Abstract
A family of 13 related but divergent vsp genes was recently found in the chromosome of the bovine pathogen Mycoplasma bovis. The vsp genomic locus was shown to undergo high-frequency rearrangements and to mediate phenotypic switching of variable lipoprotein antigens (Vsps) on the mycoplasma cell surface. Here we report that the vsp gene repertoire is subject to changes. Genetic analysis of M. bovis clonal isolates displaying distinct Vsp phenotypes showed that an intergenic recombination event between two closely related members of the vsp gene family, the formerly expressed vspA gene and the vspO gene, led to the formation of a new chimeric and functional vsp gene, vspC. The 5′ end of the recombination event was identified within the highly conserved vsp-upstream region, while the 3′ end was localized within the first repetitive domain (RA1) present in both vspA and vspO structural genes. As a result, the vspC gene is an embodiment of the following domains: an N-terminus-encoding region linked to the highly conserved vsp-upstream region provided by the vspO gene; and a C-terminus-encoding region and the more distal and divergent vsp-upstream region acquired from the vspA gene. The generation of chimeric genes encoding surface antigens may provide an important element of genetic variation and an additional source of antigenic diversification within the mycoplasma population.
One of the most effective strategies used by bacterial pathogens to avoid host immune recognition is the ability to rapidly change their surface antigenic repertoire and vary their immunogenicity (22, 25, 30). A common theme for generating and maintaining population diversity is the utilization of genetic systems consisting of multiple related variable genes organized as a gene family. Oscillation at high frequency of each individual gene between on and off expression states (phase variation), in conjunction with the ability of each gene to produce distinct size variants (size variation), allow the generation of numerous combinations of antigenic phenotypes even in a small, clonal population of bacteria, such as the limiting inoculum that initiates an infection (23, 25, 30).
Mycoplasmas represent the smallest self-replicating life forms on earth and phylogenetically are related to gram-positive eubacteria (23). Their remarkably small genomes, the lack of a rigid cell wall, and the absence of many enzymatic pathways generate an image of impotent microorganisms. However, many mycoplasma species are recognized as the etiological agents of human and animal diseases, causing in many cases acute and chronic infections with a wide range of complications (31). The successful persistence of pathogenic mycoplasmas within their host environments and their ability to evade the host immune system for a long period of time have been attributed in part to the fact that the mycoplasmas, like other well-established bacterial pathogens, possess a remarkable capability for rapid diversification of their cell surface antigens (23, 37). In most cases, surface antigenic variation in the mycoplasmas is achieved by multigene families encoding surface lipoproteins, as has been shown for Mycoplasma hyorhinis (38), Mycoplasma pulmonis (5), Mycoplasma synoviae (17), Mycoplasma gallisepticum (2), and Mycoplasma bovis (14).
M. bovis, a bovine pathogen that causes mastitis, pneumonia, and arthritis in cows and calves (10, 20), expresses several highly immunogenic and abundant variable surface lipoprotein antigens designated Vsps. The three members of this family identified so far (VspA, VspB, and VspC) (3) have been shown to possess the following features: (i) independent high-frequency phase variation, (ii) independent high-frequency size variation, (iii) membrane anchorage via an N-terminal domain and a surface-exposed C-terminal region, (iv) extensive repetitive domains extending from the N terminus to the C terminus, and (v) regions of shared epitopes. These three Vsps were identified as distinct translational products based on their monoclonal antibody (MAb) and polyclonal antibody epitope profiles and on their characteristic structural fingerprint patterns of degradation at carboxypeptidase Y cleavage sites (3).
Notably, although VspA, VspB, and VspC were shown to be three distinct translational products expressed on the mycoplasma cell surface, coexpression of VspA and VspC in a single clonal isolate was not observed. The extensive Vsp phenotypic switching in M. bovis was also shown to be associated with high-frequency chromosomal rearrangements occurring within the vsp genomic locus (13). Recently, the vsp genomic locus from an M. bovis clonal isolate coexpressing the VspA and the VspB lipoproteins was identified and characterized (14). A cluster of 13 related but divergent single-copy vsp genes comprising the vsp locus was identified. Interestingly, however, sequence analysis as well as Southern blot hybridiztions of genomic DNA failed to detect the vspC gene within the vsp locus or elsewhere on the chromosome of the M. bovis clonal isolate expressing the VspA lipoprotein.
The aim of this study was to identify and characterize the vspC gene in clonal isolates expressing this product and discern the meaning of its absence in clonal isolates expressing VspA. This report provides evidence that the vsp genomic repertoire of M. bovis is subject to changes. We show that an intergenic recombination event occurring at a high frequency between two members of the vsp gene family, vspA and vspO, results in the generation of a new and chimeric vsp gene, vspC.
MATERIALS AND METHODS
Bacterial strains, vectors, and plasmids.
Clonal isolates of M. bovis type strain PG45 expressing a 63-kDa product of VspA (clone 7) and 75- and 79-kDa products of VspC (clones 168 and 166, respectively) have been described elsewhere (3, 13). Clonal isolate 182, expressing a 75-kDa VspC product, was isolate and purified as previously described (3). The geographic origin and site of isolation of M. bovis PG45 were previously described (3). All clonal isolates were propagated at 37°C in a modified standard mycoplasma broth medium as previously described (3). The Escherichia coli strain used was DH5αMCR (Gibco BRL Life Technologies, Inc., Gaithersburg, Md.). Recombinant clones were constructed in the plasmid vector pBluescript II KS(+) (Stratagene, La Jolla, Calif.). Recombinant plasmid pKO35, carrying the vspO gene, was constructed by cloning the PCR-amplified vspO gene from M. bovis PG45 clonal isolate 7 into the plasmid vector pBS. Two oligonucleotides, 5′-CTGCTTAGTTGAGTGTTGTTCC-3′ and 5′-CCTGGGTAACAGATGCAA-3′, containing HindIII restriction sites at their ends, were used for PCR amplification and cloning of the vspO gene (14). Expression of M. bovis vspC and vspO genes in E. coli was performed by the T7 polymerase-promoter system of Tabor and Richardson (33) as previously described (13). E. coli strain DH5αMCR (pGP1-2) was used as a host for expression of mycoplasma proteins under T7 promoter control.
Chemicals, media, and growth conditions.
E. coli cultures for plasmid isolation were grown with shaking at 37°C in Luria-Bertani broth (28). E. coli cultures for expression of mycoplasma proteins under T7 promoter control (33) were grown at 30°C with shaking in M9 medium (28) supplemented with a mixture of all amino acids. Restriction enzymes, T4 ligase, and T4 polynucleotide kinase were purchased from MBI Fermentas (Amherst, N.Y.) and used according to the recommendations of the manufacturer. 5-Bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal), ampicillin, kanamycin, and rifampin were purchased from Sigma Chemicals, St. Louis, Mo. [γ-32P]ATP and [α-32P]CTP were purchased from Amersham, Little Chalfont, United Kingdom.
SDS-PAGE and Western immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (12). Samples were prepared by heating at 100°C for 5 min in sample buffer (2% SDS, 5% [vol/vol] 2-mercaptoethanol, 10% [vol/vol] glycerol, 62.5 mM Tris [pH 6.8]). Proteins were separated in 9% acrylamide gels and transferred to nitrocellulose membrane filters (0.45-μm pore size; Schleicher & Schuell, Dassel, Germany) by the method of Towbin et al. (35). Blots were incubated for 1 h at room temperature with phosphate-buffered saline containing 3% bovine serum albumin (Sigma) and then incubated overnight at 4°C with the primary antibodies diluted in phosphate-buffered saline (PBS) containing 20% (vol/vol) fetal calf serum. After three washes in PBS buffer, blots were incubated for at least 2 h at room temperature in peroxidase-conjugated goat antiserum to mouse immunoglobulin M or to mouse immunoglobulin G (Jackson ImmunoResearch Laboratories, West Grove, Pa.; Nordic, Tilburg, The Netherlands). For detection, enzyme substrates 4-chloro-1-naphthol (Aldrich, Steinheim, Germany) and o-dianisidine (Sigma) were used.
DNA preparation and manipulation.
Genomic DNAs from type strain M. bovis PG45 and from M. bovis clonal isolates were extracted and purified by the method of Marmur (15). Plasmid isolation, restriction endonuclease digestions, and gel electrophoresis of DNA and proteins were performed as previously described (3, 13, 14).
Oligonucleotides; labeling and hybridization conditions.
vsp sequence-specific oligonucleotides were synthesized at the interdepartmental facility of the Hebrew University-Hadassah Medical School on a model 380B DNA synthesizer (Applied Biosystems, Inc., Foster City, Calif.). A sequence 18 nucleotides (nt) long, 5′-GGACAAGGCACATCAGCT-3′, was designated ro-2; a sequence 35 nt long, 5′-GCTTTTATTTAGTTCTTAATACTTCATATAATAAA-3′, was designated cas-2; a sequence 20 nt long, 5′-GCCTTGATCTGTATTTTCGC-3′, was designated nt-2; and a sequence 20 nt long, 5′-GTTAGTTCCTGCACCTTGTT-3′, was designated ra-4. The conditions for oligonucleotide labeling and hybridization as well as for DNA hybridization have been described elsewhere (13, 14).
Cloning of the vsp locus from VspA and VspC clonal isolates.
Genomic libraries were constructed with the bacteriophage FIX II/Xho I vector (Stratagene) with partially digested Sau3A chromosomal fragments from M. bovis PG45 clonal isolate 7, expressing a 63-kDa product of VspA, or from M. bovis PG45 clonal isolate 168, expressing a 75-kDa product of VspC (Fig. 1A, lanes 1 and 2, respectively). Agar plates containing approximately 2 × 103 PFU were overlaid for 10 min with nitrocellulose filters (0.45-μm pore size; Schleicher & Schuell, Dassel, Germany). Plaques were screened by colony blot hybridization with 32P-labeled recombinant plasmid pKA63 carrying the vspA gene (13) as a probe for the VspA isolate or with recombinant plasmid pKCl75 carrying the vspC gene (see Results) as a probe for the VspC isolate. The conditions for DNA labeling and hybridization have been described elsewhere (13, 14). Positive phages were picked, replated at low density, and rescreened. After three rounds of plaque purification, positive phages were isolated for further analysis.
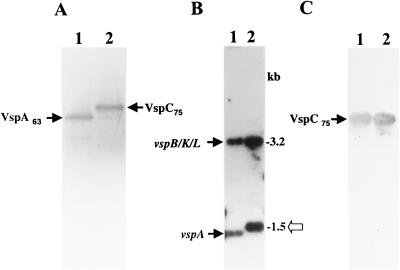
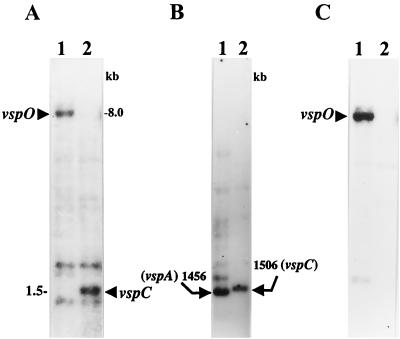
FIG. 1.
(A) Western blot analysis of two M. bovis clonal isolates expressing the VspA (lane 1, clone 7) and VspC (lane 2, clone 168) products. Whole organisms were subjected to SDS-PAGE and immunoblotted with MAb 1E5 (2). The VspA63 and VspC75 products are indicated. (B) Identification of the M. bovis vspC gene. Four-microgram aliquots of chromosomal DNAs of the isolates were digested with HindIII, subjected to Southern blot hybridization, and probed with the γ-32P-labeled ra-4 oligonucleotide. HindIII genomic fragments carrying the vspB, vspK, and vspL genes or the vspA gene are indicated by labeled arrows (14). Molecular size markers are shown on the right. An open arrow marks a 1.5-kb HindIII genomic fragment present in the VspC variant. (C) Expression in E. coli of the recombinant vspC gene. E. coli cells expressing under selective induction of the T7 promoter control the recombinant plasmid pKC75 carrying the vspC gene were separated by SDS-PAGE and immunoblotted with MAb 1E5 (lane 1). Total mycoplasma proteins of the M. bovis VspC clonal isolate (A, lane 2) were used as a positive control (lane 2). The recombinant VspC product expressed in E. coli as well as the authentic VspC product expressed in the mycoplasma are indicated by arrows.
PCR.
PCRs were carried out in 100-μl volumes containing 10 ng of template DNA, 4 U of ExSel DNA polymerase in 1× S-T Exsel buffer (MBI Fermentas), 2 mM MgSO4, 2 mM mix of deoxynucleoside triphosphates, and 500 ng of each primer. PCR amplifications were performed using a PC-Personal cycler (Biometra, Gottingen, Germany) programmed for 31 cycles. For amplifying the 2.1-kb genomic fragment, primers 5′-TGTGGTCAAACCTATGGTTAG-3′ and 5′-GCTTGTTCTCTTTGACCCAC-3′ (designated Pk-1 and P5-1, respectively [see Fig. 3B]) were used with the following cycling conditions: an initial cycle of 3-min denaturation at 95°C, 90 s of annealing at 54°C, and 150 s of polymerase extension at 72°C, followed by 30 cycles of 30 s at 95°C, 45 s at 54°C, and 2 min at 72°C. For amplifying the 1.5-kb genomic fragment, primers P5-1 and nt-2 (see Fig. 3A) were used with the following cycling conditions: initial denaturation for 3 min at 95°C, 90 s of annealing at 58°C, and 2 min of polymerase extension at 72°C, followed by 30 cycles of 30 s at 95°C, 30 s at 58°C, and 90 s at 72°C. The reaction mixtures were then incubated for a 10-min extension step at 72°C and allowed to cool slowly at 4°C. The resultant PCR products were purified by High Pure filter columns (Boehringer Mannheim GmbH, Indianapolis, Ind.) and directly sequenced.
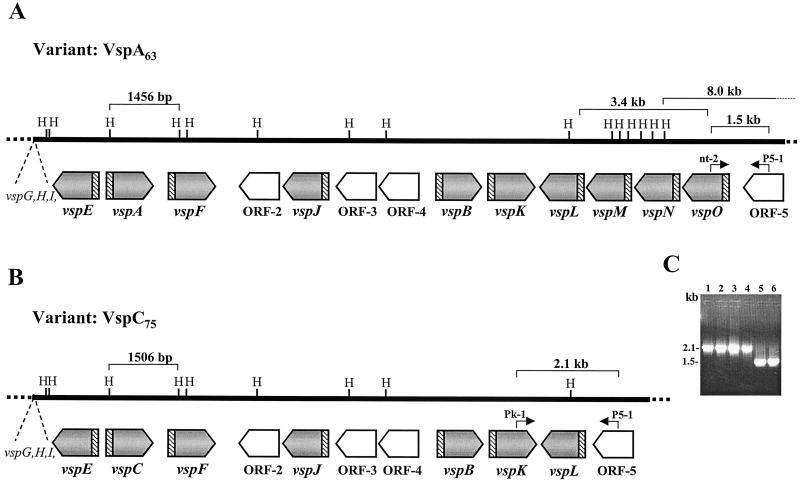
FIG. 3.
Comparison of the M. bovis vsp genomic loci from the VspA clonal isolate expressing a 63-kDa VspA protein (A) and from a VspC clonal isolate expressing a 75-kDa product (B). The locations and orientations of the vsp genes in each locus are shown by shaded and labeled arrows. Four non-vsp ORFs (ORF-2 to ORF-5) are shown by open labeled arrows. Positions of HindIII (H) restriction sites are marked. The highly conserved vsp-upstream region is shown by hatched blocks. Locations of a 1,456-bp HindIII fragment carrying the vspA gene in the VspA isolate, of a 1,506-bp HindIII fragment carrying the vspC gene in the VspC isolate, and of a 3.4-kb fragment carrying vspM, vspN, and part of the vspO gene of the VspA isolate that is missing in the VspC isolate are indicated by brackets. The 5′ end of an 8.0-kb HindIII genomic fragment (Fig. 5A, lane 1) in the VspA isolate is marked. Locations of two sets of PCR primers (P5-1/nt-2 and P5-1/Pk-1) as well as sizes of the resultant PCR products (1.5 and 2.1 kb, respectively) are marked. (C) PCR amplification of M. bovis isolates. PCR primers P5-1 and Pk-1 were used to amplify the corresponding genomic regions of isolates 168 (VspC75) (lane 1), 166 (VspC79) (lane 2), and 182 (VspC75) (lane 3) and of M. bovis type strain PG45 (lane 4). PCR primers P5-1 and nt-2 were used to amplify the corresponding genomic region of isolates 7 (VspA63) (lane 5) and M. bovis PG45 (lane 6). Sizes of the PCR products are indicated.
DNA sequence analysis.
DNA sequence analysis of both strands was performed by the dideoxy-chain termination method (29). The T7 promoter sequence and the T3 sequence located on the pKS vector, as well as vsp-related sequences, were used as primers. Sequencing was done using a model ABI PRISMA 377 automatic sequencer dye-terminator cycle sequencer (Perkin-Elmer, Foster City, Calif.). Sequence data were analyzed using the computer software AssemblyLIGN and MacVector 6.0.
Nucleotide sequence accession number.
The nucleotide sequence of the vspC gene has been assigned GenBank accession number AF224060.
RESULTS
Identification, genomic localization, and characterization of the vspC gene in a clonal isolate of M. bovis.
Genetic analysis of an M. bovis PG45 clonal isolate coexpressing the VspA and VspB lipoproteins revealed that the vspC gene was absent in this isolate (14). However, other M. bovis clonal isolates, expressing distinct VspC products, were clearly identified and characterized (3, 13).
To address this issue, two clonal isolates of M. bovis type strain PG45, one expressing the VspC product (Fig. 1A, lane 2) and the second expressing the VspA product (Fig. 1A, lane 1), were chosen for further analysis. Restricted genomic DNAs from these isolates were subjected to Southern blot hybridization (Fig. 1B) with the ra-4 oligonucleotide as a probe (13). The ra-4 oligonucleotide was chosen as the preferred probe for the identification of the vspC gene, as earlier studies have shown that the VspA and the VspC products exhibit remarkably similar polypeptide structures and regions of shared epitopes (3, 27). The high structural similarity was most profound near the C-terminal end, a region consisting of identical repetitive coding sequences. This repetitive domain, designated RA4, represents a significant portion of the vspA gene and was shown by Southern blot analysis to be localized in two distinct HindIII genomic fragments: a 1.45-kb HindIII carrying the vspA gene; and a 3.2-kb fragment carrying the vspB, vspK, and vspL genes (Fig. 1B, lane 1) (14). Interestingly, in addition to the 3.2-kb fragment that was observed in both isolates, the VspC isolate possesses an HindIII fragment which is slightly larger than the vspA-bearing fragment in the isolate expressing the VspA product (Fig. 1B, lane 2 and lane 1, respectively). No other strongly hybridizing fragments that might carry the vspC gene were detected in the VspC variant (Fig. 1B). These findings, along with our earlier observations indicating that VspA63 and VspC75 are structurally remarkably similar (3), led us to focus on the 1.5-kb HindIII fragment of the VspC variant for further study.
The 1.5-kb HindIII fragment was excised from the gel and subcloned into the plasmid vector pKS. Each orientation of this 1.5-kb fragment was cloned separately relative to the T7 promoter located on pKS, generating the recombinant plasmids pKCl75 and pKC275. Both recombinant plasmids were expressed in E. coli using the T7 RNA polymerase promoter system (33). A polypeptide band of 75 kDa was synthesized in E. coli by the recombinant pKCl75 clone (Fig. 1C, lane 1). The size of the expressed protein in E. coli was similar to that of the authentic VspC protein expressed in the mycoplasma (Fig. 1C, lane 2), indicating that the 1.5-kb HindIII genomic fragment contained the complete vspC gene.
The nucleotide sequence of the 1.5-kb HindIII fragment bearing the vspC gene was determined. Within the sequenced fragment a single open reading frame (ORF) containing 1,101 nt, starting with an ATG initiation codon and terminating at a TAA stop codon, was identified. More than 80% of the VspC molecule was composed of reiterated sequences extending from the N terminus to the C terminus of the VspC protein. Four distinct internal regions of repetitive sequences as tandem in-frame blocks were identified. Analysis of the VspC deduced amino acid sequence revealed a remarkable homology to the VspA lipoprotein (Fig. 2). The only differences between VspC and VspA were 24 additional amino acid residues and six amino acid substitutions in the VspC N-terminal region and a deletion of one of the RA4 repetitive units within the C terminus of the VspC molecule (Fig. 2).
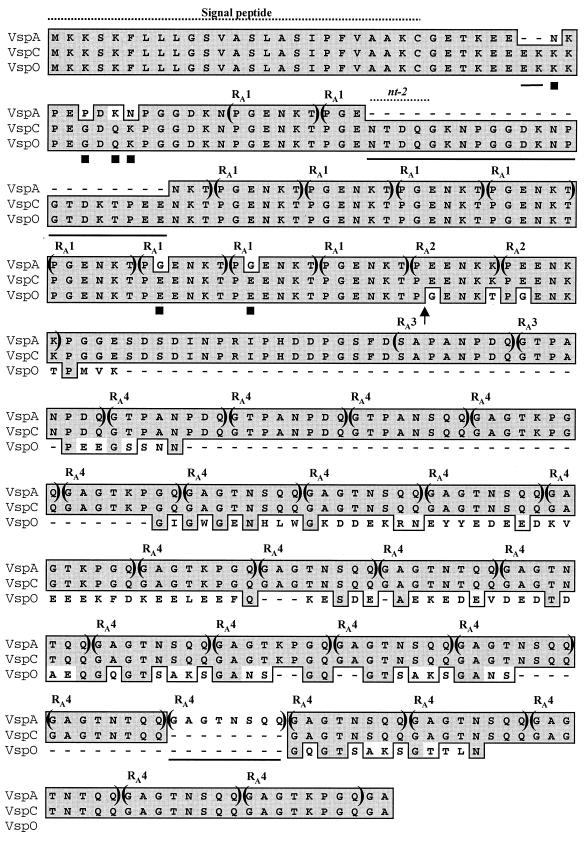
FIG. 2.
Alignment of the deduced amino acid sequences of VspA, VspC, and VspO. Identical amino acid residues are shown by shaded boxes. The solid lines under amino acid sequences within the N-terminal region represent residues that are missing in VspA but are present in VspO and VspC. Six solid squares below amino acid residues indicate amino acid substitutions found within the N-terminal region of VspA. Four distinct in-frame repetitive amino acid sequence domains within the Vsp molecules are indicated within parentheses and labeled RA1, RA2, RA3, and RA4. An arrow shows the end of the region which exhibits 100% homology between VspO and VspC and the beginning of the region displaying 100% homology between VspC and VspA. Positions of the nt-2 oligonucleotide and of the conserved Vsp lipoprotein signal peptide are shown by labeled broken lines.
The next step was to determine the location of vspC in the genome of the VspC clonal variant. The vsp locus from the VspC isolate was cloned, sequenced, and compared to its counterpart of the VspA isolate. Comparison of the two vsp loci revealed two important findings. First, in the VspC isolate, vspC was situated upstream of vspF and downstream of vspE (Fig. 3B). In other words, the vspC gene has replaced the original vspA gene present in the variant expressing the VspA protein (Fig. 3A). Second, a 3.4-kb genomic fragment, carrying the vspM gene, the vspN gene, and part of the vspO structural gene, that was positioned downstream of the vspL gene and upstream of ORF-5 in the VspA clonal isolate (Fig. 3A) was missing from the vsp locus of the VspC variant (Fig. 3B).
At this point, we examined whether the deletion of the three vsp genes observed in the variant expressing the VspC protein could be also found in other VspC variants as well as among cells of the original culture of type strain PG45. Two additional VspC clonal isolates and the original culture of M. bovis PG45 were analyzed by PCR. Two primers, representing sequences complementary to the vspK gene and to ORF-5 (designated, Pk-1 and P5-1, respectively [Fig. 3B]) were used in PCR to amplify the genomic region between the two corresponding genes in which the deletion has occurred. As a control, the P5-1 primer together with another primer (designated nt-2), which represents sequences complementary to the vspO gene, were used to amplify the genomic region between the vspO gene and ORF-5, a region that is unique to the VspA isolate and absent in the VspC isolate (Fig. 3A). A single PCR product of 2.1 kb was detected in all three VspC clonal isolates (Fig. 3C, lanes 1 to 3), while a single PCR product of 1.5 kb was detected in the VspA isolate (Fig. 3C, lane 5). Importantly, the same PCR products were also obtained when the two distinct sets of PCR primers were used to amplified the genomic DNA from the original M. bovis PG45 culture (Fig. 3C, lanes 4 and 6, respectively), indicating the existence of both populations within the original strain.
Intergenic recombination between vspA and vspO generates a chimeric and functional gene, vspC.
The findings that the variant expressing the VspC product contains the vspC gene but not the vspA gene and vice versa were consistent with our inability to detect by Southern blot hybridization the vspC gene in the genomes of variants expressing the VspA product (14) and with the fact that coexpression of VspA and VspC products was not observed in M. bovis. These findings raised the possibility that an intergenic recombination event might have occurred between the vspA gene and other vspA-related sequences and led to the generation of a new coding sequence, namely, the vspC gene. We therefore compared the vspC nucleotide sequence and its deduced peptide with all other known vsp gene and protein sequences (14). Comparison of the vspC gene with one member of the vsp gene family, vspO, provided the first evidence for the occurrence of such a recombination event. A region of 390 nt starting from the initiation codon was 100% identical between vspC and vspO. This region, which encodes the N-terminal 130 amino acid residues of the two Vsp proteins (Fig. 2), included the 24 amino acid residues that were missing from VspA as well as the six amino acid differences between VspA and VspC (Fig. 2).
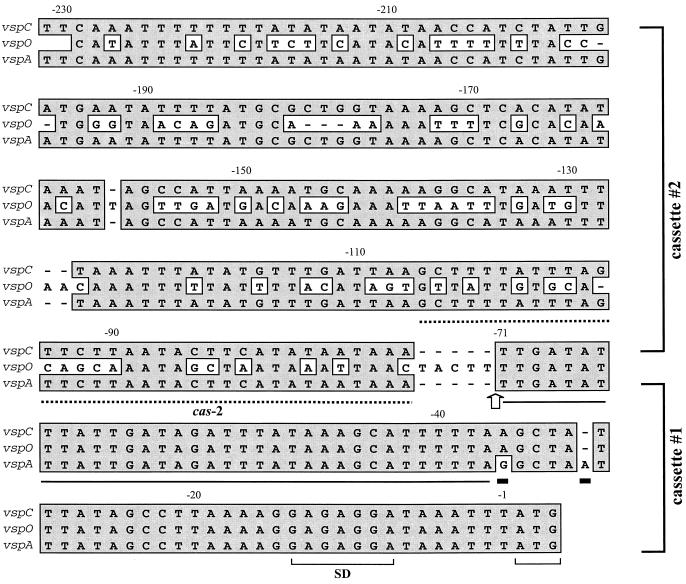
Additional data pointing to the occurrence of a recombination event between vspA and vspO were obtained when the nucleotide sequence of the vspC-upstream region was compared with those of all known vsp genes (14). The vsp-upstream region was recently shown to be highly conserved among all vsp genes and to possess two cassettes. Cassette 1, a 71-bp segment upstream of the ATG initiation codon, exhibited 98% nucleic acid identity among all vsp genes; cassette 2, about 160 bp, exhibited a more divergent sequence (14). As shown in Fig. 4, cassette 1 of the vspC was found to be 100% identical to cassette 1 of the vspO and could be distinguished from that of vspA by the presence of distinctive nucleotide signatures (Fig. 4). Interestingly, however, cassette 2 of vspC showed 100% sequence identity to that of vspA and was clearly different from that of vspO (Fig. 4).
FIG. 4.
Nucleotide sequence alignment of regions 5′ of the structural genes vspA, vspC, and vspO. Identical nucleotides are highlighted by shaded boxes. An arrow at nt -71 indicates the end of the region displaying 100% homology between vspO and vspC genes and the beginning of the region of 100% homology between vspC and vspA. This arrow also marks the junction between the two-vsp upstream cassettes 1 and 2 (14). Solid rectangles below nucleotides represent two nucleotide differences which are unique to the vspA upstream region and served as vspA fingerprints. The position of the cas-2 oligonucleotide, used as a probe, is shown by a labeled broken line. Nucleotides representing a putative ribosome binding site (SD) and the initiation codon ATG are shown by brackets. A 34-bp sequence in which the 5′ end of the recombination event between vspA and vspO has occurred is underlined.
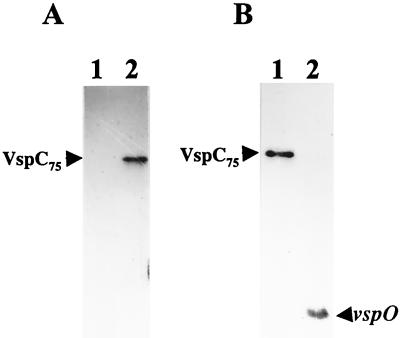
Experimental evidence supporting the generation of the vspC gene through an intergenic recombination event between vspA and vspO was obtained by monitoring the presence and size of the vspA- and vspO-bearing fragments in the genomes of the two clonal variants described in Fig. 1A and 3. Two synthetic oligonucleotides were used in Southern blot hybridization with HindIII-digested genomic DNAs of the two clonal variants. The first oligonucleotide, designated nt-2, was complementary to the N-terminus-encoding region of the vspO gene. This sequence was also found in the N-terminal region of the vspC gene but not in the vspA gene (Fig. 2). Thus, this probe can identify the vspO N-terminus-encoding region as well as the corresponding region in the vspC gene. The second oligonucleotide, designated cas-2, was complementary to vspA cassette 2, which was also found in the chimeric vspC gene (Fig. 4). This probe can monitor the corresponding genomic fragment carrying vspA cassette 2 in both clonal isolates.
As expected, an approximately 8-kb HindIII genomic fragment carrying the vspO gene (14) was identified by the nt-2 oligonucleotide probe in the VspA isolate (Fig. 5A, lane 1; Fig. 3). However, in the VspC clonal isolate the vspO N-terminus-encoding region was identified on a 1.5-kb HindIII fragment shown in this study to carry the complete vspC gene (Fig. 5A, lane 2; Fig. 3). In parallel, by using the cas-2 probe, vspA cassette 2, present on a 1,456 bp HindIII fragment carrying the vspA gene in the VspA isolate (Fig. 5B, lane 1; Fig. 3), was identified on the 1,506-bp HindIII fragment carrying the vspC gene in the VspC isolate (Fig. 5B, lane 2; Fig. 3).
FIG. 5.
Monitoring the recombination event between the vspA and vspO genes. For Southern blot hybridization of the two clonal isolates depicted in Fig. 1A and 3, genomic DNAs were digested with HindIII restriction enzyme and probed with the nt-2 oligonucleotide probe, which represents sequences complementary to the N-terminus-encoding region common to vspC and vspO (A), with vspA cassette 2-specific probe cas-2 (B), or with vspO-specific probe ro-2 (C). HindIII genomic fragments bearing the vspO or vspC gene are marked by labeled arrows.
The acquisition of the N-terminus-encoding region of the vspO gene by the corresponding region of the vspC gene was also examined at the protein level using MAb 2A8, previously shown to recognize VspC but not VspA (Fig. 6A) (4). As was shown in this study, the only differences between the VspA and VspC amino acid sequences were confined to the N-terminal region (Fig. 2), which presumably contains the epitope recognized by MAb 2A8. Since the vspC N-terminus-encoding region was completely acquired from the vspO gene, we expressed in E. coli the recombinant vspO gene from the VspA isolate as well as the recombinant vspC gene and examined their reactivities with MAb 2A8 by Western blot analysis. Both recombinant VspC protein and recombinant VspO protein (Fig. 6B, lanes 1 and 2, respectively) were clearly recognized by MAb 2A8, indicating the acquisition of the N-terminus-encoding region of the vspO gene into the hybrid vspC gene. The formation of the chimeric vspC gene through a recombination event occurring between vspA and vspO is schematically demonstrated in Fig. 7. A 34-bp sequence within the conserved cassette 1 upstream of the vspA and vspO genes (designated A1 and O1, respectively) was identified as the putative 5′ site of the recombination event (Fig. 4 and 7). As a result, vspA cassette 2 (A2) was fused to the vspO cassette 1 (O1), generating the A2-O1-vspC-upstream region. The 3′ end of the recombination event was localized at the last repeat within the RA1 repetitive domain, which is common to the vspA and vspO genes (Fig. 7). The identification of the exact 3′ end within the RA1 repetitive domain was possible due to the presence of a few nucleotide substitutions that served as distinctive fingerprints for the involved genes (Fig. 7) (14).
FIG. 6.
(A) Western blot analysis of the VspA and VspC clonal isolates. Total cell proteins from the VspC (lane 2) and VspA (lane 1) isolates were immunoblotted with MAb 2A8 (27). The authentic VspC 75-kDa protein band is indicated by an arrow. (B) Expression in E. coli of recombinant vspC and vspO genes. E. coli cells expressing, under selective control of the T7 promoter, the recombinant plasmid pKC75 carrying the vspC gene (lane 1) or the recombinant plasmid pKO35 carrying the vspO gene were separated by SDS-PAGE and immunoblotted with MAb 2A8. The recombinant VspC and the VspO products (75 and 35 kDa, respectively) are marked by labeled arrows. Notably, the size of the recombinant VspO product expressed in E. coli does not represent the complete vspO coding sequence due to the presence of two UGA residues at the C terminus (which encode the amino acid tryptophan in mycoplasmas). No UGA codons were found within the vspC gene.
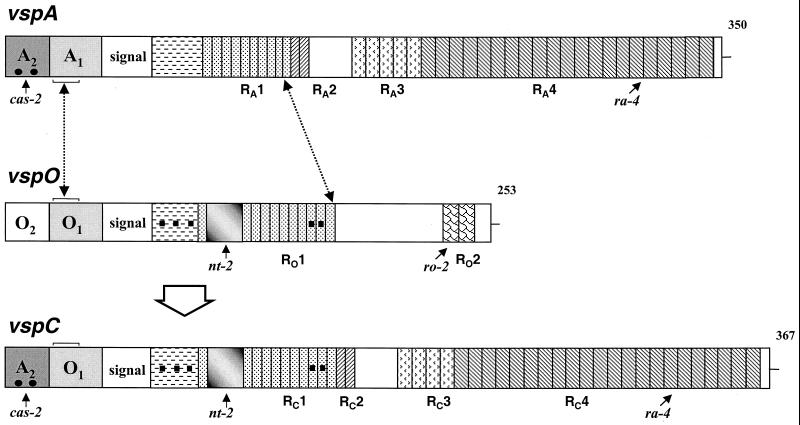
FIG. 7.
Schematic representation of the generation of the vspC gene by an intrachromosomal recombination between vspA and vspO. Structures of vspA, vspO, and vspC are presented schematically by aligned rectangles. Each vsp gene is flanked 5′ by a highly conserved noncoding region composed of two cassettes, labeled 1 and 2 with the letter of the corresponding gene. A block labeled signal represents a highly homologous sequence encoding a lipoprotein signal peptide. In-frame reiterated coding sequences extending from the N terminus to the C terminus of the Vsp proteins and encoding periodic amino acid sequences are shown by differently hatched blocks. Distinctive repetitive domains within each Vsp are labeled with R and the letter of the corresponding vsp gene. Repetitive units present in more than one Vsp molecule are similarly hatched. Numbers on the right indicate the length of each Vsp polypeptide chain. Small black squares denote distinctive nucleotide changes which exist in vspO and vspC but not in vspA, while solid dots represent a distinctive signature present in vspA and vspC but not in vspO. A shaded block represents a 66-bp sequence present in vspO and vspC but absent in vspA. Small labeled arrows mark the locations of the cas-2, ro-2, nt-2, and ra-4 oligonucleotides used as probes in Southern blot experiments. The position of a 34-bp sequence, within cassette 1 of the vspA, vspO, and vspC genes, which served as the putative 5′-end site for the recombination event is marked by a broken arrow and brackets. The 3′ end of the recombination event, which is located within the repetitive domains RA1 and RO1 of the vspA and the vspO genes, is marked by a broken line.
The vspC gene is therefore an embodiment of the following domains: an N-terminus-encoding region linked to an upstream region (cassette 1) provided by the vspO gene; and a C-terminus-encoding region and a more distal upstream region (cassette 2) acquired from the vspA gene.
Generation of the chimeric vspC gene is a nonreciprocal event.
The recombination event between vspA and vspO has led on one hand to the generation of the chimeric and functional gene vspC, which was shown to undergo independent high-frequency phase variation and to be highly immunogenic (3; Y. Ron, I. Lysnyansky, and D. Yogev, unpublished results), but on the other hand to a loss of genetic material containing three vsp genes (vspM, vspN, and vspO). This was evident when the vsp locus of the VspC variant was cloned and sequenced (Fig. 3). Thus, the generation of the vspC gene appears to be a nonreciprocal event, and the reciprocal product, which should consist of the N-terminus-encoding region of the vspA gene and of the C-terminus-encoding region of the vspO gene, was not generated during this event (Fig. 3 and 7). To examine the possibility that a reciprocal product might still be present elsewhere in the chromosome of the VspC isolate, three synthetic oligonucleotides representing unique sequences of the vspM, vspN, and vspO structural genes (14) (designated rm-2, 49n, ro-2, respectively) were used as probes in Southern blot hybridization with HindIII-digested genomic DNAs of the two variants depicted in Fig. 1A and 3. An example of such hybridization using the vspO-specific oligonucleotide (ro-2) probe is shown in Fig. 5C. The vspO probe identified, as expected, the 8.0-kb HindIII genomic fragment carrying the vspO gene in the genome of the variant expressing the VspA product (Fig. 5C, lane 1; Fig. 3). However, no vspO-related genomic fragments were observed in the genome of the VspC variant (Fig. 5C, lane 2). Similarly, no vspM- or vspN-related genomic fragments were observed in the genome of the VspC variant by using the vspM- or vspN-specific probe (data not shown). Thus, the recombination event between the vspA and the vspO genes which led to the generation of the vspC gene did not produce a reciprocal product but caused a deletion within the vsp genomic locus.
DISCUSSION
In previous studies, systematic analysis of several isogenic clonal lineages of M. bovis type strain PG45 allowed the isolation of clonal variants exhibiting discrete Vsp phenotypes (3, 13). These phenotypes included single as well as combinatorial expression of three distinct major immunogens: VspA, VspB, and VspC. Although each Vsp was shown to undergo independent high-frequency phase variation, an isolate coexpressing both VspA and VspC lipoproteins was not observed. Moreover, genetic analysis of an isolate coexpressing both VspA and VspB failed to detect the vspC gene within the vsp locus or elsewhere in the M. bovis chromosome (14).
The present study revealed that vspC is a chimeric gene generated by an intragenic recombination between two closely related vsp genes: the formerly expressed vspA gene and the vspO gene, both positioned within the vsp locus at a distance of 13.4 kb (14). Examination of the 5′ and the 3′ ends of the rearranged region revealed that vsp homologous sequences were utilized. A 34-bp sequence within the highly conserved cassette 1 common to all vsp-upstream regions (14) was identified as the potential 5′ site for the recombination event (Fig. 4 and 7), while identical reiterated sequences (RA1) within the coding regions of vspA and vspO served as potential recombination sites at the 3′ end (Fig. 7). It should be noted that the 34-bp sequence within the conserved cassette 1 was also identified as a potential site for site-specific DNA inversion events that were found to mediate VspA and VspC phase variation (I. Lysnyansky and D. Yogev, unpublished results; Y. Ron, I. Lysnyansky, and D. Yogev, unpublished results). Recombination events generating chimeric vsp genes may be initiated by a cleavage within the 34-bp sequence, while the downstream recombination might then occur at any site bearing sufficient sequence similarity, including sites within the vsp genes themselves, as was shown in this study for the formation of the vspC gene.
As to the deletion of a genomic fragment during the generation of the chimeric vspC gene, in Borrelia burgdorferi, deletion of gene sequences during the formation of chimeric gene fusions by intramolecular recombination between two osp genes was demonstrated (26). A deletion of DNA sequences was also observed in Borrelia hermsii during activation of the vmp pseudogene (24). The vspA and vspO genes are localized within the vsp locus in opposite orientations. Their opposite genomic orientations argue against a looping-out configuration as a mechanism that would generate a deletion, as was shown for example for the ospA and ospB genes of B. burgdorferi (26), and suggest a nonconservative recombination by as yet an unknown mechanism. Collectively, the presence of highly homologous sequences 5′ to all vsp genes and the recurrence of similar reiterated sequences within the coding region of several vsp genes (14) suggest that these regions might serve as potential sites for intrachromosomal recombination events (19, 32) that could occur between other vsp genes. Therefore, a large repertoire of chimeric vsp genes can potentially be generated, affecting the vsp genomic and antigenic repertoire.
Generation and expression of chimeric vsp genes might provide an important element of genetic variation and additional source of antigenic diversification within the mycoplasma population, which of course increases the microorganism's flexibility to deal with the immunologic problem posed by the host. However, it also raises a question regarding the benefit of the final outcome, at least in the case of the vspC gene. Experimental evidence obtained in this study indicated that the generation of the vspC gene by intrachromosomal recombination between two vsp homologs (vspA and vspO) led to irreversible loss of genetic material within the vsp locus. First, sequence analysis of the vsp locus from the VspC variant has shown that three vsp genes are missing in that isolate (Fig. 3). Second, Southern blot analysis using vsp-specific oligonucleotides could not detect within the vsp locus or elsewhere in the chromosome of the VspC variant a reciprocal product containing the vspA N-terminus-encoding region and the vspO C terminus (Fig. 5C). Third, PCR analysis of the original M. bovis type strain PG45 has clearly shown the presence of cells within the population harboring a deletion of three vsp genes in the vsp locus along with cells carrying the complete vsp locus (Fig. 3C). Analysis of two additional VspC clonal isolates expressing VspC products of different sizes has also shown the presence of a deleted vsp locus.
Why would an organism with limited genetic material undergo a terminal event leading to a loss of coding sequences? One possibility is that during growth in culture the vsp locus is not under selective pressure to maintain a particular gene configuration. The lack of selective pressure on one hand, and the presence of highly homologous sequences, within the upstream vsp regions as well as sites within the vsp genes themselves, on the other hand, allow the occurrence of apparently a wide range of recombination events. Some of these in vitro rearrangement events may generate, within the entire population, distinct cells harboring chimeric vsp genes and/or deletions within the vsp locus. In culture, in the absence of a selective pressure these variants survive and can be isolated as was shown for the VspC variant. However, after inoculation into the animal host, when selective pressure is restored, natural selection of antigenic phenotypes needed for adapting the host environment or evading its defense mechanisms is likely to take place in vivo. Subpopulations of M. bovis cells that possess particular vsp gene configurations and express the appropriate antigenic presentations will survive, while other phenotypes will die. While the frequency of appearance of the VspC phenotype during in vitro growth was measured to be 10−3 to 10−5 per cell per generation (3, 13), the frequency of occurrence of the chimeric vspC gene in vivo in the bovine is unknown. It is therefore possible that the VspA and the deleted VspC phenotypes confer different selective advantages during in vitro growth and passage and thus do not reflect the frequency or the precise nature of that event in vivo. Nevertheless, generation of chimeric vsp genes may still be part of a survival strategy of the mycoplasma and underscores the efficient way mycoplasmas may utilize their limited genetic material when confronted with the host environment.
Bacterial pathogens utilize a wide diversity of molecular mechanisms to vary the antigenic characteristics of their cell surface (1, 6, 21, 23, 25, 30, 34, 36, 38). In many, the presence of a family of related genes favors the occurrence of DNA rearrangements mediating on/off switching mechanisms and operating at high frequency (5, 8, 16, 23, 30). The possibility of creating functional chimeric gene fusions among homologs within a gene family is another important consequence of DNA rearrangements affecting the antigenic presentation of the microorganism (18). For example, recombination between sapA homologs of Campylobacter fetus leads to expression of a divergent S-layer protein (7, 9, 36). In B. burgdorferi (26) or B. hermsii (11), an intragenic recombination between osp or vmp genes, respectively, generates a chimeric gene fusion and is considered an additional mechanism for antigenic variation.
The high rate of DNA rearrangements observed in the chromosome of M. bovis (13), as well as in M. pulmonis (5), places the minute mycoplasma chromosome as one of the most dynamic and variable genomes known. The vsp gene family of M. bovis represents a complex system in which three distinct ways to achieve surface diversity are independently utilized: (i) high-frequency on/off switching of individual Vsps, (ii) generation of numerous Vsp size variants, and (iii) formation of chimeric vsp genes encoding variable surface lipoproteins. Although the frequency of occurrence of each process in vivo is not known, the combination of these molecular traits might provide an important element of genetic variation within the mycoplasma population contributing to rapid evolution of the variable antigen gene repertoire.
ACKNOWLEDGMENTS
This study was supported in part by the German-Israeli Foundation for Scientific Research and Development, by research grant IS-3126-99 from The United States-Israel Binational Agricultural Research and Development Fund, and by the Israel Academy of Sciences and Humanities Foundation.
REFERENCES
- 1.Barbour A G, Burman N, Carter C J, Kitten T, Bergstrom S. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol. 1991;5:489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 2.Baseggio N, Glew M D, Markham P F, Whithear K G, Browning G F. Size and genomic location of the pMGA multigene family of Mycoplasma gallisepticum. Microbiology. 1996;142:1429–1435. doi: 10.1099/13500872-142-6-1429. [DOI] [PubMed] [Google Scholar]
- 3.Behrens A, Heller M, Kirchhoff H, Yogev D, Rosengarten R. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect Immun. 1994;62:5075–5084. doi: 10.1128/iai.62.11.5075-5084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beier T, Hotzel H, Lysnyansky I, Grajetzki C, Heller M, Rabeling B, Yogev D, Sachse K. Intraspecies polymorphism of vsp genes and expression profiles of variable surface protein antigens (Vsps) in field isolates of Mycoplasma bovis. Vet Microbiol. 1998;63:189–203. doi: 10.1016/s0378-1135(98)00238-7. [DOI] [PubMed] [Google Scholar]
- 5.Bhugra B, Voelker L L, Zou N, Yu H, Dybvig K. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol Microbiol. 1995;18:703–714. doi: 10.1111/j.1365-2958.1995.mmi_18040703.x. [DOI] [PubMed] [Google Scholar]
- 6.Borst P, Greaves D R. Programmed gene rearrangements altering gene expression. Science. 1987;235:658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin J, Blaser M J. Molecular mechanisms of Campylobacter fetus surface layer protein expression. Mol Microbiol. 1997;26:433–440. doi: 10.1046/j.1365-2958.1997.6151958.x. [DOI] [PubMed] [Google Scholar]
- 8.Dybvig K. DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol. 1993;10:465–471. doi: 10.1111/j.1365-2958.1993.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 9.Garcia M M, Lutze-Wallace C L, Denes A S, Eaglesome M D, Holst E, Blaser M J. Protein shift and antigenic variation in the S-layer of Campylobacter fetus subsp. venerealis during bovine infection accompanied by genomic rearrangement of sapA homologs. J Bacteriol. 1995;177:1976–1980. doi: 10.1128/jb.177.8.1976-1980.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasper D E. The role of Mycoplasma in bovine mastitis. J Am Vet Med Assoc. 1982;181:158–162. [PubMed] [Google Scholar]
- 11.Kitten T, Barrera A V, Barbour A G. Intragenic recombination and a chimeric outer membrane protein in the relapsing fever agent Borrelia hermsii. J Bacteriol. 1993;175:2516–2522. doi: 10.1128/jb.175.9.2516-2522.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Lysnyansky I, Rosengarten R, Yogev D. Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J Bacteriol. 1996;178:5395–5401. doi: 10.1128/jb.178.18.5395-5401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lysnyansky I, Sachse K, Rosenbusch R, Levisohn S, Yogev D. The vsp locus of Mycoplasma bovis: gene organization and structural features. J Bacteriol. 1999;181:5734–5741. doi: 10.1128/jb.181.18.5734-5741.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 16.Meyer T F, Gibbs C P, Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- 17.Noormohammadi A H, Markham P F, Kanci A, Whithear K G, Browning G F. A novel mechanism for control of antigenic variation in the haemagglutinin gene family of Mycoplasma synoviae. Mol Microbiol. 2000;35:911–923. doi: 10.1046/j.1365-2958.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- 18.Pays E. Pseudogenes, chimaeric genes and the timing of antigen variation in African trypanosomes. Trends Genet. 1989;5:389–391. doi: 10.1016/0168-9525(89)90181-9. [DOI] [PubMed] [Google Scholar]
- 19.Petes T D, Hill C W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- 20.Pfützner H, Sachse K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev Sci Tech Off Int Epizoot. 1996;15:1477–1494. doi: 10.20506/rst.15.4.987. [DOI] [PubMed] [Google Scholar]
- 21.Plasterk R H A, Simon M I, Barbour A G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985;318:257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- 22.Rainey P B, Moxon E R, Thompson I P. Intraclonal polymorphism in bacteria. Adv Microb Ecol. 1993;13:263–300. [Google Scholar]
- 23.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Restrepo B I, Carter C J, Barbour A G. Activation of a vmp pseudogene in Borrelia hermsii: an alternative mechanism of antigenic variation during relapsing fever. Mol Microbiol. 1994;13:287–299. doi: 10.1111/j.1365-2958.1994.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 25.Robertson B D, Meyer T F. Antigenic variation in bacterial pathogens. Symp Soc Gen Microbiol. 1992;49:61–73. [Google Scholar]
- 26.Rosa P A, Schwan T, Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992;6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 27.Sachse K, Helbig J H, Lysnaynsky I, Grajetzki C, Muller W, Jacobs E, Yogev D. Epitope mapping of immunogenic and adhesive structures in repetitive domains of Mycoplasma bovis variable surface lipoproteins. Infect Immun. 2000;68:680–687. doi: 10.1128/iai.68.2.680-687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifert H S, So M. Genetic mechanisms of bacterial antigenic variation. Microbiol Rev. 1988;52:327–336. doi: 10.1128/mr.52.3.327-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simecka J W, Davis J K, Davidson M K, Ross S E, Stadtländer C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 391–416. [Google Scholar]
- 32.Smith G R. Hotspots of homologous recombination. Experientia. 1994;50:234–241. doi: 10.1007/BF01924006. [DOI] [PubMed] [Google Scholar]
- 33.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thon G, Baltz T, Eisen H. Antigenic diversity by the recombination of pseudogenes. Genes Dev. 1989;3:1247–1254. doi: 10.1101/gad.3.8.1247. [DOI] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tummuru M K R, Blaser M J. Rearrangement of sapA homologs with conserved and variable regions in Campylobacter fetus. Proc Natl Acad Sci USA. 1993;90:7265–7269. doi: 10.1073/pnas.90.15.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise K S, Yogev D, Rosengarten R. Antigenic variation. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 473–489. [Google Scholar]
- 38.Yogev D, Rosengarten R, Watson-McKown R, Wise K S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5′ regulatory sequences. EMBO J. 1991;10:4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]