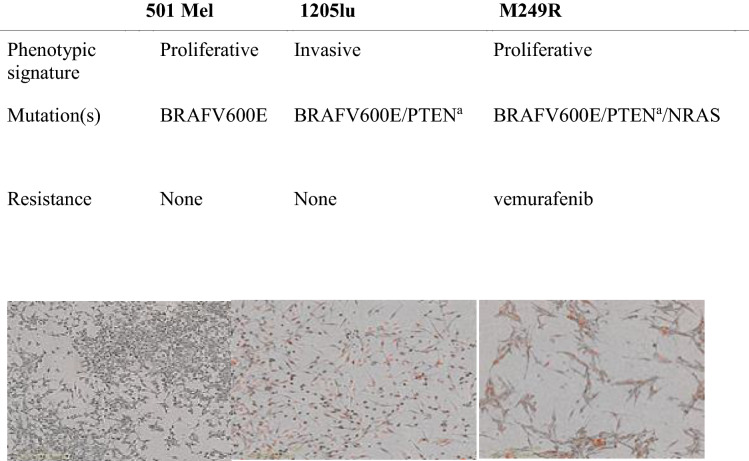
S1P1-RM were prepared at different concentrations ranging from subtherapeutic to supratherapeutic levels, specifically 0.5, 1.6, 5.5, 18, and 60 µM compared to the control group (0 µM). We have determined the therapeutic concentration from the therapeutic doses at 1.6–4.06 µM [19], 0.48–3.9 µM [20], 4.3–43 µM [21], and 0.57–2.27 µM [20] for fingolimod, siponimod, ponesimod, and ozanimod, respectively (Table 2). Cells and reagents for melanoma cell lines along with their maintenance were previously described in the literature [22]. Three cell lines harboring the activating oncogenic BRAF, a serine/threonine protein kinase mutation found in 50% of melanomas [23], were used in the experiments: 501Mel cells (from Dr. R. Halaban, Yale University School of Medicine, New Haven, CT, USA), M249R cells (from Dr. R. Lo, UCLA Dermatology, Los Angeles, CA, USA), and 1205LU cells (from Rockland, USA). These cell lines display distinct gene expression patterns and phenotypic behavior (Table 3): 501Mel cells show a predominantly proliferative and melanocytic differentiation phenotype, 1205LU cells are an invasive and de-differentiated state [22], and M249R are NRAS-mutated resistant to vemurafenib. Cell lines were used within 6 months between the resuscitation and experimentation and were authenticated via short tandem repeat (STR) profiling (Eurofins Genomics). The cells were routinely tested for the absence of mycoplasma by PCR. The experiments using melanoma cells derived from human tissue samples were conducted according to the principles of the Declaration of Helsinki. They had institutional approval (agreement no. 2137 from the French ministère de l’Enseignement supérieur et de la recherche). For live imaging, cells were transduced with NucLight Red lentivirus reagent (Essen Bioscience) and selected with puromycin (1 mg/ml). Culture reagent was purchased from Thermo Fisher Scientific [24]. Cell growth assays using a live cell imager were used to determine melanoma cell viability in response to ponesimod, ozanimod, siponimod, and fingolimod. Different nuclear-labeled fluorescent melanoma cells were treated with various concentrations of S1P1-RM, using clinical doses in people with MS for in vitro cell cultures. Cell proliferation was monitored with the IncuCyte ZOOM live cell microscope (Essen Bioscience), and images were taken every 4 h over 6 days. Confluency and number of nuclei (red cell objects) were quantified by the IncuCyte software. Normal melanocyte proliferation was measured by counting DAPI-stained nuclei.
Table 2.
Characteristics of S1P1-RM modulators
| Drug | Name | MW (g/mol) | Oral use (mg) | S1P1R | Study/Trial | FDA approval date | Indications | Reported skin cancer in clinical trial |
|---|---|---|---|---|---|---|---|---|
| Fingolimod |
Gilenya® FTY20 |
307.5 | 0.5–1.25 |
S1PR1 S1PR3 S1PR4 S1PR5 |
FREEDOMS FREEDOMS II [3] |
March 2019 |
RRMS SPMS | 0.2–6.2% |
| Siponimod |
Mayzent® BAF 312 |
516.5 | 0.25–2 |
S1PR1 S1PR5 |
BOLD [15] EXPAND [16] |
March 2019 |
RRMS SPMS |
0–2.6% |
| Ozanimod |
Zeposia® RPC 1063 |
404.5 | 0.23–0.92 |
S1PR1 S1PR5 |
RADIANCE [17] SUNBEAM [18] |
March 2020 |
RRMS SPMS |
0–0.45% |
| Ponesimod |
Ponvory® ACT128800 |
460.9 | 2–20 | S1PR1 |
OPTIMUM [11] |
March 2021 |
RRMS | 0–0.9% |
MW molecular weight, RRMS relapsing–remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis, FDA US Food and Drug Administration
Table 3.
Characteristics of melanoma cell lines
VigiBase® Query
Since 1978, the World Health Organization (WHO) mandates the Uppsala Monitoring Centre (UMC) to monitor worldwide drug safety. Data issued by each of the 130 national pharmacovigilance networks feed into the UMC database. VigiBase® [25] collects spontaneous reports, ensuring the preservation of the anonymity of patients and notifiers.
Sociodemographic characteristics (age, sex, notifier’s country) and details concerning the reported effect (suspected drugs, concomitant drugs, adverse drug reaction, and seriousness) are collected.
We queried VigiBase® for all notified cases of melanoma, and the following drugs: fingolimod, siponimod, ponesimod, and ozanimod, from November 14, 1967 (first cases reported) to November 20, 2022. In practice, the melanoma reports were defined by the Medical Dictionary for Regulatory Activities (MedDRA, version 25.1), with “melanoma of skin” as High Level Term (HLT) and ocular melanoma as Preferred Term (PT) [26].
Statistical Analysis
Numeric data were expressed as median (IQR) and discrete data as frequencies (percentages). The Shapiro–Wilk test and Levene’s test assessed the normality and heteroskedasticity of data. Differences in the confluence between experiences were assessed with the Mann–Whitney’s test for two groups and the Kruskal–Wallis’s test for three or more groups. If the null hypothesis of the Kruskal–Wallis’s test was rejected, post hoc pairwise analyses were performed using Dunn–Bonferroni’s test considering p value adjustment for multiple comparisons. Alpha risk was set to 5% (α = 0.05). Statistical analysis was performed using EasyMedStat (version 3.19; www.easymedstat.com) and GraphPad Prism software version 9.4.1 (GraphPad Software, San Diego, CA, USA).
Results
Proliferation of All Melanoma Cell Lines Under S1P1-RM at Therapeutic Doses
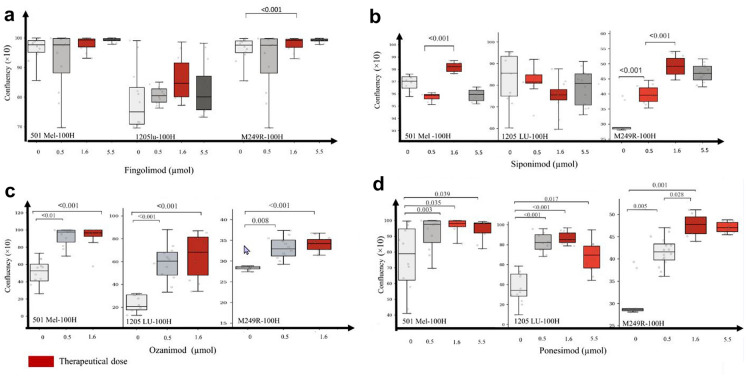
At therapeutic doses, median confluence increased overall for all lineages (Table 4): + 122% for ozanimod (p < 0.001), + 71% for ponesimod (p < 0.001), + 67% for siponimod (NS), and + 41% for fingolimod (p = 0.094).
Table 4.
Median confluence increased overall for all lineages at therapeutic doses
| Experience | Proliferation median (IQR) All lineages |
P value compared to the control |
|---|---|---|
| Ozanimod control | 28.6 (10.8) | – |
| Ozanimod 1.6 µM | 63.5 (53.9) | < 0.001 |
| Ponesimod control | 38.7 (31.2) | – |
| Ponesimod 1.6 µM | 85.7 (24.8) | < 0.001 |
| Ponesimod 5.5 µM | 81.9 (48.5) | < 0.001 |
| Fingolimod control | 45.6 (69.2) | – |
| Fingolimod 1.6 µM | 67.1 (60.2) | 0.094 |
| Siponimod control | 85.6 (67.6) | – |
| Siponimod 0.5 µM | 81.5 (53.1) | 0.71 |
| Siponimod 1.6 µM | 75.6 (43.3) | 0.231 |
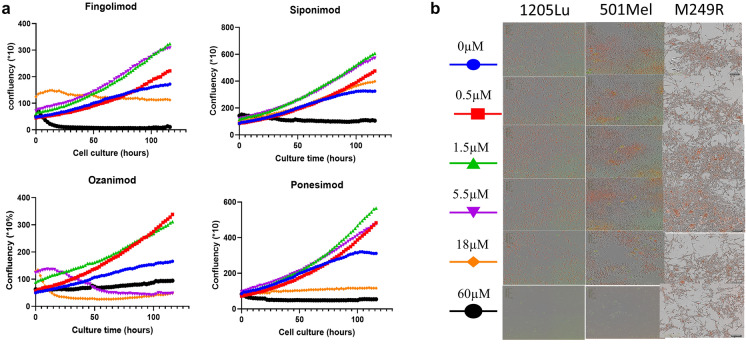
At supratherapeutic concentrations, the drugs crystallized, resulting in a lack of adherence of cells. We could not detect if low confluency was due to cell death or high proliferation provoked by cell detachment (Figs. S1 and S2 in the supplementary material). A saturation point of confluency was reached at those supratherapeutic concentrations for all four drugs, resulting in a ceiling effect. Confluence decreased at 18 concentrations in all lineages but siponimod (Fig. 1 and Fig. S2).
Fig. 1.
Confluences of 501Mel, 1205LU, and M249R under treatment with S1P1-RM over time. a Fingolimod, siponimod, ozanimod, and ponesimod induced growth of the non-metastatic 501Mel BRAF mutant melanoma cells, the metastatic 1205LU BRAF mutant, and M249R melanoma cells. Growth curves of 501Mel, 1205LU BRAF, and M249R melanoma cells labeled with the NucLight nuclear reagent were treated with vehicle (DMSO) or the indicated doses of S1P1-RM. Data were acquired in triplicate for 6 days using the live-cell imager IncuCyte. b Microphotographs showing the survival of S1P1-RM on the morphology of 501Mel cells (red nuclei, above) or the metastatic 1205LU BRAF mutant melanoma cells (below) at the end of the experimental course
Proliferation of Melanoma Cell Lines Under S1P1-RM at Therapeutic Doses
Median confluence values increased from 24.7 (IQR 3) to 34.6 (IQR 4) in M249R cell lines treated with fingolimod at therapeutic doses (p < 0.001) but did not increase in 501Mel (p = 0.285) and 1205LU (p = 0.069). Similar results were observed for siponimod-treated cells, with no increase in proliferation in 501Mel (p = 0.553) and 1205LU (p = 0.553) but increased confluency in M249R (p < 0.001). Conversely, second-generation S1P1-RM ozanimod and ponesimod-treated cells increased confluency in 501Mel, 1205LU, and M249R cell lines (p < 0.001).
Dose–Response Relationship
We observed a dose–response effect for therapeutic doses of ponesimod on M249R lineages with increasing confluences between 0 and 0.5 µM [28.6 (0.5) vs 41.6 (3.6), p = 0.005] and between 0.5 and 1.6 µM [41.6 (3.6) vs 47.8 (3.8), p = 0.028].
Our experiments did not observe such a dose–response effect for other drugs on cell lineages (Fig. 2).
Fig. 2.
Dose effect of S1P1-RM on different melanoma cells lines. a Confluences of 501Mel, 1205LU, and M249R cell lines after 100 h of treatment with fingolimod at 0 µM, 0.5 µM, 1.6 µM, and 5.5 µM. Proliferation was stable from 0 to 5.5 µM in 501Mel cells line (p = 0.17). Median values of 1205LU cell line confluences steadily increased 45.62 (IQR 27.57), 57.84 (IQR 10.04), 67.11 (IQR 25.43), and 56.95 (IQR 25.32) (p = 0.13). Differences of proliferation were found at 0.5 µM vs 0 µM (p = 0.006), 1.6 µM vs 0 µM (p < 0.001), 5.5 µM vs 0 µM (p < 0.001), and 5.5 µM vs 0.5 µM (p = 0.014) in M249R. b Proliferation of 501Mel under siponimod was not linear from 0.5 to 5.5 µM but confluences increased significantly at 1.6 µM vs 0.5 µM (p < 0.001) whereas proliferation was stable from 0 to 5.5 µM, and no significant differences were found in 1205 cell lines. Proliferation of M249R cell lines increased for 1.6 µM vs 0 µM (p < 0.001), 1.6 µM vs 0.5 (p < 0.001), and 0.5 µM vs 0 µM (p < 0.001) under siponimod treatment. c Proliferation of 501Mel was not linear under ozanimod from 0.5 to 5.5 µM but confluences increased significantly at 1.6 µM vs 0.5 µM (p < 0.001). Proliferation was stable from 0 to 5.5 µM in 1205LU whereas proliferation of M249R cell lines increased for 1.6 µM vs 0 µM (p < 0.001), 1.6 µM vs 0.5 (p < 0.001), and 0.5 µM vs 0 µM (p < 0.001). d Under ponesimod treatment, proliferation of 501Mel increased at 0.5 µM vs 0 µM (p = 0.035), 1.6 µM vs 0 µM (p = 0.003), and 5.5 vs 0 µM (p = 0.039). Proliferation of 1205LU increased at 0.5 µM vs 0 µM (p < 0.001), 1.6 µM vs 0 µM (p < 0.001), 5.5 µM vs 0 µM (p = 0.017), and 5.5 µM vs 0.5 µM (p = 0.046). Proliferation of M249R increased at 0.5 µM vs 0 µM (p = 0.005), 1.6 µM vs 0 µM (p < 0.001), 1.6 µM vs 0.5 µM (p = 0.028)
Characteristics of the Reports
As of November 20, 2022, 418 cases of melanomas were collected in VigiBase®, of which 412 were associated with fingolimod and 6 with siponimod. There is no case of melanoma reported with ponesimod or ozanimod to date in Vigibase®. Most cases concerned women: 286 (68.4%) among all reported melanomas. The most represented age range was the 45-to-64-year group, with 158 (37.8%) cases, with a median age of 47 years. More than a quarter of the notifications came from the USA (122 reports, 29.2%). Nearly all melanoma cases, 397 (95.0%) were deemed serious, with 346 reports (82.8%) medically serious and 4 fatal cases (1%). In 6 cases, melanoma was an ocular melanoma, the other preferred term reported was malignant melanoma. The most frequent co-reported MedDRA clinical terms were melanocytic nevus in 26 reports (6.2%), basocellular carcinoma in 21 reports (5.0%), and skin lesions in 17 reports (4.1%).
Discussion
There are increasing reports in the literature about the development of cutaneous melanoma following exposure to MS DMTs [3, 5, 12, 27–33] (Table 5) [33]. Indeed, previous studies [10] published real-world data that demonstrated an increased incidence of basal cell or squamous cell carcinoma or melanoma in those exposed to fingolimod and siponimod. However, the number of cases remains low compared to populational estimates, not reaching statistical significance. The occurrence of melanoma in these patients could be coincidental [28]. The relationship between cutaneous melanoma and exposure to MS DMTs is biologically plausible but approaches including the impact of these medications within in vitro studies of melanoma cell lines are lacking [34]. We tested the effects of incremental concentrations of S1P1-RM (0, 0.5, 1.6, 5.5, 18, or 60 μM) on a panel of human melanoma cell lines (501Mel and 1205LU) sensitive to treatment and M249R resistant to treatment. Proliferative melanoma cells (501Mel, M249R) rapidly form tumors, whereas invasive melanoma cells (1205LU) take weeks longer to initiate tumor growth. Melanoma cells resistant to the BRAF inhibitor are characterized by a lower proliferation rate [35].
Table 5.
Reported cases of melanoma after fingolimod treatment in patients with multiple sclerosis
| Cases sex (M/F)/age (years) | Melanoma type | Time of treatment (months) | Fingolimod (mg/day) | Skin phototype | Evolution | Familial history | Source |
|---|---|---|---|---|---|---|---|
| 1 case | NA | 24 | 1.25 | NA | Treatment stopped | NA | [3] |
| 1 case | NA | 36 | 1.25 | NA | NA | NA | [29] |
| 3 cases | NA | NA | 0.5 | NA | NA | NA | [5] |
| F/41 |
Melanoma Ex-nevo |
57 | 1.25 | II | Treatment stopped | NA | [27] |
| M/51 | SSM | 48 | 0.5 | NA | Treatment stopped | NA | [30] |
| 1F/41 | SSM | NA | 0.5 | II | Treatment stopped | NA | [12] |
| 1 F/52 | SSM | 61 | 0.5 | > 50 nevi | Treatment stopped | No history of skin cancer | [28] |
| 1 case | NA | NA | 0.5 | NA | NA | NA | [7] |
| 1 F/44 | SSM | 32 | NA | NA | NA | NA | [31] |
| 1 F/38 | SSM | 15 | NA | NA | NA | NA | [31] |
| 1 F/44 | SSM | 12 | NA | NA | NA | NA | [31] |
| 1 M/32 | SSM | 31 | NA | NA | NA | NA | [31] |
| 1 F/45 | SSM | 20 | NA | NA | NA | NA | [31] |
| 1 F/51 | Nodular melanoma | 48 | 0.5 | I | Treatment stopped | Melanoma of her mother | [13] |
| 1 F/52 | Unclassifiable malignant melanoma | 36 | NA | II | Treatment stopped | No personal or family history of melanoma | [8] |
| 1 F/48 | Thin cutaneous melanoma | 84 | NA | NA | NA | Numerous nevi and reported of a familiarity with epithelial tumors | [34] |
F female, M male, MS multiple sclerosis, SSM superficial spreading malignant melanoma, NA not applicable
Our findings indicate that ponesimod and ozanimod increase melanoma cell proliferation irrespective of their oncogenic status, phenotypic behavior, and therapeutic susceptibility. Ozanimod and ponesimod, the new generation of S1P1-RM modulators, have been marketed as having greater receptor selectivity, conveying better efficacy and safety, with no notable malignant events reported so far in the pilot studies [36]. Unexpectedly, these more recently approved S1P1-RM showed higher proliferation and dose effects in melanoma cell lines than with fingolimod and siponimod. Our results also support a dose–concentration effect as melanoma cell proliferation was associated with increased concentrations of ponesimod. In the phase III OPTIMUM clinical trial, five people with MS receiving ponesimod developed malignant skin cancers (two were basal cell carcinomas, two with excision of preexisting benign lesions (nevus), and one with malignant melanoma) compared with one patient with basal cell carcinoma in the teriflunomide group [11, 32].
Fingolimod and siponimod increase the proliferation of M249R cell lines, which are resistant to treatment with BRAF and NRAS co-mutation and known to rapidly form tumors. A previous study found that fingolimod treatment, starting from a concentration of 0.5 µM, significantly impaired LCP-Mel proliferation whereas it did not affect the proliferation of both GR-Mel and WM115 cells (human melanoma cell lines) until a concentration of 5 µM fingolimod was reached [34]. In this study, treatment was performed only for 48 h whereas we observed differences in 100 h and impairing was identified at high doses of fingolimod.
Several theories have described how S1P1-RM modulators may be related to skin cancer [10]: for example, fewer circulating lymphocytes possibly needed to identify and eventually eliminate malignant cells, activation of the IL-6/JAK/STAT3 identified to have a protumorigenic effect, and the relationship with tumor microenvironment influencing the secretion of VEGF-A [31].
Our study is the first to test S1P1-RM, including ponesimod, on various human melanoma cell lines for 6 days at different concentrations ranging from 0 to 5.5 µM. We could not analyze confluences for the supratherapeutic doses (18 and 60 µM).
This study has limitations. In vitro phenomena are often difficult to replicate in vivo as we do not have the dynamic interplay of immunosurveillance, loss of in vivo microenvironment, stromal, vascular, and immune cellular populations [37]. Therefore, we have added clinical reports in our review of the literature. We could have tested S1P1-RM on primary human melanocyte cells, but those cells do not proliferate as melanoma cell lines.
The results of this study, if correct, should alert patients to a potential increased risk of skin cancers, and the use of sunscreen or protective coverings to limit the impact of ultraviolet light exposure should be considered. The restricted use of S1P1-RM in people with MS who have a prior history of skin malignancies may also be considered. Monitoring measures such as skin examination at baseline to screen for precancerous skin lesions or additional risk factors, regular dermatologic monitoring, and patient education for regular self-skin controls are indicated for patients with MS under treatment with S1P1-RM.
Conclusion
Complementary studies are needed to evaluate whether the class of S1P1-RM drugs results in an increased propensity for risk of melanoma or other skin cancers given the presence of atypical skin lesions. Pending further evidence, we asked healthcare professionals to report any suspected adverse reactions involving skin malignancies and to consult the risk management plan submitted to European medical agencies before S1P1-RM administration. We suggest that skin examinations be regularly performed with close dermatological surveillance before and during therapy, and upon the identification of any suspicious lesions. People with MS treated with S1P1-RM should be advised to follow the usual guidelines for people at high risk of skin cancer until further in vitro clinical research clarifies the link between these drugs and the potential risk of melanoma development. These findings need to be further evaluated, including evidence from people within real-world prospective cohorts from other regions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Mickael Ohanna and Marie Hirondelle for their help in the laboratory using the IncuCyte ZOOM live cell microscope. We have no source of financial support for this work.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Caroline Ruetsch-Chelli, Darin Okuda, Fanny Rocher, Christine Lebrun-Frenay, Sophie Tartare-Deckert and Marcel Deckert contributed to the conception and design of the study, data acquisition and data analysis, drafted and critically reviewed the manuscript. Caroline Ruetsch-Chelli, Darin Okuda, Christine Lebrun-Frenay contributed substantially to acquire data, and commented on, revised, and approved the final version to the data acquisition.
Disclosures
Caroline Ruetsch-Chelli, Darin Okuda, Fanny Rocher, Christine Lebrun-Frenay, Sophie Tartare-Deckert and Marcel Deckert have nothing to declare.
Compliance with Ethics Guidelines
The experiments using melanoma cells derived from human tissue samples were conducted according to the principles of the Declaration of Helsinki. They had institutional approval (agreement no. 2137 from the French ministère de l’Enseignement supérieur et de la recherche).
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4:1–27. https://www.nature.com/articles/s41572-018-0041-4. [DOI] [PubMed]
- 2.Fronza M, Lorefice L, Frau J, Cocco E. An overview of the efficacy and safety of ozanimod for the treatment of relapsing multiple sclerosis. Drug Des Devel Ther. 2021;15:1993–2004. doi: 10.2147/DDDT.S240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappos L, Radue E-W, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 4.McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021;398:1184–1194. doi: 10.1016/S0140-6736(21)00244-0. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 6.Kappos L, O’Connor P, Radue E-W, et al. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84:1582–1591. doi: 10.1212/WNL.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–1084. doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 8.Michiels Y, Bugnon O, Michiels J-F, Mazellier S. Detection of a new melanoma in a patient treated with fingolimod. BMJ Case Rep. 2019;12:e227951. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6506067/. [DOI] [PMC free article] [PubMed]
- 9.Cree BAC, Goldman MD, Corboy JR, et al. Efficacy and safety of 2 fingolimod doses vs glatiramer acetate for the treatment of patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA Neurol. 2021;78:48–60. doi: 10.1001/jamaneurol.2020.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamatellos V-P, Rigas A, Stamoula E, Lallas A, Papadopoulou A, Papazisis G. S1P receptor modulators in multiple sclerosis: detecting a potential skin cancer safety signal. Mult Scler Relat Disorders. 2022;59. https://www.msard-journal.com/article/S2211-0348(22)00196-1/fulltext. [DOI] [PubMed]
- 11.Kappos L, Fox RJ, Burcklen M, et al. Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol. 2021;78:558–567. doi: 10.1001/jamaneurol.2021.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haebich G, Mughal A, Tofazzal N. Superficial spreading malignant melanoma in a patient on fingolimod therapy for multiple sclerosis. Clin Exp Dermatol. 2016;41:433–434. doi: 10.1111/ced.12770. [DOI] [PubMed] [Google Scholar]
- 13.Velter C, Thomas M, Cavalcanti A, et al. Melanoma during fingolimod treatment for multiple sclerosis. Eur J Cancer. 2019;113:75–7. https://linkinghub.elsevier.com/retrieve/pii/S0959804919302084. [DOI] [PubMed]
- 14.Cohen JA, Khatri B, Barkhof F, et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry. 2016;87:468–475. doi: 10.1136/jnnp-2015-310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappos L, Li DKB, Stüve O, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurol. 2016;73:1089–1098. doi: 10.1001/jamaneurol.2016.1451. [DOI] [PubMed] [Google Scholar]
- 16.Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18:1021–1033. doi: 10.1016/S1474-4422(19)30238-8. [DOI] [PubMed] [Google Scholar]
- 18.Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18:1009–1020. doi: 10.1016/S1474-4422(19)30239-X. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg MM. Multiple sclerosis review. P T. 2012;37:175–84. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3351877/. [PMC free article] [PubMed]
- 20.Jalkh G, Abi Nahed R, Macaron G, Rensel M. Safety of newer disease modifying therapies in multiple sclerosis. Vaccines. 2021;9:12. https://www.mdpi.com/2076-393X/9/1/12. [DOI] [PMC free article] [PubMed]
- 21.Tong J, Zou Q, Chen Y, et al. Efficacy and acceptability of the S1P receptor in the treatment of multiple sclerosis: a meta-analysis. Neurol Sci. 2021;42:1687–1695. doi: 10.1007/s10072-021-05049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard CA, Lecacheur M, Ben Jouira R, et al. A feed-forward mechanosignaling loop confers resistance to therapies targeting the MAPK pathway in BRAF-mutant melanoma. Cancer Res. 2020;80:1927–1941. doi: 10.1158/0008-5472.CAN-19-2914. [DOI] [PubMed] [Google Scholar]
- 23.Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519–527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebrun-Frenay C, Berestjuk I, Cohen M, Tartare-Deckert S. Effects on melanoma cell lines suggest no significant risk of melanoma under cladribine treatment. Neurol Ther. 2020;9:599–604. doi: 10.1007/s40120-020-00204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Ther Innov Regul Sci. 2008;42:409–419. [Google Scholar]
- 26.MedDRA Hierarchy | MedDRA. https://www.meddra.org/how-to-use/basics/hierarchy.
- 27.Conzett KB, Kolm I, Jelcic I, et al. Melanoma occurring during treatment with fingolimod for multiple sclerosis: a case report. Arch Dermatol. 2011;147:991–992. doi: 10.1001/archdermatol.2011.212. [DOI] [PubMed] [Google Scholar]
- 28.Robinson CL, Guo M. Fingolimod (Gilenya) and melanoma. BMJ Case Rep. 2016;2016:bcr2016217885. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5237781/. [DOI] [PMC free article] [PubMed]
- 29.Comi G, O’Connor P, Montalban X, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler. 2010;16:197–207. doi: 10.1177/1352458509357065. [DOI] [PubMed] [Google Scholar]
- 30.Havla JB, Pellkofer HL, Meinl I, Gerdes LA, Hohlfeld R, Kümpfel T. Rebound of disease activity after withdrawal of fingolimod (FTY720) treatment. Arch Neurol. 2012;69:262–264. doi: 10.1001/archneurol.2011.1057. [DOI] [PubMed] [Google Scholar]
- 31.Killestein J, Leurs CE, Hoogervorst ELJ, et al. Five cases of malignant melanoma during fingolimod treatment in Dutch patients with MS. Neurology. 2017;89:970–2. https://n.neurology.org/content/89/9/970. [DOI] [PubMed]
- 32.Albinet V, Bats M-L, Huwiler A, et al. Dual role of sphingosine kinase-1 in promoting the differentiation of dermal fibroblasts and the dissemination of melanoma cells. Oncogene. 2014;33:3364–73. https://www.nature.com/articles/onc2013303. [DOI] [PubMed]
- 33.Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs. 2018;32:939–949. doi: 10.1007/s40263-018-0564-y. [DOI] [PubMed] [Google Scholar]
- 34.Carbone ML, Lacal PM, Messinese S, et al. Multiple sclerosis treatment and melanoma development. Int J Mol Sci. 2020;21:2950. [DOI] [PMC free article] [PubMed]
- 35.Hoek KS, Eichhoff OM, Schlegel NC, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Can Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 36.Bravo GÁ, Cedeño RR, Casadevall MP, Ramió-Torrentà L. Sphingosine-1-phosphate (S1P) and S1P signaling pathway modulators, from current insights to future perspectives. Cells. 2022;11:2058. doi: 10.3390/cells11132058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marie Vincent K, Postovit L-M. Investigating the utility of human melanoma cell lines as tumour models. Oncotarget. 2017;8:10498–509. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5354675/. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.