Abstract
Per- and polyfluoroalkyl substances (PFAS) are environmentally persistent endocrine-disrupting chemicals associated with long-term health outcomes. PFAS are transferred from maternal blood to human milk, an important exposure source for infants, and understanding of this transfer is evolving. We characterized concentrations of 10 PFAS in human milk (n = 426) and compared milk-to-plasma concentrations of 9 PFAS among a subset of women with paired samples (n = 294) from the New Hampshire Birth Cohort Study using liquid chromatography-isotope dilution tandem mass spectrometry. We examined the relationship between perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in plasma versus milk and fit linear regression models to assess relationships between milk PFOA and PFOS and participant characteristics. The median plasma PFOA concentration was 0.94 ng/mL (interquartile range, IQR, 0.59–1.34) and that of PFOS was 2.60 ng/mL (IQR 1.80–3.90); the median milk PFOA concentration was 0.017 ng/mL (IQR 0.012–0.027) and that of PFOS was 0.024 ng/mL (IQR 0.016–0.036). PFOA and PFOS plasma and milk concentrations showed correlations of ρ = 0.83 and 0.77, respectively (p < 0.001). Parity, previous lactation, week of milk collection, and body mass index were inversely associated with milk PFAS. We estimate that even among our general population cohort, some infants (∼6.5%) are exposed to amounts of PFAS via milk that may have long-term health impacts.
Keywords: per- and polyfluoroalkyl substances, human milk, endocrine-disrupting chemicals, breastfeeding
Short abstract
This study shows that maternal plasma concentrations of PFOA and PFOS correlate with milk concentrations of PFOA and PFOS, indicating a transfer of these chemicals from maternal plasma to milk.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of environmentally persistent chemicals used in oil- and water-resistant consumer products, firefighting foams, and industrial surfactants.1 PFAS have been detected in air, biota, water, and soil across the globe,2 and adult humans are exposed via contaminated food and water.3,4 Some PFAS accumulate in the body and are mobilized into human milk during periods of lactation via both active and passive transport from maternal plasma.5,6 For human infants, an important pathway of PFAS exposure is through human milk.7−9
While the rates of maternal-to-infant PFAS transfer are low compared to those of other persistent environmental toxicants,10 there is evidence that early life exposure to even small amounts of PFAS can have lasting health effects.11 Epidemiologic studies show that early life exposure to PFAS via the placenta and human milk may be associated with reduced birthweight and early life growth,8,12−14 attention deficit hyperactivity disorders,15 and delayed communication development.16 Over the lifetime, concentrations of some PFAS in blood are associated with decreased vaccine immune response, thyroid and kidney dysfunction, renal and testicular cancer, elevated cholesterol, decreased breastfeeding duration, elevated liver enzymes, and hypertensive disorders of pregnancy.5,17−24 Multiple studies have described concentrations of PFAS in maternal blood and human milk individually, but limited research has described concentrations in maternal blood and milk of the same subjects.25,26 Furthermore, sample sizes of previous studies have been limited.25,26 Our study describes the concentrations of PFAS in paired maternal plasma and milk samples among 294 participants in the New Hampshire Birth Cohort Study (NHBCS), a rural pregnancy and birth cohort.
Presently, there are no established minimum risk levels (MRLs) for infants or tolerable PFAS intake values for human milk, although the European Food Safety Authority (EFSA) 2020 determined PFAS milk concentrations corresponding to a maternal tolerable weekly intake (TWI) level for four legacy PFAS.27 Our study seeks to compare human milk PFAS concentrations in our cohort to these estimated standards for tolerable PFAS exposure.
Materials and Methods
Study Population
The NHBCS is a rural, ongoing pregnancy cohort beginning in 2009 that has accrued over 2500 maternal–infant dyads with no known source of PFAS drinking water contamination. The NHBCS has been described elsewhere.28 Briefly, a series of questionnaires and medical record reviews collected extensive information on lifestyle and demographic factors, pregnancy and birth outcomes, and pregnancy and lactation history. Biological samples, including maternal plasma and human milk, were collected for analysis. The current study used data from a subset of 426 mother–infant pairs who provided milk samples between 2014 and 2019. Among these pairs, plasma PFAS had been measured in 294 mothers.29 The study was approved by the Committee for the Protection of Human Subjects at Dartmouth College, and all participants provided informed consent.
Maternal Plasma PFAS Concentrations
Maternal blood was collected from participants during pregnancy in clinic at the time of the routine antenatal oral glucose tolerance test (median 27.9 weeks, interquartile range, IQR 27.4–28.7) and processed into aliquots of plasma within 24 h of sampling. Samples were stored at −80 °C until overnight shipment on dry ice to the Centers for Disease Control and Prevention (CDC) National Center for Environmental Health laboratory where they remained stored at −80 °C until analysis. The CDC laboratory’s involvement did not constitute engagement in human subjects research. Blood plasma was analyzed for nine PFAS using on-line solid phase extraction liquid chromatography-isotope dilution tandem mass spectrometry (LC–MS/MS) as described in detail elsewhere.30
The PFAS quantified were based on the standard method used to analyze U.S. National Health and Nutrition Examination Survey (NHANES) samples and included linear perfluorooctanoate (n-PFOA), the sum of branched PFOA isomers (Sb-PFOA), linear perfluorooctane sulfonate (n-PFOS), the sum of branched (perfluoromethylheptane) sulfonate isomers (Sm-PFOS), perfluorohexane sulfonate (PFHxS), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), and 2-(N-methyl-perfluorooctane sulfonamide) acetate (MeFOSAA).31 Blanks and quality control (QC) materials were included in each batch for analysis. The limit of detection (LOD) was 0.1 ng/mL for each PFAS, and concentrations <LOD were imputed to LOD/√2.32 Due to the long biological half-lives of many PFAS, ranging from four to seven years, a single plasma sample collected during pregnancy is representative of PFAS concentrations during the entire prenatal period, although studies have indicated that levels of PFAS may fluctuate during pregnancy due to changes in maternal blood volume.33−35 Our subsample had collection dates in the late second and early third trimesters.
Human Milk PFAS Concentrations
For women who delivered after January 1, 2014, we collected pumped samples of milk from both breasts at the six-week postpartum visit (median = 6.3 weeks, IQR 6.0–6.7). Mothers were provided with written instructions for sample collection with a standardized protocol and were asked to refrain from use of soaps, lotions, or other substances prior to pumping. One 10 mL aliquot of whole milk from each breast was collected into bottles, immediately chilled, and aliquoted within 24 h.
The analysis of milk was performed by the Minnesota Children’s Health Exposure Analysis Research (CHEAR) Hub using protein precipitation followed by centrifugation, concentration, and analysis using LC–MS/MS to quantify concentrations of PFOA, PFOS, PFHxS, PFNA, PFDA, PFUnDA, perfluorobutanesulfonate (PFBS), perfluoroheptanoate (PFHpA), perfluoroheptanesulfonate (PFHpS), and perfluorohexanoate (PFHxA). In brief, a stable isotope-labeled internal standard solution and cold acetonitrile were added to a 400 μL aliquot of milk in microcentrifuge tubes. The content of the tubes was mixed, stored overnight in the freezer, centrifuged, and the supernatant was transferred to 96-well plates. The supernatant was concentrated to a volume of 200 μL at 70 °C with gentle nitrogen. After concentration, plates were mixed and centrifuged prior to analysis by LC–MS/MS (Shimadzu Prominence coupled to AB Sciex 5500Q) using electrospray ionization with multiple reaction monitoring (MRM) and retention time windows. Bovine whole milk was used to prepare matrix-matched calibration curves, and quantitation was performed via isotope dilution. A minimum of two MRM transitions were monitored for each analyte when possible, and unknowns were verified through retention time and ion ratio matching with the calibration curve. A minimum of three QC samples (a method blank, and low and high concentration QC materials) were run with each batch of 20 unknowns. The QC materials were prepared in bovine dairy milk and fortified human milk. An additional QC was run if an unknown sample required dilution, either due to a low volume of sample or an initial concentration higher than the calibration curve allowed. Batches with QC recovery outside of 70–130% were reanalyzed. Accuracy for the method, indicated by % recovery, was determined through matrix spikes (n = 8) and ranged from 72 to 103%. Precision for the method, indicated by percent relative standard deviation (%RSD), was determined by repeated measurements of in-house-prepared QC materials (n = 20) 3–18%. The limit of quantification (LOQ) ranged from 0.01 to 0.025 ng/mL for the analytes and values <LOQ were imputed to LOQ/√2.32
Statistical Analysis
Median and interquartile range (IQR) of PFAS concentrations in milk were calculated based on participants’ characteristics, including maternal age, race/ethnicity, maternal highest education level, marital status, smoking status, pre-pregnancy maternal body mass index (BMI), parity, duration of previous lactation history prior to study pregnancy, weight gain during study pregnancy, gestational age at delivery, infant sex, and infant’s postnatal age at the time of milk sample collection.
Scatter plots with Spearman correlation coefficients were created to visually assess the relationship between PFAS concentrations in maternal plasma versus human milk. We conducted sensitivity analyses in which the correlation analyses were restricted to women whose milk samples were collected prior to 10 weeks postpartum (n = 404; 94.8%) and stratified by participant characteristics (mothers of male versus female infants; primiparous versus multiparous women).
We calculated Cohen’s Kappa coefficient (95% Confidence Interval, CI) to test the reliability of PFAS concentrations categorized by quartiles in maternal plasma and milk. Values of Cohen’s Kappa less than 0.2 indicate poor agreement, 0.2–0.4 indicate fair, 0.4–0.6 indicate moderate, 0.6–0.8 indicate substantial, and values greater than 0.8 indicate almost perfect agreement.36
We performed multivariable linear regression analyses to further assess the associations of participants’ characteristics and PFAS concentrations in milk. The linear models included log2-transformed PFAS concentrations as dependent variables, with the following independent variables: maternal age (continuous, years), race/ethnicity (others versus non-Hispanic White), maternal highest education (≥ college graduate versus noncollege graduate/below), pre-pregnancy maternal BMI (continuous, kg/m2), parity (multiparous versus primiparous), duration of previous lactation history prior to study pregnancy (continuous, months), infant sex (male versus female), and infant’s postnatal age at the time of milk sample collection (continuous, weeks). Given the low variability of the marital status, smoking status, and location of recruitment among this subset of participants in the NHBCS, we did not include these variables in the final models. All participants were from areas without major known sources of PFAS exposure (e.g., biosolid application, fluorochemical plants, or military bases) as defined by the National Academies of Sciences, Engineering, and Medicine (NASEM), and so all participants were assumed to have experienced background exposure to PFAS.37 Observations with missing covariates (n = 54) were excluded from multivariable linear regression analyses. We characterized the percent difference in the PFAS concentration as (2ß – 1) × 100, where β is the estimated regression coefficient of the predictor of interest in the multivariable model.
We used Tolerance and Variance Inflation Factors (VIF) to test for multicollinearity. We used Q-Q plots to visually assess the assumptions of linear regression. The values of VIF and tolerance were close to one, indicating that there was no evidence of multicollinearity in the linear regression models. The Q-Q plots did not suggest a violation of assumptions of linear regression.
We compared milk PFAS concentrations observed in our cohort to estimated milk concentrations among mothers exposed at the EFSA 2020-proposed TWI for the sum of PFOA, PFOS, PFHxS, and PFNA of 4.4 ng/kg.30 Weekly intake of PFAS at or below this value would prevent mothers from producing milk containing PFAS concentrations that may reduce vaccine response among their children. Because the toxicokinetics and chemical structures of PFNA and PFHxS are similar to those of PFOA and PFOS, respectively, EFSA combined PFOA with PFNA and PFOS with PFHxS when considering concentrations of PFAS in milk among women at the TWI threshold of 4.4 ng/kg. EFSA 2020 derived that the sum of PFOA and PFNA (PFOA+PFNA) and the sum of PFOS and PFHxS (PFOS + PFHxS) were 0.060 and 0.073 ng/mL, respectively, in the initial milk from mothers exposed at the 4.4 ng/kg TWI.27 Given that PFOA and PFOS would decrease 7.7 and 3.1%, respectively, monthly, because of their individual half-lives, the estimated concentrations in milk at ∼6 weeks postpartum would be 0.055 and 0.071 ng/mL for PFOA+PFNA and PFOS+PFHxS, respectively.27 We used these estimates from 6 weeks postpartum as reference concentrations, reflective of the average timing of milk collection in the NHBCS, to compare to the milk concentrations observed in our cohort.
Additionally, to approximate potential PFAS exposures from human milk among infants in the NHBCS, we calculated the estimated daily intake (EDI, ng/kg/d) of PFAS by infants through human milk consumption as follows: EDI = C × V/BW, where C represents PFAS concentrations in milk (ng/mL); V represents the daily volume of milk intake, which was assigned as 750 mL/day based on average human milk daily intake in the first month of life in the United States (US)38,39 and BW represents the birth weight of infants (kg). Mean imputation was used to replace the missing values of birth weight (n = 49). Violin plots were used to assess the distribution of EDIs of PFAS.
All analyses were conducted in R 4.0.2.
Results and Discussion
Participant Characteristics
Among participants with available PFAS concentrations in milk (n = 426), 69.0% (n = 294) also had available PFAS concentrations in plasma. While only two-thirds of our participants who had human milk samples also had plasma PFAS data available, we found that the main difference between these groups was the year of milk collection. Participants in both groups had comparable demographics and median PFOA and PFOS concentrations in milk (Table S1), suggesting that this is not a source of bias in our cohort. The mean age of mothers at the time of enrollment was 32 years (standard deviation, SD = 4.5). Most participants were non-Hispanic White (n = 402; 94.4%); 71.1% participants had a college degree or above (n = 303); and 51.4% participants had a male infant (n = 219). For 90.6% of women, milk samples were collected between 5 and 10 weeks postpartum (n = 386) (Table 1).
Table 1. Distribution of Participants’ Characteristics and PFAS Concentrations in Milk in the New Hampshire Birth Cohort Study (n = 426).
| PFAS concentrations
in milk (ng/mL) |
|||
|---|---|---|---|
| participants’ characteristics | N (%) | median (IQR)b | |
| PFOA | PFOS | ||
| Maternal Age (Year) | |||
| <30 | 133 (31.2) | 0.018 [0.013, 0.027] | 0.024 [0.017, 0.034] |
| [30, 35) | 180 (42.3) | 0.018 [0.012, 0.028] | 0.023 [0.015, 0.036] |
| [35, 40) | 92 (21.6) | 0.015 [0.011, 0.022] | 0.024 [0.016, 0.033] |
| ≥ 40 | 21 (4.9) | 0.012 [0.007, 0.019] | 0.024 [0.018, 0.042] |
| Race/Ethnicity | |||
| other | 24 (5.6) | 0.015 [0.011, 0.019] | 0.019 [0.014, 0.025] |
| non-hispanic white | 402 (94.4) | 0.017 [0.012, 0.027] | 0.024 [0.016, 0.036] |
| Maternal Highest Education | |||
| noncollege graduate or below | 83 (19.5) | 0.018 [0.012, 0.025] | 0.023 [0.015, 0.031] |
| college graduate or above | 303 (71.1) | 0.017 [0.012, 0.027] | 0.024 [0.017, 0.037] |
| unknown | 40 (9.4) | 0.018 [0.011, 0.030] | 0.019 [0.013, 0.026] |
| Marital Status | |||
| married | 334 (78.4) | 0.017 [0.012, 0.026] | 0.024 [0.017, 0.037] |
| single | 42 (9.9) | 0.018 [0.013, 0.026] | 0.021 [0.015, 0.028] |
| separated/divorced/widowed | 12 (2.8) | 0.025 [0.015, 0.032] | 0.025 [0.019, 0.047] |
| unknown | 38 (8.9) | 0.018 [0.011, 0.030] | 0.017 [0.013, 0.026] |
| Smoking Status | |||
| never | 355 (83.3) | 0.017 [0.012, 0.027] | 0.024 [0.017, 0.037] |
| current | 17 (4.0) | 0.018 [0.013, 0.022] | 0.021 [0.017, 0.029] |
| former | 17 (4.0) | 0.020 [0.011, 0.027] | 0.023 [0.020, 0.030] |
| unknown | 37 (8.7) | 0.017 [0.011, 0.028] | 0.016 [0.013, 0.025] |
| Maternal Pre-Pregnancy BMI (kg/m2) | |||
| <18.5 | 11 (2.6) | 0.022 [0.016, 0.027] | 0.029 [0.020, 0.043] |
| [18.5, 25) | 238 (55.9) | 0.017 [0.012, 0.026] | 0.024 [0.017, 0.036] |
| [25, 30) | 96 (22.5) | 0.016 [0.011, 0.028] | 0.022 [0.015, 0.035] |
| ≥ 30 | 73 (17.1) | 0.019 [0.013, 0.027] | 0.024 [0.016, 0.033] |
| unknown | 8 (1.9) | 0.014 [0.010, 0.020] | 0.014 [< LOD,a 0.022] |
| Parity | |||
| 0 | 178 (41.8) | 0.024 [0.018, 0.036] | 0.031 [0.020, 0.043] |
| ≥1 | 230 (54.0) | 0.013 [< LOD, 0.019] | 0.020 [0.014, 0.029] |
| unknown | 18 (4.2) | 0.016 [0.012, 0.020] | 0.017 [0.012, 0.027] |
| Previous Lactation Duration (Month) | |||
| 0 | 182 (42.7) | 0.024 [0.018, 0.036] | 0.032 [0.021, 0.043] |
| <12 | 89 (20.9) | 0.016 [0.012, 0.019] | 0.022 [0.015, 0.031] |
| [12, 24) | 73 (17.1) | 0.012 [0.010, 0.016] | 0.022 [0.015, 0.030] |
| ≥24 | 47 (11.0) | <LOD [<LOD, 0.013] | 0.017 [0.011, 0.023] |
| Unknown | 35 (8.2) | 0.016 [0.011, 0.022] | 0.017 [0.013, 0.025] |
| Weight Gain during Pregnancy (kg) | |||
| <0 | 6 (1.4) | 0.017 [0.011, 0.035] | 0.027 [0.014, 0.043] |
| [0, 10) | 77 (18.1) | 0.018 [0.011, 0.024] | 0.022 [0.013, 0.034] |
| [10, 15) | 138 (32.4) | 0.017 [0.012, 0.027] | 0.023 [0.018, 0.033] |
| ≥ 15 | 164 (38.5) | 0.018 [0.012, 0.028] | 0.025 [0.018, 0.040] |
| unknown | 41 (9.6) | 0.014 [0.011, 0.020] | 0.019 [0.012, 0.027] |
| Gestational Age at Birth (Week) | |||
| <37 | 37 (8.7) | 0.019 [0.013, 0.027] | 0.031 [0.023, 0.045] |
| [37, 41) | 343 (80.5) | 0.017 [0.012, 0.026] | 0.023 [0.016, 0.035] |
| ≥ 41 | 46 (10.8) | 0.018 [0.011, 0.032] | 0.022 [0.015, 0.035] |
| Infant’s Sex | |||
| female | 207 (48.6) | 0.017 [0.012, 0.028] | 0.024 [0.016, 0.037] |
| male | 219 (51.4) | 0.017 [0.012, 0.026] | 0.023 [0.016, 0.034] |
| Infant’s Age at Milk Collection (Week) | |||
| <5 | 17 (4.0) | 0.020 [0.014, 0.029] | 0.035 [0.024, 0.045] |
| [5, 10) | 386 (90.6) | 0.017 [0.012, 0.027] | 0.023 [0.016, 0.035] |
| ≥ 10 | 23 (5.4) | 0.013 [< LOD, 0.017] | 0.022 [0.014, 0.025] |
Limit of Detection (LOD) for PFOA, PFOS in milk: 0.01 (ng/mL)
IQR: interquartile range.
Plasma and Milk PFAS Concentrations
The median concentration of PFOA in maternal plasma was 0.94 ng/mL and that of PFOS was 2.60 ng/mL (Table 2). Median concentrations of PFHxS and PFNA in maternal plasma were 0.70 and 0.50 ng/mL, respectively (Table 2). The following PFAS plasma concentrations were largely below the LOD and therefore were excluded from our analysis: PFDA, PFUnDA, and MeFOSAA.
Table 2. PFAS Concentrations (ng/mL) in Maternal Plasma and Milk in the New Hampshire Birth Cohort Studya.
| PFAS (ng/mL) | milk
(N = 426) |
plasma
(N = 294) |
milk-to-plasma ratio | ||
|---|---|---|---|---|---|
| N (% ≥ LODb) | median (IQR) | N (% ≥ LODc) | median (IQR) | mean (SD) | |
| PFOA | 356 (83.6) | 0.017 [0.012, 0.027] | 294 (100.0) | 0.935 [0.587, 1.335] | 0.02 (0.01) |
| PFOS | 392 (92.0) | 0.024 [0.016, 0.036] | 294 (100.0) | 2.600 [1.800, 3.900] | 0.01 (0.01) |
| PFHxS | 32 (7.5) | <LOD | 293 (99.7) | 0.700 [0.500, 1.000] | 0.01 (0.01) |
| PFNA | 29 (6.8) | <LOD | 239 (81.3) | 0.500 [0.225, 0.600] | 0.04 (0.06) |
IQR: interquartile range. SD: standard deviation.
LOD (Limit of Detection) for PFOA, PFOS, PFHxS, and PFNA in milk: 0.01 (ng/mL).
LOD for PFOA, PFOS, PFHxS, and PFNA in maternal plasma: 0.1 (ng/mL).
Our population showed plasma PFHxS and PFNA concentrations similar to those in other general population studies, including among all U.S. females in the NHANES, the Shanghai Birth Cohort, the Norwegian Mother and Child Cohort, and the Boston Project Viva cohort.40−43 Concentrations of PFAS in serum and plasma are fairly comparable,44,45 and concentrations of plasma PFNA and PFHxS in the NHBCS were equal to or higher than serum concentrations of these PFAS in Norwegian,46 French,25 and Spanish cohorts, all of which were drawn from the general population.47
The NHBCS plasma concentrations of PFOS and PFOA were lower than serum concentrations in all three other studies, as well as in the female NHANES cohort, which is representative of the general U.S. population.43 These differences may relate, at least in part, to differences in exposures among our population as compared to others or to temporal trends in exposure to PFAS among pregnant women due to phase-outs of certain chemicals, such as PFOS and PFOA. Of note, plasma concentrations of PFOA, PFOS, and PFHxS in our cohort were substantially lower than those reported in communities exposed to drinking water contaminated with PFAS, including the Swedish Ronneby cohort, where the serum geometric mean concentrations were 6.8 ng/mL for PFOA, 135 ng/mL for PFOS, and 114 ng/mL for PFHxS;48 and mothers in the C8 cohort, where serum geometric mean concentrations were 73.6 ng/mL for PFOA and 16.3 ng/mL for PFOS.49
The median concentration of PFOA in human milk was 0.017 ng/mL and that of PFOS was 0.024 ng/mL (Table 2). The following PFAS were detected in less than 8% of samples and were therefore excluded from further statistical analysis: PFHxS, PFNA, PFDA, PFUnDA, PFBS, PFHpA, PFHpS, and PFHxA.
Primiparous women had higher milk PFAS concentrations on average as compared to multiparous women, but milk PFAS concentrations did not differ by infant sex (Table 1). Overall, the concentrations of PFAS in milk in our subset of the NHBCS were lower than concentrations from general population cohorts in Sweden,50 Massachusetts,51 Germany, Hungary,52 Korea,53 and Canada.54 However, they were higher than concentrations from general population cohorts in Washington,55 Ireland,10,56 and France.25 Another study from North Carolina showed milk levels of all women below the LOD, which was higher than the LOD in our study.57 Temporal studies have indicated that milk concentrations of PFAS have been declining over time. In general, studies with higher concentrations of milk PFAS than the NHBCS all had collection dates prior to those in our cohort, which may explain differences in concentrations, with the exception of the Korean cohort.50 Collection dates among the Irish and French cohorts were similar to those in the NHBCS. Furthermore, geography has been identified as a predictor of plasma, serum, and milk PFAS concentrations,44,58 which may account for differing concentrations among cohorts.
Overall environmental quality in rural and urban areas may differ,59 and the rural location of our cohort may also have contributed to lower concentrations of PFAS in this community. In addition to known sources of major PFAS contamination for rural communities such as proximity to military bases, airports, and fluorochemical production facilities, some rural communities experience higher than background exposures due to the application of biosolids that may contain PFAS in agricultural settings that contaminate local water supplies.60 Biosolid application is an emerging source of PFAS exposure that will be important to track in the future, with some communities in rural Maine, in particular having predicted PFOA and PFOS serum concentrations far greater than the U.S. NHANES 95th percentile.60,100
Contrast of PFAS in Human Milk Versus Maternal Plasma
The average milk-to-plasma ratio was 0.04 for PFNA (SD = 0.06), 0.02 for PFOA (SD = 0.01), and 0.01 for PFOS and PFHxS (SD = 0.01) (Table 2), indicating that milk PFAS concentrations were much lower than plasma concentrations. The directionality of this ratio is expected because blood loss over the course of pregnancy and childbirth means that maternal plasma levels of PFAS after childbirth are lower than those in the second trimester, when our samples were taken.61 The majority of participants had milk PFHxS and PFNA concentrations below the LOD of 0.01 ng/mL (92.5 and 93.2%, respectively) (Table 2). Hence, the main focus of this analysis was on PFOA and PFOS.
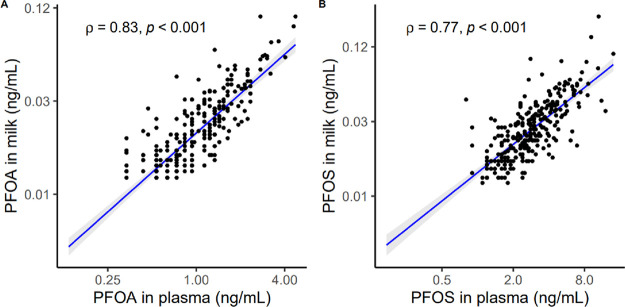
PFOA and PFOS concentrations showed strong, positive, and statistically significant correlations between maternal plasma and milk (ρ = 0.83 and 0.77, respectively) (Figure 1). Spearman correlation coefficients between plasma and milk for both PFOA and PFOS essentially remain unchanged when restricting the correlation analyses to women whose milk samples were collected prior to 10 weeks postpartum or when stratified by infant sex or parity (Figure S1). PFOA and PFOS concentrations in maternal plasma and milk showed moderate agreement across quartiles (κ = 0.47; 95% CI: [0.39, 0.54], κ = 0.46; 95% CI: [0.38, 0.53], respectively).
Figure 1.
Spearman correlation of PFAS between maternal plasma and milk in the New Hampshire Birth Cohort Study (n = 294). (A) Spearman correlation of PFOA between maternal plasma and milk; (B) Spearman correlation of PFOS between maternal plasma and milk.
Reviews of global PFAS blood and milk concentrations show that PFAS concentrations in human milk samples are lower than those in blood (plasma and serum), which is consistent with our findings.6,26,44 This finding likely relates to the relatively low transfer of these chemicals from mothers’ blood into human milk because PFAS have a high affinity for proteins, particularly albumin, unlike other persistent toxicants detected in human milk such as polychlorinated biphenyls, which are more lipophilic.6,44
While the mechanism of transfer of PFAS from blood to milk is unclear, a few theories have been posited. PFAS are structurally similar to fatty acids, which compose a substantial percentage of human milk, so it is possible that PFAS pass through the mammary epithelial membrane bound to albumin in the same manner as fatty acids.6 A different model that accounts for PFAS’ strong binding to proteins and therefore limited capacity for interaction with other molecules includes active membrane-transport mechanisms that transfer unbound PFAS into milk from maternal blood.6 Further research may help better elucidate the exact mechanism of transfer of PFAS from maternal blood into human milk.
Maternal and Infant Factors Associated with PFAS in Milk
We examined the associations of participants’ characteristics with milk PFAS concentrations, including maternal age, race/ethnicity, maternal highest education, pre-pregnancy BMI, parity, the duration of previous lactation history, infant sex, and infant’s postnatal age at the time of milk sample collection (Figure 2; Table 3). A one-month increase in the previous lactation duration prior to study pregnancy was associated with a 2% decrease in milk PFOA concentrations (95% CI: −2, −1%) and 1% decrease in milk PFOS concentrations (95% CI: −2, −1%), after adjusting for other factors (Figure 2; Table 3). Plasma PFAS concentrations also decreased with a greater duration of prior breastfeeding (ρ = −0.42 for PFOA, ρ = −0.29 for PFOS). Compared to primiparous women, milk PFOA and PFOS concentrations were, respectively, about 30% less (95% CI: −38, −20%) and 20% less (95% CI: −31, −8%) among multiparous women (Figure 2; Table 3). Plasma PFAS concentrations among primiparous women were also higher than those among multiparous women, although the milk-to-plasma ratios for primiparous and multiparous women were comparable (Table S2). Similar findings exist in other studies, which have identified inverse associations between parity and previous lactation and certain PFAS in serum42,46,53,58,62 and milk.44,54 Taken together, associations of milk PFAS with previous breastfeeding history and parity support the understanding that cross-placental transfer and lactation are important excretion routes for certain PFAS.7,9,63,64
Figure 2.
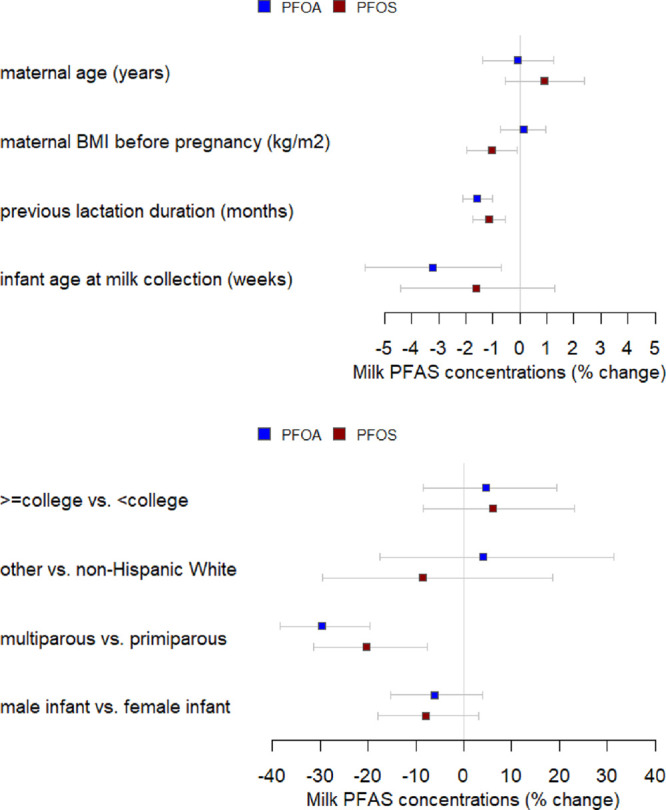
Associations between PFAS concentrations in milk and participants’ characteristics in the New Hampshire Birth Cohort Study (n = 372).
Table 3. Associations between PFAS Concentrations in Milk and Participants’ Characteristics in the New Hampshire Birth Cohort Study (n = 372)a.
| participants’ characteristics | % difference in PFAS
concentrations (95% CI) |
|
|---|---|---|
| PFOA | PFOS | |
| maternal age (years) | –0.1 (−1.4, 1.2) | 0.9 (−0.5, 2.4) |
| race/ethnicity | ||
| non-hispanic white | ref | ref |
| others | 4.1 (−17.6, 31.4) | –8.5 (−29.5, 18.7) |
| maternal highest education | ||
| noncollege graduate/below | ref | ref |
| college graduate/above | 4.6 (−8.5, 19.4) | 6.2 (−8.5, 23.1) |
| pre-pregnancy maternal BMI (kg/m2) | 0.1 (−0.7, 1.0) | –1.1 (−2.0, −0.1) |
| parity | ||
| 0 | ref | ref |
| ≥1 | –29.6 (−38.4, −19.6) | –20.4 (−31.4, −7.7) |
| previous lactation duration (months) | –1.6 (−2.1, −1.0) | –1.1 (−1.8, −0.5) |
| infant sex | ||
| female infant | ref | ref |
| male infant | –6.1 (−15.3, 4.0) | –8.0 (−17.9, 3.2) |
| infant age at milk collection (weeks) | –3.2 (−5.7, −0.7) | –1.6 (−4.4, 1.3) |
CI: confidence interval.
A one-week increase in the postpartum week of collection was associated with a 3% decrease in milk PFOA concentrations (95% CI: −6, −1%) and 2% decrease in milk PFOS concentrations (95% CI: −4, 1%) (Figure 2; Table 3), although most milk samples (94.8%) were collected before 10 weeks postpartum (Table 1). These findings echo prior research showing that earlier postpartum milk has higher concentrations of PFAS, which is thought to be related to the relatively higher concentrations of protein in earlier milk as compared to later milk.44,51 It is important to note that our milk samples were collected in a fairly narrow window (5–10 weeks) and that all samples reflected mature milk rather than colostrum or transitional milk.
The pre-pregnancy maternal BMI was not statistically significantly associated with milk PFOA concentrations (0, 95%CI: −1, 1%), but for each one-unit increase in the pre-pregnancy maternal BMI, milk PFOS decreased by 1% (95% CI: −2.0, −0.1%) (Figure 2; Table 3). Some previous studies support similar inverse associations between the BMI and PFAS in serum, with the BMI being a potential proxy for other factors such as kidney function,42,47,53,58 although the mechanism underlying an association of a higher BMI with lower milk PFAS is unknown.
Estimated PFAS Exposure among Infants
The median of concentrations in milk among our subsample of the NHBCS was 0.024 ng/mL (IQR: 0.019–0.034) for PFOA+PFNA and 0.031 ng/mL (IQR: 0.023–0.044) for PFOS+PFHxS, with the majority of observed PFAS concentrations in milk falling below the reference concentrations established from the TWI (0.055, 0.071 ng/mL for PFOA+PFNA, PFOS+PFHxS, respectively). Approximately 93.7% of observed PFOA+PFNA concentrations and 94.6% of observed PFOS+PFHxS concentrations in milk in the NHBCS were below the reference concentrations, suggesting that only a small proportion of infants in our cohort might be exposed to the estimated higher PFAS levels (6.3% (PFOA+PFNA), 5.4% (PFOS+PFHxS)) than those resulting from mothers with the TWI. A review of perinatal exposure to PFAS suggests that future research should use longitudinal studies to better investigate these early life exposures, a future research opportunity within this cohort.65
The median EDI was 3.79 ng/kg/d (IQR: 2.50–5.88) for PFOA and 5.18 ng/kg/d (IQR: 3.48–8.15) for PFOS (Figure 3), which are lower than those recently reported from a Chinese cohort and higher than those reported at 1–3 months among 50 women in a Washington state-based cohort.55,66 Our milk samples were collected at ∼6 weeks postpartum (mean = 6.7 weeks, SD = 2.1 weeks), and ideally, we would calculate EDI using birth weight and PFAS from milk collected shortly after delivery, since PFAS concentrations in milk may decrease during lactation.27 Using milk PFAS concentrations from 6 weeks may thus underestimate both the true EDI of PFAS for newborns and the proportion of infants exposed to PFAS concentrations in milk above the TWI. A recent commentary indicated that the estimated human milk concentrations of PFOS and PFOA in three Canadian and US studies were higher than recommended daily drinking water levels for children.67 While our reported milk concentrations were higher than the drinking water guidance levels, drinking water advisories do not necessarily apply to infants consuming human milk because of the difference in the matrix and unique period of growth that infants undergo between birth and 2 years. Guidance developed specifically for PFAS in human milk is urgently needed to inform public health efforts.
Figure 3.

Distribution of the estimated daily intake (EDI, ng/kg/d) for PFAS in the New Hampshire Birth Cohort Study (n = 426).
However, recent studies have suggested that even small amounts of PFAS exposure may result in long-term health impacts, leading the U.S. Environmental Protection Agency (EPA) in June 2022 to release interim drinking water health advisory levels at 0.004 and 0.02 ppt for PFOA and PFOS, respectively.68 These levels are considerably lower than the previous level of 70 ppt for the two chemicals individually or combined that the EPA set in 2016, underscoring the need to better understand the implications of subtrace low concentrations of these chemicals in human milk.
Our study is one of the first to characterize concentrations of PFAS in matched plasma and milk samples among a large birth cohort. A strength of our study is the large sample size and extent of demographic data collected as a part of the NHBCS. Although our cohort represents a vulnerable population of rural pregnant people, most participants are White, married, and nonsmokers. Therefore, our results may not be generalizable to urban populations or to populations with different racial/ethnic compositions or other sociodemographic characteristics.
While our study presents an analysis of legacy PFAS based on PFAS present in the soil and water of New Hampshire,69 we acknowledge the importance of similar analyses among populations who are exposed to shorter-chain and newer PFAS, about which less is known. Our focus on PFOA and PFOS was guided by the prevalence of these compounds in the plasma and milk of our cohort, highlighting that these legacy PFAS, while no longer manufactured, are still relevant to contemporary pregnant and lactating individuals.
In summary, we found that maternal plasma concentrations of PFOA and PFOS correlate with milk concentrations of PFOA and PFOS in matched samples. While our study confirms PFAS transfer from maternal blood into milk, the low levels of PFAS that we found in human milk as compared to in plasma suggest that protein-bound toxicants such as PFAS do not readily cross the blood–milk epithelial barrier in humans. These findings support previous research regarding the transfer of select PFAS from maternal blood into milk in a relatively larger sample size.6,26,44 Concentrations of milk PFOA and PFOS showed inverse associations with parity, previous lactation history, time of milk collection, and pre-pregnancy BMI (for PFOS only), as expected. Importantly, we estimate that among our cohort with participants’ plasma PFAS concentrations analogous to those of the general population, ∼6.5% of human milk samples’ PFAS concentration are above EFSA’s TWIs. Our research lays the groundwork for the identification of modifiable risk factors associated with greater milk PFAS that may help reduce infant perinatal exposures among mother–infant pairs in the future.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Acknowledgments
We wish to thank the NHBCS participants and the research staff who made the study possible, as well as Karin Vevang and Kitrina M. Barry for technical expertise in the analysis of PFAS in human milk. We also wish to acknowledge Kayoko Kato, Kendra Hubbard, John Eng, and Emmanuela Obi for technical assistance in quantifying plasma PFAS.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c05555.
Figure S1A. Spearman correlation of PFOA between maternal plasma and milk among women with milk collection prior to 10 weeks postpartum; Figure S1B. Spearman correlation of PFOS between maternal plasma and milk among women with milk collection prior to 10 weeks postpartum (n = 282); Figure S1C. Spearman correlation of PFOA between maternal plasma and milk among women with male infants; Figure S1D Spearman correlation of PFOS between maternal plasma and milk among women with male infants (n = 153); Figure S1E. Spearman correlation of PFOA between maternal plasma and milk among women with female infants; Figure S1F. Spearman correlation of PFOS between maternal plasma and milk among women with female infants (n = 141); Figure S1G. Spearman correlation of PFOA between maternal plasma and milk among primiparous women; Figure S1H. Spearman correlation of PFOS between maternal plasma and milk among primiparous women (n = 131); Figure S1I. Spearman correlation of PFOA between maternal plasma and milk among multiparous women; Figure S1J. Spearman correlation of PFOS between maternal plasma and milk among multiparous women (n = 161); Table S1. Comparison between participants with PFAS concentrations in plasma and milk (N = 294) and participants with missing PFAS concentrations in plasma (N = 132); and Table S2. Comparison of PFAS concentrations (ng/mL) between primiparous women (N = 178) and multiparous women (N = 230).
Author Present Address
∇ Redington-Fairview General Hospital, 46 Fairview Ave Suite 334, Skowhegan, Maine 04976, United States
Research reported in this project was supported by the National Institutes of Health through awards from the National Institute of Environmental Health Sciences under grants P01 ES022832, P42ES007373, and Minnesota CHEAR/HHEAR ES026533, the National Institute of General Medical Sciences under grant P20 GM104416, and the Office of the Director under grant UH3OD023275. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no competing financial interest.
Supplementary Material
References
- Cousins I. T.; Vestergren R.; Wang Z.; Scheringer M.; MacLAchlan M. S. The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int. 2016, 94, 331–340. 10.1016/j.envint.2016.04.044. [DOI] [PubMed] [Google Scholar]
- Kotthoff M.; Muller J.; Jurling H.; Schlummer M.; Fielder D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 2015, 22, 14546–14559. 10.1007/s11356-015-4202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck R.; Franklin J.; et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opinion of the Scientific Panel on Contaminants in the Food chain on Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA J. 2008, 653, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake B. E.; Fenton S. E. Early life exposure to per- and polyfluoroalkyl substances and latent health outcomes: A review including the placenta as a target tissue and possible driver and peri- and postnatal effects. Toxicology 2020, 443, 152565 10.1016/j.tox.2020.152565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheka-Tendenguwo L. R.; Olowoyo J. O.; Mugivhisa L. L.; Abafe O. A. Per- and polyfluoroalkyl substances in human breast milk and current analytical methods. Environ. Sci. Technol. 2018, 25, 36064–36086. 10.1007/s11356-018-3483-z. [DOI] [PubMed] [Google Scholar]
- Mogensen U. B.; Grandjean P.; Nielsen F.; Weihe P.; Butdz-Jorgensen E. Breastfeeding as an exposure pathway for perfluoroalkylates. Environ. Sci. Technol. 2015, 49, 10466–10473. 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling A. P.; Adgate J. L.; Hamman R. F.; Kechris K.; Calafat A. M.; Ye X.; Dabelea D. Pefluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: Examining mediation by maternal fasting glucose in the Healthy Start study. Environ. Health Perspect. 2017, 125, 067016 10.1289/EHP641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt I. A. L. P.; van Zelst B. D.; de Fluiter K. S.; van den Berg S. A. A.; van der Steen M.; Hokken-Koelega A. C. S. Poly- and perfluoroalkyl substances (PFAS) exposure through infant feeding in early life. Environ. Int. 2022, 164, 107274 10.1016/j.envint.2022.107274. [DOI] [PubMed] [Google Scholar]
- Pratt I.; Anderson W.; Crowley D.; Daly S.; Evans R.; Fernandes A.; Fitzgerald M.; Geary M.; Keane D.; Morrison J. J.; Reilly A.; Tlustos C. Brominated and fluorinated organic pollulutants in the breast milk of first-time Irish mothers: Is there a relationship to levels in food?. Food Addit. Contam. 2013, 30, 1788–1798. 10.1080/19440049.2013.822569. [DOI] [PubMed] [Google Scholar]
- Fenton S. E.; Reed C.; Newbold R. R. Perinatal Environmental Exposures Affect Mammary Development, Function, and Cancer Risk in Adulthood. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 455–479. 10.1146/annurev-pharmtox-010611-134659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner M.-A.; Loccisano A. E.; Morken N.-H.; Yoon M.; Wu H.; McDougall R.; Maisonet M.; Marcus M.; Kishi R.; Miyashita C.; Chen M.-H.; Hsieh W.-S.; Andersen M.; Clewell H. J. I.; Longnecker M. P. Associations of perfluoroalkyl substances (PFAS) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ. Health Perspect. 2015, 123, 1317–1324. 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaff J.; Papandonatos G. D.; Calafat A. M.; Chen A.; Lanphear B. P.; Erlich S.; Kelsey K. T.; Braun J. M. Prenatal exposure to perfluoroalkyl substances: Infant birth weight and early life growth. Environ. Epidemiol. 2018, 2, e010 10.1097/EE9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.; Mao L.; Xie J.; Zhao M.; Bai X.; Wen J.; Shen T.; Wu P. Poly- and perfluoroalkyl substance concentrations in human breast milk and their associations with postnatal infant growth. Sci. Total Environ. 2020, 713, 136417 10.1016/j.scitotenv.2019.136417. [DOI] [PubMed] [Google Scholar]
- Forns J.; Verner M.-A.; Iszatt N.; Nowack N.; Bach C. C.; Vrijheid M.; Costa O.; Andiarena A.; Sovcikova E.; Hoyer B. B.; Wittsiepe J.; Lopez-Espinosa M.-J.; Ibarluzea J.; Hertz-Picciotto I.; Toft G.; Stigum H.; Guxen M.; Liew Z.; Eggesbo M. Early life exposure to perfluoroalkyl substances (PFAS) and ADHD: A meta-analysis of nine European population-based studies. Environ. Health Perspect. 2020, 128, 057002 10.1289/EHP5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddy Z.; Hartman T. J.; Taylor E. V.; Poteete C.; Kordas K. Prenatal concentrations of perfluoroalkyl substances and early communication development in British girls. Early Human Dev. 2017, 109, 15–20. 10.1016/j.earlhumdev.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J. J.; Callahan C. L.; Calafat A. M.; Huang W.-Y.; Jones R.; Sabbisetti V. S.; Freedman N. D.; Sampson J. N.; Silverman D. T.; Purdue M. P.; Hoffman J. N. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma. J. Natl. Cancer Inst. 2020, 113, 580–587. 10.1289/isee.2020.virtual.O-SY-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham K.; Mielke H.; Fromme H.; Vilkel W.; Menzel J.; Pesier M.; Zepp F.; Willich S. N.; Weikert C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020, 94, 2131–2147. 10.1007/s00204-020-02715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake B.; Pinney S. M.; Hines E. P.; Fenton S. E.; Ferguson K. K. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ. Pollut. 2018, 242, 894–904. 10.1016/j.envpol.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P.; Heilmann C.; Weihe P.; Nielsen F.; Mogensen U. B.; Budtz-Jørgensen E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2017, 125, 077018 10.1289/EHP275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P.; Andersen E. W.; Butdz-Jorgensen E.; Nielsen F.; Molbak K.; Weihe P.; Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012, 307, 391–397. 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell R.; Crawford K. A.; Bucinca H.; Romano M. E. Endocrine-disrupting chemicals and breastfeeding duration: A review. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 388–395. 10.1097/MED.0000000000000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.; Rock S.; Stratakis N.; Eckel S. P.; Walker D. I.; Valvi D.; Cserbik D.; Jenkins T.; Xanthakos S. A.; Kohli R.; Sisley S.; Vasiliou V.; Merrill M. A. L.; Rosen H.; Conti D. V.; McConnell R.; Chatzi L. Exposure to per- and Polyfluoroalkyl Substances and Markers of Liver Injury: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2022, 130, 46001. 10.1289/EHP10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duccatman A.; Fenton S. E. Invited Perspective: PFAS and Liver Disease: Bringing All the Evidence Together. Environ. Health Perspect. 2022, 130, 041303 10.1289/EHP11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R.; Veyrand B.; Yamada A.; Berrebi A.; Zalko D.; Durand S.; Pollono C.; Marchand P.; Leblanc J.-C.; Antignac J.-P.; Le Bizec B. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ. Int. 2015, 84, 71–81. 10.1016/j.envint.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Kärrman A.; Ericson I.; Van Bavel B.; Ola Darnerud P.; Aune M.; Glynn A.; Ligneli S.; Lindström G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996-2004, in Sweden. Environ. Health Perspect. 2007, 115, 226–230. 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outcome of a public consultation on the draft risk assessment of perfluoroalkyl substances in food; EFSA Supporting Publication: EFSA (European Food Safety Authority), 2020. [Google Scholar]
- Madan J. C.; Hoen A. G.; Lundgren S. N.; Farzan S. F.; Cottingham K. L.; Morrison H. G.; Sogrin M. L.; Li H.; Moore J. H.; Karagas M. R. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016, 170, 212–219. 10.1001/jamapediatrics.2015.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M.; Heggeseth B.; Gallagher L.; Botelho J.; Calafat A.; Gilbert-Diamond D.; KAragas M. Gestational per- and polyfluoroalkyl substances exposure and infant body mass index trajectory in the New Hampshire Birth Cohort Study. Environ. Res. 2022, 215, 114418 10.1016/j.envres.2022.114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K.; Kalathil A. A.; Patel A. M.; Ye X.; Calafat A. M. Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere 2018, 209, 338–345. 10.1016/j.chemosphere.2018.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cdc.gov/exposurereport/index.html
- Hornung R. W.; Reed L. D. Estimation of average concentration in the presence of non-detectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Kato K.; Basden B. J.; Needham L. L.; Calafat A. M. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J. Chromatogr. A 2011, 1218, 2133–2137. 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- Costantine M. M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014, 5, 65. 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuanna T. D.; Savitz D. A.; Barbieri G.; Pitter G.; Jeddi M. Z.; Dapra F.; Fabricio A. S.; Russo F.; Fletcher T.; Canova C. The association between perfluoroalkyl substances and lipid profile in exposed pregnant women in the Veneto region, Italy. Ecotoxicol. Environ. Saf. 2021, 209, 111805 10.1016/j.ecoenv.2020.111805. [DOI] [PubMed] [Google Scholar]
- Landis J.; Koch G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. The National Academies Press: Washington, DC, 2022. [PubMed] [Google Scholar]
- Milk Volume . Nutrition During Lactation; Institute of Medicine Committee on Nutritional Status During Pregnancy and Lactation, National Academies Press: Washington, DC, 1991. [Google Scholar]
- 2011 Edition (Final Report)
- Zhou W.; Zhao S.; Tong C.; Chen L.; Yu X.; Yuan T.; Aimuzi R.; Luo F.; Tian Y.; Zhang J. Study ft SBC. Dietary intake, drinking water ingestion and plasma perfluoroalkyl substances concentration in reproductiev aged Chinese women. Environ. Int. 2019, 127, 487–494. 10.1016/j.envint.2019.03.075. [DOI] [PubMed] [Google Scholar]
- Starling A. P.; Engel S. M.; Whitworth K. W.; Richardson D. B.; Stuebe A. M.; Daniels J. L.; Smastuen Haug L.; Eggesbo M.; Becher G.; Sabaredzovic A.; Thomsen C.; Wilson R. E.; Travlos G. S.; Hoppin J. A.; Baird D. D.; Longnecker M. P. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ. Int. 2014, 62, 104–112. 10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv S. K.; Rifas-Shiman S. L.; Webster T. F.; Mora A. M.; Harris M. H.; Calafat A. M.; Ye X.; Gillman M. W.; Oken E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci. Technol. 2015, 49, 11849–11858. 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cdc.gov/exposurereport/pfas_early_release.html. Accessed April 3, 2022
- Jian J.-M.; Chen D.; Han F.-J.; Guo Y.; Zeng L.; Lu X.; Wang F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substance (PFAS). Sci. Total Environ. 2018, 636, 1058–1069. 10.1016/j.scitotenv.2018.04.380. [DOI] [PubMed] [Google Scholar]
- Ehresman D. J.; Froehlich J. W.; Olsen G. W.; Chang S. C.; Butenhoff J. L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176–184. 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Berg V.; Nost T. H.; Huber S.; Rylander C.; Hansen S.; Veyhe A. S.; Fuskevag O. M.; Odland J. O.; Sandanger T. M. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ. Int. 2014, 69, 58–66. 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado C.; Casas M.; Lopez-Espinosa M.-J.; Ballester F.; Basterrechea M.; Grimalt J. O.; Jimenez A.-M.; Kraus T.; Schettgen T.; Sunyer J.; Vrijheid M. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ. Res. 2015, 142, 471–478. 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Nielsen C.; Li Y.; Hammarstrand S.; Andersson E. M.; Li H.; Olsson D. S.; Engstrom K.; Pineda D.; Lindh C. H.; Fletcher T.; Jakobsson K. Serum perfluoroalkyl substances in residents following long-term drinking water contamination from firefighting foam in Ronneby, Sweden. Environ. Int. 2021, 147, 106333 10.1016/j.envint.2020.106333. [DOI] [PubMed] [Google Scholar]
- Mondal D.; Lopez-Espinosa M.-J.; Armstrong B.; Stein C. R.; Fletcher T. Relationships of Perfluorooctanoate and Perfluorooctane Sulfonate Serum Concentrations between Mother–Child Pairs in a Population with Perfluorooctanoate Exposure from Drinking Water. Environ. Health Perspect. 2012, 120, 752. 10.1289/ehp.1104538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom M.; Ehresman D. J.; Bignert A.; Butenhoff J. L.; Olsen G. W.; Chang S.-C.; Bergman A. A temporal trend study (1972-2008) of perfluorooctainesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in pooled human milk samples from Stockholm, Sweden. Environ. Int. 2011, 37, 178–183. 10.1016/j.envint.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Tao L.; Kannan K.; Wong C. M.; Arcaro K. F.; Butenhoff J. L. Perfluorinated compounds in human milk from Massachusetts, USA. Environ. Sci. Technol. 2008, 42, 3096–3101. 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- Volkel W.; Genzel-Boroviczeny O.; Demmelmair H.; Gebauer C.; Koletzko B.; Twardella D.; Raab U.; Fromme H. Perfluorooctaine sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: Results of a pilot study. Int. J. Hygiene Environ. Health. 2008, 211, 440–446. 10.1016/j.ijheh.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Lee S.; Kim S.; Park J.; Kim H.-J.; Choi G.; Choi S.; Kim S.; Kim S. Y.; Kim S.; Choi K.; Moon H. B. Perfluoroalkyl substances (PFAS) in breast milk from Korea: Time-course trends, influencing factors, and infant exposure. Sci. Total Environ. 2018, 612, 286–292. 10.1016/j.scitotenv.2017.08.094. [DOI] [PubMed] [Google Scholar]
- Rawn D. F. K.; Dufresne G.; Clement G.; Fraser W. D.; Arbuckle T. E. Perfluorinated alkyl substances in Canadian human milk as part of the Maternal-Infant Research on Environmental Chemicals (MIREC) study. Environment 2022, 831, 154888 10.1016/j.scitotenv.2022.154888. [DOI] [PubMed] [Google Scholar]
- Zheng G.; Schreder E.; Dempsey J. C.; Uding N.; Chu V.; Andres G.; Sathyanarayana S.; Salamova A. Per- and polyfluoroalkyl substance (PFAS) in breast milk: Concerning trends for current-use PFAS. Environ. Sci. Technol. 2021, 55, 7510–7520. 10.1021/acs.est.0c06978. [DOI] [PubMed] [Google Scholar]
- Pratt I. S.; Anderson W. A.; Crowley D.; Daly S. F.; Evans R. I.; Fernandes A. R.; Fitzgerald M.; Geary M. P.; KEane D. P.; Malisch R.; McBride J.; Morrison J. J.; Reilly A.; Tlustos C. Polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) in breast milk of first-time Irish mothers: Impact of the 2008 dioxin incident in Ireland. Chemosphere 2012, 88, 865–872. 10.1016/j.chemosphere.2012.03.095. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein O. S.; Fenton S. E.; Kato K.; Kuklenyik Z.; Calafat A. M.; Hines E. P. Perfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod. Toxicol. 2009, 27, 239–245. 10.1016/j.reprotox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Lauritzen H. B.; Larose T. L.; Oien T.; Odland J. O.; van de Bor M.; Jacobsen G. W.; Sandanger T. M. Factors associated with maternal serum levels of perfluoroalkyl substances and organochlorines: A descriptive study of parous women in Norway and Sweden. PLoS One 2016, 11, e0166127 10.1371/journal.pone.0166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer L. C.; Jagai J. S.; Rappazzo K. M.; Lobdell D. T. Construction of an environmental quality index for public health research. Environ. Health: A Global Access Sci. Source 2014, 13, 39. 10.1186/1476-069X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Per- and Polyfluorylalkyl Substances (PFAS) . Spills and Site Cleanup 2019, Accessed October 12, 2021.
- Lu S., Bartell S. M.. Serum PFAS Calculator for Adults, Version 1.2, 2020, www.ics.uci.edu/~sbartell/pfascalc.html.
- Upson K.; Shearston J. A.; Kioumourtzoglou M.-A. An epidemiologic review of menstrual blood loss as an excretion route for per- and polyfluoroalkyl substances. Curr. Environ. Health Rep. 2022, 9, 29–37. 10.1007/s40572-022-00332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Y.; Kim K.-N.; Shin C. H.; Lim Y.-H.; Kim J. I.; Kim B.-N.; Hong Y.-C.; Lee Y. A. The Relationship between Perfluoroalkyl Substances Concentrations and Thyroid Function in Early Childhood: A Prospective Cohort Study. Thyroid 2020, 30, 1556. 10.1089/thy.2019.0436. [DOI] [PubMed] [Google Scholar]
- Goeden H. M.; Greene C. W.; Jacobus J. A. A transgenerational toxicokinetic model and its use in derivation of Minnesota PFOA water guidance. J. Expo Sci. Environ. Epidemiol. 2019, 29, 183–195. 10.1038/s41370-018-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner M.-A.; Ngueta G.; Jensen E. T.; Fromme H.; Volkel W.; Nygaard U. C.; Granum B.; Longnecker M. P. A simple pharmacokinetic model of prenatal and postnatal exposure to perfluoroalkyl substances (PFASs). Environ. Sci. Technol. 2017, 50, 978–986. 10.1021/acs.est.5b04399. [DOI] [PubMed] [Google Scholar]
- Winkens K.; Vestergren R.; Berger U.; Cousins I. T. Early life exposure to per- and polyfluoroalkyl substances (PFASs): A critical review. Emerg. Contam. 2017, 3, 55–68. 10.1016/j.emcon.2017.05.001. [DOI] [Google Scholar]
- Zheng P.; Liu Y.; An Q.; Yang X.; Yin S.; Ma L. Q.; Liu W. Prenatal and postnatal exposure to emerging and legacy per–/polyfluoroalkyl substances: Levels and transfer in maternal serum, cord serum, and breast milk. Sci. Total Environ. 2022, 812, 152446 10.1016/j.scitotenv.2021.152446. [DOI] [PubMed] [Google Scholar]
- LaKind J. S.; Verner M.-A.; Rogers R. D.; Goeden H.; Naiman D. Q.; Marchitti S. A.; LEhmann G. M.; Hines E. P.; Fenton S. E. Current Breast Milk PFAS Levels in the United States and Canada: After All This Time, Why Don’t We Know More?. Environ. Health Perspect. 2022, 130, 25002. 10.1289/EHP10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protections Agency . Lifetime Drinking Water Health Advisories for Four Perfluoroalkyl Substance; Vol 87; Federal Register, June 21, 2022: pp. 36848–36849.
- https://www.pfas.des.nh.gov/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.