Abstract
The male preponderance in autism spectrum disorder (ASD) led to the hypothesis that aspects of female biology are protective against ASD. Females with ASD (ASD-F) report more compensatory behaviors (i.e. “camouflaging”) to overcome ASD-related social differences, which may be a mechanism of protection. No studies have examined sex-related brain pathways supporting camouflaging in ASD-F, despite its potential to inform mechanisms underlying the ASD sex bias. We used functional connectivity (FC) to investigate “sex-atypical” and “sex-typical” FC patterns linked to camouflaging in adults with ASD and examined multimodal coherence of findings via structural connectometry. Exploratory associations with cognitive/emotional functioning examined the adaptive nature of FC patterns. We found (i) “sex-atypical” FC patterns linked to camouflaging in the hypothalamus and precuneus and (ii) “sex-typical” patterns in the right anterior cingulate and anterior parahippocampus. Higher hypothalamic FC with a limbic reward cluster also correlated with better cognitive control/emotion recognition. Structural connectometry validated FC results with consistent brain pathways/effect patterns implicated in ASD-F. In summary, “male-typical” and “female-typical” brain connectivity patterns support camouflaging in ASD-F in circuits implicated in reward, emotion, and memory retrieval. “Sex-atypical” results are consistent with fetal steroidogenic/neuroinflammatory hypotheses. However, female genetics/biology may contribute to “female-typical” patterns implicated in camouflaging.
Keywords: autism, camouflaging, DTI, MRI, resting-state, sex/gender
Introduction
Autism spectrum disorder (ASD) has an estimated sex bias of 3–4: 1 males to females (Loomes et al. 2017; Posserud et al. 2021). This male preponderance suggests that sex-related biology may protect females or increase male vulnerability (Werling and Geschwind 2013). Accumulating evidence supports the female protection hypothesis, with diagnosed females showing greater ASD genetic liability than males (Ferri et al. 2018). Sex/gender-related ASD models have been put forth, characterizing adult ASD presentation, in an effort to identify mechanisms underlying the sex bias. The Extreme Male Brain model presents evidence that ASD represents the extreme end of cognitive/behavioral masculinization (Baron-Cohen 2002), while the Gender Incoherence model highlights masculinized phenotypic qualities in females with ASD (ASD-F) and feminized qualities in males with ASD (ASD-M; Bejerot et al. 2012). Both models suggest ASD-F present with a more masculine phenotype. Importantly, these models remain controversial and may be overly simplistic (Ridley 2019). For example, accumulating evidence supports viewing the brain as an individualized mosaic of both “masculine” and “feminine” biological influences interacting with environment and genetics across the lifespan, in contrast to the previously held view of a sexually dimorphic brain (Joel et al. 2015; Eliot et al. 2021). Accordingly, certain social traits in ASD-F children and adolescent are more similar to those observed in neurotypical (NT) females (e.g. social attention, linguistic discourse skills, social motivation; Lai and Szatmari 2020). Both “masculine” and “feminine” sex-related biology may be implicated in the ASD sex bias, but characterization of relevant sex-related neurobiological pathways is understudied.
Most ASD risk genes are not sex-specific, and sex-related biology is thought to act downstream of risk variants to promote female protection (Kissel and Werling 2022). Transcriptomic studies show that gene expression patterns for certain gene sets (e.g. immune/microglial-associated genes) are more similar to NT males (NT-M) than NT females (NT-F; Werling 2016; Kissel and Werling 2022), implicating “masculine” sex-related biology in ASD risk. To date, transcriptomic studies have examined bulk tissue from prefrontal, temporal, and cerebellar regions in small samples, and no studies have investigated brain regions showing pronounced sex differences (e.g. hypothalamus; McCarthy and Wright 2017). Thus, inference about brain regions implicated in the ASD sex bias remains limited. Neuroimaging is a complementary approach to transcriptomics, allowing for in vivo, whole-brain analyses on larger, more homogeneous samples. Resting-state functional MRI (rs-fMRI) and diffusion tensor imaging (DTI) can be applied to estimate functional and structural brain connections implicated in the ASD sex bias (Ecker and Murphy 2014).
A recent systematic review highlights that ASD-F show more atypical brain features than ASD-M, but some atypical features may be “protective” (Walsh et al. 2021). For example, in a large sample of children and adults, the left anterior prefrontal cortex has shown more atypical features in ASD-F (Bedford et al. 2019). However, recent evidence in a child/adolescent sample suggests that left anterior prefrontal functional connectivity (FC) with reward circuitry may buffer against ASD genetic risk in females with ASD (Hernandez et al. 2020). A novel way to elucidate brain systems underlying the sex bias in ASD is to characterize sex-related brain correlates of behaviors that show a sex bias in ASD. For example, greater use of compensatory strategies is reported in ASD-F compared with ASD-M in adolescent and adult samples (Hull et al. 2020; Tubío-Fungueiriño et al. 2020), and this compensation may be one mechanism of “protection” against ASD genetic liability in ASD-F. The primary compensatory construct investigated in adolescents and adults with ASD is “camouflaging” (e.g. behaviors to mask or overcome ASD-related social differences; Hull et al. 2017). The field is new, and the first studies quantified camouflaging as the discrepancy between observable versus “intrinsic” (e.g. self-report) ASD traits (Lai et al. 2017, 2019). The discrepancy approach has linked greater camouflaging in ASD-F adults to reduced cerebellar/medial temporal lobe volumes (Lai et al. 2017) and increased activation of the medial prefrontal cortex during self-reflection (Lai et al. 2019). However, discrepancy scores lack psychometric validation and may not accurately quantify camouflaging. More recently, the Camouflaging Autistic Traits Questionnaire (CAT-Q) was developed (Hull et al. 2018) but has yet to be linked with neuroimaging. Furthermore, no studies have examined brain connectivity camouflaging correlates, despite their translational utility (Fox et al. 2014).
Studies examining sex differences in associations between brain connectivity and measures of ASD genetic risk may be useful for informing hypotheses about “protective” sex-related brain features. A recent neuroimaging genetics study found that reward circuit FC within the left anterior prefrontal cortex was distinctly linked to greater ASD-associated risk alleles on the oxytocin receptor gene as well as reduced socio-cognitive symptom severity in child/adolescent ASD-F but not ASD-M (Hernandez et al. 2020). Thus, reward circuits may be candidate pathways supporting female protection, but the study’s seed-based methodology limits connectome-wide inference. Another recent study examined associations between FC and polygenic ASD risk, finding that greater genetic risk was linked to higher salience network FC with somatosensory cortex in child/adolescent boys, irrespective of ASD diagnosis (Lawrence et al. 2021). Again, this study also used a seed-based methodology, limiting connectome-wide inference. Furthermore, no studies to date have used structural connectivity to investigate sex differences in brain connectivity correlates of compensatory behavior or other measures of risk/protection (e.g. genetic risk scores) in ASD. Data-driven approaches are needed to advance the understanding of pathways implicated in the sex bias in ASD.
This study sought to examine sex differences in the brain connectivity correlates of camouflaging in adults with ASD using a data-driven, connectome-wide approach. The objectives were to examine “sex-atypical” and “sex-typical” FC patterns linked to camouflaging in ASD. To characterize the adaptive nature of FC patterns linked to camouflaging in adults with ASD, we examined associations with cognitive control, memory, emotion recognition, and depression/anxiety. Finally, to determine if results showed coherence across imaging modalities, we examined structural connectivity correlates of camouflaging in each group using a correlational tractography approach.
Materials and methods
Participants
The sample was derived from a larger age- and IQ-matched study (n ~ 200) examining sex differences in nonintellectually disabled, young-to-older adults with ASD and NT adults. The study began in 2015 and the CAT-Q was added in 2019. Participants were selected if they had complete CAT-Q, rs-fMRI, and DTI data, with a total of 85 participants ages 18–70 (Table 1; n = 24 ASD-F, n = 21 ASD-M, n = 20 NT-F, n = 19 NT-M). As no prior studies have investigated sex-related FC patterns linked to compensatory behavior in ASD, adequate sample size was determined from a recent study examining sex-related circuits predicting genetic risk in ASD (Lawrence et al. 2021). With a slightly larger groupwise sample than our study (e.g. n = 31, ASD-F compared with present study with n = 24, ASD-F), this study reported modest peak-cluster effect sizes (Z ~ 4; Lawrence et al. 2021), suggesting that our smaller sample will be adequate to detect sex-related brain–behavior associations.
Table 1.
Sample descriptive statistics and group differences.
| ASD | NT | ||||
|---|---|---|---|---|---|
| Females | Males | Females | Males | Difference? | |
| n | 24 | 21 | 21 | 19 | |
| Age | 39.42 (13.21) 19–60 | 41.95 (11.39) 26–64 | 43.57 (15.68) 18–65 | 48.53 (13.35) 26–70 | None |
| CAT-Q | 120.21 (30.34) 49–164 | 107.00 (23.29) 64–161 | 63.95 (24.45) 38–139 | 72.68 (13.34) 55–101 | aASD > NT, aASD-F > ASD-M |
| IQ | 110.25 (16.94) 73–148 | 104.14 (15.95) 70–131 | 108.05 (12.71) 77–132 | 107 (11.24) 92–127 | None |
| SRS-2 | 103.87 (29.56) 44–167 | 110.43 (35.45) 21–155 | 21.95 (9.67) 5–46 | 26.95 (14.45) 7–57 | b ASD>NT |
| ADOS-2 | 9.87 (2.13) 7–14 | 10.67 (3.73) 7–19 | N/A | N/A | None |
| rs-fMRI Motiond | 0.06 (0.02) 0.02–0.08 | 0.07 (0.02) 0.02–0.11 | 0.06 (0.01) 0.04–0.09 | 0.07 (0.03) 0.02–0.13 | None |
| DTI Motionc d | 0.34 (0.13) 0.16–0.61 | 0.65 (0.61) 0.19–3.01 | 0.31 (0.15) 0.12–0.64 | 0.60 (0.38) 0.30–1.63 | e male>female |
| Education (years) | 16.08 (2.32) 12–20 | 15.29 (2.90) 12–22 | 16.67 (2.22) 12–21 | 16.79 (2.15) 14–23 | f ASD<NT |
Differences were tested via ANOVA for main effects of diagnosis, sex, or their interaction. If significant, post-hoc t-tests examined pairwise group differences driving the significant main effect or interaction. *Social Responsiveness Scale—2nd Edition (SRS-2); at83 = 8.62, P < 0.001;
a t 43 = 1.62, P = 0.11,
b t 83 = 15.25, P < 0.001;
caverage root mean square displacements relative first volume;
dno group differences in percent outlier slices or framewise displacement;
e t 83 = 3.79, P < 0.001,
f F 1,81 = 3.93, P = 0.051.
gCONN average motion observed (disregarding outlier scans; threshold = 0.5).
This sample has been characterized previously (Baxter et al. 2019; Walsh et al. 2019). ASD participants were recruited using the Southwest Autism Research and Resource Center lifetime database of voluntarily enrolled individuals who consented to be contacted for future studies and presentations at local ASD community events. For all participants, snowball recruitment and community fliers were used. Existing ASD diagnoses or first-time ASD diagnoses (in cases of suspected ASD) were confirmed via the Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al. 2012) module 4, a brief case history, and the DSM-V checklist combined with clinical judgment. The ADOS-2 module 4 was administered by a research-reliable psychometrist with over 10 years ASD diagnostics experience in children and adults. Furthermore, diagnostic confirmation using the DSM-V checklist combined with clinical judgment was conducted by a psychologist with over 25 years ASD research diagnostics experience in children and adults. All participants in this sample had an ADOS-2 module 4 score exceeding diagnostic threshold (7 or higher; Table 1). NT participants were enrolled if they had (i) no self-reported suspected or diagnosed ASD, (ii) self-reported Social Responsiveness Scale—2nd Edition (SRS-2; Constantino 2012) T-scores ≤ 66, and (iii) no first-degree family history of ASD. Furthermore, no NT participants in this sample had (i) self-reported history of neuropsychiatric conditions (e.g. ADHD, learning disabilities, obsessive compulsive disorder, etc.; exception: depression/anxiety) and (ii) no current self-reported substance abuse/misuse. All participants were subject to the following exclusion criteria: (i) Kaufman Brief Intelligence Test—2nd Edition (Kaufman 2004) scores < 70, (ii) Mini Mental State Exam (Folstein et al. 1975) scores < 25, (iii) a history of head injury with loss of consciousness or neurological disorders, or (iv) current seizure disorder or use of seizure medications. Self-reported anxiety and depression were not exclusionary given high prevalence in ASD (Lever and Geurts 2016). Please see Supplementary Methods S1 for additional details regarding sample socio-demographic parameters, menopausal status, and demographic correlates of camouflaging. This study was conducted in compliance with Arizona State University’s ethical research standards and the Declaration of Helsinki 2000 revision. Participants provided Institutional Review Board approved written consent.
Behavioral measures
Camouflaging
The CAT-Q, published in 2018 (Hull et al. 2018), is a self-report questionnaire developed through interviews of the “camouflaging” experience in adults with ASD. This questionnaire quantifies behaviors used to compensate for or mask autistic traits during social interactions, scored on a 7-point Likert scale from “Strongly Disagree” to “Strongly Agree.” Sample questions include “I have tried to improve my understanding of social skills by watching other people” and “I monitor my body language or facial expressions so that I appear interested by the person I am interacting with.” The total score was used in analyses. Given that camouflaging and ASD traits are correlated (Hull et al. 2021), the SRS-2 (Constantino 2012) measured self-reported ASD traits and was used as a covariate in all analyses to account for severity-related brain differences.
Neuropsychological tests
Selected measures spanned broad executive function, memory, emotional, and psychiatric domains previously implicated in camouflaging or female protection (Livingston and Happé 2017; Allely 2019; Lai and Szatmari 2020; Morrison et al. 2020; Tubío-Fungueiriño et al. 2020; Hull et al. 2021). In brief, the Wisconsin Card Sorting Task (Memari et al. 2013), Stroop Color and Word Test (Scarpina and Tagini 2017), and Tower of London (Shallice 1982) estimated flexibility, cognitive control, and planning, respectively. The Wechsler Memory Scale—Visual Reproduction II (Wechsler 1997) and Rey Auditory Verbal Learning Task (Schmidt 1996) measured visual and verbal memory. The Toronto Alexithymia Scale (Kupfer et al. 2000) and Reading the Mind in the Eyes Task (Baron-cohen et al. 2001) measured emotional self-awareness and facial emotion recognition. Finally, the State Trait Anxiety Inventory (Spielberger 1983) and Beck Depression Inventory-II (Beck et al. 1996) measured trait anxiety and depression. See Supplementary Methods S2 for detailed measure description.
MRI parameters
Acquisition
A 3 T Philips Ingenia scanner collected anatomical, rs-fMRI, and DTI images (max. gradient strength = 5 m T/m). The anatomical sequence was 3D magnetization prepared rapid acquisition gradient echo (MPRAGE; 170 axial slices, 1.2-mm slices, 240-mm FOV, 256 × 256 acquisition matrix). Six-minute (eyes closed) rs-fMRI scans were collected via a whole-brain coverage, gradient-echo, echo-planar (EPI) series (3000 ms TR, 25 ms TE, 80° flip angle, 3-mm slices, 240-mm FOV, 64 × 64 acquisition matrix). DTI scans were acquired via a whole-brain coverage, diffusion inversion recovery sequence (7055.39 ms TR, 118.655 ms TE, 32 directions, 2500 ms/mm2 b-value, 1.41 mm in-plane resolution, 3-mm slice thickness).
Preprocessing and subject-level connectivity maps
Raw rs-fMRI images were first despiked (Wavelet Despiking Toolbox; Patel et al. 2014). Subsequent preprocessing was conducted in SPM-12 (https://www.fil.ion.ucl.ac.uk/), including (i) functional image realignment, (ii) anatomical image segmentation, skull-stripping, functional image co-registration, and (iii) DARTEL normalization to MNI space. The CONN Toolbox identified functional outliers at a conservative 0.5-mm framewise displacement threshold, followed by aCompCor confound regression (Muschellia et al. 2014), including realignment parameters/first-order derivatives, scrubbing, linear detrending, and bandpass filtering [0.008 0.1]. To facilitate data-driven selection of seeds where FC patterns were differentially linked to camouflaging as a function of sex/sex-by-diagnosis group (“sex-typical” and “sex-atypical”), we utilized the CONN Toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012) connectome-wide implementation of group multivariate voxel pattern analysis (MVPA). At the individual subject level, this procedure first applies principal components analysis to retain 64 components characterizing the most salient features of each subject’s voxel-to-voxel correlation structure, akin to subject-level dimensionality reduction applied in group-ICA (Calhoun et al. 2012). At the group-level, principal components analysis is applied jointly across all subjects, but separately for each seed(voxel)-to-voxel FC map, to retain the 15 strongest group-MVPA components (e.g. those that explain maximum intersubject variability). This procedure has been published on extensively, including in ASD (see Kelly et al. 2020 in Nature Neuroscience and Arnold Anteraper et al. 2019, Schneider et al. 2019, Anteraper et al. 2020, and Westfall et al. 2020 for other implementations of this procedure). Also, see Anteraper et al. (2019) and https://sites.google.com/ view/conn/measures/networks-voxel-level for mathematical details of the CONN Toolbox Group-MVPA procedure.
FSL (FMRIB software library, www.fmri.ox.ac.uk/fsl) was used for diffusion image preprocessing and DSI Studio (www.dsi-studio.labsolver.org) to estimate structural connectivity. In FSL, eddy correction was applied to account for head motion and eddy current distortions. Average root-mean-square displacement from the first nondiffusion weighted volume, scan-to-scan displacement, and percentage of outlier slices estimated subject-level motion (see Table 1 for group comparison). Eddy-corrected diffusion data were entered into DSI Studio to estimate quantitative anisotropy (QA). Unlike the more commonly estimated fractional anisotropy, which is thought to reflect the rate of diffusion, QA estimates the density of diffusing water in the fibers’ standard axonal direction as defined by an atlas (Yeh, Vettel, et al. 2016). This procedure applied q-space diffeomorphic reconstruction (Yeh and Tseng 2011) to a standard template to calculate the spin distribution function (Yeh et al. 2010) with a 1.25-mm diffusion sampling length and 1-mm isotropic resolution. Using the Human Connectome Project 1065 atlas, the spin distribution function magnitude in the standard fiber direction estimated voxel-wise QA, and values were concatenated within and across subjects to generate a local connectome matrix (Supplementary Methods S3 for detailed rs-fMRI and DTI preprocessing methods/connectivity map construction).
Group-level analysis
Using the CONN Toolbox, a general linear model was specified including group-wise intercepts and CAT-Q slopes with age/SRS-2 covariates (accounting for age/severity-related brain variability). “Sex-atypical” patterns (e.g. camouflaging-by-sex-by-diagnosis effects) in ASD were modeled as inverse slopes for ASD-F/NT-M vs. ASD-M/NT-F. This “sex-atypical” contrast tested for voxels where ASD-F showed camouflaging-FC associations more similar to NT-M (here-to-forth referred to as “male-typical”) and ASD-M showed camouflaging-FC associations more similar to NT-F (here-to-forth referred to as “female-typical”). “Sex-typical” patterns (e.g. camouflaging-by-sex effects) were modeled as inverse slopes for females vs. males (See Supplementary Methods S4 for contrast details). This “sex-typical” contrast tested for voxels where ASD-F showed “female-typical” camouflaging-FC associations and ASD-M showed “male-typical” camouflaging-FC associations. An F-test evaluated all 15 group-MVPA rs-fMRI components simultaneously, but separately for each contrast, to identify voxels that showed significant “sex-atypical” or “sex-typical” FC patterns (e.g. abstract multivariate representations; Shehzad et al. 2014) linked to camouflaging. Post-hoc seed-to-voxel analyses characterized FC patterns driving the significant group-MVPA clusters. Specifically, the average time course was computed for significant MVPA-derived clusters and used as a seed to produce seed-to-voxel FC maps. The same contrasts from the Group-MVPA analysis were then tested on these seed-to-voxel FC maps, separately for positive and negative effects. For all rs-fMRI analyses, a voxel height threshold of P < 0.001, uncorrected and cluster threshold of P < 0.05, and false discovery rate (FDR) corrected was used. To determine if results were driven by subgroups, we tested regression model significance using mean FC values from significant post-hoc clusters to predict residualized CAT-Q scores (partialing out age/SRS-2), with one model per MVPA cluster. Unthresholded t-maps from each MVPA post-hoc contrast were submitted to the Neurosynth Decoder (https://neurosynth.org/decode/) and word clouds were generated using R Studio Wordcloud2 for the top 10 correlated terms (based on correlation absolute values).
Group connectometry was conducted in DSI Studio (Yeh, Badre, et al. 2016) using a whole-brain regression procedure, separately across each sex-by-diagnosis group, to identify tracts significantly associated with camouflaging. Given that ASD-F shows more camouflaging than ASD-M (Hull et al. 2020) and this may be a mechanism of female protection, structural connectivity correlates of camouflaging in ASD-M and NT groups were not of primary interest but are reported in Supplementary Fig. S2. The model included CAT-Q as the study variable of interest, with age, SRS-2, and average root-mean-square displacement as nuisance covariates to account for age, symptom severity, and motion-related signal variability (given male > female group differences on this parameter; Table 1). A t-threshold of 3, corresponding to the more conservative recommended threshold in DSI Studio, was used to select QA values associated with the study variable for subsequent deterministic fiber tracking along substantial coefficients (Yeh et al. 2013). Topology-informed pruning (Yeh et al. 2019) was applied across 4 iterations to filter false tracks. For tracks exceeding a length of 40 voxels, FDR < 0.05 was estimated using a distribution of 4,000 random permutations to subject phenotypic values. Average track QA was plotted against residualized CAT-Q scores (age/SRS-2 partialed out) to visualize effects.
FC–behavior associations
For ASD-F, 2-tailed partial Pearson correlations (age/SRS-2 covariates) were calculated between behavioral measures and each FDR-corrected cluster from post-hoc seed-to-voxel results using the group-MVPA derived seeds. Again, while associations in ASD-F were of primary interest given higher self-reported use of camouflaging (Hull et al. 2020), we report behavioral associations in ASD-M in Supplementary Fig. S3.
Diagnosis-by-camouflaging effects
Diagnosis-differential camouflaging-FC associations (Supplementary Table S1; Supplementary Table S2; Supplementary Fig. S1) and behavioral associations (Supplementary Fig. S4) were explored. Given the purpose of this paper to characterize sex-related circuits underpinning camouflaging in ASD, the Methods/Results/Discussion are documented in Supplementary Materials.
Results
Functional connectivity
“Sex-atypical” (sex-by-diagnosis-by-camouflaging) effects
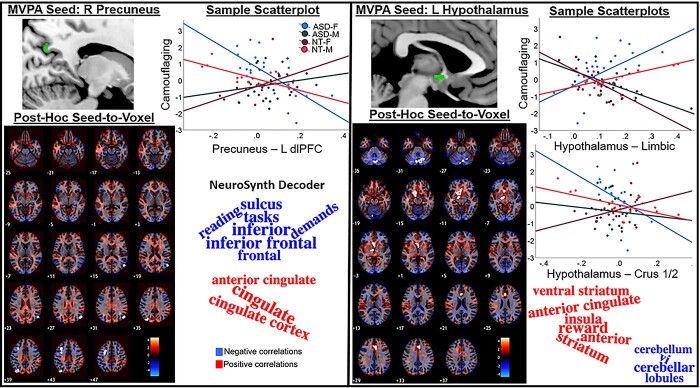
Group-MVPA revealed clusters in the precuneus and hypothalamus (Fig. 1; Table 2). For both regions, ASD-F showed “male-typical” camouflaging-FC associations. ASD-M showed inverse “female-typical” associations. For the precuneus, more positive FC with the left dorsolateral prefrontal cortex (dlPFC) and bilateral superolateral occipital cortex (sLOC; Supplementary Table S2) was linked to less camouflaging in ASD-F/NT-M and inverse patterns in ASD-M/NT-F. For the hypothalamus, more positive FC with a left lateralized limbic cluster (basal forebrain/left orbitofrontal cortex, left ventral striatum/thalamus/substantia nigra) and the right anterior cingulate cortex (ACC) as well as more negative FC with a cerebellar cluster (bilateral crus 2/1, right lobule 6) was associated with higher levels of camouflaging in ASD-F/NT-M and lower in ASD-M/NT-F.
Fig. 1.
MVPA-derived clusters showing “sex-atypical” FC patterns predicting camouflaging in ASD. ASD-F showed “male-typical” and ASD-M showed “female-typical” camouflaging-FC associations. Post-hoc seed-to-voxel results display unthresholded t-maps with FDR-surviving clusters outlined in white contours. Scatterplots show group-wise linear effects for the most significant clusters surviving FDR-correction for both positive and negative contrasts (if significant) plotted against CAT-Q scores (adjusted to account for age- and severity-related variability). Word clouds were generated by submitting unthresholded t-maps from post-hoc MVPA seed-to-voxel contrast to the Neurosynth decoder and plotting the top 10 terms (based on correlation absolute values). *right (R); left (L).
Table 2.
Sex-atypical and sex-typical camouflaging-FC associations in ASD revealed via group-MVPA.
| Cluster details | Cluster-level inference | a Groupwise: Predicts CAT-Q? | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex-atypical patterns | Cluster (x, y, z) | Size | Size P-FDR | Size P-unc | Peak P-unc | F (15,61) | ASD-F | ASD-M | NT-F | NT-M |
| R Precuneus | 16, −64, 34 | 23 | 0.021 | 0.000 | 1.000 | 3.54 | b Yes | No | No | c Yes |
| L Hypothalamus | −4, 0, −6 | 17 | 0.048 | <0.0001 | 1.000 | 2.19 | d Yes | e Yes | f Yes | g Yes |
| Sex-typical patterns | ||||||||||
| R dACC | 8, 26, 28 | 56 | <0.0001 | 0.000 | 1.000 | 4.83 | h Yes | No | No | iYes |
| R aPaHC | 22, −14, −36 | 29 | 0.003 | <0.0001 | 0.021 | 2.03 | No | No | i Yes | j Yes |
aMean clusterwise FC from significant MVPA post-hoc seed-to-voxel clusters was entered into regression to predict residualized CAT-Q (partialing out age/SRS-2 variance) for each group.
b F (3,20) = 6.08, P = 0.004.
c F (3,15) = 10.49, P = 0.001.
d F (3,20) = 4.35, P = 0.016.
e F (3,17) = 4.70, P = 0.014.
f F (3,17) = 5.13, P = 0.010.
g F (3,15) = 5.33, P = 0.011.
h F (10,13) = 3.10, P = 0.030.
i F (7,13) = 3.22, P = 0.033.
j F (7,11) = 3.24, P = 0.040; right (R); left (L).
k F (10,8) = 8.72, P = 0.003.
“Sex-typical” (sex-by-camouflaging) effects
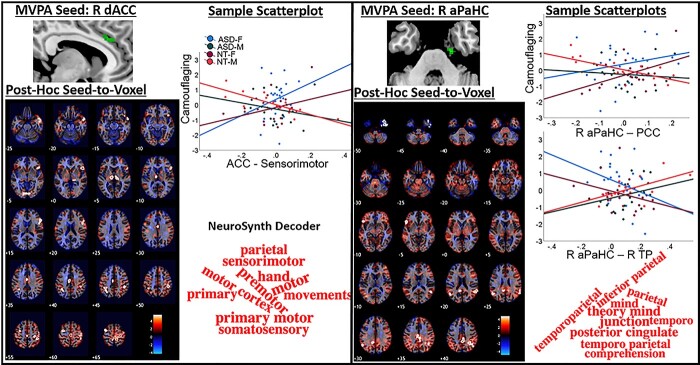
This contrast revealed significant MVPA clusters in the right anterior cingulate and right anterior parahippocampus (aPaHC; Fig. 2; Table 2). Greater right anterior cingulate FC with bilateral sensorimotor, lateral parietal, thalamic, and lingual regions; right ventral prefrontal cortex and anterior temporal cortex; left dlPFC; and mid-cingulate cortex (MCC; Supplementary Table S2) was linked to more camouflaging in females and less in males. For the right aPaHC, higher FC with bilateral parietal and left ventral prefrontal cortex as well as lower FC with the right temporal pole (TP) was associated with more camouflaging in females and less in males.
Fig. 2.
MVPA-derived clusters showing “sex-typical” FC patterns predicting camouflaging in ASD. ASD-F showed “female-typical” and ASD-M showed “male-typical” camouflaging-FC associations. Post-hoc seed-to-voxel results display unthresholded t-maps with FDR-surviving clusters outlined in white contours. Scatterplots show groupwise linear effects for the most significant clusters surviving FDR-correction for both positive and negative contrasts (if significant) plotted against CAT-Q scores (adjusted to account for age- and severity-related variability). Word clouds were generated by submitting unthresholded t-maps from post-hoc MVPA seed-to-voxel contrast to the Neurosynth decoder and plotting the top 10 terms (based on correlation absolute values). *right (R); dorsal ACC (dACC); posterior cingulate cortex (PCC).
Behavior associations
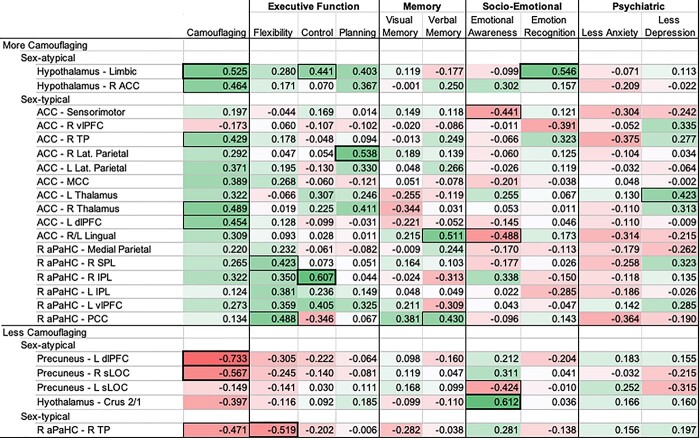
More positive FC that was linked to greater camouflaging in ASD-F was generally also linked to better executive functioning (Fig. 3). Furthermore, hypothalamic-limbic FC was the strongest positive predictor of camouflaging and was also positively linked to cognitive control and facial emotion recognition. Specific to the right anterior cingulate, more positive FC was linked to greater camouflaging but poorer emotional self-awareness. For patterns where more positive FC was linked to less camouflaging, there was also a general pattern, albeit nonsignificant, where more positive FC was associated with poorer executive functioning.
Fig. 3.
Exploratory behavioral associations in ASD-F to examine the adaptive nature of sex-related FC patterns implicated in camouflaging. Coefficients are partial Pearson correlations with SRS-2/age covariates. Dark bold boxes indicate P ≤ 0.01 and thin outlines P ≤ 0.05. For all subscales, positive correlations (green) indicate that more positive FC is linked to more camouflaging, better performance on cognitive/emotional tasks or attenuated anxiety/depression. In contrast, negative correlations (red) indicate that more positive FC is linked to less camouflaging, worse performance on cognitive/emotional tasks or more anxiety/depression. Correlations were inverted (multiplied by −1) if higher scores on a cognitive/memory/psychiatric symptoms scale indicated worse performance/symptoms. *Camouflaging Autistic Traits Questionnaire Total Score (camouflaging); Wisconsin Card Sorting Task Perseverative Errors (flexibility); Stroop Color and Word Test Interference Score (control); Tower of London Total Correct (planning); Weschler Memory Test—Visual Reproduction Delayed Recall (verbal memory); Rey Auditory Verbal Learning Task A7 (verbal memory); Toronto Alexithymia Scale—26 (emotional awareness); Reading the Mind in the Eyes Task (emotion recognition); State Trait Anxiety Inventory Trait Score (anxiety); Beck Depression Inventory—II (depression); right (R); left (L); ventrolateral prefrontal cortex (vlPFC); lateral parietal (lat. Parietal); superior parietal lobule (SPL); inferior parietal lobule (IPL).
When more positive FC was linked to greater camouflaging in ASD-F, associations were inverse in ASD-M (as specified by “sex-atypical” and “sex-typical” group-MVPA contrasts). For the anterior cingulate, more positive FC was associated with less camouflaging, poorer cognitive control, better emotional self-awareness (also for more positive hypothalamic FC), and trends toward less depression/anxiety (Supplementary Fig. S3). For the aPaHC, associations with FC were nonsignificant or mixed in positive and negative directions for executive functioning, memory, and facial emotion recognition. When FC was positively associated with camouflaging in ASD-M (e.g. precuneus–right occipital; aPaHC–TP-FC), associations with other behavioral measures were largely nonsignificant.
Structural connectometry
Connectometry in ASD-F
QA was positively associated with the camouflaging variable (FDR < 0.05) in a track (total streamline count: 989) including the left anterior thalamic radiations (ATRs), forceps minor, bilateral parahippocampal cingulum, and bilateral cerebellum/vermis (Fig. 4). Negative correlations were found in a track (streamlines: 1560) comprising posterior commissural fibers (corpus callosum [CC]-body, tapetum [temporal fibers], forceps major), and the bilateral arcuate/superior longitudinal fasciculus (SLF).
Fig. 4.
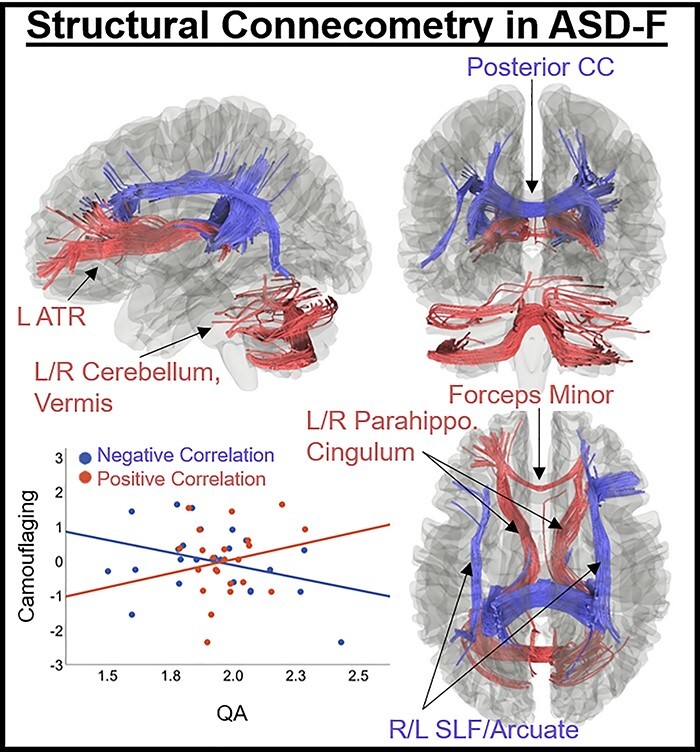
Results of connectometry in ASD-F revealing tracks where structural connectivity (QA) correlated with camouflaging (P < 0.05, FDR-corrected). Positive correlations (red) were found in a track comprising the left ATR, forceps minor, bilateral parahippocampal cingulum, and bilateral cerebellum/vermis. Negative correlations (blue) were found in the posterior corpus callosum (body/tapetum/forceps major) and bilateral arcuate/SLF. *Age, SRS-2, and DTI motion (average displacement relative to first nondiffusion volume) included as covariates in connectometry analyses.
Connectometry in ASD-M and NT groups
In ASD-M, QA was negatively associated with camouflaging only in the cerebellum (right hemisphere/vermis; 870 streamlines; Supplementary Fig. S2). In NT groups, sparse connectometry associations were observed, mostly localized to cerebellar tracts. NT-F showed positive associations in the right cerebellum and CC-tapetum (streamlines: 35), and NT-M showed positive associations in the middle cerebellar peduncle and left frontal aslant tract (streamlines: 22).
Discussion
Using a data-driven, connectome-wide analytic approach, we found distinct sex-related brain connectivity patterns associated with camouflaging in ASD-F vs. ASD-M. For ASD-F, “male-typical” FC patterns were found in the precuneus and hypothalamus and “female-typical” patterns in the right anterior cingulate and aPaHC. Hypothalamic-limbic FC was significantly associated with camouflaging across each group (ASD-F, ASD-M, NT-F, and NT-M), with “male-typical” positive associations in ASD-F and “female-typical” negative associations in ASD-M. This finding may suggest that “gender incoherent” (Bejerot et al. 2012) FC between the brain’s hormone center and reward circuitry contributes to sex-biased camouflaging patterns in ASD. In ASD-F, more positive hypothalamic-reward circuit FC may be an adaptive compensatory pattern given its (i) positive association with camouflaging, (ii) its link to better cognitive control and facial emotion recognition, and (iii) absent associations with depression/anxiety. Groupwise structural connectometry revealed substantial tracts associated with camouflaging in ASD-F, and sparse correlates outside of the cerebellum for ASD-M and NT groups. Furthermore, tracts implicated in camouflaging in ASD-F showed correlation patterns that were largely consistent with FC results and implicated overlapping circuits.
Sex-related FC patterns linked to camouflaging
More positive hypothalamic FC with reward centers was associated with more camouflaging in ASD-F (“male-typical”) and less camouflaging in ASD-M (“female-typical”). In ASD-F, higher hypothalamic-limbic FC also was linked to better cognitive control and facial emotion recognition. The hypothalamus is a sexually dimorphic structure (McCarthy 2016), responsible for neuroendocrine regulation of the autonomic nervous system (Saper and Lowell 2014). Although high-resolution spatial inference should be interpreted with caution, the hypothalamic region identified in this study overlapped most substantially with the paraventricular nucleus of the hypothalamus (Neudorfer et al. 2020), implicated in sex-differential regulation of social behavior (Rigney et al. 2021). Prior research highlights a complex interplay between hypothalamic neuropeptides (e.g. oxytocin, vasopressin) and sex hormone concentrations in socio-emotional processing (Kret and de Gelder 2012). When considered in this context of this study, ASD-related etiological factors may also impact hypothalamic function, potentially driving social processes that are more “male-typical” in ASD-F and “female-typical” ASD-M. Previous evidence has implicated reward centers in hormonally mediated female protection in ASD, such that more ASD-associated oxytocin receptor allele variants uniquely predict more positive left prefrontal-reward center FC in ASD-F, and this FC pattern is linked to reduced ASD socio-cognitive difficulties (Hernandez et al. 2020). Furthermore, reward centers play a role in sex-differential development of social behavior (Walker et al. 2017), motivated recruitment of cognitive control (Botvinick and Braver 2015), and emotional learning (Likhtik and Johansen 2019). The limbic reward cluster comprised dopaminergic regions implicated in reward processing (e.g. nigrostriatal pathway; Likhtik and Johansen 2019) and a hub of the cholinergic systems implicated in state arousal (e.g. basal forebrain; Záborszky et al. 2018). Future research is warranted to examine the sex-specific influence of hormones on major neurotransmitter systems in ASD as well as associated brain and behavioral/physiological features, in particular, social behavior, reward processing, and arousal. Finally, more positive hypothalamic-cerebellar FC was linked to less camouflaging in ASD-F (“male-typical”) and more camouflaging in ASD-M (“female-typical”). This circuit plays a role in implicit socio-emotional processing (Clausi et al. 2017), and our data suggest “sex-atypical” recruitment of hypothalamic-cerebellar FC for camouflaging in ASD.
The right precuneus also showed “sex-atypical” FC patterns linked to camouflaging in ASD. Greater FC with the left dlPFC and bilateral sLOC was associated with less camouflaging in ASD-F (“male-typical”) and inverse patterns in ASD-M (“female-typical”). The precuneus is a hub of the default mode network (DMN) implicated in task-negative reflective states and dynamic switching with task-positive networks (Riemer et al. 2020). Diverse term associations yielded by the Neurosynth Decoder may reflect the precuneus’ role in communication between task-positive and task-negative networks. The precuneus has also been extensively implicated as a sex hormone-sensitive region (Rehbein et al. 2020; Tan et al. 2020). Together, “sex-atypical” FC correlates of camouflaging highlight a complex interplay between sex-related biology and ASD etiology, potentially resulting in recruitment of “male-typical” FC patterns to support camouflaging in ASD-F and “female-typical” recruitment in ASD-M.
Right anterior cingulate FC with sensorimotor regions was a “sex-typical” FC pattern linked to camouflaging in ASD, such that more positive FC was linked to more camouflaging in females and less in males. The anterior cingulate is a hub of the salience network, involved in stimulus salience processing (Seeley et al. 2007). Altered sensory processing is a hallmark of ASD (Baum et al. 2015), and salience network FC with sensorimotor cortex has been implicated in the ASD sex bias (Lawrence et al. 2021). Specifically, irrespective of diagnosis, boys but not girls showed a relationship such that increasing polygenic ASD risk predicted greater salience network FC with sensorimotor cortex, suggesting that female biology may protect against ASD-risk gene mediated alterations in sensorimotor processing. Future research is warranted examining how this male penetrant feature of increased salience network-sensorimotor FC in ASD may reduce compensatory behaviors in ASD-M but increase them in ASD-F. We also found that greater anterior cingulate-sensorimotor FC was associated with poorer emotional self-awareness in ASD-F but greater emotional self-awareness in ASD-M. The salience network plays a role in emotional and empathic processing (Lamm et al. 2011), and FC with the sensorimotor cortex is implicated in somatic representation of emotions (Avenanti et al. 2005; Lindquist and Barrett 2012). Animal studies have shown that estrogen and testosterone inversely impact emotion context generalization (Asok et al. 2019), and it remains unknown how sex hormones uniquely influence emotion learning in ASD.
“Sex-typical” camouflaging-FC associations in ASD were also found in the right aPaHC. More positive FC with regions of the DMN (e.g. precuneus) was linked to more camouflaging in females and less in males. The parahippocampus acts as a hub linking the DMN with the medial temporal memory system (Ward et al. 2014) and may process stimulus familiarity (Kafkas and Montaldi 2018). Greater right parahippocampal-precuneus FC has been associated with better episodic remembering (Sheldon et al. 2016). From a neurochemical perspective, engagement of the right aPaHC/anterior cingulate and precuneus during memory tasks in women is altered by estrogen and anticholinergic drugs (Dumas et al. 2012), suggesting that these regions may constitute neuromodulatory hubs, where hormones and neurotransmitters interact to influence state arousal.
Coherence of structural connectivity results
Structural connectivity associations with camouflaging were substantial in ASD-F, and largely absent outside of cerebellar tracts for ASD-M and NT groups. Furthermore, structural connectivity correlates of camouflaging in ASD-F broadly aligned with sex-related circuits identified in FC results. For example, higher structural connectivity in limbic and cerebellar tracts was linked to greater camouflaging in ASD-F. The left-dominant ATRs, anterior commissural fibers, and the medial cingulum form part of the limbic pathway connecting the hypothalamus with emotion, cognitive control, reward, and cerebellar circuits (Grodd et al. 2020). These tract connections are coherent with “sex-atypical” FC results implicating hypothalamic FC with orbitofrontal/ventral striatal, anterior cingulate, and cerebellar regions. Furthermore, the direction of effects was largely consistent, where higher connectivity was associated with more camouflaging in ASD-F. In addition, the cingulum was implicated in positive structural connectivity-camouflaging associations in ASD-F. This tract connects medial regions of the DMN (Sandhu et al. 2021) and the parahippocampus (Lin et al. 2021), which is consistent with “sex-typical” results showing that higher right anterior parahippocampal FC with the DMN was linked to more camouflaging in ASD-F. Finally, higher structural connectivity in parietal tracts connecting medial/lateral parietal and occipital cortex (Hofer and Frahm 2006) to precentral (Ramos-Fresnedo et al. 2019) and dorsolateral prefrontal regions (Catani et al. 2012) was associated with less camouflaging in ASD-F. This finding is largely consistent with “sex-atypical” FC results in the right precuneus, where greater FC with superolateral occipital and dlPFC was associated with less camouflaging in ASD-F. The consistent direction of camouflaging-connectivity associations and coherence of circuits implicated across FC and structural connectivity analyses increases confidence in FC findings, suggesting that they are not influenced by scanner-related confound variables (e.g. head motion).
Sex-related biological mechanisms
The clusters identified in MVPA analyses—including the hypothalamus, precuneus, right anterior cingulate, and aPaHC—are all sex-hormone sensitive regions (Dumas et al. 2012; Tan et al. 2020). Albeit not consistently replicated (May et al. 2021), various studies have linked elevated prenatal sex steroid exposure to ASD (Ingudomnukul et al. 2007; Auyeung et al. 2013; Baron-Cohen et al. 2015, 2020; Simantov et al. 2021). Prenatal sex steroid exposure in female mice results in “masculinized” brain features (McCarthy et al. 2017). Thus, such exposure would be expected to result in some brain features being more “male-typical” in ASD-F, in alignment with observed connectivity patterns in this study. It should be noted that elevated prenatal sex steroid exposure has not been consistently linked to ASD (May et al. 2021), and other prenatal neuroinflammatory triggers (other than sex steroids) may result in similar “masculine” features (Barrientos et al. 2019; Breach et al. 2021). Furthermore, other explanations are plausible and may not be mutually exclusive. For example, sex steroid influence cannot be easily disentangled from other aspects of sex-related biology. A recent study highlights the X-chromosome’s privileged influence on neuroanatomical variation (Mallard et al. 2021). Regions linked to enriched X-chromosome morphological influence overlap with the “sex-typical” right anterior cingulate/aPaHC and the “sex-atypical” precuneus nodes linked to camouflaging in this study. It is plausible that processes such as escape from X inactivation may preserve function in sex-related pathways affected by ASD risk genes (Ferri et al. 2018). The results of this study suggest a role for hormonally mediated processes in sex-biased compensatory behavior in ASD, but other aspects of sex-specific biology (e.g. sex chromosomes) may also play a role and require further investigation.
Limitations
The small study sample and high model complexity are a limitation. However, the biological plausibility of findings revealed via data-driven methods is encouraging. To ensure results are not sample-specific, replication is needed. Furthermore, the sample age range was broad (18–70 years) and inclusion of pre-/postmenopausal women and younger/older men suggests diverse circulating sex hormone levels. However, larger hormonal variance may improve sensitivity to sex-related effects in these dimensional analyses. Given that brain connectivity patterns linked to ASD vary across ages/developmental stages (Olson et al. 2020; Walsh et al. 2021), testing whether results extend to younger cohorts is an important future direction. Furthermore, the exploratory investigations of the adaptive nature of camouflaging FC patterns via correlations with behavior were uncorrected for multiple comparisons. Future investigations would benefit from larger samples with rich phenotypic data to validate the current results. Finally, it should be noted that this study is not advocating the need to compensate for/camouflage social differences in ASD. We acknowledge that camouflaging is associated with poorer mental health in ASD (Tubío-Fungueiriño et al. 2020). Instead, the purpose of this study was to capitalize on observed sex difference in self-reported camouflaging in ASD due to its potential sensitivity to neurobiological differences related to the sex bias.
Conclusion
This study is the first to characterize sex-related connectivity correlates of compensatory social behaviors in ASD (e.g. camouflaging) using a data-driven, connectome-wide neuroimaging approach. This study lends insight into the ASD sex bias, suggesting both “male-typical” and “female-typical” brain connectivity patterns play a role in camouflaging in ASD-F. Circuits liked to greater camouflaging in ASD-F were associated with reward (“sex-atypical” pathway), emotion, and memory retrieval (“sex-typical” pathways). Intriguingly, more positive FC between the hypothalamus and limbic reward circuits was linked to more camouflaging, better cognitive control/emotion recognition, and showed no association with depression/anxiety in ASD-F, suggesting that this “male-typical” FC pattern may be particularly adaptive. The observed “male-typical” patterns supporting camouflaging in ASD-F are consistent with fetal neuroinflammatory hypotheses (e.g. fetal steroidogenic hypothesis; Ingudomnukul et al. 2007; Auyeung et al. 2013; Baron-Cohen et al. 2015, 2020; Simantov et al. 2021; see Barrientos et al. 2019; Breach et al. 2021 for other neuroimmune hypotheses), given that such exposure in females would result in “male-typical” brain features (McCarthy and Wright 2017). However, these hypotheses remain controversial, and more research is needed to characterize environmental processes that may result in “sex-atypical” brain and behavioral features in ASD. Furthermore, the growing literature on sex differences in the brain suggests that both “masculine” and “feminine” processes interact with individual genetics and environment across development in a time-sensitive manner to produce an individual’s brain “mosaic” (Joel et al. 2015; Joel and McCarthy 2017; Eliot et al. 2021). In keeping, the “female-typical” FC patterns linked to camouflaging suggest that female sex chromosomes or reproductive biology (e.g. ovarian hormones) also influence social compensatory behavior in ASD-F and, potentially, the broader sex bias in ASD. Further research is warranted investigating sex-differential brain and behavioral development in ASD across the lifespan, especially during sensitive windows of hormone transition, and its relationship to sex-specific biology (e.g. hormones, genetics). Furthermore, research characterizing broader sex-related neurochemical and hormonal influences (and their interaction) on FC and behavior in ASD is needed, in particular focusing on reward, cognitive, emotion, memory, and social circuits/behavior.
Supplementary Material
Acknowledgments
We are grateful to our participants who made this study possible and to Sharmeen Maze for her dedication to pristine MRI data collection.
Contributor Information
Melissa J M Walsh, College of Health Solutions, Arizona State University, Tempe, AZ 85281, USA.
Broc Pagni, College of Health Solutions, Arizona State University, Tempe, AZ 85281, USA.
Leanna Monahan, College of Health Solutions, Arizona State University, Tempe, AZ 85281, USA.
Shanna Delaney, College of Health Solutions, Arizona State University, Tempe, AZ 85281, USA.
Christopher J Smith, Southwest Autism Research and Resource Center, Phoenix, AZ 85006, USA.
Leslie Baxter, Mayo Clinic of Arizona, Phoenix, AZ 85054, USA.
B Blair Braden, College of Health Solutions, Arizona State University, Tempe, AZ 85281, USA.
Funding
The data collection was supported by the National Institute of Mental Health [1K01MH116098], Department of Defense [AR140105], and the Arizona Biomedical Research Commission [ADHS16-162413]. The conceptualization, formal analysis, and manuscript preparation was supported by the National Institute of Mental Health [1F31MH122107; 1K01MH116098]. The funding sources had no involvement in the research or preparation of this article. Conflict of interest statement. The authors report no competing interests.
References
- Allely CS. Understanding and recognising the female phenotype of autism spectrum disorder and the “camouflage” hypothesis: a systematic PRISMA review. Adv Autism. 2019:5:14–37. [Google Scholar]
- Anteraper SA, Guell X, D’Mello A, Joshi N, Whitfield-Gabrieli S, Joshi G. Disrupted cerebrocerebellar intrinsic functional connectivity in young adults with high-functioning autism spectrum disorder: a data-driven, whole-brain, high-temporal resolution functional magnetic resonance imaging study. Brain Connect. 2019:9:48–59. [DOI] [PubMed] [Google Scholar]
- Anteraper SA, Collin G, Guell X, Scheinert T, Molokotos E, Henriksen MT, Mesholam-Gately R, Thermenos HW, Seidman LJ, Keshavan MS, et al. Altered resting-state functional connectivity in young children at familial high risk for psychotic illness: a preliminary study. Schizophr Res. 2020:216:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Kandel ER, Rayman JB. The neurobiology of fear generalization. Front Behav Neurosci. 2019:12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV, Baron-Cohen S. Prenatal and postnatal hormone effects on the human brain and cognition. Pflug Arch Eur J Physiol. 2013:465:557–571. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005:8:955–960. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002:6:248–254. [DOI] [PubMed] [Google Scholar]
- Baron-cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “reading the mind in the eyes” test revised version: a study with normal adults, adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiat. 2001:42:241–251. [PubMed] [Google Scholar]
- Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Cohen AS, Chakrabarti B, Ruta L, Lombardo MV. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015:20:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Tsompanidis A, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah M, Cohen A, Pohl A. Foetal oestrogens and autism. Mol Psychiatry. 2020:25:2970–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Brunton PJ, Lenz KM, Pyter L, Spencer SJ. Neuroimmunology of the female brain across the lifespan: plasticity to psychopathology. Brain Behav Immun. 2019:79:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum SH, Stevenson RA, Wallace MT. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog Neurobiol. 2015:134:140–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LC, Nespodzany A, Walsh MJM, Wood E, Smith CJ, Braden BB. The influence of age and ASD on verbal fluency networks. Res Autism Spectr Disord. 2019:63:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996:67:588–597. [DOI] [PubMed] [Google Scholar]
- Bedford SA, Park MTM, Devenyi GA, Tullo S, Germann J, Patel R, Anagnostou E, Baron-Cohen S, Bullmore ET, Chura LR, et al. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol Psychiatry. 2019:25:614–628. [DOI] [PubMed] [Google Scholar]
- Bejerot S, Eriksson JM, Bonde S, Carlström K, Humble MB, Eriksson E. The extreme male brain revisited: gender coherence in adults with autism spectrum disorder. Br J Psychiatry. 2012:201:116–123. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol. 2015:66:83–113. [DOI] [PubMed] [Google Scholar]
- Breach MR, Dye CN, Joshi A, Platko S, Gilfarb RA, Krug AR, Franceschelli DV, Galan A, Dodson CM, Lenz. Maternal allergic inflammation in rats impacts the offspring perinatal neuroimmune milieu and the development of social play, locomotor behavior, and cognitive flexibility. Brain Behav Immun. 2021:95:269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2012:2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, Murphy DG, Thiebaut de Schotten M. Beyond cortical localization in clinico-anatomical correlation. Cortex. 2012:48:1262–1287. [DOI] [PubMed] [Google Scholar]
- Clausi S, Iacobacci C, Lupo M, Olivito G, Molinari M, Leggio M. The role of the cerebellum in unconscious and conscious processing of emotions: a review. Appl Sci (Switz). 2017:7:21–24. [Google Scholar]
- Constantino JN, Gruber CP. The social responsiveness scale, second edition (SRS-2). Los Angeles, CA: Western Psychological Services; 2012.
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol treatment altered anticholinergic-related brain activation during working memory in postmenopausal women. NeuroImage. 2012:60:1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Murphy D. Neuroimaging in autism-from basic science to translational research. Nat Rev Neurol. 2014:10:82–91. [DOI] [PubMed] [Google Scholar]
- Eliot L, Ahmed A, Khan H, Patel J. Dump the “dimorphism”: comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci Biobehav Rev. 2021:125:667–697. [DOI] [PubMed] [Google Scholar]
- Ferri SL, Abel T, Brodkin ES. Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep. 2018:20:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975:12:189–198. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Mallar Chakravarty M, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A. 2014:111:E4367–E4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodd W, Kumar VJ, Schüz A, Lindig T, Scheffler K. The anterior and medial thalamic nuclei and the human limbic system: tracing the structural connectivity using diffusion-weighted imaging. Sci Rep. 2020:10:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Lawrence KE, Padgaonkar NT, Inada M, Hoekstra JN, Lowe JK, Eilbott J, Jack A, Aylward E, Gaab N, et al. Imaging-genetics of sex differences in ASD: distinct effects of OXTR variants on brain connectivity. Transl Psychiatry. 2020:10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited-comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006:32:989–994. [DOI] [PubMed] [Google Scholar]
- Hull L, Petrides KV, Allison C, Smith P, Baron-Cohen S, Lai M-C, Mandy W. “Putting on my best normal”: social camouflaging in adults with autism Spectrum conditions. J Autism Dev Disord. 2017:47:2519–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull L, Mandy W, Lai MC, Baron-Cohen S, Allison C, Smith P, Petrides KV. Development and validation of the camouflaging autistic traits questionnaire (CAT-Q). J Autism Dev Disord. 2018:49:819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull L, Lai MC, Baron-Cohen S, Allison C, Smith P, Petrides K, v., Mandy W. Gender differences in self-reported camouflaging in autistic and non-autistic adults. Autism. 2020:24:352–363. [DOI] [PubMed] [Google Scholar]
- Hull L, Petrides K, v., Mandy W. Cognitive predictors of self-reported camouflaging in autistic adolescents. Autism Res. 2021:14:523–532. [DOI] [PubMed] [Google Scholar]
- Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm Behav. 2007:51:597–604. [DOI] [PubMed] [Google Scholar]
- Joel D, McCarthy MM. Incorporating sex as a biological variable in neuropsychiatric research: where are we now and where should we be? Neuropsychopharmacology. 2017:42:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, Shefi N, Pool J, Urchs S, Margulies DS, et al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci U S A. 2015:112:15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. How do memory systems detect and respond to novelty? Neurosci Lett. 2018:680:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS KN. 2004. KBIT-2: Kaufman brief intelligence test. 2nd ed. Upper Saddle River (NJ): Pearson Education, Inc. [Google Scholar]
- Kelly E, Meng F, Fujita H, Morgado F, Kazemi Y, Rice LC, Ren C, Escamilla CO, Gibson JM, Sajadi S, et al. Regulation of autism-relevant behaviors by cerebellar–prefrontal cortical circuits. Nat Neurosci. 2020:23:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel LT, Werling DM. Neural transcriptomic analysis of sex differences in autism spectrum disorder: current insights and future directions. Biol Psychiatry. 2022:91:53–60. [DOI] [PubMed] [Google Scholar]
- Kret ME, de Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012:50:1211–1221. [DOI] [PubMed] [Google Scholar]
- Kupfer J, Brosig B, Brähler E. Testing and validation of the 26-item Toronto alexithymia scale in a representative population sample. Z Psychosom Med Psychother. 2000:46:368–384. [DOI] [PubMed] [Google Scholar]
- Lai MC, Szatmari P. Sex and gender impacts on the behavioural presentation and recognition of autism. Curr Opin Psychiatry. 2020:33:117–123. [DOI] [PubMed] [Google Scholar]
- Lai M, Lombardo MV, Ruigrok ANV, Chakrabarti B, Auyeung B, Szatmari P, Happé F, Baron-cohen S, MRCA C. Quantifying and exploring camouflaging in men and women with autism. Autism. 2017:21:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Chakrabarti B, Ruigrok ANV, Bullmore ET, Suckling J, Auyeung B, Happé F, Szatmari P, Baron-Cohen S, et al. Neural self-representation in autistic women and association with compensatory camouflaging. Autism. 2019:23:1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011:54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Hernandez LM, Fuster E, Padgaonkar NT, Patterson G, Jung J, Okada NJ, Lowe JK, Hoekstra JN, Jack A, et al. Impact of autism genetic risk on brain connectivity: a mechanism for the female protective effect. Brain. 2021. https://academic.oup.com/brain/advance-article-abstract/doi/10.1093/brain/awab204/6288451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever AG, Geurts HM. Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. J Autism Dev Disord. 2016:46:1916–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Johansen JP. Neuromodulation in circuits of aversive emotional learning. Nat Neurosci. 2019:22:1586–1597. [DOI] [PubMed] [Google Scholar]
- Lin YH, Dhanaraj V, Mackenzie AE, Young IM, Tanglay O, Briggs RG, Chakraborty AR, Hormovas J, Fonseka RD, Kim SJ, et al. Anatomy and white matter connections of the parahippocampal gyrus. World Neurosurg. 2021:148:e218–e226. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Barrett LF. A functional architecture of the human brain: insights from emotion. Trends Cogn Sci. 2012:16:533–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston LA, Happé F. Conceptualising compensation in neurodevelopmental disorders: reflections from autism spectrum disorder. Neurosci Biobehav Rev. 2017:80:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, Polmear W, Mandy L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017:56:466–474. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule, second edition (ADOS-2) manual (Part I): Modules 1–4. Torrance, CA: Western Psychological Services; 2012.
- Mallard TT, Liu S, Seidlitz J, Ma Z, Moraczewski D, Thomas A, Raznahan A. X-chromosome influences on neuroanatomical variation in humans. Nat Neurosci. 2021:24:1216–1224. [DOI] [PubMed] [Google Scholar]
- May T, Yi KLJ, Loveland KL, Vollenhoven B, Williams K. Overlap of autism and conditions associated with atypical sex hormone levels or response: a systematic review and meta-analysis. Res Autism Spectr Disord. 2021:80:1–22. [Google Scholar]
- McCarthy MM. Multifaceted origins of sex differences in the brain. Philos Trans R Soc Lond B Biol Sci. 2016:371:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL. Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biol Psychiatry. 2017:81:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM, Lenz KM. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat Rev Neurosci. 2017:18:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memari AH, Ziaee V, Shayestehfar M, Ghanouni P, Mansournia MA, Moshayedi P. Cognitive flexibility impairments in children with autism spectrum disorders: links to age, gender and child outcomes. Res Dev Disabil. 2013:34:3218–3225. [DOI] [PubMed] [Google Scholar]
- Morrison KE, DeBrabander KM, Jones DR, Ackerman RA, Sasson NJ. Social cognition, social skill, and social motivation minimally predict social interaction outcomes for autistic and non-autistic adults. Front Psychol. 2020:11:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschellia J, Nebelb MB, Caffoa BS, Barberb AD, Pekard JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. NeuroImage. 2014:96:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorfer C, Germann J, Elias GJB, Gramer R, Boutet A, Lozano AM. A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Sci Data. 2020:7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LA, Mash LE, Linke A, Fong CH, Müller RA, Fishman I. Sex-related patterns of intrinsic functional connectivity in children and adolescents with autism spectrum disorders. Autism. 2020:24:2190–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vértes PE, Ersche KD, Suckling J, Bullmore ET. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. NeuroImage. 2014:95:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posserud M, Solberg BS, Engeland A, Haavik J, Klungsøyr K. Male-female ratios in autism spectrum disorders by age, intellectual disability and attention deficit/hyperactivity disorder. Acta Psychiatr Scand. 2021:144:635–646. [DOI] [PubMed] [Google Scholar]
- Ramos-Fresnedo A, Segura-Duran I, Chaichana KL, Pillai JJ. Supratentorial white matter tracts. In: Chaichana K, Quinones-Hinojosa A, eds. Comprehensive overview of modern surgical approaches to intrinsic brain tumors. Cambridge, MA: Academic Press; 2019. pp. 23–35 [Google Scholar]
- Rehbein E, Hornung J, Sundström Poromaa I, Derntl B. Shaping of the female human brain by sex hormones – a review. Neuroendocrinology. 2020:111:183–206. [DOI] [PubMed] [Google Scholar]
- Ridley R. Some difficulties behind the concept of the ‘extreme male brain’ in autism research. A theoretical review. Res Autism Spectr Disord. 2019:57:19–27. [Google Scholar]
- Riemer F, Grüner R, Beresniewicz J, Kazimierczak K, Ersland L, Hugdahl K. Dynamic switching between intrinsic and extrinsic mode networks as demands change from passive to active processing. Sci Rep. 2020:10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigney N, Whylings J, De Vries GJ, Petrulis A. Sex differences in the control of social investigation and anxiety by vasopressin cells of the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 2021:111:521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu Z, Tanglay O, Young IM, Briggs RG, Bai MY, Larsen ML, Conner AK, Dhanaraj V, Lin YH, Hormovas J, et al. Parcellation-based anatomic modeling of the default mode network. Brain Behav. 2021:11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Lowell BB. The hypothalamus. Curr Biol. 2014:24:R1111–R1116. [DOI] [PubMed] [Google Scholar]
- Scarpina F, Tagini S. The stroop color and word test. Front Psychol. 2017:8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Rey auditory verbal learning test: a handbook. Los Angeles (CA): Western Psychological Services; 1996 [Google Scholar]
- Schneider MA, Spritzer PM, Minuzzi L, Frey BN, Syan SK, Fighera TM, Schwarz K, Costa ÂB, da Silva DC, Garcia CCG, et al. Effects of estradiol therapy on resting-state functional connectivity of transgender women after gender-affirming related gonadectomy. Front Neurosci. 2019:13:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007:27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments in planning. Phil Trans R Soc Lond. 1982:298:199–209. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly C, Reiss PT, Cameron Craddock R, Emerson JW, McMahon K, Copland DA, Xavier Castellanos F, Milham MP. A multivariate distance-based analytic framework for connectome-wide association studies. NeuroImage. 2014:93:74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Farb N, Palombo DJ, Levine B. Intrinsic medial temporal lobe connectivity relates to individual differences in episodic autobiographical remembering. Cortex. 2016:74:206–216. [DOI] [PubMed] [Google Scholar]
- Simantov T, Pohl A, Tsompanidis A, Weir E, Lombardo MV, Ruigrok A, Smith P, Allison C, Baron-Cohen S, Uzefovsky F. Medical symptoms and conditions in autistic women. Autism. 2021:26:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-trait anxiety inventory: STAI. Palo Alto, CA: Consulting Psychologists Press; 1983.
- Tan GCY, Chu C, Lee YT, Tan CCK, Ashburner J, Wood NW, Frackowiak RS. The influence of microsatellite polymorphisms in sex steroid receptor genes ESR1, ESR2 and AR on sex differences in brain structure. NeuroImage. 2020:221:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubío-Fungueiriño M, Cruz S, Sampaio A, Carracedo A, Fernández-Prieto M. Social camouflaging in females with autism spectrum disorder: a systematic review. J Autism Dev Disord. 2020:51:2190–2199. [DOI] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ. Adolescence and reward: making sense of neural and behavioral changes amid the chaos. J Neurosci. 2017:37:10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJM, Baxter LC, Smith CJ, Braden BB. Age group differences in executive network functional connectivity and relationships with social behavior in adults with autism spectrum disorder. Res Autism Spectr Disord. 2019:63:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJM, Wallace GL, Gallegos SM, Braden BB. Brain-based sex differences in autism spectrum disorder across the lifespan: a systematic review of structural MRI, fMRI, and DTI findings. NeuroImage Clin. 2021:31:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014:35:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale-third edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Werling DM. The role of sex-differential biology in risk for autism spectrum disorder. Biol Sex Differ. 2016:7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013:26:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall DR, Anteraper SA, Chaddock-Heyman L, Drollette ES, Raine LB, Whitfield-Gabrieli S, Kramer AF, Hillman CH. Resting-state functional connectivity and scholastic performance in preadolescent children: a data-driven multivoxel pattern analysis (mvpa). J Clin Med. 2020:9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. CONN: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012:2:125–141. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Tseng WYI. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. NeuroImage. 2011:58:91–99. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Wedeen VJ, Tseng WYI. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010:29:1626–1635. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WYI. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013:8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Badre D, Verstynen T. Connectometry: a statistical approach harnessing the analytical potential of the local connectome. NeuroImage. 2016:125:162–171. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Vettel JM, Singh A, Poczos B, Grafton ST, Erickson KI, Tseng WYI, Verstynen TD. Quantifying differences and similarities in whole-brain white matter architecture using local connectome fingerprints. PLoS Comput Biol. 2016:12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Panesar S, Barrios J, Fernandes D, Abhinav K, Meola A, Fernandez-Miranda JC. Automatic removal of false connections in diffusion MRI tractography using topology-informed pruning (TIP). Neurotherapeutics. 2019:16:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L, Gombkoto P, Varsanyi P, Gielow MR, Poe G, Role LW, Ananth M, Rajebhosale P, Talmage DA, Hasselmo ME, et al. Specific basal forebrain–cortical cholinergic circuits coordinate cognitive operations. J Neurosci. 2018:38:9446–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.