Abstract
Background
Patient-reported outcome measures (PROMs) are recommended for assessing patient-centered outcomes in inflammatory bowel disease (IBD). The main aims were to assess the level of participation in an electronic PROM (ePROM) data collection system among patients with IBD, and evaluate reliability and validity of the resulting scores.
Methods
Patients included in the IBD registry of Maccabi Healthcare Services, a state-mandated healthcare provider for over 2.6 million people in Israel, were invited to complete the IBD-Control measure and a general health item, with follow-up ePROMs at 3 and 6 months including a global rating of change item. Descriptive statistics were used to compare patient characteristics by participation rate, and assess survey completion time. Initial scores were assessed for internal consistency reliability using Cronbach's alpha. Test–retest reliability was assessed using the intraclass correlation coefficient from paired scores of patients identified as unchanged between the initial and first follow-up. Construct validity was assessed by the ability of IBD-control scores to discriminate between patient sub-groups in expected ways. Empirical validity was assessed using ePROM score correlations with laboratory markers of disease activity. Score coverage was also assessed.
Results
A total of 13,588 patients were invited to participate [Mean age = 49 years (SD = 17); females = 51%]. Participation rate was 31.5%. Participants compared to non-participants were slightly older, were more likely to be female, to have a history of biologic treatment, to have higher socio-economic status, and to be more experienced in the usage of the digital patient portal. Median survey completion time was approximately 1:30 min. Internal consistency and test–retest reliability were 0.86 and 0.98, respectively. Scores discriminated between patient sub-groups in clinically expected ways, with expected correlations to laboratory markers of disease activity. A notable ceiling effect was observed (> 15%) for IBD-Control scores.
Conclusions
Feasibility, reliability, and validity of the ePROM system was supported for measuring the level of perceived disease control in patients diagnosed with IBD in Israel. Additional research is needed to identify ways to increase patient participation, assess clinical implications of the identified measurement ceiling of the IBD-control, and evaluate the added value of the derived scores in support of clinical decision making.
Keywords: IBD control, Patient reported outcome measure, Patient participation, Reliability, Validity
Background
Patient-reported outcome measures (PROMs) translate the patient’s experience into a measurable construct that can be used to monitor perceived health status over time [1, 2]. PROMs have been recommended for assessing patient-centered outcomes in Inflammatory bowel disease (IBD) combined with objective measures of inflammation [3, 4]. However, implementation of PROMs in routine practice is challenging, requiring patient compliance and integration of patients’ perception into clinical assessments and decision making processes. To maximize patient compliance and physician participation, reliable and valid short PROMs were developed [5], including the IBD-Control used in this study [6].
The IBD-Control, developed by Bodger et al. [6], is comprised of 13 items (questions) and a visual analogue scale (IBD-Control-VAS). Eight of the 13 items are used for scoring (IBD-Control-8). The IBD-Control was found to be reliable, valid against more complex health related quality of life tools including the UK version of the IBDQ [7] and the EuroQol (EQ-5D) [8], and sensitive for measuring overall disease control from the patient’s perspective [6]. The IBD-Control was recommended for use in pragmatic clinical trials [3], and as a single PROM included within a minimum standard set of patient-centered outcome measures for IBD [9]. Digital platforms have been suggested as appropriate means for electronic PROMs (ePROMs) data collection [10], offering data integration into electronic medical records with minimal burden, driving the aims of this study.
Methods
Aim
This study aimed to assess the implementation of a self-reported digital PROM data collection system among patients with IBD within a large nationwide state-mandated healthcare provider in Israel, Maccabi Healthcare Services (MHS), and test reliability and validity of the resulting scores.
Design and setting
A prospective observational cohort study (longitudinal survey design).
Participants and data collection period
Patients aged 18 or older who were registered in the MHS's IBD registry [11–13] were invited to participate during April 2019. A detailed description of the development and validation of the IBD registry algorithm has been published previously [11]. Briefly, the ascertainment of IBD cases utilizes three validated algorithms: (1) for identifying patients with a diagnosis of IBD; (2) for detecting the date of disease diagnosis, and (3) for identifying Crohn's Disease (CD) versus ulcerative colitis (UC) versus unclassified-IBD (IBD-U). The algorithms utilize two main criteria: (1) a combination of IBD-related ICD-9 codes when more than one code exists in the electronic health record; or (2) a combination of ICD-9 codes with at least three purchases of IBD-related medications with at least a 3-month interval from first to last purchase (sensitivity 89%, specificity 99%, positive predictive value [PPV] 92%, negative predictive value [NPV] 99%). IBD type was established according to the majority of CD/UC-specific codes out of the three most recent healthcare contacts, or the most recent contact when fewer than three were recorded (sensitivity 92%, specificity 97%, PPV 97%, NPV 92%). Only patients with a documented date of disease diagnosis were included. IBD-U type was identified according to a third algorithm, based on a specific code which exists for this condition in MHS [11–13]. No exclusion criteria were applied. Patients who completed an initial ePROM were invited to complete follow-up PROMs at 3 and 6 months.
Outcome measures
The ePROMs administered included 3 domains: (1) The general health item from the Patient-Reported Outcomes Measurement Information System (PROMIS) global measure [14]; (2) The IBD-Control-8 and the IBD-Control-VAS [6]; and (3) at follow-up, a Global Rating of Change (GRoC) item with a 15-point scale for the degree of change (-7 to + 7), with zero representing no change [15].
Survey administration process
Patients were invited to participate via a text message using the MHS patient portal messaging system, including a reminder after 3 working days, and thereafter, 2 additional reminders at one-week intervals. After successful identification on the secured patient portal, a landing page presented information about the study and the estimated completion time (2 min), inviting patients to complete the ePROM. Patients were informed that their survey data would not be shared with care providers, but would remain available to them, enabling self-tracking and sharing with their physician at their discretion. Four selections were available on the landing page: (1) participate, (2) postpone participation to a later time, (3) decline participation, or (4) decline stating they are not diagnosed with IBD. Selecting ‘participate’ was considered as agreement to participate in the study, and no other consent was required. After completion, a summary screen was presented including the IBD-Control-8 total score and score direction (higher scores = better IBD control). No other clinical interpretations or recommendations were provided. Available validated translations were obtained from the measure developers for the PROMIS global health PROM. The IBD-Control was translated into Hebrew, Russian, and Arabic by a professional translation team using validated methods [16].
Analyses
Patient sample
Health and demographic baseline patient characteristics were summarized by IBD type (CD, UC, or IBD-U) using distribution or dispersion measures as appropriate. Variables were years since the patient was included in the IBD registry, age, sex, biologic treatment, and socioeconomic-status (SES). Biologic treatment was considered as a single surrogate marker for disease severity, categorized as a binary (yes/no) variable defined as having ever purchased at least one biologic/small molecule drug including: Vedolizumab, Infliximab, Adalimumab, Ustekinumab, Golimumab, Tofacitinib, or Certolizumab pegol. SES levels, built for commercial purposes by Points Location Intelligence, were defined by residential areas ranked from 1 (lowest) to 10, and categorized by tertiles into low (1–5), medium (6–7) and high (8–10), and correlated highly with SES measured by the Israel Central Bureau of Statistics [17]. P-values for statistically significant differences were estimated using Chi-square tests for comparisons of categorical data and analysis of variance for comparisons of continuous data. However, due to the large cohort, statistically significant differences need to be interpreted with caution.
Participation rate
Participation rate was operationally defined as the percentage of patients reaching the landing page, stratified by full or partial completion, or by reasons for declining to participate. Participation was tested separately for the initial survey and for the two follow-up surveys, and by patient subgroups offering insights on differences in patient attributes by participation. Variables included age groups, sex, IBD type (CD, UC, or IBD-U), use of biologic treatment, SES, and digital platform usage during the past 12 months, including no use, or one of four digital usage levels defined by quartiles of digital log counts. To assess the potential for patient participation bias, an effect size was calculated as the standardized difference in participation rates between participants and non-participants for the variables listed above [18]. An effect size below 0.2 was considered as representing a non-meaningful difference [19]. Additionally, a multivariable logistic regression was used to estimate the likelihood of participating while accounting for all factors above.
PROM scores and completion time
PROM scores were assessed by survey type (initial or follow-up) and domain (general health and IBD-Control). Score values (mean, SD, median), as well as survey completion time, were also assessed. Survey completion time was assessed for all complete surveys with a completion time between 30 s and 1 h, assuming times outside these limits represented outliers, or surveys completed over multiple instances.
Reliability of point estimates and change scores
Internal consistency reliability for the IBD-Control-8 was assessed using initial scores with Cronbach's alpha. The standard error of measurement (SEM) was calculated by multiplying the standard deviation by the squared-root of 1-(minus) the reliability estimate, in this case Cronbach's alpha [20]. Different confidence intervals (CIs) were computed including the 68% CI, which is equivalent to 1 SEM, and 80%, 90%, and 95% CIs. Reliability of change scores was assessed using the minimal detectable change (MDC), reflecting the minimal amount of change that is beyond measurement error, at different levels of confidence. Since change involves at least two measured points, reliability-based estimates of MDC were calculated by multiplying the SEM of the difference (SEMdifference = SEM * square-root of 2) by the appropriate Z-value [20]. Test–retest reliability was assessed using the intraclass correlation coefficient (ICC) from pairs of IBD-Control-8 scores (initial and first follow-up) of patients identified as unchanged between these two measurement points [21]. Unchanged patients were defined as those that had a GRoC score at their first follow-up ePROM of − 2 to + 2, reflecting change that is less than minimally important to patients [22].
Validity
Empirical validity was assessed by testing associations between the IBD-Control-8 scores and two related scores including the IBD-Control-VAS and general health scores. Since all ePROM assessed have the same direction (higher = better), we expected positive moderate correlations or higher, which in the context assessed here, were determined to be above 0.3 [23]. We also expected a higher correlation within domain (IBD-Control-8 and IBD-Control-VAS), compared to correlations between each of these to the general health domain. Additionally, correlations of IBD-Control-8 scores with laboratory markers of inflammation and disease activity, including albumin, hemoglobin, and calprotectin, were tested at 15 days before or after the date of the ePROM. Calprotectin performance may differ between UC and CD; therefore, we analyzed these groups separately [24]. Low significant correlations in a clinically logical direction were expected. Since we were not aware of known differences between CD and UC regarding correlations of PROMs and laboratory markers, we considered these analyses exploratory rather than hypothesis driven. To account for ordinal level ePROM scores, Spearman’s rank correlations were used.
Discriminant validity was assessed by testing if IBD scores discriminated between patient groups in expected clinical patterns. Although existing evidence on associations between self-assessed IBD disease control and patient demographic and health characteristics are unclear, given previous reports, we expected higher IBD-Control for patients who were older, were males, were diagnosed with UC, and had never purchased biological medications (lower severity) [25–28]. Group differences were tested for the initial IBD-scores using ANOVA.
Score coverage was used to assess floor and ceiling effects. We defined maximally acceptable floor and ceiling effects as 15% of sample scores in the minimum or maximum score of the IBD-Control-8 and the general health question, and the minimum or maximum range of 0–5 and 95–100, respectively, for the IBD-Control-VAS [29, 30].
All analyses were performed using IBM SPSS, version 25.0.0.1 [31] and Stata version 14 [32].
Results
Patient sample
A total of 13,588 patients were invited to participate [Mean age (SD) = 48.9 (16.6); females = 50.5%; Table 1]. Compared to patients diagnosed with UC, those diagnosed with CD were on average 5 years younger, less likely to be female, and more likely to have a history of biologic and small molecule treatment use indicative of higher levels of disease severity. The distributions of SES levels were similar between IBD types. For patients who responded to the initial survey (n = 4280), the majority selected to respond in Hebrew (93.6%), followed by 3.4%, 2.6%, and 0.4% for patients responding in Russian, English, and Arabic, respectively.
Table 1.
Patient sample by IBD type
| Patient characteristics | CD n = 6917 | UC n = 6118 | Unclassified n = 553 | Total N = 13,588 | P |
|---|---|---|---|---|---|
|
Years in IBD registrya Median (Min–Max) 25th; 75th percentiles |
9 (0–19) 4; 15 |
11 (0–19) 5; 17 |
8 (0–19) 4; 13 |
10 (0–19) 5; 15 |
< 0.001 |
|
Age: Mean (SD)Median (Min–Max) Median (Min–Max) 25th;75th percentiles |
45.9 (15.9) 44.7 (19–100) 33.0; 56.4 |
52.1 (16.7) 51.2 (19–101) 39.4; 64.3 |
51.5 (18.0) 49.8 (19–102) 37.0; 64.5 |
48.9 (16.6) 47.8 (19–102) 35.7; 60.7 |
< 0.001 |
| Age groups: | < 0.001 | ||||
| 18–45 | 3512 (50.8) | 2155 (35.2) | 206 (37.3) | 5873 (43.2) | |
| > 45–65 | 2422 (35.0) | 2493 (40.8) | 212 (38.3) | 5127 (37.7) | |
| Over 65 | 983 (14.2) | 1470 (24.0) | 135 (24.4) | 2588 (19.1) | |
| Sex: | < 0.001 | ||||
| Female | 3381 (48.9) | 3167 (51.8) | 315 (57.0) | 6863 (50.5) | |
| Male | 3536 (51.1) | 2951 (48.2) | 238 (43.0) | 6725 (49.5) | |
| Biologic treatmentb | < 0.001 | ||||
| Yes | 2538 (36.7) | 742 (12.1) | 88 (15.9) | 3368 (24.8) | |
| No (never) | 4379 (63.3) | 5376 (87.9) | 465 (84.1) | 10,220 (75.2) | |
| SES | 0.769 | ||||
| 1 to 5 (low) | 1685 (24.4) | 1443 (23.6) | 132 (23.9) | 3260 (24.0) | |
| 6 to 7 (moderate) | 2610 (37.7) | 2355 (38.5) | 211 (38.2) | 5176 (38.2) | |
| 8 to 10 (high) | 2606 (37.7) | 2305 (37.7) | 207 (37.4) | 5118 (37.8) | |
| Missing | 16 (0.2) | 15 (0.2) | 3 (0.5) | 34 (0.9) |
Values are n (column %) unless noted otherwise. P-values for statistically significant differences were estimated using Chi-square tests for comparisons of categorical data and analysis of variance for comparisons of continuous data
aYear of inclusion in the IBD registry at the start of 2019. Zero represents less than 1 year within the registry
bBiologic and small molecules treatment was defined as having purchased at least one biologic medication including: Vedolizumab, Infliximab, Adalimumab, Ustekinumab, Golimumab, Tofacitinib, or Certolizumab
CD Crohn's disease; UC Ulcerative Colitis; IQR inter quartile range; SD standard deviation; SES socioeconomic status
Participation rate
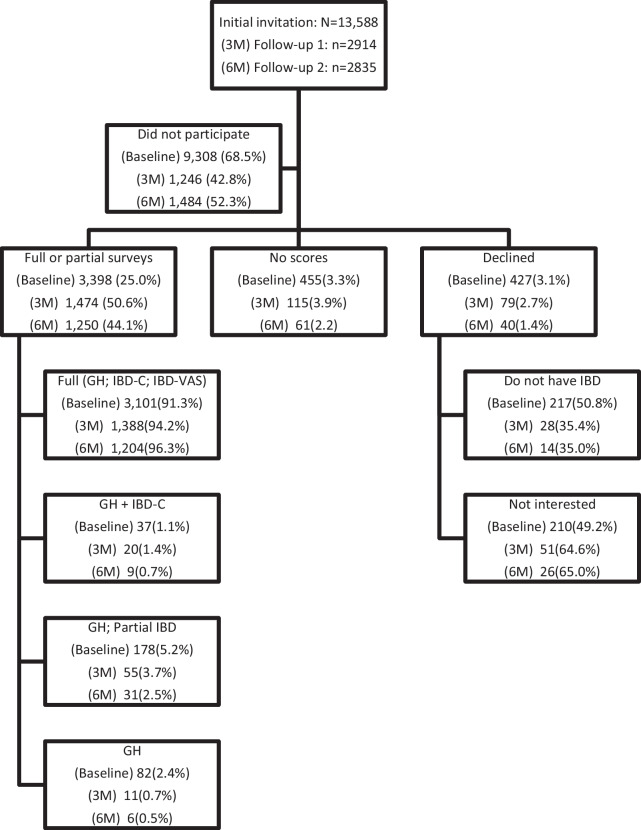
Participation rates for the initial survey by age, sex, IBD type, IBD severity, SES levels and digital platform usage are presented in Table 2. The overall participation rate was 31.5%. All standardized differences were < 0.2, except for the 'low' SES category and all except 'moderate' digital usage categories. Results from the multivariable logistic model indicated that patients were more likely to participate if they were older, had not received biologic treatment, had a moderate (compared to low) SES level, and had moderate or higher levels of digital usage. A more detailed illustration of participation in the initial survey (baseline) and the two follow-up surveys are illustrated in the Fig. 1. Overall, participation rates for the first and second follow-up surveys from those who responded to the previous survey administration were 57% and 48%, respectively. The percentage of patients with no scores ranged from 2.2 to 3.9%, and the percentage of patients who declined participation decreased between the initial and the 2nd follow-up survey from 3.1% to 1.4%.
Table 2.
Patient characteristics by participation in the initial survey
| Patient characteristics | Participated n = 4280 |
Did not participate n = 9308 |
Standardized differenceb | Odds ratioc (95% CI) |
|---|---|---|---|---|
|
Age: Mean (SD) Median (Min to Max) |
49.7 (15.1) 49.1 (19–95) |
48.5 (17.3) 47.7 (19–102) |
0.07 | NA |
| Age groups | ||||
| 18–45 | 1648 (38.5) | 4225 (45.4) | 0.14 | REF |
| > 45–65 | 1866 (43.6) | 3261 (35.0) | 0.18 | 1.9 (1.7–2.1) |
| Over 65 | 766 (17.9) | 1822 (19.6) | 0.04 | 1.6 (1.5–1.8) |
| Sex | ||||
| Female | 2304 (53.8) | 4559 (49.0) | 0.10 | REF |
| Male | 1976 (46.2) | 4749 (51.0) | 1.0 (0.9–1.1) | |
| IBD type | ||||
| CD | 2182 (51.0) | 4735 (50.9) | < 0.01 | REF |
| UC | 1938 (45.3) | 4180 (44.9) | 0.01 | 1.1 (1.0–1.1) |
| Unspecified | 160 (3.7) | 393 (4.2) | 0.03 | 0.9 (0.7–1.1) |
| Biologic treatment a | ||||
| Yes | 1136 (26.5) | 2232 (24.0) | 0.06 | REF |
| No (bio-naïve) | 3144 (73.5) | 7076 (76.0) | 1.1 (1.0–1.2) | |
| SES | ||||
| 1 to 5 (low) | 769 (18.0) | 2491 (26.8) | 0.21 | REF |
| 6 to 7 (moderate) | 1708 (39.9) | 3468 (37.3) | 0.05 | 1.2 (1.1–1.3) |
| 8 to 10 (high) | 1792 (41.9) | 3326 (35.7) | 0.13 | 1.1 (1.0–1.2) |
| Missing | 11(0.3) | 23 (0.2) | 0.02 | 1.0 (0.5–2.1) |
| Digital usage count (past year) | ||||
| None | 38 (0.9) | 1450 (15.6) | 0.55 | 0.1 (0.1–0.2) |
| Low (1 to 19) | 519 (12.1) | 2573 (27.6) | 0.40 | REF |
| Moderate (20 to 46) | 1004 (23.5) | 1926 (20.7) | 0.07 | 2.7 (2.4–3.0) |
| High (47 to 94) | 1218 (28.5) | 1823 (19.6) | 0.21 | 3.6 (3.2–4.1) |
| Very high (95 or more) | 1501 (35.1) | 1536 (16.5) | 0.43 | 5.8 (5.1–6.6) |
Values are n (column %) unless noted otherwise
Total percentages may range between 99.9–100.1 due to rounding
aBiologic treatment was defined as having purchased at least one biologic medication including: Vedolizumab, Infliximab, Adalimumab, Ustekinumab, Golimumab, Tofacitinib, or Certolizumab
bThe absolute standardized differences was calculated as described by Austin [18] Standardized differences below 0.2 were considered non-meaningful
CI confidence level, NA not applicable, REF reference group, SES Socioeconomic status, CD Crohn's disease; UC Ulcerative Colitis
cOdds ratios are mutually adjusted for all variables in the table, estimated from a multivariable logistic regression that modeled the likelihood of participating compared to the reference group
Fig. 1.
Participation in the initial and two follow-up surveys. Participation rate was operationally defined as the percentage of patients selecting the web link on the invitation text message and reaching the landing page after a successful identification. Percentages are from the level above for the corresponding survey number. For example, at baseline, 68.5% of patients did not participate, with 25.0% (3398/13,588) having full or partial survey completion, 3.3% reached the landing page but did not complete any survey item therefor had no scores, and 3.1% declined participation selecting reasons of not having IBD or not interested to participate, summing up to an overall participation rate of 31.5%. For those with full or partial survey completion, the distribution of score combination is shown for those with all three scores (global health, IBD-control, and IBD-VAS), or partial score combinations. 3 M first follow-up at three months; 6 M second follow-up at six months; IBD Inflammatory bowed disease; GH General health score from the Patient-Reported Outcomes Measurement Information System (PROMIS) general health item; IBD-C IBD-Control-8 scores; IBD-VAS IBD visual analog scale scores
PROM scores and completion time
Score counts, summary values, and overall completion time by survey type (initial or follow-up) are presented in Table 3. From 6122 surveys collected, 5759 had complete IBD-Control-8 scores. Median survey completion time for initial, first follow-up, and second follow-up surveys were all approximately 1:30 min.
Table 3.
Scores by survey and domain and survey completion time
| Domain | Initial survey | Follow-up 1 | Follow-up 2 |
|---|---|---|---|
| General Health | |||
| Counts | 3398 | 1474 | 1250 |
| Median (25th; 75th percentiles) | 3(3; 4) | 3(2; 4) | 3(3; 4) |
| Min–max | 1–5 | 1–5 | 1–5 |
| IBD-Control-8 | |||
| Counts | 3138 | 1408 | 1213 |
| Median (25th; 75th percentiles) | 13(8; 15) | 13(8; 16) | 14(9; 16) |
| Min–max | 0–16 | 0–16 | 0–16 |
| IBD-Control VAS | |||
| Counts | 3101 | 1388 | 1204 |
| Median (25th; 75th percentiles) | 74(50; 90) | 72(49; 89) | 76(51.5; 90) |
| Min–max | 0–100 | 0–100 | 0–100 |
| Total survey completion time (minutes)a | |||
| Countsb | 3047 | 1360 | 1175 |
| Median (25th; 75th percentiles) | 1:28(1:11; 1:56) | 1:34(1:15; 2:05) | 1:35(1:15; 2:08) |
IBD inflammatory bowel disease; VAS visual analog scale
aCompletion time reflect to total time needed to complete the full survey
bCounts include surveys with a completion time between 30 s and 1 h, assuming times outside these limits represented outliers, or surveys completed over multiple instances
Reliability of point estimates and change scores
Internal consistency reliability for the IBD-Control-8 was 0.86. The SEM was 1.7 points. Reliability of point estimates at 80%, 90%, and 95% levels of confidence were 2.2, 2.8, and 3.4 points, respectively. MDC at 68%, 80%, 90%, and 95% levels of confidence were 2.4, 3.1, 4.0, and 4.8 points, respectively. IBD-Control-8 test–retest reliability (ICC) using scores from 918 patients identified as unchanged was 0.968 (95%CI = 0.963–0.972).
Validity
Empirical validity
Bi-variate correlation coefficients between IBD-Control-8 scores, IBD-Control-VAS scores, and general health scores, were all above 0.6. As hypothesized, all correlations were positive, with a higher correlation found between IBD-Control-8 and IBD-Control-VAS (Spearman's rank correlation = 0.77) compared to correlations between each of these to the general health scores ranging from 0.63 to 0.64. All correlation coefficients were significant (P < 0.001). IBD-Control-8 correlations with laboratory markers of inflammation and disease activity were in the expected directions (Table 4). Correlations were overall low but significant for most tests, with the highest correlation observed between IBD-Control-8 scores and fecal calprotectin for patients with UC.
Table 4.
IBD-Control-8 score correlations with laboratory markers
| CD | UC | |
|---|---|---|
| Albumin | .192**(375) | .187**(232) |
| Calprotectin | − .106(143) | − .314*(41) |
| Hemoglobin | .139**(530) | .213**(352) |
Values are Spearman's rank correlation coefficients (n)
Time between the date of the ePROM and the laboratory test = + / − 15 days
*P < 0.05; **P < 0.01
CD Crohn's disease; UC Ulcerative Colitis
Discriminant validity
IBD scores discriminated between patient groups in expected clinical patterns (Table 5), with higher IBD-Control found for patients who were older, were males, were diagnosed with UC, and had never purchased biological medications.
Table 5.
Discriminant validity
| Patient characteristic | Model (ANOVA) | Marginal means (IBD-Control-8) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Groups | N | % | F(df) Prob > F |
b | 95% CI | |
| Age | 18–45 | 1267 | 40.4% |
15.5(2) P < 0.001 |
10.6 | 10.3 | 10.8 |
| 45–65 | 1368 | 43.6% | 11.2 | 11.0 | 11.5 | ||
| 65 to max | 503 | 16.0% | 11.9 | 11.5 | 12.3 | ||
| Gender | Male | 1443 | 46.0% |
29.77(1) P < 0.001 |
11.6 | 11.3 | 11.8 |
| Female | 1695 | 54.0% | 10.7 | 10.4 | 10.9 | ||
| IBD type | CD | 1626 | 51.8% |
19.8(2) P < 0.001 |
10.6 | 10.4 | 10.8 |
| UC | 1427 | 45.5% | 11.6 | 11.4 | 11.9 | ||
| Unclassified | 85 | 2.7% | 10.8 | 9.8 | 11.8 | ||
| *Biologic treatment | No | 2225 | 70.9% |
163.2(1) P < 0.001 |
11.7 | 11.6 | 11.9 |
| Yes | 913 | 29.1% | 9.4 | 9.1 | 9.7 | ||
Group differences were tested for the initial IBD-Control scores (N = 3138)
Marginal means are for IBD-Control-8 scores (0–16 scale)
*Biologic treatment was defined as having purchased at least one biologic medication including: Vedolizumab, Infliximab, Adalimumab, Ustekinumab, Golimumab, Tofacitinib, or Certolizumab
b beta coefficient, df degrees of freedom, CD Crohn's disease; UC Ulcerative Colitis
Score coverage
Floor and ceiling effects for IBD-Control-8 scores, IBD-Control-VAS scores, and general health scores, for the initial and the two follow-up surveys, are presented in Table 6. Floor effects were all below 15%, with negligible floor effects for the IBD-Control-8 and IBD-Control-VAS scores (< 2%). IBD-Control-8 and IBD-Control-VAS scores had notable ceiling effects ranging from 17 to 30%.
Table 6.
Score coverage
| Floor and ceiling effects (%) | |||
|---|---|---|---|
| Initial survey | Follow-up 1 | Follow-up 2 | |
| General Health (min/max) | 6.9/14.0 | 8.4/11.1 | 4.6/15.6 |
| IBD-Control-8 (min/max) | 1.4/22.7 | 1.8/25.1 | 1.2/30.0 |
| IBD-Control-VAS (0–5/95–100) | 1.7/19.9 | 1.8/16.6 | 0.9/19.3 |
Values are in percent (Floor/Ceiling)
Floor and ceiling effects were defined as the minimum or maximum score of the IBD-Control-8 scores (0 and 16) and the general health scores (1 or 5), respectively, and the minimum or maximum range of 0–5 and 95–100, respectively, for the IBD-Control-VAS
Discussion
We describe in this report the feasibility and measurement properties of an ePROM platform among IBD patients in a real-world setting. The relatively high response rate along with extremely short completion time, attest to its feasibility and potential for implementation in routine clinical practice and research initiatives. Essential psychometric properties of reliability and validity of the generated IBD-Control-8 scores were supported, increasing confidence in their precision and potential capacity to serve as a viable and valid source of information for patients and clinicians. These results should be interpreted within the context of the population tested, including mostly Hebrew speaking IBD patients in Israel.
Participation rate was 31.5% for the initial survey, increasing up to 48–57% for follow-up surveys. Over 90% of patients who started the survey completed the full set of scores including the general health item, IBD-Control-8, and IBD-Control-VAS. These participation rates are encouraging given that the framework of this study did not include any direct patient-clinician interaction related to the ePROM data collection process. Studies assessing ePROM participation rates, usually within a clinical trial or before scheduled clinical visits, reported participation rates ranging from 33 to 74% [33, 34], suggesting a potential for improved participation rates when ePROMs are implemented within a clinical setting. Recent evidence exists of improved healthcare management, physician–patient communication, and symptom detection following routine clinical use of PROMs data [35]. This may encourage physicians to engage their patients in routine PROM completion to enable self-monitoring and assist clinical decision making. The feasibility of an ePROM platform as used for this study is supported by previous findings [36], suggesting this approach could be scalable for wide range of portals and apps among IBD patients in other healthcare systems. However, the lower participation rates observed among patients with lower SES levels, or those less experienced with the use of digital portals, suggests a potential barrier of ePROMs implementation within populations that are often at risk of having lower health status. This emphasizes the need for ePROM implementation studies to assess their usability in different patient populations.
A key element to successful implementation of PROMs data collection is low survey administration burden. Survey completion time in our study was roughly 1:30 min and was similar to the timing reported by Bodger et al. [6] 1:15 min. We consider these results to not pose a barrier to patients when considering participating in ePROMs data collection. Older age has also been reported as an additional barrier to digital PROM participation [37]. Our results did not identify important differences in mean age by participation (standardized difference = 0.07). Also, standardized differences in rates of patients by age groups between participants and non-participants were all < 0.2, suggesting age was not a critical barrier for ePROM completion, as suggested previously [36].
The reliability estimates provided may help clinicians assess measurement error associated with a point estimate or a change score. For example, reliability estimates show that there is a 90% confidence that the true patient score falls within + / − 2.8 IBD-Control-8 points on the 0–16 scale. As an example, if used in conjunction with a threshold value of 13 that has been suggested to represent a state of quiescent (high level of IBD control) [6], only a perfect score of 16 (13 + 2.8) would provide this level of confidence that the patient has in fact been quiescent. Additionally, results suggest 4 or 5 change points are needed to represent true change at a 90% or 95% confidence, respectively.
Correlations between ePROM scores with several laboratory tests that may indicate disease activity or severity were low and in the expected directions, supporting the validity of the IBD-Control-8 scores. Interestingly, although correlations of albumin and hemoglobin with IBD-Control-8 were similar between CD and UC, calprotectin correlations were higher for UC compared to CD. Overall, this is not surprising as fecal calprotectin correlates better with the level of inflammation in UC than in CD [38]. Correlations between objective markers of inflammation and disease activity, and subjective measures of disease control, are not expected to be high as they assess two related but distinct constructs. Patient-perceived global control of disease in IBD may reflect both inflammatory and non-inflammatory manifestations of disease, co-existing functional symptoms and impacts of medication. Therefore, these results need to be interpreted with caution. The key use-case for the PROM is to serve as an additional marker of health status. Those reporting sub-optimal scores may have non-inflammatory drivers of their self-assessed IBD control rating, but they still have health needs to be addressed. Thus, a formal clinical assessment combined with objective tests is needed to distinguish between those with active inflammation and those with other reasons for sub-optimal PROM scores.
The main strength of this study was the large number of patients selected from a generalizable IBD registry. Also, the use of an easily accessible mobile-based digital platform to collect patient self-reported outcomes offers a novel method to improve patient centered care.
However, this study has some notable limitations. Initial surveys were completed fully or partially by 25% to 31% of all of the target patient population, respectively. Although these participation rates could be considered high given that ePROMs were not part of a clinical interaction, they also pose a potential patient participation bias that might distort the assessment of the true patient population of interest. This bias may lie in the survey's electronic administration mode, a limitation supported by our finding of higher likelihood to participate for those more experienced with overall digital usage. This result highlights the need for future studies assessing the impact of a patient's 'digital profile' on ePROM feasibility. An important strength of the MHS setting is that it harbors full demographic and health data on both responders and non-responders, offering an excellent opportunity to study the potential of response bias. Some study patients were classified as ‘IBD-U’ or indeterminate colitis [39]. Currently, there is a lack of data on its epidemiology, clinical course, reclassification trends, and treatment responses. Using PROM data may help better understand these patients’ characteristics from a patient-centered perspective. Finally, score coverage results revealed a notable ceiling effect of IBD-Control-8 and IBD-Control-VAS scores. Additional studies are needed to assess whether the measured ceiling effect reflects a true positive state of IBD-control, or a psychometric limitation.
Conclusion
The ePROM platform assessed was found feasible and suitable for clinical integration and research initiatives for patients with IBD in Israel, providing reliable and valid measures of the level of perceived disease control. This allows for an integration of ePROMs data within the electronic medical record, offering clinicians an improved ability to monitor levels of IBD control from the patient’s perspective.
Acknowledgements
We thank Dr. Eyal Zimlichman and Mr. Alex Galper from the Sheba Medical Center, Tel Hashomer, Israel, for their help and cooperation with the IBD-Control Hebrew translation. We thank the Maccabi Healthcare Services Information Technology Department, with special thanks to Mr. Shlomi Shmilovich for his continued support and management of the digital platform used in this study. Finally, we thank Prof. Varda Shalev for initiating the PROMs project at the Maccabitech Institute for Research & Innovation, which enabled this study.
Abbreviations
- CD
Crohn’s disease
- CI
Confidence interval
- ePROM
Electronic patient-reported outcome measures
- GRoC
Global rating of change
- IBD
Inflammatory bowel disease
- IBD-U
Unclassified IBD
- ICC
Intraclass correlation coefficient
- MHS
Maccabi Healthcare Services
- MDC
Minimal detectable change
- PROMs
Patient-reported outcome measures
- SD
Standard deviation
- SEM
Standard error of measurement
- SES
Socioeconomic-status
- UC
Ulcerative colitis
- VAS
Visual analogue scale
Author contributions
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. DD, CW, GC, KB and MW, have contributed to the conception and design of the study, analysis and interpretation, drafting, and final approval of the submitted manuscript. ST, and KB contributed interpretation of data, drafting, critically revising and final approval of the submitted manuscript.
Funding
This work was supported by Takeda Israel.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The MHS research committee and the institutional review board approved the study (IRB#: 0103-18-BBL) and provided an exempt status from the need to complete a formal consent form as described above.
Consent for publication
Not applicable. The manuscript, including related data, figures and tables, has not been previously published and is not under consideration elsewhere.
Competing interests
This project was supported by an institutional grant from Takeda Pharmaceutics to Maccabi Healthcare Services and did not include the medical writing by the Maccabi authors. Tsukinovsky is an employee of Takeda Pharmaceuticals. Takeda’s employees do not have any stock or stock options. Deutscher, Weil, and Chodick do not have any conflicts of interest. Waterman and Kariv provide consultation for Takeda Pharmaceutics, Petach Tikva, Israel. All authors declare they have no other financial or conflicts of interests related to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Matti Waterman and Revital Kariv have contributed equally
Contributor Information
Daniel Deutscher, Email: deutsch_d@mac.org.il.
Clara Weil, Email: weil_c@mac.org.il.
Gabriel Chodick, Email: hodik_g@mac.org.il.
Sveta Tsukinovsky, Email: Sveta.Tsukinovsky@takeda.com.
Keith Bodger, Email: kbodger@liverpool.ac.uk.
Matti Waterman, Email: m_waterman@rambam.health.gov.il.
Revital Kariv, Email: kariv_r@mac.org.il.
References
- 1.Bingham CO, 3rd, Noonan VK, Auger C, Feldman DE, Ahmed S, Bartlett SJ. Montreal Accord on Patient-Reported Outcomes (PROs) use series—Paper 4: patient-reported outcomes can inform clinical decision making in chronic care. J Clin Epidemiol. 2017;89:136–141. doi: 10.1016/j.jclinepi.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter I, Goncalves-Bradley D, Ricci-Cabello I, Gibbons C, Gangannagaripalli J, Fitzpatrick R, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. J Comp Eff Res. 2016;5(5):507–519. doi: 10.2217/cer-2015-0014. [DOI] [PubMed] [Google Scholar]
- 3.Bojic D, Bodger K, Travis S. Patient Reported Outcome Measures (PROMs) in inflammatory bowel disease: new data. J Crohns Colitis. 2017;11(suppl_2):S576–S85. [DOI] [PubMed]
- 4.de Jong ME, Taal E, Thomas PWA, Romkens TEH, Jansen JM, West RL, et al. Cross-cultural translation and validation of the IBD-control questionnaire in The Netherlands: a patient-reported outcome measure in inflammatory bowel disease. Scand J Gastroenterol. 2021;56(2):155–161. doi: 10.1080/00365521.2020.1857430. [DOI] [PubMed] [Google Scholar]
- 5.de Jong MJ, Huibregtse R, Masclee AAM, Jonkers D, Pierik MJ. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16(5):648–63 e3. [DOI] [PubMed]
- 6.Bodger K, Ormerod C, Shackcloth D, Harrison M, Collaborative IBDC. Development and validation of a rapid, generic measure of disease control from the patient's perspective: the IBD-control questionnaire. Gut. 2014;63(7):1092–1102. doi: 10.1136/gutjnl-2013-305600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung WY, Garratt AM, Russell IT, Williams JG. The UK IBDQ-a British version of the inflammatory bowel disease questionnaire. development and validation. J Clin Epidemiol 2000;53(3):297–306. [DOI] [PubMed]
- 8.EuroQol G. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands) 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 9.Kim AH, Roberts C, Feagan BG, Banerjee R, Bemelman W, Bodger K, et al. Developing a Standard Set of Patient-Centred Outcomes for Inflammatory Bowel Disease-an International, Cross-disciplinary consensus. J Crohns Colitis. 2018;12(4):408–418. doi: 10.1093/ecco-jcc/jjx161. [DOI] [PubMed] [Google Scholar]
- 10.O'Connell S, Palmer R, Withers K, Saha N, Puntoni S, Carolan-Rees G, et al. Requirements for the collection of electronic PROMS either "in clinic" or "at home" as part of the PROMs, PREMs and Effectiveness Programme (PPEP) in Wales: a feasibility study using a generic PROM tool. Pilot Feasibility Stud. 2018;4:90. doi: 10.1186/s40814-018-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman MY, Leventer-Roberts M, Rosenblum J, Zigman N, Goren I, Mourad V, et al. Development and validation of novel algorithms to identify patients with inflammatory bowel diseases in Israel: an epi-IIRN group study. Clin Epidemiol. 2018;10:671–681. doi: 10.2147/CLEP.S151339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kariv R, Turner D, Rosenblum J, Morad V, Zigman N, Friedman M, et al. Establishing a registry for inflammatory bowel disease patients in maccabi healthcare services—joint project between hospitals, Epi-Iirn Group and Community Medicine. Harefuah. 2018;157(10):655–659. [PubMed] [Google Scholar]
- 13.Ludvigsson JF, Andersson M, Bengtsson J, Eberhardson M, Fagerberg UL, Grip O, et al. Swedish Inflammatory Bowel Disease Register (SWIBREG)—a nationwide quality register. Scand J Gastroenterol. 2019;54(9):1089–1101. doi: 10.1080/00365521.2019.1660799. [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15. [DOI] [PubMed]
- 16.Eremenco SL, Cella D, Arnold BJ. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof. 2005;28(2):212–232. doi: 10.1177/0163278705275342. [DOI] [PubMed] [Google Scholar]
- 17.Israel Central Bureau of Statistics. Characterization and classification of geographic units by the socioeconomic level of the population 2008. Publication No. 1530. Jerusalem, Israel; 2013.
- 18.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988. xxi, 567 p.
- 20.Stratford PW. Getting more from the literature: Estimating the standard error of measurement from reliability studies. Physiother Can. 2004;56:27–30. doi: 10.2310/6640.2004.15377. [DOI] [Google Scholar]
- 21.Riddle DL, Stratford PW. Is this change real? Interpreting patient outcomes in physical therapy. Philadelphia: F.A. Davis Co.; 2013. [Google Scholar]
- 22.Deutscher D, Cook KF, Kallen MA, Werneke MW, Hayes D, Mioduski JE, et al. Clinical interpretation of the neck functional status computerized adaptive test. J Orthop Sports Phys Ther. 2019;49(12):875–886. doi: 10.2519/jospt.2019.8862. [DOI] [PubMed] [Google Scholar]
- 23.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 24.Bathe AL, Mavropoulou E, Mechie NC, Petzold G, Ellenrieder V, Kunsch S, et al. Impact of faecal calprotectin measurement on clinical decision-making in patients with Crohn's disease and ulcerative colitis. PLoS ONE. 2019;14(10):e0223893. doi: 10.1371/journal.pone.0223893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenstein I, Herrmann E, Filmann N, Zosel C, Tacke W, Bock H, et al. Female patients suffering from inflammatory bowel diseases are treated less frequently with immunosuppressive medication and have a higher disease activity: a subgroup analysis of a large multi-centre, prospective, internet-based study. J Crohns Colitis. 2011;5(3):203–210. doi: 10.1016/j.crohns.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Lissoos T, Dieyi C, Null KD. Development and validation of an inflammatory bowel disease severity index using US administrative claims data: a retrospective cohort study. Inflamm Bowel Dis. 2021;27(8):1177–1183. doi: 10.1093/ibd/izaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greuter T, Manser C, Pittet V, Vavricka SR, Biedermann L, on behalf of Swiss Ibdnet aowgotSSoG. Gender differences in inflammatory bowel disease. digestion. 2020;101 Suppl 1:98–104. [DOI] [PubMed]
- 28.Park KT, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen K, et al. The cost of inflammatory bowel disease: an initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis. 2020;26(1):1–10. doi: 10.1093/ibd/izz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Wamper KE, Sierevelt IN, Poolman RW, Bhandari M, Haverkamp D. The Harris hip score: Do ceiling effects limit its usefulness in orthopedics? Acta Orthop. 2010;81(6):703–707. doi: 10.3109/17453674.2010.537808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Version 25.0 ed. Armonk, NY: IBM Corp.
- 32.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP2015.
- 33.Borowsky PA, Kadri OM, Meldau JE, Blanchett J, Makhni EC. The Remote Completion Rate of Electronic Patient-Reported Outcome Forms Before Scheduled Clinic Visits-A Proof-of-Concept Study Using Patient-Reported Outcome Measurement Information System Computer Adaptive Test Questionnaires. J Am Acad Orthop Surg Glob Res Rev. 2019;3(10). [DOI] [PMC free article] [PubMed]
- 34.Howard JS, Toonstra JL, Meade AR, Whale Conley CE, Mattacola CG. Feasibility of conducting a web-based survey of patient-reported outcomes and rehabilitation progress. Digit Health. 2016;2:2055207616644844. doi: 10.1177/2055207616644844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Licqurish SM, Cook OY, Pattuwage LP, Saunders C, Jefford M, Koczwara B, et al. Tools to facilitate communication during physician-patient consultations in cancer care: an overview of systematic reviews. CA Cancer J Clin. 2019;69(6):497–520. doi: 10.3322/caac.21573. [DOI] [PubMed] [Google Scholar]
- 36.Karsten MM, Speiser D, Hartmann C, Zeuschner N, Lippold K, Kiver V, et al. Web-based patient-reported outcomes using the international consortium for health outcome measurement dataset in a major German University Hospital: observational study. JMIR Cancer. 2018;4(2):e11373. doi: 10.2196/11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millar MM, Elena JW, Gallicchio L, Edwards SL, Carter ME, Herget KA, et al. The feasibility of web surveys for obtaining patient-reported outcomes from cancer survivors: a randomized experiment comparing survey modes and brochure enclosures. BMC Med Res Methodol. 2019;19(1):208. doi: 10.1186/s12874-019-0859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, et al. C-Reactive Protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802–820. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 39.Burisch J, Zammit SC, Ellul P, Turcan S, Duricova D, Bortlik M, et al. Disease course of inflammatory bowel disease unclassified in a European population-based inception cohort: an Epi-IBD study. J Gastroenterol Hepatol. 2019;34(6):996–1003. doi: 10.1111/jgh.14563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.