Abstract
One of the most characteristic clinical features in cutaneous leishmaniasis is the development of nodules followed by ulcerations at the site of infection. Leishmania amazonensis-infected mice show similar ulcerative lesions. Leishmania-infected severe combined immunodeficiency (SCID) mice, however, have been shown to develop nonulcerative nodules. In the present study, the roles of T cells in ulceration were examined using SCID mice in cell reconstitution experiments. After development of nonulcerative nodules, SCID mice were inoculated with splenocytes from either Leishmania-infected or naive immunocompetent mice, resulting in ulceration in all mice. When naive splenocytes were depleted of CD4+, CD8+, or B220+ cell populations and the remaining cells were injected into Leishmania-infected SCID mice after the development of nodules, only SCID mice inoculated with splenocytes depleted of CD4+ cells did not show ulceration. The evidence obtained in this study clearly shows that the CD4+ cell population is indispensable for ulceration in leishmaniasis lesions of SCID mice.
Leishmaniasis is a complex disease with differing symptoms, including cutaneous, mucocutaneous, and visceral manifestations, and is one of the most widespread disease in tropical and subtropical countries. A hallmark of cutaneous leishmaniasis is the development of papules or nodules followed by ulceration at the site of promastigote infection. Cutaneous leishmaniasis, while not fatal, produces long-lasting ulcers and leaves scars upon healing.
Leishmaniasis is caused by obligate intracellular protozoan parasites of the genus Leishmania which replicate inside the parasitophorous vacuoles of infected macrophages. Most experimental investigations of leishmaniasis so far have focused almost exclusively on the cellular and molecular mechanisms involved in controlling protozoan proliferation. In this context, CD4+ T cells have been shown to play important roles in protection against Leishmania major infection (11, 12, 19).
The roles of CD4+ T cells in pathological changes in cutaneous leishmaniasis are not well understood, although they are known to be predominant inducers of pathological changes in other infectious diseases (1, 5, 8, 13). The recent increase of unusual clinical features in patients coinfected with human immunodeficiency virus (HIV) such as nonulcerative cutaneous lesions (3, 5, 16) has prompted us to investigate the role of CD4+ T cells in pathological changes in cutaneous leishmaniasis. In the present study, we demonstrate that CD4+ cells are indispensable for ulcer development in L. amazonensis-infected SCID mice.
MATERIALS AND METHODS
Mice.
C.B-17/lcrJcl-scid (SCID), C.B-17/lcrJcl (C.B-17), and BALB/cAJcl (BALB/c) were bred and housed in the specific-pathogen-free animal care facility of the Central Institute for Experimental Animals, Kanagawa, Japan. Age (8-week-old)- and sex (male)-matched mice were used throughout the experiments.
Parasite infection.
Promastigotes of L. amazonensis (MPRO/BR/72/M1845) were cultured at 23°C in medium 199 (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (FBS). The organisms were harvested from stationary-phase cultures, centrifuged, and washed with Hanks' balanced salt solution (HBSS) (Nissui Pharmaceutical Co., Ltd.). Mice were inoculated with 107 infective promastigotes subcutaneously at the base of the tail. Lesion development was visually monitored by weekly measurement with a direct-reading vernier caliper gauge and was expressed as the dimensions of the nodule base (width by length).
Cell preparation and adoptive transfer of immunocompetent cells.
For transfusion of splenocytes, spleens were removed aseptically from naive or L. amazonensis-infected C.B-17 mice at week 10 after infection. Spleens were minced and passed through a sterile nylon 200-mesh screen. Red blood cells were lysed using 144 mM NH4Cl solution. The splenocytes were washed and suspended in HBSS, and 0.5 ml of the suspension containing 107 or 108 cells was transferred intraperitoneally into L. amazonensis-infected SCID mice at week 10 after infection.
Depletion of lymphocyte subsets from splenocytes was performed using a magnetic cell sorter system. Splenocytes were prepared from spleens of BALB/c mice as described above, and 108 cells in 5 ml of RPMI-1640 supplemented with 5% heat-inactivated FBS (RPMI-5–FBS) were incubated for 30 min with 50 μg of anti-CD4 (clone RM4-5; Pharmingen, San Diego, Calif.), anti-CD8 (clone 53-6.7; Pharmingen), or anti-B220 (clone DNL-1.9; Pharmingen) rat monoclonal antibody. Cells were washed with RPMI-5–FBS and gently mixed for 10 min with sheep anti-rat immunoglobulin G-coated magnetic beads (Dynabeads; Robbins Scientific, Mountain View, Calif.). The mixture was incubated for 5 min in a magnetic field. After washing, cells were suspended in 5 ml of HBSS. All procedures were carried out at 4°C. Five hundred-microliter aliquots of cell suspensions of splenocytes depleted of different lymphocyte subsets were transferred intraperitoneally into L. amazonensis-infected SCID mice at week 13 after infection. The efficacy of depletion was examined by testing aliquots of cells by flow cytometry (Profile; Coulter, Japan Scientific Instruments Co., Ltd., Tokyo) using anti-CD8 (clone YST169.4) conjugated with fluorescein isothiocyanate (Coulter), anti-CD4 (clone YST191.1) conjugated with fluorescein isothiocyanate (Coulter), and anti-B220 (clone RA-6B2) conjugated with phycoerythrin (Coulter).
Histopathological examination.
For histopathological examinations, mice were euthanized at days 8 and 12 after transfer of immunocompetent naive splenocytes, and the skin lesions were removed from the SCID mice. Half of each tissue sample was fixed using 10% buffered formalin and was embedded in paraffin. The block was then sectioned at 5-μm thickness and stained with hematoxylin-eosin.
RESULTS
Development of skin lesions in C.B-17 and SCID mice.
The nodules in both C.B-17 and SCID mice appeared by week 3 after infection and enlarged thereafter, showing no signs of resolving. There was no significant difference between lesion sizes in C.B-17 and SCID mice until week 10 after infection (data not shown). In the lesions in C.B-17 mice, an ulcer was formed at the top of the nodule by week 6 after infection, whereas lesions of SCID mice exhibited quite a different appearance. Cutaneous lesions of SCID mice did not form any ulcers and increased in height until week 10 after infection. Since SCID mice lack functional T and B cells, it was suggested that these cells might be essential for ulcer formation in cutaneous leishmaniasis.
Ulcer formation in L. amazonensis-infected SCID mice inoculated with immunocompetent splenocytes.
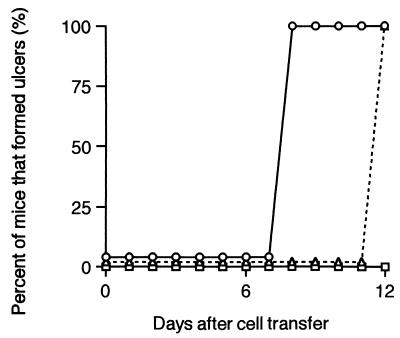
At week 10 after infection with L. amazonensis, naive or L. amazonensis-infected C.B-17 splenocytes were intraperitoneally transferred into infected SCID mice. Nodules of the SCID mice which received 107 splenocytes from C.B-17 mice infected with L. amazonensis were ulcerated on day 8 after transfer (Fig. 1). In SCID mice which were inoculated with the same number of naive C.B-17 splenocytes, ulceration occurred on day 12 after transfer.
FIG. 1.
Percentage of C.B-17-scid (SCID) mice that developed ulcers after adoptive transfer of C.B-17 splenocytes. SCID and C.B-17 mice were subcutaneously infected with 107 L. amazonensis promastigotes. At week 10 after infection, SCID mice were intraperitoneally inoculated with splenocytes (107) from L. amazonensis-infected C.B-17 (○) (n = 4) or naive C.B-17 mice (▵) (n = 4). SCID mice which were infected with L. amazonensis and did not receive any splenocytes were also examined (□) (n = 4). The development of cutaneous lesions was examined visually every week, and the percentage of the mice that developed ulcers on the nodules is indicated. Data are representative of two experiments.
Similar experiments were performed using a dose of 108 splenocytes (data not shown). All SCID mice inoculated with splenocytes from L. amazonensis-infected C.B-17 mice showed ulcer formation in 4 days. In the mice inoculated with 108 naive C.B-17 splenocytes, ulcer formation began on day 8 after cell transfer, and all the mice had ulceration in 9 days.
Histopathological changes in the skin lesions after splenocytes inoculation.
As we reported previously (21), in ulcerated nodules of C.B-17 mice, the epidermis had been desquamated and the dermis was infiltrated with mononuclear and polymorphonuclear cells. The subcutaneous tissue was infiltrated with mononuclear and polymorphonuclear cells as well as vacuolated histiocytes which contained many amastigotes. Nonulcerated nodules of SCID mice appeared to differ from ulcerated nodules of C.B-17 mice. In nodules of SCID mice, the epidermis was thickened, and the dermis was infiltrated with few mononuclear and polymorphonuclear cells. In the subcutaneous tissue, there was an accumulation of vacuolated histiocytes containing a large number of amastigotes, although few mononuclear and polymorphonuclear cells were observed.
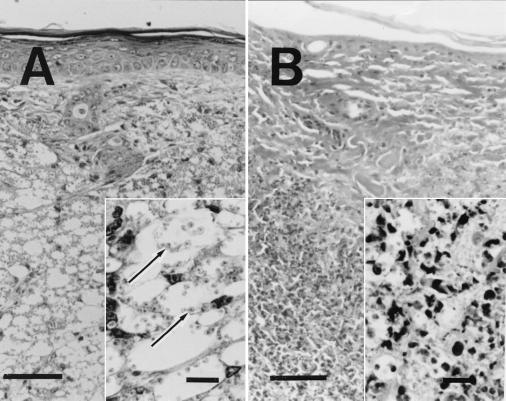
After the transfer of immunocompetent naive splenocytes to infected SCID mice on day 8, when the ulcer had not yet formed, the epidermis and dermis did not appear to differ from those of the animals before transfer (Fig. 2A). In the subcutaneous tissue, however, in addition to the accumulation of vacuolated histiocytes containing a large number of amastigotes, some areas were infiltrated with both mononuclear and polymorphonuclear cells, suggesting the occurrence of cellular reactions (Fig. 2A, inset).
FIG. 2.
Light micrographs of cutaneous lesions of C.B-17-scid (SCID) mice after intraperitoneal transfer of C.B-17 splenocytes. Paraffin-embedded sections were prepared from the lesions of SCID mice on days 8 and 12 after transfer of naive C.B-17 splenocytes and stained with hematoxylin and eosin. Scale bars indicate 50 μm (10 μm for insets). (A) Cutaneous lesion of L. amazonensis-infected SCID mice on day 8 after transfer of naive C.B-17 splenocytes. No infiltration of mononuclear and polymorphonuclear cells was observed in the epidermis and the dermis. In the subcutaneous tissue, there was an accumulation of vacuolated histiocytes containing a large number of amastigotes. (Inset) Some area of subcutaneous tissue were infiltrated with both mononuclear and polymorphonuclear cells in addition to vacuolated histiocytes containing a large number of amastigotes. Arrows indicate the amastigotes. (B) Cutaneous lesion of L. amazonensis-infected SCID mice on day 12 after transfer of naive C.B-17 spleen cells. The epidermis had fallen off. The dermal and subcutaneous tissues were infiltrated with mononuclear cells, polymorphonuclear cells, and histiocytes showing extensive necrosis. (Inset) Few parasites were seen in subcutaneous tissue where many infiltrated cells showed degradation.
Changes of cellular responses in the nodules of SCID mice were apparent on day 12 after transfer of immunocompetent naive splenocytes. When the ulcer had just formed, the epidermis had been desquamated, and the dermis was infiltrated with mononuclear and polymorphonuclear cells (Fig. 2B). The subcutaneous tissue was filled with infiltrated mononuclear cells, polymorphonuclear cells, and histiocytes. In the dermis and subcutaneous tissues, many infiltrated cells showed degradation, and only few Leishmania parasites were seen (Fig. 2B, inset). Histiocytes in the marginal areas of the ulcer, however, were vacuolated and enlarged and contained a large number of amastigotes.
Ability of CD4+ cells, CD8+ cells, or B220+ cells to induce ulcer formation in leishmaniasis.
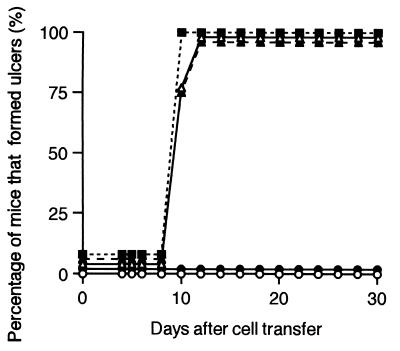
To analyze which type of cell population in immunocompetent splenocytes can trigger the necrotic responses in leishmaniasis cutaneous lesions, we examined the ability of naive splenocytes depleted of CD4+, CD8+, or B220+ cells to induce ulcers in cutaneous lesions. The efficacy of depletion was assessed by flow cytometry analysis. The proportion of cells remaining after the depletion of each subpopulation was 0.8% for CD4+ cells, 0.2% for CD8+ cells, and 0.8% for B220+ cells in a typical experiment. The remaining subpopulations of cells were transferred into L. amazonensis-infected SCID mice.
Figure 3 shows the course of ulcer formation in the nodules of SCID mice which were inoculated with various cells on week 13 after infection with L. amazonensis. The leishmaniasis nodules in SCID mice inoculated with naive splenocytes depleted of CD8+ cells became reddish on day 8 after transfer, and on day 10 after transfer, ulcers developed at the tops of the nodules in all mice. SCID mice inoculated with naive splenocytes depleted of B220+ cells or nontreated naive splenocytes showed the reddish nodules on day 8 to 9 after transfer, and on day 11 after transfer, all the mice showed ulcer formation. In contrast, SCID mice inoculated with naive splenocytes depleted of CD4+ cells did not form ulcers at the lesions even at day 30 after transfer and instead had nonulcerated lesions similar to those of nontreated L. amazonensis-infected SCID mice. None of the lesions of SCID mice inoculated with various cells showed any signs of healing until the end of the experiment (30 days after transfer).
FIG. 3.
The effects of transferred splenocyte populations on ulcer formation in C.B-17-scid (SCID) leishmaniasis lesions. SCID mice were subcutaneously infected with 107 L. amazonensis promastigotes at the tail base. At week 13 after infection, SCID mice were intraperitoneally inoculated with nontreated BALB/c splenocytes (▵) (n = 4) or splenocytes depleted of CD4+ (●) (n = 4), CD8+ (■) (n = 4), or B220+ (▴) (n = 4); cells were prepared as described in Materials and Methods. L. amazonensis-infected SCID mice which did not receive any splenocytes (○) (n = 4) were also examined. The cutaneous lesions were visually examined every week, and the percentage of the mice that developed ulcers on the nodules is indicated. Data are representative of two experiments.
DISCUSSION
The pivotal role of CD4+ T cells in liver granuloma formation has been thoroughly documented in both listeriosis and mycobacteriosis (8, 13). Consistently, it has been reported that SCID mice infected with L. donovani or Schistosoma mansoni could not form granuloma in liver lesions (1, 5). In L. amazonensis infection, even in the absence of T and B cells, SCID mice developed cutaneous nodules consisting mainly of vacuolated macrophages containing amastigotes. This finding suggests T-cell-independent mechanisms of macrophage accumulation in cutaneous leishmaniasis in contrast to T-cell-dependent macrophage accumulation observed in visceral leishmaniasis (10).
The formation of nonulcerative nodules in SCID mice after the infection with L. amazonensis is consistent with previous reports using L. major (7, 23) and suggests that ulcer formation in cutaneous nodules in leishmaniasis is a lymphocyte-dependent reaction. Therefore, we investigated lymphocyte subsets which might play pivotal roles in ulcer formation using a SCID mouse model. Previous work using SCID mice has shown the relative ease of reconstitution of the immune system with immunocompetent splenocytes containing functional T and B cells (23). Hence, we examined the ability of immunocompetent splenocytes to induce ulcer formation in cutaneous leishmaniasis lesions by transferring them into L. amazonensis-infected SCID mice. After the transfer of either naive or sensitized immunocompetent splenocytes, ulceration was observed in the nodules of infected SCID mice. The ability of immunocompetent splenocytes to induce ulceration was abolished only when CD4+ cells were depleted. These results strongly suggest that the addition of immunocompetent splenocytes is sufficient to induce ulcers in cutaneous leishmaniasis lesions in SCID mice and that CD4+ cells are indispensable for ulcer formation.
A heavy accumulation of mononuclear cells was noted in the SCID lesions after transfer of immunocompetent splenocytes. In L. major-infected SCID mice reconstituted with a T-cell line, migration of donated cells to the sites of infection was observed (9). In murine cutaneous leishmaniasis caused by L. mexicana, a selective accumulation of T cells in the cutaneous lesions has also been reported (18). Taken together, our observations also suggest migration of CD4+ cells to the lesions. Although expression of cytokines at the local site of infection was not investigated in this study, expression of certain cytokines was detected in the cutaneous lesions (unpublished data). Therefore, the transferred cells might trigger the ulcer development directly by secreting cytokines and/or indirectly by affecting other cells at the site of infection.
Recently, Soong et al. (20) reported that L. amazonensis infection caused no cutaneous lesions in the C57BL/6J mice with targeted disruption of the major histocompatibility complex class II gene or recombination-activating gene 2. The differences between the findings and our results are of interest. One critical difference is that the two studies used mice with different genetic backgrounds. We previously reported that recombination-activating gene 2 knockout mice with BALB/c background developed cutaneous lesions when infected with L. amazonensis (21). Furthermore, Soong et al. inoculated the parasites into the footpad (20). Sites of infection may be related to the susceptibility of animals (14). Therefore, the different routes of infection used might lead to the different results seen.
The typical clinical aspects of cutaneous leishmaniasis are the development of nodules and ulcer formation at the site of infection. Recently, however, human leishmaniasis with nonulcerative cutaneous lesions has been reported (6, 16). HIV in AIDS patients reduces the CD4+ T-cell number (14). The present data may explain the nonulcerative cutaneous lesions in human cases of Leishmania and HIV double infection. Nonulcerative cutaneous lesions have also been reported in diffuse cutaneous leishmaniasis (DCL) patients who are infected with L. mexicana complex including L. amazonensis (4). The lesions in DCL patients with numerous nonulcerative lesions with high numbers of parasites and few lymphocytes (4) are similar to the lesions observed in the mice lacking functional T cells (21, 22). Moreover, DCL patients are characterized by the lack of helper T-cell responses against Leishmania antigen (2, 15). Since SCID mice are deficient for functional T cells, they do not have any T-cell responses against Leishmania antigens. The present data from cell transfer experiments in which the SCID mice showed ulcer formation only when they received splenocytes with CD4+ cells may suggest pivotal roles of CD4+ cells in formation of nonulcerative nodules in DCL patients.
In conclusion, when L. amazonensis-infected SCID mice received immunocompetent splenocytes intraperitoneally, nonulcerative cutaneous lesions ulcerated. However, only when splenocytes depleted of CD4+ cells were transferred did ulceration fail to occur at the cutaneous nodules of SCID mice. Therefore, CD4+ cells appear to be indispensable for ulcer formation in cutaneous lesions of leishmaniasis in SCID mice.
ACKNOWLEDGMENTS
This work was supported by Grants-in-Aid for Scientific Research (B) (08456149) and for Scientific Research (A) (10041190) from the Ministry of Education, Sciences, Culture, and Sports, Japan.
We thank K. Hioki and S. Endo for supplying the animals, and U. A. K. Kara and D. Jankovic for critically reviewing the manuscript.
REFERENCES
- 1.Amiri P, Locksley R M, Parslow T G, Sadick M, Rector E, Ritter D, McKerrow J H. Tumor necrosis factor α restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 2.Castes M, Cabrera M, Trujillo D, Convit J. T-cell subpopulations, expression of interleukin-2 receptor, and production of interleukin-2 and gamma interferon in human American cutaneous leishmaniasis. J Clin Microbiol. 1988;26:1207–1213. doi: 10.1128/jcm.26.6.1207-1213.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cnudde F, Raccurt C, Boulard F, Teron-Aboud B, Nicolas M, Juminer B. Diffuse cutaneous leishmaniasis with visceral dissemination in an AIDS patient in Guadeloupe, West Indies. AIDS. 1994;8:559–560. [PubMed] [Google Scholar]
- 4.Convit J, Pinardi M E, Rondon A J. Diffuse cutaneous leishmaniasis: a disease due to an immunological defect of the host. Trans R Soc Trop Med Hyg. 1972;66:603–610. doi: 10.1016/0035-9203(72)90306-9. [DOI] [PubMed] [Google Scholar]
- 5.Curry A J, Kaye P M. Recombinant interleukin-1α augment granuloma formation and cytokine production but not parasite clearance in mice infected with Leishmania donovani. Infect Immun. 1992;60:4422–4426. doi: 10.1128/iai.60.10.4422-4426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da-Cruz A M, Machado E S, Menezes J A, Rutowitsch M S, Coutinho S G. Cellular and humoral immune responses of a patient with American leishmaniasis and AIDS. Trans R Soc Trop Med Hyg. 1992;86:511–512. doi: 10.1016/0035-9203(92)90089-u. [DOI] [PubMed] [Google Scholar]
- 7.Guy R A, Belosevic M. Response of scid mice to establishment of Leishmania major infection. Clin Exp Immunol. 1995;100:440–445. doi: 10.1111/j.1365-2249.1995.tb03719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hänsch H C R, Smith D A, Mielke M E A, Hahn H, Bancroft G J, Ehlers S. Mechanisms of granulona formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Int Immunol. 1996;8:1299–1310. doi: 10.1093/intimm/8.8.1299. [DOI] [PubMed] [Google Scholar]
- 9.Holaday B J, Sadick M D, Wang Z, Reiner S L, Heinzel F P, Parslow T G, Locksley R M. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J Immunol. 1991;147:1653–1658. [PubMed] [Google Scholar]
- 10.Kay P M, Bancroft G J. Leishmania donovani infection in scid mice: lack of tissue response and in vivo macrophage activation correlates with failure to trigger natural killer cell-derived gamma interferon production in vitro. Infect Immun. 1992;60:4335–4342. doi: 10.1128/iai.60.10.4335-4342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liew F Y, O'Donnell C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 12.Locksley R M, Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991;12:A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- 13.Mielke M E A, Ehlers S, Hahn H. T-cell subsets in delayed-type hypersensitivity, protection and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988;56:1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabors G S, Farrell J P. Site-specific immunity to Leishmania major in SWR mice: the site of infection influences susceptibility and expression of the antileishmanial immune response. Infect Immun. 1994;62:3655–3662. doi: 10.1128/iai.62.9.3655-3662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen E A, Neva F A, Barral A, Correa-Coronas R, Bogaert-Diaz H, Martinez D, Ward F E. Monocyte suppression of antigen-specific lymphocyte responses in diffuse cutaneous leishmania patients from the Dominican republic. J Immunol. 1984;132:2603–2606. [PubMed] [Google Scholar]
- 16.Pharoah P D P, Ponnighaus J M, Chavula D, Lucas S B. Two cases of cutaneous leishmaniasis in Malawi. Trans R Soc Trop Med Hyg. 1993;87:668–670. doi: 10.1016/0035-9203(93)90282-u. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg Y J, Anderson A O, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking? Immunol Today. 1998;19:10–17. doi: 10.1016/s0167-5699(97)01183-3. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez M A, Cáceres-Dittmar G, Oriol O, Mosca W, Kraal G, Tapia F J. Epidermal Langerhans cells and dendritic epidermal T cells in murine cutaneous leishmaniasis. Immunocytochemical study. Acta Microscopica. 1993;2:180–187. [Google Scholar]
- 19.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine models of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 20.Soong L, Chang C-H, Sun J, Longley B J, Ruddle N H, Flavell R A, McMahon-Pratt D. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J Immunol. 1997;158:5374–5383. [PubMed] [Google Scholar]
- 21.Terabe M, Kuramochi T, Hatabu T, Ito M, Ueyama Y, Katakura K, Kawazu S, Onodera T, Matsumoto Y. Non-ulcerative cutaneous lesion in immunodeficient mice with Leishmania amazonensis infection. Parasitol Inter. 1998;48:47–53. doi: 10.1016/s1383-5769(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 22.Terabe M, Hatabu T, Takahashi H, Ito M, Onodera T, Matsumoto Y. Leishmania amazonensis infection in nude mice. Exp Anim. 1999;48:119–123. doi: 10.1538/expanim.48.119. [DOI] [PubMed] [Google Scholar]
- 23.Varkila K, Chatelain R, Leal L M, Coffman R L. Reconstitution of C.B-17 scid mice with BALB/c T cells initiates a T helper type 1 response and renders them capable of healing Leishmania major infection. Eur J Immunol. 1993;23:262–268. doi: 10.1002/eji.1830230141. [DOI] [PubMed] [Google Scholar]