Abstract
The equine gastrointestinal tract is a self-sufficient fermentation system, housing a complex microbial consortium that acts synergistically and independently to break down complex lignocellulolytic material that enters the equine gut. Despite being strict herbivores, equids such as horses and zebras lack the diversity of enzymes needed to completely break down plant tissue, instead relying on their resident microbes to carry out fibrolysis to yield vital energy sources such as short chain fatty acids. The bulk of equine digestion occurs in the large intestine, where digesta is fermented for 36–48 h through the synergistic activities of bacteria, fungi, and methanogenic archaea. Anaerobic gut dwelling bacteria and fungi break down complex plant polysaccharides through combined mechanical and enzymatic strategies, and notably possess some of the greatest diversity and repertoire of carbohydrate active enzymes among characterized microbes. In addition to the production of enzymes, some equid-isolated anaerobic fungi and bacteria have been shown to possess cellulosomes, powerful multi-enzyme complexes that further enhance break down. The activities of both anaerobic fungi and bacteria are further facilitated by facultatively aerobic yeasts and methanogenic archaea, who maintain an optimal environment for fibrolytic organisms, ultimately leading to increased fibrolytic microbial counts and heightened enzymatic activity. The unique interactions within the equine gut as well as the novel species and powerful mechanisms employed by these microbes makes the equine gut a valuable ecosystem to study fibrolytic functions within complex communities. This review outlines the primary taxa involved in fibre break down within the equine gut and further illuminates the enzymatic strategies and metabolic pathways used by these microbes. We discuss current methods used in analysing fibrolytic functions in complex microbial communities and propose a shift towards the development of functional assays to deepen our understanding of this unique ecosystem.
Keywords: Equine, Microbiome, Gastrointestinal tract, Fibre, CAZyme, Anaerobic fungi, Health
Introduction
The equine hindgut is a complex naturally occurring fermentation system of powerful lignocellulolytic microbes. ‘Fibre’ (complex plant polysaccharides) degradation is an essential process in equine digestion, allowing the liberation of vital energy sources. Despite consuming a strictly plant based diet, equids lack the diversity of enzymes needed to break down complex plant polymers alone and have evolved symbiotic relationships with resident gut microorganisms to produce the enzymes that facilitate plant matter degradation [1]. These microbes ferment complex carbohydrates in plant material into short chain fatty acids, contributing 60–70% of the horse’s daily energy requirements [2].
An understanding of the equine hindgut microbial ecosystem and the parameters that control and affect it can greatly facilitate our understanding of host-microbe interactions and how we can optimise the functionality of resident microbes and ultimately maximise energy yield within this environment. Our understanding of the role of bacteria in hindgut fermentation is progressing [3–8], however, knowledge of the function of other microbial communities, including archaea and fungi, in the hindgut is still lagging [9]. This review outlines the fibrolytic microbial community composition of the equine hindgut and elucidates the enzymatic processes and metabolic pathways that allow the break down of complex polysaccharides, as well as detailing current and future techniques to assist further understanding of the mechanisms and functional genes underlining these processes.
The equine digestive habitat
Anatomy of the equine gastrointestinal tract
The majority of studies undertaken on herbivore digestive systems, and particularly the microbial communities residing in them, have been done in ruminants, specifically bovine and ovine. The horse gut shares several similarities with the ruminant gut, having combined caecal and colonic regions, with both being heavily dependent on their gut microbiota for digestion and nutrition [10]. Horses are monogastric herbivores, however, and do not regurgitate digesta for further break down like foregut fermenting ruminants, having a greater dependence on resident hindgut microbes for fermentation and digestion [11]
The equine gastrointestinal tract (GIT) is aerobic to anaerobic from anterior to posterior, due to the intake of oxygen with feeding and the subsequent utilization of most of this oxygen by aerobic fermenters prior to reaching the hindgut [5]. The horse’s stomach is the smallest of any livestock or domestic animal relative to its size, having a capacity of only 9–18 L [12]. Most food degradation takes place in the large intestine which makes up over 60% of the equine GIT. Upon entry to the large intestine, 85–95% of cell wall derived carbohydrates are undigested [12]. Digesta enters the large intestine approximately 3 h after feeding and is fermented for 36–48 h in the caecum [11]. The caecum sits at a pH of 6.3–7.5, ideal for the growth of a plethora of anaerobic bacteria, fungi and protozoa [11]. Additionally, neurological signalling during feeding times trigger the caecum to increase in capacity and mobility to enhance microbial-digesta interactions, allowing microbes to efficiently degrade difficult plant biomass and subsequently synthesise vital energy sources for the animal [11].
Equine gut microbiome
The dependence of equids on their gut microbiota and the consequent importance of this population has led to the viewing of the host and its microbes as one unit when evaluating health and host phenotype, referred to as the ‘holobiont’ (a host and its microbiota) [13]. Microbial colonization of the equine gut is generally through diet, supplements, coprophagy (faecal consumption) or other methods of ingestion [14–16]. Studies on the meconium (first defecation) of newborn foals have found that their early GIT microbiome largely reflects bacteria found in the maternal milk [17] with other studies showing the equine microbiome generally begins to stabilise between one and two months of age [18]. A database has recently been compiled to analyse taxon-associated host phenotypes and is available at http://addagma.omicsbio.info/ to allow users to make biologically relevant queries about microbial related trends in equine health and disease [19]. The database summarises experimental observations found between domestic animals and their gut microbiota, however also highlights the lack of studies assessing these relationships in equids, with studies related to horse microbial phenotypes being significantly fewer than those of cattle, pigs and chickens [19]. The entire equine GIT can contain up to 1015 bacterial cells [9], and studies have reported as many as 104 fungal zoospores/mL of caecal content [11], although these numbers do not necessarily equate to their functional value in the gut. The most important end product of fermentative processes carried out by these microbes are volatile fatty acids, such as propionate, acetate and butyrate [20] which are vital energy sources for horses, as well as carbon dioxide, water, methane, vitamins and several amino acids [21].
Fibre degradation in the equine gut is primarily carried out by anaerobic bacteria and fungi, facilitated by facultatively aerobic yeasts and methanogenic archaea [22]. For many years protozoa were additionally considered contributors to fibre degradation in the equine gut due to their ability to rapidly adhere to and colonize plant tissue [23, 24]. An older study [25] showed their potential to enhance bacterial degradation of pectin and hemicelluloses, particularly arabinogalactan and galactomannan components [25]. However a later investigation found little impact of these microbes on plant digestion and fibrolysis, and concluded that they have a minimal role in directly degrading plant matter [9]. Bacteriophages have also been suggested to influence the fitness of intestinal cellulolytic bacteria and support colonisation, although do not have a direct role in fibre break down [26].
Diet
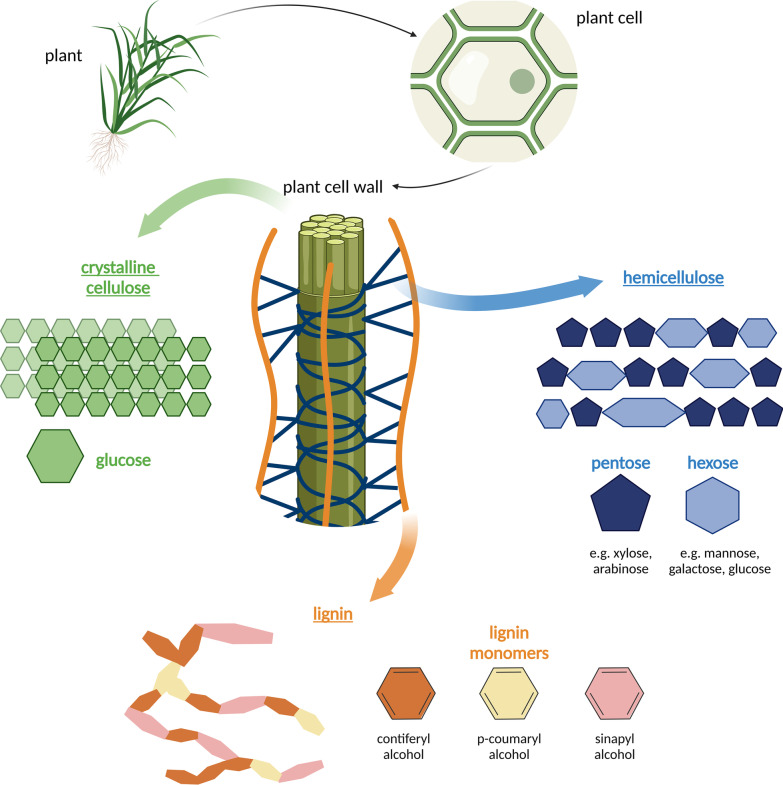
Being herbivores, equids source their nutrition from plants, either through wild forages or commercial feeds. Plant cell walls are composed of lignocellulose, a structure made of two carbohydrate polymers, cellulose and hemicellulose, and an aromatic heteropolymer, lignin, to bind the polysaccharides together (relative abundances of approximately 45%, 30% and 25% respectively) (see Fig. 1) [27].
Fig. 1.
Structure of the main components of plant biomass (cellulose, hemicellulose, and lignin). All components contain amorphous areas and variable structures and will not always present as the structures depicted above. (Adapted from [28]). Figure made in BioRender
Cellulose, the major component of plant biomass, is one of the most abundant polysaccharides on earth [29]. Cellulose is composed of closely stacked disaccharide cellobiose fibres linked by β (1–4)-glycosidic bonds, organised in crystalline structures with scattered amorphous areas. Despite being a relatively simple compound, the proximity of these fibres to each other makes accessing the bio-nutrients of cellulose difficult. Hemicellulose molecules are the second most abundant plant polysaccharide and present as polymers of pentoses and/or hexoses, interconnected by covalent hydrogen bonds [28]. The main structures of hemicellulose are xylan, xyloglucan, and galactomannan which vary in their structure and makeup depending on their respective sugars. Sugars in hemicellulose are linked similarly to cellobiose fibres by β (1–4)-glycosidic bonds and, occasionally, β (1–3)-glycosidic bonds [30]. Finally, lignin is an amorphous heterogenous polymer composed of three aromatic alcohols [28]. The quantity and distribution of these aromatic alcohols varies between plant species. Lignin is associated with hemicellulose and cellulose by ester and hydrogen bonds. It is these bonds between the different structural components of plant biomass, as well as the rigidity and complexity of individual components that create the overall resistance to degradation of lignocellulose complexes.
Unsurprisingly, the composition of plant structure and contribution to herbivore feed has had a demonstrated effect on the microbiome composition and overall fibrolytic capacity of the equine gut [14, 31–34]. Forages available for grazing equids change substantially across the globe and throughout the year, leading to corresponding changes in feed nutrient value and digestibility. Generally speaking, grasses grown during a rainy season are higher quality and more nutrient dense, while a dry season brings increased lignin in plants, the least digestible fibre component of plants, and decreased nitrogenous content [35]. Feeds harvested during dry climatic conditions generally lead to reduced fibre utilization due to the low nitrogenous content of this feed meaning there are less nitrogenous precursors for the synthesis of microbial compounds such as enzymes [35]. The effects of this may be partially overcome with nitrogenous dietary supplementation which has been shown to stimulate gut fibrolytic activity and increase degradation of low quality fibre [35].
Julliand and Grimm [14] provide an overview on the impact of diet on the equine hindgut microbiome. Generally, studies show diets high in starch have adverse effects on microbial populations in the equine gut, reducing cellulolytic bacterial counts and overall fibrolytic capacity [34, 36, 37]. Such feed types are common among domesticated pet horses in the form of pelleted horse feeds high in barley, oats and corn [37]. Correspondingly, these high concentrate diets can lead to an increase in amylolytic bacteria, the primary one being Lactobacillus species [38], overpopulation of which can lead to gut acidosis and ultimately colitis and laminitis [3, 39, 40]. High fibre diets, in contrast, seem to maximise the fibrolytic capacity of the equine gut, leading to increased cellulolytic and xylanolytic bacterial counts [32, 41], as well as decreased concentrations of bacteria typically associated with laminitis induction, such as Lactobacillus [4]. While these studies highlight apparent trends between forage composition and microbial populations, other studies have suggested the reaction of the horse gut microbiome to changes in plant structure to be largely individualised between horses, with implications on metabolic health remaining to be elucidated [42].
Fibre degrading enzymes
The complete break down of plant biomass requires the presence of multiple enzymes to target different components of lignocellulose. Carbohydrate degradation involves two steps, the first being hydrolysis of plant polysaccharides, and the second being the fermentation of the resulting simple sugars into short chain fatty acids [14].
‘CAZymes’ are carbohydrate active enzymes which catalyze the break down and assembly of glycoconjungates and glycans [43]. Currently there are six main CAZyme classes, glycoside hydrolases (GHs), glycosyl transferases (GTs), polysaccharide lyases (PLs), carbohydrate esterases (CEs), carbohydrate binding modules (CBM) and auxiliary activities (AAs) which are redox enzymes that facilitate CAZyme function. These enzymes are classified according to the different mechanisms in which they break down substrates, as well as their three-dimensional folding structure characteristics and protein sequence similarities [44]. An overview of different CAZymes is available at http://www.cazy.org/ which has recently been updated and reviewed [45].
Endoglucanases, exoglucanases and β-glucosidases are the three main cellulase groups that act synergistically and simultaneously to break down cellulose using hydrolysis [46]. Endoglucanases target amorphous areas of the crystalline cellulose matrix and cut into them, producing a chain end that exoglucanases are then able to bind to and cleave, releasing the cellobiose fibres and individual glucose monomers [46]. Finally, the cellobiose is degraded to glucose monomers by β-glucosidases [46]. While hydrolysing cellulases are the prominent degraders of cellulose, other cellulases exist which utilize other modes of action, such as cellodextrinases and cellobiose phosphorylases, which use phosphorylation-mediated cleavage, or oxidoreductases which use an oxidative mode of action. Various microorganisms can produce cellulases, including bacteria, fungi, metazoan and some animals like termites, snails and crayfish [47–49].
Several enzymes are involved in the break down of hemicellulose which act specifically on the different glycol units and glycosidic bonds. These hemicellulases include endo- and exo-β-glucanases and xylanases, polygalacturonases, pectin methyl esterases, β-mannanases, feruloyl esterases, pectin and pectate lyases and arabinofuranosidases [50]. Hemicellulases are primarily produced by saprophytic microbes isolated from decaying plant and animal material [51].
Current research on lignin degradation has focused on the role of fungi in this process [52], however bacteria have also been demonstrated to produce enzymes enabling lignin break down [53]. The enzymes primarily responsible for lignin break down are laccases and peroxidases and can be generally divided into two main groups; lignin modifying enzymes and lignin degrading auxiliary enzymes, the latter of which are unable to degrade lignin on their own but are necessary to complete the degradation process. These proteins primarily fall under the ‘auxiliary activities’ family classification within the CAZy database. Lignin degradation is an oxidative process, therefore any microbes involved in this process within the equine gut are likely rare and difficult to detect due to the prominent anaerobicity of the equine hindgut [54]. Further culture-independent research is needed to further elucidate microbial methods of lignin break down within the equine GIT.
CAZymes are a growing focus in a range of research fields due to their diverse industrial applications and environmental distribution. Anaerobic gut dwelling bacteria and fungi possess some of the greatest diversity and repertoire of CAZymes amongst characterized microbes [55, 56], and the ecosystem of the equine may even serve as an environment for horizontal gene transfer of CAZymes between organisms, as demonstrated in ruminant species [57]. Comprehensive gene cataloguing of the faeces from eleven endurance trained horses found 137 different CAZymes through shotgun sequencing methods, the majority (85.4%) belonging to glycoside hydrolase and polysaccharide lyase families [58], highlighting the wealth of fibre degrading enzymes in the horse gut.
The equine hindgut also houses microorganisms that are exceptional because they can produce ‘cellulosomes’ which are multi-enzyme complexes made up of multiple CAZymes. Cellulosomes co-ordinate lignocellulolytic enzymes of similar functions to colocalise within the complex for enhanced degradation [59]. Cellulosomal enzymes are bound to a non-enzymatic membrane-anchoring protein, a scaffoldin, via a modular dockerin domain attached to the enzymes which interact and bind with cohesion proteins on the scaffoldin [60, 61], thus creating dynamic and powerful catabolic complexes (see Fig. 2).
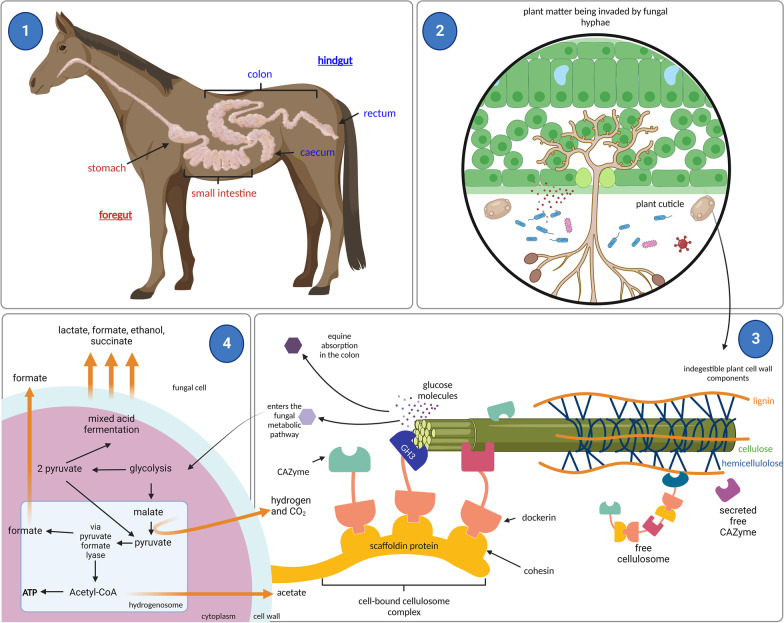
Fig. 2.
The role of fungi in plant break down and metabolism. 1 Schematic diagram of the equine digestive system (red text indicates the foregut, blue text indicates the hindgut). The majority of hindgut digestion occurs in the caecum. 2 Plant matter in the caecum is invaded by penetrative hyphae of anaerobic fungi. 3 Overview of the enzymatic activity of anaerobic fungi (adapted from [62]). Anaerobic fungi degrade plant biomass within the equine caecum through several enzymatic strategies; free carbohydrate active enzymes (CAZymes), cell bound cellulosome complexes and free cellulosomes secreted by the cell. Cell bound cellulosome example given is of glycoside hydrolase 3 which converts cellulose to monosaccharide glucose molecules via β-glucoside activity. These glucose molecules can then be absorbed in the equine gut or enter the fungal metabolic pathway. 4 Example of energy metabolism of Piromyces sp. E2 (adapted from [63]). Glucose molecules enter the glycolysis pathway, the product of which are two pyruvate molecules which either enter a mixed acid fermentation in the cytosol, or the hydrogenosome for ATP generation. Major by-products of fungal energy are indicated by the thick orange arrows. Figure made in BioRender
Fungi
Anaerobic fungi
Anaerobic fungi are believed to be major contributors to fibrolysis and hindgut fermentation [22], found to represent at least 20% of microbial biomass in the rumen (63). Anaerobic fungi are significantly better degraders of plant cell walls compared to bacteria, due to their larger repertoire of fibre degrading enzymes and cellulosomes and their ability to mechanically invade plant tissue with penetrative hyphae [56, 62, 64]. Yet, this niche group of microorganisms remains greatly understudied within herbivore research. Neocallimastigomycota is the only described anaerobic fungi phylum and their production of lignocellulolytic enzymes are central to a range of agricultural, biogeochemical, and nutritional processes [62]. First identified in the herbivore system as zooflagellates, early research on anaerobic fungi was centralised around their role in breaking down feeds [65, 66]. These fungi have since been isolated from diverse and extreme environments, including in bedrock deep in the earths biosphere [67]. The uniqueness of this group of fungi stretches beyond their ability to survive under the anaerobic conditions of the equine gut, and has propelled research into understanding their taxonomy, enzymology, morphology, and diversity in host animals. Hess et al. provides a comprehensive overview of past, present and future research on these distinctive organisms [62].
All described anaerobic fungi are members of the phyla Neocallimastigomycota, which contains a single order (Neocallimastigales) and family (Neocallimastigaceae). Within this family are 20 recognized genera, eleven of which have only been characterised in the last five years [68–71]. To date, at least six genera have been isolated from the equine gut (Piromyces [72–79], Orpinomyces [72, 80], Neocallimastix [72–74, 80], Anaeromyces [72, 74, 80], Caecomyces [72–74, 77] and Khoyollomyces [68, 73, 80]), with several more uncharacterised species also being found with the development of next generation sequencing technologies [73, 74, 80]. A list of publicly available whole genomes of anaerobic fungi was compiled by Hess et al. [62] and included one horse habituating organism, Piromyces finnis [81].
Anaerobic fungi obtain energy from breaking down plant carbohydrates using cellulolytic enzymes, prominently CAZymes. An overview of CAZymes found in well-characterized equine microbes are shown in Table 1. The functional diversity of CAZymes in gut dwelling fungi is impressive and far exceeds that of species currently used in cellulolytic cocktails for biotechnological purposes, such as Aspergillus niger and Trichoderma resii [59, 82]. In fact, early-branching anaerobic fungi encode the largest number of biomass-degrading enzyme genes found in nature to date, with the genome of a strong plant-degrading anaerobic fungus typically harbouring 200–300 CAZyme specific genes [44, 81]. This has been attributed to early horizontal gene transfer of bacterial hemicellulases to anaerobic fungi [81], providing anaerobic fungi a more diverse repertoire of enzymes compared to later diverging fungi which lack substrate catabolism diversity [56]. As such, anaerobic fungi have demonstrated efficient digestion of all major components of plant wall material including cellulose, xylan and galactomannan [83]. In ruminant GITs, Neocallimastigomycota have been found to be responsible for the fermentation of 18–63% of untreated plant biomass [56, 84, 85] despite only being approximately 8% of the gut microbiome biomass [86]. In bovines, the removal of anaerobic fungi through treatment with cycloheximide and tetronasin was shown to reduce intake of low quality feed to 70% [87]. This is likely as the mechanical disruption and subsequent enzymatic break down by anaerobic fungi would ordinarily speed up feed break down and allow more rapid clearance of digesta [22, 87], underlining their important role in the digestion of crude lignocellulose and subsequently host nutrition.
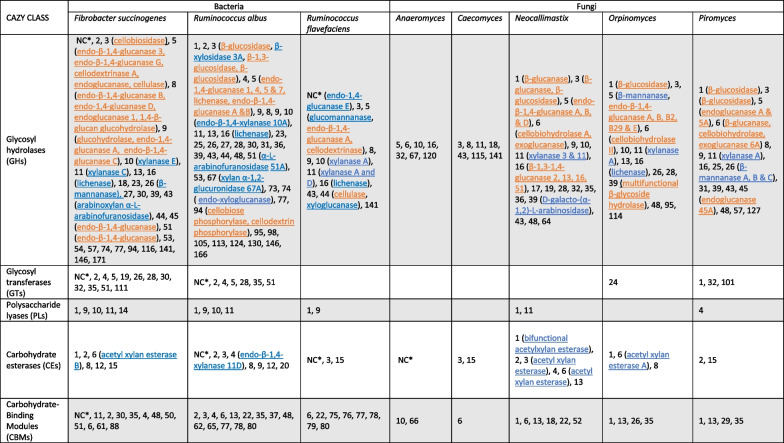
Table 1.
CAZyme families from prominent fibre degrading microbes isolated from the equine gut
Numbers under taxa indicate CAZyme families found within the corresponding CAZy class in each taxon. Fungal CAZymes found are heterologously expressed within each genus. Where enzyme functionality has been characterized, the protein name is written in brackets after the CAZy family number. Fibrolytic activities in blue are involved in hemicellulose degradation, while activities in orange are primarily cellulose degrading. Note- this is not an exhaustive list of fibrolytic microbes found in the equine gut, but rather a summary of those for whom enzymology has been described
*NC = CAZymes “non classified” CAZymes; i.e. not yet assigned to a family
In addition to their enzymatic potential, anaerobic fungi offer an added benefit in fibrolysis through mechanical agitation and hyphal invasion of plant tissue [59]. Helium ion micrographs of the equine derived anaerobic fungi P. finnis presented by Lillington et al. [59], demonstrated the dynamic mechanical and enzymatic break down of plants by anaerobic fungi including capturing of surface localised cellulosomes. Images revealed fungal rhizoids invasively covering grass particles, enhancing surface coverage and access to carbon sources. Also visible was hyphal penetration of plant substrate to access trapped carbon [59]. Hyphal tips, furthermore, have a high concentration of fibrolytic enzymes, the activity of which increase nutrient availability for other cellulolytic microbes including bacteria [62].
The simple monomers from lignocellulose degradation, such as glucose, are metabolised by fungi though a type of mixed acid fermentation (i.e., heterofermentative processes), where degradation of carbohydrates leads to the production of hydrogen, carbon dioxide, formate, acetate, succinate, and ethanol as by-products [21] (see Fig. 2 for an overview of anaerobic fungi plant break down and metabolism). The bulk of fungal metabolism is conducted by hydrogenosomes, an evolved version of the membrane bound organelle mitochondria used by respiration dependent organisms [21, 88, 89]. Rather than carrying out oxidative phosphorylation, this structure produces ATP through substrate-level phosphorylation under anoxic conditions, producing hydrogen as an end-product [21]. This hydrogen is considered vital for the growth of methanogenic archaea and bacteria [21], highlighting the importance of anaerobic fungi in the equine GIT microbial ecosystem.
Donkeys have been demonstrated to have increased fibre degradation abilities compared to horses [90] which is possibly explained by a study [73] comparing the microbial populations of different Equidae species, which found donkeys to have a six-fold greater anaerobic fungal loads compared to horses, as determined using quantitative polymerase chain reaction methods. Another key finding from that research was the strong correlation of diet with the types and concentrations of anaerobic fungi identified. The composition of the mycobiome was found to have a higher association with diet than species types, (e.g., zebra, horse, donkey) [73], highlighting the importance of equine nutrition in mycobiome development.
Past and present investigations into the equine hindgut have revealed its distinction from other anaerobic fungi ecosystems, both functionally and taxonomically. Several studies have revealed the presence and dominance of the genera Khoyollomyces (formally known as AL1), in the equine hindgut, having been found almost exclusively in equines [39, 44, 53, 55]. The type species Khoyollomyces ramosus (khyollo meaning horse, myces meaning fungi) was initially cultivated from zebra and equine faeces [68]. Isolates of K. ramosus were observed to produce monoflagellated zoospores and develop highly branched rhizoids when encysted [68]. The extensive rhizoidal branching of this species may explain its increased disruption of plant tissue compared to other anaerobic fungal species [59, 83], however this is yet to be investigated. The recent cultivation and, therefore, characterization of this genus explains why its role in the equine gut, and potentially novel enzymatic and metabolic pathways, are currently unknown.
The adaptation of anaerobic fungi to the gut environment of horses has been further emphasised through metabolic studies into equine derived fungi. Perhaps the most well documented genera of anaerobic fungi in herbivorous mammals is Piromyces spp., including their production of highly effective cellulolytic enzymes. Interestingly, metabolic differences have been reported between strains of Piromyces isolated from equid and rumen guts, with equine derived strains possessing higher fibre degrading capabilities and overall faster growth rates [91]. After 25 h of growth on soluble sugars, fungal biomass (mg/ mL) from equine derived strains (ponies and donkeys) was three-fold higher than that of rumen strains grown on the same substrate. Digesta spends a shorter amount of time in the equine caecum compared to the rumen foregut, and therefore equine-strains of Piromyces have likely adapted to induce plant cell wall break down at an increased rate [91]. Of particular significance within the Piromyces genus is P. equi, a fungus isolated from equines that was found through protein and enzyme assays in conjunction with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis, to possess a major exoglucanase- Cel6A. Cel6A is one of the most widely studied cellulolytic enzymes as it is capable of fully digesting cellulose [92, 93], a key example of the powerful fibrolytic enzymes at play in equine digestion.
Several uncultivated anaerobic fungal clades have also been identified in the equine gut, including NG1, NG2, NG3, NG5, NG7, DT1, KF1, SK1 and SK3 [73, 74, 80]. However, many limitations currently exist in both traditional and molecular identification of anaerobic fungi, including their stringent cultivation requirements, A-T and repeat rich genome, unknown ploidy, lack of a reliable DNA barcode and complex physiology [94]. Additionally, reference sequences for these taxa are not well represented in publicly available databases for both their taxonomy and functionality. Tackling these technical issues is essential to improving our understanding of the role these microbes play in fibrolysis.
Thermophilic fungi
Only recently has it been demonstrated that oxidative cleavage of cellulose occurs in the equine gut via aerobic thermophilic fungi [95]. Thermophiles are a type of extremophile microbe that can survive relatively high temperatures, over 100 °C for some Eubacterial and methanogenic Archean species [96, 97], or up to 60 °C for thermophilic fungi, the only eukaryotes demonstrated to grow at such high temperatures [98]. Thermophilic fungi, similar to anaerobic fungi, are a point of interest due to their rapid growth rates and high cellulolytic activity and have been isolated from herbivore faeces on several occasions, including from the horse [99, 100]. However, it was initially thought that the fungi colonized faecal content post defecation and consequently thrived in the warm conditions.
Studies that uncovered this process [98, 99] noted that gut dwelling anaerobic fungi seemingly lack auxiliary activity 9 family of enzymes (“AA9”) including lytic polysaccharide monooxygenases. These enzymes cleave cellulose fibres via C1 and C4 oxidation [101], making fibres more readily digestible by other microbial groups. The study successfully isolated C1 and C4- oxidized cellulose from both horse faeces and directly from the horse stomach and subsequently isolated three thermophilic fungal species which were cultivated at 50 °C; Chaetomium thermophilum, Thermoascus aurantiacus and Scytalidium thermophilum [95]. All three species when isolated directly from equine digesta were found to express AA9 enzymes, confirming their role in cellulose cleavage within the equine stomach. The study went on to postulate that these aerobic fungi grow in the anterior region of the horse gut where oxygen is consumed, consequently facilitating the anaerobic conditions of the lower gut [95]. Notably, enzyme activity of thermophilic organisms is often maximised at temperatures higher than that of the equine gut, and as such, it remains to be investigated how actively involved they are in plant break down within the equine GIT.
Facultatively aerobic yeasts
The role and presence of yeasts in the equine hindgut is even less explored than anerobic fungi, which are rarely reported as residents of this ecosystem. All yeasts belong to two phyla; Ascomycota and Basidiomycota within the Dikarya subkingdom [102]. Yeasts are highly ubiquitous in the environment, found in a variety of habitats as pathogens, transients, or symbionts [103, 104]. Although some yeast species enter the equine gut, they are often considered non-functional transients in this eco-system, despite their ability to survive in this niche environment [105]. Yeasts found within herbivore digestive tracts are not strictly anaerobic like their mould and bacterial counterparts but instead considered ‘facultatively aerobic’, being able to survive with little to no oxygen [105]. This is due to mitochondrial adaptations in the form of deletions, mutations or duplications of mitochondrial DNA or nuclear DNA involved in oxidative phosphorylation [106]. These species, often referred to in the literature as ‘petites’, are respiration deficient but viable, and unable to grow on non-fermentable substrates such as ethanol or glycerol [106].
Much research on yeasts in herbivore digestion has been in insects, however the insect hindgut is more adapted to re-absorption of amino acids rather than plant break down, and there is otherwise little overlap between the GIT of these and horses. Some yeasts found in the aforementioned studies include genera also isolated from the equine gut, including some Candida and Saccharomyces species [105, 107]. Yeasts isolated from the gut of insect herbivores, namely termites and beetles, have been shown to produce extracellular enzymes, particularly xylan- and arabinogalactan-hydrolysing glycosidases, to break down hemicellulose and detoxify toxins in the herbivore diet [108–110]. These findings, although not demonstrated in equine derived yeasts, allude to the role of GIT yeasts in contributing to plant break down in conjunction with their other microbial counterparts.
Fibrolytic bacteria
Bacteria are the most well documented microbial group within the equine GIT ecosystem, and undoubtably have important roles in fibre degradation and host health. While the cellulase diversity of anaerobic fungi is greater, cellulolytic bacteria are often found in higher concentrations throughout the GIT and have faster growth rates, consequently often being found to have higher cellulase counts [111]. Gut dwelling bacteria are well studied in mammalian hosts, having several proven roles in immunoregulation and other cell regulatory mechanisms [112]. Their fibrolytic role has been well established in the rumen caecum [113–123] and to a lesser extent, the equine hindgut [3–8].
The functionality of gut dwelling bacteria can broadly be classified into proteolytic, lactate-using, glycolytic and cellulolytic bacteria, the latter of which are mainly composed of species from the phyla Actinobacteria, Firmicutes, Proteobacteria and Bacteroidetes [12, 124]. Few cellulolytic bacteria have been isolated from the GIT of equines through culture dependent methods [25], possibly due to their slow growth rate and purported long lag phase to initiate substrate digestion [125]. Additionally, it is estimated that 33–80% of bacteria in the gut of herbivores are oxygen sensitive, including most cellulolytic bacteria [9] further challenging attempts to culture them and conduct functional evaluation. Current estimations of cellulolytic and hemicellulolytic bacterial populations in the equine hindgut range from 104 to 108 cells/ mL and 106 to 108 cells/ mL respectively, showing their dynamic functionality in breaking down different plant wall components within the hindgut [126].
Few studies have undertaken enzymatic profiling of equine fibrolytic bacteria. Genome annotation is a common method for evaluating the enzyme richness of microbial species, however, it is especially difficult for environmental and otherwise complex samples, due to the large diversified amount of data yielded from these analyses, and general absence of environmental species in current databases [127–130]. Through shotgun metagenomic sequencing, Gilory et al. [110], recovered 123 metagenome assembled genomes (MAGs) belonging to archaea and bacteria from five horse faecal samples. The bacteria detected were predominantly members of the phyla Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria, and collectively possessed a diverse array of polymer degrading enzymes [111]. Each bacterial MAG possessed on average 69 different CAZymes with Bacteroidota phyla members having the largest CAZyme repertoire. The majority of reported CAZymes belonged to the glycosyl hydrolase family (51%), indicating the potent role of these microbes in the break down of complex carbohydrates [111].
The enzymatic profiles of different microbial groups within the equine gut typically shows trends of anaerobic fungi being the primary degraders of cellulose, while fibrolytic bacteria target the degradation of hemicellulose. This functional specificity has particularly been demonstrated in the rumen. One such study assessed kingdom specific functionalities within the rumen microbiome through placing nylan bags containing switch grass directly into the rumen of two fistulated cows [131]. Metatranscriptomics on the rumen incubated bags revealed bacterial populations were primarily responsible for hemicellulose degradation, with the proteome of Fibrobacter succinogenes in particular containing a wealth of CAZymes from hemicellulose prominent families (GH11, GH51 and GH94). The proteome and transcriptome of rumen fungi however appeared better equipped for the degradation of cellulose structures with enzymes from the glycoside hydrolase family GH48 being the most abundant CAZymes detected [131].
The enhanced hemicellulolytic catabolism of equine caecal bacteria has been further demonstrated through their substantial growth on xylan rich media, the main carbohydrate component of hemicellulose, and subsequent poor colonization on cellulose rich media [6]. The xylanase activity of the dominant horse caecal isolate, Enterococcus casseliflavus, was also superior to that of isolates from buffalo and horse dung. The same study also found growth of an unidentified ligninolytic bacterium on lignin. Other cellulolytic bacteria isolated from horse faeces with substantial hydrolytic capacity include Paenibacillus polymyxa, Enterobacter cloacae and Escherichia coli, the former of which yielded the highest cellulolytic activity of those isolated [7].
Firmicutes are the largest phylum represented in the equine GIT, ranging from 40 to 90% in different sections [132]. Within this phylum is the class Clostridia which contains the obligately anaerobic family Lachnospiraceae. Lachnospiraceae are regarded as core microbiome members across most animals [133–135], and are recognised for their ability to degrade a variety of plant polysaccharides [136], resulting in the production of short chain fatty acids such as butyrate which has a demonstrated protective effect on colonocytes and serves as their main energy source [137]. The families Ruminococcaceae and Fibrobacteraceae are also members of Clostridia, and while often only representing a small portion of the equine microbiome, have been found as core members in the equine gut [9, 138]. Ruminococcus and Fibrobacter are the two main genera involved in cellulose degradation in the equine gut, specifically, Ruminococcus albus, Ruminococcus flavefaciens and Fibrobacter succinogenes, and which are often used as markers for cellulolytic activity in herbivore gut studies [23] (see Table 1 for an overview of functional enzymes).
Ruminococci are one of few bacteria isolated from the gut with the ability to produce cellulosomes [139, 140]. As previously mentioned, the use of functionally organizing CAZymes into a cellulosomal network greatly enhances the fibrolytic capacity of an organism. R. flavefaciens has frequently been identified as the main cellulolytic bacterial species in the equine gut, ranging in concentrations throughout the lower GIT of between 2 and 9.7% of overall bacterial populations [3, 141]. Assembly of a cellulosomal complex from R. flavefaciens revealed a diversity of CAZyme family domains, including glycosyl hydrolase, carbohydrate esterase and pectate lyase binding domains, however the function of around 30% of these proteins remained unknown [140]. A unique feature of the cellulosome of R. flavefaciens is its adaptor scaffoldin, ‘ScaC’, which possesses the ability to bind to different dockerin groups and consequently modulate enzyme integration into the cellulosome complex based on functional needs [142]. Furthermore, the cellulosome of R. flavefaciens showed preferential recruitment and appendage to hemicellulases, highlighting the prominent role of bacteria in hemicellulose degradation [142].
The ability of bacterial cellulosomes networks to tightly adhere to plant fibres within the gut has been recently revealed [143], further enhancing microbe-substrate interactions. Ruminococcus champanellensis, a cellulolytic bacterium isolated from the human gut, employs cellulosomes as a mechanism to anchor itself to plant substrates and withstand the motility of the GIT [143]. Through modelling and molecular dynamic simulations, researchers were able to show the cohesion-dockerin complex of these cellulosomes were able to regulate the adhesion of bacteria to substrates under different environmental conditions such as pH and high stress, with the protein bond becoming stronger as force increased [143]. No studies have yet reported the isolation of this Ruminoccous species from the equine gut, however the presence of close relatives and other cellulosomal producing species suggests this is a mechanism likely employed by equine cellulolytic bacteria as well [144].
Several studies looking into the occurrence of equine metabolic disorders analysed fluctuations on cellulolytic bacteria under different conditions [8, 38, 39]. A case study on fifteen horses treated with different antibiotics, showed a remarkable decrease in the presence of cellulolytic bacteria (> 99% decrease), following treatment, through culture-based methods [8]. After antibiotic withdrawal, the levels of cellulolytic bacteria in treated horses remained substantially lower than in control horses, and continued to decrease for a week after withdrawal [8]. Subsequently, this decrease in beneficial GIT bacteria allowed colonization of potentially pathogenic bacteria, including Salmonella and Clostridium difficile which are common causes of diarrhea and other equine GIT disorders [8]. A decrease in cellulolytic bacteria in the equine large intestine was shown to correspond with a decrease in the production of acetate, indicative of decreased fibre degradation [38].
Microbial synergism
Microbial synergism is defined as the mutually beneficial increase in the productivity or growth of a microbial community resulting from the metabolic interactions of two or more microorganisms, to which their combined effect is greater than the sum of their separate abilities [145, 146]. This is generally achieved through nutritional interdependence, whereby one species can utilize the products of another, minimizing by-products of the producer while providing nutrition to the feeder [147, 148].
Fungi and bacteria both have independent, but synergistic, fibre degrading roles in the equine hindgut, enhanced by their interactions with one another and other microbes present. As previously described, anaerobic fungi are the primary colonizers of plant biomass within the equine gut, their mechanical disruption of plant cuticles [83] enabling fibrolytic bacteria access to plant fibre which they would not otherwise be able to ferment [33]. The positive effects of anaerobic fungi on bacterial populations have particularly been demonstrated through administration of anaerobic fungal cultures as feed additives. Paul et al. [149], fed cultures of the anaerobic fungus Piromyces sp. FNG5 (previously isolated from wild bull faeces) to buffaloes and showed significant increases in cellulolytic and hemicellulolytic bacterial counts, as well as increases in resident fungal populations [149]. Increases in fibrolytic microbial populations was concomitant with increased volatile fatty acid production and increased xylanase, cellulase, protease, and acetyl and feruloyl esterase activities [150]. This is consistent with other studies demonstrating that the addition of fibrolytic fungi, to animal feed, increases bacterial populations two-fold and subsequently enzyme activities and feed digestibility within the gut [151, 152].
Yeast supplementation has been shown to have beneficial effects on rumen bacterial populations through increased production of short chain fatty acids and vitamins [153, 154], however results in equines have been less consistent. Garber, Hastie and Murray [16] have compiled a list of studies that use the yeast Saccharomyces cerevisiae as a probiotic in equines. Of five studies conducted, two recorded little to no effect on bacterial populations analysed from either the gut or faeces [155, 156], and the other three noted variable effects [103–105]. In response to yeast supplementation, one study noted decreased Streptococci in the colon and increased Lactobacillus in the caecum [157], while another study recorded overall decreased Lactobacilli counts in faeces [158]. The fifth study recorded reduced F. succinogenes populations in faeces but otherwise no effect on bacterial populations or equine fibre digestibility [159]. Ultimately it remains unclear whether yeasts in the equine GIT can produce substantial changes in the digestibility of plant substrates. A deeper understanding of the role of yeasts within the equine gut would greatly facilitate development of a more suitable feed supplement to assist plant matter break down.
The symbiotic relationship between anaerobic fungi and methanogenic archaea has been a point of interest in herbivore digestive systems [160], however little work has been done on understanding this relationship within the equine gut, with archaea only being first isolated from the equine gut in 1996 [161]. The diversity of methanogenic archaea within the equine gut varies along the GIT and depending on host animal, with the horse hindgut showing a predominance for Methanobacterium-like sequences, and the donkey hindgut housing more Methanocorpusculum-like sequences [162]. The production of methane by methanogenic archaea within the herbivore gut is a by-product of fibre digestion [163, 164]. Hydrogen is produced by anaerobic fungi and bacteria and is reduced to methane by methanogens, allowing maintenance of low hydrogen levels within the gut, to consequently maintain thermodynamic requirements for anaerobic fermentation [165].
In addition to the beneficial waste removal by methanogenic archaea, their interactions with anaerobic fungi have also demonstrated increased transcription of important CAZymes, ultimately enhancing fibrolytic capacity [166]. Co-cultivation of the bulbous anaerobic fungi Caecomyces churrovis and the methanogen Methanobacterium bryantii resulted in a stable culture which was then evaluated metabolically and transcriptionally [166]. Genome analysis found that almost 1% of all adenines were methylated, with at least 6% of the methylated genes belonging to CAZymes [166]. DNA methylation is an epigenetic process in which methyl groups are added to a DNA molecule, which can change the activity of a DNA segment through state specific control of gene expression [167]. Furthermore, co-culturing of the anaerobic fungi and methanogen resulted in a proportional increase of CAZyme gene expression, the majority of which were either carbohydrate binding modules, fungal dockerin domains or of unknown function [166]. Some cellulases contain an active domain site for binding to carbohydrate binding modules [168], with those bound to a carbohydrate binding module consistently demonstrating enhanced cellulose attachment [169–171]. Carbohydrate binding modules have diverse ligand specificity and can maintain enzyme–substrate proximity, ultimately leading to prolonged and steady enzyme–substrate interactions and increased enzyme concentrations on the polysaccharide surface [169, 172]. These enhanced interactions can increase the hydrolytic activity of enzymes on soluble substrates [173], however deeper investigation is needed on their role in plant break down within an intestinal setting.
Future directions: towards developing a functional assay
Understanding the composition and functionality of fibrolytic microbial communities within the equine hindgut is of great importance in equine nutrition and to optimise energy yield from plant matter. Gastrointestinal disturbances induced by changes in the gastric microbiome (‘dysbioses’), brought on by disease, change of diet, antibiotic use, age or other factors, can result in compositional shifts of the equine microbiome, resulting in fermentation dysfunction and ultimately metabolic disorders [174]. In the instance of the equine gut, identifying microbes present within this environment and the repertoire of enzymes they possess can help researchers and veterinarians alike better understand the taxa associated with a healthy or diseased animal, and on a larger scale, can help researchers underpin the important taxa involved in complex fibre degradation.
It is evident through elucidation of the fibrolytic capacity of the equine gut that more detailed investigations of this community require a holistic understanding of both taxonomy and functionality, with an emphasis on enzymology of particular importance in crucial functions involved in fibre break down. While taxonomically identifying bacteria and fungi from complex microbial communities has made significant headway in recent years, there are still several drawbacks that limit its usability in fully unravelling the complexity of ecosystems like the equine gut. Kauter et al. [132] provided an overview of current taxonomic methods used for analysing this microenvironment, and further evaluated the sequencing technologies available. Taxonomically, some of the major issues hindering identification of this community include, database limitations [128], substantial size polymorphism for anaerobic fungal DNA barcodes (up to 13% polymorphism between clones of a single Piromyces strain [175]) and low intra-species taxonomic resolution when using short read sequencing [73]. Identifying important functions and their corresponding genes within a mixed community has the potential to improve our understanding of the break down of complex polysaccharides in plant material within the equine gut and the specific genes involved in these processes.
In recent years, shotgun metagenomics has provided a wealth of insight into the functionality of complex microbial communities, highlighting the varying functional roles of different taxa and how they work together independently and synergistically [176]. Peng et al. [176], recently profiled the bacterial and fungal communities from goat faeces, targeting the taxa and enzymes responsible for lignocellulose break down. Briefly, faecal pellets were cultured anaerobically on a range of enrichment substrate media (alfalfa stems, canary grass, xylan or sugarcane bagasse) with varying antibiotic treatments to bias the growth of anaerobic fungi alone, or anaerobic fungi and methanogenic archaea. Shotgun metagenomics on samples yielded 18 eukaryotic derived MAGs, the annotated CAZymes which were categorized into either cellulose,- hemicellulose-, or pectin/ ester-degrading. The total number of lignocellulose-active genes from eukaryotic derived MAGs was significantly higher than that of prokaryotic derived MAGs, with a number being found only in fungi, indicating the incredible untapped potential of anaerobic fungal enzymes [176]. Through co-culturing microbial groups on different media and analysing metabolic outputs, the study was also able to assess the functional performance of different microbial consortia in degrading lignocellulose. In line with previous studies, it was found that the fungi- and archaea-dominated community not only degraded several substrates better than bacteria-dominated consortia, but also had significantly higher levels of cellulosomal CAZymes compared to the bacterial consortia.
Large scale phenotypic assays are another valuable method in uncovering key taxa involved in important targeted functions, such as fibre degradation. Recently, 1031 fungal strains acquired from a diversity of habitats were used in a large-scale multi-phenotyping assay to determine their ability to degrade non-natural industrial compounds such as plastics and dyes [177]. This assay involved culturing each strain on six different media with different degradation-resistant compounds, to determine their usability as industrial biocatalysts. Their ability to grow on these media and utilise the given substrates was determined by evaluating different degradation phenotypes depending on the substrate being utilized including change of media colour, oxidization, fungal growth, and halo clearing. The study demonstrated significant functional diversity within taxonomic ranks, with all families having at least one strain with high degradation abilities. Such findings highlight the importance of identifying fungal isolates to low taxonomic ranks (genera and species level) to fully evaluate the role of the taxa present. A focus on enzymology rather than taxonomy may also allow the elucidation of rare taxa involved in fibre break down through production of prominent CAZymes involved in fibre degradation.
Explorative microarrays, such as the ‘FibroChip’, are emerging molecular tools that utilise transcriptomics to detect genes and their genetic variants from mixed community samples [178]. Such tools can rapidly and relatively inexpensively monitor in parallel the functional potential of a high number of samples, with current analyses proving easier and quicker than other metatranscriptomics and lengthy gene annotation studies [178]. The FibroChip microarray was composed of thousands of probes targeting hundreds of CAZyme genes from eight families that were believed to have important and complementary roles in cellulose and hemicellulose degradation [GH5, 9, 10, 11, 43 and 48 and CE1 and 6]. The study was ultimately able to validate the ability of the FibroChip to detect differential gene expression in bacteria and was even able to identify differential gene expression between clone isolates cultivated on different substrates. The FibroChip was further used to analyse total RNA isolated from the rumen fluid of a cannulated cow, and while there were gaps in the enzymology of fungi, ultimately proved a valuable stepping stone towards simultaneously taxonomically and functionally analysing complex fibrolytic communities [178].
With the evolving use of long read sequencing platforms such as Nanopore Sequencing from Oxford Nanopore Technologies and single-molecule-real-time sequencing (SMRT) from Pacific Biosciences, the speed and accuracy at which we can screen genetic material from microbial communities for enzymatic genes and taxonomic barcodes is continuing to improve [179, 180]. At the moment, both technologies can rapidly generate millions of full-length reference sequences (over 10 kb) within a couple of hours [181], however come at the cost of increased error rates compared to more traditional sequencing platforms [182]. SMRT sequencing has been used to build fungal genomes for functional annotation, unveiling novel fungal genera from the equine gut [68] and elucidating the complexities of the cellulosomal complex from P. finnis [81]. The application of these technologies in large scale enzyme screening has not yet been undertaken, however with continued research would likely prove an invaluable tool in the simultaneous taxonomic and functional assessment of complex microbial ecosystems, in conjunction with traditional phenotypic assays.
Conclusion
The equine gut houses a range of unique fungi, bacteria, archaea, and other microbial groups which act synergistically to break down complex plant fibres that enter the digestive system. The interdependent and symbiotic interactions occurring within the equine gut, including sequential metabolic handoffs and epigenetic alterations of functional gene expression, mark this microbial community as both incredibly complex and powerful in its fibrolytic capacity. Understanding the microbes present within this ecosystem, particularly anaerobic fungi and cellulolytic bacteria, and their functional genes, would have widespread industrial, veterinary and research applications.
Acknowledgements
Not applicable.
Abbreviations
- DNA
Deoxyribonucleic acid
- GIT
Gastrointestinal tract
- SMRT
Single-molecule-real-time sequencing
- Kb
Kilobases
- MAG
Metagenome assembled genome
Author contributions
Wrote the paper: GW, MB, TR, MR, BC. Graphical figures: GW. All authors read and approved the final manuscript.
Funding
This work was funded by Quantal Bioscience Pty Ltd and the Tasmanian Institute of Agriculture, University of Tasmania. The funding bodies did not influence data interpretation in preparation of the manuscript.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
BC, MB and GW declare their involvement in the brand equiGI, a commercial microbiome testing service provided by Quantal Bioscience. TR and MR declare no competing financial or non-financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Georgia Wunderlich, Email: georgia.wunderlich@quantalbioscience.com.
Michelle Bull, Email: michelle.bull@quantalbioscience.com.
Tom Ross, Email: tom.ross@utas.edu.au.
Michael Rose, Email: m.rose@utas.edu.au.
Belinda Chapman, Email: belinda.chapman@quantalbioscience.com.
References
- 1.Trinci APJ, Davies DR, Gull K, Lawrence MI, Bonde Nielsen B, Rickers A, et al. Anaerobic fungi in herbivorous animals. Mycol Res. 1994;98(2):129–152. doi: 10.1016/S0953-7562(09)80178-0. [DOI] [Google Scholar]
- 2.Vermorel M, Martin-Rosset W. Concepts, scientific bases, structure and validation of the French horse net energy system (UFC) Livest Prod Sci. 1997;47(3):261–275. doi: 10.1016/S0301-6226(96)01410-8. [DOI] [Google Scholar]
- 3.Julliand V, de Vaux A, Millet L, Fonty G. Identification of Ruminococcus flavefaciens as the predominant cellulolytic bacterial species of the equine cecum. Appl Environ Microbiol. 1999;65(8):3738–3741. doi: 10.1128/AEM.65.8.3738-3741.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willing B, Vörös A, Roos S, Jones C, Jansson A, Lindberg JE. Changes in faecal bacteria associated with concentrate and forage-only diets fed to horses in training. Equine Vet J. 2009;41(9):908–914. doi: 10.2746/042516409X447806. [DOI] [PubMed] [Google Scholar]
- 5.Al Jassim RAM, Scott PT, Krause D, Denman S, McSweeney CS. Cellulolytic and lactic acid bacteria in the gastrointestinal tract of the horse. Recent Adv Anim Nutr Aust. 2005;15:155–163. [Google Scholar]
- 6.Wahyudi A, Cahyanto MN, Soejono M, Bachruddin Z. Potency of lignocellulose degrading bacteria isolated from buffalo and horse gastrointestinal tract and elephant dung for feed fiber degradation. J Indones Trop Anim Agric. 2010;35(1):34–41. doi: 10.14710/jitaa.35.1.34-41. [DOI] [Google Scholar]
- 7.Shakarami MH, Mohammadabadi T, Motamedi H, Sari M, Teimouri YA. Isolation and identification of cellulolytic bacteria from gastrointestinal tract of Arabian horse and investigation of their effect on the nutritional value of wheat straw. J Appl Microbiol. 2019;127(2):344–353. doi: 10.1111/jam.14251. [DOI] [PubMed] [Google Scholar]
- 8.Harlow BE, Lawrence LM, Flythe MD. Diarrhea-associated pathogens, Lactobacilli and cellulolytic bacteria in equine feces: responses to antibiotic challenge. Vet Microbiol. 2013;166(1):225–232. doi: 10.1016/j.vetmic.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Julliand V, Grimm P. Horse species symposium: the microbiome of the horse hindgut: history and current knowledge1. J Anim Sci. 2016;94(6):2262–2274. doi: 10.2527/jas.2015-0198. [DOI] [PubMed] [Google Scholar]
- 10.Blackmore TM, Dugdale A, Argo CM, Curtis G, Pinloche E, Harris PA, et al. Strong stability and host specific bacterial community in faeces of ponies. PLoS ONE. 2013;8(9):e75079. doi: 10.1371/journal.pone.0075079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dicks LMT, Botha M, Dicks E, Botes M. The equine gastro-intestinal tract: an overview of the microbiota, disease and treatment. Livest Sci. 2014;1(160):69–81. doi: 10.1016/j.livsci.2013.11.025. [DOI] [Google Scholar]
- 12.Santos AS, Rodrigues MAM, Bessa RJB, Ferreira LM, Martin-Rosset W. Understanding the equine cecum-colon ecosystem: current knowledge and future perspectives. Animal. 2011;5(1):48–56. doi: 10.1017/S1751731110001588. [DOI] [PubMed] [Google Scholar]
- 13.Simon JC, Marchesi JR, Mougel C, Selosse MA. Host-microbiota interactions: from holobiont theory to analysis. Microbiome. 2019;7(1):5. doi: 10.1186/s40168-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julliand V, Grimm P. The impact of diet on the hindgut microbiome. J Equine Vet. 2017;1(52):23–28. doi: 10.1016/j.jevs.2017.03.002. [DOI] [Google Scholar]
- 15.Crowell-Davis SL, Houpt KA. Coprophagy by foals: effect of age and possible functions. Equine Vet J. 1985;17(1):17–19. doi: 10.1111/j.2042-3306.1985.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 16.Garber A, Hastie P, Murray JA. Factors influencing equine gut microbiota: current knowledge. J Equine Vet. 2020;1(88):102943. doi: 10.1016/j.jevs.2020.102943. [DOI] [PubMed] [Google Scholar]
- 17.Quercia S, Freccero F, Castagnetti C, Soverini M, Turroni S, Biagi E, et al. Early colonisation and temporal dynamics of the gut microbial ecosystem in Standardbred foals. Equine Vet J. 2019;51(2):231–237. doi: 10.1111/evj.12983. [DOI] [PubMed] [Google Scholar]
- 18.Lindenberg F, Krych L, Kot W, Fielden J, Frøkiær H, van Galen G, et al. Development of the equine gut microbiota. Sci Rep. 2019;9(1):14427. doi: 10.1038/s41598-019-50563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Lei B, Zhang Q, Lei Y, Li C, Li X, et al. ADDAGMA: a database for domestic animal gut microbiome atlas. Comput Struct Biotechnol J. 2022;20:891–898. doi: 10.1016/j.csbj.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argenzio RA, Southworth M, Stevens CE. Sites of organic acid production and absorption in the equine gastrointestinal tract. Am J Physiol. 1974;226(5):1043–1050. doi: 10.1152/ajplegacy.1974.226.5.1043. [DOI] [PubMed] [Google Scholar]
- 21.Hackstein JHP, Baker SE, van Hellemond JJ, Tielens AGM. Hydrogenosomes of anaerobic fungi: an alternative way to adapt to anaerobic environments. In: Tachezy J, editor. Hydrogenosomes and Mitosomes: Mitochondria of Anaerobic Eukaryotes. Cham: Springer International Publishing; 2019. pp. 159–175. [Google Scholar]
- 22.Edwards JE. Equine anaerobic fungi: key taxa of central importance to dietary fibre degradation. In: Small Things: European Equine Health & Nutrion Congress. 2019. pp. 23–31.
- 23.Bonhomme-Florentin A. Essais de culture in vitro des Cycloposthiidae, ciliés commensaux de l’intestin du cheval. Rôle de ces ciliés dans la dégradation de la cellulose. Protistologica (Paris) 1969;5:519–522. [Google Scholar]
- 24.Bonhomme-Florentin A. Attachement des Ciliés du caecum de Cheval aux fragments végétaux.—Dégradation des chloroplastes.—Attachement des bactéries aux Ciliés du caecum. Reprod Nutr Develop. 1985;25(1):127–139. [PubMed]
- 25.Bonhomme-Florentin A. Degradation of hemicellulose and pectin by horse caecum contents. Br J Nutr. 1988;60(1):185–192. doi: 10.1079/BJN19880087. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert RA, Dagar SS, Kittelmann S, Edwards JE. Advances in the understanding of the commensal Eukaryota and viruses of the herbivore gut. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.619287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makhuvele R, Ncube I, Jansen van Rensburg EL, La Grange DC. Isolation of fungi from dung of wild herbivores for application in bioethanol production. Braz J Microbiol. 2017;48:648–655. doi: 10.1016/j.bjm.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houfani AA, Anders N, Spiess AC, Baldrian P, Benallaoua S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—a review. Biomass Bioenerg. 2020;1(134):105481. doi: 10.1016/j.biombioe.2020.105481. [DOI] [Google Scholar]
- 29.Kosheleva YP, Trofimov SY. Characteristics of the biochemical composition of plant litter at different stages of decomposition (according to thermal analysis data) Biol Bull. 2008;35(1):64–69. doi: 10.1134/S106235900801010X. [DOI] [PubMed] [Google Scholar]
- 30.Pérez J, Munoz-Dorado J, De la Rubia T, Martinez J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol. 2002;5(2):53–63. doi: 10.1007/s10123-002-0062-3. [DOI] [PubMed] [Google Scholar]
- 31.Moore BE, Dehority BA. Effects of diet and hindgut defaunation on diet digestibility and microbial concentrations in the cecum and colon of the horse. J Anim Sci. 1993;71(12):3350–3358. doi: 10.2527/1993.71123350x. [DOI] [PubMed] [Google Scholar]
- 32.Muhonen S, Sadet-Bourgeteau S, Julliand V. Effects of differences in fibre composition and maturity of forage-based diets on the microbial ecosystem and its activity in equine caecum and colon digesta and faeces. Animals. 2021;11(8):2337. doi: 10.3390/ani11082337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grenet E, Breton A, Barry P, Fonty G. Rumen anaerobic fungi and plant substrate colonization as affected by diet composition. Anim Feed Sci Technol. 1989;26(1–2):55–70. doi: 10.1016/0377-8401(89)90006-0. [DOI] [Google Scholar]
- 34.Bulmer LS, Murray JA, Burns NM, Garber A, Wemelsfelder F, McEwan NR, et al. High-starch diets alter equine faecal microbiota and increase behavioural reactivity. Sci Rep. 2019;9(9):18621. doi: 10.1038/s41598-019-54039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Detmann E, Paulino MF, Mantovani HC, de Filho SCV, Sampaio CB, de Souza MA, et al. Parameterization of ruminal fibre degradation in low-quality tropical forage using Michaelis-Menten kinetics. Livest Sci. 2009;126(1):136–146. doi: 10.1016/j.livsci.2009.06.013. [DOI] [Google Scholar]
- 36.Warzecha CM, Coverdale JA, Janecka JE, Leatherwood JL, Pinchak WE, Wickersham TA, et al. Influence of short-term dietary starch inclusion on the equine cecal microbiome. J Anim Sci. 2017;95(11):5077–5090. doi: 10.2527/jas2017.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harlow BE, Donley TM, Lawrence LM, Flythe MD. Effect of starch source (corn, oats or wheat) and concentration on fermentation by equine faecal microbiota in vitro. J Appl Microbiol. 2015;119(5):1234–1244. doi: 10.1111/jam.12927. [DOI] [PubMed] [Google Scholar]
- 38.Julliand V, de Fombelle A, Drogoul C, Jacotot E. Feeding and microbial disorders in horses: Part 3—effects of three hay:grain ratios on microbial profile and activities. J Equine Vet. 2001;21(11):543–546. doi: 10.1016/S0737-0806(01)70159-1. [DOI] [Google Scholar]
- 39.Garner HE, Moore JN, Johnson JH, Clark L, Amend JF, Tritschler LG, et al. Changes in the caecal flora associated with the onset of laminitis. Equine Vet J. 1978;10(4):249–252. doi: 10.1111/j.2042-3306.1978.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 40.Weese J, Rousseau J. Evaluation of Lactobacillus pentosus WE7 for prevention of diarrhea in neonatal foals. J Am Vet Med Assoc. 2005;1(226):2031–2034. doi: 10.2460/javma.2005.226.2031. [DOI] [PubMed] [Google Scholar]
- 41.Dougal K, de la Fuente G, Harris PA, Girdwood SE, Pinloche E, Geor RJ, et al. Characterisation of the faecal bacterial community in adult and elderly horses fed a high fibre, high oil or high starch diet using 454 pyrosequencing. PLoS ONE. 2014;9(2):e87424. doi: 10.1371/journal.pone.0087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez A, Sharma AK, Grev A, Sheaffer C, Martinson K. The horse gut microbiome responds in a highly individualized manner to forage lignification. J Equine Vet. 2021;1(96):103306. doi: 10.1016/j.jevs.2020.103306. [DOI] [PubMed] [Google Scholar]
- 43.Wardman JF, Bains RK, Rahfeld P, Withers SG. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat Rev Microbiol. 2022 doi: 10.1038/s41579-022-00712-1. [DOI] [PubMed] [Google Scholar]
- 44.Barrett K, Jensen K, Meyer AS, Frisvad JC, Lange L. Fungal secretome profile categorization of CAZymes by function and family corresponds to fungal phylogeny and taxonomy: example Aspergillus and Penicillium. Sci Rep. 2020;20(10):5158. doi: 10.1038/s41598-020-61907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drula E, Garron ML, Dogan S, Lombard V, Henrissat B, Terrapon N. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 2022;50(D1):D571–D577. doi: 10.1093/nar/gkab1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma A, Tewari R, Rana SS, Soni R, Soni SK. Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotechnol. 2016;179(8):1346–1380. doi: 10.1007/s12010-016-2070-3. [DOI] [PubMed] [Google Scholar]
- 47.Ndlovu TM, Van Wyk JPH. Isolation of cellulase enzyme from brown garden snail (Cornu aspersum) for the saccharification of waste paper materials. MethodsX. 2019;1(6):1030–1035. doi: 10.1016/j.mex.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue XM, Anderson AJ, Richardson NA, Anderson AJ, Xue GP, Mather PB. Characterisation of cellulase activity in the digestive system of the redclaw crayfish (Cherax quadricarinatus) Aquaculture. 1999;180(3):373–386. doi: 10.1016/S0044-8486(99)00213-6. [DOI] [Google Scholar]
- 49.Tokuda G, Watanabe H. Hidden cellulases in termites: revision of an old hypothesis. Biol Lett. 2007;3(3):336–339. doi: 10.1098/rsbl.2007.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakeer S, Varma M, Sharma J, Mattoo F, Gupta D, Singh J, et al. Metagenomic analysis of the fecal microbiome of an adult elephant reveals the diversity of CAZymes related to lignocellulosic biomass degradation. Symbiosis. 2020;81(3):209–222. doi: 10.1007/s13199-020-00695-8. [DOI] [Google Scholar]
- 51.Yi Y. Tiny bugs play big role: Microorganisms’ contribution to biofuel production. In: Lü X, editor. Advances in 2nd Generation of Bioethanol Production. Woodhead Publishing; 2021. pp. 113–136. [Google Scholar]
- 52.Cragg SM, Beckham GT, Bruce NC, Bugg TD, Distel DL, Dupree P, et al. Lignocellulose degradation mechanisms across the tree of life. Curr Opin Chem Biol. 2015;1(29):108–119. doi: 10.1016/j.cbpa.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janusz G, Pawlik A, Sulej J, Świderska-Burek U, Jarosz-Wilkołazka A, Paszczyński A. Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev. 2017;41(6):941–962. doi: 10.1093/femsre/fux049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ufarté L, Potocki-Veronese G, Cecchini D, Tauzin AS, Rizzo A, Morgavi DP, et al. Highly promiscuous oxidases discovered in the bovine rumen microbiome. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lillington SP, Leggieri PA, Heom KA, O’Malley MA. Nature’s recyclers: anaerobic microbial communities drive crude biomass deconstruction. Curr Opin Biotechnol. 2020;1(62):38–47. doi: 10.1016/j.copbio.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 56.Solomon KV, Haitjema CH, Henske JK, Gilmore SP, Borges-Rivera D, Lipzen A, Brewer HM, Purvine SO, Wright AT, Theodorou MK, Grigoriev IV. Early-branching gut fungi possess a large, comprehensive array of biomass-degrading enzymes. Science. 2016;351(6278):1192–1195. doi: 10.1126/science.aad1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park T, Wijeratne S, Meulia T, Firkins JL, Yu Z. The macronuclear genome of anaerobic ciliate Entodinium caudatum reveals its biological features adapted to the distinct rumen environment. Genomics. 2021;113(3):1416–1427. doi: 10.1016/j.ygeno.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Mach N, Midoux C, Leclercq S, et al. Mining the equine gut metagenome: poorly-characterized taxa associated with cardiovascular fitness in endurance athletes. Commun Biol. 2022;5:1032. doi: 10.1038/s42003-022-03977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lillington SP, Chrisler W, Haitjema CH, Gilmore SP, Smallwood CR, Shutthanandan V, et al. Cellulosome localization patterns vary across life stages of anaerobic fungi. mBio. 2021;12(3):e00832–e921. doi: 10.1128/mBio.00832-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doi RH. Cellulases of mesophilic microorganisms: cellulosome and noncellulosome producers. Ann N Y Acad Sci. 2008;1125(1):267–279. doi: 10.1196/annals.1419.002. [DOI] [PubMed] [Google Scholar]
- 61.Fontes CM, Gilbert HJ. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu Rev Biochem. 2010;79:655–681. doi: 10.1146/annurev-biochem-091208-085603. [DOI] [PubMed] [Google Scholar]
- 62.Hess M, Paul SS, Puniya AK, van der Giezen M, Shaw C, Edwards JE, et al. Anaerobic fungi: past, present, and future. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.584893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boxma B, Voncken F, Jannink S, Van Alen T, Akhmanova A, Van Weelden SWH, et al. The anaerobic chytridiomycete fungus Piromyces sp. E2 produces ethanol via pyruvate:formate lyase and an alcohol dehydrogenase E. Mol Microbiol. 2004;51(5):1389–1399. doi: 10.1046/j.1365-2958.2003.03912.x. [DOI] [PubMed] [Google Scholar]
- 64.Gruninger RJ, Puniya AK, Callaghan TM, Edwards JE, Youssef N, Dagar SS, et al. Anaerobic fungi (phylum Neocallimastigomycota): advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol Ecol. 2014;90(1):1–17. doi: 10.1111/1574-6941.12383. [DOI] [PubMed] [Google Scholar]
- 65.Gold JJ, Brent Heath I, Bauchop T. Ultrastructural description of a new chytrid genus of caecum anaerobe, Caecomyces equi gen. nov., sp. Nov., assigned to the Neocallimasticaceae. Biosystems. 1988;21(3):403–415. doi: 10.1016/0303-2647(88)90039-1. [DOI] [PubMed] [Google Scholar]
- 66.Hooker CA, Lee KZ, Solomon KV. Leveraging anaerobic fungi for biotechnology. Curr Opin Biotechnol. 2019;1(59):103–110. doi: 10.1016/j.copbio.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Drake H, Ivarsson M. The role of anaerobic fungi in fundamental biogeochemical cycles in the deep biosphere. Fungal Biol Rev. 2018;32(1):20–25. doi: 10.1016/j.fbr.2017.10.001. [DOI] [Google Scholar]
- 68.Hanafy RA, Lanjekar VB, Dhakephalkar PK, Callaghan TM, Dagar SS, Griffith GW, et al. Seven new Neocallimastigomycota genera from wild, zoo-housed, and domesticated herbivores greatly expand the taxonomic diversity of the phylum. Mycologia. 2020;112(6):1212–1239. doi: 10.1080/00275514.2019.1696619. [DOI] [PubMed] [Google Scholar]
- 69.Hanafy RA, Elshahed MS, Liggenstoffer AS, Griffith GW, Youssef NH. Pecoramyces ruminantium, gen. nov., sp. nov., an anaerobic gut fungus from the feces of cattle and sheep. Mycologia. 2017;109(2):231–243. doi: 10.1080/00275514.2017.1317190. [DOI] [PubMed] [Google Scholar]
- 70.Joshi A, Lanjekar VB, Dhakephalkar PK, Callaghan TM, Griffith GW, Dagar SS. Liebetanzomyces polymorphus gen. et sp. nov., a new anaerobic fungus (Neocallimastigomycota) isolated from the rumen of a goat. MycoKeys. 2018;40:89–110. doi: 10.3897/mycokeys.40.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stabel M, Hanafy RA, Schweitzer T, Greif M, Aliyu H, Flad V, et al. Aestipascuomyces dupliciliberans gen. nov., sp. nov., the first cultured representative of the uncultured sk4 clade from aoudad sheep and alpaca. Microorganisms. 2020;8(11):1734. doi: 10.3390/microorganisms8111734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang J, Park H. Nucleotide and protein researches on anaerobic fungi during four decades. J Anim Sci Technol. 2020;62(2):121–140. doi: 10.5187/jast.2020.62.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edwards JE, Shetty SA, Van Den Berg P, Burden F, Van Doorn DA, Pellikaan WF, et al. Multi-kingdom characterization of the core equine fecal microbiota based on multiple equine (sub) species. Anim Microb. 2020;2(1):1–16. doi: 10.1186/s42523-020-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liggenstoffer AS, Youssef NH, Couger MB, Elshahed MS. Phylogenetic diversity and community structure of anaerobic gut fungi (phylum Neocallimastigomycota) in ruminant and non-ruminant herbivores. ISME J. 2010;4(10):1225–1235. doi: 10.1038/ismej.2010.49. [DOI] [PubMed] [Google Scholar]
- 75.Seppälä S, Solomon KV, Gilmore SP, Henske JK, O’Malley MA. Mapping the membrane proteome of anaerobic gut fungi identifies a wealth of carbohydrate binding proteins and transporters. Microb Cell Fact. 2016;15(1):212. doi: 10.1186/s12934-016-0611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Heath IB, Bauchop T. Piromyces mae and Piromyces dumbonica, two new species of uniflagellate anaerobic chytridiomycete fungi from the hindgut of the horse and elephant. Can J Bot. 1990;68(5):1021–1033. doi: 10.1139/b90-129. [DOI] [Google Scholar]
- 77.Orpin CG. Isolation of cellulolytic phycomycete fungi from the caecum of the horse. Microbiology. 1981;123(2):287–96. doi: 10.1099/00221287-123-2-287. [DOI] [PubMed] [Google Scholar]
- 78.Breton A, Dusser M, Gaillard-Martinie B, Guillot J, Millet L, Prensier G. Piromyces rhizinflata nov. sp., a strictly anaerobic fungus from faeces of the Saharian ass: a morphological, metabolic and ultrastructural study. FEMS Microbiol Lett. 1991;82(1):1–8. doi: 10.1111/j.1574-6968.1991.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 79.Gaillard-Martinie B, Breton A, Dusser M, Julliand V. Piromyces citronii sp. nov., a strictly anaerobic fungus from the equine caecum: a morphological, metabolic, and ultrastructural study. FEMS Microbiol Lett. 1995;130(2–3):321–326. doi: 10.1111/j.1574-6968.1995.tb07738.x. [DOI] [Google Scholar]
- 80.Mura E, Edwards J, Kittelmann S, Kaerger K, Voigt K, Mrázek J, et al. Anaerobic fungal communities differ along the horse digestive tract. Fungal Biol. 2019;123(3):240–246. doi: 10.1016/j.funbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Haitjema CH, Gilmore SP, Henske JK, Solomon KV, de Groot R, Kuo A, et al. A parts list for fungal cellulosomes revealed by comparative genomics. Nat Microbiol. 2017;2(8):1–8. doi: 10.1038/nmicrobiol.2017.87. [DOI] [PubMed] [Google Scholar]
- 82.Seppälä S, Wilken STE, Knop D, Solomon KV, O’Malley MA. The importance of sourcing enzymes from non-conventional fungi for metabolic engineering and biomass breakdown. Metab Eng. 2017;44:45–59. doi: 10.1016/j.ymben.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Joblin KN, Naylor GE. Fermentation of woods by rumen anaerobic fungi. FEMS Microbiol Lett. 1989;65(1–2):119–122. doi: 10.1111/j.1574-6968.1989.tb03608.x. [DOI] [Google Scholar]
- 84.Orpin CG. The rumen flagellate Piromonas communis: its life-history and invasion of plant material in the rumen. J Gen Microbiol. 1977;99(1):107–117. doi: 10.1099/00221287-99-1-107. [DOI] [PubMed] [Google Scholar]
- 85.Youssef NH, Couger MB, Struchtemeyer CG, Liggenstoffer AS, Prade RA, Najar FZ, et al. The genome of the anaerobic fungus Orpinomyces sp. strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Appl Environ Microbiol. 2013. [DOI] [PMC free article] [PubMed]
- 86.Theodorou MK, Mennim G, Davies DR, Zhu WY, Trinci APJ, Brookman JL. Anaerobic fungi in the digestive tract of mammalian herbivores and their potential for exploitation. Proc Nutr Soc. 1996;55(3):913–926. doi: 10.1079/PNS19960088. [DOI] [PubMed] [Google Scholar]
- 87.Gordon GLR, Phillips MW. Removal of anaerobic fungi from the rumen of sheep by chemical treatment and the effect on feed consumption and in vivo fibre digestion. Lett Appl Microbiol. 1993;17(5):220–223. doi: 10.1111/j.1472-765X.1993.tb01451.x. [DOI] [Google Scholar]
- 88.Tielens AG, Hellemond JJV. Anaerobic mitochondria: properties and origins. In: Martin WF, Müller M, editors. Origin of Mitochondria and Hydrogenosomes. Springer; 2007. p. 85–103.
- 89.Hackstein JH, Tjaden J, Huynen M. Mitochondria, hydrogenosomes and mitosomes: products of evolutionary tinkering! Curr Genet. 2006;50(4):225–245. doi: 10.1007/s00294-006-0088-8. [DOI] [PubMed] [Google Scholar]
- 90.Cuddeford D, Pearson RA, Archibald RF, Muirhead RH. Digestibility and gastro-intestinal transit time of diets containing different proportions of alfalfa and oat straw given to Thoroughbreds, Shetland ponies, Highland ponies and donkeys. Anim Sci. 1995;61(2):407–417. doi: 10.1017/S1357729800013953. [DOI] [Google Scholar]
- 91.Julliand V, Riondet C, de Vaux A, Alcaraz G, Fonty G. Comparison of metabolic activities between Piromyces citronii, an equine fungal species, and Piromyces communis, a ruminal species. Anim Feed Sci Technol. 1998;70(1–2):161–168. doi: 10.1016/S0377-8401(97)00043-6. [DOI] [Google Scholar]
- 92.Harhangi HR, Freelove ACJ, Ubhayasekera W, Van Dinther M, Steenbakkers PJM, Akhmanova A, et al. Cel6A, a major exoglucanase from the cellulosome of the anaerobic fungi Piromyces sp. E2 and Piromyces equi. Biochimica et Biophysica Acta (BBA) Gene Struct Expr. 2003;1628(1):30–39. doi: 10.1016/S0167-4781(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 93.Koivula A, Ruohonen L, Wohlfahrt G, Reinikainen T, Teeri TT, Piens K, et al. The active site of cellobiohydrolase Cel6A from Trichoderma reesei: the roles of aspartic acids d221 and d175. J Am Chem Soc. 2002;124(34):10015–10024. doi: 10.1021/ja012659q. [DOI] [PubMed] [Google Scholar]
- 94.Edwards JE, Forster RJ, Callaghan TM, Dollhofer V, Dagar SS, Cheng Y, et al. PCR and Omics based techniques to study the diversity, ecology and biology of anaerobic fungi: insights, challenges and opportunities. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu N, Yu W, Guo X, Chen J, Xia D, Yu J, et al. Oxidative cleavage of cellulose in the horse gut. Microb Cell Fact. 2022;21(1):38. doi: 10.1186/s12934-022-01767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hutchinson MI, Powell AJ, Herrera J, Natvig DO. New perspectives on the distribution and roles of thermophilic fungi. In: Tiquia-Arashiro SM, Grube M, editors. Fungi in Extreme Environments: Ecological Role and Biotechnological Significance. Cham: Springer International Publishing; 2019. pp. 59–80. [Google Scholar]
- 97.Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, et al. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci U S A. 2008;105(31):10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tansey MR, Brock TD. The upper temperature limit for eukaryotic organisms. Proc Natl Acad Sci. 1972;69(9):2426–2428. doi: 10.1073/pnas.69.9.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li DC, Lu M, Li YL, Lu J. Purification and characterization of an endocellulase from the thermophilic fungus Chaetomium thermophilum CT2. Enzyme Microb Technol. 2003;33(7):932–937. doi: 10.1016/S0141-0229(03)00245-X. [DOI] [Google Scholar]
- 100.Li AN, Yu K, Liu HQ, Zhang J, Li H, Li DC. Two novel thermostable chitinase genes from thermophilic fungi: cloning, expression and characterization. Biores Technol. 2010;101(14):5546–5551. doi: 10.1016/j.biortech.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 101.Vaaje-Kolstad G, Forsberg Z, Loose JS, Bissaro B, Eijsink VG. Structural diversity of lytic polysaccharide monooxygenases. Curr Opin Struct Biol. 2017;44:67–76. doi: 10.1016/j.sbi.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 102.Dujon BA, Louis EJ. Genome diversity and evolution in the budding yeasts (Saccharomycotina) Genetics. 2017;206(2):717–750. doi: 10.1534/genetics.116.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yurkov A. Temporal and geographic patterns in yeast distribution. In: Buzzini P, Lachance MA, Yurkov A, editors. Yeasts in Natural Ecosystems: Ecology. Springer; 2017. p. 101–30.
- 104.Gabaldón T, Naranjo-Ortíz MA, Marcet-Houben M. Evolutionary genomics of yeast pathogens in the Saccharomycotina. FEMS Yeast Res. 2016;16(6):fow064. doi: 10.1093/femsyr/fow064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Uden N, Sousa LDC, Farinha M. On the intestinal yeast flora of horses, sheep, goats and swine. Microbiology. 1958;19(3):435–445. doi: 10.1099/00221287-19-3-435. [DOI] [PubMed] [Google Scholar]
- 106.Contamine V, Picard M. Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol Mol Biol Rev. 2000;64(2):281–315. doi: 10.1128/MMBR.64.2.281-315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lorliam W, Akaracharanya A, Jindamorakot S, Suwannarangsee S, Tanasupawat S. Characterization of xylose-utilizing yeasts isolated from herbivore faeces in Thailand. ScienceAsia. 2013;39(1):26. doi: 10.2306/scienceasia1513-1874.2013.39.026. [DOI] [Google Scholar]
- 108.Schäfer A, Konrad R, Kuhnigk T, Kämpfer P, Hertel H, König H. Hemicellulose-degrading bacteria and yeasts from the termite gut. J Appl Bacteriol. 1996;80(5):471–478. doi: 10.1111/j.1365-2672.1996.tb03245.x. [DOI] [PubMed] [Google Scholar]
- 109.Suh SO, Marshall CJ, Mchugh JV, Blackwell M. Wood ingestion by passalid beetles in the presence of xylose-fermenting gut yeasts. Mol Ecol. 2003;12(11):3137–3145. doi: 10.1046/j.1365-294X.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 110.Molnár O, Wuczkowski M, Prillinger H. Yeast biodiversity in the guts of several pests on maize; comparison of three methods: classical isolation, cloning and DGGE. Mycol Prog. 2008;7(2):111–123. doi: 10.1007/s11557-008-0558-0. [DOI] [Google Scholar]
- 111.Gilroy R, Leng J, Ravi A, Adriaenssens EM, Oren A, Baker D, et al. Metagenomic investigation of the equine faecal microbiome reveals extensive taxonomic diversity. PeerJ. 2022;23(10):e13084. doi: 10.7717/peerj.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quigley EMM. Gut bacteria in health and disease. Gastroenterol Hepatol (N Y) 2013;9(9):560–569. [PMC free article] [PubMed] [Google Scholar]
- 113.Koike S, Kobayashi Y. Fibrolytic rumen bacteria: their ecology and functions. Asian Australas J Anim Sci. 2009;22(1):131–138. doi: 10.5713/ajas.2009.r.01. [DOI] [Google Scholar]
- 114.Varel VH, Jung HJG. Influence of forage phenolics on ruminal fibrolytic bacteria and in vitro fiber degradation. Appl Environ Microbiol. 1986;52(2):275–280. doi: 10.1128/aem.52.2.275-280.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Michalet-Doreau B, Fernandez I, Peyron C, Millet L, Fonty G. Fibrolytic activities and cellulolytic bacterial community structure in the solid and liquid phases of rumen contents. Reprod Nutr Dev. 2001;41(2):187–194. doi: 10.1051/rnd:2001122. [DOI] [PubMed] [Google Scholar]
- 116.Koike S, Pan J, Kobayashi Y, Tanaka K. Kinetics of in sacco fiber-attachment of representative ruminal cellulolytic bacteria monitored by competitive PCR. J Dairy Sci. 2003;86(4):1429–1435. doi: 10.3168/jds.S0022-0302(03)73726-6. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi Y, Shinkai T, Koike S. Ecological and physiological characterization shows that Fibrobacter succinogenes is important in rumen fiber digestion—review. Folia Microbiol. 2008;53(3):195–200. doi: 10.1007/s12223-008-0024-z. [DOI] [PubMed] [Google Scholar]
- 118.Xie X, Yang C, Guan LL, Wang J, Xue M, Liu JX. Persistence of cellulolytic bacteria Fibrobacter and Treponema after short-term corn stover-based dietary intervention reveals the potential to improve rumen fibrolytic function. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shinkai T, Ueki T, Kobayashi Y. Detection and identification of rumen bacteria constituting a fibrolytic consortium dominated by Fibrobacter succinogenes. Anim Sci J. 2010;81(1):72–79. doi: 10.1111/j.1740-0929.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 120.Shinkai T, Kobayashi Y. Localization of ruminal cellulolytic bacteria on plant fibrous materials as determined by fluorescence in situ hybridization and real-time pcr. Appl Environ Microbiol. 2007;73(5):1646–1652. doi: 10.1128/AEM.01896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miron J, Ben-Ghedalia D, Morrison M. Adhesion mechanisms of rumen cellulolytic bacteria. J Dairy Sci. 2001;84(6):1294–1309. doi: 10.3168/jds.S0022-0302(01)70159-2. [DOI] [PubMed] [Google Scholar]
- 122.Russell JB, Muck RE, Weimer PJ. Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol Ecol. 2009;67(2):183–197. doi: 10.1111/j.1574-6941.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- 123.Castillo-González A, Burrola-Barraza M, Domínguez-Viveros J, Chávez-Martínez A. Rumen microorganisms and fermentation. Arch Med Vet. 2014;46(3):349–361. doi: 10.4067/S0301-732X2014000300003. [DOI] [Google Scholar]
- 124.Liu L, Huang WC, Liu Y, Li M. Diversity of cellulolytic microorganisms and microbial cellulases. Int Biodeterior Biodegrad. 2021;1(163):105277. doi: 10.1016/j.ibiod.2021.105277. [DOI] [Google Scholar]
- 125.McDonald JE, Rooks DJ, McCarthy AJ. Methods for the isolation of cellulose-degrading microorganisms. Meth Enzymol. 2012;510:349–74. doi: 10.1016/B978-0-12-415931-0.00019-7. [DOI] [PubMed] [Google Scholar]
- 126.Sadet-Bourgeteau S, Julliand V. La diversité de l’écosystème microbien du tractus digestif équin. INRAE Product Anim. 2012;25(5):407–418. doi: 10.20870/productions-animales.2012.25.5.3228. [DOI] [Google Scholar]
- 127.Tringe SG, Rubin EM. Metagenomics: DNA sequencing of environmental samples. Nat Rev Genet. 2005;6(11):805–814. doi: 10.1038/nrg1709. [DOI] [PubMed] [Google Scholar]
- 128.Tedersoo L, Anslan S, Bahram M, Kõljalg U, Abarenkov K. Identifying the ‘unidentified’ fungi: a global-scale long-read third-generation sequencing approach. Fungal Diversity. 2020;103(1):273–293. doi: 10.1007/s13225-020-00456-4. [DOI] [Google Scholar]
- 129.Nielsen HB, Almeida M, Juncker AS, Rasmussen S, Li J, Sunagawa S, et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat Biotechnol. 2014;32(8):822–828. doi: 10.1038/nbt.2939. [DOI] [PubMed] [Google Scholar]
- 130.Ijaq J, Chandrasekharan M, Poddar R, Bethi N, Sundararajan VS. Annotation and curation of uncharacterized proteins- challenges. Front Gen. 2015 doi: 10.3389/fgene.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hagen LH, Brooke CG, Shaw CA, Norbeck AD, Piao H, Arntzen MØ, et al. Proteome specialization of anaerobic fungi during ruminal degradation of recalcitrant plant fiber. ISME J. 2021;15(2):421–434. doi: 10.1038/s41396-020-00769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kauter A, Epping L, Semmler T, Antao EM, Kannapin D, Stoeckle SD, et al. The gut microbiome of horses: current research on equine enteral microbiota and future perspectives. Anim Microb. 2019;1(1):1–15. doi: 10.1186/s42523-019-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut Lachnospiraceae. Microorganisms. 2020;8(4):573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anderson CJ, Koester LR, Schmitz-Esser S. Rumen epithelial communities share a core bacterial microbiota: a meta-analysis of 16S rRNA gene Illumina MiSeq sequencing datasets. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.625400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cotta M, Forster R. The family Lachnospiraceae, including the genera Butyrivibrio,Lachnospira and Roseburia. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The Prokaryotes; Springer. 2006; p. 1002–21.
- 136.Boutard M, Cerisy T, Nogue PY, Alberti A, Weissenbach J, Salanoubat M, et al. Functional diversity of carbohydrate-active enzymes enabling a bacterium to ferment plant biomass. PLoS Genet. 2014;10(11):e1004773. doi: 10.1371/journal.pgen.1004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roediger WEW. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83(2):424–429. doi: 10.1016/S0016-5085(82)80339-9. [DOI] [PubMed] [Google Scholar]
- 138.Ze X, Le Mougen F, Duncan SH, Louis P, Flint HJ. Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes. 2013;4(3):236–240. doi: 10.4161/gmic.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bule P, Alves VD, Israeli-Ruimy V, Carvalho AL, Ferreira LMA, Smith SP, et al. Assembly of Ruminococcus flavefaciens cellulosome revealed by structures of two cohesin-dockerin complexes. Sci Rep. 2017;7(1):759. doi: 10.1038/s41598-017-00919-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hastie P, Mitchell K, Murray JA. Semi-quantitative analysis of Ruminococcus flavefaciens, Fibrobacter succinogenes and Streptococcus bovis in the equine large intestine using real-time polymerase chain reaction. Br J Nutr. 2008;1(100):561–568. doi: 10.1017/S0007114508968227. [DOI] [PubMed] [Google Scholar]
- 142.Bule P, Alves VD, Leitão A, Ferreira LMA, Bayer EA, Smith SP, et al. Single binding mode integration of hemicellulose-degrading enzymes via adaptor scaffoldins in Ruminococcus flavefaciens cellulosome. J Biol Chem. 2016;291(52):26658–26669. doi: 10.1074/jbc.M116.761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Z, Liu H, Vera AM, Bernardi RC, Tinnefeld P, Nash MA. High force catch bond mechanism of bacterial adhesion in the human gut. Nat Commun. 2020;11(1):4321. doi: 10.1038/s41467-020-18063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujimori S. Humans have intestinal bacteria that degrade the plant cell walls in herbivores. World J Gastroenterol. 2021;27(45):7784–7791. doi: 10.3748/wjg.v27.i45.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dehority BA. Effects of microbial synergism on fibre digestion in the rumen. Proc Nutr Soc. 1991;50(2):149–159. doi: 10.1079/PNS19910026. [DOI] [PubMed] [Google Scholar]
- 146.Schink B. Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek. 2002;81(1):257–261. doi: 10.1023/A:1020579004534. [DOI] [PubMed] [Google Scholar]
- 147.Miura H, Horiguchi M, Ogimoto K, Matsumoto T. Nutritional interdependence among rumen bacteria during cellulose digestion in vitro. Appl Environ Microbiol. 1983;45(2):726–729. doi: 10.1128/aem.45.2.726-729.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wolin MJ, Miller TL, Stewart CS. Microbe-microbe interactions. In: Hobson PN, Stewart CS, editors. The Rumen Microbial Ecosystem. Springer; 1997. pp. 467–91. [Google Scholar]
- 149.Paul SS, Deb SM, Punia BS, Singh D, Kumar R. Fibrolytic potential of anaerobic fungi (Piromyces sp.) isolated from wild cattle and blue bulls in pure culture and effect of their addition on in vitro fermentation of wheat straw and methane emission by rumen fluid of buffaloes. J Sci Food Agric. 2010;90(7):1218–1226. doi: 10.1002/jsfa.3952. [DOI] [PubMed] [Google Scholar]
- 150.Paul SS, Kamra DN, Sastry VRB, Sahu NP, Agarwal N. Effect of administration of an anaerobic gut fungus isolated from wild blue bull (Boselaphus tragocamelus) to buffaloes (Bubalus bubalis) on in vivo ruminal fermentation and digestion of nutrients. Anim Feed Sci Technol. 2004;115(1):143–157. doi: 10.1016/j.anifeedsci.2004.01.010. [DOI] [Google Scholar]
- 151.Arambel MJ, Wiedmeier RD, Walters JL. Influence of donor animal adaptation to added yeast culture and/or Aspergillus oryzae fermentation extract on in vitro rumen fermentation. Nutr Rep Int. 1987;35(3):433–437. [Google Scholar]
- 152.Lee SS, Ha JK, Cheng KJ. Influence of an anaerobic fungal culture administration on in vivo ruminal fermentation and nutrient digestion. Anim Feed Sci Technol. 2000;88(3):201–217. doi: 10.1016/S0377-8401(00)00216-9. [DOI] [Google Scholar]
- 153.Moya D, Ferret A, Blanch M, Fuentes MC, Fandiño JI, Calsamiglia S. Effects of live yeast (Saccharomyces cerevisiae) and type of cereal on rumen microbial fermentation in a dual flow continuous culture fermentation system. J Anim Physiol Anim Nutr. 2018;102(6):1488–1496. doi: 10.1111/jpn.12975. [DOI] [PubMed] [Google Scholar]
- 154.Elghandour MMY, Tan ZL, Abu Hafsa SH, Adegbeye MJ, Greiner R, Ugbogu EA, et al. Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: a review. J Appl Microbiol. 2020;128(3):658–674. doi: 10.1111/jam.14416. [DOI] [PubMed] [Google Scholar]
- 155.Taran FMP, Gobesso A, Gonzaga IVF, Françoso R, Centini TN, Moreira CG, et al. Effects of different amounts of Saccharomyces cerevisiae supplementation on apparent digestibility and faecal parameters in horses fed high-roughage and high-concentrate diets. Livest Sci. 2015;1:186. [Google Scholar]
- 156.Jouany JP, Medina B, Bertin G, Julliand V. Effect of live yeast culture supplementation on hindgut microbial communities and their polysaccharidase and glycoside hydrolase activities in horses fed a high-fiber or high-starch diet. J Anim Sci. 2009;87(9):2844–2852. doi: 10.2527/jas.2008-1602. [DOI] [PubMed] [Google Scholar]
- 157.Gobesso AAO, Pombo GV, Costa RL, Pereira YS, Feltre K. Effect of yeast supplementation on digestibility, fecal microbiota and serum endotoxin levels in non-exercising and exercising horses. Livest Sci. 2018;215:21–24. doi: 10.1016/j.livsci.2017.10.023. [DOI] [Google Scholar]
- 158.Medina B, Girard ID, Jacotot E, Julliand V. Effect of a preparation of Saccharomyces cerevisiae on microbial profiles and fermentation patterns in the large intestine of horses fed a high fiber or a high starch diet. J Anim Sci. 2002;80(10):2600–2609. doi: 10.2527/2002.80102600x. [DOI] [PubMed] [Google Scholar]
- 159.Murray JAMD, Brown S, O’Shaughnessy P, Monteiro A, Warren H, Hastie PM. Effect of live yeast culture supplementation on fibrolytic and saccharolytic bacterial populations in the feces of horses fed a high-fiber or high-starch diet. J Equine Vet. 2017;51:41–45. doi: 10.1016/j.jevs.2016.12.009. [DOI] [Google Scholar]
- 160.St-Pierre B, Wright ADG. Diversity of gut methanogens in herbivorous animals. Animal. 2013;1(7):49–56. doi: 10.1017/S1751731112000912. [DOI] [PubMed] [Google Scholar]
- 161.Morvan B, Rieu-Lesme F, Fonty G, Gouet P. In vitro Interactions between rumen H2-producing cellulolytic microorganisms and H2-utilizing acetogenic and sulfate-reducing bacteria. Anaerobe. 1996;2(3):175–180. doi: 10.1006/anae.1996.0023. [DOI] [Google Scholar]
- 162.Murru F, Fliegerova K, Mura E, Mrázek J, Kopečný J, Moniello G. A comparison of methanogens of different regions of the equine hindgut. Anaerobe. 2018;1(54):104–110. doi: 10.1016/j.anaerobe.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 163.Evans PN, Boyd JA, Leu AO, Woodcroft BJ, Parks DH, Hugenholtz P, et al. An evolving view of methane metabolism in the Archaea. Nat Rev Microbiol. 2019;17(4):219–232. doi: 10.1038/s41579-018-0136-7. [DOI] [PubMed] [Google Scholar]
- 164.Jensen BB. Methanogenesis in monogastric animals. Environ Monit Assess. 1996;42(1):99–112. doi: 10.1007/BF00394044. [DOI] [PubMed] [Google Scholar]
- 165.Li Y, Meng Z, Xu Y, Shi Q, Ma Y, Aung M, et al. Interactions between anaerobic fungi and methanogens in the rumen and their biotechnological potential in biogas production from lignocellulosic materials. Microorganisms. 2021;9(1):190. doi: 10.3390/microorganisms9010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown JL, Swift CL, Mondo SJ, Seppala S, Salamov A, Singan V, et al. Co-cultivation of the anaerobic fungus Caecomyces churrovis with Methanobacterium bryantii enhances transcription of carbohydrate binding modules, dockerins, and pyruvate formate lyases on specific substrates. Biotechnol Biofuels. 2021;10(14):234. doi: 10.1186/s13068-021-02083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacol. 2013;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Várnai A, Mäkelä MR, Djajadi DT, Rahikainen J, Hatakka A, Viikari L. Carbohydrate-binding modules of fungal cellulases: occurrence in nature, function, and relevance in industrial biomass conversion. In: Sariaslani S, Gadd GM, editors. Advances in Applied Microbiology. Academic Press; 2014. pp. 103–65. [DOI] [PubMed] [Google Scholar]
- 169.Ståhlberg J, Johansson G, Pettersson G. A new model for enzymatic hydrolysis of cellulose based on the two-domain structure of cellobiohydrolase I. Bio/Technology. 1991;9(3):286–290. [Google Scholar]
- 170.Tomme P, Van Tilbeurgh H, Pettersson G, van Damme J, Vandekerckhove J, Knowles J, et al. Studies of the cellulolytic system of Trichoderma reesei QM 9414: analysis of domain function in two cellobiohydrolases by limited proteolysis. Eur J Biochem. 1988;170(3):575–581. doi: 10.1111/j.1432-1033.1988.tb13736.x. [DOI] [PubMed] [Google Scholar]
- 171.Van Tilbeurgh H, Tomme P, Claeyssens M, Bhikhabhai R, Pettersson G. Limited proteolysis of the cellobiohydrolase I from Trichoderma reesei: separation of functional domains. FEBS Lett. 1986;204(2):223–227. doi: 10.1016/0014-5793(86)80816-X. [DOI] [Google Scholar]
- 172.Reinikainen T, Ruohonen L, Nevanen T, Laaksonen L, Kraulis P, Jones TA, et al. Investigation of the function of mutated cellulose-binding domains of Trichoderma reesei cellobiohydrolase I. Proteins Struct Funct Bioinf. 1992;14(4):475–482. doi: 10.1002/prot.340140408. [DOI] [PubMed] [Google Scholar]
- 173.Limón MC, Margolles-Clark E, Benítez T, Penttilä M. Addition of substrate-binding domains increases substrate-binding capacity and specific activity of a chitinase from Trichoderma harzianum. FEMS Microbiol Lett. 2001;198(1):57–63. doi: 10.1016/S0378-1097(01)00124-0. [DOI] [PubMed] [Google Scholar]
- 174.Dougal K, de la Fuente G, Harris PA, Girdwood SE, Pinloche E, Newbold CJ. Identification of a core bacterial community within the large intestine of the horse. PLoS ONE. 2013;8(10):e77660. doi: 10.1371/journal.pone.0077660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Edwards JE, Hermes GDA, Kittelmann S, Nijsse B, Smidt H. Assessment of the accuracy of high-throughput sequencing of the ITS1 region of Neocallimastigomycota for community composition analysis. Front Microbiol. 2019;10:2370. doi: 10.3389/fmicb.2019.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peng X, Wilken SE, Lankiewicz TS, Gilmore SP, Brown JL, Henske JK, et al. Genomic and functional analyses of fungal and bacterial consortia that enable lignocellulose breakdown in goat gut microbiomes. Nat Microbiol. 2021;6(4):499–511. doi: 10.1038/s41564-020-00861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Navarro D, Chaduli D, Taussac S, Lesage-Meessen L, Grisel S, Haon M, et al. Large-scale phenotyping of 1,000 fungal strains for the degradation of non-natural, industrial compounds. Commun Biol. 2021;4(1):1–10. doi: 10.1038/s42003-021-02401-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Comtet-Marre S, Chaucheyras-Durand F, Bouzid O, Mosoni P, Bayat A, Peyret P, et al. FibroChip, a functional dna microarray to monitor cellulolytic and hemicellulolytic activities of rumen microbiota. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rhoads A, Au KF. PacBio sequencing and its applications. Genom Proteom Bioinf. 2015;13(5):278–289. doi: 10.1016/j.gpb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kono N, Arakawa K. Nanopore sequencing: review of potential applications in functional genomics. Dev Growth Differ. 2019;61(5):316–326. doi: 10.1111/dgd.12608. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y, Zhao Y, Bollas A, Wang Y, Au KF. Nanopore sequencing technology, bioinformatics and applications. Nat Biotechnol. 2021;39(11):1348–1365. doi: 10.1038/s41587-021-01108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Loit K, Adamson K, Bahram M, Puusepp R, Anslan S, Kiiker R, Drenkhan R, Tedersoo L. Relative Performance of MinION (Oxford Nanopore Technologies) versus Sequel (Pacific Biosciences) Third-Generation Sequencing Instruments in Identification of Agricultural and Forest Fungal Pathogens. Appl Environ Microbiol. 2019;85(21):e01368–19. doi: 10.1128/AEM.01368-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.