Abstract
Study Design
Systematic review
Background: Considering the infiltrative nature of intramedullary astrocytoma, the goal of surgery is to have a better patient related outcome.
Objective
To compare the overall survival (OS) and neurologic outcomes of complete vs incomplete surgical resection for patients with intramedullary astrocytoma.
Methods
A comprehensive search of MEDLINE, CENTRAL and EMBASE was conducted by two independent reviewers. Individual patient data (IPD) analysis and multivariate Cox Proportional Hazard Model was developed to measure the effect of surgical strategies on OS, post-operative neurological improvement (PNI), and neurological improvement in the last follow up (FNI).
Results
We included 1079 patients from 35 studies. Individual patient data of 228 patients (13 articles) was incorporated into the integrative IPD analysis. Kaplan-Meier survival analysis showed complete resection (CR) significantly improved OS in comparison with the incomplete resection (IR) (log-rank test, P = .004). In the multivariate IPD analysis, three prognostic factors had significant effect on the OS: (1) Extent of Resection, (2) pathology grade, and (3) adjuvant therapy. We observed an upward trend in the popularity of chemotherapy, but CR, IR, and radiotherapy had relatively stable trends during three decades.
Conclusion
Our study shows that CR can improve OS when compared to IR. Patients with spinal cord astrocytoma undergoing CR had similar PNI and FNI compared to IR. Therefore, CR should be the primary goal of surgery, but intraoperative decisions on the extent of resection should be relied on to prevent neurologic adverse events. Due to significant effect of adjuvant therapy on OS, PNI and FNI, it could be considered as the routine treatment strategy for spinal cord astrocytoma.
Keywords: astrocytoma, intramedullary, spinal, tumor
Introduction
Intramedullary astrocytomas constitute 2-4% of central nervous system tumors and 30% of all intramedullary spinal cord tumor. They have an incidence of .74 to 1.27 per 1,00,000 people each year.1,2 The therapeutic approaches for intramedullary astrocytoma include surgical resection with or without adjuvant radiotherapy and chemotherapy. Due to the infiltrative nature of astrocytomas, the optimal extent of tumor resection to achieve better outcomes while concurrently preserving neurologic functions and quality of life remains controversial. Some factors predicting a poor prognosis include high and low extremes of age, incomplete resection, severe neurologic deficit, and ineffective adjuvant therapies. 3
To date, the treatment most used for intramedullary astrocytoma mainly involves surgical intervention, which would be accompanied by adjuvant therapies for histopathologically confirmed malignant tumors. The surgical interventions include gross total resection (GTR), near total resection (NTR), subtotal resection (STR), or biopsy. 4 When a tumor does not provide a favorable resection plane, a NTR or STR may be the only feasible option. 4 Intramedullary astrocytomas more commonly involve white matter which results in asymmetrical cord expansion with secondary involvement of adjacent parenchyma. This would obscure the tumor margin and causes potential threat to the peripheral normal tissue during surgical resection. More aggressive surgical resection may present some adverse effects such as neurological deterioration, longer hospitalization time, and patient discomfort. 5
The aim of this systematic review is to compare overall survival (OS) and neurologic outcomes between specific surgical strategies for patients with intramedullary astrocytoma.
Methods
A detailed protocol has been published in PROSPERO (CRD42018103513) to study “Biopsy vs resection for intramedullary spinal cord tumors” and a systematic review specifically on intramedullary ependymoma has been performed according to the protocol. 6 Simultaneously, we conducted a systematic review of intramedullary astrocytoma based on the same protocol and the records identified by searching databases. In the section below, we provided a summary of the method used. Our systematic review was done according to the PRISMA 2009 Checklist. 7
Search Strategy
Electronic searches of MEDLINE (1946 to present), CENTRAL, and EMBASE (1980 to present) were performed comprehensively. We conducted searches based on the Medical Subject Headings (MeSH) terms for ‘Spinal Cord Tumors’, ‘Extent of Tumor Resection, and ‘Biopsy’. In addition, we reviewed the references of the included studies for potentially eligible studies. We set no date and language limit in the literature review and non-English language papers were translated by Google online translation tool.
Selection Method
We reviewed all published papers relevant to intramedullary spinal cord tumors using the search strategy. After removal of duplications, four reviewers (??) independently screened the titles and abstracts of studies in a manner allowing each article to be tested by two reviewers. Afterwards, one reviewer (??) assessed the full text of the papers for eligibility criteria. The second reviewer (??) resolved any discrepancies in inclusions or exclusions.
Eligibility Criteria
All published original papers that reported the extent of surgical resection and follow-up data in patients suffering from intramedullary astrocytoma were included in this study. Papers regarding patients with extra medullary tumors (filum terminale and cauda equina tumors), history of prior surgery, and lacking data of extent of surgical resection or follow-up were excluded. In the case of two or more papers of the same database, the paper with a higher number of cases and more complete data was included. Additionally, to eliminate effect of the surgical experience on the outcome, publications with less than 10 cases were excluded.
Main Outcomes
We evaluated the frequency of all-cause mortality and postoperative survival via OS. Due to heterogeneity of definitions of progression-free survival (PFS) in the included studies such as a lack of distinction between local progression, recurrence, and distant metastases; we decided not to include PFS as an outcome. Moreover, we determined functional outcomes via two parameters: post-operative neurologic improvement (PNI) and follow-up neurologic improvement (FNI). For functional outcomes, three different classification grading systems including McCormick, Frankel, and American Spinal Injury Association (ASIA) have been used in the included studies. The data of functional neurologic status at pre-operative, post-operative, and last follow-up were collected for each case irrespective of the different classification methods. To make methods comparable, Frankel and ASIA grades A + B, C, D, and E were considered equal to McCormick grade 4, 3, 2, and 1, respectively.8,9
Data Extraction
We inserted data into a pre-designed data collection form. The database contained general information of the articles, studies information, participants, astrocytoma characteristics, the extent of initial tumor resection, adjuvant treatment, length of follow-up, OS, and pre-and post-operative neurologic scores.
Age was grouped into ≤18 years and >18 years. The Extent of Resection (EOR) was dichotomized into two categories: Complete Resection (CR) and Incomplete Resection (IR). The absence of any residual tumor evidence reported by the neurosurgeons intraoperatively and/or postoperatively via neuroimaging was classified as CR. In contrast, subtotal resection, partial resection, near-total resection, and biopsy were classified as IR. Tumor location was classified as cervical and thoracolumbar according to the highest spinal level involved. Tumor pathology was classified based on the WHO tumor grading system into low-grade astrocytoma (grades 1 and 2), anaplastic astrocytoma (grade 3) and glioblastoma multiform (grade 4). We considered both grade 3 and grade 4 astrocytoma as high-grade astrocytoma. Tumor extension was divided into two subgroups based on the number of segments involved: short segment (<3) and long segment (≥3). Also, the pre-operative neurological status was divided into high-grade dysfunction (standardized score ≥3) and low-grade dysfunction (standardized score <3).
Assessment of Methodological Quality
All papers selected for inclusion in integrative analysis were subjected to appraise by Joanna Briggs Institute (JBI) critical appraisal tool for descriptive/case series studies.
Statistical Analysis
Meta-Analysis
All the included studies were case series. We performed a meta-analysis to assess the prognostic potential of surgery types (CR vs IR). To improve the meta-analysis power, we defined the eligibility criteria for studies to be included into the meta-analysis as (1) group sizes (CR and IR) needed to be at least three and (2) at least one outcome needed to have happened in each group. Fixed-effect inverse variance model was used to estimate pooled Hazard Ratio (HR) and associated 95% Confidence Interval (CI) from OS data. The heterogeneity of studies was calculated using I2 statistics.
Integrative Analysis
We carried out statistical analysis on papers that fully met the inclusion criteria and contained individual patients’ data (IPD). Kaplan-Meier analysis was used to analyze OS (IR vs CR). PFS was not accounted for in the integrative analysis due to the small sample size and ambiguity of the definition of progression as the distinction of progression and recurrence is not completely clear in most of the included studies. OS was compared in the CR and IR groups using the Log-rank test. Events including all-cause mortality and neurologic improvement rates were compared in IR and CR groups using the chi-square test. In univariate analysis, we estimated the impact of potential prognostic variables including age, sex, EOR, tumor length, tumor pathology grade, tumor location, pre-operative neurologic score, and adjuvant therapy on OS and FNI. Multivariate Cox Proportional Hazard Model was developed to measure the effect of CR (vs IR) on outcomes of interest (OS, PNI, and FNI) after adjusting for each potential confounding variables including age, sex, tumor pathologic grade, tumor length, tumor location, pre-operation neurologic status, and adjuvant therapy. The HR with a 95% CI for each of the variables was estimated. To account for the variability in surgeon approaches and treatment plans, we conducted a paper-adjusted analysis. We carried out the analysis using Stata (StataCorp. 2011. Stata Statistical Software: Release 14. College Station, Texas, USA). In this analysis, P < .05 was considered statistically significant.
Ethical Approval
The Ethics Committee of Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences, approved this study, with the reference number 98-02-38-379.
Results
Results of the Search
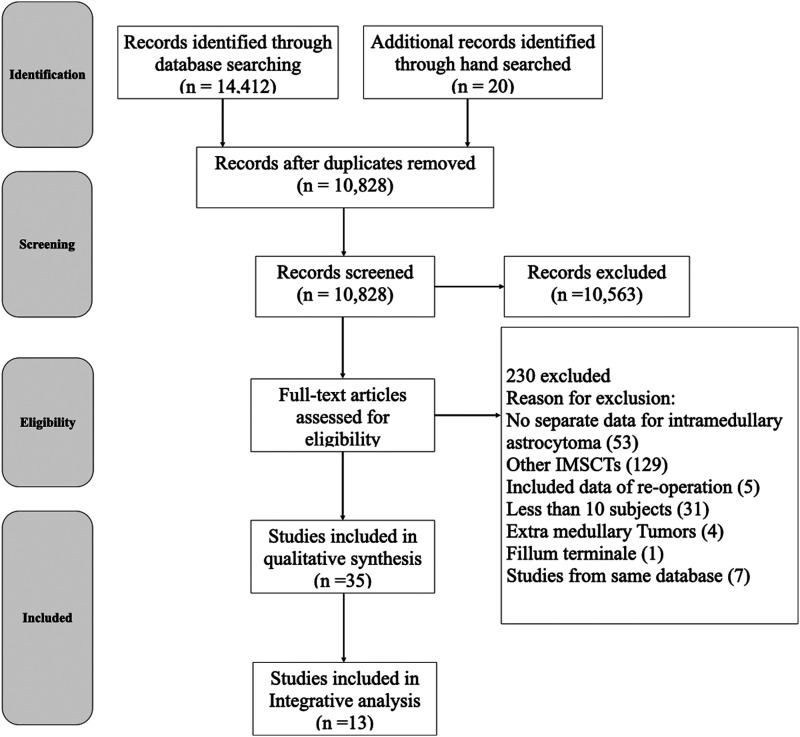
We retrieved a total of 14,432 publications through database searching and hand searching. After removal of 3604 duplicated articles, screening of the titles and abstracts of 10,828 articles was performed and 10,563 articles were excluded (Figure 1). After reviewing the full text of the remaining 265 articles, 35 articles met our inclusion criteria (Table 1). Among the 230 articles excluded, 53 articles did not report data of surgery-related outcomes for astrocytoma separately, 129 articles focused on other intramedullary spinal cord tumors (IMSCTs), five articles included data of re-operation, 31 articles reported less than 10 cases, four articles addressed extra medullary tumors, and one article reported tumors located in the filum terminal region. We also excluded seven articles due to their data being partially retrieved from identical databases.10-16 For the 35 included articles, qualitative analysis was done with respect to the important variables. Thirteen articles containing individual patient data underwent integrative analysis.
Figure 1.
Study flow diagram. IMSCT, Intramedullary spinal cord tumors.
Table 1.
Clinical Information of Included Studies.
| Author& Year | No | Extent of Resection | Adjuvant Therapy | Recurrence | Mortality | Follow-up Neurologic Improvement | Mean FU (month) | High-Grade Pathology | Integrative Analysis | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Santi et al 2003 10 | 36 | 7 CR 27 IR (16B) 2 NR |
17 RT 7 CH |
10 | 36 | NR | 17 a | 34 | * |
| 2 | Robinson et al 2005 11 | 14 | 1 CR 13 IR (7B) |
10 RT 0 CH |
0 | 3 | 3 | 122.4 | 0 | * |
| 3 | McGirt et al 2008 12 | 35 | 12 CR 23 IR |
15 RT 14 CH |
7 | 3 | NR | 38 | 35 | * |
| 4 | Raco et al 2010 13 | 22 | 2 CR 20 IR (2B) |
15 RT 14 CH |
0 | 22 | NR | 18.7 | 22 | * |
| 5 | Guss et al 2013 14 | 29 | 15 CR 13 IR 1 NR |
10 RT (2NR) 13 CH |
NR | 4 | NR | 52 a | 5 | * |
| 6 | Rossitch et al 1990 15 | 12 | 4 CR 8 IR (5B) |
6 RT NR CH |
4 | 2 | NR | 126 | 0 | * |
| 7 | Cheng et al 2017 1 | 14 | 4 CR 10 IR (5B) |
9 RT 12 CH |
NR | 14 | NR | 15 a | 14 | * |
| 8 | Ardeshiri et al 2013 2 | 22 | 16 CR 6 IR (2B) |
NR | NR | 1 | 0 (9 NR) | 21 | 4 | * |
| 9 | Allen et al 1998 16 | 13 | 3 CR 10 IR (3B) |
10 RT 13 CH |
7 | 6 | NR | 76 a | 13 | * |
| 10 | Karikari et al 2011 17 | 21 | 3 CR 18 IR (3B) |
NR | 10 | NR | 1 | 41.8 | 4 | * |
| 11 | Merchant et al 2000 18 | 11 | 5 CR 6 IR (3B) |
6 RT | 2 | 1 | 1 | 11 | 0 | * |
| 12 | Przybylski et al 1997 19 | 18 | 5 CR 13 IR (6B) |
9 RT 0 CH |
0 | 3 | 4 | 132 | 7 | * |
| 13 | Nishio et al 2000 20 | 10 | 0 CR 10 IR (5B) |
6 RT 4 CH |
NR | 4 | 2 | 51.2 | 4 | * |
| 14 | Eroes et al 2010 21 | 15 | 5 CR 10 IR (4B) |
2 RT 0 CH |
0 | 0 | 3 | NR | 0 | |
| 15 | Matsuyamaet al 2009 22 | 12 | 1 CR 11 IR (7B) |
NR | NR | NR | 3 | 148.8 | NR | |
| 16 | Bostrom et al 2014 23 | 11 | 1 CR 10 IR (3B) |
4 RT 4 CH |
1 | 4 | 1 | NR | 3 | |
| 17 | Cooper et al 1989 24 | 18 | 9 CR 9 IR (0B) |
18 RT | NR | 12 | NR | NR | 7 | |
| 18 | Klekamp et al 2013 25 | 76 | 15 CR 61 IR (13B) |
NR | 32 | NR | NR | NR | NR | |
| 19 | Lam et al 2012 26 | 48 | 6 CR 35 IR (8B) 3 NOS |
38 RT (CH NR) | NR | 41 | NR | 120 | 48 | |
| 20 | Cui al. 2017 27 | 21 | 4 CR 17 IR (9B) |
4 RT 1 CH |
NR | NR | 9 | 32.23 | 5 | |
| 21 | Zou et al 2018 28 | 94 | 21 CR 73 IR (23B) |
54 RT 53 CH |
NR | 74 | 2 | 24 a | 48 | |
| 22 | Minehanet al. 2009 29 | 136 | 22 CR 114 IR (80B) |
102 RT 18 CH |
NR | NR | NR | 124.8 | 27 | |
| 23 | Zorlu et al 2005 30 | 24 | 24 IR (14B) | 24 RT (CH NR) | NR | 17 | 21 | 39 a | 4 | |
| 24 | Zileli et al 1996 31 | 15 | 2 CR 13 IR (6B) |
10 RT | 2 | NR | NR | NR | 8 | |
| 25 | Abdel-Wahab et al 2006 32 | 57 | 13 CR 40 IR 4 NR |
39 RT | NR | 24 | 14 34 NR |
21 a | 1 (46 NR) | |
| 26 | Bansal et al 201233 | 23 | 5 CR 18 IR (0B) |
NR | 0 | 0 | 2 | NR | 7 | |
| 27 | Kahn et al 201134 | 18 | 18 IR (10B) | 18 RT 4 CH |
3 | 8 | NR | NR | 14 (1 NR) | |
| 28 | Yang et al 200935 | 62 | 24 CR 38 IR (0B) |
39 RT | 9 | 8 | 37 | NR | 6 | |
| 29 | Nakamura et al 200836 | 23 | 7 CR 14 IR (10B) (2 NR) |
NR | NR | 13 | 3 | NR | 12 | |
| 30 | Sandler et al 201137 | 21 | 3 CR 18 IR (11B) |
15 RT | NR | 5 | NR | 41 a | 2 (1 NR) | |
| 31 | Fornari et al 199838 | 10 | 2 CR 8 IR (0B) |
NR | 3 | 3 | NR | NR | 3 | |
| 32 | Hejazi et al 199839 | 29 | 25 CR 4 IR (0B) |
2 RT | 1 | 0 | 23 | NR | 2 | |
| 33 | Hardison et al 198740 | 23 | 1 CR 22 IR |
5RT | 6 | NR | NR | NR | 6 | |
| 34 | Hulshof et al 199341 | 13 | 13 IR (8B) | 12 RT | 6 | NR | NR | NR | 3 | |
| 35 | Bouffet et al 199842 | 73 | 11 CR 62 IR (9B) |
37 RT 9 CH |
17 | 22 | NR | 50 a | 24 |
Abbreviations: CR, Complete resection, IR, Incomplete resection, B, Biopsy, NR, Not reported, CH, Chemotherapy, RT, Radiotherapy.
aMedian months of follow-up.
Description of Studies and Demographic Features
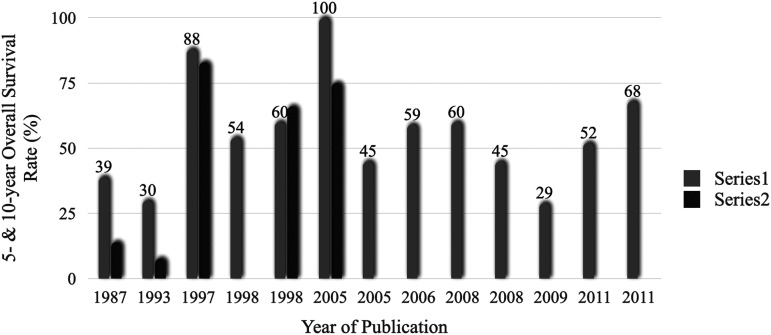
We include 1079 patients from 35 studies. Sample sizes ranged between 10 and 136 cases. All the publications were case series with a single-to-multiple institution ratio of 3.3:1. Clinical information regarding all the included studies is available in Table 1. 5-year and 10-year survival and the corresponding trends over three decades are depicted in Figure 2 and Supplementary Table 1. Individual patient data of 228 patients collected from 13 articles has been incorporated into the integrative analysis. The methodological quality of the selected papers is presented in Table 2. 11 out of 13 studies had more than a 7/10 score from the JBI checklist and were assumed to have low risk of bias. Characteristics of the included patients were summarized in Table 3. The proportion of patients younger or equal to 18 years was 45% and 43% of the patients were female.
Figure 2.
Trends in the rate of patients who survived ≥5 (Series 1) and ≥10 years (Series 2) after diagnosis. Boxes above the bar show 5-years-overall survival rate.
Table 2.
Joanna Briggs Institute Critical Appraisal Checklist for 13 Studies Incorporated Into the Integrative Analysis.
| Author& Year | Clear Inclusion Criteria | Standard Measurement | Valid Methods | Consecutive Inclusion | Complete Inclusion | Clear Reporting of Demographics | Clear Reporting of Clinical Information | Clear Reporting of Outcomes | Clear Reporting of Clinic Demographic | Appropriate Statistical Analysis | Sum (/10) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Santi et al 2003 10 | Yes | Yes | Yes | No | No | Yes | No | Yes | Unclear | Yes | 6 |
| 2 | Robinson et al 2005 11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | 9 |
| 3 | McGirt et al 2008 12 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | 9 |
| 4 | Raco et al 2010 13 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 5 | Guss et al 2013 14 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | 9 |
| 6 | Rossitch et al 1990 15 | Yes | Yes | Yes | Unclear | Unclear | No | Yes | Yes | Yes | No | 6 |
| 7 | Cheng et al 2017 1 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 8 | Ardeshiri et al 2013 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | 9 |
| 9 | Allen et al 1998 16 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | No | Yes | 8 |
| 10 | Karikari et al 2011 17 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| 11 | Merchant et al 2000 18 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 9 |
| 12 | Przybylski et al 1997 19 | Yes | Yes | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 13 | Nishio et al 2000 20 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 9 |
Table 3.
Characteristics of Patients Included in Integrative Analysis.
| Demographic | Number of Pts |
|---|---|
| Number of patients | 230 |
| Age, mean ± SEM | 26.28 |
| ≤18 | 103 |
| 18< | 127 |
| Gender | |
| Female | 84 |
| Male | 111 |
| Tumor location | |
| Cervical | 104 |
| Thoracolumbar | 106 |
| Holocord | 7 |
| Pathology | |
| Low-grade | 78 |
| High-grade† | 130 |
| Complete resection | 67 |
| Incomplete resection | 161 |
| Biopsy | 51 |
| Adjuvant therapy | |
| Radiotherapy | 130 |
| Chemotherapy | 73 |
| Preoperative neurologic dysfunctional situation | |
| Low grade | 97 |
| High grade‡ | 49 |
| Post-operative neurologic status | |
| Improved | 15 |
| Not improved | 106 |
| Follow up neurologic status | |
| Improved | 11 |
| Not improved | 69 |
| Tumor length | |
| <3 | 54 |
| 3≤ | 100 |
| Follow up, mean ± SD, month | 58.89 |
| Recurrence | 47 |
| Death | 109 |
Among the 67 patients who underwent CR, 37% were female and 64% were affected by high-grade astrocytoma. In this group, 36% of patients died in the follow-up period (mean: 67.71 months). The 5- and 10-year OS rates were 71% for both in this group. Additionally, among patients who were examined for neurologic status, 5% and 10% experienced PNI and FNI, respectively.
In contrast, 212 patients underwent IR, 24% of which had a biopsy. 45% were female and 66% had high-grade pathology. Mortality was 57% in the IR group in the follow-up period (mean: 50.80 months), and the 5- and 10-year OS rates were 49% and 45%, respectively. Among patients who were examined for neurologic status, 15% and 13% of the patients experienced PNI and FNI, respectively.
Trends in Treatment Strategies
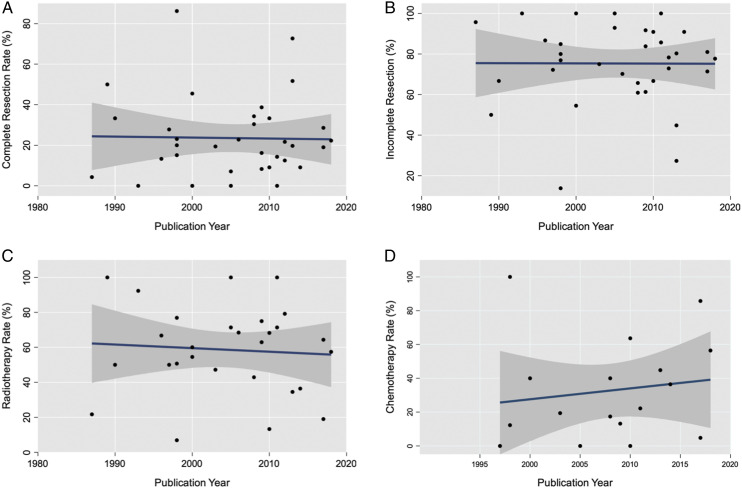
We attempted to assess the trend of treatment strategies with intramedullary astrocytoma according to years of selection of patients in each study. However, this was not possible statistically. So, as an alternative option, we used the years of publication. We observed an upward trend in the popularity of chemotherapy, but CR, IR, and RT had relatively stable trends. The trends are demonstrated in Figure 3.
Figure 3.
Trends in the management of patients with intramedullary astrocytoma, within 1987 - 2018, for complete resection (A), incomplete resection (B), radiotherapy (C), chemotherapy (D).
Meta-Analysis
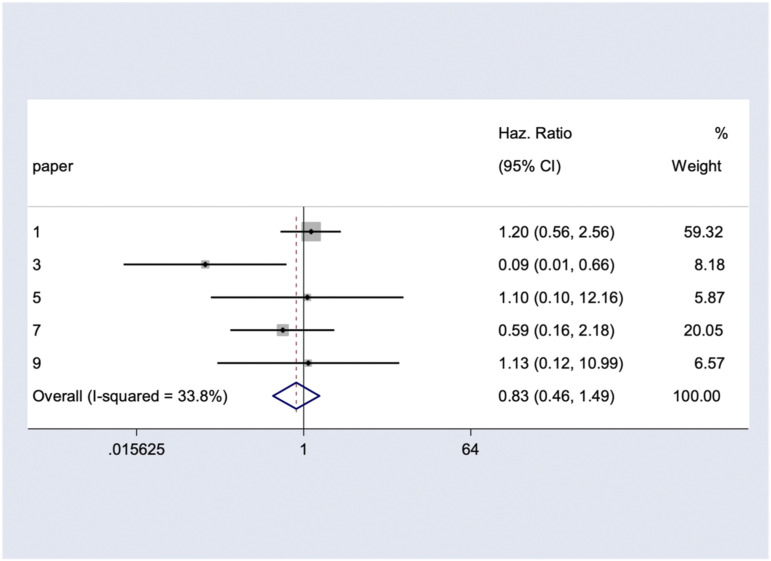
Five series involving 107 participants provided data for meta-analysis of OS. Combining the results, the pooled estimate HR for all-cause mortality among astrocytoma patients who underwent CR vs IR was .83 (95% CI: .46 - 1.49) which did not reach the statistically significant threshold (P = .196) (Figure 4). There was moderate heterogeneity among the included studies (I2 = 33.8%). Further, the age- and grade-adjusted HR were .625 (95% CI: .361 - 1.082, P =.094) and .674 (95% CI: .387 - 1.170, P = .162), respectively. Our studies do not fulfill the criteria of meta-analyses for PNI and FNI.
Figure 4.
The pooled hazard ratio of complete resection (versus incomplete resection) for overall survival.
Integrative Analysis
Overall Survival
Due to the relatively small sample size in long-term follow-up, we restricted our survival analysis to patients with less than 180 months of follow-up. The all-cause mortality rate in CR and IR groups was 17/46 and 87/139, respectively. Compared to the IR group, there was significantly lower mortality risk in the CR group (RR =.59; 95% CI: .41 - .83; P = .002). Mean time to death is estimated at 111.01 ± 11.9 and 60.48 ± 5.72 months in CR and IR groups, respectively. Kaplan-Meier survival analysis showed CR significantly improves OS in comparison with the IR (log-rank test, P = .004) (Figure 5). To elucidate the prognostic impact of other selected variables, univariate analysis by the Cox model was performed. We found that the following variables have a significant association with OS; age, tumor length, pathology grade, and pre-operation neurologic status (Table 4). On the other hand, multivariate analysis of the variables demonstrated that high pathology grade and adjuvant therapy were significant prognostic factors for OS (pathology grade: HR = 4.96; 95% CI: 2.82 - 8.27; P < .01; adjuvant therapy: HR = .30; 95% CI: .10 - .91; P = .03). Sex also shows a trend toward significant but did not reach the statistical significance threshold (HR = 1.89; 95% CI: .91 - 3.88; P = .08) (Table 5). Paper-adjusted Cox proportional hazard model to adjust for unknown/unmeasured confounder (ie, surgical experience, institution setting, etc.) showed that CR led to significantly longer OS (HR: .577, 95% CI: .346 - .960, P = .034) (Table 6). This EOR association with OS remained significant when applied by Cox model in multiple analysis with paper, age, gender, tumor pathology grade, pre-operation neurologic score and adjuvant therapy adjusted analysis (Table 6).
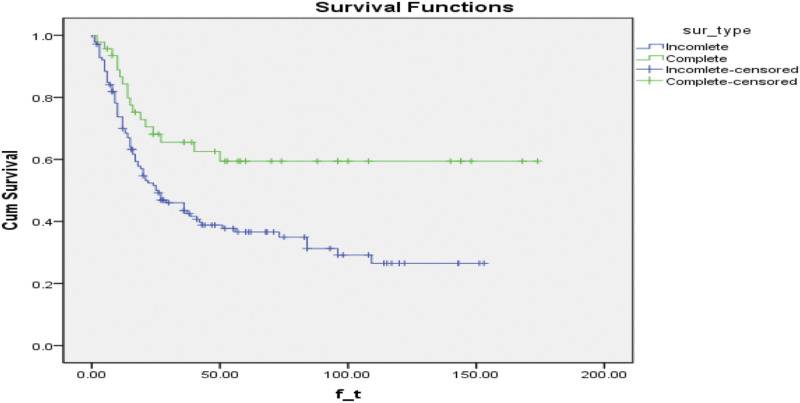
Figure 5.
Kaplan Meier estimates of overall survival for patients with astrocytoma as stratified by extent of resection (P value per Log-rank test = .004). Cum, Cummulative; sur, survival.
Table 4.
Univariate Analysis of the Association of Potential Variables with Overall Survival and Follow-up Neurologic Improvement.
| Overall Survival | Follow-up Neurologic Improvement | |||||
|---|---|---|---|---|---|---|
| Variables | (Crude) HR | 95% CI | P-Value | (Crude) HR | 95% CI | P-Value |
| ≤18 vs >18 | ||||||
| Age | 1.02 | (1.01-1.03) | .000 | 1.01 | (.98-1.05) | .34 |
| Female vs male | ||||||
| Sex | 1.004 | (.67-1.48) | .98 | .57 | (.14-2.24) | .42 |
| <3 segments vs ≥3 segments | ||||||
| Tumor length | .54 | (.33-.88) | .01 | .62 | (.18-2.19) | .46 |
| High vs low | ||||||
| Pathology grade | 13.92 | (6.68-29.00) | .000 | 1.02 | (.26-3.93) | .97 |
| Cervical vs thoracolumbar | ||||||
| Tumor location | .73 | (.49-1.08) | .11 | .73 | (.17-3.12) | .67 |
| High vs low | ||||||
| Pre-operative neurologic score | 1.53 | (1.19-1.98) | .001 | .65 | (.32-1.30) | .23 |
| Yes vs no | ||||||
| Adjuvant therapy | 1.30 | (.71-2.35) | .38 | .13 | (.02-.56) | .006 |
Abbreviation: HR, Hazard Ratio.
Table 5.
Multivariate Analysis of the Association of Potential Variables with Overall Survival and Follow up Neurologic Improvement.
| Overall Survival | Post-Operative Neurological Improvement | Follow-up Neurologic Improvement | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | (Crude) HR | 95% CI | P-Value | (Crude) HR | 95% CI | P-Value | (Crude) HR | 95% CI | P-Value |
| Complete resection vs incomplete resection | |||||||||
| Extent of resectio | .51 | (.19-1.37) | .18 | .33 | (.03-3.07) | .33 | .45 | (.03-5.30) | .53 |
| >18 vs ≤18 | |||||||||
| Age | 1.01 | (.99-1.03) | .38 | .99 | (.95-1.03) | .53 | .99 | (.95- 1.03) | .52 |
| Male vs female | |||||||||
| Sex | 1.89 | (.91 -3.88) | .084 | .73 | (.206-2.59) | .63 | .39 | (.07- 2.23) | .29 |
| <3 vs. ≥3 segments | |||||||||
| Tumor length | .71 | (.32- 1.60) | .41 | .41 | (.08-2.10) | .29 | .49 | (.05-4.89) | .54 |
| High vs low | |||||||||
| Pathology grade | 4.96 | (2.82- 8.72) | .000 | 1.36 | (.50- 3.68) | .543 | 1.25 | (.24-6.52) | .79 |
| Cervical vs thoracolumbar | |||||||||
| Tumor location | .80 | (0.35- 1.81) | .59 | ||||||
| High vs low | |||||||||
| Pre-operative neurologic score | 1.30 | (.86- 1.96) | .21 | .66 | (.25- 1.76) | .41 | |||
| Yes vs no | |||||||||
| Adjuvant therapy | .30 | (.10- .91) | .033 | .18 | (.04-.79) | .024 | .09 | (.01- .63) | .015 |
Abbreviation: HR, Hazard Ratio. Statistically significance value set as P<0.05.
Table 6.
Multivariate Association of Extent of Resection With Overall Survival if Adjusted for Other Potential Variables and Paper.
| Overall Survival | ||||||
|---|---|---|---|---|---|---|
| Variable | (Non-paper Adjusted) HR | 95% CI | P-Value | (Paper Adjusted) HR | 95% CI | P-Value |
| Complete resection vs incomplete resection | ||||||
| EOR | .59 | (.36-.97) | .03 | .57 | (.34-.96) | .03 |
| ≤18 vs >18 | ||||||
| Age-adjusted EOR | .70 | (.42-1.16) | .17 | .59 | (.35-.98) | .04 |
| Female vs male | ||||||
| Gender-adjusted EOR | .62 | (.37-1.03) | .06 | .57 | (.34-.98) | .04 |
| <3 segments vs ≥3 segments | ||||||
| Tumor length-adjusted EOR | .63 | (.33-1.18) | .15 | .56 | (.29-1.07) | .08 |
| High vs low | ||||||
| Pathology grade-adjusted EOR | .55 | (.33-.91) | .02 | .62 | (.37-1.04) | .07 |
| Cervical vs thoracolumbar | ||||||
| Tumor location-adjusted EOR | .67 | (.40-1.10) | .11 | .60 | (.36-1.007) | .05 |
| High vs low | ||||||
| Pre-operative neurologic score-adjusted EOR | .40 | (.18-.89) | .02 | .31 | (.13-.69) | .005 |
| Yes vs no | ||||||
| Adjuvant therapy-adjusted EOR | .50 | (.27-.90) | .02 | .55 | (.29-1.03) | .06 |
Abbreviations: EOR, Extent of resection; HR, Hazard Ratio.
Post-Operative Neurological Improvement and Follow-up Neurological Improvement:
We collected PNI and FNI data in 121 and 80 patients, respectively. PNI was experienced in 13 out of 83 patients and 2 out of 38 patients in CR and IR groups, respectively. There was no significant difference in PNI between CR and IR groups (HR = .73; 95% CI: .20 - 2.64, P = .64) (Table 7). 8 out of 58 patients in the CR group and 3 out of 22 patients in the IR group experienced FNI. There was no statistically significant difference in FNI between CR and IR group (HR = .25; 95% CI: .03 - 2.001; P = .19) (Table 7). Univariate analysis for other potential prognostic factors revealed that only adjuvant therapy had a statistically significant effect on FNI (HR = .13; 95% CI: .02 - .56; P = .006) (Table 4). On the other hand, in multivariate analyses of PNI and FNI, only adjuvant therapy for both outcomes showed significant benefit (PNI: HR = .18; 95% CI: .04 - .79; P = .02; FNI: HR = .09; 95% CI: .01 - .63; P = .01) (Table 5). We identified that according to paper adjusted Cox model, there is no significant correlation between EOR and PNI (HR: 1.01; 95% CI 0.25 - 4.04, P =.98) or FNI (HR: .47; 95% CI 0.05 - 4.07, P =.49) (Table 7). Besides, multiple analysis by the Cox model adjusting for paper, age, gender, tumor length, tumor pathology grade, pre-operation neurologic score and adjuvant therapy have found no association between EOR and PNI or FNI (Table 7).
Table 7.
Multivariate Association of Extent of Resection With Follow-up Neurological Improvement Adjusted for Other Potential Variables and Paper.
| Follow-up Neurological Improvement | ||||||
|---|---|---|---|---|---|---|
| Variable | (Non-Paper Adjusted) HR | 95% CI | P-Value | (Paper Adjusted) HR | 95% CI | P-Value |
| Complete resection vs incomplete resection | ||||||
| EOR | .25 | (.03-2.001) | .19 | .47 | (.05-4.07) | .49 |
| ≤18 vs >18 | ||||||
| Age-adjusted EOR | .27 | (.03-2.15) | .21 | .42 | (.04-3.81) | .44 |
| Female vs male | ||||||
| Sex-adjusted EOR | .37 | (.04-3.13) | .37 | .73 | (.07-6.91) | .78 |
| <3 segments vs ≥3 segments | ||||||
| Tumor length-adjusted EOR | .32 | (.04-2.62) | .29 | 1.69 | (.15-18.11) | .66 |
| High vs low | ||||||
| Pathology grade-adjusted EOR | .25 | (.03-1.99) | .19 | .48 | (.05-4.23) | .51 |
| High vs low | ||||||
| Pre-operative neurologic score-adjusted EOR | .29 | (.03-2.36) | .25 | .49 | (.05-4.30) | .52 |
| Yes vs no | ||||||
| Adjuvant therapy-adjusted EOR | .39 | (.04-3.22) | .38 | .75 | (.08-6.91) | .80 |
Abbreviations: EOR, Extent of resection; HR, Hazard Ratio.
Resectability
Univariate analysis showed no significant correlation between EOR and tumor location, pathology grade, and tumor length. Likewise, after controlling for the effect of covariates in the logistic regression model, similar results were achieved. Of note, in logistic regression analysis, we found that the rate of CR may decrease in cervical location tumors (OR = .72; 95% CI: .30 - 1.70, P =.54) and increase in higher grade pathology (OR = 1.59; 95% CI: .63 - 4.00, P: .31), however, both were not statistically significant (Table 8).
Table 8.
Respectability: Univariate (A) and multivariate (B) Association of EOR with Tumor Location, Tumor Length, and Pathology Grade.
| Extent of resection | |||
|---|---|---|---|
| (A) | |||
| Variables | Odds Ratio | 95% CI | P-value |
| Cervical vs Thoracolumbar | |||
| Tumor location | 1.09 | (.60-1.98) | .77 |
| High vs low | |||
| Pathology grade | 1.06 | (.54-2.08) | .84 |
| <3 vs ≥3 segments | |||
| Tumor length | 1.00 | (.91-1.10) | .88 |
| (B) | |||
| Covariate | Odds ratio | 95% CI | P-value |
| Cervical vs thoracolumbar | |||
| Tumor location | .72 | (.30-1.70) | .45 |
| High vs low | |||
| Pathology grade | 1.59 | (.63- 4.00) | .31 |
| <3 vs ≥3 segments | |||
| Tumor length | 1.03 | (.89-1.19) | .64 |
Discussion
Intramedullary spinal cord astrocytoma is a rare tumor, leading to limited high-quality data on its treatment and prognostic factors. The dominance of single-institutional studies (>75% of studies) and lack of randomized trials and prospective studies in our review is due to this rarity and practical and methodological difficulties in conducting surgical trials. To the best of our knowledge, this is the most comprehensive study on intramedullary astrocytomas comparing survival and functional outcomes between complete and incomplete resection using IPD meta-analysis. In addition, the current review is the only integrative analysis using IPD to assess resectability.
In this systematic review, we included 228 individual patients from 13 articles in our integrative analysis. The mean age at presentation (26 years) and the male predominance in our review are consistent with two other reports.17,18 In this study, neither cervical (48%) nor thoracolumbar (49%) held a predominance, which was consistent with findings of the systematic review conducted by Hamilton et al which reported 44% and 55% incidence for cervical and thoracolumbar tumors, respectively. 18 Our main outcomes were OS, PNI, and FNI. CR rate was 23% in all included studies and 27% in studies that were included in our integrative analysis. In the Hamilton et al 18 study, the CR rate was 22.2% which is in accordance with our results. 18 Azad et al 17 reported the CR rate in the pediatric group to be 39%. 17 This difference may be explained by our broad inclusion criteria of both adult and pediatric patients.
Based on the meta-analysis of five eligible studies, CR did not show statistically significant superiority on OS over IR (HR: .83, 95% CI: .46 - 1.49). Age and tumor grade may confound the survival beneficial effect of CR in some instances, but this effect was maintained when adjusted for age and tumor grade. We could not perform a meta-analysis on other outcomes due to lack of eligible data.
Using IPD analysis, Kaplan-Meier analysis showed a statistically significant benefit of the CR over IR to improve OS. This implies that there may be some confounders in the meta-analysis that were eliminated using IPD analysis. In the multivariate IPD analysis, there were three prognostic factors which showed statistically significant effect on the OS: EOR, pathology grade, and adjuvant therapy. This is in concordance with previous systematic reviews.17,18 In a multi-center analysis on high-grade intramedullary astrocytoma by Wong et al, when compared to other surgery strategies, CR was associated with a significantly prolonged 10-year OS in univariate analysis but lost its significance in multivariate analysis. 19 This difference between our results and this study might be explained with smaller studied population (n = 88) in Wong’s study and different confounding factors considered in our study. In an integrative survival analysis of spinal GBM, the extent of resection had no significant effect on OS. 20 This difference might be explained by shorter follow up (18 months) in this study.
Multivariate IPD analysis adjusted for potential unknown/unmeasured confounders using paper adjusted analysis showed that beneficial effect of CR on survival was independent of age, gender, pathology grade, pre-operative neurologic score, and adjuvant treatment. This is in contrast with the theory that the improved outcome is due to a higher incidence of CR in lower pathology grades. 21 Another notable finding was that CR loses its favorable effect on OS when it was adjusted for tumor length and tumor location (Table 6). Explaining this finding becomes even more difficult considering that the aforementioned factors along with tumor grade had no significant effect on the resectability of the tumor in both univariate and multivariate analysis (Table 8). This obscurity might be resolved with an increase in study size as the P-value and 95% CI came very close to significance in our paper adjusted multivariate analysis (Table 6).
The result of this study shows that PNI and FNI were not significantly affected by CR compared to IR. This is an important finding as it shows CR does not negatively impact post-operative neurological status. This may be explained by the double-edge razor effect of EOR on PNI and FNI, as CR may injure the normal tissue around the tumor. On the other hand, IR may cause pressure effect due to tumor growth or wounded glioma effect secondary to significant vasogenic edema. This is somehow opposed to previous conceptions that due to the infiltrative nature and lack of plane of dissection in astrocytoma, more aggressive surgeries could lead to a negative impact on the patient’s postoperative neurological status.22,23 However, in two series of intramedullary anaplastic astrocytoma patients,24,25 CR was not associated with significant neurological decline which is concordant with our results. Pooled analysis of 125 articles (691 patients) by Montano showed there are two significant predictors for PNI and FNI in IMSCT, pre-op functional status and CR. 26 An important point of the Montano analysis was that ependymomas, hemangioblastomas and cavernomas had a better functional outcome compared to astrocytomas, which lead the author to concluded that there is no indication to attempt CR in high-grade astrocytoma due to the infiltrative growth pattern of this tumor that leads to a higher surgical morbidity. But our analysis revealed that CR do not increase PNI and FNI. It is important to note that this finding does not indicate that aggressive surgical strategies can be implemented at a relatively minimal functional cost, but rather it implies the surgeon’s intraoperative decision on whether to attempt CR is reasonable.
Another important finding was that post-operative adjuvant therapy had a significantly favorable effect on both FNI and PNI in both univariate and multivariate analysis. Postoperative adjuvant therapy also had a significant favorable effect on OS in multivariate analysis. This finding shows that adjuvant therapy not only increases the patient’s chances of survival but can also help to significantly increase the patient’s quality of life. The potential benefit of adjuvant therapy on PNI represent a potential confounder in this area because PNI was assessed before starting the adjuvant therapy. This may be due to the strategy for optimal safe resection that prevents aggressive total resection in favor of reducing the mass effect and edema of the tumor. In other word, considering that the adjuvant therapy would eliminate the small residual tumor, the neurosurgeon would not perform aggressive resection which could introduce neurological deterioration. There are level IIa and IIb evidence in favor of post-operative radiotherapy and chemotherapy for residual or recurrent intramedullary astrocytoma, respectively. 27 Hamilton et al 18 in a multivariate analysis of 57 articles (3022 patients) revealed radiotherapy increased the risk of mortality in low-grade IMSCT (HR for OS 5.20, P < .01), but decreased mortality in high-grade IMSCT (HR for OS 2.46, P < .01). 18 We also found that although better preoperative neurological status correlated with better survival, it had no such correlation with FNI and PNI in both univariate and multivariate analysis. This is also a peculiar finding which is in stark contrast with previous studies28-30 which reported the opposite regarding PNI and FNI. However, all these previous studies were case series with limited size.
Trend analysis during three decades regarding treatment strategies showed that major technical improvement in micro-neurosurgery could not improve the EOR in recent decades (Figure 3). We observed an upward trend in the popularity of chemotherapy, but CR, IR, and RT had relatively stable trends (Figure 3). To the best of our knowledge, this was the first report on the trend of surgical strategies in intramedullary astrocytoma.
The result of this study showed that neither tumor location, tumor length, nor even histopathology grade could predict resectability of the spinal cord astrocytoma (Table 8). But in the logistic regression analysis, we found that the rate of CR may decrease in cervical location tumors (OR = .72; 95% CI: .30 - 1.70, P: .54) and increase in higher grade pathology (OR = 1.59; 95% CI: .63 - 4.00, P: .31), however, both were not statistically significant. In a multi-center retrospective series by Parker et al on 95 Intramedullary astrocytoma patients, a positive correlation (P = .002) was found between cervical location of the tumor and CR. The authors attributed this to the larger volume of the spinal cord in that region which decreases tumor volume compared to cord volume ratio.10,31 However, CR rates differed between different hospitals in that study. To the best of our knowledge the current review is the only integrative analysis using individual patient data to assess respectability.
This review had several limitations, most importantly the retrospective and un-randomized nature of all its included studies. This was not unexpected as intramedullary astrocytoma is a rare tumor which makes conducting randomized prospective studies extremely difficult. In addition, different treatment plans and different surgeon experiences could have affected our results. We tried to mitigate this problem by conducting paper-adjusted analysis but undoubtedly this does not remove all heterogeneity risks present here. Also, heterogeneity among definitions restricted our ability to make the most of the available data. For example, different papers regarded any or all local progression, recurrence, and distant metastases as a progression which rendered us unable to make a unified definition of progression-free survival as an outcome. In addition, definition of the EOR was biased regarding intraoperative judgement of the surgeon or post-operative neuroimaging. Further large-scale multi-center randomized trials would be helpful to confirm this study’s results. Such a desired Randomised Controlled Trial (RCT) needs specific consideration in study design and randomization. When there is no clear line between tumor and spinal cord, preoperative randomization is not possible and ethical because patients without a clear line need to undergo the I. R procedure. This challenge may be addressed by intra-operative randomization when patients give informed consent preoperatively, but randomization occurs intra-operatively, once there is the certainty that both procedures can be performed. 32 An alternative design can be a combination of preoperative random allocation and distinguishable surgery line. In other words, patients with a clear line between tumor and spinal cord will stay on the preoperative random allocation, whereas patients without a clear line between tumor and spinal cord will be allocated to the I. R group. Although this design cannot replace a well-designed RCTs, however, acceptable evidence will be attained by applying appropriate statistical analysis methods, such as multivariable analysis, propensity score analysis, instrument variable analysis or sensitivity analysis. It is important to value findings from such studies, when conducting RCTs is impractical or unethical.
Conclusion
Our study showed that CR can improve OS when compared to IR. Using multivariate IPD analysis, there were three prognostic factors for OS: EOR, pathology grade, and adjuvant therapy. Patient with spinal cord astrocytoma who had undergone CR had similar PNI and FNI to patient with IR. Therefore, CR should be the primary goal of surgery, but intraoperative decision-making on the extent of resection should still be relied upon to prevent neurologic adverse events. Due to significant effect of adjuvant therapy on OS, PNI and FNI, it could be considered as the routine treatment strategy for spinal cord astrocytoma.
Supplemental Material
Supplementary Material for Complete Versus Incomplete Surgical Resection in Intramedullary Astrocytoma: Systematic Review with Individual Patient Data Meta-Analysis by Mehdi Golpayegani, Maryam Edalatfar, Ayat Ahmadi, Mohsen Sadeghi-Naini, Farhad Salari, Sara Hanaei, Farhad Shokraneh, Zahra Ghodsi, Alex R. Vaccaro, and Vafa Rahimi-Movaghar in Global Spine Journal.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences [grant number is 98-02-38-43254].
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Alex R. Vaccaro https://orcid.org/0000-0002-8073-0796
Vafa Rahimi-Movaghar https://orcid.org/0000-0001-7347-8767
References
- 1.Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: A review. Curr Neurol Neurosci Rep. 2011;11(3):320-328. [DOI] [PubMed] [Google Scholar]
- 2.Hsu S, Quattrone M, Ostrom Q, Ryken TC, Sloan AE, Barnholtz-Sloan JS. Incidence patterns for primary malignant spinal cord gliomas: A surveillance, epidemiology, and end results study. J Neurosurg Spine. 2011;14(6):742-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milano MT, Johnson MD, Sul J, et al. Primary spinal cord glioma: A surveillance, epidemiology, and end results database study. J Neurooncol. 2010;98(1):83-92. [DOI] [PubMed] [Google Scholar]
- 4.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700-708. [DOI] [PubMed] [Google Scholar]
- 5.Matsuyama Y, Sakai Y, Katayama Y, et al. Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection: Clinical article. J Neurosurg Spine. 2009;10(5):404-413. [DOI] [PubMed] [Google Scholar]
- 6.Salari F, Golpayegani M, Sadeghi-Naini M, et al. Complete versus incomplete surgical resection in intramedullary ependymomas: A systematic review and meta-analysis. Global Spine J. 2021;11(5):761-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X, Lou S, Huang S, Chen H, Liu J. Primary spinal cord glioblastoma multiforme: A retrospective study of patients at a single institution. World Neurosurg. 2017;106:113-119. [DOI] [PubMed] [Google Scholar]
- 9.Ardeshiri A, Chen B, Hütter B-O, et al. Intramedullary spinal cord astrocytomas: The influence of localization and tumor extension on resectability and functional outcome. Acta Neurochir. 2013;155(7):1203-1207. [DOI] [PubMed] [Google Scholar]
- 10.Parker F, Campello C, Lejeune JP, et al. [Intramedullary astrocytomas: A French retrospective multicenter study]. Neurochirurgie. 2017;63(5):402-409. [DOI] [PubMed] [Google Scholar]
- 11.Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56(5):972-981; discussion 972-981. [PubMed] [Google Scholar]
- 12.Kutluk T, Varan A, Kafalı C, et al. Pediatric intramedullary spinal cord tumors: A single center experience. Eur J Paediatr Neurol. 2015;19(1):41-47. [DOI] [PubMed] [Google Scholar]
- 13.Innocenzi G, Raco A, Cantore G, Raimondi AJ. Intramedullary astrocytomas and ependymomas in the pediatric age group: A retrospective study. Childs Nerv Syst. 1996;12(12):776-780. [DOI] [PubMed] [Google Scholar]
- 14.Innocenzi G, Salvati M, Cervoni L, Delfini R, Cantore G. Prognostic factors in intramedullary astrocytomas. Clin Neurol Neurosurg. 1997;99(1):1-5. [DOI] [PubMed] [Google Scholar]
- 15.Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM. Spinal cord astrocytoma: Pathological and treatment considerations. J Neurosurg. 1995;83(4):590-595. [DOI] [PubMed] [Google Scholar]
- 16.Xiao R, Abdullah KG, Miller JA, et al. Molecular and clinical prognostic factors for favorable outcome following surgical resection of adult intramedullary spinal cord astrocytomas. Clin Neurol Neurosurg. 2016;144:82-87. [DOI] [PubMed] [Google Scholar]
- 17.Azad TD, Pendharkar AV, Pan J, et al. Surgical outcomes of pediatric spinal cord astrocytomas: Systematic review and meta-analysis. J Neurosurg Pediatr. 2018;22(4):404-410. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton KR, Lee SS, Urquhart JC, Jonker BP. A systematic review of outcome in intramedullary ependymoma and astrocytoma. J Clin Neurosci. 2019;63:168-175. [DOI] [PubMed] [Google Scholar]
- 19.Wong AP, Dahdaleh NS, Fessler RG, et al. Risk factors and long-term survival in adult patients with primary malignant spinal cord astrocytomas. J Neurooncol. 2013;115(3):493-503. [DOI] [PubMed] [Google Scholar]
- 20.Konar SK, Maiti TK, Bir SC, Kalakoti P, Bollam P, Nanda A. Predictive factors determining the overall outcome of primary spinal glioblastoma multiforme: An integrative survival analysis. World Neurosurg. 2016;86:341-348. [DOI] [PubMed] [Google Scholar]
- 21.Ogunlade J, Wiginton JG, Elia C, Odell T, Rao SC. Primary spinal astrocytomas: A literature review. Cureus. 2019;11(7):e5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcés-Ambrossi GL, McGirt MJ, Mehta VA, et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11(5):591-599. [DOI] [PubMed] [Google Scholar]
- 23.Samartzis D, Gillis CC, Shih P, O’Toole JE, Fessler RG. Intramedullary spinal cord tumors: Part II-management options and outcomes. Global Spine J. 2016;6(2):176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGirt MJ, Goldstein IM, Chaichana KL, Tobias ME, Kothbauer KF, Jallo GI. Extent of surgical resection of malignant astrocytomas of the spinal cord: Outcome analysis of 35 patients. Neurosurgery. 2008;63(1):55-61; discussion 60-61. [DOI] [PubMed] [Google Scholar]
- 25.Soo Kim M, Kee Chung C, Choe G, Han Kim I, Jib Kim H. Intramedullary spinal cord astrocytoma in adults: Postoperative outcome. J Neurooncol. 2001;52(1):85-94. [DOI] [PubMed] [Google Scholar]
- 26.Montano N, Papacci F, Trevisi G, Fernandez E. Factors affecting functional outcome in patients with intramedullary spinal cord tumors: Results from a literature analysis. Acta Neurol Belg. 2017;117(1):277-282. [DOI] [PubMed] [Google Scholar]
- 27.Tobin MK, Geraghty JR, Engelhard HH, Linninger AA, Mehta AI. Intramedullary spinal cord tumors: A review of current and future treatment strategies. Neurosurg Focus. 2015;39(2):E14. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed R, Menezes AH, Awe OO, Torner JC. Long-term disease and neurological outcomes in patients with pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr. 2014;13(6):600-612. [DOI] [PubMed] [Google Scholar]
- 29.Karikari IO, Nimjee SM, Hodges TR, et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: A single-center experience with 102 patients. Neurosurgery. 2015;76(suppl 1):S4-S13; discussion S13. [DOI] [PubMed] [Google Scholar]
- 30.Beneš V, Barsa P, Benes V, Suchomel P. Prognostic factors in intramedullary astrocytomas: a literature review. Eur Spine J. 2009;18(10):1397-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messerer M, Maduri R, Daniel RT, Parker F. Surgery for intramedullary astrocytomas: Does tumour location matter? Acta Neurochir. 2017;159(5):937-938. [DOI] [PubMed] [Google Scholar]
- 32.Farrokhyar F, Karanicolas PJ, Thoma A, et al. Randomized controlled trials of surgical interventions. Ann Surg. 2010;251(3):409-416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material for Complete Versus Incomplete Surgical Resection in Intramedullary Astrocytoma: Systematic Review with Individual Patient Data Meta-Analysis by Mehdi Golpayegani, Maryam Edalatfar, Ayat Ahmadi, Mohsen Sadeghi-Naini, Farhad Salari, Sara Hanaei, Farhad Shokraneh, Zahra Ghodsi, Alex R. Vaccaro, and Vafa Rahimi-Movaghar in Global Spine Journal.