Abstract
Antimicrobial-resistance (AMR) genes can be transferred between microbial cells via horizontal gene transfer (HGT), which involves mobile and integrative elements such as plasmids, bacteriophages, transposons, integrons and pathogenicity islands. Bacteriophages are found in abundance in the microbial world, but their role in virulence and AMR has not fully been elucidated in the Enterobacterales . With short-read sequencing paving the way to systematic high-throughput AMR gene detection, long-read sequencing technologies now enable us to establish how such genes are structurally connected into meaningful genomic units, raising questions about how they might cooperate to achieve their biological function. Here, we describe a novel ~98 kbp circular P1-bacteriophage-like plasmid termed ph681355 isolated from a clinical Salmonella enterica serovar Typhi isolate. It carries bla CTX-M-15, an IncY plasmid replicon (repY gene) and the ISEcP1 mobile element and is, to our knowledge, the first reported P1-bacteriophage-like plasmid (phage-plasmid) in S . enterica Typhi. We compared ph681355 to two previously described phage-plasmids, pSJ46 from S . enterica serovar Indiana and pMCR-1-P3 from Escherichia coli , and found high nucleotide similarity across the backbone. However, we saw low ph681355 backbone similarity to plasmid p60006 associated with the extensively drug-resistant S . enterica Typhi outbreak isolate in Pakistan, providing evidence of an alternative route for bla CTX-M-15 transmission. Our discovery highlights the importance of utilizing long-read sequencing in interrogating bacterial genomic architecture to fully understand AMR mechanisms and their clinical relevance. It also raises questions regarding how widespread bacteriophage-mediated HGT might be, suggesting that the resulting genomic plasticity might be higher than previously thought.
Keywords: bacteriophage, Illumina, Nanopore, phage-plasmid, plasmid, Salmonella enterica serovar Typhi
Data Summary
All fastq files and assemblies were submitted to the National Center for Biotechnology Information (NCBI). All data can be found under BioProject PRJNA248792 – https://www.ncbi.nlm.nih.gov/bioproject/PRJNA248792. Strain-specific details can be found in Methods under data deposition.
Impact Statement.
Whole-genome sequencing has revolutionized the way we identify and characterize antimicrobial-resistance (AMR) genes and regions/elements in pathogens. Illumina sequencing coupled with Nanopore sequencing and careful data curation allows the mining of pathogen genomes to detect, characterize and track novel mobile elements involved in AMR transmission. In this study, we have identified a circular P1-bacteriophage-like plasmid (termed phage-plasmid) harbouring a bla CTX-M-15 gene conferring extended-spectrum β-lactamase resistance in Salmonella enterica serovar Typhi. It is the first time, to our knowledge, that such a DNA element has been described in this organism. There is increasing evidence from the literature to show that the horizontal spread of AMR genes mediated by bacteriophages and bacteriophage-like plasmid elements is much more common than previously envisioned. This current study shows the potential ability of using Nanopore sequencing for the detection and characterization of these elements, highlighting the importance of including long-read sequence data for the screening and surveillance of mechanisms involved in AMR transmission. Understanding AMR carriage and transmission patterns provides information to support appropriate clinical management and inform implementation of public-health control measures.
Introduction
Salmonella enterica subspecies enterica serovar Typhi is the causative agent of typhoid fever and is associated with an estimated 11 million infections and 116 000 deaths globally each year [1]. The majority of this disease burden is concentrated in South Asia and other low to middle income countries (LMICs) [1].
Growing rates and spread of antimicrobial resistance (AMR) pose a threat to the effective empirical treatment and control of typhoid fever. Third-generation cephalosporins (extended spectrum β-lactams) are frequently used in the treatment of typhoid fever. The emergence and spread of extensively drug-resistant (XDR) S . enterica Typhi in Pakistan, and now globally, is a public-health concern as it has left azithromycin (one of the last oral antibiotics available) and meropenem (carbapenem) as the only options available for treatment [2–8] (https://emergency.cdc.gov/han/2021/han00439.asp).
Resistance to cephalosporins is principally mediated by acquisition of a plasmid carrying extended-spectrum β-lactamase (ESBL) genes, the most prevalent of which are the CTX-M type ESBLs [9]. Recently bla CTX-M-15 and bla CTX-M-55 present on a transmissible ~84 kbp IncY incompatibility group plasmid (p60006) were shown to be associated with extended-spectrum β-lactam resistance in XDR outbreak S . enterica Typhi isolates from Pakistan [2, 3]. We are also now observing chromosomal integration of ESBL genes and the loss of the IncY plasmid in these XDR S . enterica Typhi isolates, demonstrating evolution of the AMR drug region throughout the outbreak [3].
Mobile and integrative genetic elements, including plasmids, bacteriophages, transposons, integrons and pathogenic islands, are important vehicles of horizontal gene transfer (HGT) enabling transmission of genetic information between bacteria [10]. Plasmids are considered the most common and important genetic element to spread ESBLs between bacterial strains, but studies have shown the mobilization or transfer of AMR genes by bacteriophages in various bacterial species, including Escherichia coli and non-typhoidal Salmonella (NTS) [11–15], and reported a mcr-1 gene in E. coli and a bla CTX-M-27 gene in NTS present on bacteriophage-like IncY elements (phage-plasmid) that were 97 and 104 kbp in size, respectively. In another report [13], a 115 kbp circular P1-like bacteriophage harbouring a bla SHV-12 element in E. coli was characterized. P1-like bacteriophages are known to replicate in their host as independent low copy number plasmid-like elements [13].
Recent advances in sequencing technologies, especially long-read sequencing, now enable us to identify and characterize novel bacteriophage/plasmid-like elements, as well as look at their genetic diversity [16–18]. Long-read sequencing has also enabled the ability to characterize the genetic architecture of individual mobile integrative elements harbouring AMR determinants [19–23], which is essential in order to characterize the precise biological mechanisms underpinning HGT mechanisms.
In this study, we describe a circular P1-bacteriophage-like plasmid (phage-plasmid) harbouring a bla CTX-M-15 gene and IncY plasmid replicon (repY) isolated from a clinical S . enterica Typhi isolate from a traveller returning to the UK from Iraq. To the best of our knowledge, this is the first time such a genomic element, and the implications of its presence towards AMR acquisition and maintenance, have been described for S . enterica Typhi. That is especially relevant due to the current heavy burden of the disease, and the pathogenic potential that S . enterica Typhi strains carrying AMR might present in the future.
Methods
Strain selection and details
A laboratory-confirmed S . enterica Typhi isolate termed 681 355 was referred to the Gastrointestinal Bacterial Reference Unit (GBRU), UK Health Security Agency (UKHSA) [formally Public Health England (PHE)] in January 2019. This isolate was from a traveller returning to the UK from Iraq. Epidemiological information and phylogenetic analysis of this isolate have previously been described by Godbole et al. [24]. Ethical approval for the detection of gastrointestinal bacterial pathogens from faecal specimens, or the identification, characterization and typing of cultures of gastrointestinal pathogens, submitted to GBRU is not required as it is covered by UKHSA’s surveillance mandate.
Antimicrobial-susceptibility testing
Antimicrobial-susceptibility testing was performed on this isolate as described by Chattaway et al. [25]. Minimum inhibitory concentrations (MICs) were determined by agar dilution using Mueller–Hinton agar for the standard panel of antibiotics recommended for Salmonella spp. by the European Committee on Antimicrobial Susceptibility Testing (EUCAST); breakpoints and screening concentration criteria were used for interpretation of results as described by EUCAST (2020; https://www.eucast.org/).
DNA extraction, library preparation, Illumina sequencing and data processing
Genomic DNA was extracted from S . enterica Typhi culture using the QIAsymphony system (Qiagen). The sequencing library was prepared using the Nextera XP kit (Illumina) for sequencing on the HiSeq 2500 instrument (Illumina), run with the fast protocol. fastq reads were processed using Trimmomatic v0.27 [26] to remove bases with a Phred score of <30 from the leading and trailing ends, with reads <50 bp after quality trimming discarded.
Genotyping and in silico AMR typing
Sequence type (ST) and serovar were determined from reads using most (v1.0) as described by Tewolde et al. [27] and eBURST group (eBG) as described by Achtman et al. [28].
Resistance genes for the S . enterica Typhi isolate used in the study were detected using GeneFinder (https://github.com/phe-bioinformatics/gene_finder), a customized algorithm that uses Bowtie2 (v2.3.5.1) [29] to align reads to a set of reference sequences, and SAMtools (v1.8) [30], to generate an mpileup file, as previously described [31]. Briefly, the data are parsed based on read coverage of the query sequence (100 %), consensus base-call on variation (>85 %) and the nucleotide identity (>90 %) to determine the presence of the reference sequence or nucleotide variation within that sequence. β-Lactamase variants were determined with 100 % identity using the reference sequences downloaded from ResFinder [32] or the National Center for Biotechnology Information (NCBI) β-lactamase data resources (https://www.ncbi.nlm.nih.gov/pathogens/beta-lactamase-data-resources). Known acquired resistance genes and resistance-conferring mutations relevant to β-lactams, fluroquinolones, aminoglycosides, chloramphenicol, macrolides, sulphonamides, tetracyclines, trimethoprim, rifamycins and fosfomycin were included in the analysis [33, 34].
DNA extraction, library preparation, Nanopore sequencing and data processing
High-molecular mass DNA was extracted from S . enterica Typhi isolate 681 355 using the Fire Monkey HMW DNA extraction kit (RevoluGen) and DNA concentration was determined via Qubit (Thermofisher Scientific), as previously described [23]. Library preparation was performed using the rapid barcoding kit (SQK-RBK004) (Oxford Nanopore Technologies). The prepared library was loaded onto a FLO-MIN106 R9.4.1 flow cell (Oxford Nanopore Technologies) and sequenced using the MinION system (Oxford Nanopore Technologies) for 72 h.
Data produced in a raw FAST5 format was basecalled using Guppy v3.2.6 Fast model (Oxford Nanopore Technologies) into fastq format. Read de-multiplexing, quality control, trimming and filtering were completed as described elsewhere [35] with the only modification being bases=490 Mbp, to generate approximately 100× coverage of a Salmonella genome (approximately 4.9 Mbp).
De novo assembly, correction, re-orientation and annotation
The filtered Nanopore fastq file with the 100× coverage of longest reads was assembled using Flye v2.8 [36] with default parameters enabled. Correction (polishing) of the assembly was performed in a modified two-step process described previously [35, 37]. Firstly, Pilon v1.22 [38] was used with Illumina fastq reads as the query dataset with the use of bwa v0.7.17 [39] and SAMtools v1.7 [30]. Secondly, Racon v1.3.3, [40] also using bwa v0.7.17 [39] and SAMtools v1.7 [30], was used again with the Illumina fastq reads. As the chromosome was circular and closed, it was re-orientated to start at the dnaA gene (GenBank accession no. NC_000913) from E. coli K-12, using the --fixstart parameter in Circlator v1.5.5 [41]. Prokka v1.13 [42] was used to annotate the final assembly.
In silico plasmid typing and comparison of ph681355 and replicon to publicly available sequences
The plasmid replicon was identified for each non-chromosomal contig within the final assembly using PlasmidFinder v2.1 [43] with the Enterobacteriaceae , minimum identity=90 % and minimum coverage=90 % parameters set. brig [44] was used to compare ph681355 to the bla CTX-M-27 S . enterica serovar Indiana Chinese SJ46 phage-plasmid (GenBank accession no. NC_031129), E. coli bacteriophage P1 (accession no. AF234172), the mcr-1 E. coli phage-plasmid (accession no. KX880944) and plasmid p60006 (accession no. LT906492) isolated from a Pakistan XDR S . enterica Typhi outbreak isolate that also harboured bla CTX-M-15. Parameters used included -perc_identity=90 and -e value=1×10-10. The coding sequences (CDSs) were annotated using Prokka v1.13 [42] as stated in the previous section, with AF234172 acting as a reference for CDS and gene annotation. The repY genes from the above plasmids were compared to the repY gene from ph681355 also using blastn [45].
Detection and characterization of ph681355 structural variation
To determine whether multiple isoforms were present within the Nanopore reads, the Nanopore fastq reads for the sample were aligned to the finalized assembly using Minimap2 v2.17 [46] and SAMtools v0.7.17 [30]. The alignment was visualized using Integrative Genomics Viewer (igv) v2.12.3 [47] and the breakpoints of each isoform were identified. Once breakpoints were identified relative to each isoform, those positions were used with SAMtools v0.7.17 [30] to isolate reads that aligned and spanned across both ends of each breakpoint (i.e. spanned the homologous region in question). Any reads that aligned across a given set of breakpoints had to share the same size as it existed in the fastq file, and not be clipped within the alignment, to be considered. From here, the relative proportions of reads aligning to each isoform were calculated.
Data deposition
Illumina and Nanopore fastq files and polished assembly for S . enterica Typhi isolate 681 355 are available from the NCBI under BioProject PRJNA248792. The SRA (sequence read archive) accession numbers are as follows: Illumina fastq – SRR8554071; Nanopore fastq – SRR16296518. The GenBank accession numbers are CP083411 for the chromosome and CP083412 for ph681355 (phage-plasmid).
Results
Sample 681355 genome statistics and genotyping
Isolate 681 355 was confirmed to be S . enterica Typhi ST1, a member of serovar Typhi eBURST group 13 (eBG13). Previous phylogenetic analysis confirmed that this strain sat within the dominant global H58 haplotype, but it did not cluster with the recent XDR S . enterica Typhi outbreak strains in Pakistan [2, 24]. Nanopore sequencing and processing produced a final genome of two contigs, one chromosome (4 782 729 kbp) and one of 98 174 kbp (ph681355).
Phenotypic and genotypic resistance to extended-spectrum β-lactam
S . enterica Typhi isolate 681 355 was found to have minimum inhibitory concentrations (MICs) to the following antimicrobials (in µg µl−1): amoxicillin [>128 (R)], ciprofloxacin [0.06 (R)], ceftriaxone [>64 (R)], cotrimoxazole [1 (S)], ertapenem [0.25 (S)] and azithromycin [<2 (S)] as observed by Chattaway et al. [25]. Genotypic mapping of AMR determinants did not reveal the presence of carbapenem, fosfomycin and azithromycin resistance, but showed the presence of bla CTX-M-15, a point mutation in gyrA [83:S-F] and IncY plasmid replicon (repY) genes.
Characterization and comparison of ph681355
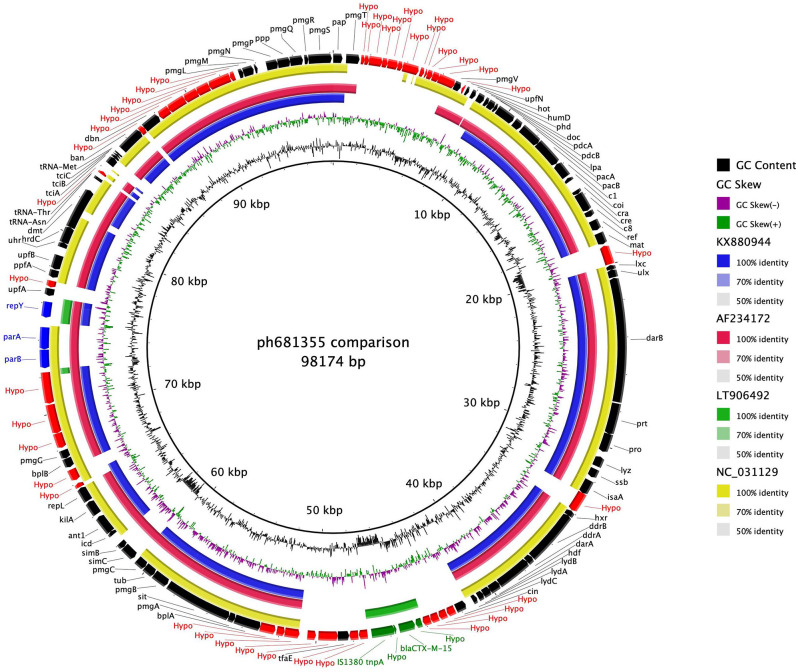
ph681355 is ~98 kbp in size. The ph681355 contig was annotated to contain 118 CDSs, of which 8 (7 %) are maintenance genes (Fig. 1). A 3.5 kbp ISEcpl-bla CTX-M-15-tnpA gene resistance cassette was confirmed to be present on ph681355. Plasmid typing using PlasmidFinder determined the replicon type to be IncY plasmid (repY) (Fig. 1). The repY gene from ph681355 matched bacteriophage P1 (GenBank accession no. AF234172), phage-plasmid PMCR-1-P3 (accession no. KX880944) and plasmid p60006 (accession no. LT906492) repY genes 100, 88 and 100 % at the nucleotide level, respectively. Notably, repY is absent from phage-plasmid SJ46 (NC_031129) from the S . enterica serovar Indiana isolate from China.
Fig. 1.
brig plot of ph681355 as the reference versus PMCR-1-P3 phage-plasmid shown as the blue ring (GenBank accession no. KX880944) – E coli plasmid; bacteriophage P1 as the red ring (accession no. AF234172); plasmid p60006 as the green ring (accession no. LT906492) – S . enterica Typhi; and finally SJ46 phage-plasmid (accession no. NC_031129) shown as the yellow ring. Also shown are CDSs (genes) on the outer ring, with green showing AMR cassette, blue showing plasmid maintenance genes, black showing bacteriophage-associated genes and red showing hypothetical proteins.
Sequence analysis of ph681355 compared to publicly available P1 bacteriophage from E. coli (AF234172), phage-plasmids SJ46 from S . enterica serovar Indiana (NC_031129) and PMCR-1-P3 from E. coli (KX880944) showed 79.7, 78.0 and 78.5% nucleotide similarity, respectively, across the sequenced region (Fig. 1). Hence, ph681355 (like SJ46 and PMCR-1-P3) was characterized as a chimeric element termed as phage-plasmid (phage and bacterial genes present). The main region of variation was the absence of the 3.5 kbp ISEcpl-bla CTX-M-15-tnpA resistance gene cassette in bacteriophage P1, phage-plasmids SJ46 and PMCR-1-P3 (Fig. 1).
There was only a 4.9 % sequence similarity between ~98 kbp phage-plasmid ph681355 and the ~84 kbp plasmid p60006 (LT906492), although the repY plasmid replicon gene and ISEcpl-bla CTX-M-15-tnpA resistance cassette were present in both (Fig. 1). Our analysis shows that the ESBL resistance is carried by the same 3.5 kbp ISEcpl-bla CTX-M-15-tnpA resistance gene cassette but that different mechanisms are involved in the transmission of ESBL resistance.
Detection and characterization of structural variation on ph681355
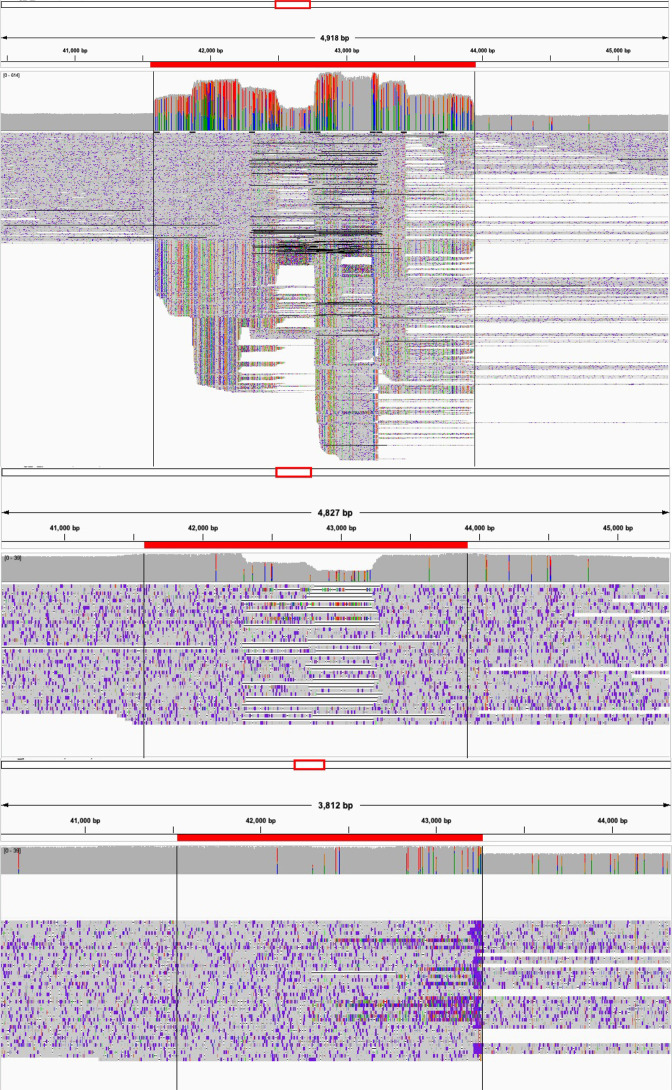
When confirming the validity of the ph681355 contig, it was noted that some Nanopore reads were clipped in the alignment at the same loci as the mobile genetic element in which the bla CTX-M-15 gene is located (Fig. 2). Artificial (in silico) removal of the bla CTX-M-15 mobile genetic element also showed Nanopore reads aligning across this region with the absence of clipping (Fig. 2). This suggests that there are two isoforms of the ph681355 within the single Nanopore read set, one set (approximately 40 % of reads) confirming the presence of the mobile genetic element within ph681355 and a second set (approximately 60 %) aligning correctly (with no clipping) suggesting the absence of the mobile genetic element from ph681355.
Fig. 2.
Integrative Genomics Viewer (igv) visualization of the alignment of reads for the region of ph681355 where the bla CTX-M-15 is located (top). Also showing the same alignment with only reads spanning across the bla CTX-M-15 mobile genetic element (middle). Finally, showing the alignment of reads with the bla CTX-M-15 mobile genetic element removed in silico (bottom). The red line above each part of the figure (top, middle, bottom) indicates the tnpA-Hypo-bla-Hypo element.
Discussion
The transmission of ESBL resistance is increasing in Enterobacterales mainly due to the presence of bla CTX-M and bla SHV class genes [3, 13–15, 48, 49], and highlighted by the recent XDR S . enterica Typhi outbreak isolates in Pakistan that have subsequently spread globally [2, 3, 25, 50]. The bla CTX-M-15 gene responsible for ESBL resistance was present on a ~84 kbp IncY plasmid (p60006). Nair et al. [3] showed the rapid evolution of bla CTX-M-15 resistance mechanisms with the loss of the plasmid and the integration of bla CTX-M-15 into various regions of the chromosome of XDR S . enterica Typhi isolates.
In this study, a S . enterica Typhi isolate (681355) from a patient returning from Iraq had a bla CTX-M-15 gene present on an extrachromosomal element harbouring a plasmid replication repY gene. Phylogenetic analysis conducted by Godbole et al. [24] showed that this Iraqi S . enterica Typhi strain belonged to the global H58 haplotype but did not cluster with the XDR S . enterica Typhi outbreak Pakistani isolates [2].
Using Nanopore sequencing data, we identified a ~98 kbp extrachromosomal element (ph681355) that was ~14 kbp larger than plasmid p60006 described in the XDR S . enterica Typhi outbreak isolates from Pakistan [2]. ph681355 shared only the repY gene and the 3.5 kbp ISEcpl-bla CTX-M-15-tnpA gene cassette with p60006 (Fig. 1).
Multiple modes for the rapid transmission of AMR and virulence genes involving mobile genetic elements have been widely described [51, 52]. More recently, studies have used long-read sequence data to demonstrate the involvement of P1- and P7-like bacteriophage elements in AMR gene transfer [13–15]. These bacteriophage-like elements (phage-plasmid) have both the bacteriophage-like lytic replication (repL) and plasmid replication (rep) genes, which we observed in ph681355 [13–15] (Fig. 1).
However, a phage-plasmid had only been described once in S . enterica serovar Indiana [14] and, hence, to our knowledge ph681355 is the first one described in S . enterica Typhi. ph681355 is a chimeric molecule that consists of a bacteriophage backbone, a plasmid replicon (rep gene), a drug-resistance region(s)/gene(s) (e.g. a ISEcpl-bla CTX-M-15-tnpA gene cassette) and a lysogenized P1 bacteriophage sequence [49] that have resulted from recombination of integrative elements on plasmids and prophage (chromosomally integrated, lysogenized bacteriophages) genomes.
Our findings provide supporting increasing evidence of the role played by viral vectors in the vertical and horizontal transfer of AMR genes and mobile elements between bacteria [13–15, 49]. Phage-plasmids of sizes between 90 and 120 kbp have been described in other Enterobacterales , such as Citrobacter , Enterobacter and Pantoea [17], and it has been suggested that the repY replicon in both plasmids and phage-plasmids is associated with strains harbouring ESBL genes [13, 53]. However, the bla CTX-M-27 gene in the S . enterica serovar Indiana isolate [14] was present on a 104 kbp repA-like phage-plasmid and a bla CTX-M-15 gene was present on a 94 kbp IncF1A phage-plasmid in Klebsiella pneumoniae [49]. Phage-plasmids of different compatibility groups can be involved in the transmission of additional resistance determinants such as colistin (mcr-1) [15].
Nanopore sequencing provided extra context, revealing two isoforms of the same phage-plasmid structure, with and without a bla CTX-M-15 mobile genetic element (Fig. 2). Our observation suggests an ongoing process, whereby AMR genes can be dynamically acquired and lost depending on the evolutionary pressures surrounding the phage-plasmid and its host.
We are beginning to detect previously undescribed elements of AMR transmission, such as phage-plasmids, due to our ability to assemble complete genomes via long-read sequencing. Understanding the structure of such genomic elements is essential to fully elucidate the biological mechanisms of AMR and their clinical relevance. For instance, with long-read sequencing data we are now able to detect structural variants within the reads of a single bacterial culture [54]. This is showcased by our observation that in a proportion of the culture the bla CTX-M-15 resistance cassette is missing from the phage-plasmid (Fig. 2).
However, technical difficulties arise from genome assembly, which at times requires manual curation to ensure the validity of these assemblies for downstream analyses [37, 55, 56]. The same is true for accurate prophage and phage-plasmid detection and annotation directly from assemblies. Even from the abundant short-read sequence data (e.g. Illumina) and rapidly increasing long-read data, prophages and phage-plasmids sequences can often be missed [56, 57]. This is exacerbated by the number of in silico bacteriophage detection tools, which produce differing results from methodologies varying from reference-based detection through to machine-learning approaches [56, 58–63].
In conclusion, this study demonstrates the use of modern sequencing and data curation techniques in the successful detection, characterization and tracking of novel mobile elements involved in AMR transmission. We show the potential role of phage-plasmids in the capture and spread of ESBL resistance genes in S . enterica Typhi, in agreement with an increasing body of evidence showing the importance of horizontal spread of AMR genes mediated by bacteriophages and phage-plasmids [13]. Our findings also demonstrate the utility of long-read sequencing for non-typeable plasmids and phage-plasmids in detection and surveillance procedures once adequate sequencing capabilities become available. Future work will include the screening of gastrointestinal bacterial pathogens at UKHSA for the presence of repL and other relevant genes to detect the involvement of phage-plasmids in the transmission of AMR genes.
Funding information
C.J./D.R.G. and M.A.C./P.R. are affiliated to the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Pathogens and NIHR HPRU in Genomics and Enabling Data at the University of Liverpool and the University of Warwick, respectively (NIHR200892), in partnership with the UKHSA, and are based at the UKHSA. M.T.B. is affiliated to the NIHR HPRU in Healthcare Associated Infections and Antimicrobial Resistance at the University of Oxford, in partnership with the UKHSA. In addition, G.C.L. and E.V.W. were funded by the Biotechnology and Biological Sciences Research Council (BBSRC); Institute Strategic Programme Microbes in the Food Chain BB/R012504/1 and its constituent project BBS/E/F/000PR10348. The views expressed are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care nor the UKHSA.
Author contributions
Conceptualization – S.N. Formal analysis – D.R.G., M.T.B., S.N. Investigation – D.R.G., M.T.B., M.A.C., G.C.L., E.V.W., P.R., C.J., S.N. Writing – original draft – S.N., D.R.G. Writing – review and editing – D.R.G., M.T.B., M.A.C., G.C.L., E.V.W., P.R., C.J., S.N.
Conflicts of interest
The authors declare there are no conflicts of interest.
Footnotes
Abbreviations: AMR, antimicrobial resistance; CDS, coding sequence; ESBL, extended-spectrum β-lactamase; HGT, horizontal gene transfer; NCBI, National Center for Biotechnology Information; NIHR, National Institute for Health Research; ST, sequence type; UKHSA, UK Health Security Agency; XDR, extensively drug resistant.
The GenBank/EMBL/DDBJ accession numbers for the chromosome and ph681355 phage-plasmid sequences of Salmonella enterica serovar Typhi isolate 681355 are CP083411 and CP083412, respectively. All supporting data, code and protocols have been provided within the article or through supplementary data files.
References
- 1.Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, et al. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the global burden of disease study 2017. Lancet Infect Dis. 2019;19:369–381. doi: 10.1016/S1473-3099(18)30685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, et al. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio. 2018;9:e00105-18. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair S, Chattaway M, Langridge GC, Gentle A, Day M, et al. ESBL-producing strains isolated from imported cases of enteric fever in England and Wales reveal multiple chromosomal integrations of blaCTX-M-15 in XDR Salmonella Typhi. J Antimicrob Chemother. 2021;76:1459–1466. doi: 10.1093/jac/dkab049. [DOI] [PubMed] [Google Scholar]
- 4.Godbole GS, Day MR, Murthy S, Chattaway MA, Nair S. First report of CTX-M-15 Salmonella Typhi from England. Clin Infect Dis. 2018;66:1976–1977. doi: 10.1093/cid/ciy032. [DOI] [PubMed] [Google Scholar]
- 5.Nizamuddin S, Ching C, Kamal R, Zaman MH, Sultan F. Continued outbreak of ceftriaxone-resistant Salmonella enterica serotype Typhi across Pakistan and assessment of knowledge and practices among healthcare workers. Am J Trop Med Hyg. 2021;104:1265–1270. doi: 10.4269/ajtmh.20-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi S, Naveed AB, Yousafzai MT, Ahmad K, Ansari S, et al. Response of extensively drug resistant Salmonella Typhi to treatment with meropenem and azithromycin, in Pakistan. PLoS Negl Trop Dis. 2020;14:e0008682. doi: 10.1371/journal.pntd.0008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herdman MT, Karo B, Dave J, Katwa P, Freedman J, et al. Increasingly limited options for the treatment of enteric fever in travellers returning to England, 2014–2019: a cross-sectional analytical study. J Med Microbiol. 2021;70:001359. doi: 10.1099/jmm.0.001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC Extensively Drug-Resistant Salmonella Typhi Infections among U.S. Residents without International Travel, CDC Health Advisory, CDCHAN-00439 ( https://emergency.CDC.gov/han/2021/han00439.asp) Atlanta, GA: Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 9.Cantón R, González-Alba JM, Galán JC. CTX-M enzymes: origin and diffusion. Front Microbiol. 2012;3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aviv G, Tsyba K, Steck N, Salmon-Divon M, Cornelius A, et al. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environ Microbiol. 2014;16:977–994. doi: 10.1111/1462-2920.12351. [DOI] [PubMed] [Google Scholar]
- 11.Goh S, Hussain H, Chang BJ, Emmett W, Riley TV, et al. Phage ϕC2 mediates transduction of Tn6215, encoding erythromycin resistance, between Clostridium difficile strains. mBio. 2013;4:e00840-13. doi: 10.1128/mBio.00840-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyder SL, Streitfeld MM. Transfer of erythromycin resistance from clinically isolated lysogenic strains of Streptococcus pyogenes via their endogenous phage. J Infect Dis. 1978;138:281–286. doi: 10.1093/infdis/138.3.281. [DOI] [PubMed] [Google Scholar]
- 13.Billard-Pomares T, Fouteau S, Jacquet ME, Roche D, Barbe V, et al. Characterization of a P1-like bacteriophage carrying an SHV-2 extended-spectrum β-lactamase from an Escherichia coli strain. Antimicrob Agents Chemother. 2014;58:6550–6557. doi: 10.1128/AAC.03183-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Li W, Jiang G-Z, Zhang W-H, Ding H-Z, et al. Characterization of a P1-like bacteriophage carrying CTX-M-27 in Salmonella spp. resistant to third generation cephalosporins isolated from pork in China. Sci Rep. 2017;7:40710. doi: 10.1038/srep40710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Feng Y, Liu F, Jiang H, Qu Z, et al. A phage-like IncY plasmid carrying the mcr-1 gene in Escherichia coli from a pig farm in China. Antimicrob Agents Chemother. 2017;61:e02035-16. doi: 10.1128/AAC.02035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl A, Battesti A, Ansaldi M. Prophages in Salmonella enterica: a driving force in reshaping the genome and physiology of their bacterial host? Mol Microbiol. 2019;111:303–316. doi: 10.1111/mmi.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilcrease EB, Casjens SR. The genome sequence of Escherichia coli tailed phage D6 and the diversity of Enterobacteriales circular plasmid prophages. Virology. 2018;515:203–214. doi: 10.1016/j.virol.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler BA, Kazakov AE, Zhong C, Liu H, Kutter E, et al. The genetic basis of phage susceptibility, cross-resistance and host-range in Salmonella . Microbiology. 2021;167:001126. doi: 10.1099/mic.0.001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patchanee P, Chokesajjawatee N, Santiyanont P, Chuammitri P, Deeudom M, et al. Characterisation of Salmonella enterica clones carrying mcr-1 plasmids in meat products and patients in Northern Thailand using long read sequencing. Int J Food Microbiol. 2021;358:109314. doi: 10.1016/j.ijfoodmicro.2021.109314. [DOI] [PubMed] [Google Scholar]
- 20.Locke RK, Greig DR, Jenkins C, Dallman TJ, Cowley LA. Acquisition and loss of CTX-M plasmids in Shigella species associated with MSM transmission in the UK. Microb Genom. 2021;7:000644. doi: 10.1099/mgen.0.000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sia CM, Greig DR, Day M, Hartman H, Painset A, et al. The characterization of mobile colistin resistance (mcr) genes among 33 000 Salmonella enterica genomes from routine public health surveillance in England. Microb Genom. 2020;6:000331. doi: 10.1099/mgen.0.000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greig DR, Dallman TJ, Hopkins KL, Jenkins C. MinION nanopore sequencing identifies the position and structure of bacterial antibiotic resistance determinants in a multidrug-resistant strain of enteroaggregative Escherichia coli . Microb Genom. 2018;4:000213. doi: 10.1099/mgen.0.000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WWY, Mattock J, Greig DR, Langridge GC, Baker D, et al. Characterization of a pESI-like plasmid and analysis of multidrug-resistant Salmonella enterica Infantis isolates in England and Wales. Microb Genom. 2021;7:000658. doi: 10.1099/mgen.0.000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godbole G, McCann N, Jones SM, Dallman TJ, Brown M. Ceftriaxone-resistant Salmonella Typhi in a traveller returning from a mass gathering in Iraq. Lancet Infect Dis. 2019;19:467. doi: 10.1016/S1473-3099(19)30176-8. [DOI] [PubMed] [Google Scholar]
- 25.Chattaway MA, Gentle A, Nair S, Tingley L, Day M, et al. Phylogenomics and antimicrobial resistance of Salmonella Typhi and Paratyphi A, B and C in England, 2016–2019. Microb Genom. 2021;7:000633. doi: 10.1099/mgen.0.000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tewolde R, Dallman T, Schaefer U, Sheppard CL, Ashton P, et al. MOST: a modified MLST typing tool based on short read sequencing. PeerJ. 2016;4:e2308. doi: 10.7717/peerj.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achtman M, Wain J, Weill F-X, Nair S, Zhou Z, et al. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica . PLoS Pathog. 2012;8:e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day MR, Doumith M, Do Nascimento V, Nair S, Ashton PM, et al. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Salmonella enterica serovars Typhi and Paratyphi. J Antimicrob Chemother. 2018;73:365–372. doi: 10.1093/jac/dkx379. [DOI] [PubMed] [Google Scholar]
- 32.Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75:3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day M, Doumith M, Jenkins C, Dallman TJ, Hopkins KL, et al. Antimicrobial resistance in Shiga toxin-producing Escherichia coli serogroups O157 and O26 isolated from human cases of diarrhoeal disease in England, 2015. J Antimicrob Chemother. 2017;72:145–152. doi: 10.1093/jac/dkw371. [DOI] [PubMed] [Google Scholar]
- 34.Sadouki Z, Day MR, Doumith M, Chattaway MA, Dallman TJ, et al. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Shigella sonnei isolated from cases of diarrhoeal disease in England and Wales, 2015. J Antimicrob Chemother. 2017;72:2496–2502. doi: 10.1093/jac/dkx170. [DOI] [PubMed] [Google Scholar]
- 35.Greig DR, Jenkins C, Gharbia SE, Dallman TJ. Analysis of a small outbreak of Shiga toxin-producing Escherichia coli O157:H7 using long-read sequencing. Microb Genom. 2021;7:000545. doi: 10.1099/mgen.0.000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37:540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 37.Yara DA, Greig DR, Gally DL, Dallman TJ, Jenkins C. Comparison of Shiga toxin-encoding bacteriophages in highly pathogenic strains of Shiga toxin-producing Escherichia coli O157:H7 in the UK. Microb Genom. 2020;6:000334. doi: 10.1099/mgen.0.000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaser R, Sović I, Nagarajan N, Šikić M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt M, Silva ND, Otto TD, Parkhill J, Keane JA, et al. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 2015;16:294. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 43.Carattoli A, Hasman H. PlasmidFinder and in silico pMLST: identification and typing of plasmid replicons in whole-genome sequencing (WGS) Methods Mol Biol. 2020;2075:285–294. doi: 10.1007/978-1-4939-9877-7_20. [DOI] [PubMed] [Google Scholar]
- 44.Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 46.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Argimón S, Nagaraj G, Shamanna V, Sravani D, Vasanth AK, et al. Circulation of third-generation cephalosporin resistant Salmonella Typhi in Mumbai, India. Clin Infect Dis. 2022;74:2234–2237. doi: 10.1093/cid/ciab897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin J, Ko KS. A plasmid bearing the bla(CTX-M-15) gene and phage P1-like sequences from a sequence type 11 Klebsiella pneumoniae isolate. Antimicrob Agents Chemother. 2015;59:6608–6610. doi: 10.1128/AAC.00265-15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.François Watkins LK, Winstead A, Appiah GD, Friedman CR, Medalla F, et al. Update on extensively drug-resistant Salmonella serotype Typhi infections among travelers to or from Pakistan and report of ceftriaxone-resistant Salmonella serotype Typhi infections among travelersI to Iraq – United States, 2018–2019. MMWR Morb Mortal Wkly Rep. 2020;69:618–622. doi: 10.15585/mmwr.mm6920a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hensel M. Evolution of pathogenicity islands of Salmonella enterica . Int J Med Microbiol. 2004;294:95–102. doi: 10.1016/j.ijmm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 52.Suez J, Porwollik S, Dagan A, Marzel A, Schorr YI, et al. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One. 2013;8:e58449. doi: 10.1371/journal.pone.0058449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hrabák J, Empel J, Bergerová T, Fajfrlík K, Urbásková P, et al. International clones of Klebsiella pneumoniae and Escherichia coli with extended-spectrum β-lactamases in a Czech hospital. J Clin Microbiol. 2009;47:3353–3357. doi: 10.1128/JCM.00901-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzgerald SF, Lupolova N, Shaaban S, Dallman TJ, Greig D, et al. Genome structural variation in Escherichia coli O157:H7. Microb Genom. 2021;7:000682. doi: 10.1099/mgen.0.000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clokie MRJ, Kropinski AM. Bacteriophages: Methods and Protocols. New York: Humana Press; 2009. [Google Scholar]
- 56.Sirén K, Millard A, Petersen B, Gilbert MTP, Clokie MRJ, et al. Rapid discovery of novel prophages using biological feature engineering and machine learning. NAR Genom Bioinform. 2021;3:lqaa109. doi: 10.1093/nargab/lqaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durrant MG, Li MM, Siranosian BA, Montgomery SB, Bhatt AS. A bioinformatic analysis of integrative mobile genetic elements highlights their role in bacterial adaptation. Cell Host Microbe. 2020;27:140–153. doi: 10.1016/j.chom.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song W, Sun H-X, Zhang C, Cheng L, Peng Y, et al. Prophage Hunter: an integrative hunting tool for active prophages. Nucleic Acids Res. 2019;47:W74–W80. doi: 10.1093/nar/gkz380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kieft K, Zhou Z, Anantharaman K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome. 2020;8:90. doi: 10.1186/s40168-020-00867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akhter S, Aziz RK, Edwards RA. PhiSpy: a novel algorithm for finding prophages in bacterial genomes that combines similarity- and composition-based strategies. Nucleic Acids Res. 2012;40:e126. doi: 10.1093/nar/gks406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amgarten D, Braga LPP, da Silva AM, Setubal JC. MARVEL, a tool for prediction of bacteriophage sequences in metagenomic bins. Front Genet. 2018;9:304. doi: 10.3389/fgene.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arndt D, Marcu A, Liang Y, Wishart DS. PHAST, PHASTER and PHASTEST: tools for finding prophage in bacterial genomes. Brief Bioinform. 2019;20:1560–1567. doi: 10.1093/bib/bbx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Starikova EV, Tikhonova PO, Prianichnikov NA, Rands CM, Zdobnov EM, et al. Phigaro: high-throughput prophage sequence annotation. Bioinformatics. 2020;36:3882–3884. doi: 10.1093/bioinformatics/btaa250. [DOI] [PubMed] [Google Scholar]