Abstract
Background: Superior capsule reconstruction (SCR) is an option for the treatment of massive, irreparable rotator cuff tears. However, which materials yield the strongest constructs remains undetermined. Purposes: We sought to investigate whether SCR with polytetrafluoroethylene (PTFE) or human dermal allograft (HDA), 2 or 3 glenoid anchors, and suture or minitape resulted in better failure load properties at the patch-glenoid interface. Methods: We conducted a biomechanical study in 30 glenoid-sided SCR repairs in Sawbones models divided into 5 groups. Each was pulled to failure to assess mode of failure, peak load (N), stiffness (N/mm), yield load (N), peak energy (N m), and ultimate energy (N m). The 5 groups were as follows: group 1—PTFE, 2 anchors, and suture; group 2—PTFE, 2 anchors, and minitape; group 3—HDA, 2 anchors, and suture; group 4—HDA, 2 anchors, and minitape; group 5—PTFE, 3 anchors, and minitape. Results: Repairs failed by button-holing of suture/minitape. Group 5 had greater peak load, stiffness, yield load, and peak energy (384 ± 62 N; 24 ± 3 N/mm; 343 ± 42 N; 4 ± 2 N m) than group 3 (226 ± 67 N; 16 ± 4 N/mm; 194 ± 74 N; 2 ± 1 N m) or group 4 (274 ± 62 N; 17 ± 4 N/mm; 244 ± 50 N; 2 ± 1 N m) and greater ultimate energy (8 ± 3 N m) than all other groups. Conclusions: This biomechanical study of SCR repairs in Sawbones models found that yield load was greater in PTFE than HDA, 3 anchors were better than 2, and minitape was no better than suture.
Keywords: biomechanics, superior capsular reconstruction, synthetic, arthroscopy, irreparable rotator cuff tear
Massive, irreparable rotator cuff tears are difficult injuries to treat, and there are various approaches to their management. These have included debridement, tendon transfers [8,11,16,29], interposition grafts [2,4,5,13–15,22,24,31,32,34,41–43,45,47,48], shoulder arthroplasty [17,23,30,35,40,49], and superior capsule reconstruction (SCR). Mihata et al [28] pioneered SCR in 2012, using autologous fascia lata graft anchored to the glenoid medially and to greater tuberosity laterally in a biomechanical study of 8 human cadaveric shoulders. This study compared the superior stability of cadaveric shoulders in 5 conditions, using intact and torn supraspinatus groups as controls. Fascia lata SCR was effective in restoring superior humeral head stability, reducing subacromial contact pressure, and improving the range of motion at the glenohumeral joint.
Clinically, however, human dermal allograft (HDR) has become popular in SCR [21]. The main advantage of using HDR is it circumvents harvesting of autologous fascia lata and the associated donor site morbidity. Ex vivo biomechanical studies that compared fascia lata with HDR found that when grafts were of similar thickness, HDR was equally as effective as fascia lata in increasing the acromiohumeral distance and reducing subacromial contact pressures [10,46].
Synthetic patches also circumvent autologous harvest. In a clinical study of 35 patients treated with Teflon (polytetrafluoroethylene [PTFE]) SCR, Okamura et al [33] compared single-layer PTFE with triple-layer PTFE in 15 and 20 patients, respectively, with a minimum 2-year follow-up. Both groups experienced improvements in American Shoulder and Elbow Surgeons Shoulder (ASES) scores, visual analog scale score for motion pain, and strength in abduction. However, those in the triple-layer group experienced greater acromiohumeral distance (6 mm vs 9 mm) at 1-year post-operation and active elevation (107° vs 142°) at final follow-up than those in the single-layer group. Although synthetic patch SCR has shown promising results, how synthetic PTFE patches compare with HDR with respect to failure properties in SCR remains undetermined.
While various morphological patterns of graft failure exist [6], several clinical studies have identified through postoperative magnetic resonance imaging that graft failures most commonly failed at the glenoid interface, followed in frequency by intrasubstance tearing [3,9,12,18,39]. A number of authors used 2 glenoid anchors as described in both clinical and biomechanical studies by Mihata et al [26,28], whereas others used 3 glenoid anchors [7,19,36,44]. Pogorzelski et al [38] found that SCR with 3 glenoid anchors had greater pullout strength than SCR with 4 anchors in 36 cadaveric shoulders. However, to our knowledge, there has been no study to determine whether there is any biomechanical advantage of using 3 versus 2 anchors at the glenoid.
A previous study by our institution found that tape had higher peak failure loads compared with suture rotator cuff repairs in ovine shoulders [20]. However, the potential benefits of minitape versus suture have not been investigated with respect to SCR.
We hypothesized that with respect to mode of failure and peak failure load, that synthetic PTFE patches would be superior to HDR, 3 glenoid anchors would be superior to 2 glenoid anchors, and minitape would be superior to suture. The aims of this study, therefore, were to determine whether using (1) PTFE or HDR, (2) suture or minitape, and (3) 2 or 3 glenoid-sided anchors would result in different mode of failure, peak failure load, and stiffness properties in SCR.
Methods
We conducted a biomechanical study in 30 glenoid-sided SCR repairs in Sawbones models (#1050, large left scapula, foam cortical shell with cancellous material, with vise attachment block). We sought to determine mode of failure, peak load (N), stiffness (N/mm), yield load (N), peak energy (N m), and ultimate energy (N m) in 3 comparisons: 2.87-mm-thick synthetic PTFE patches (PTFE Felt) versus 1.27- to 1.78-mm thick HDR (GraftJacket, MaxForce Extreme; 1.9–2.5 mm thickness, 40 mm × 70 mm); 2 versus 3 anchors (SwiveLock; Arthrex; 4.75 mm); and suture (No. 2 FiberWire) versus minitape (MINITAPE).
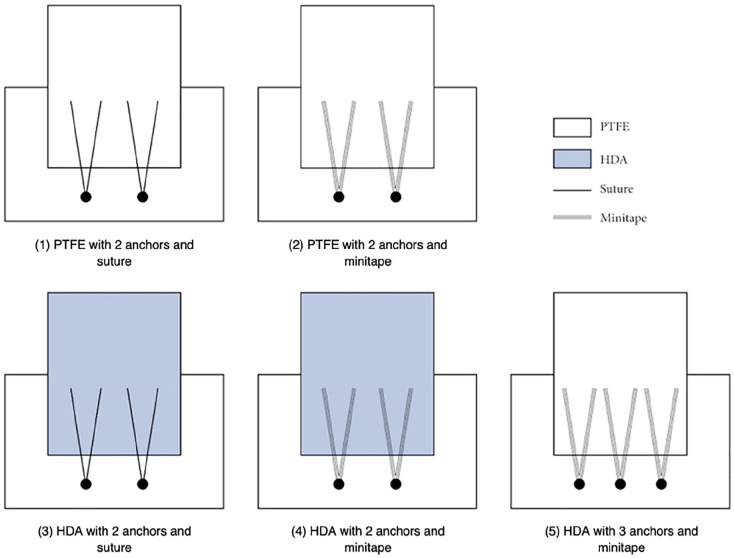
We divided the 30 glenoid-sided SCR repairs into 5 groups: group 1—PTFE, 2 anchors, and suture; group 2—PTFE, 2 anchors, and minitape; group 3—HDA, 2 anchors, and suture; group 4—HDA, 2 anchors, and minitape; group 5—PTFE, 3 anchors, and minitape (Fig. 1). Sawbones models were chosen as they provided controlled bone density in the context of this load-to-failure study. Polytetrafluoroethylene was selected as it is an accessible material that has shown promising 2-year outcomes in both functional tests and patient-reported outcome measures [33]. Human dermal allograft was selected because it has become the most commonly used graft in SCR [21]. Suture was selected as it is the conventional fixation used in SCR, and minitape was selected to investigate for a biomechanical difference between the 2.
Fig. 1.
Schema of superior capsule reconstruction repair groups 1–5; n = 6 for each group. PTFE polytetrafluoroethylene, HDA human dermal allograft.
A total of 18 PTFE and 12 HDR grafts were cut to 30 mm × 50 mm patches for surgical repair method testing. The strips of human dermal allografts were rehydrated according to the manufacturer’s instructions by soaking in 0.9% normal saline for at least 1 hour before being cut, fabricated, and repaired for testing on the same day. The scapular part of 15 Sawbones scapulae was sawn off, leaving rectangular blocks that accommodated 2 SCR repairs each.
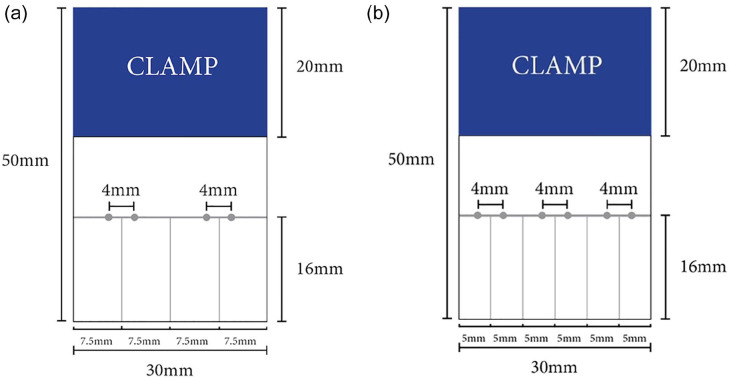
A template (Fig. 2a) was created for the standardization of 24 patches for 2-glenoid anchor SCR repairs (groups 1–4). Four guide holes were made with a 3-0 tapered needle, with 2 holes for each inverted mattress suture/minitape. Another template (Fig. 2b) was created for the standardization of 6 patches for the 3-glenoid anchor SCR group (group 5). Six guide holes were made with a 3-0 tapered needle for 3 inverted mattress sutures/minitapes.
Fig. 2.
Template used for the preparation of (a) 2-anchor and (b) 3-anchor repairs.
Sutures/minitapes were passed using the inverted mattress technique (4 mm apart, 16 mm from medial end of the graft) using a suture passer (Scorpion; Arthrex) through the premarked patches (Fig. 1). Two holes were tapped 15 mm apart for groups 1 to 4, and 3 holes were tapped 10 mm apart for group 5 in Sawbones blocks using a 4.7-mm SwiveLock punch. Each free end of the suture/minitape was cut to 25 mm and then passed through a SwiveLock anchor. The anchor was tapped into the premade holes and screwed into the Sawbones block.
This study used a custom testing apparatus that incorporated 2 clamps: 1 stationary vice to hold the Sawbones block and 1 clamp for the graft, which was connected to a loadcell (HFG 110, Transducer Techniques) mounted on a separate vice. A digital caliper (RS193-252, Mitutoyo) was attached to the mobile vice for linear position measurements. Modes of failure were recorded by video for each repair.
The protocol used was as per previous investigations [1,20,24,37,42,48]. In brief, the Sawbones block was secured in the stationary vice, and the patch graft was secured by a 20-mm bite of the clamp in the dynamic component of the testing apparatus (Fig. 2). The repairs were tested with the direction of pull through the longitudinal axis of the graft, perpendicular to the anchors. Each repair was preloaded with 10 N for 30 seconds as previously established [1,20,24,37,42,48] and then progressively pulled to failure at a rate of 1 mm/second. This protocol was repeated for all 5 groups.
Modes of failure were recorded by video for each repair. Peak load was the maximum load value recorded during testing (N). Stiffness was the resistance to deformation (N/mm). Yield load was the load at which elastic deformation became permanent or “plastic” deformation (N). Peak energy was the area under the load-displacement curve up to the yield load (N m). Ultimate energy was the entire area under the load-displacement curve.
Sample size was set at 6 in accordance with a power calculation (α = 0.05, power = 0.80) that determined a minimum of 4 samples were required. Differences in peak load (N), stiffness (N/mm), yield load (N), peak energy (N m), and ultimate energy (N m) were analyzed by 1-way analysis of variance with correction for multiple comparisons using the Tukey method. P < .05 was considered statistically significant.
The reliability of using this setup was evaluated in an unpublished biomechanical study. The reliability of using ImageJ (Ver. 1.51, National Institutes of Health) to measure the predefined parameters was assessed using 12 measurements for the length on superior and lateral views between 2 markings on a patch, which was preloaded at 10 N for 30 seconds. Rater 1 and Rater 2 measured the lengths independently using ImageJ. Two-way random-effects intraclass correlation coefficients (ICCs) were calculated using SPSS. The reliability of using ImageJ for measurement was excellent, with inter-rater reliability ICC = 0.93 and intra-rater reliability ICC = 0.99.
Results
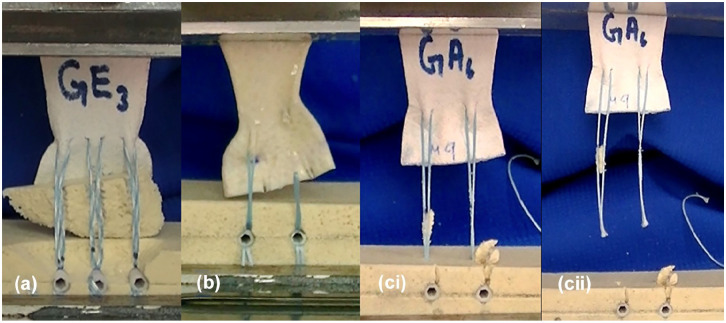
The most common mode of failure in 2-anchor repairs (groups 1–4) was button-holing of the suture/minitape through the graft (Fig. 3a), whereas the most common mode of failure in 3-anchor repairs (group 5) was avulsion of the Sawbones by the suture/minitape (Fig. 3b). Suture/minitape cutout through the Sawbones (Fig. 3ci) and eventual pullout from the anchors (Fig. 3cii) were the remaining modes of failure that were observed. The peak load (N), stiffness (N/mm), yield load (N), and peak energy (N m) values were significantly greater in group 5 (PTFE, 3 anchors, and minitape) than in either group 3 (HDA, 2 anchors, and suture) or group 4 (HDA, 2 anchors, and minitape). The ultimate energy (N m) was significantly greater in group 5 than in groups 1 to 4, respectively (Table 1).
Fig. 3.
Modes of failure: (a) avulsion of Sawbones, (b) button-holing at the right suture human dermal allograft interface, (ci) suture cutout through Sawbones and (cii) eventual suture pullout from anchors.
Table 1.
Most common mode of failure (number of samples that failed in stated mechanism/number of samples), peak load (N), stiffness (N/mm), yield load (N), peak energy (N m), and ultimate energy (N m).
| (1) PTFE, 2 anchors, suture |
(2) PTFE, 2 anchors, minitape |
(3) HDA, 2 anchors, suture |
(4) HDA, 2 anchors, minitape |
(5) PTFE, 3 anchors, minitape |
Significant differences between groups (P < .05) | |
|---|---|---|---|---|---|---|
| Most common mode of failure | Button-holing (4/6) | Button-holing (6/6) | Button-holing (6/6) | Button-holing (6/6) | Avulsion (3/6) |
Statistical analysis was not performed. |
| Peak load (N) | 293 ± 65 | 291 ± 36 | 226 ± 67 | 274 ± 62 | 384 ± 62 | (3) vs (5) (4) vs (5) |
| Stiffness (N/mm) | 22 ± 3 | 22 ± 3 | 16 ± 4 | 17 ± 4 | 24 ± 3 | (3) vs (5) (4) vs (5) |
| Yield load (N) | 268 ± 70 | 264 ± 33 | 194 ± 74 | 244 ± 50 | 343 ± 42 | (3) vs (5) (4) vs (5) |
| Peak energy (N m) | 3 ± 1 | 3 ± 1 | 2 ± 1 | 2 ± 1 | 4 ± 2 | (3) vs (5) (4) vs (5) |
| Ultimate energy (N m) | 4 ± 1 | 4 ± 1 | 2 ± 1 | 3 ± 1 | 8 ± 3 | (1) vs (5) (2) vs (5) (3) vs (5) (4) vs (5) |
Data are mean ± SD. PTFE polytetrafluoroethylene, HDA human dermal allograft.
In group 1, 4/6 repairs failed by button-holing, and 2/6 repairs failed by suture cut-out. In group 2, 6/6 repairs failed by button-holing. In group 3, 6/6 repairs failed by button-holing. In group 4, 6/6 repairs failed by button-holing. In group 5, 3/6 repairs failed by avulsion of the Sawbones, 2/6 repairs failed by button-holing, and 1/6 repairs failed by consecutive cutting out of each of the minitapes.
Group 5 had a significantly greater peak load (384 ± 62 N) than group 3 (226 ± 67 N; P = .037) or group 4 (274 ± 62 N; P = .006) (Fig. 4). Group 5 had a significantly greater stiffness (24 ± 3 N/mm) than group 3 (16 ± 4 N/mm; P = .004) or group 4 (17 ± 3 N/mm; P = .044) (Fig. 5). Group 5 had a significantly greater yield load (343 ± 42 N) than group 3 (194 ± 74 N; P = .001) or group 4 (244 ± 50 N; P = .038) (Fig. 6). Group 5 had a significantly greater peak energy (4 ± 2 N m) than group 3 (2 ± 1; P = .006) or group 4 (2 ± 1 N m; P = .044) (Fig. 7a). Group 5 had a significantly greater ultimate energy (8 ± 3 N m) than group 1 (4 ± 1 N m; P = .041), group 2 (4 ± 1 N m; P = .028), group 3 (2 ± 1 N m; P = .001), or group 4 (3 ± 1 N m; P = .002) (Fig. 7b).
Fig. 4.
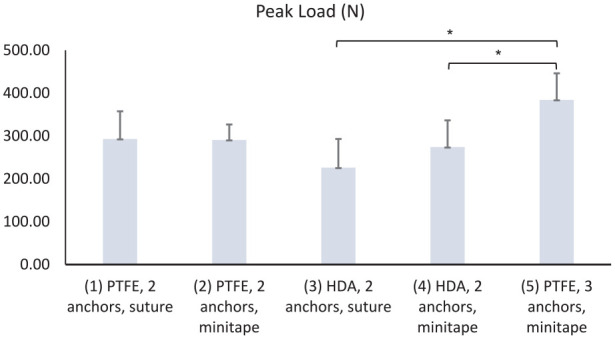
Peak failure load (N) for groups 1–5. PTFE polytetrafluoroethylene, HDA human dermal allograft.
*P < .05 calculated with 1-way analysis of variance using the Tukey multiple comparisons test.
Fig. 5.
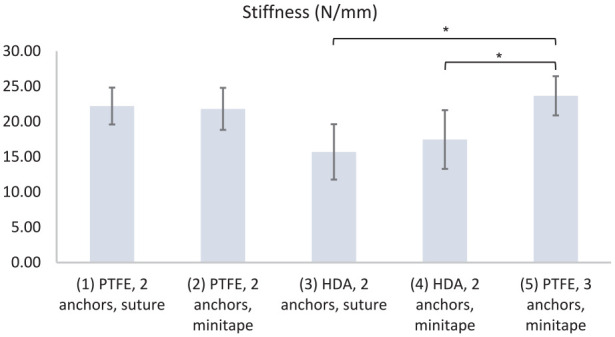
Stiffness (N/mm) for groups 1–5. PTFE polytetrafluoroethylene, HDA human dermal allograft.
*P < .05 calculated with 1-way analysis of variance using the Tukey multiple comparisons test.
Fig. 6.
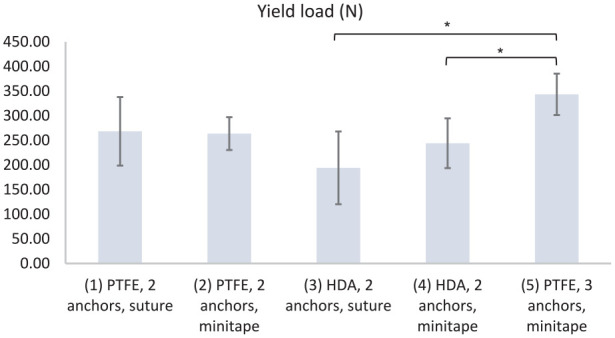
Yield load (N) for groups 1–5. PTFE polytetrafluoroethylene, HDA human dermal allograft.
*P < .05 calculated with 1-way analysis of variance using the Tukey multiple comparisons test.
Fig. 7.
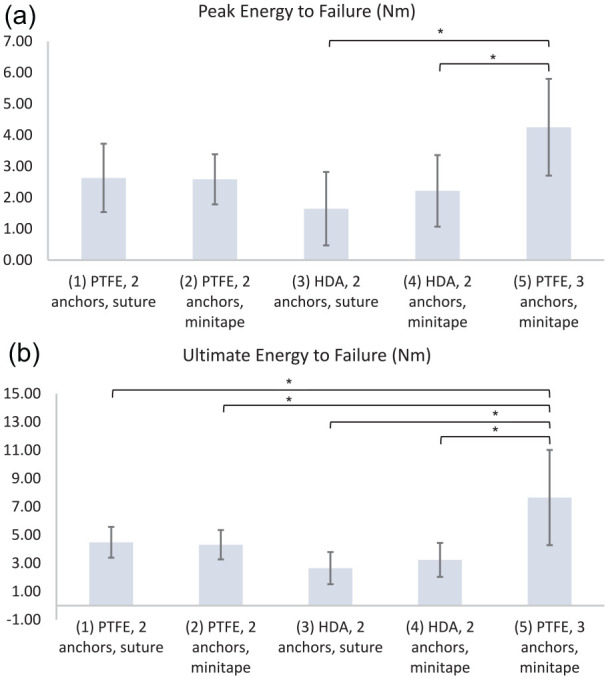
(a) Peak energy (N m) and (b) ultimate energy for groups 1–5. PTFE polytetrafluoroethylene, HDA human dermal allograft.
*P < .05 calculated with 1-way analysis of variance using the Tukey multiple comparisons test.
Discussion
Our major finding in this biomechanical study was that SCR with PTFE, 3 anchors, and minitape demonstrated a significantly greater peak load, ultimate energy, stiffness, and yield load than HDA with 2 anchors and suture or HDA with 2 anchors and minitape at the glenoid interface. Polytetrafluoroethylene with 3 anchors and minitape also demonstrated a significantly greater ultimate energy than all other groups. Our hypotheses, that PTFE would be superior to HDA and that 3 anchors would be superior to 2 anchors, were affirmed, whereas our hypothesis that suture would be superior to minitape was refuted.
This study has some limitations. First, we focused on one side of the repair and did not analyze the patch-greater tuberosity interface. However, we focused on the glenoid interface because of the tendency for SCR to fail at the glenoid in the clinical setting [3,9,12,18,39]. Second, we used Sawbones instead of cadaveric models, and as with any ex vivo biomechanical study, we were unable to account for any biological changes that would occur clinically. The focus of this study was on the material properties of the graft itself. Finally, a larger sample size may have revealed more subtle differences between groups.
Peak load was the greatest load before failure, peak energy was the energy absorbed by the repair up to the yield point, and ultimate energy was the energy that was absorbed by the repair until it failed completely. While these are important material properties, stiffness, the resistance to elastic deformation, and yield load, the load at which elastic deformation becomes plastic deformation, are more clinically relevant properties when one considers the repetitive, low-grade stresses that occur in vivo. Because superior capsule reconstruction is a static stabilizer, its efficacy is diminished as it loses tension at higher angles of abduction [27]. Stiffer grafts with higher yield loads are less susceptible to plastic deformation, thereby preserving the graft’s ability to buttress the humeral head and reduce superior translation and subacromial contact pressure [25].
Among 2-anchor repairs, the most common mode of failure was by button-holing of the suture or minitape through the patch, which occurred in 6/6 samples for all 2-anchor groups except for the PTFE, 2 anchors, and suture group, in which suture cutout occurred in 2/4 samples. This demonstrated that 2 anchor repairs failed at the patch-suture interface in preference to bony damage. The most common mode of failure in the PTFE with 3 anchors and minitape group was by avulsion of Sawbones, which occurred in 3/6 samples, followed by button-holing. A potential clinical implication of glenoid avulsion would be destruction of bone stock, resulting in increased difficulty if revision arthroplasty is needed.
Among the groups that used 2 anchors, there were no significant differences in peak load, ultimate energy, peak energy, stiffness, or yield load between PTFE and HDR, or between suture and minitape.
In conclusion, glenoid-sided superior capsule reconstruction with PTFE, 3 anchors, and minitape demonstrated greater peak load, peak energy, stiffness, and yield load than either HDR with 2 anchors and suture or minitape, and greater ultimate energy than all other groups. The most common mode of failure was button-holing of suture/minitape through the graft. There were no differences between suture and minitape in 2-anchor repairs.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: George A.C. Murrell, MD, DPhil, reports relationships with Smith & Nephew, the Journal of Shoulder and Elbow Surgery, and Shoulder and Elbow. The other authors declare no potential conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent: Informed consent was not required for this biomechanical study.
Level of Evidence: Level V, Biomechanical Study.
Required Author Forms: Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
ORCID iDs: Ryan S. Ting  https://orcid.org/0000-0003-0106-3129
https://orcid.org/0000-0003-0106-3129
Ron Rosenthal  https://orcid.org/0000-0002-5423-9530
https://orcid.org/0000-0002-5423-9530
George A. C. Murrell  https://orcid.org/0000-0002-8251-1327
https://orcid.org/0000-0002-8251-1327
References
- 1. Andres BM, Lam PH, Murrell GAC. Tension, abduction, and surgical technique affect footprint compression after rotator cuff repair in an ovine model. J Shoulder Elbow Surg. 2010;19(7):1018–1027. 10.1016/j.jse.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 2. Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17(suppl 1):35s–39s. 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 3. Badman BL, Baessler AM, Moor M. Short-term clinical outcomes and comparison of ultrasound versus magnetic resonance imaging of superior capsular reconstruction. Arthrosc Sports Med Rehab. 2020;2(3):e229–e235. 10.1016/j.asmr.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8–15. 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 5. Bektaşer B, Oçgüder A, Solak S, Gönen E, Yalçın N, Kılıçarslan K. Free coracoacromial ligament graft for augmentation of massive rotator cuff tears treated with mini-open repair. Acta Orthop Traumatol Turc. 2010;44(6):42630–42634. 10.3944/aott.2010.2423. [DOI] [PubMed] [Google Scholar]
- 6. Bernstein JM, Perez JR, Shah HA, et al. MRI of superior capsular reconstruction. RadioGraphics. 2020;40(2):454–467. 10.1148/rg.2020190074. [DOI] [PubMed] [Google Scholar]
- 7. Burkhart SS, Pranckun JJ, Hartzler RU. Superior capsular reconstruction for the operatively irreparable rotator cuff tear: clinical outcomes are maintained 2 years after surgery. Arthroscopy. 2020;36(2):373–380. 10.1016/j.arthro.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 8. Camp CL, Elhassan B, Dines JS. Clinical faceoff: irreparable rotator cuff tears in young, active patients: tendon transfer versus superior capsular reconstruction? Clin Orthop Relat Res. 2018;476(12):2313–2317. 10.1097/CORR.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell AL, Baron SL, Pham H, Gyftopoulos S, Meislin R, Samim M. MRI of superior capsular reconstruction graft and associated short-term clinical outcomes in patients with massive irreparable rotator cuff tears. Clin Imaging. 2021;70:74–80. 10.1016/j.clinimag.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 10. Cline KE, Tibone JE, Ihn H, et al. Superior capsule reconstruction using fascia lata allograft compared with double- and single-layer dermal allograft: a biomechanical study. Arthroscopy. 2021;37(4):1117–1125. 10.1016/j.arthro.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 11. Delaney RA, Kadow TR, Garcia D, Minorini R, Baratz ME, Lin A. Latissimus dorsi tendon transfer vs. superior capsular reconstruction for treatment of irreparable rotator cuff tears: a retrospective comparison study with short-term clinical results. JSES Open Access. 2019;3(4):249. 10.1016/j.jses.2019.10.064. [DOI] [Google Scholar]
- 12. Emerson CP, Balazs GC, Lee SC, Dines JS, Jose J, Greditzer H. Magnetic resonance imaging of the failed superior capsular reconstruction. Clin Imaging. 2020;60(2):172–176. 10.1016/j.clinimag.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 13. Encalada-Diaz I, Cole BJ, Macgillivray JD, et al. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months’ follow-up. J Shoulder Elbow Surg. 2011;20(5):788–794. 10.1016/j.jse.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872–879. 10.1177/0363546512475204. [DOI] [PubMed] [Google Scholar]
- 15. Hirooka A, Yoneda M, Wakaitani S, et al. Augmentation with a Gore-Tex patch for repair of large rotator cuff tears that cannot be sutured. J Orthop Sci. 2002;7(4):451–456. 10.1007/s007760200078. [DOI] [PubMed] [Google Scholar]
- 16. Kadow TR, Meredith SJ, Garcia D, et al. Latissimus dorsi tendon transfer and superior capsular reconstruction for irreparable, posterosuperior rotator cuff tears. Arch Bone Jt Surg. 2021;9(1):44–49. 10.22038/abjs.2020.50854.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khair MM, Gulotta LV. Treatment of irreparable rotator cuff tears. Curr Rev Musculoskelet Med. 2011;4(4):208–213. 10.1007/s12178-011-9098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacheta L, Horan MP, Schairer WW, et al. Clinical And MRI outcomes after arthroscopic superior capsule reconstruction with human dermal allograft for irreparable posterosuperior rotator cuff tears a minimum two year follow-up. Orthop J Sports Med. 2020;8(3, suppl 2):2325967120. 10.1177/2325967120S00123. [DOI] [PubMed] [Google Scholar]
- 19. Lee CS, Reddy M, Scott B, Curtis D, Amirouche F, Athiviraham A. Suture tape-reinforced human dermal allograft used for superior capsule reconstruction demonstrates improved ability to withstand elongation. Arthrosc Sports Med Rehabil. 2020;2(5):e511–e515. 10.1016/j.asmr.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu RW, Lam PH, Shepherd HM, Murrell GAC. Tape versus suture in arthroscopic rotator cuff repair: biomechanical analysis and assessment of failure rates at 6 months. Orthop J Sports Med. 2017;5(4):2325967117701212. 10.1177/2325967117701212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makovicka JL, Chung AS, Patel KA, Deckey DG, Hassebrock JD, Tokish JM. Superior capsule reconstruction for irreparable rotator cuff tears: a systematic review of biomechanical and clinical outcomes by graft type. J Shoulder Elbow Surg. 2020;29(2):392–401. 10.1016/j.jse.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 22. Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33(6):907–911. 10.1177/0363546504271500. [DOI] [PubMed] [Google Scholar]
- 23. Matsen FA, III. Clinical practice. Rotator-cuff failure. N Engl J Med. 2008;358(20):2138–2147. 10.1056/NEJMcp0800814. [DOI] [PubMed] [Google Scholar]
- 24. McKeown A, Beattie RF, Murrell GA, Lam PH. Biomechanical comparison of expanded polytetrafluoroethylene (ePTFE) and PTFE interpositional patches and direct tendon-to-bone repair for massive rotator cuff tears in an ovine model. Shoulder Elbow. 2016;8(1):22–31. 10.1177/1758573215601815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mihata T, Bui CNH, Akeda M, et al. A biomechanical cadaveric study comparing superior capsule reconstruction using fascia lata allograft with human dermal allograft for irreparable rotator cuff tear. J Shoulder Elbow Surg. 2017;26(12):2158–2166. 10.1016/j.jse.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 26. Mihata T, Lee TQ, Hasegawa A, et al. Five-year follow-up of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. J Bone Joint Surg Am. 2019;101(21):1921–1930. 10.2106/jbjs.19.00135. [DOI] [PubMed] [Google Scholar]
- 27. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical effect of thickness and tension of fascia lata graft on glenohumeral stability for superior capsule reconstruction in irreparable supraspinatus tears. Arthroscopy. 2016;32(3):418–426. 10.1016/j.arthro.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 28. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248–2255. 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 29. Milano G, Saccomanno MF, Colosio A, et al. Arthroscopic superior capsule reconstruction with doubled autologous semitendinosus tendon graft. Arthrosc Tech. 2020;9(11):e1665–e1672. 10.1016/j.eats.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore DR, Cain EL, Schwartz ML, Clancy WG., Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392–396. 10.1177/0363546505281237. [DOI] [PubMed] [Google Scholar]
- 31. Nada AN, Debnath UK, Robinson DA, Jordan C. Treatment of massive rotator-cuff tears with a polyester ligament (Dacron) augmentation: clinical outcome. J Bone Joint Surg Br. 2010;92(10):1397–1402. 10.1302/0301-620x.92b10.24299. [DOI] [PubMed] [Google Scholar]
- 32. Neumann JA, Zgonis MH, Rickert KD, et al. Interposition dermal matrix xenografts: a successful alternative to traditional treatment of massive rotator cuff tears. Am J Sports Med. 2017;45(6):1261–1268. 10.1177/0363546516683945. [DOI] [PubMed] [Google Scholar]
- 33. Okamura K, Abe M, Yamada Y, et al. Arthroscopic superior capsule reconstruction with Teflon felt synthetic graft for irreparable massive rotator cuff tears: clinical and radiographic results at minimum 2-year follow-up. J Shoulder Elbow Surg. 2021;30(3):625–634. 10.1016/j.jse.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 34. Ozaki J, Fujimoto S, Masuhara K, Tamai S, Yoshimoto S. Reconstruction of chronic massive rotator cuff tears with synthetic materials. Clin Orthop Relat Res. 1986;202:173–183. [PubMed] [Google Scholar]
- 35. Pandey V, Jaap Willems W. Rotator cuff tear: a detailed update. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2015;2(1):1–14. 10.1016/j.asmart.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pennington WT, Bartz BA, Pauli JM, Walker CE, Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34(6):1764–1773. 10.1016/j.arthro.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 37. Peters KS, Lam PH, Murrell GA. Repair of partial-thickness rotator cuff tears: a biomechanical analysis of footprint contact pressure and strength in an ovine model. Arthroscopy. 2010;26(7):877–884. 10.1016/j.arthro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 38. Pogorzelski J, Muckenhirn KJ, Mitchell JJ, et al. Biomechanical comparison of 3 glenoid-side fixation techniques for superior capsular reconstruction. Am J Sports Med. 2018;46(4):801–808. 10.1177/0363546517745626. [DOI] [PubMed] [Google Scholar]
- 39. Ravenscroft MJ, Riley JA, Morgan BW, Sandher DS, Odak SS, Joseph P. Histological incorporation of acellular dermal matrix in the failed superior capsule reconstruction of the shoulder. J Exp Orthop. 2019;6(1):21. 10.1186/s40634-019-0189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rockwood CA, Jr, Williams GR, Jr, Burkhead WZ, Jr. Débridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77(6):857–866. 10.2106/00004623-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 41. Ronquillo JC, Fpoa MD, Lam P, Murrell GAC. Arthroscopic ePTFE patch repair for irreparable rotator cuff tears: part II: preliminary clinical results. Tech Should Elbow Surg. 2013;14(2):33–41. [Google Scholar]
- 42. Ronquillo JC, Lam P, Murrell GAC. Arthroscopic ePTFE patch repair for irreparable rotator cuff tears: part I: surgical technique and biomechanical comparison of 2-suture techniques at the patch to tendon interface. Tech Should Elbow Surg. 2013;14(2):29–32. [Google Scholar]
- 43. Sano H, Mineta M, Kita A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310–316. 10.1007/s00776-010-1453-5. [DOI] [PubMed] [Google Scholar]
- 44. Scheiderer B, Kia C, Obopilwe E, et al. Biomechanical effect of superior capsule reconstruction using a 3-mm and 6-mm thick acellular dermal allograft in a dynamic shoulder model. Arthroscopy. 2020;36(2):355–364. 10.1016/j.arthro.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 45. Seker V, Hackett L, Lam PH, Murrell GAC. Evaluating the outcomes of rotator cuff repairs with polytetrafluoroethylene patches for massive and irreparable rotator cuff tears with a minimum 2-year follow-up. Am J Sports Med. 2018;46(13):3155–3164. 10.1177/0363546518801014. [DOI] [PubMed] [Google Scholar]
- 46. Shah SS, Kontaxis A, Jahandar A, et al. Superior capsule reconstruction using a single 6-mm-thick acellular dermal allograft for massive rotator cuff tears: a biomechanical cadaveric comparison to fascia lata allograft. J Shoulder Elbow Surg. 2021;30:2166–2176. 10.1016/j.jse.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 47. Shepherd HM, Lam PH, Murrell GAC. Synthetic patch rotator cuff repair: a 10-year follow-up. Shoulder Elbow. 2014;6(1):35–39. 10.1111/sae.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sunwoo JY, Lam PH, Murrell GAC. A comparison of two arthroscopic techniques for interpositional polytetrafluoroethylene patch repair for massive irreparable rotator cuff tears: speed and biomechanics. HSS J. 2018;14(2):186–191. 10.1007/s11420-018-9607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89(4):786–791. 10.2106/jbjs.F.00315. [DOI] [PubMed] [Google Scholar]