Abstract
Intro:
The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) proposed “Biotypes,” subgroups of psychosis cases with neuro-cognitive homology. Neural activity unbound to stimulus processing (nonspecific or intrinsic activity) was important for differentiating Biotypes, with Biotype-2 characterized by high nonspecific neural activity. A precise estimate of intrinsic activity (IA) was not included in the initial Biotypes characterization. This report hypothesizes intrinsic activity is a critical differentiating feature for psychosis Biotypes.
Method:
Participants were recruited at B-SNIP sites and included probands with psychosis (schizophrenia, schizoaffective disorder, bipolar I disorder), their first-degree biological relatives, and healthy persons (N = 1338). Probands were also sub-grouped by psychosis Biotype. 10-sec inter-stimulus intervals during an auditory paired-stimuli task were used to quantify intrinsic activity from 64 EEG sensors. Single-trial power and connectivity measures at empirically derived frequency bands were quantified. Multivariate discriminant and correlational analyses were used to summarize variables that efficiently and maximally differentiated groups by conventional diagnoses and Biotypes and to determine their relationship to clinical and social functioning.
Results:
Biotype-1 consistently exhibited low IA, and Biotype 2 exhibited high IA relative to healthy persons across power frequency bands (delta/theta, alpha, beta, gamma) and alpha band connectivity estimates. DSM groups did not differ from healthy persons on any IA measure.
Discussion:
Psychosis Biotypes, but not DSM syndromes, were differentiated by intrinsic activity; Biotype-2 was uniquely characterized by an accentuation of this measure. Neurobiologically defined psychosis subgroups may facilitate the use of intrinsic activity in translation models aimed at developing effective treatments for psychosisrelevant deviations in neural modulation.
Keywords: Intrinsic activity, B-SNIP, Biotypes, DSM, Psychosis, Connectivity
1. Introduction
The Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) evaluated whether DSM psychosis diagnoses are neuro-biologically distinctive [1,2]. There were statistical tendencies for schizophrenia (SZ), schizoaffective disorder (SAD), and bipolar disorder with psychosis (BDP) to differ in predictable directions on multiple measures of brain function and structure. But these syndromes were also characterized by heterogeneity within and overlap between subgroups on every evaluated purported biomarker ([3–8]). Reorganizing of cases based on similarity of biomarker features yielded three psychosis Biotypes [9] that were neurobiologically distinctive and better predictors of external validating measures [6,10].
A critical differentiating feature of the Biotypes was a composite measure of neural response to sensory events summarized as “sensorimotor reactivity.” Biotype-1 showed deficient abilityto generate neural responses to sensory stimuli and Biotype-2 had accentuated neural activity during auditory stimulation tasks; however, Biotype-3 had only mild deviations from healthy on these measures. Biotype-2’s evoked response amplitudes (e.g., n100, p200, p300 ERPs) to auditory stimulation were similar to healthy people, but other measures related to ongoing activity (not specifically stimulus-related) were significantly elevated [9,11]. One such bio-factor (integrated bio-markers) initially called “intrinsic activity” is more accurately described as “ongoing high frequency activity”. This bio-factor was comprised of preparatory and induced high frequency signals from oddball and paired-stimulus tasks [9].
The concept of intrinsic activity (IA) is implicated in the neurophysiology of the psychoses [11,12]. Ongoing and unstructured neural activity accounts for 95% of the brain’s energy expenditure [13]. Translational models purport that diminished signal-to-noise ratios are associated with problems identifying stimulus salience, presenting a promising way of identifying physiological mechanisms for psychosis manifestation [12]. Differences in intrinsic activity between Biotype-1 and Biotype-2 could reveal important information about the neurobiological correlates of these psychosis subgroups.
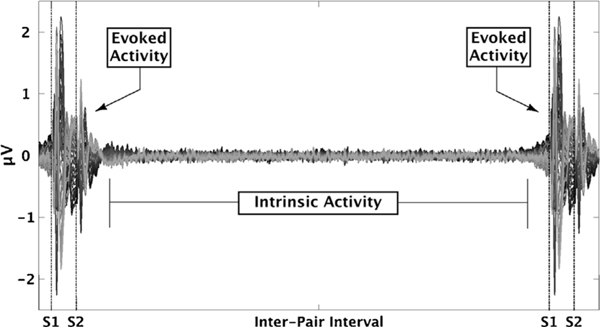
A direct quantification of ongoing, or intrinsic, neural activity was not included in the original Biotypes biomarker analyses. However, such information is available within the context of the auditory paired-stimuli paradigm. During this task, stimuli are presented in pairs separated by 500-ms followed by a 10-sec inter-pair interval (Fig. 1). Paired-stimuli variables used in Biotypes creation came from the period 200-ms before the first stimulus to 400-ms after the second stimulus, leaving at least 9-s of inter-pair interval available for separate IA quantification. During this period, participants are awaiting the next stimulus pair, but have no structured task or processing requirements. In contrast to the original induced activity bio-factor, this unstructured period provides a different and perhaps better index of ongoing neural activity.
Fig. 1.
Intrinsic Activity. Butterfly plot of mean voltage response averaged over all trials and all healthy participants (N =218). Evoked activity is in response to a paired click stimuli (500 ms interval between clicks). Intrinsic activity was quantified using the intervals between each evoked response.
Our interest was to improve a theoretically and practically important psychosis subgroup difference on level of IA that could be used in subsequent translational and treatment outcome investigations. The purpose of these analyses was to evaluate the following hypotheses: (i) DSM psychosis subgroups do not differ on level of IA; (ii) Biotype-1 cases have reduced levels of IA compared to all other groups; and (iii) Biotype-2 cases have enhanced levels of IA compared to all other groups; and (iv) the initial bio-factors used to characterize exuberant neural activity in Biotype-2 are highly correlated with a more direct (least constrained) index of IA.
2. Methods
2.1. Participants
Laboratory data collection, participant recruitment, and interviews were completed at the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) consortium sites (complete details on recruitment, clinical, and demographic characteristics can be found in [1]). Data analysis was performed at the University of Georgia in Athens, Georgia. Probands with psychosis (N = 531), their first-degree relatives (N = 589), and demographically comparable healthy people (N = 218) were fully clinically characterized [1]. Probands were assessed with the Structured Clinical Interview for DSM-IV-TR [14] while their relatives were given the Structured Interview for DSM-IV Personality Disorders [15] to measure psychosis spectrum personality traits. Those probands that met DSM-IV criteria for either SZ, SAD, or BDP were administered the Wide Range Achievement Test (WRAT; [16]), Positive and Negative Symptom (PANSS; [17]), Montgomery-Asberg Depression Rating (MADRS; [18]), Young Mania Rating (YMRS; [19]), Global Assessment of Functioning (GAF; [14]), and Birchwood Social Functioning (SFS; [20]) scales ([1]; see Table 1a–b, Supplemental Tables 1a–b). Healthy participants had no personal history of lifetime psychotic disorders, and no first-degree relatives with a history of psychotic or bipolar disorder as assessed by Family History Research Diagnostic Criteria [21]. The majority of probands and a small subset of their relatives were taking psychotropic medication (Supplemental Tables 2a–d). There were minimal associations between clinical and/or medication variables and biomarker outcomes [9]. The project was approved by IRBs at the participating institutions. All participants provided informed consent prior to inclusion after they obtained a complete description of the study.
Table 1.
Sociodemographic and Clinical characteristics of Probands and HC. 1a) Sample characteristics by Biotype constructs. 1b) Sample characteristics by conventional diagnoses.
| Sample Characteristics by Biotype Constructs | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| HC (n = 218) | B1 (n = 148) | B2 (n = 169) | B3 (n = 214) | Test Statistic | P Value | |
|
| ||||||
| Sociodemographic Characteristics | ||||||
| Age, Years, Mean (SD) | 37.43 (12.39) | 37.42 (13.26) | 35.29 (12.22) | 35.01 (12.48) | F6.1330 = 9.927 | <.001 |
| Sex/Male, n(%) | 93 (42.7) | 77 (52.0) | 86 (50.9) | 114 (53.3) | X26 = 43.08 | <.001 |
| Handedness, n(%) | X26 = 13.40 | 0.341 | ||||
| Right | 189 (86.7) | 117 (80.7) | 149 (88.7) | 180 (84.9) | ||
| Left | 26 (11.9) | 23 (15.5) | 16 (9.5) | 29 (13.7) | ||
| Ambidextrous | 3 (1.4) | 5 (3.4) | 3 (1.8) | 3 (1.4) | ||
| Ethnicity/Hispanic, n(%) | 21 (9.6) | 15 (10.1) | 18 (10.7) | 14 (6.5) | X26 = 11.798 | 0.067 |
| Race, n(%) | ||||||
| White | 148 (67.9) | 69 (46.6) | 116 (68.6) | 150 (70.1) | X26 = 57.194 | <.001 |
| African American | 56 (25.7) | 77 (52.0) | 50 (29.6) | 60 (28.0) | X26 = 61.901 | <.001 |
| Other | 4 (1.8) | 3 (2.0) | 2 (1.2) | 3 (1.4) | X26 = 1.221 | 0.976 |
| Education, years, Mean (SD) | 15.26 (2.62) | 12.41 (2.08) | 13.16 (2.32) | 14.15 (2.31) | F6.1327 = 27.85 | <.001 |
| Clinical Characteristics, Mean (SD) | ||||||
| Age of Illness Onset, Years | - | 17.55 (6.99) | 17.55 (7.76) | 17.38 (7.90) | F6,760 = 12.346 | <.001 |
| Age of First Hospitalization, Years | - | 21.47 (7.72) | 23.11 (8.35) | 23.73 (8.83) | F6,541=1.222 | 0.3 |
| No. of Lifetime Hospitalizations | - | 7.24 (7.18) | 5.89 (6.55) | 4.69 (5.43) | F6,497 = 2.835 | <.05 |
| PANSS | ||||||
| Total | - | 64.33 (17.96) | 64.99 (17.65) | 60.12 (16.50) | F6,593 = 5.163 | <.001 |
| Positive Subscale | - | 16.41 (5.55) | 16.29 (5.77) | 14.93 (5.15) | F6,594 = 4.025 | <.005 |
| Negative Subscale | - | 16.27 (5.88) | 15.45 (5.27) | 13.92 (5.30) | F6,594 = 6.510 | <.001 |
| General Symptoms Subscale | - | 31.68 (9.18 | 33.30 (9.20) | 31.28 (8.73) | F6,595 = 3.498 | <.005 |
| YMRS | - | 5.49 (5.98) | 6.38 (6.22) | 5.73 (5.97) | F6,656 = 3.812 | <.005 |
| MADRS | - | 10.01 (9.73) | 11.54 (9.15) | 10.52 (5.97) | F6,656 = 6.174 | <.001 |
| GAF | 86.52 (6.60) | 49.09 (12.08) | 52.66 (13.63) | 56.04 (13.68) | F6,1315 = 232.947 | <.001 |
| WRAT-4 IQ | 103.45 (14.20) | 90.01 (13.79) | 94.85 (13.92) | 105.36 (14.03) | F6,1310 = 31.905 | <.001 |
|
| ||||||
| Sample Characteristics by Conventional Diagnoses | ||||||
|
| ||||||
| HC (n = 218) | SZ (n = 223) | SAD (n = 130) | BD (n = 178) | Test Statistic | P Value | |
|
| ||||||
| Sociodemographic Characteristics | ||||||
| Age, Years, Mean (SD) | 37.43 (12.39) | 35.22 (12.78) | 36.51 (12.20) | 35.92 (12.81) | F6.1330 = 9.728 | <.001 |
| Sex/Male, n(%) | 93 (42.7) | 148 (66.4) | 57 (43.8) | 72 (40.4) | X26 = 73.830 | <.001 |
| Handedness, n(%) | X26 = 16.581 | 0.166 | ||||
| Right | 189 (86.7) | 185 (84.5) | 112 (86.2) | 149 (84.7) | ||
| Left | 26 (11.9) | 30 (13.7) | 12 (9.2) | 26 (14.8) | ||
| Ambidextrous | 3 (1.4) | 4 (1.8) | 6 (4.6) | 1 (0.6) | ||
| Ethnicity/Hispanic, n(%) | 21 (9.6) | 19 (8.5) | 15 (11.5) | 13 (7.3) | ||
| Race, n(%) | ||||||
| White | 148 (67.9) | 112 (50.2) | 87 (66.9) | 136 (76.4) | X26 = 62.266 | <.001 |
| African American | 56 (25.7) | 104 (46.6) | 47 (36.2) | 36 (20.2) | X26 = 66.695 | <.001 |
| Other | 4 (1.8) | 4 (1.8) | 1 (0.80) | 3 (1.7) | X26 = 2.931 | 0.818 |
| Education, years, Mean (SD) | 15.26 (2.62) | 12.78 (2.25) | 14.20 (2.37) | 36.51 (12.20) | F6,1327 = 23.092 | <.001 |
| Clinical Characteristics, Mean (SD) | ||||||
| Age of Illness Onset, Years | - | 18.12 (6.16) | 17.45 (8.91) | 14.20 (2.37) | F6,760 = 12.483 | <.001 |
| Age of First Hospitalization, Years | - | 22.19 (7.05) | 23.60 (9.59) | 23.10 (8.89) | F5,541=0.621 | 0.684 |
| No. of Lifetime Hospitalizations | - | 5.11 (4.97) | 6.00 (7.59) | 6.75 (6.80) | F5,498=1.923 | 0.089 |
| PANSS | ||||||
| Total | - | 67.04 (17.31) | 53.13 (13.59) | 68.98 (16.61) | F6,593 = 21.018 | <.001 |
| Positive Subscale | - | 16.98 (5.48) | 12.67 (4.30) | 17.96 (5.15) | F6,594 = 10.831 | <.001 |
| Negative Subscale | - | 17.00 (5.86) | 11.99 (3.76) | 15.97 (5.23) | F6,594 = 21.178 | <.001 |
| General Symptoms Subscale | - | 33.10 (9.05) | 28.47 (7.99) | 35.06 (8.86) | F6,595 = 10.927 | <.001 |
| YMRS | - | 5.84 (5.80) | 5.21 (5.89) | 6.86 (6.59) | F6,656 = 4.145 | <.001 |
| MADRS | - | 8.63 (8.27) | 10.46 (9.30) | 14.51 (10.39) | F6,656 = 11.367 | <.001 |
| GAF | 86.52 (6.60) | 49.19 (12.37) | 61.02 (12.54) | 48.63 (11.73) | F6,1314 = 261.329 | <.001 |
| WRAT-4 IQ | 103.45 (14.20) | 94.96 (16.19) | 101.72 (13.57) | 97.03 (15.19) | F6,1310 = 9.278 | <.001 |
2.2. Procedures
Across sites, testing and recording conditions were similar; stimulus presentation and recording equipment were identical. Experimenters were trained and continually monitored across sites to ensure data collection procedures were comparable. Previous studies confirmed there were no group by site effects on the EEG data [7].
2.3. Stimuli
Participants sat in a shielded booth during the paired-stimuli task, and listened to 150 binaural broadband auditory click pairs (4-ms duration at 75 dB with a 500-ms interclick interval) occurring every 9–10 for an average of 9.5 s. Clicks were presented through etymotic ear inserts. Participants were told to count click pairs [7].
2.4. EEG recording
Electroencephalogram (EEG) from 64 sensors was recorded following previously published methods from the B-SNIP consortium ([7]; Supplemental Methods).
2.4.1. Data processing
EEG data were pre-processed following previously published methods ([7], Supplemental methods). After artifact removal, data from 500 ms after the second click of each trial and 500 ms before the next trial were extracted. Epochs containing activity ±125 μV at any sensor at any time point were excluded from any subsequent analysis. Any participant that did not have at least 30 epochs (270 s) was excluded from subsequent analysis.
2.5. Time-frequency transformation
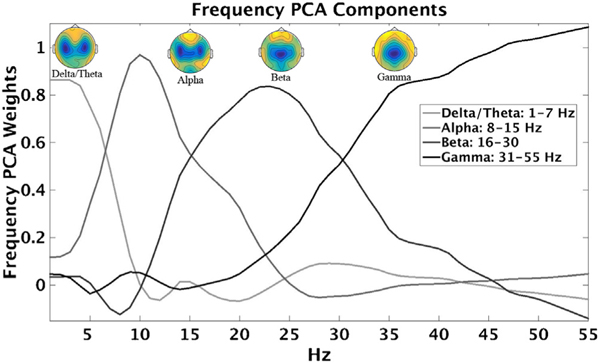
Data were transformed into the time-frequency (TF) domain using the following approach. In EEGlab, FFTs were computed on 50% overlapping Hanning tapered windows (1–55 Hz, 1000 ms steps, 1 Hz resolution) for each 9-second inter-pair epoch, resulting in 17 time bins per epoch [500–8500 ms in 500 ms bins] [40,47]. Power values (squared absolute values of complex FFT outputs) were then converted to decibels (10*log10). To determine the stability of the Power values across time, intraclass correlation was calculated across time bins for each sensor and frequency using all participants’ data. Since all ICCs were > 0.96, power values were averaged over time bins. In order to capture maximum explanatory variance across variables, avoid information redundancy, and reduce the number of statistical comparisons, frequency data reduction was accomplished by principal component analysis (PCA, see Supplemental Methods; Fig. 2). The 55 frequencies were reduced to four primary bands (97% variance accounted): delta/theta (1–7 Hz), alpha (8–15 Hz), beta (16–30 Hz), and gamma (31–55 Hz), as shown in Fig. 2. An additional spatial PCA (variance range: 37–49%) was performed on each frequency band in order to reduce the data from 64 sensors to one virtual sensor ([7,8]; Supplemental Methods).
Fig. 2.
Empirically derived frequency bands. Each line represents the factor pattern matrix results for each frequency (1–55 Hz) for each component. Topographies show averaged neural response from all participants for each frequency component.
2.6. Connectivity analyses
Organization of brain activity within and between brain regions is an important compliment to assessing the magnitude of intrinsic activity [22]. To assess this additional feature, we used the debiased weighted phase-lag index (dbWPLI) as computed in Fieldtrip (Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, The Netherlands: https://www.ru.nl/neuroimaging/fieldtrip) [39]. We used all 2016 sensor pairs from the concatenated complex output of the FFTs and all time bins from each epoch (1–55 Hz; output is averaged connectivity across all epochs). The dbWPLI method minimizes associations that may result from erroneous inflation of EEG connectivity caused by volume conduction [38]. It does so by calculating an unbiased index of phase synchronization (2 or more signals oscillating with similar phase angles) between two time series that are then weighted by the magnitude of the imaginary component of the cross-spectrum [22,23,50]. The cross spectrum describes how much linear information of one signal is explained by another paired signal to estimate association between the two. The index values range from 0 to 1; 0 indicates the absence of phase-lagged coupling and 1 indicates the strongest possible coupling [22,23]. After dbWPLI calculation, the frequency PCA weights (delta/theta (1–7 Hz), alpha (8–15 Hz), beta (16–30 Hz), and gamma (31–55 Hz) derived from the Power values were applied to each sensor pair for each participant in order to compare responses across the same frequency bands.
2.7. Statistical analysis
All statistics were performed in SPSS Statistics version 23 (Armonk, NY: IBM Corp.) [42]. The following analyses were performed on each Power PCA component. Each PCA component’s age effects were calculated using a linear regression analysis on healthy participants [24]. Components with significant age effects were adjusted for all participants by subtracting the product of the linear regression coefficient and age for each individual (see Supplemental Methods). Analysis of variance (ANOVA) was used to evaluate for group differences, separately for DSM and Biotype. Each component was analyzed with a 2 (gender) X 4 (DSM: [HC, SZ, SAD, BDP]; Biotype: [HC, B1, B2, B3]) mixed model. This analysis was also performed for HC vs relatives groups (DSM: [HC, SZR, SADR, BDPR]; Biotype: [HC, B1R, B2R, B3R]). Tukey post hoc tests were used to probe significant effects in the omnibus ANOVAs. In order to account for type-1 error inflation, a significant threshold of 0.0125 was set for these ANOVA comparisons. In order to determine if there was an interaction between DSM and Biotype designation, an additional DSM by Biotype ANOVA was performed using only psychosis probands ([SZ, SAD, BDP] vs. [B1, B2, B3]).
dBWPLI:
For each of the 2016 sensor pair connections at each frequency band component (4), a 1-way ANOVA was performed separately for DSM and for Biotype proband groups and separately for HC vs DSM and Biotype relative groups. Due to the large number of statistical tests, each set of ANOVAs was run 5000 times (bootstrap procedure) with group membership randomly shuffled at each step (sampling with replacement). Probability estimates of the actual F-values were then calculated as the proportion of randomly generated F-values greater than the actual estimate [7]. To correct for the effect of multiple comparisons, the resulting distributions of p-values were converted with the false discovery rate procedure [25] to adjusted p-values, which minimized falsely rejected null hypotheses to 5%. The resulting set of significant sensors pairs were then averaged. All statistical steps used to compare Power by groups were performed on the averaged dBWPLI values.
2.8. Post hoc analyses: canonical correlation and discriminant analysis
To summarize variables that efficiently differentiated groups based on intrinsic neural responses, EEG Power/dbWPLI variables significant in the group comparisons were subjected to canonical discriminant analysis (CDA) ([7]; supplemental methods) separately by DSM [HC, SZ, SAD, and BDP] and Biotype [HC, B1, B2, and B3]. For each significant canonical variate, means and standard error of the mean were generated. A post hoc Tukey’s B test was performed to identify homogenous sub-groupings. To parsimoniously evaluate the relationships between the significant neural components and clinical measures (GAF, SFS, PANSS Negative, PANSS Positive, PANSS General, YMRS, MADRS), canonical correlation analyses (CCA) across all psychosis proband groups were performed ([26]; Supplemental Methods).
Finally, we also used Pearson correlations to quantify associations between IA measures from the above analyses and the two bio-factors on which Biotype-2 had exuberant activity (what we previously called the ‘intrinsic activity’ and ‘P200’ bio-factors; [9]) across all proband participants. This allowed us to test if those bio-factors were specifically indexing intrinsic neural activity.
3. Results
3.1. Probands: biotypes versus HC
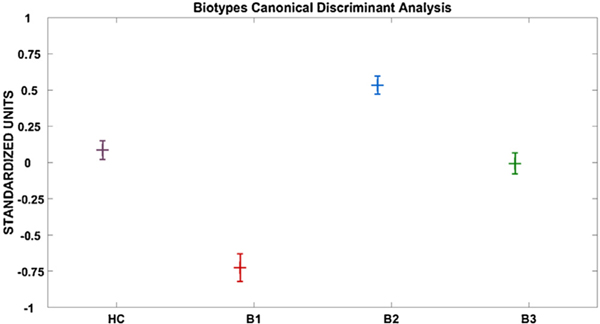
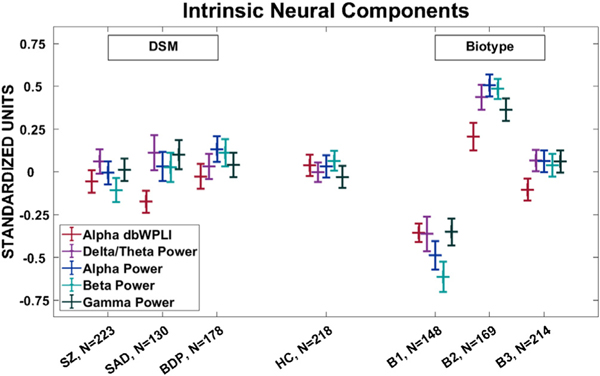
Probands grouped by the Biotype versus HC detected a significant group effect for Power in each of the 4 frequency components [delta/theta: F(3, 745) = 18.233, p < .001; alpha: F(3, 745) = 29.570, p < .001; beta: F(3, 745) = 39.232, p < .001; gamma: F(3, 745) = 16.736, p < .001]. For each frequency band Tukey B follow up tests identified three unique subgroups of B2> [HC/B3] > B1 (see Fig. 3; Supplemental Fig. 1a–d).
Fig. 3.
Canonical Variate means. Shown is a pattern of B2 > HC/B3 > B1. The variate correlated the strongest with Alpha and Beta Power components. HC, Healthy Comparison subjects (n = 218); B1, Biotype-1 (n = 148); B2, Biotype-2 (n = 169); B3, Biotype-3 (n = 214). Values are in standardized units. Error bars = SEM.
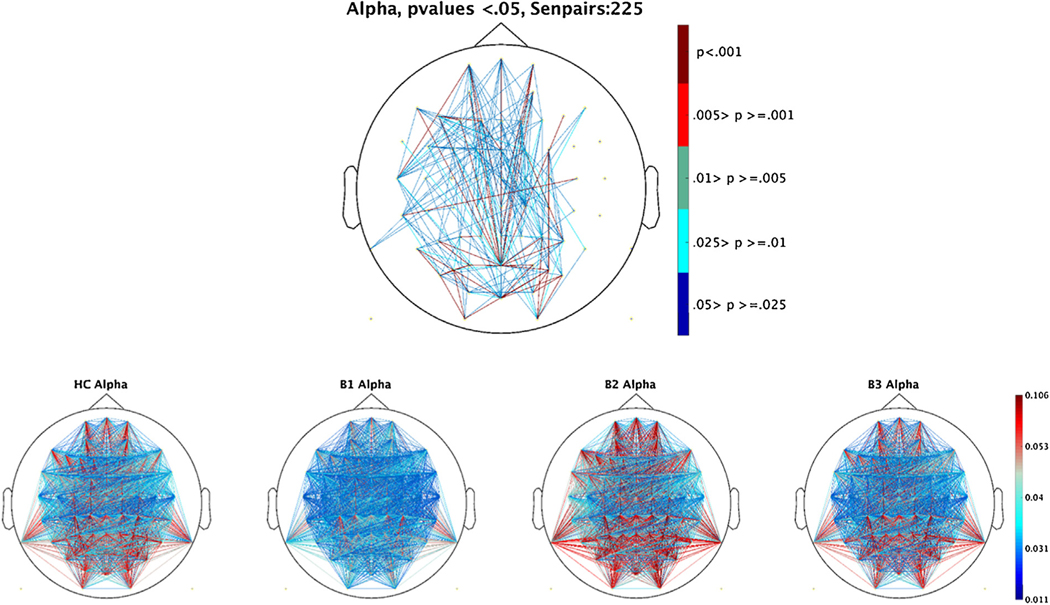
For dbWPLI, only the alpha frequency component showed an above chance number of sensor pairs (225) that remained significant after FDR correction. In alpha, the Biotype versus HC model detected a significant between-groups effect [F(3, 745) = 11.063, p < .001]. The Tukey B follow up tests identified three unique subgroups of B2> [HC/B3] > [HC/B1] (see Fig. 4).
Fig. 4.
Debiased Weighted Phase lab index for each sensor pair (2016) after Power frequency PCA weights had been applied to the data. A) FDR corrected significant (p < .05) sensor pair group ANOVA results by biotype sub-grouping. B) Group averages by biotype for the alpha frequency band (8–15 Hz). Values range from 0–1. 0 indicates the absence of phase-lagged coupling and 1 indicates the strongest possible coupling. Due to its non-normal distribution, scaling is based off of distribution percentiles from the grand average responses across groups. Tick marks indicate 25th percentiles.
3.1.1. Probands: DSM diagnosis versus HC
Probands grouped by the DSM versus HC did not detect significant between-group effects for Power in any of the 4 frequency components [delta/theta: F(3, 745) = .364, p = .779; alpha: F(3, 745) = .694, p = .556; beta: F(3, 745) = 1.867, p = .134; gamma: F(3, 745) = .546, p = .651]. For dbWPLI, no sensor pairs survived FDR correction when grouped by DSM diagnosis (see Fig. 4; Supplemental Figs. 1 and 3).
3.1.2. Probands: interaction between DSM diagnosis and biotypes
The follow up DSM by Biotype ANOVAs using only the psychosis probands did not show any significant interactions for Power or dbWPLI components: (delta/theta, alpha, beta, and gamma Power, alpha dbWPLI) [F(4,739)>.752, p’s>.171].
3.1.3. Relatives: biotypes versus HC
Relatives grouped by the Biotypes versus HC model did not detect significant between group effects for Power in three power frequency components [delta/theta: F(3, 803) = .590, p = .621; alpha: F(3, 803) = 1.838, p = .139; gamma: F(3, 803) = 2.093, p = 0.100] (see Supplemental Figs. 2 and 3). There was a significant group difference at beta [F(3, 803) = 3.061, p < .05], B1R being significantly different from B2R (p < .05). For dbWPLI, no sensor pairs survived FDR correction when grouped by Biotype.
3.1.4. Relatives: DSM versus HC
Relatives grouped by the DSM versus HC did not detect significant between group effects for Power in any of the 4 power frequency components [delta/theta: F(3, 803) = 1.295, p = .275; alpha: F(3, 803) = 1.107, p = .345; beta: F(3, 803) = .614, p = .606; gamma: F(3, 803) = 1.076, p = .358] (see Supplemental Fig. 2a–d). For dbWPLI, no sensor pairs survived FDR correction when grouped by DSM diagnosis.
3.2. Canonical discriminant analysis
3.2.1. Probands: biotypes versus HC
The 5 components that showed significant group differences (delta/theta, alpha, beta, gamma Power, and alpha dbWPLI) were used in the CDA to efficiently summarize group differentiations (HC, B1, B2, B3; no variables significantly differentiated the DSM groups). Only the first variate (= .387, Wilks’ Lambda = .833, Chi = 136.1, df = 15, p < .001) was statistically significant (see Table 2 for canonical loadings). The canonical variate (plotted in Fig. 5, values in standardized units) was associated with higher beta (r = .944), and alpha Power (r = .810), and showed a pattern of B2 (mean = .528, SEM = .063) >HC (mean = .089, SEM = .066), B3 (mean = .008, SEM = .072) > B1 (mean= −.747, SEM = .094). A follow up Tukey B post-hoc test identified three homogenous sub-groups: B2 > HC/B3> B1. Correlations between each component and the canonical variate are provided in Table 2.
Table 2.
Canonical Discriminant Analysis Results: Biotypes Variate 1.
| Eigenvalue | % of Variance | Cumulative % | Canonical Correlation | |
|---|---|---|---|---|
|
| ||||
| Biotypes Variate 1: | 0.176 | 89.5 | 89.5 | 0.387*** |
| Wilks’ Lambda | Chi-Square | Df | Sig. | |
| Correlations: | 0.833 | 136.055 | 15 | <0.001 |
| Delta/Theta STP | Alpha STP | Beta STP | Gamma STP | Alpha dbWPLI | |
|
| |||||
|---|---|---|---|---|---|
| Group Centroids: | −0.011 | −0.043 | 1.037 | −0.151 | 0.31 |
| N | Mean | SD | Lower 95% | Upper 95% | |
|
| |||||
|---|---|---|---|---|---|
| HC | 218 | 0.089 | 0.971 | −0.04 | 0.219 |
| B1 | 148 | −0.747 | 1.144 | −0.932 | −0.561 |
| B2 | 169 | 0.528 | 0.823 | 0.403 | 0.653 |
| B3 | 214 | 0.008 | 1.05 | −0.133 | 0.149 |
Note:
p <.05
p< .01
p < .001.
Fig. 5.
Final Component values for each of the 5 significant components (Delta, Alpha, Beta, & Gamma Power and Alpha dbWPLI). See supplemental Figs. 1 and 2 for frequency and spatial PCA weights. Each component showed a highly similar pattern for DSM (HC and probands showed a similar response) and Biotype groupings (B2 > HC/B3> B1). A) Values sorted by DSM categories. HC, Healthy Comparison subjects (n = 218); SZ, probands with schizophrenia (n = 223); SAD, probands with schizoaffective disorder (n = 130); BDP, probands with bipolar disorder I with psychosis (n = 178). B) HC, Healthy Comparison subjects (n = 218); B1, Biotype-1 (n = 148); B2, Biotype-2 (n = 169); B3, Biotype-3 (n = 214). Values are in standardized units. Error bars = SEM.
3.2.2. DSM probands & DSM, biotypes relatives versus HC
Since there were no significant results for DSM probands or relatives, and only one for Biotype relatives, no post-hoc CDAs were performed.
3.3. Canonical correlations
Only the first canonical variate was significant [canonical correlation = 0.40, Wilk’s lambda = 0.76, F(40, 1585) = 2.61, p < .001]. Correlation loadings for each variable with its canonical variate are provided in Supplemental Table 3 (see, e.g. [27]). The loadings indicate current general psychosis-related clinical features are positively and most closely associated with magnitude of neural activity in lower frequency ranges (beta, alpha, and delta/theta).
3.3.1. Canonical correlations by groups
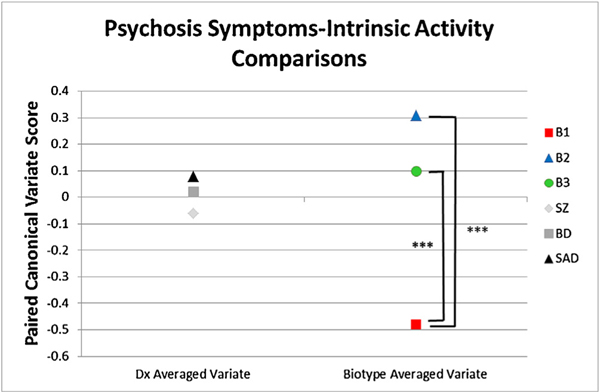
In order to find the strength of the CCA associations as function of group membership, the canonical coefficients for the intrinsic activity variate and clinical symptom variate were averaged together for each individual. This step was taken since both variates index the same construct. High averaged coefficients mean high intrinsic neural activity was associated with more psychosis symptoms, and low averaged coefficients mean low intrinsic neural activity was associated with fewer psychosis symptoms (see Fig. 6). The DSM groups did not differ on magnitude of the averaged coefficients (F(2, 373) = 0.900, p = 0.408). The Biotype groups, however, significantly differed on the averaged coefficients (F(2, 373) = 30.55, p < .001). According to Tukey’s b post-hoc test, B1 and B2 (p < .001), and B1 and B3 (p < .001), significantly differed. B2 and B3 did not differ significantly on strength of the relationship between intrinsic activity and general psychosis symptoms (p = .066). The pattern of group differences indicates that the high B2 intrinsic activity was associated with increased current psychosis symptoms, while the low B1 intrinsic activity was associated with fewer current psychosis symptoms.
Fig. 6.
Average of the two canonical correlation variates using probands only (82% of proband sample). One variate was comprised of EEG variables (Delta/Theta, Alpha, Beta, Gamma Power, and Alpha dbWPLI) and one variate was comprised of clinical variables (GAF, SFS, YMRS, MADRS, WRAT, PANSS (Positive, Negative, General)). Coefficients are listed in supplemental Table 3. Values are for participants when organized by DSM diagnoses and biotype. Significant differences are shown with asterisks for B1 < B3 and B1 < B2.
3.3.2. Correlation of IA measures with bio-factors
Across the proband sample the IA measures across frequency bands were strongly correlated with the ongoing high frequency (previously called “intrinsic activity”) bio-factor [delta/theta: r = 0.558, p < .001; alpha: r=0.614, p < .001; beta: r=0.741, p < .001; gamma: r=0.640, p < .001]. The IA measures had a weaker correlation with the “p200” bio-factor: delta/theta: r=0.136, p < .005; alpha: r=0.239, p < .001; beta: r=0.302, p < .001; gamma: r=0.317, p < .001.
4. Discussion
Multiple lines of evidence support the proposition that level of intrinsic neural activity is important for understanding psychosis neurophysiology [11,12,28]. Differences in IA across multiple neural oscillation frequencies or within specific neural oscillatory ranges may be important translational biomarkers, especially for studying mechanisms supporting specific pharmacological interventions ([12]; Spencer et al., 2014). The biomarker panel used in developing psychosis Biotypes by B-SNIP [9] did not include a direct index of IA, although Biotype-2 was characterized by exuberant neural activity on two bio-factors. The “intrinsic activity” (really “ongoing high-frequency”) bio-factor was significantly more highly correlated with the direct measures of IA described in this paper than with the “p200” bio-factor. Additionally, this direct measure of IA distinguished groups as well as, or better than, the bio-factor statistically used to maximally differentiate groups (Glass Δ present in Table 3). This outcome, therefore, yields the possibility of more specific target engagement for interventions aimed at moderating a physiological deviation associated with a subset of psychosis features
Table 3.
Glass Δ Effect Sizes.
| B1 | Biotype B2 | B3 | SZ | DX BD | SAD | |
|---|---|---|---|---|---|---|
|
| ||||||
| Delta/Theta STP | −0.412 | 0.537 | 0.059 | 0.075 | 0.047 | 0.133 |
| Alpha STP | −0.544 | 0.495 | 0.032 | −0.04 | 0.106 | 0.001 |
| Beta STP | −0.802 | 0.482 | −0.013 | −0.197 | 0.052 | −0.042 |
| Gamma STP | −0.359 | 0.41 | 0.115 | 0.045 | 0.071 | 0.14 |
| Alpha dbWPLI | −0.018 | 0.008 | −0.007 | −0.004 | −0.003 | −0.01 |
Consistent with expectations based on Clementz et al. [9], intrinsic neural activity was high in Biotype-2, low in Biotype-1, and similar to healthy levels in Biotype-3. The unique contribution of this report is that these differences spanned frequency bands but were most prominent in lower bands, including measures of power and distributed sensor-sensor connectivity. The gamma band is most closely associated with local sensory processing [29], so it is unsurprising neural activity in this frequency range contributed less to group discriminations using data collected during an inter-trial interval. Intrinsic activity measures were also associated with level of current psychosis symptoms: Biotype-2 showing high IA-high current symptoms and Biotype-1 showing low IA-low current psychosis symptoms, while Biotype-3 reflected healthy comparisons. When grouped by DSM subgroups there were minimal between-groups differences on intrinsic neural activity, and there was no clear relationship between level of IA and current psychosis symptoms (see Fig. 6). There was also little indication that IA is a unique biomarker for any specific DSM psychosis syndrome used in this report.
Psychosis syndromes have shown differences from healthy persons on non-specific neural activity and correspondingly low signal-to-noise ratios using multiple measurement schemes, paradigms, and quantification methods (Butler et al., 2001; Clementz & Blumendfeld, 2001; Clementz et al., 2004; Clementz et al., 2008, [30]; Hudgens- Haney et al., 2017; Krishnan et al., 2005; [48] 1; [31,32]; Winterer et al., 2000, 2006). Most of these reports involved exclusive evaluation of SZ cases (for an exception see [33]), with findings driving theories of how intrinsic activity could be an important translational biomarker for an aspect of this psychosis syndrome [12,28]. The present report supports this proposition, but adds that deviant intrinsic activity can manifest in divergent ways within SZ, and, more explicitly, may transcend psychosis syndromes. Biotype-2 cases, with high IA, are about 45% SZ, while Biotype-1 cases, with low IA, are about 60% SZ [9]. No interaction between Biotype and DSM was found in our analysis. The conclusions drawn about intrinsic activity as a biomarker for SZ, therefore, may be dependent on the type of SZ subsample being recruited into each individual research project.
Neural oscillations measured with EEG derive from coordinated ensemble activity comprising thousands of neurons, with distinct frequencies typically associated with different functions and neural architectures [41]. For instance, lower frequency oscillations (e.g., theta, alpha) are associated with cortico-cortical communication between distant brain regions. Theta and alpha oscillations are both theorized to be associated with and/or support various higher-level cognitive operations (see, e.g., Narayanan et al., 2014, 2015; [28]). In addition, alpha oscillations may be associated with coordinating activity during idling states of the brain, perhaps best characterized as the “default mode” [34]. It is perhaps unsurprising that distributed sensor-sensor associations were only observed in this frequency range. The observation that enhanced connectivity was only observed among Biotype-2 s, however, is consistent with a neuronal hyper-excitation model that may decrease the stability of cortical networks selected to support current behavioral requirements (e.g., [11]). Alternatively, low levels of neuronal activity among Biotype-1 s in frequency ranges supporting distant cortico-cortical communication may cause difficulties modulating behavior as functions of changing behavioral contexts [12]. Given that beta oscillations may be a gross index of cortical excitability [35], exuberant activity in Biotype-2 and reduced activity among Biotype-1 cases in this frequency range highlight differing functional cortical properties in these groups.
There are some limitations to be considered when evaluating this study. First, like most studies of mid-course psychosis, most probands were medicated (see Supplementary Table 1). However, the Biotype subgroups were similarly medicated, so it is unlikely pharmacological interventions account for between-groups differences. Second, there are differences in Biotype subgroup sample sizes, but this is a function of the apparent distribution of such cases in mid-course psychosis given that subgroups were empirically derived based on biomarker profiles. Third, although the number of participating relatives was large, there was only approximately one biological relative per proband, which is far from complete sampling of first-degree relatives. It is possible that bias in those relatives willing to participate accounted for minimal differences between relatives and healthy subjects on IA. Finally, the current measure was extracted from the paired-stimuli task data, a task that was used in Biotypes construction. The purpose of the present report, however, was not to use intrinsic activity as an external validator but to clarify and examine the range of IA differences between psychosis subgroups to refine future Biotype construction and target engagement efforts. These effects spanned frequency ranges, indicating that it may be critical to understand commonalities in generation of neural oscillations rather than focusing on a specific frequency range when building neurophysiological models of psychosis that incorporate intrinsic neural activity [28].
Supplementary Material
Acknowledgements
Thank you to the numerous researchers and clinicians who contributed to participant recruitment and data collection, and most of all to the participants for contributing their time and effort to the study.
Funding
National Institutes of Health (NIH) R01s:MH077945,MH077862, MH077851, MH078113, MH085485.
Footnotes
Declaration of Competing Interest
OF Thomas, DA Parker, RL Trotti, JE McDowell, E Ivleva ES Gershon, JA Sweeney, MS Keshavan, GD Pearlson, CA Tamminga, and SK Keedy report no conflicts of interests, financial or otherwise. BA Clementz has served as a consultant for Astellas.
Narayanan et al. [48] used a subset of the present sample (about 83%) and also forced SAD cases into the SZ and BDP subgroups based on depressive or manic subtype, respectively. They also used different data (a separate “resting EEG” period that was only available in a subset of B-SNIP participants) and a different frequency band extraction method (ICA). Nevertheless, the overall conclusion that low frequency activity deviations account for SZ-BDP effects is similar to the outcome of this study.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.bionps.2019.100002.
References
- [1].Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA, Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP), (Am. J. Psychiatry 170 (11) (2013) 1263–1274. [DOI] [PubMed] [Google Scholar]
- [2].Keshavan MS, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA, Reimagining psychoses: an agnostic approach to diagnosis, (Schizophr. Res 146 (1-3) (2013) 10–16. [DOI] [PubMed] [Google Scholar]
- [3].Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz BA, Thaker G, Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum, (Schizophr. Bull 40 (Suppl. 2) (2014) S131–S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pearlson G, Clementz BA, Sweeney JA, Keshavan M, Tamminga CA, Does biology transcend the symptom-based boundaries of psychosis? (Psychiatr. Clin. North Am 39 (2) (2016) 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study, (Am. J. Psychiatry 170 (11) (2013) 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ivleva EI, Clementz BA, Dutcher AM, Arnold SJM, Jeon-Slaughter H, Aslan S, Witte B, Poudyal G, Lu H, Meda SA, Pearlson GD, Sweeney JA, Keshavan MS, Tamminga CA, Brain structure biomarkers in the psychosis biotypes: findings from the Bipolar-Schizophrenia Network for Intermediate Phenotypes, (Biol. Psychiatry 82 (1) (2017) 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hamm JP, Ethridge LE, Boutros NN, Keshavan MS, Sweeney JA, Pearlson GD, Tamminga CA, Clementz BA, Diagnostic specificity and familiarity of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum, (J. Psychophysiol 51 (4) (2014) 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA, Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder, (Biol. Psychiatry 77 (2) (2015) 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA, Identification of distinct psychosis biotypes using brain-based biomarkers, (Am. J. Psychiatry 173 (4) (2016) 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meda SA, Clementz BA, Sweeney JA, Keshavan MS, Tamminga CA, Ivleva EI, Pearlson GD, Examining functional resting-state connectivity in psychosis and its subgroups in the Bipolar-Schizophrenia Network on Intermediate Phenotypes cohort, (Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1 (6) (2016) 488–497. [DOI] [PubMed] [Google Scholar]
- [11].Hudgens-Haney ME, Ethridge LE, Knight JB, McDowell JE, Keedy SK, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA, Clementz BA, Intrinsic neural activity differences among psychotic illnesses, (J. Psychophysiol 54 (8) (2017) 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rolls ET, Loh M, Deco G, Winterer G, Computational models of schizophrenia and dopamine modulation in the prefrontal cortex, (Nat. Rev. Neurosci 9 (9) (2008) 696–709. [DOI] [PubMed] [Google Scholar]
- [13].Raichle ME, The restless brain: how intrinsic activity organized brain function, (Philos. Trans. Biol. Sci 370 (1668) (2015) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].First MB, Spitzer RL, Gibbon M, Williams JBW, Structured Clinical Interview for DSM-IV Axis I Disorders, American Psychiatric Press, Washington, DC, 1997. [Google Scholar]
- [15].Zanarini MC, Frankenburg FR, Sickel AE, Yong L, The Diagnostic Interview for DSM-IV Personality Disorders (DIP DIV), McLean Hospital, Belmont, MA, 1996. [Google Scholar]
- [16].Wilkinson GS, Robertson GJ, Wide Range Achievement Test 4 Professional Manual, Psychological Assessment Resources, Lutz, 2006. [Google Scholar]
- [17].Lançon C, Auquier P, Nayt G, Reine G, Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS), (Schizophr. Res 42 (2000) 231–239. [DOI] [PubMed] [Google Scholar]
- [18].Montgomery SA, Asberg M, A new depression scale designed to be sensitive to change, (Br. J. Psychiatry 134 (1979) 382–389. [DOI] [PubMed] [Google Scholar]
- [19].Young RC, Biggs JT, Ziegler VE, Meyer DA, A rating scale for mania: reliability, validity and sensitivity, (Br. J. Psychiatry 133 (1978) 429–435. [DOI] [PubMed] [Google Scholar]
- [20].Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S, The social functioning scale: the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients, (Br. J. Psychiatry 157 (6) (1990) 853–859. [DOI] [PubMed] [Google Scholar]
- [21].Andreasen NC, Endicott J, Spitzer RL, Winokur G, The family history method using diagnostic criteria: reliability and validity, (Arch. Gen. Psychiatry 34 (1977) 1229–1235. [DOI] [PubMed] [Google Scholar]
- [22].Wang J, Ethridge L, Mosconi MW, White SP, Binder DK, Pedapati E, Erickson C, Byerly MJ, Sweeney JA, A resting EEG study of neocortical hyperexcitability and altered functional connectivity in fragile X syndrome, (J. Neurodev. Disord 9 (11) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CMA, An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias, (NeuroImage 55 (4) (2011) 1548–1565. [DOI] [PubMed] [Google Scholar]
- [24].Dukart J, Schroeter ML, Mueller K, Alzheimer’s Disease Neuroimaging Initiative, Age correction in dementia – matching to a healthy brain, (PLoS One 6 (7) (2011) 1–9 e22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hochberg Y, Benjamini Y, More powerful procedures for multiple significance testing, (Stat. Med 9 (7) (1990) 811–818. [DOI] [PubMed] [Google Scholar]
- [26].Lambert ZV, Wildt AR, Durand RM, Redundancy analysis: an alternative to canonical correlation and multivariate multiple regression in exploring interest associations, (Psychol. Bull 104 (2) (1988) 282–289. [Google Scholar]
- [27].Hair JF Jr., Anderson RE, Tatham RL, Black WC, Multivariate Data Analysis, 5th ed, Prentice Hall, New Jersey, 1998. [Google Scholar]
- [28].Spencer KM, Time to be spontaneous: A renaissance of intrinsic brain activity in psychosis research? (Biol. Psychiatry 76 (6) (2014) 434–435. [DOI] [PubMed] [Google Scholar]
- [29].Kaiser J, Lutzenberger W, Human gamma-band activity: a window to cognitive processing, (NeuroReport 16 (3) (2005) 243–247. [DOI] [PubMed] [Google Scholar]
- [30].Ethridge L, Moratti S, Gao Y, Keil A, Clementz BA, Sustained versus transient brain responses in schizophrenia: the role of intrinsic neural activity, (Schizophr. Res 133 (1-3) (2011) 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW, Neural synchrony indexes disordered perception and cognition in schizophrenia, (Proc. Natl. Acad. Sci. U. S. A 101 (49) (2004) 17288–17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA, Diminished parietal cortex activity associated with poor motion direction discrimination performance in schizophrenia, (Cereb. Cortex 20 (7) (2010) 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kam JW, Bolbecker AR, O’Donnell BF, Hetrick WP, Brenner CA, Resting state EEG power and coherence abnormalities in bipolar disorder and schizophrenia, (J. Psychiatr. Res 47 (12) (2013) 1893. –1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M, Electrophysiological signatures of resting state networks in the human brain, (Proc. Natl. Acad. Sci. U. S. A 104 (32) (2007) 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rangaswamy M, Poriesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, Rohrbaugh J, O’Connor SJ, Kuperman S, Reich T, Begleiter H, Beta power in the EEG of alcoholics, (Biol. Psychiatry 52 (8) (2002) 831–841. [DOI] [PubMed] [Google Scholar]
- [38].Van Diessen E, Numan T, van Dellen E, van der Kooi AW, Boersma M, Hofman D, van Lutterveld R, van Dijk BW, van Straaten ECW, Hillebrand A, Stam CJ, Opportunities and methodological challenges in EEG and MEG resting state functional brain network research, (Clin. Neurophysiol 126 (8) (2015) 1468–1481. [DOI] [PubMed] [Google Scholar]
- [39].Toolbox Fieldtrip, Donders Institute for Brain, Cognition and Behavior, Radboud University, Netherlands, 2010. https://www.ru.nl/neuroimaging/fieldtrip. [Google Scholar]
- [40].Freeman W, Quiroga RQ, Frequency analysis, in: Freeman W, Quiroga RQ (Eds.), Imaging Brain Function With EEG: Advanced Temporal and Spatial Analysis of Electroencephalographic Signals, Springer Nature, Switzerland, 2013, pp. 21–36. [Google Scholar]
- [41].Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK, Large-scale cortical correlation structure of spontaneous oscillatory activity, (Nat. Neurosci 15 (6) (2012) 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].IBM Corp, IBM SPSS Statistics for Windows. Version 24.0. Armonk, N.Y., (2016). [Google Scholar]
- [47].Mitra PP, Pesaran B, Analysis of dynamic brain imaging data, (Biophys. J 76 (2) (1999) 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Narayanan B, O’Neil K, Berwise C, Stevens MC, Calhoun VD, Clementz BA, Tamminga CA, Sweeney JA, Keshavan MS, Pearlson GD, Resting state electroencephalogram oscillatory abnormalities in schizophrenia and psychotic bipolar patients and their relatives from the bipolar and schizophrenia network on intermediate phenotypes study, (Biol. Psychiatry 76 (6) (2014) 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Siegel M, Donner TH, Engel AK, Spectral fingerprints of large-scale neuronal interactions, (Nat. Rev. Neurosci 13 (2) (2012) 121–134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.