Abstract
Sickle cell disease (SCD) is a common genetic blood disorder associated with acute and chronic pain, progressive multi-organ damage and early mortality. Recent advances in technologies to manipulate the human genome, a century of research and the development of techniques enabling the isolation, efficient genetic modification, and re-implantation of autologous patient hematopoietic stem cells (HSCs), mean that curing most patients with SCD could soon be a reality in wealthy countries. In parallel, ongoing research is pursuing more facile treatments, such as in vivo-delivered genetic therapies and new drugs that can eventually be administered in low- and middle-income countries where most SCD patients reside.
The lifelong burden of inherited hemoglobin disorders
Hemoglobinopathies are autosomal recessive disorders caused by mutations that impair hemoglobin function or production. Sickle cell disease (SCD) [1] and β-thalassemia [2], are common β-hemoglobinopathies affecting the HBB gene, which encodes the β-globin subunit of adult hemoglobin (HbA, α2β2). Hundreds of different mutations cause β-thalassemia, which is associated with impaired production of β-globin. Sickle cell disease (SCD) is associated with only a few mutations, the major one being p.Glu6Val, which creates an abnormal protein with pathological characteristics. Hemoglobinopathy mutations are common in tropical regions where malaria is endemic, including parts of Africa where sickle cell disease (SCD) is most prevalent. In 1949 Haldane explained this distribution by proposing that heterozygosity for thalassemia mutations alters the physiology of red blood cells (RBCs) to confer resistance to malaria infection. [3,4]. Soon afterward, Allison extended this hypothesis to include SCD [5]. β-hemoglobinopathy carrier states are now recognized as one of the most prominent examples of genetic selection in humans [5–7].
While the SCD carrier state is largely asymptomatic, homozygosity typically causes recurrent acute pain episodes, chronic pain, progressive damage to key organs including kidney, lungs, heart and brain, and reduces life expectancy [1]. It is estimated that more than 350,000 children with SCD are born each year world-wide, with the highest incidence in equatorial Africa [8,9]. This review focuses on SCD, although the key therapeutic concepts also apply to β-thalassemia. That is, both diseases can be alleviated or cured by correction of the HBB mutation, replacement of the defective HBB gene with a functional one, or reactivation of fetal hemoglobin (HbF, α2γ2) in post-natal RBCs.
100 years of globin research
In 1911 James Herrick, a Professor of Medicine in Chicago, identified sickle-shaped red blood cells in a blood smear from a student of Caribbean ancestry [10,11]. In 1949, SCD was recognized as an autosomal recessive Mendelian disorder by Neel and Beet [12,13]. In the same year, Linus Pauling dubbed SCD a ‘molecular disease’ after noting that globin protein from patient RBCs exhibited altered electrophoretic mobility [14]. Focussed research by Vernon Ingram over the next ten years identified a Glutamic acid to Valine substitution at amino acid position 6 of mature β-globin protein, first reported in 1956 [15]. We now know that the specific mutation converts the Glutamic acid codon GAG to a Valine codon, GTG [16]. This is referred to as either p.Glu6Val or p.Glu7Val with the latter accounting for the amino-terminal methionine that is cleaved from the nascent protein. The 3-dimensional structure of hemoglobin was deduced using X-ray crystallography by Max Perutz and John Kendrew in 1960 [17,18]. The mutation reduces the charge in a key area of the β-globin chain, causing the HbS tetramer (α2βS2) to form stiff, rigid polymers at low oxygen concentration [19,20]. The polymerization of HbS induces the formation of fragile, sticky, sickle-shaped RBCs, triggering a complex pathophysiology leading to the clinical manifestations of SCD [1,8].
First generation therapies
In low-income regions where SCD is most prevalent, mainly Africa, at least half of SCD patients die before age 5 [21–25]. Medical advances, including newborn screening, immunizations, antibiotics, and RBC transfusions with iron chelation, have greatly extended the lifespan of children with SCD in high-income countries [26]. However, most patients still suffer from significant symptoms and die prematurely, typically in their mid-40s [27]. The first effective drug for SCD, hydroxyurea, was shown in 1995 to reduce the incidence of painful vaso-occlusive crises by 50% [28]. We now know that hydroxyurea also reduces the frequency of SCD-related hospitalizations, organ damage and death, in part by inducing HbF and reducing neutrophil counts [29–33]. However, hydroxyurea does not eliminate entirely the clinical manifestations of SCD in any patient and responses vary between patients [33–35].
Allogeneic hematopoietic stem cell transplantation (HSCT) from donors with normal HBB alleles or SCD trait is the only approved cure for SCD, with disease-free survival rates of 90–95% in patients who receive HSCs from HLA-matched sibling donors [36–38]. As such donors are available for only 20% of SCD patients, optimizing the safety and efficacy of alternative donor sources is a major research goal. Complications of allogeneic HSCT include graft-versus-host disease and graft rejection, which can occur even with HLA-matched donors. All current HSCT protocols require treating the patient with one or more myelotoxic agents to clear the bone marrow niche for occupancy by donor HSCs. Complications associated with this “bone marrow conditioning” include bleeding, organ damage and potentially lethal infections [39].
Improved understanding of globin gene regulation fuels new therapies
Red blood cells carry out the specialized job of transporting oxygen from the lungs to peripheral tissues. The major oxygen carrier is hemoglobin, a tetramer composed of α-like and β-like subunits that make up more than 95% of soluble RBC protein [18]. Human chromosome 11 harbors multiple tandemly arrayed, developmentally regulated β-like globin genes that generate different forms of hemoglobin across ontogeny. During late gestation, γ-globin (encoded by HBG1 and HBG2) is predominantly expressed, giving rise to HbF. Around birth, γ-globin expression switches to β-globin (HBB), causing a shift from HbF to HbA, or HbS (α2βS2) in the case of SCD [40–42]. The perinatal γ-to-β-globin switch is partially inhibited in hereditary persistence of fetal hemoglobin (HPFH) a benign genetic condition in which postnatal RBCs express HbF at levels >30% [43]. It has been known for more than 40 years that individuals who co-inherit HPFH and SCD exhibit few or no symptoms of the latter [43–46]. This can occur in individuals who are compound heterozygotes for “deletional” HPFH, which is caused by kb-scale deletions that eliminate the HBB gene, combined with a single HBBS allele on the other chromosome. Alternatively, symptoms may be alleviated in individuals with homozygous HBBS who harbor “non-deletional” HPFH caused by point mutations or small deletions in the γ-globin gene promoter. Thus, researchers across the world have sought to understand the mechanisms that control globin gene expression in order to manipulate this process for treating SCD and β-thalassemia. Hard-won results of these studies, along with advances in modern genetics, have produced general insights into the biology of gene expression and elucidated new therapeutic strategies [47–52].
In 2007–2008, genome wide association studies showed that common variants in the BCL11A gene are associated with post-natal γ-globin de-repression [53–55]. The BCL11A gene encodes a repressor protein that accounts for approximately 50% of postnatal gene silencing [56–58]. In 2016, a second repressor, ZBTB7A/LRF, was identified [59]. Around birth, BCL11A and ZBTB7A/LRF bind to different regions of the γ-globin promoter and directly silence transcription in an additive fashion. Remarkably, several HPFH variants induce HbF by disrupting BCL11A or ZBTB7A/LRF binding motifs in the γ-globin promoter [60]. One can imagine the γ-globin gene as a cart parked on a slope with just the handbrake and a brick holding it in place. Removing either repressor or its binding site in the promoter is enough to start the cart rolling. By the same analogy, other HPFH variants that create new binding sites for erythroid transcriptional activators, such as GATA1, KLF1 or TAL1, can nudge the cart and get expression of γ-globin going [60].
New tools for genetic manipulation advance new, potentially curative therapies
The development of new tools for manipulating genetic material has been pivotal to enabling new therapeutic strategies. All of these are based on autologous HSCT, which includes isolation of patient HSCs, ex vivo genetic manipulation, followed by reintroduction after administrating bone marrow conditioning to facilitate engraftment of the modified cells. The field is advancing rapidly as the tools for genetic manipulation become more efficient and precise. Figure 1 summarizes the different genome modification strategies to treat SCD. These strategies are discussed further below.
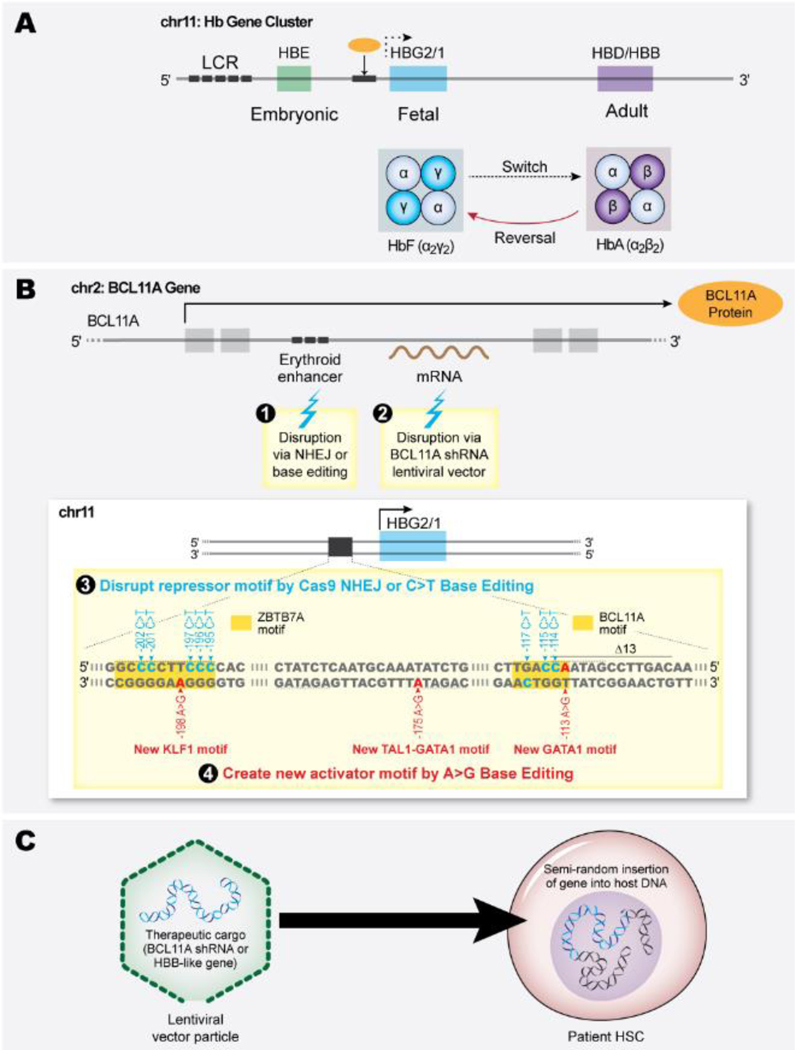
Figure 1:
Therapeutic genetic manipulation of sickle cell disease (SCD) patient hematopoietic stem cells (HSCs). (A) The developmentally regulated β-like globin gene cluster. Two fetal γ-globin genes (HBG2 and HBG1 shown here as a single HBG2/1 gene) are expressed during late gestation, resulting in the production of fetal hemoglobin (HbF, α2γ2) in red blood cells (RBCs). Around birth, γ-globin expression declines and is replaced by adult globins, predominantly β-globin (HBB) but also small amounts of δ-globin HBD shown here as a single HBD/B gene), resulting in a shift from HbF to HbA (α2β2) (and small amounts of HbA2 (α2δ2) in healthy individuals, or to HbS (α2βS2) in the case of SCD. The switch from HbF to HbA is highly regulated by BCL11A, a transcription repressor that binds to the HBG2/1 promoters (orange circle represents BCL11A protein, black box underneath represents the HBG2/1 promoters where it binds). Inhibiting the γ- to β-globin switch can alleviate β-hemoglobinopathies. (B) Induction of HbF by interfering with the expression or binding of the transcriptional repressors BCL11A or ZBTB7A to the HBG2/1 promoters (their binding motifs are highlighted in orange in panel 3). Panel 3 shows the γ-globin gene (HBG1 and HBG2) promoter. Around birth, BCL11A expression increases, and the protein binds a cognate motif (TGACC) to inhibit γ-globin transcription. Strategies for manipulation of autologous SCD patient HSCs include disruption of an erythroid-specific BCL11A gene enhancer via genome-editing nuclease–mediated NHEJ or base editing (1), transduction with a lentiviral vector (LV) that drives erythroid-specific expression of a BCL11A shRNA (2), disruption of a BCL11A/ZBTB7A binding motif in the HBG2/1 promoters via NHEJ or C>T base editors (3). Other genetic strategies for HbF induction use base editors to generate naturally occurring hereditary persistence of fetal hemoglobin variants that create new binding motifs for transcriptional activators KLF1 (RCCNCNCCCN), TAL1-GATA1 (CAGNTGNNNNNNNNNWGATAR) or GATA1 (WGATAR) (4). (C) Lentiviral vector gene therapy. SCD HSCs are transduced with lentiviral vector particles encoding erythroid-specific expression cassettes for a β-like globin gene or BCL11A shRNA. The LV integrates semi-randomly into the host HSC genome and is expressed in erythroid progeny. The β-like globin binds endogenous α-globin to generate functional HbA; the shRNA inhibits BCL11A expression to induce γ-globin transcription and raise HbF levels.
Manipulation of gene expression using lentiviral vectors.
Early approaches to genetic manipulation used information gleaned through studies of the β-globin promoter and the large multi-component enhancer termed Locus Control Region (LCR) to design lentiviral vectors capable of driving high-level, sustained, RBC-specific expression of β-globin or a related protein [61,62]. Patient HSCs are transduced ex vivo with lentiviral vector particles encoding a β-like globin gene expression cassette [63,64]. Iterative clinical trials combined with many technical improvements to enhance the collection of HSCs and their transduction have optimized this approach over the past decade. A recent publication describes these improvements and shows promising early results in the largest clinical study to date [64].
With the discovery of BCL11A and ZBTB7A/LRF, researchers rapidly turned their attention to disabling either repressor as a means of re-awakening fetal globin gene expression. BCL11A was the favoured target as it does not appear to regulate other essential red cell genes [57,65–67]. In contrast, disruption of ZBTB7A/LRF impairs erythroid development by interfering with the expression of cell survival genes [68]. One approach has been to combine lentiviral vectors with RNA interference technology using short hairpin RNAs (shRNAs) that anneal to BCL11A mRNA to stimulate its degradation and impair its translation [65,69,70]. Knowledge from previous efforts in globin gene therapy allowed the rapid design and delivery of lentiviral vectors that express anti-BCL11A shRNA specifically in RBC precursors. This strategy led to a reduction in BCL11A expression and induction of RBC HbF, with early clinical benefits [71].
First generation genome editing
Genome editing employs programmable nucleases to introduce targeted, sequence-specific double stranded DNA breaks that are subsequently repaired in cells by one of two different mechanisms [72,73]. Non-homologous end joining (NHEJ), which is most frequently employed by HSCs, introduces small insertion/deletion mutations (indels) that can inactivate genes or genetic regulatory elements. Alternatively, DSBs can be resolved by homology directed repair (HDR) through recombination with a suitable DNA template that is cointroduced with the genome editing nuclease. This approach can incorporate precise nucleotide changes. The most versatile and widely used system for genome editing is CRISPR/Cas9, which is programmed by a single guide RNA (sgRNA) with sequence complementarity to the DNA target site.
The original genome wide association study and careful follow-up studies identified a BCL11A enhancer element that is required for full expression in the RBC lineage, but not in other cells where BCL11A has essential roles [74,75]. Introduction of ribonucleoprotein (RNP) consisting of Cas9 nuclease complexed to a sgRNA targeting the BCL11A erythroid enhancer into HSCs caused high-frequency indels and induction of HbF in RBC progeny [74,76,77]. A clinical trial using this strategy has demonstrated efficient enhancer impairment, accompanied by the expected reduction in BCL11A expression, elevation of RBC HbF, and amelioration of SCD symptoms [78].
A potentially more focused therapeutic approach is to disrupt repressor binding sites in the γ-globin promoter, while leaving the repressors themselves, and any other genes they might regulate, unaffected [79–85]. In the γ-globin promoter, BCL11A binds the DNA motif TGACC at around position −114, while ZBTB7A/LRF binds the motif GCCCCTTCCC at position −200 [60,66,85]. Equipping Cas9 genome editors with specific sgRNAs can disrupt these elements, prevent repressor binding, and alleviate γ-globin repression to induce HbF. This approach has been demonstrated to efficiently modify primary human HSCs and is now entering clinical trials.
Efforts are also underway to convert the mutant SCD codon (GAG, Glutamic acid) to normal (GTG, Valine) via Cas9-mediated HDR. Converting sickle HBB to normal HBB is the most desirable curative approach [86–91]. However, several problems must be overcome. First, co-delivery of the HDR template along with Cas9 and targeting sgRNA is technically challenging and potentially toxic to HSC. Second, HDR occurs at substantially lower frequency than NHEJ in HSCs; in the range of 10–20%, which may be limiting for therapeutic efficacy. Finally, efforts to repair the SCD codon by HDR are complicated by competing NHEJ, which creates indels that disrupt the HBB coding region, thereby creating β-thalassemia-like mutations. Nonetheless, preclinical studies have refined this procedure for genetic correction of SCD and clinical trials are now underway or imminent.
Base editors
Base editors consist of a catalytically impaired version of Cas9 that creates single stranded DNA breaks (“Cas9 nickase”) fused to engineered deaminase domains capable of installing precise sgRNA-directed A-to-G or C-to-T transitions via DSB-independent mechanisms [92–94]. Both adenine and cytosine base editors have been used to induce RBC HbF by creating point mutations in the γ-globin (HBG2/1) promoters that eliminate BCL11A or ZBTB7A/LRF repressor binding, or by introducing HPFH variants that create new binding sites for transcriptional activators, such as TAL1, KLF1 or GATA1 [95–98]. Of note, the latter modifications require precise base pair substitutions that cannot be achieved via Cas9induced NHEJ. In contrast to genome editing nucleases that create unpredictable variable sized indels, base editors are more precise in their action, which is likely to induce HbF more consistently across RBC populations.
No current base editor exists for generating the T-to-A transversion required to convert the SCD mutant codon from GTG to normal GAG. However, adenosine base editors have been designed to convert the SCD codon to GCG (Ala), which generates Hemoglobin Makassar, a naturally occurring non-sickling variant [99,100]. High-frequency conversion of the HbS mutation to Hb Makassar has been demonstrated in HSCs from SCD patients and a mouse SCD model with marked reduction of RBC sickling and associated pathologies, suggesting that this approach could be an effective therapy.
A newer method of genetic modification termed prime editing uses an RNA template and reverse transcriptase to guide the repair of a Cas9 nickase-induced single stranded DNA break [92,100]. Prime editing is extremely versatile with the capacity to introduce any chosen modification at virtually any target site in the genome. Correction of the SCD codon by prime editing has been achieved in heterologous cell lines [101]; current research is focused on optimizing this procedure in HSCs.
Genotoxicities associated with genetic modifications
All strategies for genetic modification carry potential risks. Retroviral vectors insert semi-randomly into the genome and can potentially activate proto-oncogenes or inactivate tumor suppressors [102,103]. In early gene therapy trials for immunodeficiencies, gamma-retroviral vectors derived from Moloney Murine Leukemia Virus caused leukemia in some individuals by activating the expression of a cellular proto-oncogene near the vector integration site [62,104]. This severe adverse effect led to the development of safer lentiviral vectors, which are derived from the HIV-1 retrovirus. Lentiviral vectors currently in use for hemoglobinopathy gene therapy have been extensively modified and tested to minimize insertional oncogene activation, although this remains a theoretical possibility. Among more than 100 SCD and β-thalassemia patients who have received β-like globin replacement gene therapy with lentiviral vectors, one developed a dominant HSC clone linked to insertional activation of HMG2A, a transcription factor associated with solid tumor formation in humans and HSC expansion in non-human primates [105–107]. The patient remained stable over 15 months and has not been reported to develop leukemia or persistent clonal haematopoiesis.
Thus far, no β-hemoglobinopathy patients treated with lentiviral vector gene therapy have developed leukemia caused by insertional activation of proto-oncogenes. However, a recent study of 44 individuals with SCD who were treated with β-globin encoding lentiviral vector, 2 developed acute myeloid leukemia (AML) 3- and 5.5-years post-therapy [64]. In one case, the AML contained no vector DNA, and the malignancy was attributed to busulfan conditioning [108]. In the second individual, AML blasts had vector insertion in VAMP4, which is not a known oncogene. Extensive analysis indicated that insertional mutagenesis was not the cause for leukemia, given the integration site, the low level of transgene expression in blast cells, and the lack of effect on expression of surrounding genes [109]. Thus, the cause for these 2 cases of gene therapy-associated AML is unknown.
Patients with SCD may be particularly susceptible to developing AML after allogeneic or autologous HSCT. Of 76 adult patients who received allogeneic HSCT for SCD, 3 who received low-intensity non-myeloablative conditioning developed graft failure, followed by AML arising from autologous cells [110]. Analysis of two cases revealed TP53 loss-offunction mutations that were present at low level in hematopoietic cells before HSCT and in the leukemic blasts that developed subsequently. Somatic mutations in TP53 and other pre-cancerous genes in HSCs are associated with clonal haematopoiesis of indeterminant potential (CHIP) which occurs in older non-SCD individuals, usually after age 50 years [111,112]. Compared to the general population, individuals with SCD have an increased rate of AML, although the absolute risk remains low [113,114]. Additionally, hematopoietic stresses associated with SCD may increase the incidence of CHIP at earlier ages [115,116]. Based on these findings, it is hypothesized that allogeneic HSCT or autologous gene therapy for SCD promotes the expansion and malignant transformation of HSCs harbouring CHIP mutations [117,118]. One way to test this hypothesis will be to screen SCD patient hematopoietic cells for CHIP mutations serially, before and after allogeneic HSCT or autologous HSC gene therapy.
Genome editing to modify somatic DNA is associated with several potential genotoxicities [119–123]. Genome editing nucleases, including Cas9, can create unintended double strand DNA breaks (DSB) followed by indel formation at off-target sites, usually in genomic regions with partial homology to the sgRNA. Additionally, Cas9 DSBs at on- or off-target sites can cause chromosomal rearrangements, aneuploidy or chromothripsis with TP53 activation and the potential for malignant transformation [124–130]. Off-target indels are likely to be clinically silent, while chromosome-level abnormalities are likely to eliminate the affected cell. However, inadvertent oncogene activation or tumor suppression gene inactivation resulting in malignant transformation is a theoretical possibility.
Base editors can reduce some of the problems associated with Cas9 nuclease by acting through mechanisms that are DSB-independent. However, base editors can generate rare DSBs, albeit at lower rates than conventional Cas9 [92–94]. Moreover, base editors can create off-target mutations by modifying sites with partial homology to the targeting sgRNA or by modifying “bystander” nucleotides that are near the target. In addition, the deaminase module associated with base editors can generate unpredictable sgRNA-independent “spurious” mutations in DNA or RNA [131,132].
Current research is focused on developing sensitive assays to detect unintended genome editing-induced mutations and creating modified versions of Cas9 and base editors to enhance their activity and specificity at sgRNA-directed target sites [119–121, 123, 133–140]. While all genome editing tools can produce unintended modifications, the biological consequences are largely unknown and must be examined carefully in preclinical studies and ultimately in clinical trials.
How much is enough?
One important question regarding genetic induction of HbF or correction of the SCD mutation is whether the desired DNA modification can be achieved in a sufficiently high proportion of HSCs to prevent sickling of most or all RBCs for optimal therapeutic efficacy. Since non-sickling RBCs have a substantial survival advantage over ones that sickle, genetic correction of all patient HSCs may not be necessary. Supporting this principle, allogeneic HSCT studies for SCD using normal donors or SCD heterozygotes have shown that donor HSC chimerism as low as 20% can result in 100% donor-derived RBCs with substantial clinical benefits [141–143]. Therefore, heterozygous genetic correction of the SCD codon in 20% of autologous HSC may be sufficient for clinical improvement or even cure. Requirements for therapeutic induction of HbF are more complex since one must consider the total level of HbF, its distribution in the RBC population and the fraction of modified HSCs [45,46,52]. Based on clinical observations of individuals with HPFH, >30% HbF expressed homogeneously in all RBCs (i.e., pancellularly) seems to be a reasonable therapeutic benchmark. Recently released data from new clinical trials provides an initial basis for comparison [71,78]. However, long-term clinical and laboratory follow-up are essential to evaluate how well genetic therapies ameliorate progressive multi-organ damage associated with SCD.
Focussing on every step along the therapeutic pathway
As of April 2022, at least 11 different gene therapy or gene editing clinical trials are currently running based on clinicaltrials.gov (summarized in Table). Most trials utilize lentiviral vectors or Cas9 nuclease. One trial is using zinc finger nuclease (ZFN) [144,145] and another is using the Cas9-like nuclease Cpf1 [146]. Clinical trials utilizing base editors to induce HbF or convert the SCD codon from Val to Ala to generate Hb Makassar are planned to open in 2022.
Table 1:
Gene therapy and genome editing clinical trials for SCD
| Strategy | Modality | Sponsoring Agent | Clinical trial ID Status | Estimated participants | Results |
|---|---|---|---|---|---|
| β-Like globin gene replacement | LentiViral Vector (LV) | ||||
| GLOBE1 anti-sickling β-globin (βAS3) LV | Assistance Publique–Hopitaux de Paris | NCT03964792 Phase I, II | 10 | No results posted | |
| Lenti/G-bAS3-FB anti-sickling β-globin (βAS3) LV | University of California, Los Angeles | NCT02247843 Phase I, II | 6 | No results posted | |
| LentiGlobin BB305 anti-sickling β-globin (βA-T87Q) LV | Bluebird bio | NCT02140554 Phase I, II | 50 | 25 SCD (group C) participants followed for 3–25 months. Most had near-pancellular expression of HbAT87Q ≥6 months after therapy with 99.5% mean reduction in the annualized VOC+ACS rate overall | |
| LentiGlobin BB305 anti-sickling β-globin (βAS3) LV | Bluebird bio | NCT04293185 Phase III | 35 | No results posted | |
| ARU-1801 γ-globin G16D LV) | Cincinnati Children’s Hospital Medical Center | NCT02186418 Phase I, II | 10 | 3 SCD patients, follow-up 9–30 months, with sustainable expression of HbF and clinical improvement | |
| BCH-BB694 anti-BCL11A shRNA LV | Boston Children’s Hospital | NCT03282656 Phase I, II | 10 | 6 SCD participants infused, follow-up 7–29. months. No patient has had a VOC, ACS, or stroke since the treatment. | |
| Nuclease/Target | |||||
| HbF Induction | Cas9 disruption of BCL11A erythroid enhancer via NHEJ | CRISPR Therapeutics; Vertex Pharmaceuticals Incorporated | NCT03745287 Phase I, II, III | 45 | 2 SCD participants with follow-up of 3 and 12 months have had no SCD-related VOCs since infusion of gene-modified cells; 5 β-thalassemia participants with follow-up of 3–15 months are transfusion independent. |
| BCL11A (BIVV003)-Targeted zinc finger disruption of BCL11A erythroid enhancer via NHEJ (PRECIZN-1) | Bioverativ, a Sanofi company | NCT03653247 Phase I, II | 8 | 4 of 4 treated participants showed higher Hb levels and elevated HbF expression with clinical improvement over 2 years follow up. 1 serious VOC in 1 patient approximately 9 months post therapy | |
| OTQ923/HIX763 Cas9 disruption of BCL11A erythroid enhancer via NHEJ or Cas9 disruption of BCL11A binding motif in γ-globin promoter via NHEJ | Novartis/Intellia | NCT04443907 Phase I, II | 30 | No results posted | |
| EDIT-301 Cpf1 disruption of BCL11A binding motif in γ-globin promoter via NHEJ | Editas Medicine, Inc. | NCT04853576 Phase I, II | 40 | No results posted | |
| HbS mutation repair | GPH101 Hb β6: GTG->GAG via Cas9 HDR | Graphite Bio | NCT04819841 Phase I, II | 15 | No results posted |
| CRISPR_SCD001 Hb β6: GTG->GAG via Cas9 HDR | UCSF Benioff Children’s Hospital Oakland | NCT04774536 Phase I, II | 9 | No resuits posted | |
All clinical trials for SCD listed on ClinicalTrials.gov as of April 2022 are shown. Some trials also include patients with β-thalassemia. ACS, acute chest syndrome; NHEJ, non-homologous end joining; HDR: homology directed repair; LV: Lentiviral vector; VOC, vaso-occlusive crisis; shRNA: short hairpin RNA, CRISPR: Clustered regularly insterspaced short palindromic repeats, HbF: fetal hemoglobin.
While scientific attention has focussed on the gene modification aspects of HSCT therapy for SCD via lentiviral vector transduction or genome editing, every step in the pathway must be optimised to achieve overall success (Figure 2). Essential components of autologous HSCT therapy for SCD include:
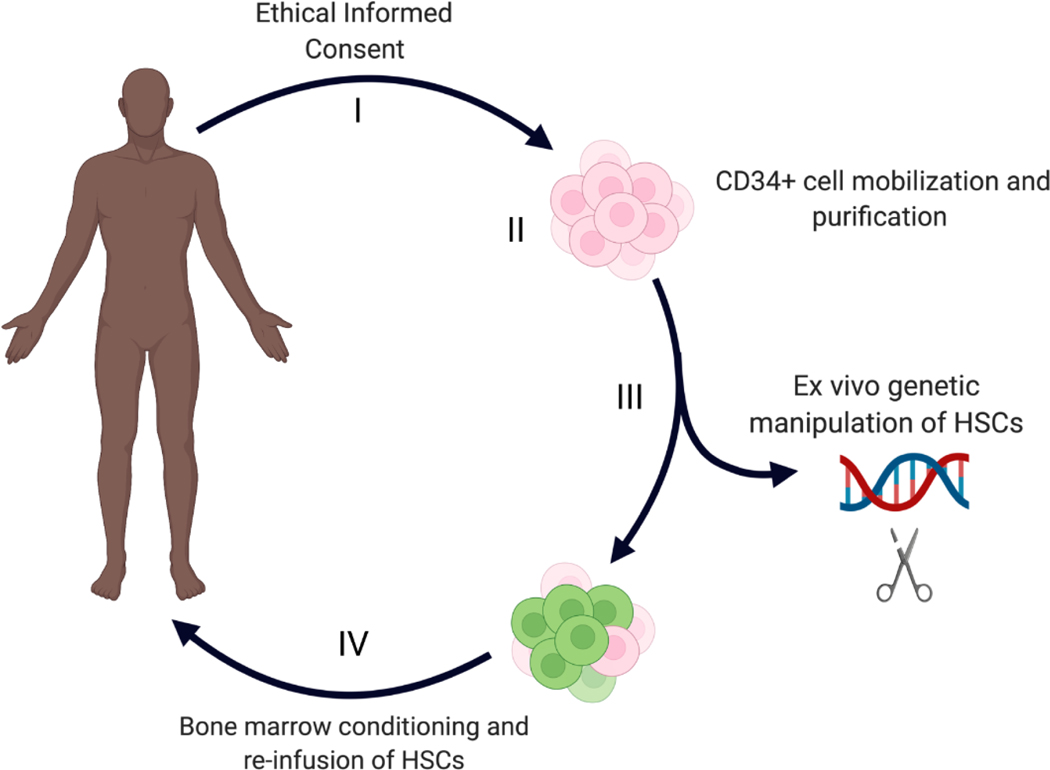
Figure 2:
Four critical steps in genetic therapies to treat SCD. (I) The informed consent process, including education of patients and families followed by written consent; (II) mobilization, apheresis collection and purifications of CD34+ hematopoietic stem and progenitor cells (HSPCs); (III) Ex vivo genetic manipulation of HSCs to express functional β-globin or induce HbF expression; (IV) Administration of bone marrow conditioning followed by infusion of the modified HSPCs.
Informed consent. Like most individuals with serious diseases, many SCD patients do not fully understand gene therapy protocols in sufficient detail to provide informed consent [147]. This is complicated further by a general lack of trust among SCD patients and current health care systems and the failure of SCD providers to establish optimal communication with their patients [148]. Hence, research efforts are focused on identifying and fulfilling the educational needs of individuals with SCD and their families who are considering curative therapies.
Isolating SCD patient HSCs for in vitro genetic manipulation. For most clinical applications of HSCT, HSCs are mobilized into the circulation by treatment with granulocyte colony stimulating factor (G-CSF), followed by apheresis collection. However, the administration of G-CSF to individuals with SCD has been associated with severe complications and is therefore, contraindicated [149]. Therefore, SCD patient HSCs are obtained by mobilizing them with the CXCR4 chemokine receptor antagonist plerixafor, followed by apheresis collection and immunomagnetic bead enrichment for the HSC surface antigen CD34 [150–154]. Autologous HSCT protocols require the infusion of at least 2 × 106 modified CD34+ cells per kg, although at least 5-fold more cells are required to account for manufacturing losses during genetic manipulation and for storage of unmanipulated back-up HSCs [78,151,154]. This usually necessitates multiple rounds of HSC mobilization and collection. Therefore, efforts are underway to develop improved HSC mobilization agents. For example, GRO-beta is a chemokine (CXCR2 agonist) given alone or in combination with plerixafor and appears to mobilize long-term bone marrow-repopulating HSCs more effectively than GCSF or plerixafor alone [155,156].
Bone marrow conditioning. Current autologous HSCT protocols employ myelotoxic agents, mainly busulfan, to clear the hematopoietic niche for engraftment by genetically manipulated HSCs [37,39,64,71,78,157]. This treatment can produce severe toxicities including organ damage, immunodeficiency, infertility, and death [39]. Individuals with baseline organ damage due to the chronic effects of SCD and/or transfusion-related iron overload are more susceptible to busulfan toxicities, and therefore ineligible for many clinical studies. Current research efforts are investigating new bone marrow conditioning agents with reduced toxicity, mainly by employing antibodies that target resident HSCs [157–166].
Democratizing genetic therapies for SCD
We have come a long way toward generating a one-time ‘genetic surgery’ treatment of HSCs from patients with SCD. However, this therapy involves hospitalization, mobilization and harvesting of blood stem cells, their genetic modification at scale, cytotoxic bone marrow conditioning, then re-implantation and monitoring. This requires sophisticated health care systems that are not available in most countries where β-hemoglobinopathies are common. So, what is the fastest way to offer novel, more effective therapies equitably across the world? Possibilities to consider are “in vivo” gene therapy and improved, mechanism-based drug therapies.
In vivo gene therapy
“In vivo” gene therapy refers to a process by which gene modifying proteins or nucleic acids are delivered to HSCs after intravenous or intraosseous injection. This has been achieved successfully for some non-hematopoietic diseases. For example, viral vectors or lipid nanoparticles that deliver gene modifying proteins or nucleic acids specifically to hepatocytes can successfully treat haemophilia and other rare genetic disorders [104,160,167]. Subretinal injection of adeno-associated viral vector particles can treat genetic retinopathies [168,169]. In vivo genetic modification of HSCs may be more challenging, due to the rarity of this population and the lack of cell type-specific antigens expressed at high level. However, research is ongoing [170]. For example, promising results have been achieved in mice by using granulocyte colony stimulating factor to mobilize HSCs into the circulation, followed by intravenous injection of viral vector particles expressing Cas9 or base editors and sgRNA [97,171,172].
Mechanism-based drug therapies
Several recently approved drugs target various aspects of SCD pathophysiology by inhibiting HbS polymerization, RBC oxidant stress or neutrophil-mediated inflammation [32,173–176]. While these drugs represent substantial progress, they are expensive and only partially effective. A highly effective drug that can be manufactured inexpensively and distributed to millions of individuals with SCD across the world represents a therapeutic “holy grail”. Considering that SCD is essentially cured by co-inheritance of HPFH in many individuals [43–45], robust pharmacological induction of HbF is an attractive possibility. Now that BCL11A is established as a key γ-globin repressor in adult RBCs, studies to understand its physiological regulation may elucidate new drugs to inhibit its function [48]. For example, depletion of kinase HRI or its downstream effector, the transcriptional activator ATF4, have been shown to reduce BCL11A expression [177–179]. Therefore, pharmacological inhibitors of HRI kinase could potentially induce HbF in adult erythroid precursors. Transcriptional repression by BCL11A is mediated by its interaction with the NuRD co-repressor complex. Expression of the essential NuRD component CHD4 depends on the transcription factor ZNF410 [180,181]. Remarkably, CHD4 is the only gene regulated by ZNF410 in erythroid cells. Thus, inactivating ZNF410 could induce HbF. It may also be possible to induce HbF by using proteolysis targeting chimeric (PROTAC) technology to devise a bifunctional small molecule that links an erythroid-expressed E3 ubiquitin ligase to BCL11A or other negative regulators of γ-globin transcription, to stimulate their proteasomal degradation [182]. Notably, ZNF410 and HRI were identified by CRISPR/Cas9-based screening methods. Ongoing genetic screens using Cas9 and base editors are continuing to identify new proteins and regions of the genome that can be targeted for induction of HbF [183,184].
Concluding Remarks:
More than 60 years after SCD was recognized as “the first molecular disease”, we are now positioned to make impactful improvements in patient care, including curative therapies and substantially improved drugs. These new opportunities arose from decades of scientific studies to define the structure and regulation of the human β-like globin genes, along with the development of technologies to manipulate the genome therapeutically and to screen for new therapeutic targets. Promising new strategies include the development of new drugs to safely raise HbF to therapeutic levels in all RBCs and approaches to correct the SCD mutation or bypass its deleterious effects through genetic manipulation of autologous patient HSCs.
Rapid advancement has been achieved through collaborations between basic scientists, clinical researchers, caregivers, the biopharmaceutical industry and individuals with SCD. The long-term goal of SCD research is to restore normal life and longevity to all patients through novel genetic and pharmacological approaches. We are not there yet. Although numerous new drugs for SCD show signs of efficacy, no panaceas exist. And while numerous curative therapies based on allogeneic or autologous HSCT are demonstrated to be effective, none are without risk or universally available. Many questions remain (see Outstanding Questions box), and it is likely that the best therapeutic approaches for SCD are yet to be developed. However, the field is sparked by new discoveries, investigators and patients are highly motivated, and the path toward our destination is now in sight.
Outstanding Questions Box.
How can we best balance the potential risks and benefits of sickle cell disease gene therapy for individual patients?
How can we educate SCD patients and their family members to evaluate their personalized risks and benefits of gene therapy?
What are the most measurable and biologically relevant pre-clinical approaches to evaluate for low-level but potentially deleterious genotoxicities of genetic therapies?
Is it possible to develop more efffective SCD drugs that can be administered continuously and safely over the entire lifespan beginning in children?
Is it possible to develop safe and effective in-vivo hematopoietic stem cell genome editing approaches that can be delivered via intravenous or intra bone marrow injection?
Are individuals with SCD at increased risk for developing Clonal Hematopoiesis of Indeterminant Potential (CHIP) and does this increase the risk of leukemia following hematopoietic stem cell transplantation?
Highlights.
β-hemoglobinopathies result from homozygous or compound heterozygous β-globin HBB gene mutations that affect the function or production of the β-globin subunit of adult-type hemoglobin (α2β2).
Persistance of high-level γ-globin HBG gene expression after birth resulting in the production of fetal hemoglobin (α2γ2) can alleviate β-hemoglobinopathies.
β-hemoglobinopathies are among the world’s most prevalent disorders due to evolutionary selection for malaria resistance in heterozygous carriers.
Sickle cell disease (SCD), caused mainly by homozygosity for the HBB p.Glu6Val mutation, was the first genetic disorder characterised at a molecular level.
Now, more than 60 years later, numerous mechanism-based somatic gene therapies are being examined in clinical trials on small numbers of patients.
Ultimately the broad scientific knowledge gained by studying globin biology will inform the development of effective drugs that can be administered to many SCD individuals worldwide.
Acknowledgements
This work was supported by funding from the Australian National Health and Medical Research Council APP1164920 (M.C.), and by a US National Institutes of Health award P01 HL053749 (M.J.W.), the St. Jude Collaborative Research Consortium (M.J.W.), the Doris Duke Foundation (for aspects of this study that did not involve mice, M.J.W.), the American Lebanese Syrian Associated Charities (M.J.W.) and the Assisi Foundation of Memphis (M.J.W.). All figures were created with Biorender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kato GJ, et al. (2018) Sickle cell disease. Nat. Rev. Dis. Primers 4,18010. [DOI] [PubMed] [Google Scholar]
- 2.Taher AT, et al. (2021) β-Thalassemias. N. Engl. J. Med 384, 727–743. [DOI] [PubMed] [Google Scholar]
- 3.Haldane JBS (1949) The rate of mutations of human genes. Hereditas 35, 267–273. [Google Scholar]
- 4.Aidoo M. et al. (2002) Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 359, 1311–1312. [DOI] [PubMed] [Google Scholar]
- 5.Allison AC (1954) Protection afforded by sickle-cell trait against subtertian malareal infection. Br. Med. J 1(4857), 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onwubalili JK (1983) Sickle-cell anaemia: an explanation for the ancient myth of reincarnation in Nigeria. Lancet 2(8348), 503–505. [DOI] [PubMed] [Google Scholar]
- 7.Allison AC (1964) Polymorphism and natural selection in human populations. Cold Spring Harb. Symp. Quant. Biol 129,137–149. [DOI] [PubMed] [Google Scholar]
- 8.Piel FB et al. (2017) Sickle Cell Disease. N. Engl. J. Med 376, 1561–1573. [DOI] [PubMed] [Google Scholar]
- 9.Piel FB et al. (2013) Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Medicine 10(7):e1001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrick JB (1910) Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia Arch. Intern. Med. (Chic) 6, 517–521. [Google Scholar]
- 11.Savitt TL and Goldberg MF (1989) Herrick’s 1910 case report of sickle cell anemia. The rest of the story. JAMA 261, 266–271. [PubMed] [Google Scholar]
- 12.Neel JV (1949) The Inheritance of Sickle Cell Anemia. Science 110, 64–66. [DOI] [PubMed] [Google Scholar]
- 13.Beet EA (1947) The genetics of the sickle-cell trait in a Bantu tribe. Ann. Eugen. 14, 279–284. [DOI] [PubMed] [Google Scholar]
- 14.Pauling L. and Itano HA (1949) Sickle cell anemia a molecular disease. Science 110, 543–548. [DOI] [PubMed] [Google Scholar]
- 15.Ingram VM (1956) A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature.178, 792–794. [DOI] [PubMed] [Google Scholar]
- 16.Marotta CA et al. (1977) Human beta-globin messenger RNA. III. Nucleotide sequences derived from complementary DNA. J. Biol. Chem 252, 5040–5053. [PubMed] [Google Scholar]
- 17.Kendrew JC et al. (1960) Structure of myoglobin: A three-dimensional Fourier synthesis at 2 A. resolution. Nature 185, 422–427. [DOI] [PubMed] [Google Scholar]
- 18.Perutz MF (1960) Structure of hemoglobin. Brookhaven Symp. Biol 13, 165–83 [PubMed] [Google Scholar]
- 19.Eaton WA and Hofrichter J. (1990) Sickle cell hemoglobin polymerization. Adv. Protein Chem 40, 63–279. [DOI] [PubMed] [Google Scholar]
- 20.Eaton WA and Bunn HF (2017) Treating sickle cell disease by targeting HbS polymerization. Blood 129, 2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosse SD et al. (2011) Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am. J. Prev. Med 41(6 Suppl 4):S398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makani J. et al. (2007) Sickle cell disease in Africa: burden and research priorities. Ann. Trop. Med. Parasitol 101, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serjeant GR (2005) Mortality from sickle cell disease in Africa. BMJ 330, 432–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wonkam A. et al. (2021) Lancet Haematol. 8(10):e677–e678. doi: 10.1016/S2352-3026(21)00268-4 [DOI] [PubMed] [Google Scholar]
- 25.Williams TN Sickle Cell Disease in Sub-Saharan Africa. Hematol. Oncol. Clin. North Am 30, 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner K. et al. (2016) Survival in adults with sickle cell disease in a high-income setting. Blood 128, 1436–1438. [DOI] [PubMed] [Google Scholar]
- 27.Lanzkron S. et al. (2013) Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public Health Rep. 128, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charache S. et al. , (1995) Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N. Engl. J. Med 332, 1317–1322. [DOI] [PubMed] [Google Scholar]
- 29.Nevitt SJ et al. (2017) Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst. Rev 4:CD002202. doi: 10.1002/14651858.CD002202.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strouse JJ and Heeney MM, (2012) Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatr. Blood Cancer 59, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yawn BP, et al. (2014) Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 312, 1033–1048. [DOI] [PubMed] [Google Scholar]
- 32.Rai P. and Ataga KI (2020) Drug therapies for the management of sickle cell disease. F1000Res. doi: 10.12688/f1000research.22433.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charache S. et al. (1996) Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore). 75, 300–326. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers GP et al. (1990) Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N. Engl. J. Med 322, 1037–1045. [DOI] [PubMed] [Google Scholar]
- 35.Lanzkron S. et al. (2008) Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann. Intern. Med 148, 939–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de la Fuente J. et al. (2020)The role of haematopoietic stem cell transplantation for sickle cell disease in the era of targeted disease-modifying therapies and gene editing. Lancet Haematol. 7(12):e902–e911. doi: 10.1016/S2352-3026(20)30283-0 [DOI] [PubMed] [Google Scholar]
- 37.Eapen M. et al. (2019) Effect of donor type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: a retrospective multicentre, cohort study. Lancet Haematol. 6(11):e585–e596. doi: 10.1016/S2352-3026(19)30154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gluckman E. et al. (2017) Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood 129, 1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciurea SO and Andersson BS (2009) Busulfan in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant 15, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terrenato L. et al. (1981) The switch from haemoglobin F to A: the time course of qualitative and quantitative variations of haemoglobins after birth. Br. J. Haematol 47, 31–41. [DOI] [PubMed] [Google Scholar]
- 41.Wood WG et al. (1977) Developmental biology of human hemoglobins. Prog. Hematol 10, 43–90. [PubMed] [Google Scholar]
- 42.Alter BP and Nathan DG (1982) A cellular model for hemoglobin switching. Birth Defects Orig. Artic. Ser 18, 111–116. [PubMed] [Google Scholar]
- 43.Forget BG (1988) Molecular basis of hereditary persistence of fetal hemoglobin. Ann. N. Y. Acad. Sci 850, 38–44. [DOI] [PubMed] [Google Scholar]
- 44.Ngo DA et al. (2012) Fetal haemoglobin levels and haematological characteristics of compound heterozygotes for haemoglobin S and deletional hereditary persistence of fetal haemoglobin. Br. J. Haematol 156, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinberg MH (2020) Fetal hemoglobin in sickle hemoglobinopathies: high HbF genotypes and phenotypes. J. Clin. Med 9, 3782 doi: 10.3390/jcm9113782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinberg MH (2020) Fetal hemoglobin in sickle cell anemia. Blood 136, 2392–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papayannopoulou T. (2020) Control of fetal globin expression in man: new opportunities to challenge past discoveries. Exp. Hematol 92, 43–50. [DOI] [PubMed] [Google Scholar]
- 48.Vinjamur DS et al. (2018) Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br. J. Haematol 180, 630–643. [DOI] [PubMed] [Google Scholar]
- 49.Rosanwo TO and Bauer DE (2021) Editing outside the body: Ex vivo gene-modification for β-hemoglobinopathy cellular therapy. Mol. Ther 29, 3163–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DA and Esrick E. (2021) Investigational curative gene therapy approaches to sickle cell disease. Blood Adv. 5, 5452 doi: 10.1182/bloodadvances.2021005567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abraham AA and Tisdale JF (2021) Gene therapy for sickle cell disease: moving from the bench to the bedside. Blood 138, 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doerfler PA et al. (2021) Genetic therapies for the first molecular disease. J. of Clin. Invest 131(8)doi: 10.1172/jci146394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lettre G. et al. (2008) DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc. Natl Acad. Sci. U S A 105, 11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uda M. et al. (2008) Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc. Natl Acad. Sci. U S A 105, 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menzel S. et al. (2007) A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat. Genet 39, 1197–1199. [DOI] [PubMed] [Google Scholar]
- 56.Xu J. et al. (2010) Transcriptional silencing of {gamma}-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 24, 783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sankaran VG et al. (2008) Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 322, 1839–1842. [DOI] [PubMed] [Google Scholar]
- 58.Xu J. et al. (2013) Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc. Natl Acad. Sci. U S A 110, 6518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masuda T. et al. (2016) Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science 351, 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wienert B. et al. (2018) Wake-up sleepy gene: reactivating fetal globin for β-hemoglobinopathies. Trends Genet. 134, 927–940. [DOI] [PubMed] [Google Scholar]
- 61.Magrin E. et al. (2019) Lentiviral and genome-editing strategies for the treatment of β-hemoglobinopathies. Blood 134, 1203–1213. [DOI] [PubMed] [Google Scholar]
- 62.Naldini L. (2019) Genetic engineering of hematopoiesis: current stage of clinical translation and future perspectives. EMBO Mol. Med 11(3)doi: 10.15252/emmm.201809958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Locatelli F. et al. (2022) Betibeglogene autotemcel gene therapy for non-β0/β0 genotype β-thalassemia. N. Engl. J. Med 386, 415–427. [DOI] [PubMed] [Google Scholar]
- 64.Kanter J. et al. (2022) Biologic and clinical efficacy of lentiglobin for sickle cell disease. N. Engl. J. Med 386, 617–628. [DOI] [PubMed] [Google Scholar]
- 65.Brendel C. et al. (2016) Lineage-specific BCL11A knockdown circumvents toxicities and reverses sickle phenotype. J.Clin. Invest 126, 3868–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu N. et al. (2018) Direct promoter repression by BCL11A controls the fetal to adult hemoglobin switch. Cell 173, 430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Psatha N. et al. (2018) Disruption of the BCL11A erythroid enhancer reactivates fetal hemoglobin in erythroid cells of patients with β-thalassemia major. Mol. Ther. Methods Clin. Dev 10, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maeda T. et al. (2009) LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis. Dev. Cell 17, 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brendel C. et al. (2020) Preclinical evaluation of a novel lentiviral vector driving lineage-specific BCL11A knockdown for sickle cell gene therapy. Mol. Ther. Methods Clin. Dev 17, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guda S. et al. (2015) miRNA-embedded shRNAs for lineage-specific BCL11A knockdown and hemoglobin F induction. Mol. Ther 23, 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esrick EB et al. (2021) Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N. Engl. J. Med 384, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doudna JA (2020) The promise and challenge of therapeutic genome editing. Nature. 578, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doudna JA and Charpentier E. (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1258096. doi: 10.1126/science.1258096 [DOI] [PubMed] [Google Scholar]
- 74.Canver MC et al. (2015) BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 527, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauer DE et al. (2013) An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 342, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang KH et al. (2017) Long-term engraftment and fetal globin induction upon BCL11A gene editing in bone-marrow-derived CD34(+) hematopoietic stem and progenitor cells. Mol. Ther. Methods Clin. Dev 4, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y. et al. (2019) Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med 25, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frangoul H. et al. (2021) CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med 384, 252–260. [DOI] [PubMed] [Google Scholar]
- 79.Métais JY et al. (2019) Genome editing of HBG1 and HBG2 to induce fetal hemoglobin. Blood Adv. 3, 3379–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weber L. et al. (2020) Editing a γ-globin repressor binding site restores fetal hemoglobin synthesis and corrects the sickle cell disease phenotype. Sci. Adv 6(7)doi: 10.1126/sciadv.aay9392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Humbert O. et al. (2019) Therapeutically relevant engraftment of a CRISPR-Cas9-edited HSC-enriched population with HbF reactivation in nonhuman primates. Sci. Transl. Med 11(503)doi: 10.1126/scitranslmed.aaw3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Traxler EA et al. (2016) A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med 22, 987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye L. et al. (2016) Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc. Natl Acad. Sci. U S A 113, 10661–10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lux CT et al. ( 2019) TALEN-mediated gene editing of HBG in human hematopoietic stem cells leads to therapeutic fetal hemoglobin induction. Mol. Ther. Methods Clin. Dev 12, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martyn GE et al. (2018) Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat. Genet 50, 498–503. [DOI] [PubMed] [Google Scholar]
- 86.Dever DP et al. (2016) CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature 539, 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoban MD et al. (2015) Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood 125, 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park SH et al. (2019) Highly efficient editing of the beta-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res. 47, 7955–7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pickar-Oliver A. and Gersbach CA (2019) The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell. Biol 20, 490–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lattanzi A. et al. (2021) Development of beta-globin gene correction in human hematopoietic stem cells as a potential durable treatment for sickle cell disease. Sci. Transl. Med 13(598)doi: 10.1126/scitranslmed.abf2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin RM et al. (2019) Highly efficient and marker-free genome editing of human pluripotent stem cells by CRISPR-Cas9 RNP and AAV6 donor-mediated homologous recombination. Cell Stem Cell. 24, 821–828. [DOI] [PubMed] [Google Scholar]
- 92.Anzalone AV et al. (2020) Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol 38, 824–844. [DOI] [PubMed] [Google Scholar]
- 93.Gaudelli NM et al. (2017) Programmable base editing of A• T to G• C in genomic DNA without DNA cleavage. Nature 551, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Komor AC et al. (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L. et al. (2020) Reactivation of γ-globin expression through Cas9 or base editor to treat β-hemoglobinopathies. Cell Res. 30, 276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng J. et al. (2020) Therapeutic base editing of human hematopoietic stem cells. Nat. Med 26, 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li C. et al. (2021) In vivo HSPC gene therapy with base editors allows for efficient reactivation of fetal γ-globin in β-YAC mice. Blood Adv. 5, 1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ravi NS et al. (2022) Identification of novel HPFH-like mutations by CRISPR base editing that elevate the expression of fetal hemoglobin. Elife. doi: 10.7554/eLife.65421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chu SH et al. (2021) Rationally designed base editors for precise editing of the sickle cell disease mutation. CRISPR J. 4, 169–177. [DOI] [PubMed] [Google Scholar]
- 100.Newby GA et al. (2021) Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature 595, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anzalone AV et al. (2019) Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cesana D. et al. (2014) Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther 22, 774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baum C. et al. (2006) Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum. Gene Ther 17, 253–263. [DOI] [PubMed] [Google Scholar]
- 104.Kohn DB (2018) Gene therapy for blood diseases. Curr. Opin. Biotechnol 60, 39–45. [DOI] [PubMed] [Google Scholar]
- 105.Cavazzana-Calvo M. et al. (2010) Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 467, 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonner MA et al. (2021) 3’ UTR-truncated HMGA2 overexpression induces non-malignant in vivo expansion of hematopoietic stem cells in non-human primates. Mol. Ther. Methods Clin. Dev 21, 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mansoori B. et al. (2021) HMGA2 as a critical regulator in cancer development. Genes (Basel) 12(2)doi: 10.3390/genes12020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsieh MM et al. (2020) Myelodysplastic syndrome unrelated to lentiviral vector in a patient treated with gene therapy for sickle cell disease. Blood Adv. 4, 2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goyal S. et al. (2022) Acute myeloid leukemia case after gene therapy for sickle cell disease. N. Engl. J. Med 386, 138–147. [DOI] [PubMed] [Google Scholar]
- 110.Ghannam JY et al. (2020) Baseline TP53 mutations in adults with SCD developing myeloid malignancy following hematopoietic cell transplantation. Blood 135, 1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zink F. et al. (2017) Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130, 742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Acuna-Hidalgo R. et al. (2017) Ultra-sensitive sequencing identifies high prevalence of clonal hematopoiesis-associated mutations throughout adult life. Am. J. Hum. Genet 101, 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brunson A. et al. (2017) Increased risk of leukemia among sickle cell disease patients in California. Blood 130, 1597–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seminog OO et al. (2016) Risk of individual malignant neoplasms in patients with sickle cell disease: English national record linkage study. J. R. Soc. Med 109, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liggett LA et al. (2022) Clonal hematopoiesis in sickle cell disease. J. Clin. Invest 132(4) doi: 10.1172/JCI156060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pincez T. et al. (2021) Clonal hematopoiesis in sickle cell disease. Blood 138, 21482152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stonestrom AJ and Levine RL (2022) The hematopoietic saga of clonality in sickle cell disease. J. Clin. Invest 132(5)doi: 10.1172/JCI158251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DeBaun MR and Clayton EW (2017) Primum non nocere: the case against transplant for children with sickle cell anemia without progressive end-organ disease. Blood Adv. 1, 2568–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim D. et al. (2017) Genome-wide target specificities of CRISPR RNA-guided programmable deaminases. Nat. Biotechnol 35, 475–480. [DOI] [PubMed] [Google Scholar]
- 120.Kim D. et al. (2019). Evaluating and enhancing target specificity of gene-editing nucleases and deaminases. Annu. Rev. Biochem 88,191–220. [DOI] [PubMed] [Google Scholar]
- 121.Kim DY et al. (2020) Unbiased investigation of specificities of prime editing systems in human cells. Nucleic Acids Res. 48, 10576–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsai SQ and Joung JK (2016) Defining and improving the genome-wide specificities of CRISPR-Cas9 nucleases. Nat. Rev. Genet 17, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng Y. and Tsai SQ Illuminating the genome-wide activity of genome editors for safe and effective therapeutics. Genome Biol. 19(1):226. doi: 10.1186/s13059-018-1610-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leibowitz ML et al. (2021) Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet 53, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Enache OM et al. (2020) Cas9 activates the p53 pathway and selects for p53inactivating mutations. Nat. Genet 52, 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haapaniemi E. et al. (2018) CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med 24, 927–930. [DOI] [PubMed] [Google Scholar]
- 127.Ihry RJ et al. (2018) p53 inhibits CRISPR–Cas9 engineering in human pluripotent stem cells. Nat. Med 24, 939–946. [DOI] [PubMed] [Google Scholar]
- 128.Kosicki M. et al. (2018) Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotech 36, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Turchiano G. et al. (2021) Quantitative evaluation of chromosomal rearrangements in gene-edited human stem cells by CAST-Seq. Cell Stem Cell. 28, 1136–1147 e5. [DOI] [PubMed] [Google Scholar]
- 130.Boutin J. et al. (2021) CRISPR-Cas9 globin editing can induce megabase-scale copyneutral losses of heterozygosity in hematopoietic cells. Nat. Commun 12, 4922. doi: 10.1038/s41467-021-25190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grünewald J. et al. (2019) Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 569, 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou C. et al. (2019) Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 571, 275–278. [DOI] [PubMed] [Google Scholar]
- 133.Doman JL et al. (2020) Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat. Biotechnol 38, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jeong YK et al. (2020) Current status and challenges of DNA base editing tools. Mol. Ther 28, 1938–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim D. et al. (2019) Genome-wide target specificity of CRISPR RNA-guided adenine base editors. Nat. Biotechnol 37, 430–435. [DOI] [PubMed] [Google Scholar]
- 136.Yu Y. et al. (2020) Cytosine base editors with minimized unguided DNA and RNA off-target events and high on-target activity. Nat. Commun 11(1)doi: 10.1038/s41467-02015887-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zuo E. et al. (2020) A rationally engineered cytosine base editor retains high on-target activity while reducing both DNA and RNA off-target effects. Nat. Methods 17, 600604. [DOI] [PubMed] [Google Scholar]
- 138.Liang M. et al. (2020) AcrIIA5 suppresses base editors and reduces their off-target effects. Cells 9(8):1786. doi: 10.3390/cells9081786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coelho MA et al. (2020) CRISPR GUARD protects off-target sites from Cas9 nuclease activity using short guide RNAs. Nat. Commun 11(1):4132. doi: 10.1038/s41467-020-17952-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naeem M. et al. (2020) Latest developed strategies to minimize the off-target effects in CRISPR-Cas-mediated genome editing. Cells. 9(7):1608. doi: 10.3390/cells9071608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abraham A. et al. (2017) Unrelated umbilical cord blood transplantation for sickle cell disease following reduced-intensity conditioning: results of a phase I trial. Biol. Blood Marrow Transplant 23, 1587–1592. [DOI] [PubMed] [Google Scholar]
- 142.Fitzhugh CD (2017) At least 20% donor myeloid chimerism is necessary to reverse the sickle phenotype after allogeneic HSCT. Blood 130, 1946–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walters MC et al. (2001) Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol. Blood Marrow Transplant 7, 665–673. [DOI] [PubMed] [Google Scholar]
- 144.Hoban MD et al. (2015) Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood 125, 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carroll D. (2011) Genome engineering with zinc-finger nucleases. Genetics 188, 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li X. et al. (2018) Base editing with a Cpf1-cytidine deaminase fusion. Nat. Biotechnol 36, 324–327. [DOI] [PubMed] [Google Scholar]
- 147.Kraft SA et al. (2018) Beyond consent: building trusting relationships with diverse populations in precision medicine research. Am. J. Bioeth 18, 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rose Marie Martinez, Henrietta Awo Osei-Anto, Marie McCormick editors, Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. The National Academies Press; 2020:522. [PubMed] [Google Scholar]
- 149.Fitzhugh CD et al. (2009). Granulocyte colony-stimulating factor (G-CSF) administration in individuals with sickle cell disease: time for a moratorium? Cytotherapy 11, 464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boulad F. et al. (2018) Safety and efficacy of plerixafor dose escalation for the mobilization of CD34(+) hematopoietic progenitor cells in patients with sickle cell disease: interim results. Haematologica 103, 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Esrick EB et al. (2018) Successful hematopoietic stem cell mobilization and apheresis collection using plerixafor alone in sickle cell patients. Blood Adv. 2, 2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lagresle-Peyrou C. et al. (2018) Plerixafor enables safe, rapid, efficient mobilization of hematopoietic stem cells in sickle cell disease patients after exchange transfusion. Haematologica 103, 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Uchida N. et al. (2020) Safe and efficient peripheral blood stem cell collection in patients with sickle cell disease using plerixafor. Haematologica 105(10):e497. doi: 10.3324/haematol.2019.236182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leonard A. et al. (2021) Disease severity impacts plerixafor-mobilized stem cell collection in patients with sickle cell disease. Blood Adv. 5, 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fukuda S. et al. (2007) The chemokine GROβ mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood 110, 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hoggatt J. et al. (2018) Rapid mobilization reveals a highly engraftable hematopoietic stem sell. Cell 172, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palchaudhuri R. et al. (2016) Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat. Biotechnology 34, 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burke JM et al. (2003) Cytoreduction with iodine-131-anti-CD33 antibodies before bone marrow transplantation for advanced myeloid leukemias. Bone Marrow Transplant 32, 549–556. [DOI] [PubMed] [Google Scholar]
- 159.Burtner CR et al. (2015) 211Astatine-conjugated monoclonal CD45 antibody-based nonmyeloablative Conditioning for stem cell gene therapy. Hum. Gene Ther 26, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gao C. et al. (2019) Nongenotoxic antibody-drug conjugate conditioning enables safe and effective platelet gene therapy of hemophilia A mice. Blood Adv. 3, 2700–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kornblit B. et al. (2012) Conditioning with α-emitter based radioimmunotherapy in canine allogeneic hematopoietic cell transplantation. Chimerism 3, 40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Z. et al. (2019) Hematopoietic chimerism and donor-specific skin allograft tolerance after non-genotoxic CD117 antibody-drug-conjugate conditioning in MHC-mismatched allotransplantation. Nat. Commun 10(1)doi: 10.1038/s41467-018-08202-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mawad R. et al. (2014) Radiolabeled anti-CD45 antibody with reduced-intensity conditioning and allogeneic transplantation for younger patients with advanced acute myeloid leukemia or myelodysplastic syndrome. Biol. Blood Marrow Transplant 20, 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pang WW et al. Anti-CD117 antibody depletes normal and myelodysplastic syndrome human hematopoietic stem cells in xenografted mice. Blood 133, 2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kwon HS et al. (2019) Anti-human CD117 antibody-mediated bone marrow niche clearance in nonhuman primates and humanized NSG mice. Blood 133, :2104–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yokoi T. et al. (2016) Non-myeloablative preconditioning with ACK2 (anti-c-kit antibody) is efficient in bone marrow transplantation for murine models of mucopolysaccharidosis type II. Mol. Genet. Metab 119, 232–238. [DOI] [PubMed] [Google Scholar]
- 167.Fischer A. and Hacein-Bey-Abina S. Gene therapy for severe combined immunodeficiencies and beyond. J. Exp. Med 217(2)doi: 10.1084/jem.20190607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fenner BJ et al. (2021) Gene-based therapeutics for inherited retinal diseases. Front Genet. 12:794805 doi: 10.3389/fgene.2021.794805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ong T. et al. (2019) Adeno-associated viral gene therapy for inherited retinal disease. Pharm. Res 36(2)doi: 10.1007/s11095-018-2564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rosanwo TO and Bauer DE (2021) Editing outside the body: ex vivo gene-modification for beta-hemoglobinopathy cellular therapy. Mol. Ther 29, 3163–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li C. et al. (2019) Targeted integration and high-level transgene expression in AAVS1 transgenic mice after in vivo HSC transduction with HDAd5/35++ vectors. Mol. Ther 27, 2195–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li C. et al. (2022) Safe and efficient in vivo hematopoietic stem cell transduction in nonhuman primates using HDAd5/35++ vectors. Mol. Ther. Methods Clin. Dev 24, 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ataga KI et al. (2017) Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med 376, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vichinsky E. et al. (2019) A Phase 3 Randomized trial of voxelotor in sickle cell disease. N. Engl. J. Med 381, 509–519. [DOI] [PubMed] [Google Scholar]
- 175.Niihara Y. et al. (2018) A phase 3 trial of l-Glutamine in sickle cell disease. N. Engl. J. Med 379, 226–235. [DOI] [PubMed] [Google Scholar]
- 176.Shah N. et al. (2022) Real-world effectiveness of voxelotor for treating sickle cell disease in the US: a large claims data analysis. Expert Rev. of Hematol 2022:1–7. doi: 10.1080/17474086.2022.2031967 [DOI] [PubMed] [Google Scholar]
- 177.Grevet JD et al. (2018) Domain-focused CRISPR screen identifies HRI as a fetal hemoglobin regulator in human erythroid cells. Science 361, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang P. et al. (2020) The HRI-regulated transcription factor ATF4 activates BCL11A transcription to silence fetal hemoglobin expression. Blood 135, 2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Peslak SA et al. (2020) HRI depletion cooperates with pharmacologic inducers to elevate fetal hemoglobin and reduce sickle cell formation. Blood Adv. 4, 4560–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vinjamur DS et al. (2021) ZNF410 represses fetal globin by singular control of CHD4. Nat Genet. 53(5):719–728. doi: 10.1038/s41588-021-00843-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lan X. et al. (2021) ZNF410 uniquely activates the NuRD component CHD4 to silence fetal hemoglobin expression. Mol. Cell 81, 239–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Verheul TC et al. (2020) Targeted protein degradation as a promising tool for epigenetic ppregulation of fetal hemoglobin. Chem. Med. Chem 15, 2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cheng L. et al. (2021) Single-nucleotide-level mapping of DNA regulatory elements that control fetal hemoglobin expression. Nat. Genet 53, 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yu L. (2022) Identification of novel gamma-globin inducers among all current potential erythroid druggable targets. Blood Adv. doi: 10.1182/bloodadvances.2021006802 [DOI] [PMC free article] [PubMed] [Google Scholar]