Abstract
Introduction
This study aimed to investigate the relationship between gene polymorphisms and clinical factors with the concentrations of valproic acid (VPA) in adult patients who underwent neurosurgery in China.
Methods
A total of 531 serum concentration samples at steady state were collected from 313 patients to develop a population pharmacokinetic (PPK) model. Data analysis was performed using nonlinear mixed effects modeling. Covariates included demographic parameters, biological characteristics, and genetic polymorphism. Bootstrap evaluation showed that the final model was stable. Sensitive analysis was performed to verify the relationship between gene polymorphisms and concentrations of VPA. Linear regression was used to analyze the relationship between VPA concentration, ANKK1, and daily dosage.
Results
In the recruited patients, 17 of 25 single-nucleotide polymorphism distributions were consistent with the Hardy–Weinberg equilibrium. A one-compartment model with first-order absorption and elimination was developed for VPA injections. VPA clearance was significantly influenced by three variables: sex (17.41% higher in male than female patients), body weight, and the ANKK1 gene. Typical values for the elimination clearance and the volume of central compartment were 0.614 L/min and 23.5 L, respectively. The model evaluation indicated the stable and precise performance of the final model. After sensitive analysis using Kruskal–Wallis and Mann–Whitney U tests, we found that patients with AA alleles had higher VPA concentrations than those with GG and AG alleles. Linear regression models showed that gene polymorphisms of ANKK1 had little effects on VPA concentration.
Conclusion
A PPK model of VPA in Chinese Han patients was successfully established; this can be helpful for model-informed precision-dosing approaches in clinical patient care, and for exploring the mechanism of VPA-induced weight gain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00419-8.
Keywords: Valproic acid, Population pharmacokinetic model, Neurosurgery, ANKK1, Weight gain
Key Summary Points
| Why carry out this study? |
| Our previous studies have shown that patients who use valproic acid after neurosurgery are at higher risk of liver injury, and the toxicity threshold of blood valproic acid concentration is significantly lower than stated in the instructions, making it difficult for physicians to determine the appropriate dose of valproic acid in such patients. Therefore, the pharmacokinetics of valproic acid in patients following neurosurgery must be studied. |
| What was learned from the study? |
| This is the first time that ANKK11 gene polymorphisms have been associated with the pharmacokinetics of valproic acid. |
| VPA clearance was significantly influenced by three variables: patient sex, body weight, and the ANKK1 gene, but gene polymorphisms of ANKK1 had little effect on VPA concentration. |
| The results showed that the pharmacokinetics of valproic acid in patients following neurosurgery are comparable to those in patients who did not undergo neurosurgery. |
Introduction
Valproic acid (VPA, valproate sodium) is a broad-spectrum antiepileptic drug (AED) used for the prevention and treatment of various types of epilepsy. VPA has also been used to treat bipolar disorder, schizophrenia, personality disorders, and migraines [1, 2]. However, large interindividual variability in VPA efficacy has been observed in our and in other studies [3, 4]. Blood concentration monitoring is often used to ensure the safety and effectiveness of treatment. However, a small number of patients are still at risk of treatment failure or adverse reactions, such as pancreatitis, teratogenicity, or liver injury. The metabolism of VPA is very complex and affected by many factors. The major metabolic pathways of VPA comprise glucuronidation, mitochondrial β-oxidation, and the cytochrome P450 (CYP)-mediated ω-oxidation pathway [5]. Pharmacogenetic polymorphism is an important reason for the significant individual differences in the efficacy and toxicity of VPA [6].
Pharmacogenomic studies of VPA have mainly focused on well-known genetic polymorphisms in the metabolic enzymes, such as UDP glycosyltransferase (UGT) and CYP [7, 8]. Genetic variants in leptin receptor (LEPR), α catalytic subunit of adenosine monophosphate-activated protein kinase (AMPK), and ankyrin repeat kinase domain containing 1 gene (ANKK1) have been reported in association with weight gain induced by VPA, which could influence the expression of nuclear receptors and energy homeostasis through the regulation of multiple signaling pathways [9]. The influence of LEPR variants on adverse effects induced by antipsychotic drugs is currently being investigated [10, 11]. Drug transporters also play key roles in the absorption, distribution, and excretion of therapeutic agents. The ATP binding cassette (ABC) family, a major efflux transporter superfamily, plays an important role in the pharmacokinetic/pharmacodynamic (PK/PD) variability of AEDs. Some members of the ABC superfamily participate in VPA transport, including ABCC2 [12]. In this study, 25 single-nucleotide polymorphisms (SNPs), including CYP and UGT families, were examined to identify their influence on VPA concentrations.
Post-traumatic epilepsy refers to seizures that occur after brain injury. A study in The Lancet has shown that, compared with patients who have not suffered a brain injury, the relative risk of developing epilepsy for people older than 15 years was 12.2 times higher after severe traumatic brain injury [13]. Based on the aforementioned research results, VPA has been commonly used for epilepsy prevention after neurosurgery in China. Our previous study has shown that the occurrence of liver injury after neurosurgery is associated with the concentration of VPA and gene polymorphisms [14].
Based on the above background, the present study aimed to explore the effects of genetic variants as well as other clinical factors on VPA concentration in a cohort of Chinese Han adult patients after neurosurgery. Twenty-five SNPs located in 17 genes correlated with the metabolizing enzymes and transporters of VPA were identified, and their relationships with VPA plasma concentrations were evaluated.
Methods
Study Design and Population Characteristics
This is a prospective nonintervention study. We established a population pharmacokinetic (PPK) model to assess the effect of genetic polymorphisms and other clinical factors on VPA concentrations. Between September 2019 and August 2021, 313 Chinese Han patients ≥ 18 years of age who undertook neurosurgery and were administered VPA injections at Shanxi Provincial People’s Hospital were enrolled in this study. Written informed consent was obtained from all patients or their guardians before recruitment. The study was approved by the Ethics Committee of the Shanxi Provincial People’s Hospital. To avoid the complexity of PK interactions caused by polytherapy, the influence of a single AED in combination with VPA treatment was analyzed. Therefore, the collected data correspond to monotherapies. The patients were administered 0.4 g VPA two to three times per day. The initial dose of VPA was based solely on the experience of the physician, and the physician adjusted the dose according to the patients’ clinical manifestations and VPA concentration. The steady-state drug concentrations were generally reached after five doses [15]. Furthermore, one of the characteristics of population pharmacokinetics is that it does not require the drug concentrations to be stable. Irrespective of whether the drug concentration is in an ascending phase or a steady phase, the population pharmacokinetic study can be conducted. Therefore, after at least 3 days of VPA administration, the concentration of VPA was measured. Trough concentration was defined as the concentration of the drug 30 min before the next dose and peak concentration as that 1 h after dosing.
The inclusion criteria were as follows: patients who underwent neurosurgery and received a VPA injection; ≥ 18 years of age; no presentation of history of viral or alcoholic hepatitis; and normal liver function before VPA administration. Both male and female patients were enrolled. The exclusion criteria included the following: patients who were treated with VPA for < 5 days; < 18 years of age; presentation with hepatic dysfunction before medication; history of viral or alcoholic hepatitis; patients currently suffering sepsis; incomplete clinical information; lack of patient’s VPA concentration measurement during treatment; or administration of other medications that affect VPA concentration (such as phenobarbital, cimetidine, or carbapenems).
The patients’ demographic information, including gender, age, body mass index (BMI), and medication details (VPA dosing history, medication history, and laboratory tests) was obtained through the hospital information system. According to previously published reports, SNPs have been related to VPA metabolism and toxicity [2, 7, 9, 16–29]. As such, the population that was enrolled in the final cohort was investigated for the following SNPs: ABCC2 (rs2273697), CYP2C9 (rs1799853), LEPR (rs1137101), SCN1A (rs2298771), SCN2A (rs17183814), COL1A1 (rs1800012), ANKK1 (rs1800497), ABCB1 (rs2032582), GABRA1 (rs2279020), CYP1A1 (rs2606345), AMPK (rs10789038), SH2B1 (rs3888190), CYP2C19 (rs1057910, rs12769205, rs3758581, rs4244285, rs4986893), UGT2B7 (rs12233719, rs7439366, rs7668258), UGT1A6 (rs2070959, rs28898617, rs6759892), mitochondrial DNA (mtDNA) polymerase γ (POLG) (rs3087374), and superoxide dismutase 2 (SOD2) (rs4880).
Detection of Valproic Acid
Serum concentrations of VPA were measured by fluorescence polarization immunoassay using the ARCHITECT platform (Abbott Laboratories, Abbott Park, IL, USA). The calibration curve range of this assay was between 2.0 and 150 μg/mL. The coefficient of variation assay was lower than 10%, with a sensitivity of 0.7 μg/mL.
Genotyping
Blood samples were collected from all participants, and 2% ethylenediaminetetraacetic acid (EDTA) was added to prevent coagulation. Genomic DNA was extracted from peripheral blood leukocytes using a DNA extraction kit (Tiangen Biotech Company, Beijing, China) and stored at – 80°C. Genotyping assays for the selected SNPs were designed using the MassARRAY platform (Agena Bioscience, San Diego, CA, USA). Approximately 20 ng of genomic DNA from each sample was used for genotyping. Locus-specific PCR and detection primers were designed using Assay Designer 4.0 (Sequenom, San Diego, CA). After amplification of the DNA samples via multiplex PCR, allele detection was performed using matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. After quality control, 25 SNP were analyzed in 313 individuals.
Statistical and Sensitive Analysis
VPA concentration–time data were analyzed using NONMEM (Version 7.3) integrated with Pirana [30]. The compiler was gfortran in Windows 7. The final model was validated after bootstrapping with WFN [version wfn730, http://wfn.sourceforge.net/]. Diagnostic plots were drawn using the ggplot2 package in R 3.5. The first-order conditional estimation method with interaction (FOCEI) algorithm was applied for parameter estimation. The Hardy–Weinberg equilibrium (HWE) test was performed with the appropriate chi-squared test using Perl software. P < 0.05 indicated a lack of agreement with HWE. Linear regression was used to analyze the relationship among VPA concentration, ANKK1, and daily dosage.
In addition, to verify the relationship between gene polymorphisms and VPA concentrations, we performed a sensitive analysis. To eliminate the influence of VPA dose and weight on plasma VPA concentration, CVPA was calculated and applied. The CVPA was calculated as: (VPA plasma concentration)/(daily VPA dose/weight). The patients included in this study varied in age, gender, and BMI. To eliminate these confounding factors, analyses of covariance were used for association tests between genotype and CVPA as the dependent variable, with gender, age, and BMI as the covariates. The Kruskal–Wallis test and Mann–Whitney U tests were used for multiple comparisons. Two-sided P-values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSSAU (Version 21.0; https://www.spssau.com).
Model Building
A one-compartment model was applied according to published papers [8]. Owing to Taylor expansion [31], the between subject variability (BSV) was described by an exponential model and the residual variance (RV) by the additive model (ADD):
where, Y is the measured concentration and F the corresponding predicted concentration. The symbol ε represents the residual error between F and Y, with a mean of zero and variances of σ2 (i.e., ε ~ N[0, σ2]).
The covariates were included into the model using stepwise covariate modeling (SCM) implemented in Perl-speaks-NONMEM (PsN). Significance levels (forward inclusion α = 0.01, backward elimination α = 0.005) were predefined. The relationship of continuous covariates, such as age and body weight (BW), is described by
where Pj is the model predicted value of a PK parameter P in the jth patient for a given covariate value-Covj. θcov represents a scaling factor for the influence of that covariate. Covj/Covm is the normalized transformation for Covj, where Covm is the median of covariate. TVP represents the typical parameter value.
The relationship of categorical variates, such as sex, was
where Covj is the numeric index value of the covariate and θcov represents a multiplicative factor for the influence of that covariate. For example, if θcov is negative, the effect is a decrease in the typical value; conversely, an increase in the typical value is noted for positive θcov values. The remaining parameters were as in the continuous expression.
The covariates selected by PsN were based on the maximum of the drop of objective function value. In the first step, the model with the highest drop in objective value was retained and taken forward to the next step. However, we added the criteria that the interindividual and residual variabilities should decrease. After the forward modeling run finished, we selected qualified models with a drop of objective function value (OFV) larger than 6.64 (α = 0.01, χ2, df = 1) and compared them. Only the model with the highest decrease in interindividual variabilities could be taken forward to the next step. The forward selection step stopped when there were not any increased interindividual variabilities, even if a forward model returned the required drop of objective function value (larger than 6.64).
An increase in OFV of at least 7.87 (α = 0.005, χ2, df = 1) was used as a criterion for retaining relationships during backward elimination. The final population model was the one that contained all significant covariates after the backward elimination process.
Evaluation and Validation
The bias between the final model and bootstrapping median was calculated. Goodness of fit (GOF) of observed and predicted concentrations of VPA were evaluated through graphics drawn using ggplot2. The bootstrap method was applied to assess the robustness of the final model. Five hundred datasets were generated by resampling through WFN. Bootstrapping results are reported as median parameters and 95% confidence intervals based on 2.5th and 97.5th percentiles.
Results
Patient Demographic Data and Genotyping
A total of 313 patients who underwent neurosurgery were included in the model-building group. From these patients, 531 blood samples were collected and analyzed for VPA concentrations (range, 27.83–102.21 μg/mL). VPA was administered intravenously two to three times daily. None of the samples were below the lower limit for quantification. A summary of the demographic characteristics of the patients used for model development and evaluation is provided in Table 1.
Table 1.
Demographic data of patients evaluated in the population pharmacokinetic analysis of valproic acid
| Variable | Value |
|---|---|
| No. of patients (males/females) | 313 (175/138) |
| No. of VPA concentration samples | 531 |
| Age (years) | 56 (18–86) |
| Weight (kg) | 65 (40–113) |
| BMI | 23.32 (14.69–41.51) |
| ALT (U/L) | 17.52 (3.56–38.73) |
| AST (U/L) | 18.46 (9.06–39.96) |
| Albumin (g/L) | 37.04 (19.54–49.74) |
| SCr (μmol/L) | 61.02 (20.18–495.46) |
| VPA daily dose (mg/kg) | 14.41 (7.08–30) |
| VPA serum concentration (μg/mL) | 52.15 (27.83–102.21) |
ALT alanine transaminase, AST aspartate transaminase, BMI body mass index, SCr serum creatinine, VPA valproic acid
Continuous data are presented as median (range) unless specified otherwise; categorical data, as the number of patients
Reference ranges: ALT: 0–40 U/L; AST: 0–40 U/L; SCr: 53–106 μmol/L
The frequencies of selected polymorphisms were comparable to those of published data for Chinese people. Deviations from HWE for the various SNPs were assessed using the Chi-squared test, and 17 of 25 genotypes were found to be in equilibrium (P > 0.05) (Supplementary Table 1).
Base Model
The minimal OFV of the base model was 3120.6. The one-compartment model that was parameterized in terms of the elimination clearance (CL) and volume of central compartment (V) was selected as the base model (Table 2).
Table 2.
Population pharmacokinetics of the base model
| Value | %RSE | |
|---|---|---|
| CL (L/min) | 0.67 | 1.9 |
| V (L) | 18.5 | 6.9 |
| BSV_CL | 0.258 | 6.9 |
| BSV_V | 0 fixed | – |
| RV | 9.455 | 6.4 |
BSV interindividual variability, RV residual variability, RSE relative standard error
The expression of the final model is as follows:
After forward selection and backward elimination, covariates that remained significant in the final model were sex on CL, BW on CL, and ANKK1 on V. The final model (OFV = 3058.1) showed a decrease in OFV of 62.5 points compared with that of the base model (OFV = 3120.6). The interindividual variability fluctuated from 25.8 to 24% for CL. The additive model was finally selected to describe the between-subject variability. The standard deviation was 8.983 mg/mL, which was the square root of the variance 80.7 in the final model.
The whole process of establishing the final model including stepwise forward addition and backward elimination is displayed in Table 3. No value was excluded from the final model after the backward elimination because the minimum increase of OFV was 16.7, which was larger than the required 7.87.
Table 3.
Results of model building
| Model | Covariates | OFV | DOFV | Method |
|---|---|---|---|---|
| 1 | NONE | 3120.6 | – | – |
| 2 | CL–SEX | 3084.2 | – 36.2 | Stepwise |
| 3 | V–ANKK1 | 3066.3 | – 17.9 | Stepwise |
| 4 | CL–BW | 3058.1 | – 8.2 | Stepwise |
| 5 | CL–SEX is eliminated | 3076.6 | + 18.5* | Backward |
| 6 | CL–ANKK1 is eliminated | 3074.8 | + 16.7* | Backward |
OFV minimal objective function value, DOFV drop of OFV; *DOFV was calculated using the OFV in the model (5/6) minus that of model 4 (final model). Method: stepwise inclusion or backward elimination. BW body weight
A linear regression analysis was performed to investigate the relationships among ANKK1, body weight, daily dosage, and VPA concentration. The final model expression is given below:
Model Evaluation
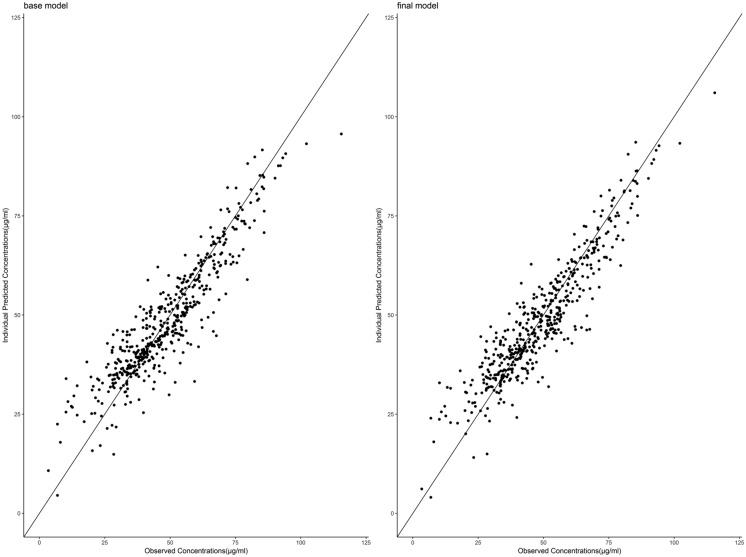
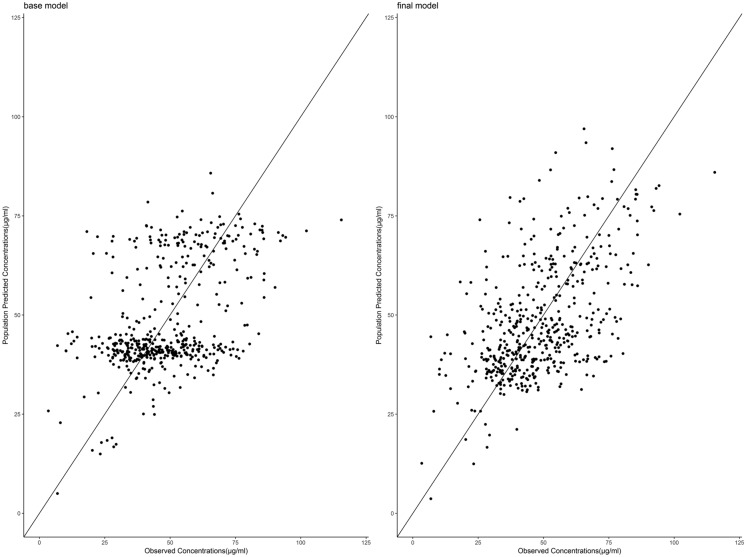
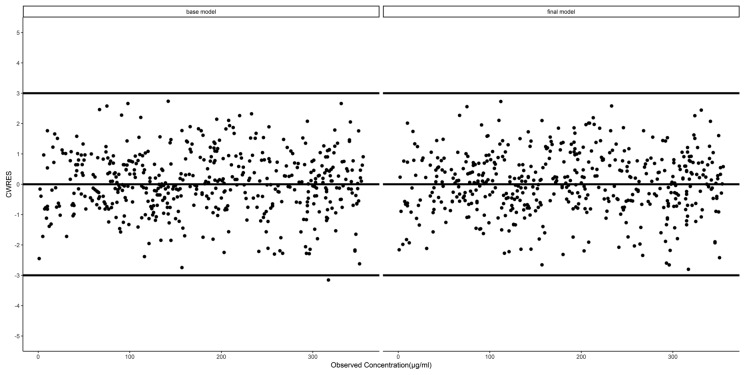
The diagnostic plots of the base and final models are presented as the observed versus predicted individual or population concentrations in Figs. 1 and 2, respectively. The conditional weighted residuals were homogeneously distributed over the observed concentrations (Fig. 3) and the majority fell within a ± 3 range.
Fig. 1.
Plot of valproate individual concentrations predicted by the final model versus measured concentrations
Fig. 2.
Plot of valproate population concentrations predicted by the final model versus measured concentrations
Fig. 3.
Conditional weighted residual (CWRES) versus observed concentration levels in the final model
Four hundred ninety-nine bootstraps were minimized successfully from 500 runs. The main parameter (CL and V) estimates in the final model were within ± 3% compared with those in the bootstrap median. Parameter estimates were within 95% confidence interval and are listed in Table 4.
Table 4.
Parameter estimates from the final population model and bootstrap validation
| Parameter | Final model | Bootstrap | |
|---|---|---|---|
| Estimate (RSE/%) | Median | 95% CI | |
| CL (L/min) | 0.614 (2.5) | 0.613 | (0.5850, 0.6460) |
| V (L) | 23.5 (9.4) | 23.5 | (19.6, 29.1) |
| CL–sex | 0.174 (24.0) | 0.175 | (0.0925, 0.266) |
| V–ANKK1 (if AG) | – 0.233 (39.2) | – 0.227 | (– 0.416, – 0.0037) |
| V–ANKK1 (if AA) | – 0.464 (17.0) | – 0.458 | (– 0.5910, – 0.2560) |
| CL–BW | 0.267 (36.1) | 0.267 | (0.0824, 0.4330) |
| BSV_CL | 0.240 (7.3) | 0.24 | (0.202, 0.275) |
| BSV_V | 0 (fixed) | − | − |
| RV | 8.983 (6.7) | 8.933 | (7.785, 10.150) |
Based on the results of the PPK model, we found that ANKK1 gene polymorphisms were associated with VPA concentration; therefore, we performed a sensitive analysis to verify the relationship between ANKK1 and CVPA. After Kruskal–Wallis and Mann–Whitney U tests, we found that patients with AA alleles had higher concentrations than those with GG and AG alleles (Table 5).
Table 5.
Results of Mann–Whitney U test
| Two independent samples | No. of patients | Median | P-value | |||
|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | |
| CVPA-AA | CVPA-GG | 54 | 121 | 4.281 | 3.383 | < 0.01 |
| CVPA-AA | CVPA-AG | 54 | 138 | 4.281 | 3.557 | < 0.05 |
| CVPA-GG | CVPA-AG | 121 | 138 | 3.383 | 3.557 | 0.256 |
Discussion
Based on our previous study, the toxicity threshold was much lower than the label for VPA in post-neurosurgery patients [14]. Therefore, for these patients, the VPA concentration has stricter requirements. On searching PubMed, Web of Science, and Embase databases, we found that there are no reports on the effects of neurosurgery on VPA metabolism. To our knowledge, this is the first PPK study to systematically investigate clinical factors and genetic polymorphisms, including metabolic enzymes (CYPs and UGTs), ABC transporters, ANKK1, and other genetic variants, on the PK of VPA in adult patients who underwent neurosurgery.
We built a reliable population model of VPA in Chinese Han adult patients. The final model, which included the effect of sex and BW on CL and ANKK1 on V, showed a significant drop in OFV (62.5). The final model indicated that the covariate of BW had a positive influence on CL by power function. The normalized transformation BW/62 could be put into simple practice. The influence of sex on CL by linear function shows that VPA metabolism is 17.41% faster in males compared with females, which is consistent with previous reports [32]. The typical value of CL is similar to that of patients who did not undergo neurosurgery [4]. This shows that the PKs of valproic acid in patients following neurosurgery are comparable to those in patients who did not undergo neurosurgery. To avoid the effect of polytherapy on VPA metabolism, our study excluded other drugs that may affect the PK of VPA. This allowed us to better understand the PK of VPA. Therefore, we speculate that the results of our study are applicable to Chinese Han patients treated solely with VPA.
The results of the PPK model showed that the volume of central compartment (V) of ANKK1 with AG alleles was reduced by 23.3% compared with that with GG alleles and the V of the AA alleles was reduced by 46.4% compared with that with GG alleles. A decrease of the volume of central compartment resulted in an increase in steady-state concentrations. The sensitive analysis showed that the patients with the AA ANKK1 alleles had higher concentrations than those with GG and AG alleles. This is the first report to study the relationship between ANKK1 and VPA concentration.
Dopamine 2 receptor gene (DRD2) polymorphisms have been found to be associated with altered perception of food reward and weight gain [33, 34]. The most commonly tested and referred to DRD2 polymorphism is ANKK1 (rs1800497). Early reports have demonstrated that in patients with epilepsy with AG and GG genotypes, VPA treatment is associated with increased weight gain compared with those with genotype AA [9]. We hypothesized that patients with AG and GG genotypes weighed more and had larger apparent volumes of distribution, resulting in lower VPA concentrations than those without these genotypes. However, linear regression models showed that gene polymorphisms of ANKK1 had little effects on VPA concentration. We presume that this may be because long-term VPA treatment is required for genetic polymorphisms to induce a considerable change in the body weight of patients; nonetheless, the treatment course of most patients included in this study was not longer than 10 days.
There are some limitations of this study. First, we only added a single antiepileptic drug to avoid the complexity of PK interactions caused by multiple-drug coadministration. However, in many clinical cases, it is necessary to combine VPA with other AEDs; the relevant covariates were not included in our model. Second, a constant coefficient of variation (CCV) model was not found for the residual model. When a combined ADD/CCV model was applied, the variance of CCV was close to the 0 boundary. As a result, the ADD model for residual variance was selected both for the base and the final model. Third, given the general assumption that VPA is not metabolized by ANKK1, the mechanism by which ANKK1 affects VPA concentration requires further study.
Conclusions
We describe in detail the PK parameters of VPA when administered alone through a PPK model; VPA CL was significantly influenced by three variables: sex (17.41% higher in male than female patients), body weight, and ANKK1. The results of the PPK model showed that the V of the ANKK1 with AG alleles was reduced by 23.3% compared with that with the GG alleles, whereas the V with the AA alleles was reduced by 46.4% compared with that with the GG alleles. Sensitive analysis showed that patients with AA alleles had higher concentrations than those with GG and AG alleles. To our knowledge, this is the first report that demonstrated that ANKK1 gene polymorphisms are associated with the PK of VPA. These findings can be useful to better understand the VPA PK and establish individual drug dosage regimens in Chinese Han adult patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Professor Hui Liao, Shuangshuang Tian and Fang Zhang for their help. We thank the participants of the study.
Funding
This research was funded by Shanxi Provincial Social Development Project (ID: 201903D321033), and the Rapid Service Fee was funded by Fundamental Research Program of Shanxi Province (ID: 202103021224384).
Author Contributions
Conceptualization: Jiuhong Ma, Xiuzhao Fan and Jinlin Guo; Methodology: Jiuhong Ma, Xinfeng Cai and Jinlin Guo; Formal analysis and investigation: Xiuzhao Fan, Xinfeng Cai, Yuanping Li and Hongming Ji; Writing—original draft preparation: Jinlin Guo; Writing—review and editing: Jiuhong Ma, Xiuzhao Fan, Xinfeng Cai, Yuanping Li and Hongming Ji; Funding acquisition: Jiuhong Ma; Resources: Hongming Ji and Yuanping Li; Supervision: Hongming Ji and Yuanping Li.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The study was approved by the Ethics Committee of the Shanxi Provincial People’s Hospital (No. 2019–36) and was conducted following the legal requirements and tenets of the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from all patients or their guardians before recruitment.
Disclosure
The authors declare no conflict of interest.
Data Availability
The data that support the findings of this study are available from the corresponding author (Professor Jinlin Guo) upon reasonable request.
Footnotes
Jiuhong Ma and Xiuzhao Fan contributed equally to this paper.
References
- 1.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10(7):685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- 2.Stewart JD, Horvath R, Baruffini E, Ferrero I, Bulst S, Watkins PB, et al. Polymerase gamma gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology. 2010;52(5):1791–1796. doi: 10.1002/hep.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haerian BS, Baum L, Tan HJ, Kwan P, Raymond AA, Saruwatari J, et al. SCN1A IVS5N+5 polymorphism and response to sodium valproate: a multicenter study. Pharmacogenomics. 2012;13(13):1477–1485. doi: 10.2217/pgs.12.127. [DOI] [PubMed] [Google Scholar]
- 4.Guo J, Huo Y, Li F, Li Y, Guo Z, Han H, et al. Impact of gender, albumin, and CYP2C19 polymorphisms on valproic acid in Chinese patients: a population pharmacokinetic model. J Int Med Res. 2020;48(8):300060520952281. doi: 10.1177/0300060520952281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo HL, Jing X, Sun JY, Hu YH, Xu ZJ, Ni MM, et al. Valproic acid and the liver injury in patients with epilepsy: an update. Curr Pharm Des. 2019;25(3):343–351. doi: 10.2174/1381612825666190329145428. [DOI] [PubMed] [Google Scholar]
- 6.Klotz U. The role of pharmacogenetics in the metabolism of antiepileptic drugs: pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 2007;46(4):271–279. doi: 10.2165/00003088-200746040-00001. [DOI] [PubMed] [Google Scholar]
- 7.Wen ZP, Fan SS, Du C, Yin T, Zhou BT, Peng ZF, et al. Influence of acylpeptide hydrolase polymorphisms on valproic acid level in Chinese epilepsy patients. Pharmacogenomics. 2016;17(11):1219–1225. doi: 10.2217/pgs-2016-0030. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Chen Y, Zhao M, Guo Y, Wang Z, Zhao L. Population pharmacokinetics of valproic acid in epileptic children: effects of clinical and genetic factors. Eur J Pharmaceut Sci. 2018;122:170–178. doi: 10.1016/j.ejps.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Wang X, Zhou Y, Ni G, Su Q, Chen Z, et al. Association of LEPR and ANKK1 gene polymorphisms with weight gain in epilepsy patients receiving valproic acid. Int J Neuropsychopharmacol. 2015;18(7):pyv021. [DOI] [PMC free article] [PubMed]
- 10.Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86(9):4434–4439. doi: 10.1210/jcem.86.9.7842. [DOI] [PubMed] [Google Scholar]
- 11.Gregoor JG, van der Weide J, Loovers HM, van Megen HJ, Egberts TC, Heerdink ER. Polymorphisms of the LEP, LEPR and HTR2C gene: obesity and BMI change in patients using antipsychotic medication in a naturalistic setting. Pharmacogenomics. 2011;12(6):919–923. doi: 10.2217/pgs.11.40. [DOI] [PubMed] [Google Scholar]
- 12.Yi JH, Cho YJ, Kim WJ, Lee MG, Lee JH. Genetic variations of ABCC2 gene associated with adverse drug reactions to valproic acid in Korean epileptic patients. Genomics Inform. 2013;11(4):254–262. doi: 10.5808/GI.2013.11.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373(9669):1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Ma J, Wang S, Li X, Ji H, Li Y, et al. Valproic acid after neurosurgery induces elevated risk of liver injury: a prospective nested case-control study. Annal Pharmacother 2021:10600280211055508. [DOI] [PubMed]
- 15.David E, Golan M, Armen H, Tashjian, Ehrin J Armstrong, April W, Armstrong. Principles of pharmacology: The pathophysiologic basic of drug therapy. The 2nd Edition ed: People's Health Publishing House; 2009
- 16.Hung CC, Ho JL, Chang WL, Tai JJ, Hsieh TJ, Hsieh YW, et al. Association of genetic variants in six candidate genes with valproic acid therapy optimization. Pharmacogenomics. 2011;12(8):1107–1117. doi: 10.2217/pgs.11.64. [DOI] [PubMed] [Google Scholar]
- 17.Mei S, Feng W, Zhu L, Yu Y, Yang W, Gao B, et al. Genetic polymorphisms and valproic acid plasma concentration in children with epilepsy on valproic acid monotherapy. Seizure. 2017;51:22–26. doi: 10.1016/j.seizure.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Ogusu N, Saruwatari J, Nakashima H, Noai M, Nishimura M, Deguchi M, et al. Impact of the superoxide dismutase 2 Val16Ala polymorphism on the relationship between valproic acid exposure and elevation of gamma-glutamyltransferase in patients with epilepsy: a population pharmacokinetic-pharmacodynamic analysis. PLoS ONE. 2014;9(11):e111066. doi: 10.1371/journal.pone.0111066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan L, Yu JT, Sun YP, Ou JR, Song JH, Yu Y. The influence of cytochrome oxidase CYP2A6, CYP2B6, and CYP2C9 polymorphisms on the plasma concentrations of valproic acid in epileptic patients. Clin Neurol Neurosurg. 2010;112(4):320–323. doi: 10.1016/j.clineuro.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Voso MT, Santini V, Finelli C, Musto P, Pogliani E, Angelucci E, et al. Valproic acid at therapeutic plasma levels may increase 5-azacytidine efficacy in higher risk myelodysplastic syndromes. Clin Cancer Res. 2009;15(15):5002–5007. doi: 10.1158/1078-0432.CCR-09-0494. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Lin XQ, Cai WK, Xu GL, Zhou MD, Yang M, et al. Effect of UGT2B7 genotypes on plasma concentration of valproic acid: a meta-analysis. Eur J Clin Pharmacol. 2018;74(4):433–442. doi: 10.1007/s00228-017-2395-z. [DOI] [PubMed] [Google Scholar]
- 22.Delacretaz A, Zdralovic A, Vandenberghe F, Saigi-Morgui N, Glatard A, Quteineh L, et al. Association of variants in SH2B1 and RABEP1 with worsening of low-density lipoprotein and glucose parameters in patients treated with psychotropic drugs. Gene. 2017;628:8–15. doi: 10.1016/j.gene.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Smith RL, Haslemo T, Refsum H, Molden E. Impact of age, gender and CYP2C9/2C19 genotypes on dose-adjusted steady-state serum concentrations of valproic acid-a large-scale study based on naturalistic therapeutic drug monitoring data. Eur J Clin Pharmacol. 2016;72(9):1099–1104. doi: 10.1007/s00228-016-2087-0. [DOI] [PubMed] [Google Scholar]
- 24.Haerian BS, Baum L, Kwan P, Tan HJ, Raymond AA, Mohamed Z. SCN1A, SCN2A and SCN3A gene polymorphisms and responsiveness to antiepileptic drugs: a multicenter cohort study and meta-analysis. Pharmacogenomics. 2013;14(10):1153–1166. doi: 10.2217/pgs.13.104. [DOI] [PubMed] [Google Scholar]
- 25.Villegas-Martinez I, de-Miguel-Elizaga I, Carrasco-Torres R, Marras C, Canteras-Jordana M, Yedra-Guzman MJ, et al. The COL1A1 SP1 polymorphism is associated with lower bone mineral density in patients treated with valproic acid. Pharmacogenet Genomics. 2016;26(3):126–32. [DOI] [PubMed]
- 26.Lakhan R, Misra UK, Kalita J, Pradhan S, Gogtay NJ, Singh MK, et al. No association of ABCB1 polymorphisms with drug-refractory epilepsy in a north Indian population. Epilepsy & Behavior. 2009;14(1):78–82. doi: 10.1016/j.yebeh.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Balan S, Sathyan S, Radha SK, Joseph V, Radhakrishnan K, Banerjee M. GABRG2, rs211037 is associated with epilepsy susceptibility, but not with antiepileptic drug resistance and febrile seizures. Pharmacogenet Genom. 2013;23(11):605–610. doi: 10.1097/FPC.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 28.Grover S, Talwar P, Gourie-Devi M, Gupta M, Bala K, Sharma S, et al. Genetic polymorphisms in sex hormone metabolizing genes and drug response in women with epilepsy. Pharmacogenomics. 2010;11(11):1525–1534. doi: 10.2217/pgs.10.120. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Fan J, Gao K, Li Z, Yi Z, Wang L, et al. Neurotrophic tyrosine kinase receptor type 2 (NTRK2) gene associated with treatment response to mood stabilizers in patients with bipolar I disorder. J Mol Neurosci. 2013;50(2):305–310. doi: 10.1007/s12031-013-9956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101(1):72–79. doi: 10.1016/j.cmpb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y. Derivation of various NONMEM estimation methods. J Pharmacokinet Pharmacodyn. 2007;34(5):575–593. doi: 10.1007/s10928-007-9060-6. [DOI] [PubMed] [Google Scholar]
- 32.Zang YN, Guo W, Niu MX, Bao S, Wang Q, Wang Y, et al. Population pharmacokinetics of valproic acid in adult Chinese patients with bipolar disorder. Eur J Clin Pharmacol. 2022;78(3):405–418. doi: 10.1007/s00228-021-03246-2. [DOI] [PubMed] [Google Scholar]
- 33.Muller DJ, Zai CC, Sicard M, Remington E, Souza RP, Tiwari AK, et al. Systematic analysis of dopamine receptor genes (DRD1-DRD5) in antipsychotic-induced weight gain. Pharmacogenomics J. 2012;12(2):156–164. doi: 10.1038/tpj.2010.65. [DOI] [PubMed] [Google Scholar]
- 34.Roth CL, Hinney A, Schur EA, Elfers CT, Reinehr T. Association analyses for dopamine receptor gene polymorphisms and weight status in a longitudinal analysis in obese children before and after lifestyle intervention. BMC Pediatr. 2013;13:197. doi: 10.1186/1471-2431-13-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Professor Jinlin Guo) upon reasonable request.