Abstract
Background:
EEG responses during auditory paired-stimuli paradigms are putative biomarkers of psychosis syndromes. The initial iteration of the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP1) showed unique and common patterns of abnormalities across schizophrenia (SZ), schizoaffective disorder (SAD), and bipolar disorder with psychosis (BDP). This study replicates those findings in new and large samples of psychosis cases and extends them to an important comparison group, bipolar disorder without psychosis (BDNP).
Methods:
Paired stimuli responses from 64-sensor EEG recording were compared across psychosis (n = 597; SZ = 225, SAD = 201, BDP = 171), BDNP (n = 66), and healthy (n = 415) subjects from the second iteration of B-SNIP. EEG activity was analyzed in voltage and in the time-frequency domain. Principal component analysis (PCA) over sensors (sPCA) was used to efficiently capture EEG voltage responses to the paired stimuli. Evoked power was calculated via a Morlet wavelet procedure. A frequency PCA divided evoked power data into three frequency bands: Low (4–17 Hz), Beta (18–32 Hz), and Gamma (33–55 Hz). Each time-course (ERP Voltage, Low, Beta, and Gamma) were then segmented into 20 ms bins and analyzed for group differences. To efficiently summarize the multiple EEG components that best captured group differences we used multivariate discriminant and correlational analyses. This approach yields a reduced set of measures that may be useful in subsequent biomarker investigations.
Results:
Group ANOVAs identified 17 time-ranges that showed significant group differences (p < .05 after FDR correction), constructively replicating B-SNIP1 findings. Multivariate linear discriminant analysis parsimoniously selected variables that best accounted for group differences: The P50 response to S1 and S2 uniquely separated BDNP from healthy and psychosis subjects (BDNP > all other groups); the S1 N100 response separated groups along an axis of psychopathology severity (HC > BDNP > BDP > SAD > SZ); the S1 P200 response indexed psychosis psychopathology (HC/BDNP > SAD/SZ/BDP); and the preparatory period to the S2 stimulus separated SZ from other groups (SZ > SAD/BDP>HC/BDNP).
Canonical correlation identified an association between the neural responses during the S1 N100, S1 N200 and S2 preparatory period and PANSS positive symptoms and social functioning. The neural responses during the S1 P50 and S1 N100 were associated with PANSS Negative/General, MADRS and Young Mania symptoms.
Conclusions:
This study constructively replicated prior B-SNIP1 research on auditory deviations observed during the paired stimuli task in SZ, SAD and BDP. Inclusion of a group of BDNP allows for the identification of biomarkers more closely related to affective versus nonaffective clinical phenotypes and neural distinctions between BDP and BDNP. Findings have implications for nosology and future translational work given that some biomarkers are shared across all psychosis and some are unique to affective syndromes.
Keywords: Psychosis, Paired-stimuli, ERP, P50, N100, Bipolar disorder, Schizophrenia
1. Introduction
An important goal for any medical discipline is to transition diagnoses from clinical syndromes to identifying biological features that improve targeting of specific treatments to specific pathophysiologies [1]. An essential step in this process is identification of objective measures capturing deviations that are relatively specific to persons with the syndrome compared to unaffected persons as well as those with other related syndromes [2]. If obtained from anatomical or physiological laboratory testing, such measures are called biomarkers but endophenotypes if they also capture inherited predisposition for the syndrome [3].
Developing biomarker-informed diagnosis is a vigorous area of investigation in psychiatry [4]. The Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP [5,6]) consortium believes that for one domain of psychiatry, psychosis syndromes, neurobiologically-informed diagnoses that would improve patient care is achievable [7,8]. B-SNIP’s initial objective was to characterize overlapping and unique biomarker features across the dimension of major psychosis syndromes (schizophrenia, SZ; schizoaffective disorder, SAD; and bipolar disorder with psychosis, BDP). This work required large samples to capture heterogeneity within and between syndromes and support required statistical analyses.
One of the most historically influential putative psychosis biomarkers comes from event-related potential (ERP) responses obtained during auditory paired-stimuli paradigms [9–14]. The typical variant involves two stimuli (S1 and S2) presented in close temporal proximity (separated by 500 ms) with pairs separated by long (9–10 s) inter-pair intervals. A traditional outcome measure from such paradigms is the ‘gating’ or suppression index, which assessed the size of an ERP (e.g., P50 or N100) to S2 compared to S1. Poor suppression means the two ERP responses are close in amplitude, and good suppression means the S2 ERP is small compared to the S1 ERP.
Numerous studies demonstrated that SZ and their first-degree biological relatives have worse P50 suppression indices than healthy subjects [9,12,13], indicating possible utility as a biomarker of risk [3]. A smaller number of studies find that cases of bipolar disorder, especially those with psychosis, also have worse suppression than healthy subjects [14–16], although not as severe as deficits reported in SZ. The true differentiation between SZ and bipolar disorder cases with and without psychosis (BDP versus BDNP) on ERP suppression indices is uncertain given the paucity of investigations comparing these three groups.
Patterson et al. [13] described the variability in P50 suppression findings across laboratories and multiple methodological uncertainties. Investigators have taken various approaches to quantification (e.g.: What to use for S1 versus S2 baseline? Peak-to-trough measures? Exclude or include grand averages with a preceding P30? Single versus multi-sensor recordings?1. Varied quantification approaches combined with questionable reliability of suppression indices both within and between sessions [13,20–22]2 lead to concerns with how P50 suppression could be easily implemented to assist diagnosis and effective treatment targeting. Multiple investigations also support the proposition that ERP responses to S1 might be an effective psychosis biomarker [24–28], and that N100 may be equally or more effective as the P50 in this regard [24,27,29], including the demonstration that S1 ERPs highly predicts S2 ERPs among both healthy and SZ subjects [24].
Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP1) consortium compared over 400 SZ and BDP cases and 470 of their first-degree relatives to 225 healthy persons using simplified voltage and frequency approaches to quantify ERP responses during a paired stimuli paradigm [28]. This was later extended to include SAD cases and their first-degree relatives in a paper that demonstrated the usefulness of a paired-stimuli paradigm for differentiating psychosis Biotypes [30]. The main outcomes of those papers were: (i) SZ-like psychosis had more high frequency activity that was not directly stimulus-related (in pre-stimulus period and that was not clearly phase locked to stimulus presentation); (ii) psychosis groups, most notably SZ psychosis, had lower gamma band activity in relation to stimulus processing; (iii) psychosis groups had lower amplitude N100 responses; (iv) SZ psychosis had a different recovery function following S1 and preparatory function preceding S2 [31,32]; and (v) the amplitude of response to S1 was the largest determinant of group differences. Even though psychosis groups had statistically significant differentiations from healthy subjects on multiple ERP measures, there were no statistically significant between-groups differences on ERP suppression measures using an established approach [28]. These findings suggest shared and unique disruptions across the psychosis spectrum in the neural substrates of basic auditory stimulus processing that are best captured by direct measures of ERP voltage and frequency responses during paired-stimuli testing.
Previous findings from the B-SNIP consortium (B-SNIP1) can be extended through replication in a large new sample (B-SNIP2), and will serve as a prelude to replication of our previously published psychosis Biotypes [30]. Uncertainties about similarities and differences between BDP and BDNP also will be addressed by including a sample of BDNP cases. The present study used multi-sensor EEG to examine neural responses during a paired-stimuli paradigm in a large, well-characterized sample of psychosis and BDNP cases from the second iteration of B-SNIP data collection (B-SNIP2). The study has five major goals: (i) replicate previous B-SNIP1 findings; (ii) determine whether auditory deviations are shared across the psychosis spectrum; (iii) determine whether individuals with bipolar I disorder with (BDP) and without psychosis (BDNP) have similar deviations; and (iv) determine the relationship between auditory deviations and clinical features. These comparisons will provide a framework for understanding how auditory processing may be biomarkers with clinical relevance across the psychosis-affective spectrum.
2. Methods and materials
2.1. Recruitment
Subjects (total n = 1078: HC = 415, BDNP = 66, BDP = 171, SAD = 201, and SZ = 225) were recruited at three Psychosis and Affective Research Domains and Intermediate Phenotypes (PARDIP) and five Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP2) consortium sites (three of which were also PARDIP sites) following previously published approaches (for details see [6] and Supplemental Methods). Subjects were grouped according to their DSM-IV-TR [33] diagnoses since PARDIP and B-SNIP are ongoing studies and subjects have not yet been classified using Biotype methods [30]. This project was approved by the Institutional Review Board of every consortium site; all subjects provided written informed consent before participation. Demographic information and clinical ratings across groups are presented in Table 1.
Table 1.
Demographics & clinical scales by group.
| HC | BDNP | BDP | SAD | SZ | Statistic | p | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | 415 | 66 | 171 | 201 | 225 | ||
| Mean age | 34.74 | 40.39 | 37.20 | 40.32 | 40.03 | F(4,1073) = 11.89*** | <.001 |
| Age SD | 12.50 | 12.16 | 11.72 | 10.98 | 11.66 | ||
| Sex (% F) | 57 | 71 | 54 | 55 | 38 | x2(4) = 31.89*** | <.001 |
| N from each site | x2(16) = 99.27*** | <.001 | |||||
| Boston | 90 | 8 | 25 | 19 | 36 | ||
| Chicago | 51 | 0 | 49 | 45 | 51 | ||
| Dallas | 81 | 25 | 40 | 36 | 46 | ||
| Georgia | 86 | 0 | 16 | 36 | 44 | ||
| Hartford | 107 | 33 | 41 | 65 | 48 | ||
| Number of trials in each waveform average by group | |||||||
| Mean | 114 | 114 | 113 | 113 | 113 | F(4,1073) = .36 .84 | |
| SD | 8.15 | 8.19 | 8.66 | 8.16 | 9.19 | ||
| Global Assessment of Function (GAF) | F(4,942) = 456.38*** | <.001 | |||||
| F(3,606) = 10.84*** | <.001 | ||||||
| N | 337 | 60 | 161 | 184 | 205 | HC > BDNP***, BDP*** > SAD*, SZ*** | |
| M | 83.42 | 57.88 | 56.73 | 53.26 | 50.51 | ||
| SD | 6.61 | 11.40 | 13.47 | 11.15 | 11.54 | ||
| Birchwood Social Functioning Scale (SFS) | F(4,985) = 158.54 | <.001 | |||||
| F(3,605) = 17.82*** | <.001 | ||||||
| N | 381 | 61 | 159 | 187 | 202 | HC > BDNP***, BDP*** > SAD***, SZ* | |
| M | 154.15 | 130.15 | 131.11 | 116.78 | 117.33 | ||
| SD | 17.34 | 20.41 | 23.65 | 22.93 | 21.32 | ||
| Positive and Negative Syndrome Scale (PANSS) Positive | F(3,592) = 22.62*** | <.001 | |||||
| N | N/A | 63 | 157 | 178 | 198 | SZ, SAD > BDP***, | |
| M | N/A | 12.76 | 13.82 | 17.85 | 17.71 | BDNP*** | |
| SD | N/A | 3.64 | 5.86 | 6.92 | 6.29 | ||
| PANSS Negative | F(3,591) = 9.22*** | <.001 | |||||
| N | N/A | 63 | 157 | 178 | 197 | SZ > BDP***, BDNP*, SAD > BDP** | |
| M | N/A | 14.57 | 13.85 | 16.32 | 17.39 | ||
| SD | N/A | 5.86 | 6.44 | 7.16 | 6.71 | ||
| PANSS General | F(3,592) = 1.28 | .28 | |||||
| N | N/A | 63 | 157 | 178 | 198 | ||
| M | N/A | 31.67 | 31.06 | 33.16 | 32.24 | ||
| SD | N/A | 8.83 | 10.59 | 9.87 | 10.00 | ||
| PANSS Total | F(3,591) = 8.18*** | <.001 | |||||
| N | N/A | 63 | 157 | 178 | 197 | SZ, SAD > BDP***, BDNP* | |
| M | N/A | 59.00 | 58.72 | 67.33 | 67.42 | ||
| SD | N/A | 15.84 | 21.12 | 21.39 | 20.15 | ||
| Montgomery-Åsberg Depression Rating Scale (MADRS) | F(3,600) = 8.90*** | <.001 | |||||
| N | N/A | 63 | 159 | 183 | 199 | SZ < SAD***, BDP***, BDNP** | |
| M | N/A | 14.79 | 14.56 | 14.01 | 9.75 | ||
| SD | N/A | 9.33 | 11.82 | 10.70 | 8.94 | ||
| Young Mania Rating Scale (YMRS) | F(3,599) = 1.64 | .18 | |||||
| N | N/A | 63 | 159 | 181 | 200 | ||
| M | N/A | 9.41 | 8.98 | 10.83 | 9.93 | ||
| SD | N/A | 7.10 | 8.82 | 7.95 | 7.28 | ||
Note: HC, Healthy Subjects; SZ, Probands with schizophrenia; SAD, Probands with schizoaffective disorder; BDP, Probands with bipolar disorder I with a history of psychotic symptoms; BDNP, Probands with bipolar disorder I without a history of psychosis symptoms.
For scales with two sets of ANOVA statistics, the first was done including HC, and the second included only probands.
p < .05
p < .01
p < .001.
Group comparisons performed with Tukey tests.
2.2. Stimuli
Recording conditions, stimulus presentation, and recording equipment were standardized across sites [28]. Participants refrained from smoking one hour prior to testing. Subjects completed the auditory paradigm while seated in a sound and electrically shielded booth. Each subject passively listened to 120 binaural broadband noise auditory click pairs. Each stimulus was 4 ms in duration, presented at 70 dB, and separated by a 9–10 s inter-trial interval, with 500 ms between each pair. The subjects had a short break after every 40 trials and were asked to keep a mental count of the number of trials.
2.3. EEG recording
Electroencephalogram (EEG) was continuously recorded from 64 silver/silver chloride sensors (impedance <10 kΩ; QuikCap, Compumedrics Neuroscan, El Paso, Texas), positioned according to the standard 10–20 EEG system with mastoids and CB1/2 locations to provide greater sampling below the canthomeatal line, with nose reference and forehead ground. Recordings were amplified (12,500X) and digitized (1000 Hz) using Neuroscan ACQUIRE and SynAmps2 recording systems (Compumedics Neuroscan).
2.4. EEG processing
EEG data were pre-processed following previously published methods [28,34]. Raw EEG data were inspected for bad sensors and artifacts. Bad sensors were interpolated (no more than 5% for any subject) using spherical spline interpolation (BESA 5.3; MEGIS Software, Grafelfing, Germany). Data were transformed to an average reference and downsampled to 500 Hz and digitally band pass filtered from .5 Hz to 55 Hz (zero-phase filter; roll-off: 6 and 48 dB/octave, respectively). Blink and cardiac artifacts were minimized using independent component analysis (EEGLAB 13.6) [35]. EEG data on each trial were then segmented into 1250-ms epochs extending from 250 ms before to 1000 ms after S1. Trials containing activity ±75 mV at any sensor were eliminated from further processing. At least 50 % of trials were accepted for all included subjects and number of trials did not differ by group (Table 1). Data from included trials were averaged for each subject to create a 64-sensor grand average ERP. Each ERP was baseline adjusted using the 100 ms pre-S1 period.
2.5. ERP principal component analysis for spatial data reduction
To use ERP data from every sensor and capture topographical distributions of evoked brain responses across time, we used spatial principal component analysis (sPCA) on the concatenated grand average from all groups (implemented in Matlab (MathWorks, Natick, Massachusetts) [36]). This resulted in one paired stimuli component (accounting for 92 % of the variance; Fig. 2). The sPCA component weights were multiplied by each subject’s grand average data, summed across sensors, and divided by the plus sum of the component weights, reducing the waveform from 64 sensors to one “virtual sensor.” This “virtual sensor” efficiently summarized the spatial distributions, minimizing the number of statistical comparisons and maximizing the signal/noise ratio of ERP data [37–39]. In order to compare results from the first iteration of B-SNIP (B-SNIP-1), we performed identical analyses on BSNIP-1 and PARDIP/BSNIP-2 subjects and compared each group’s grand average waveform between studies (HC, BDP, SAD, SZ). The within-group correlations between studies were all above r = .95 (Fig. 1).
Fig. 2.
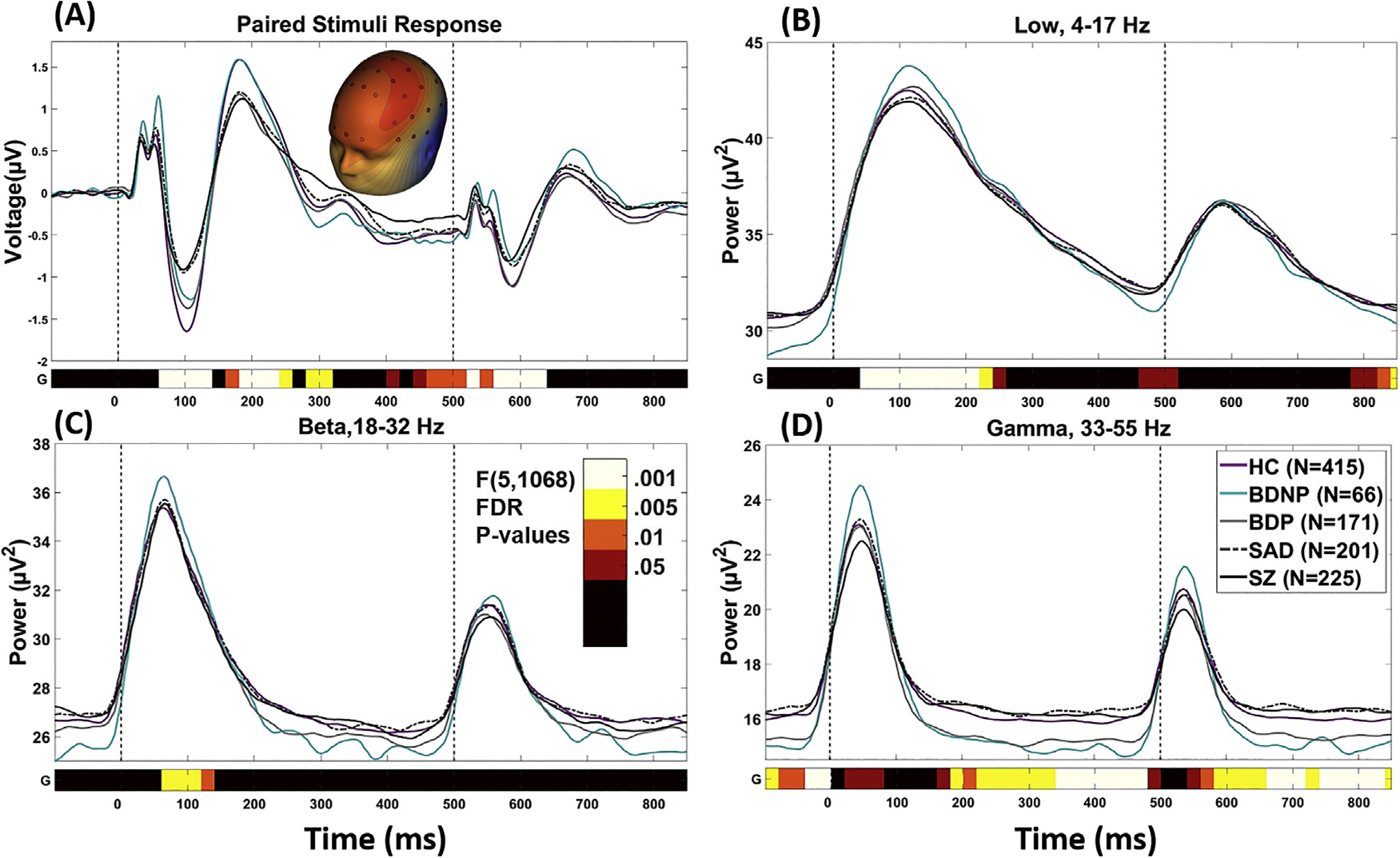
Paired stimulus grand average waveforms by group. Note that waveforms are not corrected for age, as this was done at a later stage of processing. The bottom bar indicates where there was a significant main effect of group [F(5,1068), p < .05 (after FDR correction)]. Color Bar indicates the level of significance for each 20 ms time bin. (A) is the spatial PCA component for the grand average ERP. (B), (C), and (D) are grand averages of the time-frequency transformed data from each frequency component averaged over all sensors. D is the spatial PCA component for the grand average ERP. Significant time-ranges were averaged across consecutive time-bins and were demarcated at points of polarity switches for use in post-hoc multivariate analyses. HC, Healthy Subjects; BDNP, Probands with bipolar disorder I without any history of psychotic symptoms; BDP, Probands with bipolar disorder I with a history of psychotic symptoms; SAD, Probands with schizoaffective disorder; SZ, Probands with schizophrenia.
Fig. 1.
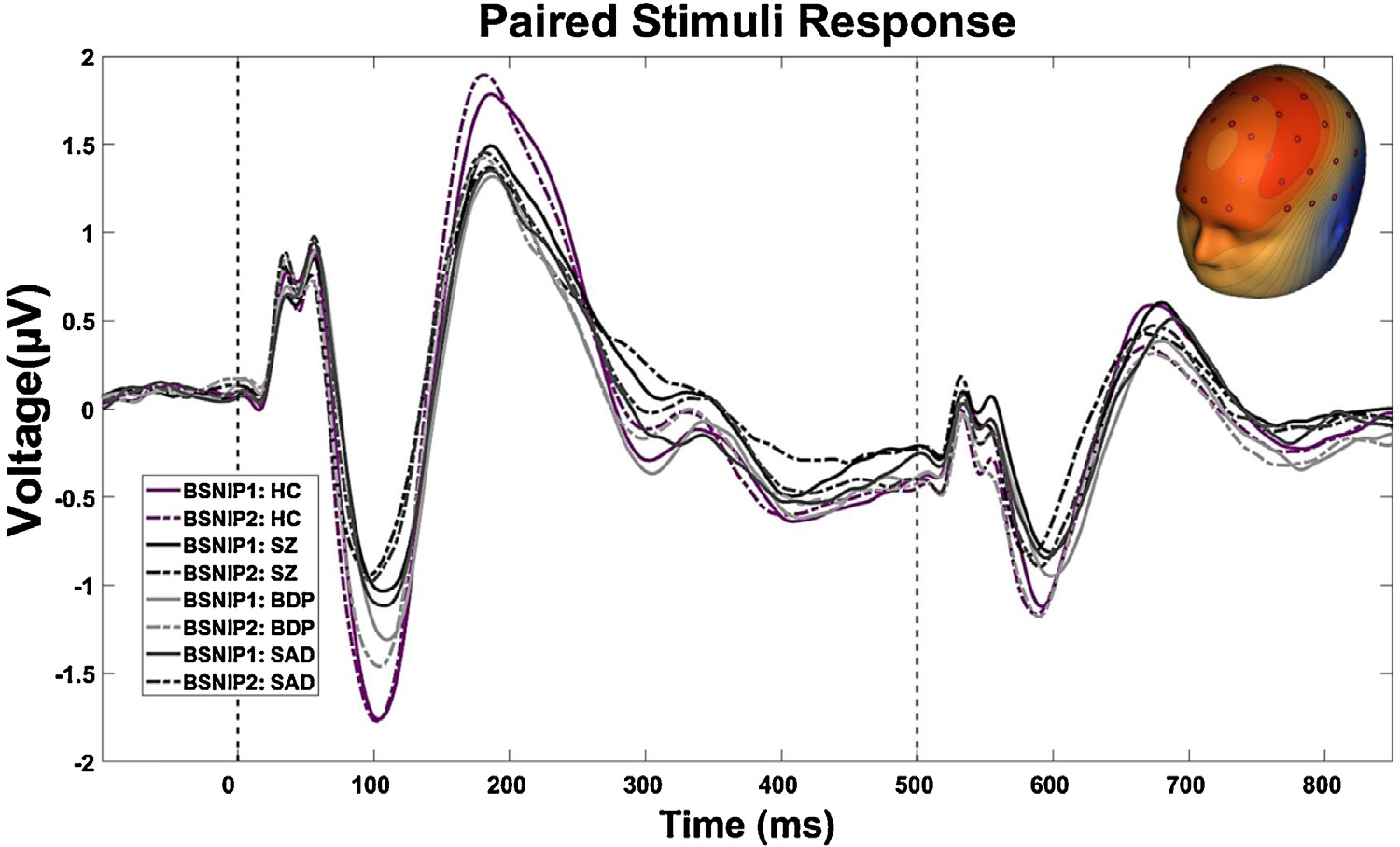
Constructive Replication of BSNIP Paired Stimuli Response Across Psychosis-Spectrum: The grand average response from all trials was calculated for BSNIP-1 and PARDIP/BSNIP-2 Subjects. Each ERP was standardized within study and then a Pearson correlation was performed between: [BSNIP1: HC (n = 198) and BSNIP2: HC (n = 415), r = .98; BSNIP1 SZ (n = 190) and BSNIP2: SZ (n = 225), r = .95; BSNIP1: BDP (n = 159) and BSNIP2: BDP (n = 171), r = .96; BSNIP1: SAD (n = 108) and BSNIP2: SAD (n = 201), r = .97]. HC, Healthy Subjects; SZ, Probands with schizophrenia; BDP, Probands with bipolar disorder I with a history of psychotic symptoms; SAD, Probands with schizoaffective disorder.
2.6. Time-frequency analysis
Data from each sensor of the grand average ERP were converted to the time-frequency (TF) domain with a modified Morlet wavelet procedure (4–55 Hz, 5-ms steps, 1 cycle at lowest to 8 cycles at highest; output ranged from −105 ms to 845 ms post-S1) [38–40]. Power values (squared absolute values of complex wavelet outputs) were then converted to decibels (10 * log10).
In order to identify frequency bands of interest we performed a frequency principal component analysis (fPCA) using the following steps: power values from each time-bin from 0 ms to 850 ms post-S1 were averaged. All subjects’ data were concatenated to create a matrix of 52 variables (4–55 Hz) and nX64 observations (where n is the number of subjects; n = 1078). An fPCA was carried out on the matrix with promax (oblique) vector rotation and Kaiser normalization [36]. Scree tests identified three components accounting for greater than 95 % of the variance across subjects and sensors. The 3 resulting fPCA components were: 1) Low frequency band (4–17 Hz); 2) Beta (18–32 Hz); and 3) Gamma (33–55 Hz) (See Supplemental Fig. 1). This result is highly consistent with previous results from BSNIP-1 [28] and captures the cortical relevant frequency bands resolvable with EEG [41]. Each fPCA component weight was multiplied by each subject’s grand average TF data at each time bin, summed across frequencies, and divided by the plus sum of the component weights, reducing the waveform from 52 frequencies (4–55 Hz) to three frequency bands. For each frequency component, all sensors where averaged to create one time-frequency waveform (Fig. 2).
2.7. Age adjustment
To test and adjust for age associations with the ERP and TF components, we performed the following steps [42]. For each ERP and TF component, data were segmented into 20 ms time bins from −105 to 845 ms and linear and quadratic regressions were performed on age using only the healthy sample. When the beta coefficients for age effects were significant (p < .05), data for all subjects at that time bin were adjusted for the healthy aging association prior to group comparisons (see also [28]).
2.8. Statistical analysis
Statistics were performed with SPSS Statistics version 23 (Armonk, NY: IBM Corp.), SAS, and custom Matlab code. For each ERP and TF component, a group X sex ANOVA was calculated on each 20 ms time bin from −105 ms to 845 ms. All ANOVA p-values within each component were adjusted using a false discovery rate method [43]. Significant effects involving group membership were averaged within adjacent time bins for each subject.
2.9. Group discrimination analyses
The above analyses yielded a large number of variables significantly differentiating groups. However, some of those variables could provide redundant information. To evaluate for this possibility, we took the following steps. First, significant effects involving group from the above ERP and TF ANOVAs were used in a linear discriminant analysis (LDA) with group as the dependent variable (HC, BDNP, BDP, SAD, and SZ). This analysis was used to identify variables that uniquely and efficiently summarized group differences. ERP variables that minimized overall Wilks’ lambda at p < .05 were entered in a stepwise fashion, leaving a parsimonious selection of neural measures that differentiated groups [38,39,44]. Second, to ensure that identified ERP and TF variables contributed to reliable and stable group separations, a jackknife procedure was performed by submitting 95 % of the total sample (n = 1024, sampling without replacement) to an LDA 1000 times [45]. Components consistently identified across iterations were used in subsequent analyses (see Supplemental Table 6 for jackknife results and Table 2 for LDA results and group comparisons for components surviving the LDA procedure).
Table 2.
Linear Discriminant Analysis Results:
| S1 P50 | S1 N100 | S1 P200 | Pre-S2 | Low (4–17 Hz) Early/Mid | Low (4–17 Hz) Pre-S2 | Gamma (33–55Hz) S2 Early | ||||||||
| Range: | 55–75 ms | 76–135 ms | 155– 255 ms | 435–515 ms | 35– 255 ms | 455–515 ms | 535– 635 ms | |||||||
| Number of time in Jacknife LDA (1000 total) | 1000 | 1000 | 979 | 870 | 1000 | 1000 | 999 | |||||||
| Omnibus ANOVA F Value | 13.51*** | 20.86*** | 10.99*** | 6.75*** | 15.92*** | 2.86* | 4.54** | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| HC (n = 415) | 0.073 | 0.74 | −1.263 | 1.01 | 1.275 | 0.95 | −0.499 | 0.460 | 41.236 | 3.87 | 31.601 | 4.07 | 17.426 | 2.92 |
| BDNP (n = 66) | 0.698 | 0.91 | −0.966 | 0.82 | 1.241 | 1.07 | −0.575 | 0.471 | 41.203 | 3.91 | 31.681 | 4.13 | 19.124 | 3.14 |
| BDP (n = 171) | 0.086 | 0.80 | −1.077 | 1.01 | 0.893 | 0.78 | −0.493 | 0.480 | 40.211 | 3.89 | 31.661 | 3.94 | 17.703 | 3.29 |
| SAD (n = 201) | 0.328 | 0.75 | −0.684 | 0.81 | 0.968 | 0.78 | −0.458 | 0.511 | 39.376 | 3.88 | 32.559 | 4.01 | 17.878 | 2.98 |
| SZ (n = 225) | 0.044 | 0.75 | −0.677 | 0.87 | 0.911 | 0.73 | −0.316 | 0.519 | 39.049 | 3.71 | 32.407 | 4.29 | 17.668 | 3.25 |
| Effect Sizes (Glass Delta) | ||||||||||||||
| BDNP | 0.84 | 0.29 | −0.04 | −0.17 | −0.01 | 0.02 | 0.58 | |||||||
| BDP | 0.02 | 0.18 | −0.40 | 0.01 | −0.26 | 0.01 | 0.09 | |||||||
| SAD | 0.34 | 0.57 | −0.32 | 0.09 | −0.48 | 0.24 | 0.16 | |||||||
| SZ | −0.04 | 0.58 | −0.38 | 0.40 | −0.57 | 0.20 | 0.08 | |||||||
| Tukey’s B | [BDNP]> [SAD]> [BDP/ HC/ SZ] | [HC/ BDNP]> [BDNP/ BDP]> [SAD/ SZ] | [HC/ BDNP]> [SAD /SZ/ BDP] | [SZ] > [SAD/ BDP/ HC/ BDNP] | [HC/ BDNP/ BDP] > [BDP/ SAD]> [SAD/ SZ] | n.s. | [BDNP]> [SAD/ BDP/ SZ/ HC] | |||||||
Note: HC, Healthy Subjects; SZ, Probands with schizophrenia; BDP, Probands with bipolar disorder I with a history of psychotic symptoms; SAD, Probands with
2.10. Clinical features analyses
To parsimoniously evaluate relationships between neural components and clinical features from the Birchwood Social Functioning Scale [46], PANSS [47], Young Mania Rating Scale [48], Montgomery-Asberg Depression Rating Scale [49], a canonical correlation analysis (CCA) across all probands was performed, with clinical features on one side and all neural variables on the other side of the equation.
3. Results
As described above, there were four time courses to analyze: (i) the PCA that integrated voltage information across all sensors to yield one “virtual sensor”, (ii) the low frequency response (4–17 Hz), (iii) the beta range response (18–32 Hz), and (iv) the gamma range response (33–55 Hz). Each of these time courses was analyzed with ANOVAs in 20 ms segments starting 105 ms before S1 and ending 345 ms following S2.The results of these FDR-adjusted analyses are illustrated in Fig. 2, and are summarized below. Effect sizes in relation to the healthy group (Glass’ Δ; in relation to healthy mean and std) are provided:
3.1. Voltage time course analyses (see Fig. 2, top row plot)
ANOVAs on the voltage time course revealed significant main effects of group for eight time-ranges. Significant time-ranges were averaged across consecutive time-bins and were demarcated at points of polarity switches. For ease of interpretation they are named in accordance with traditional ERP components.
-
S1 P50: 55–75 ms, average F(4,1068) = 10.75 (healthy mean = .073, std = .745).
BDNP (Δ = .838), SAD (Δ = .341), BDP (Δ = .018), SZ (Δ= −0.04).
-
S1 N100: 76–135 ms, average F(4,1068) = 15.79 (healthy mean= −1.263, std = 1.101).
SZ (Δ = .58), SAD (Δ = .572), BDNP (Δ = .293), BDP (Δ = .183).
-
S1 P200: 155–255 ms, average F(4,1068) = 6.19 (healthy mean = 1.275, std = .952).
BDNP (Δ= −.036), SAD (Δ= −.323), SZ (Δ= −.383), BDP (Δ= −.402).
-
S1 N200: 275–315 ms, average F(4,1068) = 4.55 (healthy mean= −.101, std = .784).
SZ (Δ = .252), SAD (Δ= −.03), BDP (Δ= −.139), BDNP (Δ= −.404)
-
S1 Late: 395–415 ms, average F(4,1068) = 6.19 (healthy mean= −.591, std = .65).
SZ (Δ = .328), SAD (Δ = .064), BDP (Δ = .06), BDNP (Δ = .052).
-
Pre-S2: 435–515 ms, average F(4,1068) = 3.97 (healthy mean= −.499, std = .46).
SZ (Δ = .399), SAD (Δ = .089), BDP (Δ = .013), BDNP (Δ= −.165).
-
S2 P50: 516–555 ms, average F(4,1068) = 4.95 (healthy mean= −.297, std = .478).
SZ (Δ = .337), BDNP (Δ = .176), SAD (Δ = .103), HC = BDP (Δ= −.061).
-
S2 N100: 556–635 ms, average F(4,1068) = 9.64 (healthy mean= −.747, std = .559).
BDNP (Δ = .463), SZ (Δ = .456), SAD (Δ = .307), BDP (Δ= −.009).
There were not significant group by sex interactions surviving FDR adjustment.
3.2. Low Frequency (4–17 Hz) time course analyses (see Fig. 2, second row plot)
ANOVAs on the low frequency time course revealed significant main effects of group for 3 time-ranges:
-
Low S1 Early/Mid: 35–255 ms, average F (4, 1068) = 10.56 (healthy mean = 41.236, std = 3.87).
BDNP (Δ= −.008), BDP (Δ= −.265), SAD (Δ= −.481), SZ (Δ= −.565).
Low Pre-S2: 455–515 ms, average F(4,1068) = 3.50 (healthy mean = 31.601, std = 4.068). SAD (Δ = .236), SZ (Δ = .198), BDNP (Δ = .02), BDP (Δ = .015).
Low S2 Late: 775–845 ms, average F(4,1068) = 4.07 (healthy mean = 30.936, std = 4.297). SAD (Δ = .198), SZ (Δ = .145), BDP (Δ = .021), BDNP (Δ= −.10).
There were not significant group by sex interactions surviving FDR correction.
3.3. Beta (18–32 Hz) time course analyses (see Fig. 2, third row plot)
ANOVAs on the Beta Frequency component revealed a significant main effect of group for one time-range:
-
S1 Early: 55–135 ms, average F(4,1068) = 5.80 (healthy mean = 18.973, std = 3.304).
BDNP (Δ = .416), SAD (Δ = .077), BDP (Δ = .046), SZ (Δ= −.007).
There were not significant group by sex interactions surviving FDR correction.
3.4. Gamma (33–55 Hz) time course analyses (see Fig. 2, bottom row plot)
ANOVAs on the Gamma Frequency component revealed significant main effects of group for 5 time-ranges:
Gamma Pre-S1: −105 to −5 ms, average F(4,1068) = 4.98 (healthy mean = 15.689, std = 2.776). SAD (Δ = .373), SZ (Δ = .251), BDP (Δ = .228), BDNP (Δ = .201).
Gamma S1 P50: 15–75 ms, average F(4,1068) = 2.87 (healthy mean = 21.764, std = 4.035). BDNP (Δ = .493), SAD (Δ = .041), BDP (Δ= −.082), SZ (Δ= −.128)
-
Gamma S1 Mid/Pre S2: 155–495 ms, average F(4,1068) = 4.85 (healthy mean = 15.404, std = 2.683).
SAD (Δ = .329), SZ (Δ = .298), BDP (Δ = .279), BDNP (Δ = .247).
-
Gamma S2 Early: 535–635 ms, average F(4,1068) = 4.10 (healthy mean = 17.426, std = 2.918).
BDNP (Δ = .582), SAD (Δ = .155), BDP (Δ = .095), SZ (Δ = .083).
-
Gamma S2 Late: 636–845 ms, average F(4,1068) = 5.52 (healthy mean = 15.381, std = 2.68).
SAD (Δ = .367), BDP (Δ = .313), BDNP (Δ = .286), SZ (Δ = .286).
There were not significant group by sex interactions surviving FDR correction.
3.5. Multivariate analyses
To efficiently summarize the multiple EEG components that best captured group differences we used multivariate linear discriminant and correlational analyses. This approach yields a reduced set of measures that may be most useful in subsequent biomarker investigations.
3.5.1. Linear discriminant analysis
The 17 ERP and TF variables that differentiated groups were used in the jackknifed linear discriminant analysis procedure to identify stable differentiating variables. Seven variables contributed to group separations; patterns of group differences and post-hoc Tukey’s B tests are detailed in Table 2 and Fig. 3. The P50 response to S1 (S1 P50 ERP) and Gamma S2 (Gamma S2 Early) uniquely separated BDNP from healthy and psychosis subjects (BDNP > all other groups); the S1 N100 (S1 ERP N100 and Low S1 Early/Mid) response separated groups along an axis of psychopathology severity (HC > BDNP > BDP > SAD > SZ); the S1 P200 (S1 ERP P200) response indexed psychosis psychopathology (HC/BDNP > SAD/SZ/BDP); and the preparatory period to the S2 (ERP Pre-S2 and Low Pre-S2) stimulus separated SZ from other groups (SZ > SAD/BDP > HC/BDNP).
Fig. 3.
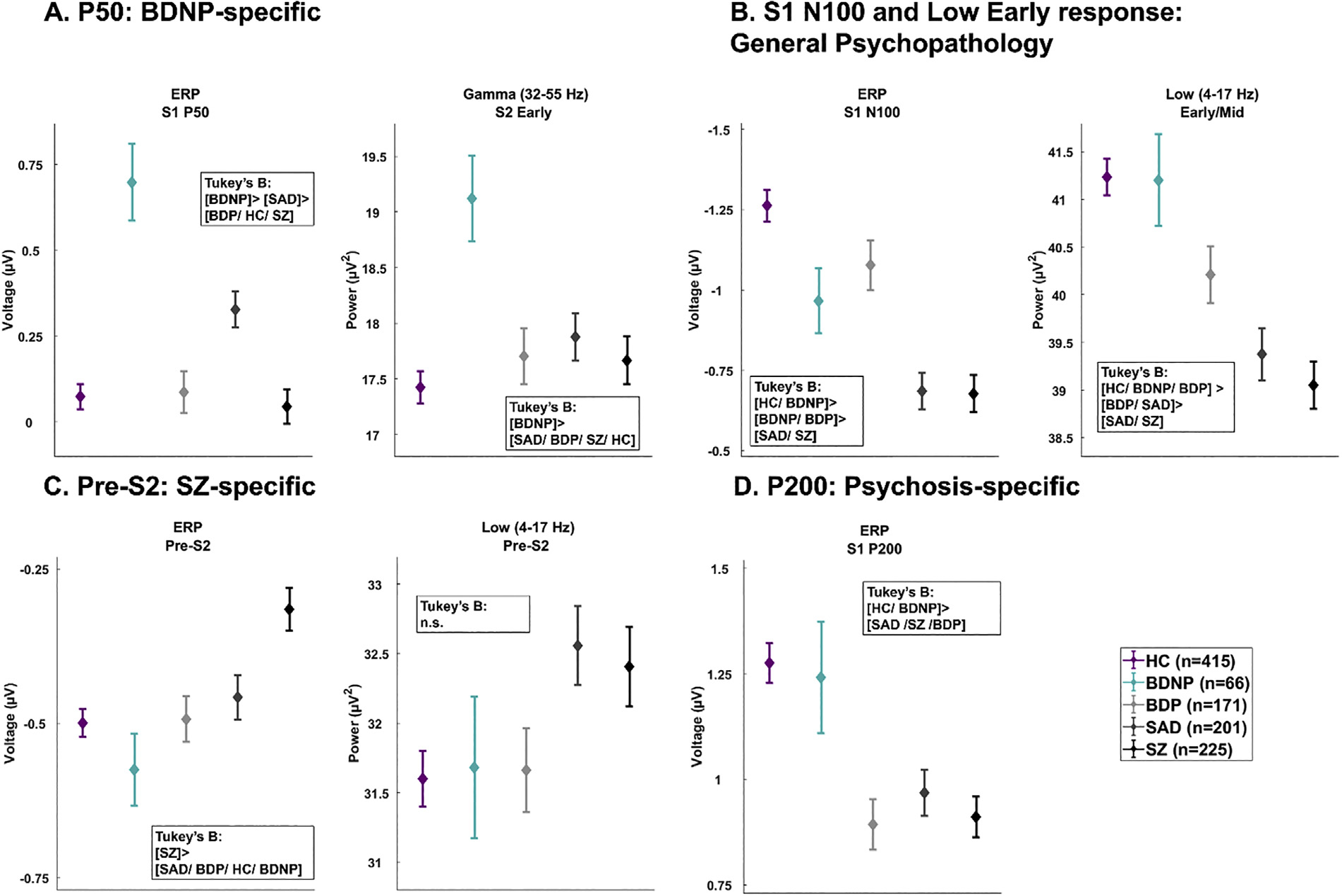
Group averages and standard errors for seven variables most parsimoniously explained patterns of group differences determined in the jackknifed linear discriminant analysis. (A) The S1 P50 and Gamma S2 Early separated BDNP from other groups. (B) The ERP N100 and Low Early response showed a patterned of general psychopathology severity. (C) The ERP Pre-S2 and Low Pre-S2 separated SZ from BDP/BDNP/HC. (D) The P200 variable separated psychosis groups from BDNP and HC. HC, Healthy Subjects; BDNP, Probands with bipolar disorder I without any history of psychotic symptoms; BDP, Probands with bipolar disorder I with a history of psychotic symptoms; SAD, Probands with schizoaffective disorder; SZ, Probands with schizophrenia.
3.5.2. Canonical correlation analysis relating ERPs and clinical features
In order capture the relationship between clinical symptoms and neural responses regardless of diagnoses we performed a CCA between all 17 significant neural variables and 6 clinical features (PANSS Positive, Negative, and General, MADRS, Young Mania, and the Birchwood social functioning scale). Two canonical correlation components were identified (Variate 1: r = .358, p < .0001; Variate 2: r = .283, p = .032). Each variate has a latent component for the neural responses and for the clinical responses. The structure matrix detailing correlations between the neural and clinical measures is embedded in Fig. 4A and B.
Fig. 4.
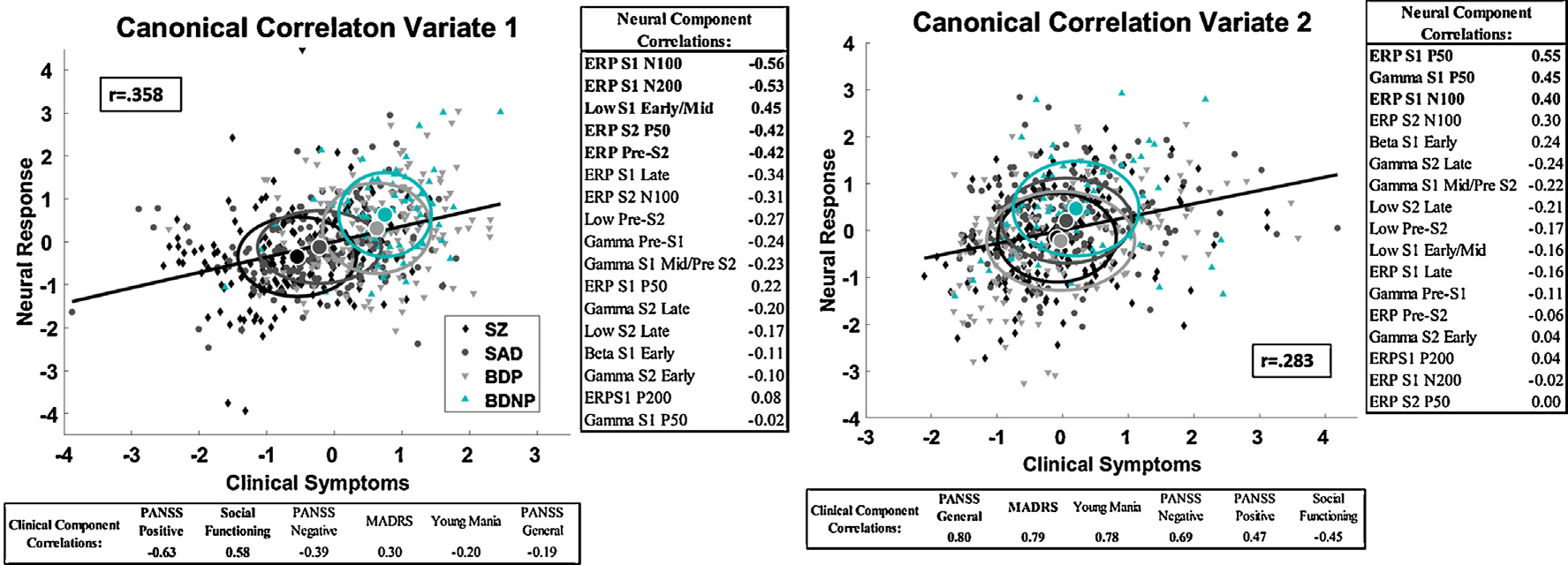
Paired Stimuli Neural Response association with current clinical syndromes.Canonical correlation analysis variates based on all probands (N with complete data = 568). Regression line, group means and standard deviations are shown. Fisher’s Z test did not find a difference in response between groups 4A) The first variate shows an association between early sensory response (N100/Low Early response) and pre-stimulus neural activity before S2 (S1 N100/Pre-S2/ Early S2 response) and current PANSS Positive symptoms and Social Functioning. 4B) The second variate shows an association between the P50 neural response and N100 response and current PANSS Genera/Negative, MADRS and Young Mania symptoms. Pearson Correlations between the neural variate and ERP components and the clinical variate and are shown. BDNP, Probands with bipolar disorder I without any history of psychotic symptoms; BDP, Probands with bipolar disorder I with a history of psychotic symptoms; SAD, Probands with schizoaffective disorder; SZ, Probands with schizophrenia.
The first CCA variate indicated an association between higher levels of PANSS Positive Symptoms and lower levels of social functioning with lower early sensory responses to S1 (ERP N100 and Low (4–17 Hz) Early response) and to the pre-stimulus and early response to S2 (S1 N200, PreS2 and S2 P50) (Fig. 4A). The second CCA variate, after removing variance associated with the first variate, was an association between higher levels of current affective and general psychosis symptoms (MADRS, Young Mania, and PANSS General) and heightened P50 (55–75 ms) response (Fig. 4B). Fisher’s Z test showed that correlations between the clinical variate and the neural variate did not differ between groups (p > .05) for CCA1 or CCA2.
4. Discussion
The present study investigated auditory ERP characteristics in a large sample across the schizophrenia-bipolar spectrum. Results clarify how psychosis, affective history, and current symptoms are related to auditory ERP components from the paired stimuli paradigm. Detailed discussion of the study outcomes and the relationship to the 5 main study goals and their importance for understanding neuro-pathological correlates of psychosis and bipolar disorder is provided below.
4.1. Goal 1: replication of B-SNIP paired stimuli results
We replicated B-SNIP1 auditory-paired stimuli findings [28]. Similarities in patterns of ERP responses across B-SNIP1 and B-SNIP2 between SZ, SAD, BDP, and healthy subjects are striking (all correlations between B-SNIP1 and B-SNIP2 grand average ERPs were greater than .95; see Fig. 1). This outcome provides a strong foundation for using paired stimuli ERP responses as psychosis syndrome biomarkers.
Remarkable similarities in response were observed between Hamm et al. [28] and the current study. The five time ranges identified in Hamm et al. [28] that best discriminated between SZ, BDP and HC had analogous variables that were significant and had similar patterns of response. SZ and BDP had high levels of gamma activity during the pre-stimulus S1 period (BSNIP1 Glass’s Δ: SZ = .36, BDP = .26; BSNIP2: SZ = .25; BDP = .23). The S1 N100 ERP response showed a similar pattern of HC > BDP > SZ (BSNIP1 Glass’s Δ: SZ = .56, BDP = .32; BSNIP2: SZ = .58; BDP = .18). The S1 Low Frequency response showed a similar pattern of HC > BDP > SZ (BSNIP1 Glass’s Δ: SZ = −.62, BDP = −.29; BSNIP2: SZ = −.57; BDP = −.27). The S1 P200 ERP indicated a shared reduction across psychosis cases from the healthy comparison group, but SZ and BDP did not differ to the same extent (BSNIP1 Glass’s Δ: SZ = −.27, BDP = −.48; BSNIP2: SZ = −.38; BDP = −.40). The S2 P50 ERP showed a similar pattern of SZ > HC > BDP (BSNIP1 Glass’s Δ: SZ = .23, BDP= −.19; BSNIP2: SZ = .34; BDP= −.06). The goal of replicating B-SNIP’s prior research directly supports psychiatry’s need for replication of large scale studies and provides crucial support for using these biological measures in efforts to identify biologically-defined sub-groups [30] and for their use in clinical applications.
4.2. Goal 2: identify biomarkers shared across the psychosis spectrum
Overlap between conventional psychosis syndromes has been shown across multiple biomarkers at many levels of analysis [6,28,30,34,39,50–52]. The present study adds nuance to these efforts through the use of multiple psychosis syndromes, while showing remarkable congruity with previous large sample investigations of paired stimuli responses. Reduced N100 responses in SZ-like psychosis are frequently reported [27–29,34,53]; the present study illustrated ERP N100 reductions in SZ and SAD and reductions in lower frequencies across all psychosis group (Fig. 3). The S1 P200 shows a reduction in all psychosis groups, but was of normal morphology in BDNP. The P200 has a unique association with stimulus classification and its independence from N100-related sensory registration and attentional demand. This pattern of findings indicates that P200 it is a psychosis specific biomarker (see also association of P200 with family history of psychosis among BDNP [54]).
4.3. Goal 3: evidence for distinct ERP responses within BDP and BDNP
Multiple measures provide evidence of partially distinct pathophysiologies for BDP and BDNP. Family studies indicate that, compared to BDNP, BDP are 2–3 times more likely to have relatives with BDP [55–57], and SZ have increased rates of BDP vs. BDNP in their relatives [56,58], which is also consistent with twin studies [57]. Transient auditory EEG responses in BD are moderated by a family history of psychosis [54], people with BD have a reduced auditory oddball response based on the current presence of psychotic symptoms [59]. BDNP differed from all other psychosis groups and HC on the S1 P50 and S2 P50 in the gamma frequency component, showing heightened responses. While some studies have found reduced P50 responses in BD [15,16], hints of S1 P50 hyper-reactivity is found in Hamm et al. [54] in BDNP without a family history of psychosis. Importantly, this provides further evidence for examining EEG responses based on the presence of psychotic history in future studies of BD.
4.4. Goal 4: identify associations between clinical features and ERPs
Identifying the relationship between clinical symptoms and auditory EEG variables in schizophrenia has been proven to be difficult despite evidence of their utility as biomarkers [60]. The present study used a multivariate and trans-diagnostic approach with a large and well-characterized sample of cases across the affective-psychosis spectrum. Additionally, the inclusion of data in both the voltage and frequency domains provided important neural information that could be related to specific clinical profiles. This approach may enhance the probability of detecting relationships between EEG and clinical variables by enhancing the range of clinical features than would be found in a single syndrome. Two major relationships were identified: (i) high positive psychosis symptoms and low social functioning are related to reduced S1 N100, early low frequency response, and the preparatory and early response periods around S2. This clinical profile is associated with more SZ-like syndromes; and (ii) higher levels of PANSS general, MADRS (depression), and mania symptoms are related to higher responses during the S1 P50, the early S2 Gamma period, and during the S1 N100. This clinical profile is associated with a more prototypical affective case with few positive psychosis symptoms.
4.5. Limitations
The strengths of our study include a large sample covering a broad range of serious psychiatric syndromes and a rich characterization of their clinical features. Among limitations, almost all patients were medicated, some of which have been linked with ERP responses (see Supplemental table 5 for correlations between EEG variables and medications), so the effect of treatment cannot be excluded definitively. There are considerations for determining if medications impacted our results. First, while the majority of psychosis individuals were actively treated with antipsychotics (SZ = 84 %, SAD = 79 %, and BDP = 65 %), many BDNP (39 %) were on antipsychotics. Second, while there was a significant overall difference in frequency of mood stabilizer and antidepressant use, BDP and BDNP had similar rates (mood stabilizer: BDP = 65 %, BDNP = 68 %; lithium: BDP = 23 %, BDNP = 26 %; antidepressant: BDP = 29 %, BDNP = 35 %). A majority of the psychosis cases were also chronic, stable outpatients. While there is evidence of paired stimuli ERP abnormalities in pre-onset and first episode psychosis, this has not been adequately assessed in BD and early psychosis groups [61,62]. Such investigations could provide a biomarker of distinct etiologies or risk profiles in the most affective psychosis cases vs. BDNP.
4.6. Conclusions
By taking a trans-diagnostic approach across the psychosis-affective spectrum, this study replicated ERP deviations related to general psychosis status, provided evidence for partially unique neurophysiologies between BDP and BDNP, and identified ERPs that differ between affective and non-affective psychosis cases. Auditory cortical disruptions during the paired stimuli tasks show disruptions across early, middle, and late sensory processing. The complex profile of shared and unique auditory cortical disruptions suggest future studies of EEG responses using biologically-derived subgroups which could enhance understanding of specific neuropathologies and their relation to the etiology of serious psychopathology.
Supplementary Material
Acknowledgments
The authors would like to thank the numerous researchers and clinicians who assisted in recruitment and data collection. Most importantly, they express gratitude to the patients who contributed their time and effort to participate in this study.
Funding
National Institute of Health, National Institute of Mental Health (NIMH): MH096942, MH078113, MH096900, MH103366, MH096913, MH077851, MH096957, MH077945, MH103368.
Footnotes
Most auditory suppression studies used a single EEG sensor (the vertex electrode). A single sensor, however, is sub-optimal for evaluating the complex dynamics of brain systems responding to sensory inputs (see, e.g., [17,18]). First, a single sensor may provide an inaccurate estimate of ‘true’ brain responses (given inter-individual differences in spatial and temporal weightings and orientations of single and multiple sources active at any one time), and may result in misinterpretations of between-groups similarities and differences on signal strength. Second, integrating activity over multiple recording sites can decrease the probability that nonspecific forms of variance are masquerading as signal [18,19]). Third, neural responses are distributed and complex phenomena that require more, not less, sophisticated technology for their precise measurement; similar to the need for MRI to improve in measurement capability to precisely capture functional anatomy.
Hall et al. [23] is unusual in this literature in suggesting higher repeatability of P50 suppression scores in a twin study. One factor that may have contributed to this studies outcome, however, is the trail selection procedures that, which included: (i) starting with Cz but using other sensors if Cz scoring was unclear; (ii) excluding runs without identifiable ERP responses within a limited a pre-determined time window; and (iii) “Blocks containing EOG activity in the 40–75 ms post-stimulus window exceeding the P50 wave or with a large negative–positive P30 complex (1.5 times bigger than the P50 wave) were also excluded from the grand average.” All of these approaches may have assisted with intra-study measurement consistency but would be difficult to implement across laboratories for biomarker diagnostic purposes and may yield outcomes that are not representative of the population of ERP responses.
Declaration of Competing Interest
DA Parker, RL Trotti, JE McDowell, SK Keedy, ES Gershon, EI Ivleva, GD Pearlson, MS Keshavan, CA Tamminga, JA Sweeney, and BA Clementz report no financial interests or potential conflicts of interest related to this project.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.bionps.2020.100014.
References
- [1].N.R. Council, Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease, Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease, The National Academies Press: Washington (DC), 2011. [PubMed] [Google Scholar]
- [2].Jablensky A, Endophenotypes in psychiatric research, in: Salzinger K, Serper MR (Eds.), Behavioral Mechanisms and Psychopathology - Advancing the Explanation of Its Nature, Cause, and Treatment, American Medical Association, USA, 2009. [Google Scholar]
- [3].Gottesman II, Gould TD, The endophenotype concept in psychiatry: etymology and strategic intentions, Am. J. Psychiatry 160 (4) (2003) 636–645. [DOI] [PubMed] [Google Scholar]
- [4].Tamminga CA, et al. , Strategies for advancing disease definition using biomarkers and genetics: the bipolar and schizophrenia network for intermediate phenotypes, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 (1) (2017) 20–27. [DOI] [PubMed] [Google Scholar]
- [5].Tamminga CA, et al. , Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum, Schizophr. Bull 40 (Suppl. 2) (2014) S131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tamminga CA, et al. , Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP), Am. J. Psychiatry 170 (11) (2013) 1263–1274. [DOI] [PubMed] [Google Scholar]
- [7].Clementz BA, Time for change in psychosis research, in: Tamminga C, Ivleva E, Reininghaus U, van Os J (Eds.), “Psychotic Disorders: Comprehensive Conceptualization and Treatments”, Oxford University Press, 2019. [Google Scholar]
- [8].Pearlson GD, et al. , Does biology transcend the symptom-based boundaries of psychosis? Psychiatr. Clin. North Am 39 (2) (2016) 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Freedman R, et al. , Neurobiological studies of sensory gating in schizophrenia, Schizophr. Bull 13 (4) (1987) 669–678. [DOI] [PubMed] [Google Scholar]
- [10].Adler LE, et al. , Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia, Biol. Psychiatry 17 (6) (1982) 639–654. [PubMed] [Google Scholar]
- [11].Bramon E, et al. , Meta-analysis of the P300 and P50 waveforms in schizophrenia, Schizophr. Res. 70 (2–3) (2004) 315–329. [DOI] [PubMed] [Google Scholar]
- [12].Clementz BA, Geyer MA, Braff DL, Poor P50 suppression among schizophrenia patients and their first-degree biological relatives, Am. J. Psychiatry 155 (12) (1998) 1691–1694. [DOI] [PubMed] [Google Scholar]
- [13].Patterson JV, et al. , P50 sensory gating ratios in schizophrenics and controls: a review and data analysis, Psychiatry Res 158 (2) (2008) 226–247. [DOI] [PubMed] [Google Scholar]
- [14].Johannesen JK, et al. , Diagnostic specificity of neurophysiological endophenotypes in schizophrenia and bipolar disorder, Schizophr. Bull 39 (6) (2013) 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cheng CH, et al. , Auditory sensory gating in patients with bipolar disorders: a meta-analysis, J. Affect. Disord. 203 (2016) 199–203. [DOI] [PubMed] [Google Scholar]
- [16].Morsel AM, et al. , Systematic review of cognitive event related potentials in euthymic bipolar disorder, Clin. Neurophysiol 129 (9) (2018) 1854–1865. [DOI] [PubMed] [Google Scholar]
- [17].Cardenas VA, et al. , A multichannel, model-free method for estimation of event-related potential amplitudes and its comparison with dipole source localization, J. Med. Eng. Technol. 19 (2–3) (1995) 88–98. [DOI] [PubMed] [Google Scholar]
- [18].Picton TW, et al. , Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria, Psychophysiology 37 (2) (2000) 127–152. [PubMed] [Google Scholar]
- [19].Picton TW, et al. , Human auditory steady-state responses: the effects of recording technique and state of arousal, Anesth. Analg 97 (5) (2003) 1396–1402. [DOI] [PubMed] [Google Scholar]
- [20].Smith DA, Boutros NN, Schwarzkopf SB, Reliability of P50 auditory event-related potential indices of sensory gating, Psychophysiology 31 (5) (1994) 495–502. [DOI] [PubMed] [Google Scholar]
- [21].Rentzsch J, et al. , Test-retest reliability of P50, N100 and P200 auditory sensory gating in healthy subjects, Int. J. Psychophysiol 67 (2) (2008) 81–90. [DOI] [PubMed] [Google Scholar]
- [22].Dalecki A, Croft RJ, Johnstone SJ, An evaluation of P50 paired-click methodologies, Psychophysiology 48 (12) (2011) 1692–1700. [DOI] [PubMed] [Google Scholar]
- [23].Hall MH, et al. , Heritability and reliability of P300, P50 and duration mismatch negativity, Behav. Genet. 36 (6) (2006) 845–857. [DOI] [PubMed] [Google Scholar]
- [24].Blumenfeld LD, Clementz BA, Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG, Clin. Neurophysiol 112 (9) (2001) 1650–1659. [DOI] [PubMed] [Google Scholar]
- [25].Clementz BA, et al. , Ear of stimulation determines schizophrenia-normal brain activity differences in an auditory paired-stimuli paradigm, Eur. J. Neurosci. 18 (10) (2003) 2853–2858. [DOI] [PubMed] [Google Scholar]
- [26].Turetsky BI, et al. , Profile of auditory information-processing deficits in schizophrenia, Psychiatry Res 165 (1–2) (2009) 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Turetsky BI, et al. , Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands, Biol. Psychiatry 64 (12) (2008) 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hamm JP, et al. , Diagnostic specificity and familiality of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum, Psychophysiology 51 (4) (2014) 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rosburg T, Boutros NN, Ford JM, Reduced auditory evoked potential component N100 in schizophrenia–a critical review, Psychiatry Res 161 (3) (2008) 259–274. [DOI] [PubMed] [Google Scholar]
- [30].Clementz BA, et al. , Identification of distinct psychosis biotypes using brain-based biomarkers, Am. J. Psychiatry 173 (4) (2016) 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Popov T, et al. , Adjusting brain dynamics in schizophrenia by means of perceptual and cognitive training, PLoS One 7 (7) (2012) e39051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Popov T, et al. , Evoked and induced oscillatory activity contributes to abnormal auditory sensory gating in schizophrenia, Neuroimage 56 (1) (2011) 307–314. [DOI] [PubMed] [Google Scholar]
- [33].American Psychiatric Association, Diagnostic Criteria From DSM-IV-TR, American Psychiatric Association, Washington, D.C, 2000. xii, 370 p. [Google Scholar]
- [34].Ethridge LE, et al. , Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder, Biol. Psychiatry 77 (2) (2015) 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Delorme A, Makeig S, EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis, J. Neurosci. Methods 134 (1) (2004) 9–21. [DOI] [PubMed] [Google Scholar]
- [36].Dien J, Khoe W, Mangun GR, Evaluation of PCA and ICA of simulated ERPs: promax vs. Infomax rotations, Hum. Brain Mapp. 28 (8) (2007) 742–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Carroll CA, et al. , Contributions of spectral frequency analyses to the study of P50 ERP amplitude and suppression in bipolar disorder with or without a history of psychosis, Bipolar Disord. 10 (7) (2008) 776–787. [DOI] [PubMed] [Google Scholar]
- [38].Ethridge LE, et al. , Neural activations during auditory oddball processing discriminating schizophrenia and psychotic bipolar disorder, Biol. Psychiatry 72 (9) (2012) 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hamm JP, et al. , Spatiotemporal and frequency domain analysis of auditory paired stimuli processing in schizophrenia and bipolar disorder with psychosis, Psychophysiology 49 (4) (2012) 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hamm JP, et al. , Pre-cue fronto-occipital alpha phase and distributed cortical oscillations predict failures of cognitive control, J. Neurosci. 32 (20) (2012) 7034–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Venables NC, Bernat EM, Sponheim SR, Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia, Schizophr. Bull 35 (4) (2009) 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dukart J, et al. , Age correction in dementia–matching to a healthy brain, PLoS One 6 (7) (2011) e22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hochberg Y, Benjamini Y, More powerful procedures for multiple significance testing, Stat. Med 9 (7) (1990) 811–818. [DOI] [PubMed] [Google Scholar]
- [44].Mardia KV, Kent JT, Bibby JM, Multivariate analysis, Probability and Mathematical Statistics, Academic Press, London; New York, 1979. xv, 521 p. [Google Scholar]
- [45].Lee H-S, Canonical correlation analysis using small number of samples, Commun. Stat. - Simul. Comput 35 (5) (2007) 973–985. [Google Scholar]
- [46].Birchwood M, et al. , The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients, Br. J. Psychiatry 157 (1990) 853–859. [DOI] [PubMed] [Google Scholar]
- [47].Lancon C, et al. , Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS), Schizophr. Res. 42 (3) (2000) 231–239. [DOI] [PubMed] [Google Scholar]
- [48].Young RC, et al. , A rating scale for mania: reliability, validity and sensitivity, Br. J. Psychiatry 133 (1978) 429–435. [DOI] [PubMed] [Google Scholar]
- [49].Montgomery SA, Asberg M, A new depression scale designed to be sensitive to change, Br. J. Psychiatry 134 (1979) 382–389. [DOI] [PubMed] [Google Scholar]
- [50].Hill SK, et al. , Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study, Am. J. Psychiatry 170 (11) (2013) 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Reilly JL, et al. , Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories, Schizophr. Bull 40 (5) (2014) 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ivleva EI, et al. , Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes, Biol. Psychiatry 82 (1) (2017) 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shelley AM, Silipo G, Javitt DC, Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction, Schizophr. Res. 37 (1) (1999) 65–79. [DOI] [PubMed] [Google Scholar]
- [54].Hamm JP, et al. , Family history of psychosis moderates early auditory cortical response abnormalities in non-psychotic bipolar disorder, Bipolar Disord. 15 (7) (2013) 774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Potash JB, et al. , The bipolar disorder phenome database: a resource for genetic studies, Am. J. Psychiatry 164 (8) (2007) 1229–1237. [DOI] [PubMed] [Google Scholar]
- [56].Kendler KS, Gruenberg AM, Tsuang MT, Psychiatric illness in first-degree relatives of schizophrenic and surgical control patients. A family study using DSM-III criteria, Arch. Gen. Psychiatry 42 (8) (1985) 770–779. [DOI] [PubMed] [Google Scholar]
- [57].Cardno AG, et al. , A twin study of schizoaffective-mania, schizoaffective-depression, and other psychotic syndromes, Am. J. Med. Genet. B Neuropsychiatr. Genet 159B (2) (2012) 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lichtenstein P, et al. , Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study, Lancet 373 (9659) (2009) 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lundin NB, et al. , Reduced electroencephalogram responses to standard and target auditory stimuli in bipolar disorder and the impact of psychotic features: analysis of event-related potentials, spectral power, and inter-trial coherence, Bipolar Disord. 20 (1) (2018) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ford JM, The difficulty in finding relationships between ERPs and clinical symptoms of schizophrenia, Clin. EEG Neurosci 49 (1) (2018) 6–7. [DOI] [PubMed] [Google Scholar]
- [61].Hamilton HK, et al. , Auditory and visual oddball stimulus processing deficits in schizophrenia and the psychosis risk syndrome: forecasting psychosis risk with P300, Schizophr. Bull 45 (5) (2019) 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hamilton HK, et al. , Association between P300 responses to auditory oddball stimuli and clinical outcomes in the psychosis risk syndrome, JAMA Psychiatry 76 (11) (2019) 1187–1197, doi: 10.1001/jamapsychiatry.2019.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


