Abstract
Background/Aims
Patients with gastroesophageal reflux disease (GERD) frequently experience nighttime heartburn and sleep disturbance. Tegoprazan is a new potassium-competitive acid blocker that can rapidly block acid secretion. This study aims to evaluate the efficacy of tegoprazan compared with esomeprazole in relieving nighttime heartburn and sleep disturbances.
Methods
Patients with erosive esophagitis, nighttime heartburn, and sleep disturbances were randomized to receive tegoprazan 50 mg or esomeprazole 40 mg for 2 weeks. The primary endpoint was time to first nighttime heartburn-free interval. The percentage of nighttime heartburn-free days was also compared between the 2 groups.
Results
A total of 46 patients were enrolled in this study. Time to the first nighttime heartburn-free interval was shorter with tegoprazan than with esomeprazole but the difference was not statistically significant (1.5 days vs 3 days, P = 0.151). The percentage of nighttime heartburn-free days was higher in the tegoprazan group but the difference was insignificant (57.8% vs 43.1%, P = 0.107). Adverse events occurred in 2 patients. They were mild in severity.
Conclusions
Tegoprazan may induce faster relief of nighttime heartburn symptoms and may improve sleep disorders associated with nighttime heartburn. Further large-scale studies are required to validate our findings.
Keywords: Gastroesophageal reflux, Heartburn, Potassium channel blockers, Proton pump inhibitors, Sleep wake disorders
Introduction
Previous studies have suggested that patients with gastroesophageal reflux disease (GERD) frequently complain of heartburn at night.1,2 Patients with GERD symptoms have increased risks of difficulties in sleep induction, nightmares, and frequent awakenings.3 This may collectively lead to reduced production at work and during daily activities.4 Daytime drowsiness has been shown to increase the risk of cardiovascular mortality, especially in women.5 Nighttime reflux symptoms have been shown to increase the risk of esophageal adenocarcinoma, asthma, and obstructive sleep apnea syndrome.6,7
Reflux events during sleep are reported to be less frequent but last longer than during daytime.7 Reflux events can lead to sleep disturbance, negatively influencing reflux events.8 The frequency of swallowing, saliva production, and gastric emptying decreases during sleep, resulting in a prolonged acid contact time of refluxate during sleep.9 Increased severity or frequency of symptoms is associated with more comorbidities, decreased quality of life, reduced work productivity, impaired daily activities, and increased health care usage in GERD patients.8
Tegoprazan is a novel potassium-competitive acid blocker (PCAB) that shows rapid and effective anti-secretory effects by reversibly binding to the H+/K+-ATPase on the parietal cell.10 Animal and clinical pharmacology studies have demonstrated that tegoprazan exhibits its acid inhibitory effect more rapidly and for a more extended period than PPIs.11,12 The rapid and long-lasting effect of tegoprazan may be beneficial for patients experiencing symptoms due to nocturnal reflux.
In this study, we evaluate the efficacy of tegoprazan compared with esomeprazole for improving nocturnal symptoms and sleep disturbances. We hypothesize that rapid inhibition of gastric acid secretion by tegoprazan could lead to faster improvement of nighttime symptoms of GERD.
Materials and Methods
Study Design
This prospective, double-blind study was conducted from May 2020 to November 2021 at 4 university hospitals. The Institutional Review Board of each hospital approved this study as follows: Incheon St. Mary’s Hospital, The Catholic University College of Medicine (OC20MSDV0003), Kangdong Sacred Heart Hospital, Hallym University College of Medicine (KANGDONG2020-01-003), Chungnam National University School of Medicine (CNUH2020-01-038), and Severance Hospital, Yonsei University College of Medicine (4-2019-1342). It was registered at ClinicalTrials.gov (NCT04309916). The investigation proceeded according to the principles of the Declaration of Helsinki and under ethical guidance for clinical studies in Korea. All patients provided written informed consent before participating in the study.
Patients
We recruited patients with a history of symptomatic GERD within 3 months and GERD-related sleep disturbances. All patients received an endoscopy within 1 month. They were enrolled if erosive esophagitis was present. Patients were excluded for the following: unable to undergo upper endoscopy; unable to write daily symptom diaries; presence of esophageal stricture, peptic ulcer obstruction, esophageal varices, Barrett’s esophagus, eosinophilic esophagitis, active peptic ulcer, or bleeding during endoscopy; symptoms of primary or secondary esophageal movement disorders; planning to perform surgery or history of receiving surgery that could affect gastric acid secretion (ie, upper gastrectomy, vagotomy, etc); diagnosed with functional dyspepsia, primary esophageal motility disorder, irritable bowel syndrome, or inflammatory bowel disease within 3 months; any condition other than GERD that could be the primary cause of sleep disturbance; known hypersensitivity to antacids, proton pump inhibitors (PPIs) and PCABs; history of malignancy within 3 years; coexisting diseases; pregnancy or lactation; nightshift work; history of alcohol or drug abuse; anticipated travel beyond 3 time zones; human immunodeficiency virus; use of PPI 14 days before enrollment; unable to discontinue sleep medication, anti-depressants, or anti-anxiety medication during the study period; or at investigator’s discretion. All patients underwent a physical examination, including vital signs, body weight, and routine laboratory evaluation.
Protocol
Patients meeting the inclusion criteria underwent a run-in period of 1 week in which antacids were allowed as rescue medication. Patients who experienced nighttime heartburn for ≥ 3 days or reported sleep disturbances due to heartburn for ≥ 2 days during the run-in period were subjected to randomization. Patients who met all study criteria were randomized in a double-blind fashion to receive tegoprazan 50 mg daily with placebo or esomeprazole 40 mg with placebo for 2 weeks. Patients were advised to take their medication before bedtime. Antacids were permitted daily in patients with persistent symptoms despite their medication. On day 1 and week 2/final visit, patients underwent an investigator assessment for 2 questionnaires: the Korean gastrointestinal symptom rating scale (KGSRS) and the Korean version of the Epworth Sleepiness Scale (KESS).13,14 Diaries were accessed and reviewed at week 2/final visit to ensure compliance. Diaries were used during the screening and treatment periods to record the presence and severity of nocturnal heartburn symptoms. Patients were provided with diaries and trained to use daily logs on the first day of the screening period. Diary entries were completed once every morning upon waking. Patients graded the severity of nighttime heartburn according to the following 5-point scale: 0 = none, 1 = mild (occasional heartburn that could be ignored without influencing sleep), 2 = moderate (heartburn that could not be overlooked and occasionally affected sleep), 3 = severe (heartburn that was present most of the night and regularly affected sleep), and 4 = very severe (constant heartburn that markedly affected sleep). Patients rated the presence of GERD-related sleep disturbance on a 2-point scale of yes or no.
Additional secondary endpoints included changes in KGSRS and KESS scores from baseline to week 2. The KGSRS is a validated questionnaire that can discriminate symptom severity and frequency of patients with GERD.13 The questionnaire consists of 16 items combined into 5 symptom clusters depicting reflux, abdominal pain, indigestion, diarrhea, and constipation. The KESS is a validated questionnaire widely used to measure a patient’s sleepiness and the requirement for medical attention.14 The test consists of 8 situations in which one can rate the tendency to become asleep on a scale of 0 to 3. All treatment-emergent adverse events (TEAEs) which occurred after participants received the investigational product were collected. The investigator rated the severity of each adverse event (AE) and assessed its relationship to the study drug.
Study Endpoints
The primary endpoint was the time to the first nighttime heartburn-free interval. Heartburn severity was recorded during nighttime hours. The definition of time in days to the first nighttime heartburn-free interval was the total number of days from the first period to the beginning of the night period during which the heartburn symptom score equaled zero.
The secondary endpoint was the rate of nights without heartburn over 2 weeks and the rate of patients with relief of GERD-related sleep disturbances over the last 7 days of treatment. Improvement of sleep disturbance was defined as more than 6 out of 7 nights without sleep disturbance due to heartburn symptoms. We also compared differences in KGSRS and KESS scores after 2 weeks from baseline between treatment groups.
Safety was evaluated by TEAEs at week 2, physical examination, vital signs, and laboratory test results.
Statistical Methods
This study was designed as a pilot study to determine the effect of tegoprazan on nighttime heartburn. Thus, we have adopted the sample size of 12 per group rule.15 Based on this rule, we set the sample size to be 23 patients for each group.
Efficacy assessments were analyzed primarily in the full analysis set (FAS). Safety assessments were analyzed in the safety set. Baseline demographic characteristics were summarized with descriptive statistics. Continuous variables were compared by Student’s t test or Mann-Whitney test depending on whether the normality assumption was satisfied. Categorical variables were compared by chi-square or Fisher’s exact test depending on whether 20% or more of cells with an expected frequency of 5 or less were found. The time to complete resolution of heartburn was compared by log-rank test. Student’s t test compared the percentage of subjects with improved sleep disorders. Changes in KGSRS score and KESS score compared to baseline were compared by Student’s t test or Mann-Whitney test depending on whether the normality was satisfied. All data were analyzed using SPSS statistical program, version 22 (IBM Corp, Armonk, NY, USA) with the significance level set at P < 0.05.
Results
Patients
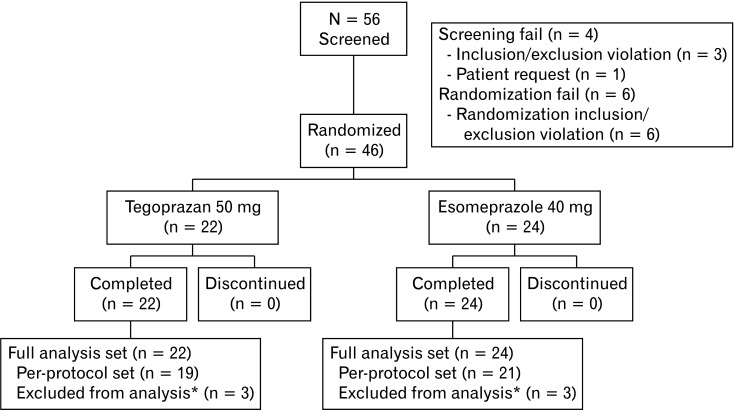
Fifty-six patients were screened and 46 were enrolled after the run-in period. These patients were included in the FAS. Three patients in each group were excluded due to major protocol violations (Fig. 1). Thus, the per-protocol set (PPS) included 19 in the tegoprazan group and 21 in the esomeprazole group. Patient demographics and baseline characteristics by treatment group are summarized in Table 1. The majority of patients were diagnosed with LA grade A esophagitis. There were no significant differences in demographics or baseline characteristics between treatment groups.
Figure 1.
Flow chart showing the selection of participant for this study. *Major protocol violation.
Table 1.
Baseline Characteristics of Patients
| Baseline characteristics | Tegoprazan (n = 22) |
Esomeprazole (n = 24) |
P-value |
|---|---|---|---|
| Age | 52.1 ± 15.5 | 52.5 ± 18.3 | 0.834a |
| Sex | |||
| Male | 11 (50.0) | 14 (58.3) | 0.571b |
| Female | 11 (50.0) | 10 (41.7) | |
| BMI (kg/m2) | 24.5 ± 4.1 | 23.9 ± 3.2 | 0.533c |
| Smoking | |||
| Yes | 3 (13.6) | 4 (16.7) | 1.000d |
| No | 19 (86.4) | 20 (83.3) | |
| Alcohol consumption | |||
| Yes | 9 (40.9) | 8 (33.3) | 0.595b |
| No | 13 (59.1) | 16 (66.7) | |
| LA grade | |||
| A | 18 (81.8) | 21 (87.5) | 0.491d |
| B | 3 (13.6) | 1 (4.2) | |
| C/D | 1 (4.6) | 2 (8.3) |
aMann-Whitney test.
bchi-square test.
cStudent’s t test.
dFisher’s exact test.
BMI, body mass index; LA, Los Angeles.
Data are presented as mean ± SD or number of patients (%).
Efficacy Outcomes
The efficacy outcomes of each group are summarized in Table 2. Time to first nighttime heartburn-free interval was shorter in patients receiving tegoprazan 50 mg daily than in those receiving esomeprazole 40 mg for both the FAS group (1.5 days vs 3.0 days, P = 0.151) and the PPS group (2.0 days vs 3.0 days, P = 0.301). However, their differences were not statistically significant. The percentage of nighttime heartburn-free days was higher in patients receiving tegoprazan 50 mg daily than in those receiving esomeprazole 40 mg for both the FAS (57.8% vs 43.1%, P = 0.107) and the PPS group (52.4% vs 42.8%, P = 0.321). However, such differences did not reach statistical significance. There was no difference in the percentage of subjects with improved sleep disorders due to nighttime heartburn within 7 days for both the FAS (54.6% vs 54.2%, P = 0.979) and the PPS group (47.4% vs 52.4%, P = 0.752). Patients in the tegoprazan group experienced an improvement in KGSRS scores for both the FAS group (–8.9 vs –5.2, P = 0.377) and the PPS group (–10.5 vs –7.9, P = 0.385). However, these differences were not statistically significant. The detailed scores of KGSRS are presented in Supplementary Table. KESS scores also improved in the tegoprazan group for both the FAS (–1.2 vs –0.5, P = 0.351) and the PPS group (–1.2 vs –0.5, P = 0.356) but did not reach statistical significance.
Table 2.
Summary of Efficacy Results
| Endpoint parameters | Tegoprazan | Esomeprazole | P-value |
|---|---|---|---|
| Time to first nighttime heartburn-free interval (day) | |||
| FAS | 1.5 (0-3.0) | 3.0 (0.5-8.0) | 0.151a |
| PPS | 2.0 (0-3.0) | 3.0 (1.0-7.0) | 0.301a |
| Percentage of nighttime heartburn-free days | |||
| FAS | 57.8 ± 30.3 | 43.1 ± 30.1 | 0.107b |
| PPS | 52.4 ± 28.7 | 42.8 ± 31.3 | 0.321b |
| Percentage of subjects with improved sleep disorders within 7 days | |||
| FAS | 54.6 (33.7-75.4) | 54.2 (34.2-74.1) | 0.979b |
| PPS | 47.4 (24.9-69.8) | 52.4 (31.0-73.7) | 0.752b |
| Change from baseline KGSRS score | |||
| FAS | –8.9 (–14.6-–3.1) | –5.2 (–10.9-0.5) | 0.377c |
| PPS | –10.5 (–17.2-–3.8) | –7.9 (–12.8-–3.0) | 0.385c |
| Change from baseline KESS score | |||
| FAS | –1.2 (–2.4-0.1) | –0.5 (–1.4-0.4) | 0.351b |
| PPS | –1.2 (–2.7-0.3) | –0.5 (–1.4-0.5) | 0.356b |
aLog rank test.
bStudent’s t test.
cMann-Whitney test.
FAS, full analysis set; PPS, per protocol set; KGSRS, Korean gastrointestinal symptom rating scale; KESS, Korean version of the Epworth Sleepiness Scale.
Data are presented as median of days (interquartile range), mean ± SD, or mean (95% CI).
Subgroup Analysis of Patients
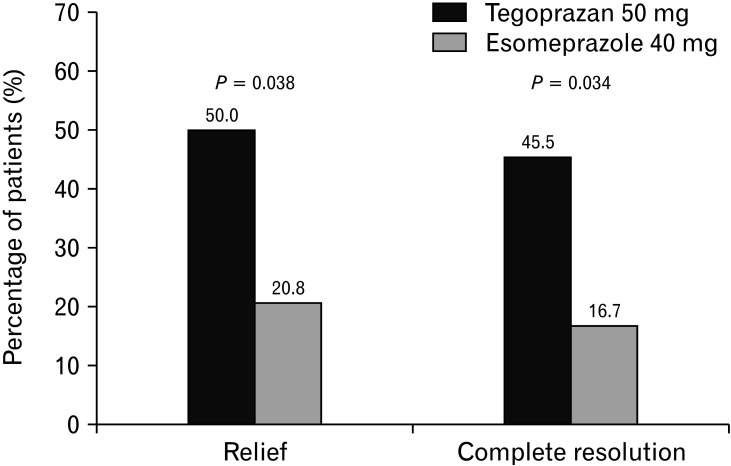
Subgroup analysis for the rate of subjects with complete resolution and relief of nighttime heartburn was performed for patients during the last 7 days of the study. Relief of nighttime heartburn was defined as a daily diary response of “none” on ≥ 6 of the previous 7 days of the study, allowing for 1 “mild” response. Complete resolution of heartburn was defined as a daily diary response of “none” for 7 consecutive days of the study. Significantly more patients receiving tegoprazan achieved complete resolution than those receiving esomeprazole for the FAS group (P = 0.038; Fig. 2). Also, more patients receiving tegoprazan achieved relief of nighttime heartburn compared to esomeprazole (P = 0.034; Fig. 2).
Figure 2.
Percentage of subjects with complete resolution and relief of nighttime heartburn in the full-analysis set.
Safety Analyses
AE occurred in 1 (4.6%) patient in the tegoprazan group and 1 (4.2%) patient in the esomeprazole group. These 2 patients who experienced AEs had events that were mild in severity. The AE in the tegoprazan group was considered irrelevant to the study drug. However, the AE in the esomeprazole group was deemed related to the study drug. There was no serious AE or death during the study.
Discussion
In our study, tegoprazan 50 mg daily showed faster symptom relief than esomeprazole 40 mg, as evidenced by the shorter time to the first nighttime heartburn-free interval. Tegoprazan was also more effective in improving nighttime heartburn symptoms, as evidenced by the higher percentage of nighttime heartburn-free days for 2 weeks. Results did not show statistical significance between the 2 groups due to the small number of patients enrolled. However, the results of this study should be a cornerstone for future studies. It is worth noting that patients enrolled in this study were diagnosed with erosive esophagitis by endoscopy. These inclusion criteria were used to restrict the inclusion of functional heartburn and reflux hypersensitivity patients in the study.
Tegoprazan is a novel PCAB approved for treating GERD, gastric ulcer, and Helicobacter pylori eradication in South Korea in 2018. Tegoprazan shows a rapid inhibition of acid secretion from the time of initial administration. Several experimental and clinical studies have demonstrated its sustained acid suppression.10-12 We hypothesize that these 2 qualities of tegoprazan may render it advantageous compared to PPIs in patients with nighttime heartburn.
The duration and quality of sleep are key components of general health and quality of life. Sleep disturbance leads to decreased sleep quality and various health-related conditions such as cognitive impairment, anxiety, depression, and an overall increase in mortality.16 Overlapping between GERD symptoms and sleep disturbances has been reported for a long time.17,18 Studies have demonstrated a bidirectional relationship between GERD and sleep, where GERD can lead to sleep deprivation. Sleep disturbance can lead to GERD, resulting in a vicious cycle.8 Therapies that improve GERD can also reduce sleep deprivation, and those that improve sleep can reduce the severity of GERD. PPIs are the mainstay of treatment for GERD. They can reduce nighttime reflux symptoms, leading to improved sleep disturbances.2,19-21 However, PPIs have some limitations for treating nighttime reflux symptoms. The maximal effect takes several days. Due to the slow onset of action, symptoms are not sufficiently relieved after the first dose of PPI.21,22 PPIs cannot completely block acid secretion at night, frequently resulting in nighttime heartburn symptoms.20,22,23
PCABs suppress acids with rapid onset and sustained action. This results in immediate heartburn relief, faster healing of erosive esophagitis, and enhanced inhibition of nighttime acid secretion than PPIs.24 PCABs may have advantages over PPIs in patients with nighttime heartburn due to their fast onset of action and potent inhibition of acid secretion.
To the best of our knowledge, only 1 study has examined the effects of vonoprazan in relieving nighttime heartburn compared with PPIs.25 That study enrolled 32 patients with reflux esophagitis and heartburn, and compared vonoprazan with lansoprazole to alleviate heartburn symptoms. The authors reported that significantly more patients achieved complete nocturnal heartburn relief with vonoprazan than with lansoprazole.
Currently, studies comparing tegoprazan with PPIs for treating nocturnal heartburn have not been reported yet. A randomized study of healthy subjects reported that tegoprazan 50 mg resulted in a rapid, potent, and sustained night-time acid suppression than vonoprazan 20 mg or esomeprazole 40 mg.26 Although, we were not able to achieve statistical significance, tegoprazan achieved shorter interval to first nighttime heartburn-free days and the percentage of nighttime heartburn-free days was higher compared to PPIs. Subgroup analysis revealed that significantly more patients achieved complete resolution and relief of nighttime heartburn symptoms. These results suggest that rapid onset of tegoprazan can result in faster relief of nighttime symptoms and that such effect is sustained over 2 weeks.
Our study has some limitations. First, a small number of patients were enrolled in our study, which prevented us from confirming statistical significance. Based on our pilot study, 60 patients need to be registered in each group to ensure a shorter duration of nighttime symptom relief for tegoprazan compared with esomeprazole. We plan to conduct a more extensive prospective study to confirm this in the future. Our study did prove that the percentage of subjects with complete resolution of nighttime heartburn was higher in the tegoprazan group. Another limitation of our study was that we did not assess the effects of the 2 medications in improving daily symptoms as we focused on enhancing nighttime symptoms.
In conclusion, tegoprazan of 50 mg once daily might be more effective than esomeprazole of 40 mg in terms of more rapid and sustained relief of nighttime heartburn symptoms. This may improve sleep quality and life quality in patients who experience nighttime heartburn.
Supplementary Material
Note: To access the supplementary table mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm22104.
Footnotes
Financial support: This study was funded in full by HK inno.N Corp, Seoul, Korea (Study No. KCAB_002).
Conflicts of interest: None.
Author contributions: Sang Kil Lee is guarantor of the article and designed the research; Joon Sung Kim, Seung In Seo, and Sun Hyung Kang contributed to the design of the study; all authors performed the research, collected and analyzed the data; Joon Sung Kim wrote the paper; Sang Kil Lee, Seung In Seo, and Sung Hyung Kang contributed to the revision of the manuscript; and all authors approved the final version of the manuscript including the authorship list.
References
- 1.Farup C, Kleinman L, Sloan S, et al. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch Intern Med. 2001;161:45–52. doi: 10.1001/archinte.161.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Shaker R, Castell DO, Schoenfeld PS, Spechler SJ. Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: the results of a gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol. 2003;98:1487–1493. doi: 10.1111/j.1572-0241.2003.07531.x. [DOI] [PubMed] [Google Scholar]
- 3.Janson C, Gislason T, De Backer W, et al. Prevalence of sleep disturbances among young adults in three European countries. Sleep. 1995;18:589–597. doi: 10.1093/sleep/18.7.589. [DOI] [PubMed] [Google Scholar]
- 4.Wahlqvist P, Carlsson J, Stålhammar NO, Wiklund I. Validity of a work productivity and activity impairment questionnaire for patients with symptoms of gastroesophageal reflux disease (WPAI-GERD)--results from a cross-sectional study. Value Health. 2002;5:106–113. doi: 10.1046/j.1524-4733.2002.52101.x. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The cardiovascular health study research group. J Am Geriatr Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 6.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 7.Jaspersen D. Extra-esophageal disorders in gastroesophageal reflux disease. Dig Dis. 2004;22:115–119. doi: 10.1159/000080309. [DOI] [PubMed] [Google Scholar]
- 8.Kurin M, Shibli F, Kitayama Y, Kim Y, Fass R. Sorting out the relationship between gastroesophageal reflux disease and sleep. Curr Gastroenterol Rep. 2021;23:15. doi: 10.1007/s11894-021-00815-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen CL, Robert JJ, Orr WC. Sleep symptoms and gastroesophageal reflux. J Clin Gastroenterol. 2008;42:13–17. doi: 10.1097/MCG.0b013e31802fc1bc. [DOI] [PubMed] [Google Scholar]
- 10.Oshima T, Miwa H. Potent potassium-competitive acid blockers: a new era for the treatment of acid-related diseases. J Neurogastroenterol Motil. 2018;24:334–344. doi: 10.5056/jnm18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi N, Take Y. Tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther. 2018;364:275–286. doi: 10.1124/jpet.117.244202. [DOI] [PubMed] [Google Scholar]
- 12.Han S, Choi HY, Kim YH, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50:751–759. doi: 10.1111/apt.15438. [DOI] [PubMed] [Google Scholar]
- 13.Kwon S, Jung JK, Hong JH, Park HS. Diagnostic validity of the Korean gastrointestinal symptom rating scale (KGSRS) in the assessment of gastro-esopahgeal reflux disease. Ewha Med J. 2008;31:73–80. doi: 10.12771/emj.2008.31.2.73. [DOI] [Google Scholar]
- 14.Cho YW, Lee JH, Son HK, Lee SH, Shin C, Johns MW. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath. 2011;15:377–384. doi: 10.1007/s11325-010-0343-6. [DOI] [PubMed] [Google Scholar]
- 15.Friede T, Kieser M. Sample size recalculation in internal pilot study designs: a review. Biom J. 2006;48:537–555. doi: 10.1002/bimj.200510238. [DOI] [PubMed] [Google Scholar]
- 16.St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the american heart association. Circulation. 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson C, Nordenstedt H, Wallander MA, et al. A population-based study showing an association between gastroesophageal reflux disease and sleep problems. Clin Gastroenterol Hepatol. 2009;7:960–965. doi: 10.1016/j.cgh.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Orr WC. Gastrointestinal functioning during sleep: a new horizon in sleep medicine. Sleep Med Rev. 2001;5:91–101. doi: 10.1053/smrv.2000.0149. [DOI] [PubMed] [Google Scholar]
- 19.Chand N, Johnson DA, Tabangin M, Ware JC. Sleep dysfunction in patients with gastro-oesophageal reflux disease: prevalence and response to GERD therapy, a pilot study. Aliment Pharmacol Ther. 2004;20:969–974. doi: 10.1111/j.1365-2036.2004.02213.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DA, Katz PO. Nocturnal gastroesophageal reflux disease: issues, implications, and management strategies. Rev Gastroenterol Disord. 2008;8:98–108. [PubMed] [Google Scholar]
- 21.Rackoff A, Agrawal A, Hila A, Mainie I, Tutuian R, Castell DO. Histamine-2 receptor antagonists at night improve gastroesophageal reflux disease symptoms for patients on proton pump inhibitor therapy. Dis Esophagus. 2005;18:370–373. doi: 10.1111/j.1442-2050.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 22.Katz PO, Castell DO, Chen Y, Andersson T, Sostek MB. Intragastric acid suppression and pharmacokinetics of twice-daily esomeprazole: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2004;20:399–406. doi: 10.1111/j.1365-2036.2004.02079.x. [DOI] [PubMed] [Google Scholar]
- 23.Sachs G, Shin JM, Hunt R. Novel approaches to inhibition of gastric acid secretion. Curr Gastroenterol Rep. 2010;12:437–447. doi: 10.1007/s11894-010-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibli F, Kitayama Y, Fass R. Novel therapies for gastroesophageal reflux disease: beyond proton pump inhibitors. Curr Gastroenterol Rep. 2020;22:16. doi: 10.1007/s11894-020-0753-y. [DOI] [PubMed] [Google Scholar]
- 25.Oshima T, Arai E, Taki M, et al. Randomised clinical trial: vonoprazan versus lansoprazole for the initial relief of heartburn in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49:140–146. doi: 10.1111/apt.15062. [DOI] [PubMed] [Google Scholar]
- 26.Yang E, Kim S, Kim B, et al. Night-time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol. 2022;88:3288–3296. doi: 10.1111/bcp.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.