Abstract
Background
Few studies have assessed the impact of race on short-term patient outcomes in the brain metastasis population. The goal of this study is to evaluate the association of race with inpatient clinical presentation, treatment, in-hospital complications, and in-hospital mortality rates for patients with brain metastases (BM).
Method
Using data collected from the National Inpatient Sample between 2004 and 2014, we retrospectively identified adult patients with a primary diagnosis of BM. Outcomes included nonroutine discharge, prolonged length of stay (pLOS), in-hospital complications, and mortality.
Results
Minority (Black, Hispanic/other) patients were less likely to receive surgical intervention compared to White patients (odds ratio [OR] 0.70; 95% confidence interval [CI] 0.66–0.74, p < 0.001; OR 0.88; 95% CI 0.84–0.93, p < 0.001). Black patients were more likely to develop an in-hospital complication than White patients (OR 1.35, 95% CI 1.28–1.41, p < 0.001). Additionally, minority patients were more likely to experience pLOS than White patients (OR 1.48; 95% CI 1.41–1.57, p < 0.001; OR 1.34; 95% CI 1.27–1.42, p < 0.001). Black patients were more likely to experience a nonroutine discharge (OR 1.25; 95% CI 1.19–1.31, p < 0.001) and higher in-hospital mortality than White (OR 1.13; 95% CI 1.03–1.23, p = 0.008).
Conclusion
Our analysis demonstrated that race is associated with disparate short-term outcomes in patients with BM. More efforts are needed to address these disparities, provide equitable care, and allow for similar outcomes regardless of care.
Keywords: brain metastases, clinical outcomes, disparities, inpatient, race
The effects of racial disparities in the US healthcare system, specifically on delivery of treatment/interventions and patient outcomes, have been well established across multiple medical and surgical specialties.1–3 In the field of oncology, there is well-documented evidence of historically marginalized patient populations, including Black and Hispanic patients, receiving disparate care and having worse outcomes than White patients.4–11 Across many common cancer subtypes including breast,6 prostate,8 colorectal,9 lung,7 cervical, pancreatic,10 and esophageal,11 Black patients typically have higher mortality rates, receive less aggressive interventions, and have worse health-related quality-of-life outcomes than White patients. While there are many studies addressing racial disparities amongst patients with primary cancer diagnoses, there is a dearth of literature on the impact of race on patients with site-specific metastases including the brain.
Brain metastases (BM) are the most common form of brain malignancy, outnumbering primary brain tumors by a factor of 10. The incidence of BM is increasing due to improvements in imaging techniques and treatment of primary tumors leading to prolonged survival.12 Approximately 9%–40% of patients with systemic malignancies will eventually be diagnosed with BM.13 Great variability exists in disease course and treatment regimens amongst patients, which contributes to disparities in clinical outcomes.14 Therefore, exploring the impact of patient characteristics on clinical outcomes provides valuable information for teams managing patients with BM.
While BM are much more common than primary brain tumors, most literature on the impact of race on patients with intracranial oncologic pathologies has been conducted in patient populations with primary brain neoplasms. These studies have found that while there is a lower incidence of primary brain tumor diagnoses amongst Black patient populations,15,16 Black patients with these diagnoses have higher mortality rates and worse outcomes than White patients.17,18 Not all studies report race-based differences in mortality rates; however, they do demonstrate that Black patients often receive surgical intervention at low rates, have longer hospital lengths of stay, and have more postoperative complications.4,15,16,19–21 While there have been strides in understanding race-based differences in interventions provided to and subsequent outcomes for patients with primary brain tumors,5,15–17 it is important to extend this literature to patients with BM as understanding race-based differences in medicine is the first step toward addressing racial disparities in health care.
Thus, the purpose of this study is to evaluate the association of race with clinical presentation, treatment, in-hospital complications, and in-hospital mortality rates for patients diagnosed with BM. The goal of this study is to provide insight into disparities among vulnerable patient populations who may require additional resources and considerations throughout their treatment. We hypothesized that in the setting of BM, Black patient populations would have less invasive procedures but also worse clinical and functional outcomes.
Methods
Data for this study were available from the US National Inpatient Sample (NIS) database, which is a national, multi-institutional database that gathers information on approximately 8 million admissions per year for patients admitted to nonfederal academic and community hospitals. Given that we used data from a national, accessible database, this study was exempt from Institution Review Board (IRB) review. Our study analyzed de-identified patient data collected between the years 2004 and 2014, including demographics, comorbidities, diagnoses, and complications. The NIS data are coded using Clinical Classification Software (CCS) diagnostic codes and International Classification of Diseases Classification 9th Revision (ICD-9) codes. The NIS database has been vetted for quality and recognized as highly reputable in previously published studies.20,21
Our sample included patients over the age of 18 with primary discharge diagnoses of metastasis to the brain (ICD-9 code 198.3). Patients meeting the inclusion criteria were stratified by race: “White”, “Black”, or “Hispanic and other”. The NIS defines race as White (Caucasian), Black (African-American), Hispanic, Asian or Pacific Islander, Native American, other, and unknown. For the purposes of this analysis, Native American, Asian or Pacific Islanders, and other were combined with Hispanic patients to create the “Hispanic and other” group. We acknowledge the limitations of this approach, but this ensured that the sample size was large enough for a reliable multivariate analysis. Those characterized as “unknown” were excluded due to the small sample size. Baseline demographic data including age, sex, primary insurance status (private, Medicare, Medicaid, other/unknown), and median household income based on patient’s ZIP code were collected. Baseline health information was documented, including smoking status, primary tumor type, and Charlson Comorbidity Index (CCI), a method of categorizing comorbidities of patients based upon ICD-9 diagnosis codes. Additionally, data referencing the admitting hospital type (hospital teaching status and hospital size) were collected. Hospitals were then categorized per the NIS database using location (rural vs. urban), teaching versus nonteaching, and size; for example, large urban teaching hospitals are those that have a minimum of 325–450 beds, depending on geographic region.22
The ICD-9 codes were used to characterize patients who underwent surgical intervention (3.01, 3.09, 03.4, 03.53, 81.00–81.08, 81.61), and CCS codes were used to classify patients who underwent radiation therapy (RT) (CCS code 211) or chemotherapy (CCS code 224). All procedures were performed during inpatient admissions. Additionally, all RTs were conventional RT and not stereotactic radiosurgery (SRS).
In-hospital complications and mortality, prolonged length of stay (75th percentile), and nonroutine discharge (discharge to site other than home) were collected. In-hospital complications collected in this dataset included sepsis (CCS code 2), decubitus ulcers (ICD-9 codes 707.01-0.9), neurological complications including seizures (ICD-9 codes 997.00-997.09 and code 780.39), pneumonia (CCS code 122), other respiratory complications (ICD-9 codes 518.5, 518.81, 518.84, and 997.3), cardiac complications (ICD-9 codes 997.1 and CCS code 410), venous thromboembolism (415.11-415.19, 453.40-2, 453.8, and 453.9), gastrointestinal complications (ICCD-0 codes 008.45, 560.1, and 997.4), urinary tract infection (ICCD-9 codes 595.0, 595.9, and 599.0), and other urinary/renal complications (ICD-9 codes 584.5, 594.9, and 997.5).
Statistical Analysis
Descriptive statistics were initially performed. Data were stratified into three groups and compared—White, Black, and Hispanic and other. Groups were compared using t-tests or chi-square tests as necessary. Multivariable logistic regression analyses were completed to evaluate the independent effect of race on outcomes; results with a probability value less than 0.100 on univariable analysis were included in the analysis. Results are displayed as odds ratios (OR) with corresponding 95% confidence intervals (CI). Probability values of less than 0.05 were recognized as statistically significant.
Results
Demographic Data
The 83,449 patients in our sample included 63,918 White; 10,224 Black; and 5,109 Hispanic, 1,957 Asian/Pacific Islanders, 340 Native Americans, and 1,901 other patients were then lumped together into our third group. Patients differed significantly in several baseline characteristics when stratified by race. Specifically, Black patients were more likely to be female (p < 0.001), and Black and Hispanic/other patients were more likely to have Medicaid (p < 0.001) while White patients were more likely to be privately insured (Table 1). Income quartile as determined by zip code (p < 0.001) and admitting hospital characteristics (hospital size and teaching hospital status) differed significantly by race (p < 0.001). Specifically, Black patients were more likely to be treated at teaching hospitals while Hispanics/other were more likely to receive care at larger facilities. Regarding baseline health status, Black patients had significantly higher CCI scores (6.7 vs. 6.6, p < 0.001), while White patients had higher rates of smoking (38.6 vs. 34.8, p < 0.001). In an additional analysis in which patients were stratified by primary tumor diagnosis within race, Black patients had relatively higher rates of lung, breast, and prostate cancer (Table 1). Additionally, there were significantly higher rates of colon and kidney cancer in patients characterized as Hispanic or other (Table 1).
Table 1.
Baseline and oncological characteristics of all patients with brain metastasis
| Variable | White | Black | Hispanic and other | p-Value |
|---|---|---|---|---|
| Average age | 63.4 | 59.8 | 59.2 | <0.001* |
| Male sex (%) | 46.6 | 39.7 | 44.9 | <0.001* |
| Insurance | ||||
| Private (%) | 48.9 | 41.0 | 36.2 | <0.001* |
| Medicare (%) | 8.5 | 23.5 | 22.9 | |
| Medicaid (%) | 37.0 | 25.8 | 32.0 | |
| Other/unknown (%) | 5.6 | 7.8 | 8.9 | |
| Income quartile | ||||
| 1st (%) | 20.9 | 50.8 | 29.8 | <0.001* |
| 2nd (%) | 25.3 | 20.6 | 22.4 | |
| 3rd (%) | 25.6 | 16.4 | 23.8 | |
| 4th (%) | 28.2 | 12.2 | 23.9 | |
| Teaching hospital (%) | 60.2 | 73.0 | 66.5 | <0.001* |
| Large hospital size (%) | 69.3 | 69.1 | 72.3 | <0.001* |
| Average Charlson comorbidity index | 6.6 | 6.7 | 6.5 | <0.001* |
| Smoking (%) | 38.6 | 34.8 | 26.6 | <0.001* |
| Primary tumor type | ||||
| Lung (%) | 47.0 | 50.3 | 37.6 | <0.001* |
| Breast (%) | 16.1 | 25.3 | 23.2 | <0.001* |
| Prostate (%) | 3.8 | 4.3 | 3.4 | 0.004* |
| Colon (%) | 5.3 | 5.5 | 6.5 | <0.001* |
| Kidney (%) | 4.4 | 2.5 | 6.0 | <0.001* |
| Other/unspecified (%) | 28.1 | 16.5 | 26.6 | <0.001* |
| Visceral metastasis (%) | 20.0 | 20.3 | 20.2 | 0.727 |
| Bone metastases (%) | 19.1 | 22.1 | 23.4 | <0.001 |
| Paralysis (%) | 0.9 | 1.3 | 1.7 | <0.001 |
*Statistically significant result.
In-hospital Mortality and Complication Rates
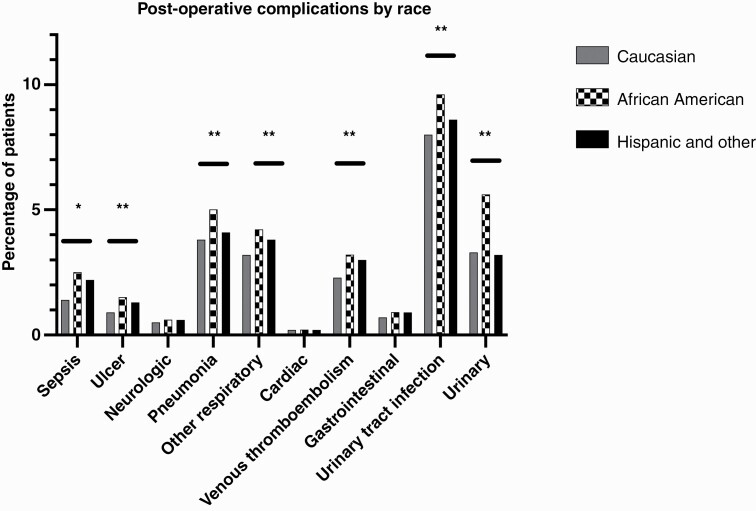
Rates of in-hospital complication (sepsis, ulcer, neurological complication, pneumonia, other respiratory complication, cardiac complication, venous thromboembolism, gastrointestinal complication, urinary tract infection, and urinary complication) were significantly higher in Black (33.1%) patients when compared to White (26.4%) and Hispanic/Other patients (27.0%) (p < 0.001) (Figure 1). Outcomes for patients presenting with BM are summarized in Table 2 and Figure 2.
Figure 1.
Differences in rates of postoperative complications by race. * indicates p > 0.01; ** indicates p < 0.001.
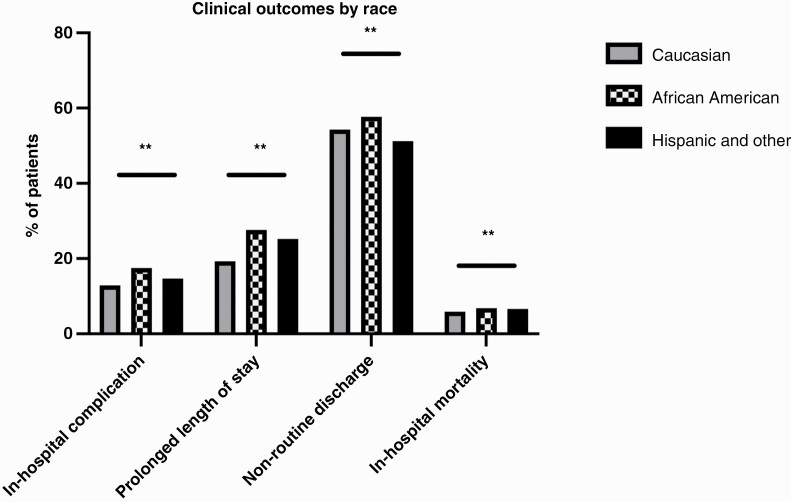
Table 2.
Treatment and outcomes for patients presenting with brain metastasis
| Variable | White | Black | Hispanic and other | p-Value |
|---|---|---|---|---|
| Surgical intervention (%) | 24.5 | 19.0 | 22.7 | <0.001* |
| Radiotherapy (%) | 14.7 | 19.6 | 15.5 | <0.001* |
| Chemotherapy (%) | 2.0 | 2.1 | 2.9 | <0.001* |
| In-hospital complication (%) | 12.9 | 17.5 | 14.7 | <0.001* |
| Prolonged length of stay (%) | 19.3 | 27.6 | 25.2 | <0.001* |
| Nonroutine discharge (%) | 54.3 | 57.7 | 51.2 | <0.001* |
| In-hospital mortality (%) | 5.9 | 6.8 | 6.6 | <0.001* |
*Statistically significant result.
Figure 2.
Differences in rates of complications by race. ** indicates p < 0.001.
Interventions
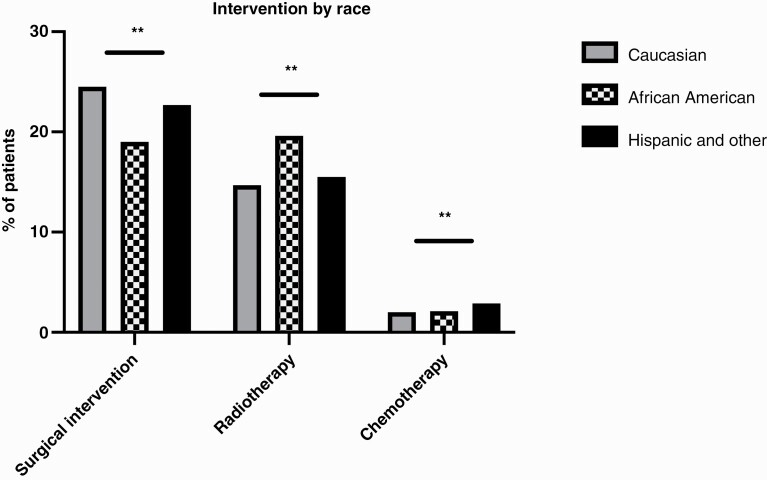
Race was associated with differing rates of surgical intervention, radiotherapy, and chemotherapy. Interventions for patients presenting with BM are summarized in Table 2 and Figure 3. Of note, there was no significant difference between rates of patients who did not receive any intervention (60.3% for White patients, 61.0% for Black patients, and 60.9% for Other patients, p = 0.302). The most common primary procedures associated with admissions in which the patient did not receive surgery, RT, or chemotherapy were imaging studies (MRI or CT scans), blood transfusions, diagnostic biopsies, and “other therapeutic procedures”.
Figure 3.
Differences in rates of intervention (surgery, radiotherapy, or chemotherapy) by race. ** indicates p < 0.001.
Multivariate Logistic Regression
Results of the multivariate regression analyses are found in Table 3. After controlling for age, sex, insurance status, income quartile, hospital teaching status, hospital size, CCI, smoking status, tumor type, presence of visceral metastases, bone metastases, and paralysis, Black and Hispanic/other patients were significantly less likely to receive surgical intervention (OR 0.69; 95% CI 0.66–0.74, p < 0.001; OR 0.89; 95% CI 0.84–0.94, p < 0.001). In addition, Black patients had significantly higher rates of in-hospital complications (OR 1.35; 95% CI 1.28–1.41, p < 0.001). Black and Hispanic/other patients were significantly more likely to have pLOS than White patients (OR 1.48; 95% CI 1.41–1.57, p < 0.001; OR 1.34; 95% CI 1.27–1.41, p < 0.001). Finally, Black patients were more likely to experience nonroutine discharge (OR 1.24; 95% CI 1.19–1.31, p < 0.001) and experienced higher rates of in-hospital mortality (OR 1.13; 95% CI 1.03–1.23, p = 0.008).
Table 3.
Multivariable associations between race and outcome for patients with brain metastasis
| Outcome | White (reference group) | Black | Hispanic and other |
|---|---|---|---|
| Surgical intervention | |||
| Odds ratio | 1.00 | 0.69 | 0.89 |
| 95% CI | 0.66–0.74 | 0.84–0.94 | |
| p-Value | <0.001* | <0.001* | |
| In-hospital complication** | |||
| Odds ratio | 1.00 | 1.35 | 1.00 |
| 95% CI | 1.28–1.41 | 0.96–1.06 | |
| p-Value | <0.001* | 0.801 | |
| Prolonged length of stay*** | |||
| Odds ratio | 1.00 | 1.48 | 1.34 |
| 95% CI | 1.41–1.57 | 1.27–1.42 | |
| p-Value | <0.001* | <0.001* | |
| Nonroutine discharge*** | |||
| Odds ratio | 1.00 | 1.25 | 0.98 |
| 95% CI | 1.19–1.31 | 0.94–1.03 | |
| p-Value | <0.001* | 0.490 | |
| In-hospital mortality** | |||
| Odds ratio | 1.00 | 1.13 | 1.03 |
| 95% CI | 1.03–1.23 | 0.93–1.13 | |
| p-Value | 0.008* | 0.523 |
*Statistically significant result; **controlled for baseline characteristics and also for surgical intervention; ***controlled for surgical intervention and complication occurrence.
Discussion
Studies have previously demonstrated that race significantly impacts clinical outcomes; however, there are few such studies for patients with BM. Our study is the largest nationwide study to evaluate the impact of race on outcomes in this patient population. We identified significant differences in baseline characteristics including age, insurance status, income quartile, characteristics of admitting hospital, comorbidity index, smoking status, and primary tumor type between races. Our results also demonstrate racial inequities when comparing rates of in-hospital mortality, complications, surgery, and prolonged length of stay.
Racial inequities associated with baseline health characteristics are well documented across many medical and surgical fields. The reasons underlying these disparities are multifactorial and often attributed to associations with other social determinants of health.23 Consistent with our findings regarding income quartile, both Black and Hispanic patients are more likely to be unemployed, experience poverty, and/or have lower rates of private insurance than their White counterparts.24–27 These lower rates of private insurance may explain the higher burden of comorbidities observed in Black patients as poor insurance coverage may result in less contact with healthcare providers and worse health maintenance over time.28
Similarly, the lower rate of surgical intervention observed for Black patients and those of other minority groups was consistent with prior studies demonstrating that minority patients receive surgical intervention as part of oncologic management less frequently than nonminority patients.29,30 Similar disparities in treatment have been observed in other intracranial conditions, such as mechanical thrombectomy and aneurysmal subarachnoid hemorrhage.21,31 Several hypotheses have been presented to explain these inequities including differences in presenting stage and prognosis and/or inherent racial bias. Consistent with the former hypothesis, we found higher rates of bone metastases and paralysis in minority patients, which suggested a higher disease burden at the time of presentation. Since more advanced BMs are often not managed with surgery, the difference in intervention received by race could reflect disparate extents of disease. In support of this hypothesis, Virnig et al. previously found that White patients were more likely to be diagnosed at earlier stages of cancer than Black patients for 31 out of 34 tumor sites.32 These later-stage disease presentations may be due to the generally lower rates of cancer screening observed in minority patient populations along with other factors including socioeconomic barriers to appropriate and timely healthcare assessments, disparities in insurance coverage, and mistrust of the healthcare system.33
However, it is also important to note that the difference in rates of surgical intervention persisted after the multivariate analysis, which controlled for bone metastasis and paralysis. This finding suggests that the stage of disease presentation alone cannot explain disparities in rates of surgical intervention and that racial bias in surgical decision-making may also play a role. Racial bias, specifically pro-White bias, has been shown to impact physician decision-making across several medical specialties.34–37 Other studies have reported that physicians unconsciously hold the belief that minorities may be less willing to receive medical procedures, and thus may be more likely to offer aggressive medical interventions to White patients.36–38 Additionally, studies have reported that rates of receipt of preventive medical care are lower in Black and other minority patients.36,37,39,40 Therefore, the effect of implicit racial bias on surgical decision-making may have reduced the likelihood that minority patients in our study received surgery for the treatment of BM.
Additionally, our study found that Black and Hispanic patients were more likely to be treated at teaching/large hospitals. While this finding is contradictory to previously published literature showing that minority patients were more likely to be treated in community hospitals, Amin et al. have reported a positive correlation between treatment in a teaching hospital and chemoradiotherapy reception.41–43 Our study found that rates of chemotherapy and radiation therapy were higher in Black and Hispanic patients; thus, this difference in interventions received may have affected the locations where patients received care.
Regarding hospital outcomes, both Black and Hispanic patients experienced higher rates of in-hospital complications and prolonged length of stay as compared to White patients. Additionally, Black patients had significantly higher rates of nonroutine discharge. These differences remained after controlling for external factors in the multivariate analysis. Racial inequities in rates of in-hospital complication rates after neurosurgical procedures have been previously reported. Using the NIS database, Nuño et al. showed nonroutine discharge, postoperative complications, and length of stay were higher for Black patients after craniotomy for brain metastasis than for White patients.20 Likewise, Mukherjee et al. reported that Black patients were more likely to experience in-hospital complications and longer length of stay compared to White patients.44 Thus, our findings are in accordance with previously published literature.
Our study also found a higher mortality rate for Black patients after both univariate and multivariate analyses, even when controlling for differences in surgical intervention. In a variety of medical specialties, especially oncology, there is compelling evidence demonstrating a survival advantage for White patients.45 In particular, prior studies show a survival advantage for White patients in lung,46 breast,47 prostate,48 gynecologic,49 and colon cancer.50 Existing literature supports race-specific differences in mortality in the brain metastasis population. For example, in a study using data from the NIS, Curry et al. found that in-hospital mortality rates for Black patients with brain metastasis after craniotomy were significantly higher than for White patients.5 Moreover, Nuño et al., also using NIS data, demonstrated that Black patients have a significantly higher rate of mortality than White patients following craniotomy for brain metastasis, with Black women being affected more than men.18 Thus, our findings of differences in mortality when stratified by race are concordant with previous findings.
While it is important to report racial inequities across various medical and surgical fields, it is critical to acknowledge that this research is only the first step in a long process to mitigate disparities in health care. Unfortunately, the reasons underlying poor outcomes for Black and Hispanic patients compared to White patients are deeply engrained in both our healthcare system and larger society. Thus, it will require systematic, thoughtful, and multilevel approaches at the societal, community, health system, and individual provider levels to dismantle the observed racial inequities. These strategies include but are not limited to government policy changes to broaden insurance access and improve equitable access to medical and community-based resources, intentional implementation of anti-racist policies throughout healthcare systems, and individual providers identifying and mitigating their own personal biases.
Limitations
Large national database studies are subject to limitations by the data reported. As such, our study is similarly limited. The NIS data are limited to inpatient admissions; thus, results may not represent the general population. Additionally, while quality assurance measures are aimed at reducing variability in how variables are reported by participating medical centers, it is inevitable that some level of inconsistency remains. We attempted to minimize this variability by reducing our data collection period to 10 years. Moreover, large databases inherently lack granularity in the reported data; therefore, our findings are meant to represent associations and shed light on potential trends rather than claim causation. Regarding limitations directly related to the findings of our study, we acknowledge that the timing, circumstance, and setting of brain metastasis diagnoses in relation to intervention received can greatly impact patient outcomes. Unfortunately, due to the nature of the database, we were not able to comment on these nuances in treatment. Similarly, we recognize that our study results may be most relevant to a selected population of patients who needed to receive treatment (surgery, RT, and/or chemotherapy) for their BM while admitted to the hospital. Generally, patients who require this level of care are likely those who are most impaired from their disease and require urgent treatment; thus, we acknowledge that our findings may not apply to patients receiving interventions in a less emergent, outpatient setting. Similarly, we used diagnoses of bone metastases and paralysis as proxies for late-stage disease and acknowledge that these are not the best proxies for the disease burden of BM. Furthermore, given the small number of patients in Hispanic and other ethnic groups included in our database, we had to combine Hispanic patients with other ethnic groups for multivariable analyses; we acknowledge this is a significant limitation as these patient populations are distinct and should ideally be analyzed separately. Finally, institutional based studies are necessary to validate our findings with more granularity.
Conclusion
Our study represents the largest database study evaluating the significance of race on the treatment and outcomes in BM. Given the high probability of morbidity and mortality in brain metastasis, prompt delivery of optimal treatment is imperative to improve health-related quality of life in patients with this diagnosis. We observed race-related differences in baseline characteristics. Additionally, after controlling for external factors, race was found to be associated with intervention received, in-hospital complications, discharge disposition, length of stay, and in-hospital mortality. This study sheds light on the importance of race in the treatment and outcomes in BM. Furthermore, incorporating race into clinical decision-making may help to lower to gaps in outcomes moving forward.
Supplementary Material
Contributor Information
Edwin McCray, Department of Neurosurgery, Spine Division, Duke University Medical Center, Durham, North Carolina, USA.
Romaric Waguia, Department of Neurosurgery, Spine Division, Duke University Medical Center, Durham, North Carolina, USA.
Rafael de la Garza Ramos, Department of Neurosurgery, Montefiore Medical Center/Albert Einstein College of Medicine, New York City, New York, USA.
Meghan J Price, Department of Neurosurgery, Spine Division, Duke University Medical Center, Durham, North Carolina, USA.
Theresa Williamson, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Tara Dalton, Department of Neurosurgery, Spine Division, Duke University Medical Center, Durham, North Carolina, USA.
Daniel M Sciubba, Department of Neurosurgery, Zucker School of Medicine at Hofstra, Long Island Jewish Medical Center and North Shore University Hospital, Northwell Health, Manhasset, New York, USA.
Reza Yassari, Department of Neurosurgery, Montefiore Medical Center/Albert Einstein College of Medicine, New York City, New York, USA.
Andrea N Goodwin, Department of Sociology, Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Peter Fecci, Department of Neurosurgery, Spine Division, Duke University Medical Center, Durham, North Carolina, USA.
Margaret O Johnson, Department of Neurosurgery, Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, North Carolina, USA.
Kaisorn Chaichana, Mayo Clinic, Jacksonville, Florida, USA.
C Rory Goodwin, Department of Neurosurgery, Spine Division, Duke University Medical Center, Durham, North Carolina, USA.
Funding
No funding source was used to support this work.
Conflict of interest statement. The authors have no disclosures relevant to the current work, nor any true/perceived conflicts of interest. Disclosures unrelated to the current work include: Edwin McCray, Rafael De la Garza Ramos, Meghan Price, Theresa Williamson, Tara Dalton, Reza Yassari, Andrea M. Goodwin, Peter E. Fecci, Kaisorn Chaichana, Romaric Waguia, Margaret Johnson: None. Daniel M. Sciubba, MD: Consultant for Baxter, DePuy-Synthes, Globus Medical, K2M, Medtronic, NuVasive, Stryker. Unrelated grant support from Baxter Medical, North American Spine Society, and Stryker. C. Rory Goodwin, MD, PhD: Received grants from the Robert Wood Johnson Harold Amos Medical Faculty Development Program, the Federal Food and Drug Administration, and the NIH/NINDS K12 NRCDP Physician Scientist Award. Consultant for Medtronic
References
- 1. Narayan MC, Scafide KN. Systematic review of racial/ethnic outcome disparities in home health care. J Transcult Nurs. 2017;28(6):598–607. [DOI] [PubMed] [Google Scholar]
- 2. Haider AH, Scott VK, Rehman KA, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216(3):482–492.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61(2):387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bytnar JA, Lin J, Shriver CD, et al. Racial differences in brain cancer characteristics and survival: an analysis of SEER data. Cancer Causes Control. 2019;30(12):1283–1291. [DOI] [PubMed] [Google Scholar]
- 5. Curry WT, Jr., Carter BS, BarkerFG, 2nd. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988-2004. Neurosurgery. 2010;66(3):427–437. [DOI] [PubMed] [Google Scholar]
- 6. DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. [DOI] [PubMed] [Google Scholar]
- 7. Lin JJ, Mhango G, Wall MM, et al. Cultural factors associated with racial disparities in lung cancer care. Ann Am Thorac Soc. 2014;11(4):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benjamins MR, Hunt BR, Raleigh SM, et al. Racial disparities in prostate cancer mortality in the 50 largest US cities. Cancer Epidemiol. 2016;44:125–131. [DOI] [PubMed] [Google Scholar]
- 9. Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30(4):401–405. [DOI] [PubMed] [Google Scholar]
- 10. Khawja SN, Mohammed S, Silberfein EJ, et al. Pancreatic cancer disparities in African Americans. Pancreas. 2015;44(4):522–527. [DOI] [PubMed] [Google Scholar]
- 11. Jinjuvadia R, Jinjuvadia K, Liangpunsakul S. Racial disparities in gastrointestinal cancers-related mortality in the U.S. population. Dig Dis Sci. 2013;58(1):236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamba N, Wen PY, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease. Neuro-Oncology. 2021;23(9):1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 14. Balsa AI, Seiler N, McGuire TG, et al. Clinical uncertainty and healthcare disparities. Am J Law Med. 2003;29(2–3):203–219. [PubMed] [Google Scholar]
- 15. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 16. Deorah S, Lynch CF, Sibenaller ZA, et al. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20(4):E1. [DOI] [PubMed] [Google Scholar]
- 17. Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003;98(3):603–609. [DOI] [PubMed] [Google Scholar]
- 18. Curry WT, Jr., BarkerFG, 2nd. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93(1):25–39. [DOI] [PubMed] [Google Scholar]
- 19. Sharma R, Johnson A, Li J, et al. Racial disparities and the acute management of severe blunt traumatic brain injury. Trauma Surg Acute Care Open. 2019;4(1):e000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nuno M, Mukherjee D, Elramsisy A, et al. Racial and gender disparities and the role of primary tumor type on inpatient outcomes following craniotomy for brain metastases. Ann Surg Oncol. 2012;19(8):2657–2663. [DOI] [PubMed] [Google Scholar]
- 21. Rinaldo L, Rabinstein AA, Cloft H, et al. Racial and ethnic disparities in the utilization of thrombectomy for acute stroke. Stroke. 2019;50(9):2428–2432. [DOI] [PubMed] [Google Scholar]
- 22. Project HCaU. HOSP_BEDSIZE-Bedsize of Hospital. 2022. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp. Accessed June 22, 2022.
- 23. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Statistics USBoL. Labor Force Statistics from the Current Population Survey. 2022. E-16 Unemployment Rates by Age, Sex, Race, and Hispanic or Latino ethniCity. https://www.bls.gov/web/empsit/cpsee_e16.htm. Accessed July 5, 2021.
- 25. Shrider EA, Kollar M, Chen F, et al. Income and Poverty in the United States: 2020 . 2021. https://www.census.gov/library/publications/2021/demo/p60-273.html. Accessed July 5, 2021.
- 26. Buchmueller TC, Levinson ZM, Levy HG, et al. Effect of the affordable care act on racial and ethnic disparities in health insurance coverage. Am J Public Health. 2016;106(8):1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berland LL, Monticciolo DL, Flores EJ, et al. Relationships between health care disparities and coverage policies for breast, colon, and lung cancer screening. J Am Coll Radiol. 2019;16(4 Pt B):580–585. [DOI] [PubMed] [Google Scholar]
- 28. Dickman SL, Himmelstein DU, Woolhandler S. Inequality and the health-care system in the USA. Lancet. 2017;389(10077):1431–1441. [DOI] [PubMed] [Google Scholar]
- 29. Du XL, Lin CC, Johnson NJ, et al. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979-2003. Cancer. 2011;117(14):3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg Oncol Clin N Am. 2012;21(3):417–437, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eden SV, Heisler M, Green C, et al. Racial and ethnic disparities in the treatment of cerebrovascular diseases: importance to the practicing neurosurgeon. Neurocrit Care. 2008;9(1):55–73. [DOI] [PubMed] [Google Scholar]
- 32. Virnig BA, Baxter NN, Habermann EB, et al. A matter of race: early-versus late-stage cancer diagnosis. Health Aff. 2009;28(1):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De la Garza Ramos R, Benton JA, Gelfand Y, et al. Racial disparities in clinical presentation, type of intervention, and in-hospital outcomes of patients with metastatic spine disease: an analysis of 145,809 admissions in the United States. Cancer Epidemiol. 2020;68:101792. [DOI] [PubMed] [Google Scholar]
- 34. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel N, Patel S, Cotti E, et al. Unconscious racial bias may affect dentists’ clinical decisions on tooth restorability: a randomized clinical trial. JDR Clin Trans Res. 2019;4(1):19–28. [DOI] [PubMed] [Google Scholar]
- 36. Breathett K, Yee E, Pool N, et al. Does race influence decision making for advanced heart failure therapies? J Am Heart Assoc. 2019;8(22):e013592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breathett K, Jones J, Lum HD, et al. Factors related to physician clinical decision-making for African-American and Hispanic patients: a qualitative meta-synthesis. J Racial Ethn Health Disparities. 2018;5(6):1215–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22(9):1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saha S, Komaromy M, Koepsell TD, et al. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999;159(9):997–1004. [DOI] [PubMed] [Google Scholar]
- 40. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hung P, Deng S, Zahnd WE, et al. Geographic disparities in residential proximity to colorectal and cervical cancer care providers. Cancer. 2020;126(5):1068–1076. [DOI] [PubMed] [Google Scholar]
- 42. Onega T, Alford-Teaster J, Wang F. Population-based geographic access to parent and satellite National Cancer Institute Cancer Center Facilities. Cancer. 2017;123(17):3305–3311. [DOI] [PubMed] [Google Scholar]
- 43. Amin S, Baine M, Meza J, et al. The impact of treatment facility type on the survival of brain metastases patients regardless of the primary cancer type. BMC Cancer. 2021;21(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mukherjee D, Patil CG, Todnem N, et al. Racial disparities in Medicaid patients after brain tumor surgery. J Clin Neurosci. 2013;20(1):57–61. [DOI] [PubMed] [Google Scholar]
- 45. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. [DOI] [PubMed] [Google Scholar]
- 46. Richards TB, Henley SJ, Puckett MC, et al. Lung cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):5079–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yedjou CG, Sims JN, Miele L, et al. Health and racial disparity in breast cancer. Adv Exp Med Biol. 2019;1152:31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steele CB, Li J, Huang B, et al. Prostate cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):5160–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rauh-Hain JA, Melamed A, Schaps D, et al. Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies. Gynecol Oncol. 2018;149(1):4–11. [DOI] [PubMed] [Google Scholar]
- 50. Jackson CS, Oman M, Patel AM, et al. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol. 2016;7(Suppl 1):S32–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.