Abstract
Background/Aims
Lactase deficiency, which has many similarities with small intestinal bacterial overgrowth (SIBO), causes various gastrointestinal symptoms. We estimate the prevalence of SIBO in patients with intestinal symptoms from dairy products and investigate the association between lactase deficiency (LD) and SIBO.
Methods
This prospective study included patients with functional intestinal symptoms from dairy product indigestion. A questionnaire on gastrointestinal symptoms, a hydrogen (H2)-methane glucose breath test (GBT) for SIBO, and lactose intolerance quick test (LQT) for LD using upper gastrointestinal endoscopy were performed.
Results
A total of 88 patients, 29 (33.0%) with severe and 36 (40.9%) with mild LD were included. Sixteen patients (18.2%) were GBT positive. Patients with LQT negativity indicating severe LD showed a higher positivity to GBT or GBT (H2) than the historic controls (27.6% vs 6.7%, P = 0.032). There was no difference in the items on the symptom questionnaire according to the presence of LD or SIBO, except for higher symptom scores for urgency in GBT-positive patients. There were more LQT-negative patients in the GBT (H2)-positive group than in the other groups (27.6% vs 10.2%, P = 0.036). Moreover, only GBT (H2)-positivity was significantly associated with a higher risk of LQT negativity in multivariate analysis (OR, 4.19; P = 0.029).
Conclusions
SIBO producing H2 is common in patients with severe LD suspected lactose intolerance. SIBO may be a new therapeutic target for managing intestinal symptoms in patients with lactose intolerance.
Keywords: Breath tests, Endoscopy, Lactase deficiency, Lactose intolerance, Small intestinal bacterial overgrowth
Introduction
Lactose is a disaccharide found in milk and is composed of galactose and glucose. Lactose intolerance (LI) refers to a condition in which malabsorption of lactose causes various symptoms. The most common type of LI is primary LI, which is caused by lactase deficiency (LD) at the brush border of duodenal mucosal cells due to various causes after weaning diet.1 LI has a similar prevalence in both men and women, but there are regional or ethnic differences. According to previous studies, Asians, Native Americans, and African Americans have the highest rates of LI (60-100%). Although the prevalence is relatively lower among Northern European populations and pure United States Caucasians, up to 22% have been reported in West,2 the gastrointestinal symptoms may still impair the quality of life.
Representative gastrointestinal symptoms that can appear in LD include diarrhea, abdominal pain, bloating, borborygmus, and flatulence.3,4 These symptoms are evoked when lactose is not absorbed from the small intestine and reaches the colon, causing osmotic diarrhea and abdominal pain, or gas production due to fermentation by the gut microbiome.5 If the aforementioned symptoms are experienced when consuming lactose-containing milk or dairy products, LD should be suspected. For the objective diagnosis of LD, a useful rapid test kit (Lactose Intolerance Quick Test [LQT]) to assess lactase activity in the intestine has been recently available in western countries. It utilizes the mucosal tissue obtained by biopsy forceps during upper gastrointestinal endoscopy.6-9
Small intestinal bacterial overgrowth (SIBO) refers to an increase in the number of bacteria or alteration of the microbial composition in the small intestine. SIBO can cause various gastrointestinal symptoms.10 A high rate of SIBO is present in patients with irritable bowel syndrome (IBS), which has symptom profiles similar to those of LI.11 In general, the gold standard for diagnosis is jejunal aspirate culture, but this is limited due to its invasiveness. Instead, the breath test is widely used for SIBO diagnosis in current clinical practice.12,13
The common feature between LD and SIBO is that both their pathophysiology involves bacteria in the gut that cause similar intestinal symptoms. There is a possibility that the 2 diseases may be correlated, but studies on the clinical characteristics of SIBO accompanying LD are lacking, and data on the use of LQT with endoscopy is insufficient. We aim to estimate the prevalence of SIBO in patients with gastrointestinal symptoms in dairy products and to investigate the clinical characteristics of patients with LD and/or SIBO.
Materials and Methods
Study Design and Population
This study was designed as a single-center prospective observational study conducted in a teaching hospital (St. Vincent’s Hospital, the Catholic University of Korea). Patients who visited our center with functional gastrointestinal symptoms such as bloating, flatus, abdominal pain, and diarrhea caused by the ingestion of dairy products from August 2017 to July 2021 were included in the study. Exclusion criteria were as follows: age under 18 or over 70 years, having undergone gastrointestinal surgery other than appendectomy, confirmed to have an accompanying organic gastrointestinal disease (peptic ulcer, gastrointestinal malignancy, gastroenteritis, colitis, etc) that may affect gastrointestinal motility evaluation within 15 days of enrollment; concomitant diseases that can significantly affect the results of the study (eg, renal failure with a serum creatinine level of 3 mg/dL or higher, hepatic diseases corresponding to Child-Pugh classification C, a history of inflammatory bowel disease such as Crohn’s diseases and ulcerative colitis), having received antibiotics within 3 months, taking drugs that may affect gastrointestinal motility (narcotic drugs, probiotics, laxatives, antacids, etc, except for those who had a drug holiday of more than 30 days), having difficulty communicating due to hearing impairment, those with major psychiatric illnesses, abnormalities in masticatory function, participation in other clinical studies, or others who were determined to be inappropriate by the research staff. The GBTs in enrolled patients were compared with 30 historical healthy controls who were enrolled for determination of the normal values for GBT at the Catholic University of Medicine in 2007.14
The primary objective of this study is to investigate the prevalence of SIBO in patients with gastrointestinal symptoms from the intake of dairy products. The secondary objectives were symptomatic profiles of the study population, clinical characteristics according to the presence of LD or SIBO, and the correlation between LD and SIBO.
This study complied with the Declaration of Helsinki, and written consent was obtained from all enrolled patients. The study was approved by the institutional review board of the Catholic University of Korea (VC17OESI0140).
Study Design
Informed consent for the study was obtained from patients who visited the clinic with a history of repeated gastrointestinal symptoms, such as bloating, flatus, abdominal pain, and diarrhea related to the ingestion of dairy products. After history taking and physical examination, a questionnaire about gastrointestinal symptoms was administered on the day of the first visit. On the second day of visit, a glucose breath test (GBT), followed by LQT using gastrointestinal endoscopy, was performed on all study participants.
During endoscopy, the presence of any other symptom-causing organic lesion was confirmed. If there was no specific abnormality, 2 post-bulbar duodenal biopsies were taken from each patient, and LQT was performed using a test kit (Biohit, Helsinki, Finland). The biopsy specimens were examined immediately and the test results were determined by an accompanying investigator.
Glucose Breath Test
A carbohydrate-free dinner and fast for at least 12 hours before GBT was recommended. All participants were required to keep the oral cavity clean by using 20 mL of chlorhexidine 0.05% 30 minutes before the GBT and limit smoking, physical exercise, sleeping, and eating until the test was complete. Each patient ingested 75 g of glucose (DIASOL-S SOLN; Taejoon Pharm Co, Ltd, Seoul, Korea), mixed with 120 mL of water, and exhaled 2 times before glucose administration and every 10 minutes up to 120 minutes after glucose administration. All exhalations were collected, and hydrogen (H2) and methane (CH4) concentrations were measured with the equipment of the breath test (the Quintron SC breathtracker; Quintron Instrument Company, Milwaukee, WI, USA). GBT positivity, indicating the presence of SIBO, was defined as meeting following criteria with an increment of H2 level ≥ 12 ppm or CH4 level ≥ 10 ppm from the baseline concentrations within 90 minutes after glucose ingestion.12,13 If the GBT result for H2 met the aforementioned criteria, it was diagnosed as “H2 positivity”; the same was true for “CH4 positivity.” If the patient is positive for both H2 and CH4, it was classified as “mixed positivity.” The GBT results of the study participants were compared with those of the historic healthy control group, which has been validated and utilized in previous studies.14-16
Lactose Intolerance Quick Test
A kit for LQT is based on a colorimetric assay developed to diagnose LD according to the level of lactase enzyme activity in a biopsy specimen.6 The kit consisted of 1 plate and 3 reagents, which were used to treat the biopsy tissue, resulting in a color reaction. The reaction was expressed in 2 steps: lactase reaction for 15 minutes and signal reaction for 5 minutes. During the lactase reaction, the lactase in the specimen decomposes the lactose substrate into the monosaccharides, glucose, and galactose. In the subsequent signal reaction, both chromogen and signal reaction solutions were added to the plate to detect the amount of glucose, and a colored mixture was formed during the process. The degree of color reaction was interpreted using a color chart enclosed in the kit. In patients with LD, the plate appeared colorless (negative), indicating a severe lactase deficiency, or light blue (weakly positive), reflecting a mild lactase deficiency, whereas a strong blue reaction (strongly positive) was observed in normolactasia (lactase activity > 10 U/g protein and lactase/saccharase ratio > 0.25).8
Intestinal Symptom Questionnaire
The validated questionnaire form included the Rome IV criteria and additional questions regarding the individual bowel symptoms.17,18 In addition, 13 questions about individual bowel symptoms experienced in the preceding 4 weeks were asked. The frequency and bothersomeness of each symptom were assessed using a 7-point scale from 0 (never) to 6 (always or extremely). Symptoms severity was evaluated by total symptom score, which was defined as the sum of symptom frequencies and bothersomeness scores. Thus, the total symptom score for each symptom ranged from 0 to 12.
Statistical Methods
R Studio was used for all data analyses (version 1.0.153; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were described as mean and SD or as the median and interquartile range if variables did not satisfy the assumption of a normal distribution. Categorical variables are described as frequencies and percentages. Student’s t test or Wilcoxon–Mann–Whitney test was used to compare continuous variables, and the chi-squared test or Fisher’s exact test was used for categorical variables. To explore the potential relationship between LQT positivity and other clinical variables, a multivariate logistic regression model was used. Statistical significance was defined as a P-value of less than 0.05 in every analysis.
Results
Characteristics of the Study Population
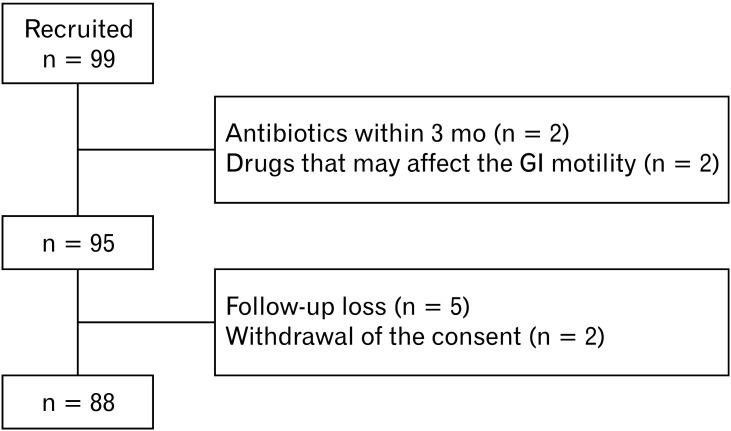
A total of 99 participants suspected of LI were initially recruited, and 4 were excluded based on exclusion criteria. In addition, 7 were dropped out due to loss to follow-up or withdrawal of consent, so the data of 88 patients were analyzed (Fig. 1). The mean age of the participants was 50.8 years. The proportion of women was higher than that of men (54.5% vs 45.5%). Hypertension was the most common comorbidity (n = 18, 20.7%), and patients with diabetes accounted for 17.0%. Approximately one-third of the study population was diagnosed with IBS using the questionnaire (n = 31, 35.2%). Negative LQT was observed in 29 participants (33.0%), and 23 (26.1%) were strongly positive. Regarding the GBT results, 16 patients (18.2%) tested positive. The demographic characteristics of the study groups are presented in Table 1. In the questionnaire of intestinal symptoms for last 4 weeks, abdominal discomfort, loose stool, bloating, flatulence, and urination corresponded to the 5 items of patients with the highest scores. Details about symptom scores are demonstrated in Table 2.
Figure 1.
A schematic diagram of the study enrollment.
Table 1.
Baseline Characteristics of Patients (N = 88)
| Variables | Mean ± SD or n (%) |
|---|---|
| Demographics | |
| Age (yr) | 50.8 ± 13.9 |
| Sex | |
| Male | 40 (45.5) |
| Female | 48 (54.5) |
| BMI (kg/m2) | 23.6 ± 3.3 |
| Medical history | |
| Hypertension | 18 (20.7) |
| Diabetes | 15 (17.0) |
| Dyslipidemia | 11 (12.5) |
| IBS on questionnaire | 31 (35.2 ) |
| LQT | |
| Negative | 29 (33.0) |
| Weak positive | 36 (40.9) |
| Strong positive | 23 (26.1) |
BMI, body mass index; IBS, irritable bowel syndrome; LQT, lactose intolerance quick test; GBT, glucose breath test; H2, hydrogen; CH4, methane.
Table 2.
Intestinal Symptom Scores of Patients
| Symptom item | Frequency(0 to 6) | Bothersomeness(0 to 6) | Total scorea(0 to 12) |
|---|---|---|---|
| Abdominal discomfort | 2.9 ± 1.7 | 2.7 ± 1.7 | 5.5 ± 3.3 |
| Hard stool | 1.4 ± 1.6 | 1.4 ± 1.7 | 2.8 ± 3.1 |
| Loose stool | 2.7 ± 1.9 | 1.9 ± 1.7 | 4.6 ± 3.4 |
| Strain | 1.9 ± 1.8 | 1.6 ± 1.8 | 3.4 ± 3.4 |
| Urgency | 2.0 ± 1.6 | 1.9 ± 1.7 | 3.9 ± 3.2 |
| Tenesmus | 2.7 ± 1.9 | 2.1 ± 2.1 | 4.8 ± 3.8 |
| Mucus | 0.7 ± 1.2 | 0.5 ± 1.0 | 1.2 ± 2.1 |
| Bloating | 3.0 ± 1.7 | 2.5 ± 2.0 | 5.5 ± 3.5 |
| Flatulence | 3.4 ± 1.5 | 1.8 ± 1.8 | 5.2 ± 3.0 |
| Chest discomfort | 1.8 ± 1.9 | 1.7 ± 2.0 | 3.5 ± 3.7 |
| Satiety | 2.6 ± 2.0 | 1.5 ± 1.7 | 4.2 ± 3.5 |
| Urination | 3.2 ± 1.8 | 1.8 ± 2.0 | 5.0 ± 3.4 |
| Nausea | 1.6 ± 1.7 | 1.4 ± 1.8 | 3.0 ± 3.4 |
aTotal score = frequency + bothersomeness.
Data are expressed as mean ± SD.
Comparison of Glucose Breath Test Profiles Between the Study Population and Healthy Controls
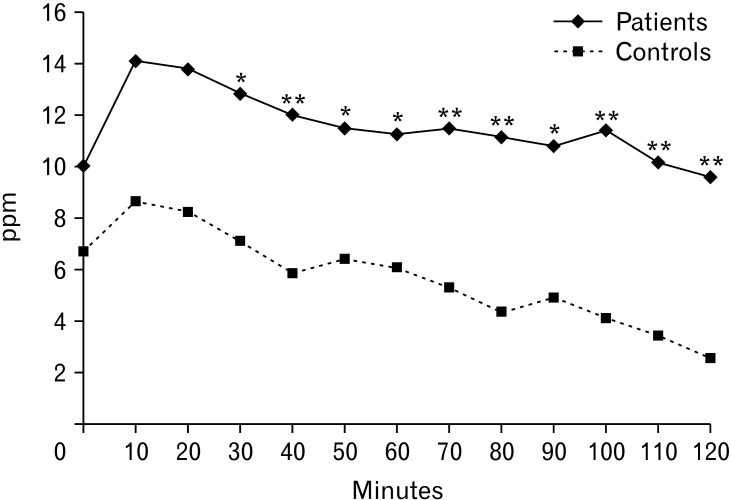
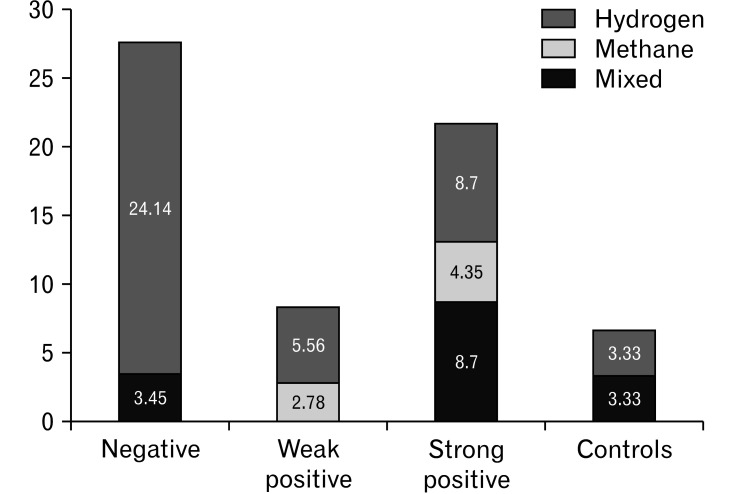
Between the study population and historical healthy controls, there were significant differences in the H2 level in the breath at every 10-minute time point from 30 minutes to 120 minutes (Fig. 2). The difference between GBT positivity in the study population and that in controls was not statistically significant (18.2% vs 6.7%, P = 0.130). Whereas the patients with negative LQT had a significantly higher GBT positivity or GBT (H2) positivity than that in controls, respectively (Table 3). Considering the subtypes of GBT, patients with negative LQT had a significant higher GBT (H2) positivity (27.6%) than those in the other 3 groups, including the weak positivity (5.6%), strong positivity (17.4%), and controls (6.7%) (P = 0.039) (Fig. 3).
Figure 2.
The profiles of glucose hydrogen breath test of the study population (solid line) and healthy historic controls (dotted line). *P < 0.05, **P < 0.01. ppm, parts per million.
Table 3.
Comparison of Glucose Breath Test Profiles Between Patients and Control Group
| GBT profiles | Patient (N = 88) |
LQT negative (n = 29) |
Control (n = 30) |
P-valuea |
|---|---|---|---|---|
| GBT positivity | 16 (18.2%) | 8 (27.6%) | 2 (6.7%) | 0.032 |
| Subtypes | ||||
| H2 (purely) | 11 (12.5%) | 7 (12.5%) | 1 (3.3%) | 0.064 |
| CH4 (purely) | 2 (2.3%) | 0 (2.3%) | 0 (0.0%) | |
| Mixedb | 3 (3.4%) | 1 (3.4%) | 1 (3.3%) | |
| H2 + Mixedb | 14 (15.9%) | 8 (27.6%) | 2 (6.7%) | 0.032 |
| CH4 + Mixedb | 5 (5.7%) | 1 (3.4%) | 1 (3.3%) | 0.981 |
aCompared between lactose intolerance quick test (LQT) negative patients and controls.
bMixed, both hydrogen and methane were positive.
GBT, glucose breath test; H2, hydrogen; CH4, methane.
Figure 3.
Glucose breath test positivity patterns in subgroups according to the lactose intolerance quick test (compared with controls).
Characteristics of Subgroups According to Glucose Breath Test Results
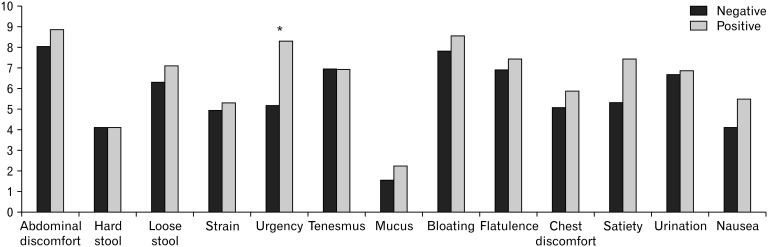
Table 4 shows the characteristics of the study population according to the GBT results. There were no significant differences in age, sex, body mass index, and LQT subtypes based on GBT positivity, whereas only LQT negativity had a tendency related to GBT positivity. As for total symptom scores (0-12 points), GBT-positive patients complained of total symptom scores for most individual items comparing to GBT negative patients, but only an item of urgency was of significance (8.31 ± 4.92 vs 5.18 ± 4.73, P = 0.020) (Fig. 4). A multivariate regression analysis showed no independent factors related with the results of GBT. LQT subtypes did not have a significant effect on total symptom scores of individual items (data not shown).
Table 4.
The Profiles of Demographic, Lactose Intolerance Quick Test Results According to the Positivity to Glucose Breath Test
| Variables | GBT | P-value | |
|---|---|---|---|
| Negativity (n = 72) | Positivity (n = 16) | ||
| Demographics | |||
| Age (yr) | 50.6 ± 13.6 | 51.4 ± 15.5 | 0.836 |
| Sex | |||
| Male | 35 (48.6) | 5 (31.3) | 0.207 |
| Female | 37 (51.4) | 11 (68.8) | |
| BMI (kg/m2) | 23.9 ± 3.4 | 22.6 ± 2.8 | 0.169 |
| LQT subtypes | |||
| Negativity | 21 (29.2) | 8 (50.0) | 0.118 |
| Weak positivity | 33 (45.8) | 3 (18.8) | |
| Strong positivity | 18 (25.0) | 5 (31.3) | |
| LQT | |||
| Negativity | 21 (29.2) | 8 (50.0) | 0.109 |
| Positivitya | 51 (70.8) | 8 (50.0) | |
aIncluded patients with lactose intolerance quick test (LQT) weak and strong positivity.
GBT, glucose breath test; BMI, body mass index.
Data are expressed as mean ± SD or n (%).
Figure 4.
Gastrointestinal symptom scores according to glucose breath test results. *P < 0.05.
Glucose Breath Test (Hydrogen) Positivity and Lactose Intolerance Quick Test-Strong Positive Group
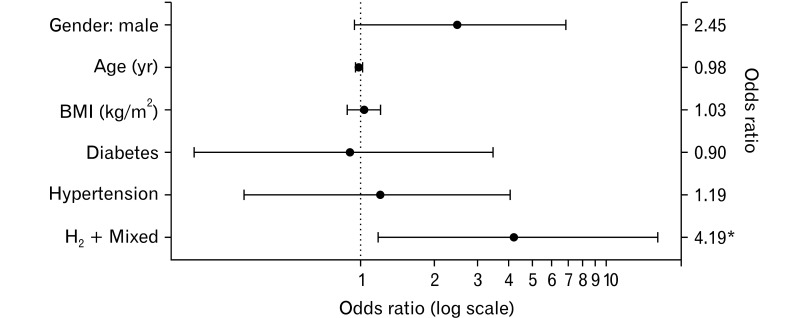
Among patients with gastrointestinal symptoms evoked by dairy products, LQT-negative participants had a significantly higher rate of GBT (H2) positivity than others (Table 5). To identify possible clinical factors related to LI, we performed a multivariate analysis of patient age, sex, body mass index, underlying diseases (diabetes, hypertension, and dyslipidemia), and GBT (H2) positivity. Multivariable logistic regression analysis showed that GBT (H2) positivity was the only significant independent factor for LQT negativity, indicating severe LD among patients with suspected LI (OR, 4.19; 95% CI, 1.18-16.20; P = 0.029). The other variables did not show significant correlations. The ORs of each factor in the multivariate model are presented in Figure 5.
Table 5.
The Profiles of Demographic, According to the Lactose Intolerance Quick Test Negativity
| Variables | LQT Negativity | P-value | |
|---|---|---|---|
| Yes (n = 29) | No (n = 59) | ||
| Demographics | |||
| Age (yr) | 48.28 ± 13.33 | 52.02 ± 14.05 | 0.236 |
| Sex | |||
| Male | 17 (58.6) | 23 (39.0) | 0.131 |
| Female | 12 (41.4) | 36 (61.0) | |
| BMI (kg/m2) | 23.85 ± 2.50 | 23.51 ± 3.62 | 0.621 |
| GBT | |||
| Total H2 | 190.34 ± 204.69 | 130.02 ± 147.45 | 0.164 |
| Total CH4 | 116.93 ± 79.88 | 98.76 ± 98.71 | 0.389 |
| Positivity (total) | 8 (27.6) | 8 (13.6) | 0.109 |
| Subtypes | |||
| H2 (purely) | 7 (24.2) | 4 (6.8) | 0.105 |
| CH4 (purely) | 0 (0.0) | 2 (3.4) | |
| Mixeda | 1 (3.4) | 2 (3.4) | |
| H2 + Mixeda | 8 (27.6) | 6 (10.2) | 0.036 |
| CH4 + Mixeda | 1 (3.4) | 4 (6.8) | 0.526 |
aMixed, both hydrogen (H2) and methane (CH4) were positive.
LQT, lactose intolerance quick test; BMI, body mass index; GBT, glucose breath test.
Data are expressed as mean ± SD or n (%).
Figure 5.
Plot for odds ratio of negativity in lactose intolerance quick test. *P < 0.05. BMI, body mass index; H2, hydrogen.
Discussion
We confirmed that SIBO producing H2 is common in patients with severe LD suspected lactose intolerance. In particular, severe LD was evaluated by LQT negativity as proven by a biopsy-based test using upper gastroduodenal endoscopy.
Although classified as different disease entities, both LD and SIBO could induce common intestinal symptoms, suggesting that gut bacteria may play an important role as a causative factor for LI. They are thought to be linked in a direct causal relationship, and the suggested pathophysiological mechanism is that the destruction of mucosal enzymes due to SIBO causes secondary LI.19 Accordingly the close relationship between SIBO and LI has been suggested in the literature. However the study lacks a direct investigation of intestinal mucosa, and secondary lactose malabsorption as well as preanalytical limitations of the breath test procedure can cause discrepant results. Although we did not investigate pathological confirmation of the intestinal mucous membrane, we could observe the statues of lactose malabsorption using intestinal tissue. A retrospective study revealed the rates of carbohydrate malabsorption in patients using the lactulose breath test, and pointed out that SIBO could be a remediable cause of malabsorption, including LI.20 Similarly, a study demonstrated the prevalence of clinical LI through a nutritional survey in patients with functional distension syndrome, and 77.0% of them were accompanied by SIBO in the H2 breath test.21 Meanwhile, another research group studied LD and SIBO in post-infectious IBS patients, and it is noteworthy that all LD patients had SIBO, although there is a limitation in that the SIBO diagnosis was made by H2 level only and not by CH4.22 In one study, SIBO was confirmed in all secondary LD cases of post-infectious IBS patients. Both SIBO and secondary LD improved after 2 weeks of probiotic treatment, suggesting that changes in the gut microbiota may help relieve LI symptoms.23 The strength of our study was both H2 and CH4 being used. Our study indicated that breath H2 was distinctly related with severe lactase deficiency.
Some studies have not shown an association between SIBO and LI. A previous study did not confirm a statistically significant association between SIBO and lactose malabsorption.24 However, it is necessary to pay attention to the interpretation of this result because the study population consisted of pediatric patients with gastrointestinal symptoms related to dairy product consumption, which was different from our study. Another study suggested that SIBO using GBT is independent of the presence of LI.25 However, the study was done by self-reported milk intolerance, which was known as a poor sensitivity in detecting LI.
As mentioned above, opinions differ in each study regarding the correlation between LI and SIBO. This discrepancy may stem from the heterogeneity of related studies. For instance, there have been ethnic, regional, and age differences in establishing the study population in previous studies. Additionally, it should be noted that inconsistencies in the definition of LI or SIBO exist. Breath tests have been widely used in both clinical and research practices for SIBO diagnosis, but every researcher applied their methods for the test progression and interpretation; types and dosages of the breath test substrate were different, and there are various ways as to which exhaled gas (H2 or CH4) was collected and utilized as the standard for SIBO diagnosis. In cases of LI diagnosis, the objectivity of diagnosis may be affected if the definition relies only on the results of symptom-based questionnaires or history-based interviews. Two noninvasive tests such as lactose H2 breath test and lactose tolerance test have been clinically used for LI. However, although invasive, the gold standard for diagnosing lactose malabsorption would be evaluating the level of lactase activity directly in the small intestine.26 There is still strong argument that the true “lactose intolerance” must include enzymatic deficiency of lactase along the brush border. To overcome these conventional limitations, we attempted to make some changes to the methodology. One such attempt was that LQT was used as a tool for LI diagnosis in our study. LQT was well known as fast, reliable, easy method with high sensitivity (95%), specificity (100%), positive predictive value (100%), and negative predictive value (98%).7 To the best of our knowledge, this study is the first attempt to find out the association between LD diagnosed by LQT and SIBO. The LQT has advantages as a tool for LI diagnosis over the conventional breath test or tolerance test. It is simpler and faster and hence, the result can be confirmed almost immediately on endoscopic examination using a biopsy specimen. Moreover, LQT is intuitive for understanding the degree of LD through color reaction, and it can be used for subgroup analysis according to LI levels. We also focused on obtaining more reliable results when conducting GBT. H2 or CH4 levels in the exhaled gas were measured twice consecutively at each time point, and the average values were obtained and utilized. For the confirmation of SIBO, both H2 and CH4 were used, so the results according to each criterion were dealt with separately. In addition, since all patients were visually checked for whether sufficient fasting was achieved through preceding upper gastrointestinal endoscopy, it was possible to minimize the confounding effect of intraluminal remnant food materials, which could have affected the breath test results.
The risk of severe LD proven by LQT was 4.19 times higher in GBT (H2) positive cases, demonstrating a high correlation between the 2 diseases. However, our data did not demonstrate that weak LQT positivity was significantly related to any subtype of GBT positivity. A previous study confirmed that LQT effectively identifies patients with severe duodenal hypolactasia beyond mild hypolactasia.7 Moreover, several reasons could be cited for this unexpected result. First, the number of enrolled patients may not have been sufficient; therefore, sufficient power could not be obtained for the analyses. Second, the LQT used in our study interpreted the degree of LD based on the color change of the reagent. Although the test kit provides reference colors for each condition, mild positivity is relatively vague, so the examiner’s subjective interpretation may have affected the results. Another possible reason is that GBT is a qualitative diagnostic tool. Generally, the cutoff level of GBT for SIBO detection is known to be > 103-105 CFU/mL in duodenal aspirates and culture.12 Gut microorganisms below the GBT cutoff level, which may be related to mild LD, could not be identified. Therefore, the association between bacterial overgrowth and mild LD could also have been underestimated. Based on the 3 points mentioned above, we anticipate that it may be worthwhile to explore the characteristics of symptoms and related factors with a large-scale design on objective judgement with an interpersonal agreement in future studies on LI with GBT. Furthermore, quantitative testing methods such as small bowel aspirate culture should be considered in future studies. In addition, the false positivity related with rapid transit should be considered during GBT in patients with LI who were expected to have high bowel movement. To reduce this, we used glucose as substrate for the breath test in this study. GBT can detect only proximal bacteria, as glucose is completely absorbed in proximal small bowel.
The symptom score of urgency was significantly high in GBT-positive compared to that in GBT-negative patients. In our study, most GBT-positive patients are hydrogen-producing bacteria. The main symptom of LI is bowel symptoms associated with high bowel movement, which indirectly suggests that it is related to H2 rather than CH4 producing SIBO being associated with constipation. Moreover, most of abdominal symptoms were more common in the GBT-positive patients whereas almost negligible in the GBT-negative patients. We need to study further with larger number of patients.
There are potential limitations of this study. We used the historical controls much less than the cases. However, the positive rate of SIBO observed in control of this study was similar with normal personal data in the literature.27 As another limitation of the study, we showed only the possibility of an association between SIBO and LD and could not evaluate the effectiveness of SIBO treatment on LI. The prevalence of LI is generally higher in Asia than in Western countries, and LI may be a more important problem in this area than before owing to the westernization of eating habits. Therefore, by recognizing the comorbidity of SIBO in patients with LI and providing appropriate treatment for SIBO, a positive effect on symptom alleviation and improvement of the quality of life for these patients can be expected beyond avoidance therapy or commonly conducted symptomatic treatments. To prove this point, an objective and accurate evaluation of the post-SIBO treatment response of LI is necessary for future related studies. Finally, there is insufficient information about the symptoms following consumption of dairy products. In the next study, it will be necessary to evaluate lactose-related gastrointestinal symptoms using a well-validated questionnaire.
In conclusion, the prevalence of SIBO in patients with gastrointestinal symptoms induced by dairy products is relatively high. Moreover, the presence of SIBO is an independent factor related to severe LD. Further research is needed to understand the role of SIBO in patients with LD demonstrating the response to SIBO treatment.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Ik Hyun Jo collected and analyzed the data, and drafted the article; Yeon-Ji Kim, Ji Min Lee, Soo Yeon Choi, and Kee Pyung Hong collected the data; and Chang-Nyol Paik designed the study, collected and analyzed the data, and drafted the article.
References
- 1.Swagerty DL, Jr, Walling AD, Klein RM. Lactose intolerance. Am Fam Physician. 2002;65:1845–1850. doi: 10.1097/01.jaa.0000586344.04372.e6. [DOI] [PubMed] [Google Scholar]
- 2.Jellema P, Schellevis FG, van der Windt DA, Kneepkens CM, van der Horst HE. Lactose malabsorption and intolerance: a systematic review on the diagnostic value of gastrointestinal symptoms and self-reported milk intolerance. QJM. 2010;103:555–572. doi: 10.1093/qjmed/hcq082. [DOI] [PubMed] [Google Scholar]
- 3.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1–4. doi: 10.1056/NEJM199507063330101. [DOI] [PubMed] [Google Scholar]
- 4.Saha M, Parveen I, Shil BC, et al. Lactose intolerance and symptom pattern of lactose intolerance among healthy volunteers. Euroasian J Hepatogastroenterol. 2016;6:5–7. doi: 10.5005/jp-journals-10018-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappello G, Marzio L. Rifaximin in patients with lactose intolerance. Dig Liver Dis. 2005;37:316–319. doi: 10.1016/j.dld.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Ojetti V, La Mura R, Zocco MA, et al. Quick test: a new test for the diagnosis of duodenal hypolactasia. Dig Dis Sci. 2008;53:1589–1592. doi: 10.1007/s10620-007-0027-7. [DOI] [PubMed] [Google Scholar]
- 7.Kuokkanen M, Myllyniemi M, Vauhkonen M, et al. A biopsy-based quick test in the diagnosis of duodenal hypolactasia in upper gastrointestinal endoscopy. Endoscopy. 2006;38:708–712. doi: 10.1055/s-2006-925354. [DOI] [PubMed] [Google Scholar]
- 8.Rao P, Rao N, Jordinson M, Scott C, Hinchcliffe C, Campbell D. Comparison of quick point-of-care test for small-bowel hypolactasia with biochemical lactase assay in children. J Pediatr Gastroenterol Nutr. 2012;54:401–403. doi: 10.1097/MPG.0b013e318231eb30. [DOI] [PubMed] [Google Scholar]
- 9.Furnari M, Bonfanti D, Parodi A, et al. A comparison between lactose breath test and quick test on duodenal biopsies for diagnosing lactase deficiency in patients with self-reported lactose intolerance. J Clin Gastroenterol. 2013;47:148–152. doi: 10.1097/MCG.0b013e31824e9132. [DOI] [PubMed] [Google Scholar]
- 10.Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol. 2007;3:112–122. [PMC free article] [PubMed] [Google Scholar]
- 11.Ghoshal UC, Shukla R, Ghoshal U. Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy. Gut Liver. 2017;11:196–208. doi: 10.5009/gnl16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the north american consensus. Am J Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrielli M, D'Angelo G, Di Rienzo T, Scarpellini E, Ojetti V. Diagnosis of small intestinal bacterial overgrowth in the clinical practice. Eur Rev Med Pharmacol Sci. 2013;17(suppl 2):30–35. [PubMed] [Google Scholar]
- 14.Sung HJ, Paik CN, Chung WC, Lee KM, Yang JM, Choi MG. Small intestinal bacterial overgrowth diagnosed by glucose hydrogen breath test in post-cholecystectomy patients. J Neurogastroenterol Motil. 2015;21:545–551. doi: 10.5056/jnm15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DB, Paik CN, Kim YJ, et al. Positive glucose breath tests in patients with hysterectomy, gastrectomy, and cholecystectomy. Gut Liver. 2017;11:237–242. doi: 10.5009/gnl16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YJ, Paik CN, Jo IH, Kim DB, Lee JM. Serum gastrin predicts hydrogen-producing small intestinal bacterial overgrowth in patients with abdominal surgery: a prospective study. Clin Transl Gastroenterol. 2020;12:e00291. doi: 10.14309/ctg.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JM, Choi MG, Oh JH, et al. Cross-cultural validation of irritable bowel syndrome quality of life in Korea. Dig Dis Sci. 2006;51:1478–1484. doi: 10.1007/s10620-006-9084-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim EJ, Paik CN, Chung WC, Lee KM, Yang JM, Choi MG. The characteristics of the positivity to the lactulose breath test in patients with abdominal bloating. Eur J Gastroenterol Hepatol. 2011;23:1144–1149. doi: 10.1097/MEG.0b013e32834b0e5c. [DOI] [PubMed] [Google Scholar]
- 19.Enko D, Rezanka E, Stolba R, Halwachs-Baumann G. Lactose malabsorption testing in daily clinical practice: a critical retrospective analysis and comparison of the hydrogen/methane breath test and genetic test (c/t-13910 polymorphism) results. Gastroenterol Res Pract. 2014;2014:464382. doi: 10.1155/2014/464382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perets TT, Hamouda D, Layfer O, et al. Small intestinal bacterial overgrowth may increase the likelihood of lactose and sSorbitol but not fructose intolerance false positive diagnosis. Ann Clin Lab Sci. 2017;47:447–451. [PubMed] [Google Scholar]
- 21.Novillo A, Peralta D, Dima G, Besasso H, Soifer L. [Frequency of bacterial overgrowth in patients with clinical lactose intolerance]. Acta Gastroenterol Latinoam. 2010;40:221–224. [Spanish] [PubMed] [Google Scholar]
- 22.Fadeeva NA, Ruchkina IN, Parfenov AI, Shcherbakov PL. [Small intestinal bacterial overgrowth as a cause of lactase deficiency]. Ter Arkh. 2015;87:20–23. doi: 10.17116/terarkh201587220-23. [Russian] [DOI] [PubMed] [Google Scholar]
- 23.Ruchkina IN, Fadeeva NA, Parfenov AI, et al. [The role of small bowel microflora in the development of secondary lactase deficiency and the possibilities of its treatment with probiotics]. Ter Arkh. 2013;85:21–26. doi: 10.17116/terarkh201587220-23. [Russian] [DOI] [PubMed] [Google Scholar]
- 24.dos Reis JC, de Morais MB, Fagundes Neto U. [Breath hydrogen test to evaluate lactose absorption and small bowel bacterial overgrowth in children]. Arq Gastroenterol. 1999;36:169–176. doi: 10.1590/S0004-28031999000400003. [Portuguese] [DOI] [PubMed] [Google Scholar]
- 25.Gupta D, Ghoshal UC, Misra A, Misra A, Choudhuri G, Singh K. Lactose intolerance in patients with irritable bowel syndrome from northern India: a case-control study. J Gastroenterol Hepatol. 2007;22:2261–2265. doi: 10.1111/j.1440-1746.2007.04986.x. [DOI] [PubMed] [Google Scholar]
- 26.Law D, Conklin J, Pimentel M. Lactose intolerance and the role of the lactose breath test. Am J Gastroenterol. 2010;105:1726–1728. doi: 10.1038/ajg.2010.146. [DOI] [PubMed] [Google Scholar]
- 27.Shah ED, Basseri RJ, Chong K, Pimentel M. Abnormal breath testing in IBS: a meta-analysis. Dig Dis Sci. 2010;55:2441–2449. doi: 10.1007/s10620-010-1276-4. [DOI] [PubMed] [Google Scholar]