Abstract
Background/Aims
Chronic psychological stress affects gastrointestinal physiology which may underpin alterations in the immune response and epithelial transport, both functions are partly regulated by enteric nervous system. However, its effects on enteric neuroplasticity are still unclear. This study aims to investigate the effects of chronic unpredictable psychological stress on intestinal motility and prominent markers of enteric function.
Methods
Adult male C57BL/6J mice were exposed to 19 day of unpredictable stress protocol schedule of social defeat and overcrowding. We investigated the effects on plasma corticosterone, food intake, and body weight. In vivo gastrointestinal motility was assessed by fecal pellet output and by whole-gastrointestinal transit (using the carmine red method). Tissue monoamine level, neural and glial markers, neurotrophic factors, monoamine signaling, and Toll-like receptor expression in the proximal and distal colon, and terminal ileum were also assessed.
Results
Following chronic unpredictable psychological stress, stressed mice showed increased food intake and body weight gain (P < 0.001), and reduced corticosterone levels (P < 0.05) compared to control mice. Stressed mice had reduced stool output without differences in water content, and showed a delayed gastrointestinal transit compared to control mice (P < 0.05). Stressed mice exhibited decreased mRNA expression of tyrosine hydroxylase (Th), brain-derived neurotrophic factor (Bdnf) and glial cell-derived neurotrophic factor (Gdnf), as well as Toll-like receptor 2 (Tlr2) compared to control (P < 0.05), only proximal colon. These molecular changes in proximal colon were associated with higher levels of monoamines in tissue.
Conclusion
Unpredictable psychological chronic stress induces region-specific impairment in monoamine levels and neuroplasticity markers that may relate to delayed intestinal transit.
Keywords: Enteric nervous system; Gastrointestinal motility; Nerve tissue proteins; Neuronal plasticity; Stress, psychological
Introduction
Chronic exposure to stress can negatively affect gastrointestinal (GI) functions1-3 and plays an important role in the development exacerbation of GI disorders as irritable bowel syndrome (IBS).4-6 IBS is defined by recurrent abdominal pain associated with altered bowel habits,7 and it is one of the most common functional GI disorders in Western countries with a significant socioeconomic impact.8 Diverse pathological factors, including physical and psychological stressors, have been proposed to disturb gut-brain bi-directional communication.3-7,9 These factors lead to gut dysmotility and enhanced visceral pain,10-12 and contribute to the development of IBS. Brain-gut axis communication involves coordinated responses of the enteric nervous system (ENS), the autonomic nervous system, the hypothalamic-pituitary-adrenal axis, and neuro-immuno endocrine mediators.13,14 Chronic psychological stress increases HPA axis and autonomic nervous system activity which promotes modulation in monoaminergic systems signaling including the dopaminergic (DA), noradrenergic (NA), and serotonergic (5-HT) circuitries involved in the regulation of gut physiology and brain-gut axis function.1,3-6,11,14,15 Much of our knowledge about chronic stress-induced dysfunction of GI physiology and brain-gut axis comes from rodent models,9 though the specific molecular mechanisms underlying stress-induced enteric neuroplasticity remain elusive.16-18 Clinical data show neuroplastic changes (increased neuronal outgrowth as well as features of enteric glial cells activation) in the colonic mucosa of IBS patients associated with visceral hypersensitivity.19,20 Different studies support the role of neuroimmune activation in psychological stress-induced visceral hyperalgesia.18,21 In line with this, we have demonstrated that chronic psychological stress in mice results in increased visceral hypersensitivity associated with up-regulation of Toll-like receptor (TLR) 4 and microglia activation within CNS, as well as increased social anxiety-like behaviour.22,23 Therefore, this study aims to investigate whether chronic psychological stress affects intestinal functions (motility and secretion) and to examine neuroplasticity and immune-related changes that may underpin such effects.
Materials and Methods
Animals
Adult male C57BL/6J mice (n = 60; 8 weeks of age) were used as experimental mice, and adult male CD-1 mice (n = 20; 9-10 weeks of age) were used as resident aggressors, and additional C57BL/6J mice (n = 8; 8-9 weeks of age) were used to train the aggressor CD-1 mice, all animals were purchased from Envigo (Huntingdon, UK). All mice were singly housed in standard mouse cages (33X15X13 cm) with only white bedding without additional enrichment, and had ad libitum access to food and tap water under a 12-hour light/dark schedule (light on at 5 AM) with standard controlled conditions (21 ± 1℃, 55 ± 10% humidity) throughout the study. None of the procedures required a fasting period. Once weekly, home cages were cleaned and equipped with new white bedding before stool collection by the same researcher. All animal care and experimental procedures were carried out following the European Community Council Directive (86/609/EEC) and approved by the Animal Experimentation and Ethics Committee.
Experimental Design
Chronic psychosocial stress procedure
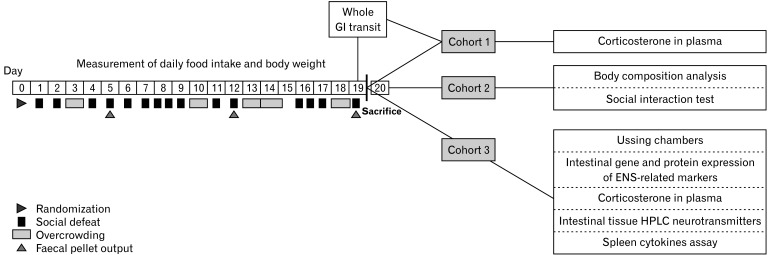
One day before starting the chronic unpredictable stress (CUS) protocol, mice were balanced based on body weight and randomly assigned to either the CUS or control group (n = 10 each). Afterwards, experimental mice were randomized into 3 separate cohorts (n = 20 each) for GI motility and secretomotory function, molecular markers, and behavior analysis (Fig. 1). CUS procedure was carried out as described previously.22 Briefly, mice were exposed to an unpredictable mixed model that comprised social defeat and overcrowding sessions throughout 19 days (Supplementary Methods).
Figure 1.
Timeline of the experimental procedure. Mice (n = 60) were exposed to 19 days of unpredictable chronic social stress paradigm. Fecal pellet were collected at day 5, 12, and 19 (indicated by arrow heads). Mice were split into 3 separated cohorts (n = 20): a sample, gastrointestinal (GI) motility and behavioral testing group. In the sample group plasma corticosterone, tissue neurotransmitters (high-performance liquid chromatography [HPLC]), mRNA and protein expression were assessed as well as ex vivo cecum ion transport (Ussing chambers). In the motility testing group red carmine dye was administered 2 hours following last stress session to assess the whole GI transit time and plasma corticosterone was determined. In the behavioral testing group, the social interaction test and body composition analysis were carried out. ENS, enteric nervous system.
Corticosterone assay
At the end of the experimental paradigm and procedures, mice were sacrificed just at the beginning of the dark phase (between 4:30 PM and 6 PM) based on our previous findings for this CUS protocol,22,23 and on the circadian pattern of plasma corticosterone concentrations, which are higher during the early dark phase. Trunk blood was collected into EDTA-coated tubes. Plasma corticosterone concentration was quantified with commercially available enzyme immunoassay kits, namely Enzo ADI-901-097 (Enzo Life Sciences, Exeter, UK, LTD) according to the manufacturer’s instructions (Supplementary Methods).
Body composition analysis
The following day of the last stress session (between 9 AM and 1 PM), fat and lean body mass were determined. Measurements were carried out with conscious mice in a 3-minute trial using the Minispec mq benchtop NMR spectrometer (Bruker Instruments, Rheinstetten, Germany).24,25
Social interaction test
The following day of the last stress session (between 9 AM and 1 PM), social avoidance behavior was assessed as described previously in the social interaction test (Supplementary Methods).22
Neurotransmitters determination
Tissue concentration of NA, DA, 5-HT, and their metabolites were quantified in the full-thickness terminal ileum, proximal and distal colon tissue from both experimental groups by high-performance liquid chromatography (HPLC) coupled to electrochemical detection, using a modification of a previously described procedure (Supplementary Methods).26
Quantitative real-time polymerase chain reaction
To determine the differential transcriptional profile associated with the regulation of the ENS functions, total RNA was extracted from full thickness terminal ileum, proximal and distal colon with the Qiagen RNeasy Lipid Mini Kit (Qiagen, Valencia, CA,USA). Complementary DNA was synthesized with 1 mg total RNA with random primers. Quantitative changes in mRNA levels were estimated by quantitative real-time polymerase chain reaction (Supplementary Methods).
Protein isolation and Western blotting
Western blot was performed to determine the protein levels of glial fibrillary acidic protein (GFAP), S100 calcium-binding protein B (S100B), and protein gene product 9.5 (PGP 9.5) from homogenized tissues from the proximal colon (Supplementary Methods).
In vivo assessment of gastrointestinal motility
Fecal pellet output and water content. Fecal pellets expelled were collected from each animal for 90 minutes during the dark phase on days 5, 12, and 19 just after the bedding change once a week, as well, the water content in feces was assessed (Supplementary Methods).
Whole gastrointestinal transit time. A cohort of experimental mice (n = 20) was administered carmine dye by oral gavaged 2 hours following the last stress, GI transit time was considered as the time between gavage and first observance of a red fecal pellet (Supplementary Methods).
Ex vivo Ussing chamber experiments
Experiments reported in this study used mouse caecum due to its optimal size and different segments of the colon were processed and used for subsequent analysis. Paired mucosa submucosa preparations from each animal (n = 6) were mounted in Ussing chambers (Harvard Apparatus, Kent, UK) (Supplementary Methods).
Spleen cytokine assays
The cytokines amount such as IL-1β, 6, 10, 12, IFN-γ, mouse keratinocyte-derived cytokine, and TNF-α on spleen cells cultured with lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate (PMA), and lipoteichoic acid (LTA) (Sigma-Aldrich, Wicklow, Ireland) was quantified using custom mouse Multi-Spot 96 Well Plates (Meso Scale Discovery, Rockville, MD, USA) according to manufacturer’s recommendations (Supplementary Methods).
Statistical Methods
Data are represented in the text and figures as mean values ± SEM. Statistical analysis was performed using repeated measures two-way ANOVA for overall effects in the analysis of body weights followed by Bonferroni post hoc test. The Student’s t test and the non-parametric Mann-Witney’s U test were used to compare 2 independent groups. Differences were considered significant at P < 0.05. All analysis was carried out with GraphPad Prism software (GraphPad Software Inc, La Jolla, CA, USA).
Results
Chronic Psychological Stress Induces a Significant Increase in Body Weight, Food Intake, and Significant Changes in Body Composition
Chronic psychological stress significantly induced an increase in body weight in mice compared with control mice, from day 10 to the end of the CUS procedure (stress: F [1332] = 71.5, P < 0.001) (stress X day: F [8332] = 4.0, P < 0.001; Supplementary Fig. 1A), consistent with the previous report.17 Thus, at the end of the experimental paradigm, stressed mice showed a higher food intake than control mice (3.7 ± 0.1 vs 4.3 ± 0.0 g, P < 0.001; n = 20) (Supplementary Fig. 1A). In CUS mice, fat mass was significantly reduced (P = 0.003, n = 20) compared to controls, while lean mass increased in stressed animals (P = 0.003, n = 20) (Supplementary Fig. 1B), which replicates earlier findings.22,23
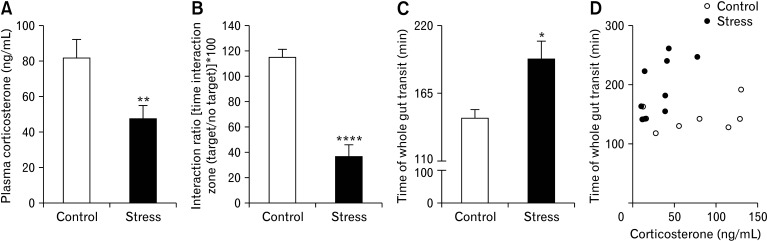
Chronic Psychosocial Stress Reduces Plasma Corticosterone Levels
CUS protocol induced a reduction in evening corticosterone levels in plasma at day 19 compared to controls (Fig. 2A).
Figure 2.
Stress had effects on behavior and induced changes in evening plasma corticosterone levels and total gastrointestinal (GI) motility. (A) Social avoidance was analyzed by social interaction test. Exposure to chronic stress caused a significant increase in social avoidance (n = 10/group). (B) Plasma corticosterone levels were measured by enzyme-linked immunosorbent assay. Exposure to chronic stress caused a significant decrease in evening plasma corticosterone levels (n = 20/group). (C) Total GI transit time was measured after gavage of red carmine in control and stress mice (n = 9-10/group); total GI transit time was significantly increased in stressed mice; (D) however, total GI transit time was not associated with plasma corticosterone levels (n = 8-9/group) (Spearman’s rank correlation). (E) Data represent mean ± SEM. *P < 0.05,**P < 0.01 ****P < 0.0001; *by t test (A, B), Mann Whitney test (C), and Spearman’s rank correlation (D) represent significant difference between stress and control groups.
Chronic Psychosocial Stress Induces Social Avoidance Behavior
Similar to our previous data,23 the animals exposed to 19 days of CUS exhibited pronounced social avoidance behaviour as reflected by an alteration in the social interaction ratio compared with the control group (Fig. 2B).
Chronic Psychosocial Stress Delays Gastrointestinal Transit
Following 5 days of CUS, the fecal water content was increased in stressed mice compared with control mice, while no differences were observed in fecal pellet counts (Table 1). However, at the end of the procedure, water content was comparable to control mice whereas stool output was significantly reduced (Table 1), indicating that CUS protocol appears to delay GI motility. Consistently, during the dark cycle, CUS mice displayed significant delay in GI transit compared to control animals (Fig. 2C) that was not associated with decreased plasma corticosterone levels (Fig. 2D).
Table 1.
Comparison of Number of Fecal Pellets and Fecal Water Content Between Groups (n = 16-20 group) Over Chronic Unpredictable Stress Procedure
| Day | Control | Stress | |||
|---|---|---|---|---|---|
| Fecal pellet output | Fecal water content (%) | Fecal pellet output | Fecal water content (%) | ||
| 5 | 2.5 ± 0.4 | 61.6 ± 1.4 | 2.6 ± 0.4 | 77.4 ± 3.7** | |
| 12 | 2.9 ± 0.4 | 65.8 ± 2.0 | 2.2 ± 0.4 | 68.8 ± 2.5 | |
| 19 | 3.1 ± 0.4 | 60.7 ± 2.1 | 1.8 ± 0.3* | 63.5 ± 2.0 | |
*P < 0.05, **P < 0.01 by Mann Whitney test.
Data represent mean ± SEM.
Stress Had No Impact on Ex Vivo Cecum Ion Transport
Under basal conditions, Cl--secretory short-circuit current (Isc) values were comparable in the cecum of both experimental groups (Supplementary Fig. 2A). Electrogenic Na+ absorption was present in the cecum of both groups, as evidenced by the amiloride-induced decrease of Isc without significant difference among them (Supplementary Fig. 2C-E). The addition of bethanechol and forskolin elicited a sustained increase in Isc that was similar between groups. To assess the CUS impact on the intestinal epithelial barrier integrity, transepithelial resistance of cecal explants among groups was analyzed, demonstrating no significant difference between stressed mice and controls (Supplementary Fig. 2B).
Chronic Psychosocial Stress Induces Changes in Enteric Neurotransmitters in Proximal Colon
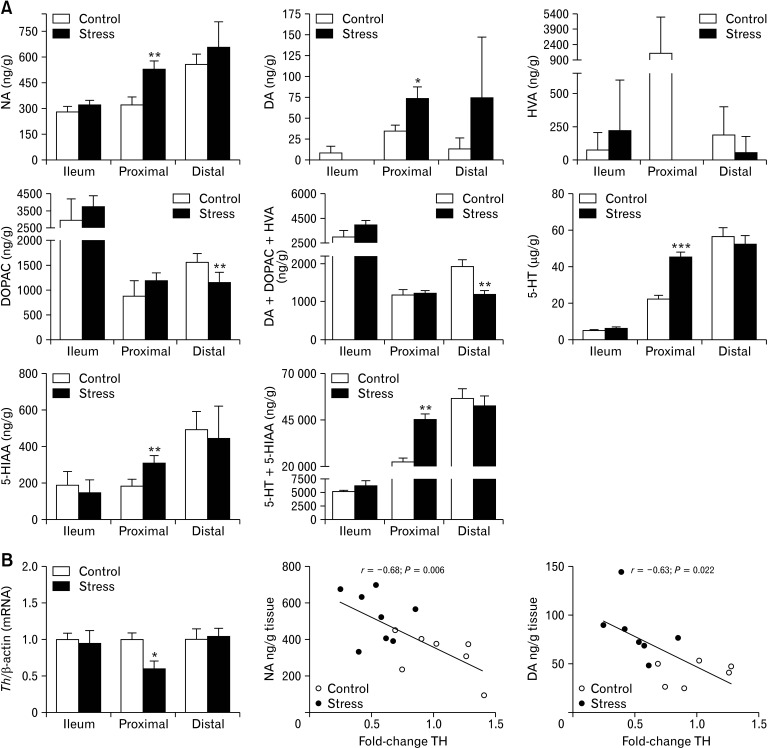
Based on our observation that chronic stress alters GI motility, we examined along different intestinal full-thickness sections the content of diverse neurotransmitters as well as the expression pattern of genes involved in their regulation. Differences in noradrenalin and dopamine (catecholamines) levels between both groups were found only in the proximal colon, where CUS mice displayed higher catecholamine content than controls (Fig. 3A). While catecholamines levels in the proximal colon were increased, we did observe reduced expression of tyrosine hydroxylase (Th), the enzyme that controls catecholamine synthesis, which was inversely related to catecholamines content in that segment (Fig. 3B), indicating a possible adaptive compensatory mechanism for biosynthesis regulation. On the other hand, CUS mice showed lower levels of the specific metabolite of DA, 4-hydroxyvanillic acid (HVA) in both colonic segments, especially in the proximal colon, where it was undetectable. Notably, CUS mice showed lower total content of DA and its metabolites, hydroxyvanillic acid, and DOPAC, in the distal colon. 5-HT and 5-hydroxyindoleacetic acid levels were higher only in the proximal colon of CUS-exposed animals (Fig. 3A), indicating increasing 5-HT turnover. Again, differential changes in the expression of key components of 5-HT system evoked by stress along the GI tract were observed. P11 was significantly up-regulated in the distal colon, whereas expression of Htr4 was up-regulated in the proximal colon. No significant change in the expression of the solute carrier family 6, Slc6a4 (Sert), was observed in any segment analysed (Fig. 4C). Overall, these findings point to a differential neurotransmission pattern in stress response along the GI tract, limited to proximal colon which may explain the motility impairment.
Figure 3.
Chronic stress induces changes in enteric neurotransmitters and down regulated the expression of tyrosine hydroxylase (Th) mRNA in proximal colon. Levels of noradrenaline (NA), dopamine (DA), serotonin (5-HT), and metabolites from dopamine and 5-HT were measured by high-performance liquid chromatography (HPLC) in ileum and colon in control and stress mice (n = 7-8/group). (A) Levels of NA, DAc, and 5-HT were significantly greater than those in control mice only in proximal colon of stressed mice. No HVA levels were detected in proximal colon of stressed mice. The total levels of Dopamine plus its metabolites (homovanillic acid [HVA] and 3,4-dihydroxyphenylacetic acid [DOPAC]) were significantly lower in distal colon of stressed mice than control. Total levels of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) were significantly greater in proximal colon of stressed mice than controls. Quantitative real-time polymerase chain reaction was used to quantify to measure transcripts encoding Th in small intestine and colon of control and stressed mice (n = 10/ group). (B) In proximal colon transcript levels of Th negatively correlated with the concentration of NA and DA. Data represent mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 by t test (A, B) and Pearson analysis (B).
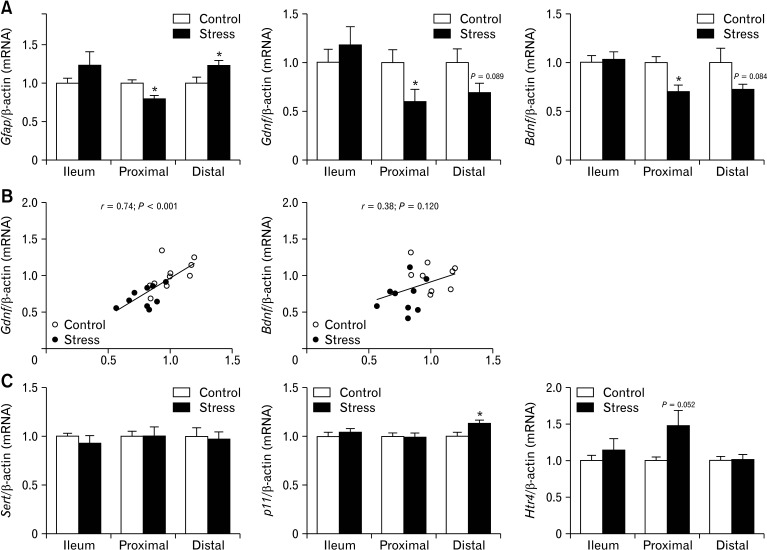
Figure 4.
Chronic stress down-regulate transcripts encoding enteric glia, neurotrophins in proximal colon. Quantitative real-time polymerase chain reaction was used to analyze glial fibrillary acidic protein (Gfap), glial cell-derived neurotrophic factor (Gdnf), and brain-derived neurotrophic factor (Bdnf) expression levels in small intestine and colon of control and stressed mice (n = 10/group). (A) Transcripts encoding Gfap, Gdnf, and Bdnf were significantly decreased in proximal colon after chronic stress when compared with control mice. (B) In proximal colon also a strong correlation between Gfap and Gdnf expression levels was observed. (C) Expression levels of serotonin transporter, solute carrier family 6 Slc6a4 (Sert), was not different between stressed and control mice, expression levels of S100 calcium binding protein A10 (p11) were significantly increased in distal colon of stress mice and the increase of serotonin receptor 4 (Htr4) receptor mRNA levels were nearly significant in proximal colon of stress mice. Data represent mean ± SEM, *P < 0.05 by t test.
Chronic Stress Induces Segmental-specific Differential Expression Patterns in Genes Associated With the Regulation and Integrity of the Enteric Nervous System
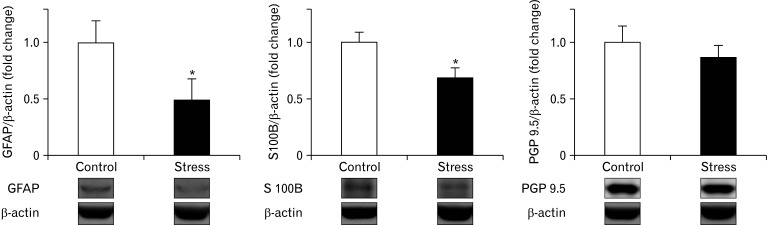
Given that CUS-induced GI motility and neurotransmitter dysfunction, we next examined if CUS could differentially modulate the expression of ENS markers involved in regulating intestinal plasticity and motility along the different intestinal segments. As shown in Figure 4A, there was a down-regulation of Gfap mRNA (glial cell marker), and neurotrophic factors (NTFs) as glial cell-derived neurotrophic factor (Gdnf) mRNA and brain-derived neurotrophic factor (Bdnf) mRNA in the proximal colon of mice exposed to CUS. Whereas the distal colon of mice exposed to CUS revealed an up regulation of Gfap mRNA and down-regulation of Gdnf and Bdnf mRNA. Interestingly, only in the proximal colon mRNA levels of Gfap were related to the mRNA levels of Gdnf (P < 0.001, Fig. 4B). No significant changes in mRNA levels of these transcripts were observed in the ileum of mice exposed to CUS. Based on our observations, we next measured the protein expression of 2 glia markers (GFAP and S100B) and neuron (PGP 9.5) in the proximal colon. Mice exposed to CUS showed a significant concomitant reduction in the protein expression of GFAP and S100B yet protein expression of PGP 9.5 tended to be reduced, though not significantly (Fig. 5).
Figure 5.
Effects of chronic stress on enteric glia population and neurons in proximal colon. Western blot analysis of specific glia markers, glial fibrillary acidic protein (GFAP) and S100 calcium-binding protein B (S-100B), and neurons (protein gene product 9.5 [PGP 9.5]) in proximal colon of stressed and control mice (n = 7-8/group). Protein fold-change was calculated for each sample with reference to the average of the target protein to β-actin ratio of the control group. The protein expression of enteric glia markers were significantly decreased in proximal colon after chronic stress when compared with control mice. Data represent mean ± SEM, *P < 0.05 by Mann Whitney test.
Chronic Psychosocial Stress Increases Lipopolysaccharide-stimulated Release of Spleen Inflammatory Mediators
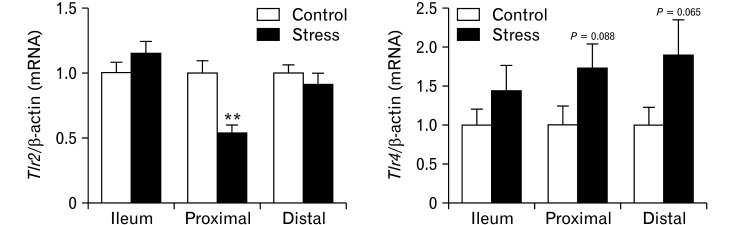
In order to examine if disruption of the ENS and intestinal dysfunction can reflect a systemic inflammatory process through TLRs activation, we evaluated the differential cytokine production in TLR-ligand-mediated in the periphery. Spleens from both groups were stimulated with a selective TLR2 and TLR4 agonist. In agreement with our previous results,24 splenocytes of CUS mice displayed increased levels of IL-12, IL-6, TNF-α, and mouse keratinocyte-derived cytokine in response to LPS, a TLR4 agonist, compared with the control group (Supplementary Table). In contrast, similar amounts of IL-6, TNF-α, IL-10, and IL-12 were produced by splenocytes upon stimulation with lipoteichoic acid and PMA in both groups (data not shown). Then, based on the potential role of TLRs signaling in the development and maintenance of a proper ENS, we analyzed the differential expression of transcripts encoding Tlr2 and Tlr4 between both experimental groups in the different intestinal segments. The proximal colon of CUS mice revealed an up regulation of Tlr2 mRNA (P < 0.01). Whereas both colonic segments of mice exposed to CUS showed a slight increase of Tlr4 mRNA (Fig. 6A). Together with the transcriptional changes induced by CUS exposure in the proximal colon of genes involved in ENS homeostasis, we wanted to examine whether an association with changes in Tlr2 expression in this segment could be observed. Interestingly, significant positive correlations were observed between all of them Table 2.
Figure 6.
Transcripts encoding Toll-like receptor 2 (Tlr2) are downregulated in the proximal colon of stressed mice and correlated with levels of transcripts encoding genes involved in enteric nervous system (ENS) function. Quantitative real-time polymerase chain reaction was used to analyze Tlr2 and Tlr4 expression levels in small intestine and colon of control and stressed mice (n = 10/group). Transcripts encoding Tlr2 were downregulated in the proximal colon of stressed mice. The increase expression levels of Tlr4 in proximal and distal colon of stressed mice did not reach statistical significance. Data represent mean ± SEM.**P < 0.01 by Mann Whitney test.
Table 2.
Pearson’s Correlation Analysis Between Tlr2 and Tlr4 Expression and Levels of Transcripts Encoding Enteric Nervous System-relevant Genes Involved in Motility in the Proximal Colon of Control and Stressed Mice
| Gene mRNA | Tlr2 mRNA | Tlr4 mRNA | |||
|---|---|---|---|---|---|
| r | P-value | r | P-value | ||
| Gfap | 0.55 | 0.002a | –0.15 | 0.564 | |
| Th | 0.77 | 0.010a | 0.03 | 0.893 | |
| Gdnf | 0.48 | 0.004a | 0.24 | 0.332 | |
| Bdnf | 0.67 | 0.003a | 0.08 | 0.745 | |
| Htr4 | –0.52 | 0.024a | 0.61 | 0.008a | |
Tlr, Toll-like receptor; Gfap, glial fibrillary acidic protein; Th, tyrosine hydroxylase; Gdnf, glial cell-derived neurotrophic factor; Bdnf, brain-derived neurotrophic factor; Htr4, serotonin receptor 4.
aP < 0.05 was considered statistically significant.
Stress-induced Changes in Neurotransmitter Levels Correlate With the Expression of Selective Genes Involved in Enteric Nervous System Homeostasis
Growing evidence highlights the critical role of neurotransmitters in the regulation of GI physiology and homeostasis maintenance. To support this idea, we analyzed the relationship in the proximal colon between amine content and the expression levels of all transcripts measured. Catecholamine content in the proximal colon of CUS inversely correlated with Gfap, Bdnf, and Tlr2 expression. In this colonic segment, we also found a significant negative correlation between 5-HT levels and Gdnf, Bdnf, and Tlr2 expression Table 3.
Table 3.
Correlation Between Neurotransmitters Concentration (Noradrenaline, Dopamine, and Serotonin) and Expression Levels of Transcripts Encoding Genes Involved in the Function of ENS in the Proximal Colon of Control and Stress Mice
| Gene mRNA | NA (ng/g) | DA (ng/g) | 5-HT (µg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |||
| Gfap | –0.54 | 0.048a | –0.41 | 0.143 | –0.48 | 0.065 | ||
| Gdnf | –0.45 | 0.092 | –0.31 | 0.253 | –0.53 | 0.034a | ||
| Bdnf | –0.57 | 0.026a | –0.49 | 0.067 | –0.70 | 0.003a | ||
| Htr4 | 0.16 | 0.576 | 0.43 | 0.105 | 0.31 | 0.245 | ||
| Tlr2 | –0.57 | 0.028a | –0.55 | 0.033a | –0.73 | 0.001a | ||
| Tlr4 | –0.06 | 0.843 | 0.47 | 0.088 | 0.14 | 0.614 | ||
NA, noradrenaline; DA, dopamine; 5-HT, serotonin; Gfap, glial fibrillary acidic protein; Gdnf, glial cell-derived neurotrophic factor; Bdnf, brain-derived neurotrophic factor; Htr4, serotonin receptor 4; Tlr, Toll-like receptor.
aP < 0.05 was considered statistically significant.
Discussion
In the present study, we provide novel molecular insights into stress-induced mechanisms evoking constipation and enteric neuroplasticity. First, in our chronic stress model, a slow gut transit is observed and accompanied by a decrease in fecal output without changes in fecal water content. Second, along the intestinal segments evaluated, chronic psychosocial stress caused a marked decrease in the expression of glial-specific molecular markers, Gfap and S100B, at the transcriptional and protein level in the proximal colon. Importantly, stressed mice exhibit also in the proximal colon a significant down-regulation of 2 neurotrophic factors; furthermore, the Gdnf expression reduction is in parallel with changes in transcription levels of Gfap. Again, only in the proximal colon of mice exposed to CUS, a significant increase in monoamines content rate compared with controls was observed. In addition, the increase of NA and DA levels is associated with a reduction in the expression of Th, the rate-limiting enzyme in catecholamines biosynthesis. However, despite the descriptive nature of this work, it could be considered as a potential constipation-predominant IBS model. Overall, our data reveal that CUS causes intestinal dysmotility related to transcriptional changes associated with the regulation of the ENS activity in an intestinal-region-specific manner. It is worth noting that, intestinal motility tests were done in both experimental groups without fasting and during the dark (active) cycle.
Emerging evidence shows that mood disorders are comorbid with functional constipation and constipation-predominant IBS. Moreover, neurological disorders are often associated with GI dysmotility,27,28 explained by the structural and neurochemical similarities that share ENS with CNS. It requires a proper network of enteric neuronal and glial cells with balanced neurotransmission for GI physiological function regulation.29,30
Chronic constipation is a multifactorial disorder with a complex pathogenesis that includes abnormalities within the ENS.31,32 In terms of animal models, different approaches have been used to provide a better understanding of the pathophysiology of constipation where changes in density and/or altered morphology of the ENS, as well as altered neurotransmission, have been found.33,34 To date, less is known about the mechanism by which chronic stress impacts normal enteric neurotransmission and both motor and sensory functions. Here, chronic stress-induced imbalance in neurotransmitters levels within the proximal colon with accompanying molecular changes that might compromise the ENS function.
Interestingly, our data show again that those mice exposed to CUS exhibited social anxiety-like behavior.23 Two animal models that resemble IBS-like features exhibited increased stress-induced neurochemical and ultrastructural changes in ENS in both the small intestine17 and distal colon,18 and an association between enteric glial cells (EGCs) activity and higher colonic contractility.18 Advances in enteric neurobiology support EGCs as important players in enteric neuroplasticity, neurotransmission,35 and mucosal immune response balance.28,36,37
Another interesting feature is their contribution to the regulation of motility patterns and their outstanding plasticity, although today remains unclear under which specific conditions they switch.38 Numerous reports show EGCs loss in colon biopsy specimens from patients with altered GI motility.20,28,39 In the present study, stressed mice exhibit different expression patterns of glial-specific molecular markers between the proximal colon and distal colon compared with controls, and changes in Gfap expression in the proximal colon are inversely correlated with the NA levels. Notably, ion transport in the caecum was not affected by chronic stress-related molecular changes in the ENS of the proximal colon.
Indeed, this result could explain that mice exposed to CUS exhibited reduced stool output without changes in water content. Although not shown in this study, in the presence of impaired intestinal transit, dysbiosis is the rule, what could determine the balance between absorption and secretion, but also cecal permeability, by multiple mechanisms, being one of the most frequent the increased levels of 5-HT and tryptamine that mediates colonic secretion through serotonin 4 receptor. Despite that we cannot associate the motility readouts with neurotransmitter concentration, increased levels of DA levels in the proximal colon might be the main factor, which explains CUS-induced intestinal dysmotility. Thus, it has been previously shown the inhibitory effect of dopamine on intestinal motility through its receptor D2.40
Of interest, preclinical studies have previously reported altered intestinal transit, whereas fecal water content41 or ion transport were unchanged after pharmacological disruption of EGCs function.42
NTFs as well as EGCs are important players in supporting neurotransmission and neuroplasticity,43 indeed colonic Bdnf modulates both intestinal motor and sensory functions.44,45 Our data suggest their role in the regulation of neurotransmission processes in the proximal colon as reflected by an inverse association between NTFs transcripts and total tissue levels of 5-HT and NA, although we do not find statistical differences in protein expression of pan-neuronal marker PGP 9.5 between groups.
The cellular and molecular pathways underlying the ENS organization and activity are unclear, yet recent studies suggest the potential role of TLR-mediated signalling.46-48 It is known that different TLRs are expressed in both CNS and ENS, regulating enteric neuronal plasticity and neurotransmission.49 Indeed, the absence of TLR2 and TLR4 signaling alters GI motility may, in part, results from their role in modulating the magnitude of motor response elicited by 5-HT in the proximal colon,50 enteric neurotransmission,47,48 and glial phenotype by regulating NTFs production. Furthermore, a significant reduction in Tlr2 expression is identified in the proximal colon of stressed mice concomitant with a reduction of Gfap, Gdnf, and Th expression, besides being associated with the tissue monoamine content.
Taken together, all these findings suggest a potential association between changes in neuroplasticity through Tlr2 and stress-induced slow intestinal transit.
Indeed, our laboratory has shown different TLRs expression profiles in colonic mucosa in 2 distinct stress animal models,51 besides in this CUS paradigm we have exhibited the contribution of TLR4-dependent mechanism to stress-induced visceral hypersensitivity through activation of microglia within the CNS.23 As well, other IBS stress-based rodent model advocated for the role of TLR4 signaling coupled with increased colonic glial activation in visceral pain.52 In this line, our study shows an increased of both Gfap and Tlr4 expression in the distal colon of stressed mice.
On the other hand, an enhanced expression of TLR2 and TLR4 on splenic macrophages has been shown in a model of repeated social defeat.53 Therefore, our group has also shown peripheral enhanced TLR4 activity associated with a visceral hyperalgesic phenotype in DIO mice.54 Our current results indicate heightened TLR4 activity of peripheral immune cells following CUS results in increased LPS-induced release of several pro-inflammatory cytokines. Finally, it is important to note that various animal models have proved how acute or repetitive exposure to homotypic stress, mediates intestinal pathology and psychiatric disorders.55-57
However, the predictive validity of those models remains disputed because in socially organized mammals, dominant stressors in natural conditions are frequently heterotypic and complex, and best represented by social experiences.58 The combination of social defeat and overcrowding that we used in this study reflects better the stress experienced by humans on a daily basis.
In addition, our study has several limitations: we did not conduct a metagenomics analysis of the intestinal microbiome to assess the impact of CUS in gut microbiota and its metabolic activity, so we cannot rule out the influence of microbiota on gut motility, ENS disruption, and secretion/absorption process through Tlr signaling; second, in our model, only male mice have been employed, and therefore, we recognize the potential sex-bias in our conclusions on the impact of chronic stress on colonic neuroplasticity and neurotransmission. Therefore, studies comparing responses between sexes are certainly warranted; third, gut motility and secretion/absorption are certainly regulated by multiple signaling pathways not analyzed in this study. Thus, further work is necessary to clarify whether other pathways participate in the regulation of gut motility in constipation.
Unfortunately, the present design was limited in exploring the relationship between stress anxiety-like behavior and the delay in GI transit time in the same cohort, as well the lack of functional characterizations analyses focused on EGCs and neurons are required in order to provide further insight into regional functional changes in motility.
Finally, further mechanistic studies are needed to determine how these transcriptional changes contribute to stress-induced visceral hypersensitivity and motility dysfunction. While our work does not provide a specific underlying mechanism, it offers an overview of how the effects of chronic stress could vary across GI tract segments. These findings might reflect the complex nature of the chronic psychological stress impact on intestinal motility. Thus, advancing our understanding of the mechanism(s) by which the ENS responds to stress serves to elucidate the biology supporting stress-related GI disorders, and requires further evaluation as therapeutic targets to counteract the consequences of chronic stress. This study would give new insights into stress-induced constipation, and further studies in constipation-predominant IBS are needed to investigate molecular changes related to the ENS integrity function.
Supplementary Materials
Note: To access the supplementary table, figures, and methods mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm22009.
Acknowledgements
We would like to thank Rachel D Moloney and Mr Patrick Fitzgerald for their technical assistance and Dr Bruno M Godinho for help with spleen stimulation studies.
Footnotes
Financial support: This study was supported in part by Fondo Europeo de Desarrollo Regional (FEDER), Fondo de Investigación Sanitaria, and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Instituto de Salud Carlos III, Subdirección General de Investigación Sanitaria, Ministerio de Economía y Competitividad: CM08/00229 and Beca Estada l’estranger (Societat Catalana Digestología) 2011 and PI19/01643 (Beatriz Lobo); PI17/01902 (Javier Santos) CIBEREHD (Javier Santos); Vall d’Hebron Institut de Recerca. FONDECYT #1181699 and #11121527; MECESUP 0608 Universidad de Chile (Caroll Beltran). The work described herein was supported by APC Microbiome Ireland, funded by Science Foundation Ireland (SFI), through the Irish Government’s National Development Plan. The authors and their work were supported by SFI (Grant No. 02/CE/B124, 07/CE/B1368, and SFI/12/RC/2273).
Conflicts of interest: The authors disclose the following: Javier Santos serves as a consultant for Noventure and discloses present or past recent scientific collaborations with Salvat, Norgine, Alfa-Sigma, Cosmo, Adare, Devintec Pharma, Pileje, and Danone. John F Cryan has been an invited speaker at meetings organized by Precision Biotics, Alkermes, and Janssen and has received research funding from Cremo, Nutricia, DuPont (IFF), and is a consultant for Alkermes and Nestlé. Timothy G Dinan has been an invited speaker at meetings organized by Servier, Lundbeck, Janssen, and AstraZeneca and has received research funding from Cremo, Nutricia. The remaining authors declare no competing interests. The study was carried out in APC Microbiome Institute Biosciences Building, University College Cork, Western Road, Cork, Ireland.
Author contributions: Beatriz Lobo conceived of the experiments; Beatriz Lobo, Mónica Tramullas, Beate-C Finger, Niall P Hyland, and John F Cryan supervised the experiments; Beatriz Lobo, Mónica Tramullas, Beate-C Finger, Kevin W Lomasney, and Caroll Beltran conducted the experiments; Beatriz Lobo wrote the initial draft of the manuscript; and Beatriz Lobo, Mónica Tramullas, Gerard Clarke, Javier Santos, John F Cryan, and Timothy G Dinan wrote subsequent drafts of the manuscript.
References
- 1.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vicario M, Guilarte M, Alonso C, et al. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav Immun. 2010;24:1166–1175. doi: 10.1016/j.bbi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Huerta-Franco MR, Vargas-Luna M, Tienda P, Delgadillo-Holtfort I, Balleza-Ordaz M, Flores-Hernandez C. Effects of occupational stress on the gastrointestinal tract. World J Gastrointest Pathophysiol. 2013;4:108–118. doi: 10.4291/wjgp.v4.i4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pigrau M, Rodiño-Janeiro BK, Casado-Bedmar M, Lobo B, Vicario M, Santos J, Alonso-Cotoner C. The joint power of sex and stress to modulate brain-gut-microbiota axis and intestinal barrier homeostasis: implications for irritable bowel syndrome. Neurogastroenterol Motil. 2016;28:463–486. doi: 10.1111/nmo.12717. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy PJ, Clarke G, O'Neill A, et al. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med. 2014;44:1553–1566. doi: 10.1017/S0033291713002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SH, Naliboff BD, Shih W, et al. Resilience is decreased in irritable bowel syndrome and associated with symptoms and cortisol response. Neurogastroenterol Motil. 2018;30:e13155. doi: 10.1111/nmo.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407. e5. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2021;160:99–114. e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Di Lorenzo C, Farrugia G, et al. Functional bowel disorders: a roadmap to guide the next generation of research. Gastroenterology. 2018;154:723–735. doi: 10.1053/j.gastro.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Salvo-Romero E, Martínez C, Lobo B, et al. Overexpression of corticotropin-releasing factor in intestinal mucosal eosinophils is associated with clinical severity in diarrhea-predominant irritable bowel syndrome. Sci Rep. 2020;10:20706. doi: 10.1038/s41598-020-77176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kano M, Muratsubaki T, Van Oudenhove L, et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7:12425. doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moloney RD, Johnson AC, O'Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF. Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther. 2016;22:102–117. doi: 10.1111/cns.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weltens N, Iven J, Van Oudenhove L, Kano M. The gut-brain axis in health neuroscience: implications for functional gastrointestinal disorders and appetite regulation. Ann N Y Acad Sci. 2018;1428:129–150. doi: 10.1111/nyas.13969. [DOI] [PubMed] [Google Scholar]
- 14.Tache Y, Larauche M, Yuan PQ, Million M. Brain and gut CRF signaling: biological actions and role in the gastrointestinal tract. Curr Mol Pharmacol. 2018;11:51–71. doi: 10.2174/1874467210666170224095741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal R, Debs LH, Patel AP, et al. Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol. 2017;232:2359–2372. doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujikawa Y, Tominaga K, Tanaka F, et al. Enteric glial cells are associated with stress-induced colonic hyper-contraction in maternally separated rats. Neurogastroenterol Motil. 2015;27:1010–1023. doi: 10.1111/nmo.12577. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Fei G, Fang X, et al. Changes in enteric neurons of small intestine in a rat model of irritable bowel syndrome with diarrhea. J Neurogastroenterol Motil. 2016;22:310–320. doi: 10.5056/jnm15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Song J, Bai T, Wang R, Hou X. Sustained pain hypersensitivity in the stressed colon: role of mast cell-derived nerve growth factor-mediated enteric synaptic plasticity. Neurogastroenterol Motil. 2018;30:e13430. doi: 10.1111/nmo.13430. [DOI] [PubMed] [Google Scholar]
- 19.Yu YB, Zuo XL, Zhao QJ, et al. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61:685–694. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]
- 20.Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. e4. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Sawicki CM, Humeidan ML, Sheridan JF. Neuroimmune interactions in pain and stress: an interdisciplinary approach. Neuroscientist. 2021;27:113–128. doi: 10.1177/1073858420914747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tramullas M, Dinan TG, Cryan JF. Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress. 2012;15:281–292. doi: 10.3109/10253890.2011.622816. [DOI] [PubMed] [Google Scholar]
- 23.Tramullas M, Finger BC, Moloney RD, et al. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry. 2014;76:340–348. doi: 10.1016/j.biopsych.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- 25.Klaus S, Rudolph B, Dohrmann C, Wehr R. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol Genomics. 2005;21:193–200. doi: 10.1152/physiolgenomics.00299.2004. [DOI] [PubMed] [Google Scholar]
- 26.Julio-Pieper M, O'Mahony CM, Clarke G, Bravo JA, Dinan TG, Cryan JF. Chronic stress-induced alterations in mouse colonic 5-HT and defecation responses are strain dependent. Stress. 2012;15:218–226. doi: 10.3109/10253890.2011.607524. [DOI] [PubMed] [Google Scholar]
- 27.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–528. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niesler B, Kuerten S, Demir IE, Schäfer KH. Disorders of the enteric nervous system-a holistic view. Nat Rev Gastroenterol Hepatol. 2021;18:393–410. doi: 10.1038/s41575-020-00385-2. [DOI] [PubMed] [Google Scholar]
- 29.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Lou J, Shan W, et al. Pathophysiologic role of neurotransmitters in digestive diseases. Front Physiol. 2021;12:567650. doi: 10.3389/fphys.2021.567650.1a49fea6b30e4fdb8328c43f352d4b99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharucha AE, Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020;158:1232–1249. e3. doi: 10.1053/j.gastro.2019.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassotti G, Villanacci V, Creţoiu D, Creţoiu SM, Becheanu G. Cellular and molecular basis of chronic constipation: taking the functional/idiopathic label out. World J Gastroenterol. 2013;19:4099–4105. doi: 10.3748/wjg.v19.i26.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang C, Wang KY, Yu Z, Xu B. Development of a novel mouse constipation model. World J Gastroenterol. 2016;22:2799–2810. doi: 10.3748/wjg.v22.i9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colucci M, Cervio M, Faniglione M, et al. Intestinal dysmotility and enteric neurochemical changes in a Parkinson's disease rat model. Auton Neurosci. 2012;169:77–86. doi: 10.1016/j.autneu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Boesmans W, Cirillo C, Van den Abbeel V, et al. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil. 2013;25:e151–e160. doi: 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- 36.Yoo BB, Mazmanian SK. The enteric network: interactions between the immune and nervous systems of the gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow AK, Gulbransen BD. Potential roles of enteric glia in bridging neuroimmune communication in the gut. Am J Physiol Gastrointest Liver Physiol. 2017;312:G145–G152. doi: 10.1152/ajpgi.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity phenotypic plasticity of glia cells in the mammalian enteric nervous system. Glia. 2015;63:229–241. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- 39.Ulmer TF, Rosch R, Mossdorf A, Alizai H, Binnebösel, Neumann U. Colonic wall changes in patients with diverticular disease-is there a predisposition for a complicated course? Int J Surg. 2014;12:426–431. doi: 10.1016/j.ijsu.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Auteri M, Zizzo MG, Amato A, Serio R. Dopamine induces inhibitory effects on the circular muscle contractility of mouse distal colon via D1- and D2-like receptors. J Physiol Biochem. 2016;73:395–404. doi: 10.1007/s13105-017-0566-0. [DOI] [PubMed] [Google Scholar]
- 41.Rao M, Rastelli D, Dong L, et al. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology. 2017;153:1068–1081. e7. doi: 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasser Y, Fernandez E, Keenan CM, et al. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol. 2006;291:G912–G927. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- 43.Liu S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol Motil. 2018;30:e13446. doi: 10.1111/nmo.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan X, Luo H, Fan H, et al. Brain-derived neurotrophic factor contributes to colonic hypermotility in a chronic stress rat model. Dig Dis Sci. 2015;60:2316–2326. doi: 10.1007/s10620-015-3695-8. [DOI] [PubMed] [Google Scholar]
- 45.Wang P, Du C, Chen FX, et al. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2016;6:20320. doi: 10.1038/srep20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarandi SS, Kulkarni S, Saha M, Sylvia KE, Sears CL, Pasricha PJ. Intestinal bacteria maintain adult enteric nervous system and nitrergic neurons via toll-like receptor 2-induced neurogenesis in mice. Gastroenterology. 2020;159:200–213. e8. doi: 10.1053/j.gastro.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brun P, Gobbo S, Caputi V, et al. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci. 2015;68:24–35. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Caputi V, Marsilio I, Cerantola S, et al. Toll-like receptor 4 modulates small intestine neuromuscular function through nitrergic and purinergic pathways. Front Pharmacol. 2017;8:350. doi: 10.3389/fphar.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forcén R, Latorre E, Pardo J, Alcalde AI, Murillo MD, Grasa L. Toll-like receptors 2 and 4 exert opposite effects on the contractile response induced by serotonin in mouse colon: role of serotonin receptors. Exp Physiol. 2016;101:1064–1074. doi: 10.1113/EP085668. [DOI] [PubMed] [Google Scholar]
- 51.McKernan DP, Nolan A, Brint EK, et al. Toll-like receptor mRNA expression is selectively increased in the colonic mucosa of two animal models relevant to irritable bowel syndrome. PLoS One. 2009;4:e8226. doi: 10.1371/journal.pone.0008226.0ee5709c37c64f76ac4beb5946299e1b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu S, Qin B, Shi A, Zhao J, Guo X, Dong L. Oxytocin inhibited stress induced visceral hypersensitivity, enteric glial cells activation, and release of proinflammatory cytokines in maternal separated rats. Eur J Pharmacol. 2018;818:578–584. doi: 10.1016/j.ejphar.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a toll-like receptor-dependent pathway. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1180–R1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- 54.Tramullas M, Finger BC, Dinan TG, Cryan JF. Obesity takes its toll on visceral pain: high-fat diet induces toll-like receptor 4-dependent visceral hypersensitivity. PLoS One. 2016;11:e0155367. doi: 10.1371/journal.pone.0155367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 56.Santos J, Benjamin M, Yang PC, Prior T, Perdue MH. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G847–G854. doi: 10.1152/ajpgi.2000.278.6.G847. [DOI] [PubMed] [Google Scholar]
- 57.Wilmes L, Collins JM, O'Riordan KJ, O'Mahony SM, Cryan JF, Clarke G. Of bowels, brain and behavior: a role for the gut microbiota in psychiatric comorbidities in irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14095. doi: 10.1111/nmo.14095. [DOI] [PubMed] [Google Scholar]
- 58.Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.