Abstract
T-cell-mediated immunity is known to play a central role in the host response to Candida albicans. T-cell clones are useful tools for the exact identification of fungal T-cell epitopes and the processing requirements of C. albicans antigens. We isolated human T-cell clones from an HLA-DRB1*1101 healthy donor by using an antigenic extract (MP-F2) of the fungus. Specific clones were T-cell receptor α/β and CD4+/CD8− and showed a T-helper type 1 cytokine profile (production of gamma interferon and not interleukin-4). The large majority of these clones recognized both the natural (highly glycosylated) and the recombinant (nonglycosylated) 65-kDa mannoprotein (MP65), an MP-F2 minor constituent that was confirmed to be an immunodominant antigen of the human T-cell response. Surprisingly, most of the clones recognized two synthetic peptides of different MP65 regions. However, the peptides shared the amino acid motif IXSXIXXL, which may be envisaged as a motif sequence representing the minimal epitope recognized by these clones. Three clones recognized natural and pronase-treated MP65 but did not detect nonglycosylated, recombinant MP65 or the peptides, suggesting a possible role for polysaccharides in T-cell recognition of C. albicans. Finally, lymphoblastoid B-cell lines were efficient antigen-presenting cells (APC) for recombinant MP65 and peptides but failed to present natural, glycosylated antigens, suggesting that nonprofessional APC might be defective in processing highly glycosylated yeast proteins. In conclusion, this study provides the first characterization of C. albicans-specific human T-cell clones and provides new clues for the definition of the cellular immune response against C. albicans.
Fungal opportunistic infections, in particular, those caused by Candida species, have gained considerable significance as a cause of morbidity and mortality. Mucosal candidiasis is frequent in immunocompromised patients, especially those infected by the human immunodeficiency virus or those affected by idiopathic CD4+ T lymphocytopenia (6, 15, 34, 44), while deep-seated candidiasis is highly prevalent in neutropenic, bone marrow transplant patients (28, 30, 53). Finally, a large incidence of vaginal infection by Candida is recorded in otherwise healthy women of premenopausal age (24).
Although some controversy exists about the final effector mechanisms of anti-Candida protection (9, 45, 46), cellular immune responses, in particular, those relying on or regulated by T lymphocytes, are generally considered of utmost importance for the induction of a protective state. In particular, this notion has been well established with experimental models of infection with Candida albicans, the most pathogenic species of the genus. This fungus is an ordinary human commensal, capable in this state of inducing persistent humoral and cellular responses in healthy subjects, as witnessed by the presence of antibodies against various cell surface constituents, intense proliferative responses of peripheral blood mononuclear cells (PBMC), and delayed-type hypersensitivity following stimulation with Candida antigens (24). All these responses are representative of an active immunization state. Thus, oral candidiasis in human immunodeficiency virus-infected subjects is believed to be caused by the acquired T-lymphocyte deficiency, and the onset of mucosal candidiasis in these patients is closely related to both numerical and functional decreases in CD4+ T lymphocytes (6, 15, 31, 37, 44, 51).
Human T-cell lines and T-cell clones (TCC) specific for C. albicans have rarely been generated and described in the literature (29, 33), although they could be useful tools in the study of immune responses to Candida. TCC could provide direct clues about the nature and requirements for antigen processing of complex natural glycoconjugates, such as the mannoproteins, which are major T-cell antigens of this fungus (10, 12, 27, 52). They could also provide direct evidence of immunodominant epitopes of the pathogen and might be useful for immunoreconstitution therapy of some forms of candidiasis (33, 51). However, we are unaware of any approach specifically devoted to understanding immune responses in candidiasis by the use of Candida-specific human TCC.
The 65-kDa mannoprotein (MP65) has long been demonstrated to be a major target of T-cell responses in humans (3, 4, 7, 10, 26, 52). Some properties of this major Candida antigen with regard to potential immunoprophylactic or immunotherapeutic activity, or even for use as an immunodiagnostic reagent, have been disclosed. However, the importance of MP65 relative to that of other C. albicans antigens in inducing a T-cell response could be derived only from a comparison with other products. In addition, little information on the antigenic availability of MP65 and its processing requirements could be obtained by use of PBMC for measurement of the response to the antigen. Some putative epitopes could be identified by studying the proliferative response of PBMC to MP65 peptides derived from tryptic digestion (26), but a full definition of the antigenic properties and epitopes of MP65 could not be established by use of the polyclonal T-cell populations represented by PBMC.
The aims of this work were to establish C. albicans antigen-specific human TCC and to examine the extent to which they recognize MP65 as a major antigen among soluble fungal products. Efforts were also made to further characterize T-cell epitopes among the most immunogenic peptides of MP65 in an attempt to define HLA restriction. Finally, we compared some processing features of natural (glycosylated) and recombinant (nonglycosylated) MP65 (32) with the aim of discovering a possible role of polysaccharides in the processing requirements and T-cell recognition of this major antigen of C. albicans.
MATERIALS AND METHODS
C. albicans antigens and MP65 synthetic peptides.
The C. albicans mannoprotein-rich fraction MP-F2 was prepared as described elsewhere (11). Briefly, MP-F2 was separated by ion-exchange chromatography with DEAE–Sephadex A-50 from a crude mannoprotein extract of C. albicans yeast cells. MP-F2 was composed of >90% mannan and 8% protein. Biochemical and immunological characterization of this antigen has been reported elsewhere (52).
Mycelial secreted mannoproteins (M-sMP), an antigenic preparation containing mannoproteins spontaneously released from C. albicans cultures grown to the mycelial form, were prepared as described elsewhere (7, 27). Pronase digestion of M-sMP (resulting in M-sMP-P) was carried out as previously described (27).
C. albicans natural, glycosylated MP65 was purified from M-sMP by immunoaffinity chromatography with a monoclonal antibody (MAb) directed against the protein moiety of the molecule as previously described (26, 27). The purified antigen was substantially free from other mannoproteins or proteins, as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, silver staining, concanavalin A detection, and immunoblotting with MAbs or polyclonal antibodies directed against other components of M-sMP (26).
Recombinant nonglycosylated MP65 of both C. albicans (Ca-rMP65) and Saccharomyces cerevisiae (Sc-rMP65) was generated as previously described (32).
The total polysaccharide and protein compositions of the different antigen preparations were determined by the phenol-sulfuric acid method and the Bio-Rad (Hercules, Calif.) protein assay, respectively, as previously described (27). None of the C. albicans antigens contained Limulus amoebocyte lysate-detectable lipopolysaccharide.
Synthetic peptides T1a, T1b, T2a, and T2b were purchased from Tana Laboratories (Houston, Tex.). They were provided as >80% pure and assessed for purity by high-pressure liquid chromatographic amino acid analysis and sequencing as previously described (26). The synthetic peptides were dissolved in 5% dimethyl sulfoxide (Sigma) and then diluted to the desired concentration in RPMI 1640 medium (Euroclone Ltd., Wetherby, United Kingdom) as previously described (26).
Reagents.
Phytohemagglutinin (PHA) was obtained from Murex (Dartford, United Kingdom). Recombinant interleukin-2 (IL-2) was a kind gift from EuroCetus (Milan, Italy), and tetanus toxoid (TT) was obtained from Chiron (Siena, Italy). Tritiated thymidine was purchased from Amersham (Little Chalfont, United Kingdom). Fluorescein isothiocyanate (FITC)-labeled anti-CD4 and anti-CD8 were purchased from Becton Dickinson (Mountain View, Calif.), and anti-major histocompatibility complex (anti-MHC) class II MAb L243 was obtained from the American Type Culture Collection, Rockville, Md. An FITC-conjugated mouse immunoglobulin G (IgG) control (Becton Dickinson) served for background determination.
Media.
RPMI 1640 medium was supplemented with 100 U of kanamycin/ml, 1 mM l-glutamine, 1 mM sodium pyruvate, and 1% nonessential amino acids (complete medium). When needed, 10% fetal calf serum (FCS; Gibco Laboratories, Grand Island, N.Y.) or 5% human serum (Sigma) was added. IL-2 was used at 10 to 100 U/ml.
MP-F2-specific TCC.
MP-F2-specific TCC were derived from PBMC of a DRB1*1101 (DR5) normal donor as previously described (40, 41). Briefly, PBMC were purified from heparinized blood on a density gradient (Lymphoprep; Nycomed Pharma AS, Oslo, Norway) and resuspended in complete medium supplemented with 5% autologous serum in the presence or absence of 5 μg of MP-F2/ml. After 5 and 10 days, 10 and 100 U of IL-2/ml, respectively, were added to the cultures. After 5 additional days, cultures showing significant cell growth were considered positive. Cells were counted and cloned by limiting dilution in the presence of 5 × 105 irradiated PBMC, 1 μg of PHA/ml, and 100 U of IL-2/ml. After 10 to 15 days, growing cultures were expanded in medium containing 100 U of IL-2/ml and finally tested for MP-F2 specificity by a proliferation assay using irradiated autologous PBMC prepulsed or not prepulsed with MP-F2 at 5 μg/ml. MP-F2-specific clones were maintained and expanded in cultures with 25- to 35-day cycles of restimulation with PHA and irradiated PBMC.
Epstein-Barr virus-transformed LCL and DC.
Lymphoblastoid B-cell lines (LCL) were established from the same donor by infecting 106 PBMC in an overnight incubation with Epstein-Barr virus-containing supernatant from the B.869 cell line and then were cultured in complete medium supplemented with 10% FCS and 0.5 μg of cyclosporine/ml. Dendritic cells (DC) were prepared by isolation on Percoll gradients of monocytes from PBMC as described previously (47). After 5 days of culturing in the presence of granulocyte-macrophage colony-stimulating factor and IL-4, cells were treated with antigen or apoptotic cells overnight (1, 2) and then were treated with lipopolysaccharide (Sigma) for 6 h to induce final differentiation. After extensive washings, cells were used as antigen-presenting cells (APC) for MP-F2-specific TCC in proliferation experiments.
Proliferation assay.
Proliferative responses were measured with triplicate cultures (220 μl) in 96-well flat-bottom plates that routinely contained 3 × 104 to 5 × 104 responder TCC and either 105 irradiated PBMC or 3 × 104 to 5 × 104 LCL as APC in 10% FCS-containing complete medium. C. albicans and S. cerevisiae antigens were used at a final concentration of 5 μg (polysaccharide for MP-F2 and M-sMP and protein for purified molecules) per milliliter. Synthetic peptides were used at 10 μg/ml. In dose-response tests, twofold dilutions from 5 to 1.25 μg/ml (mannoproteins and proteins) or from 10 to 0.312 μg/ml (peptides) were used.
In inhibition experiments, autologous LCL were incubated with peptides at 2 μg/ml for 2 h at 4°C. LCL were then washed and incubated for 1 h with decreasing concentrations of anti-HLA-DR (from 1:4 to 1:64 twofold dilutions of L243 cell culture supernatant) before TCC were added for the proliferation assay.
In all proliferation assays, tritiated thymidine was added at 5 μCi/well after 48 h of culturing, and cells were harvested 18 h later. Results were expressed as mean counts per minute in triplicate wells.
Cytokine determinations.
The production of cytokines by selected MP-F2-specific TCC, chosen from among those showing different reactivities, was measured with pooled supernatants from 0.2-ml cultures containing 5 × 105 irradiated autologous PBMC/ml and 5 × 105 TCC/ml, with or without MP-F2 at 10 μg/ml. Culture supernatants were collected after 48 h of culturing. Gamma interferon (IFN-γ) and IL-4 were measured using a commercial enzyme-linked immunosorbent assay according to manufacturer instructions (Quantikine; R&D Systems, Inc., Minneapolis, Minn.).
Phenotypic analysis.
Aliquots of 2 × 105 TCC were harvested from macrocultures, washed twice with cold 0.1% bovine serum albumin–phosphate-buffered saline, and stained with FITC-conjugated anti-CD4 or anti-CD8 MAb. An FITC-conjugated mouse IgG control (Becton Dickinson) served for background determination. After washings, cells were resuspended in medium suitable for FACScan (Becton Dickinson) analysis.
Fluorescence intensity was evaluated by computerized analysis of histograms generated from at least 5,000 viable cells (5).
RESULTS
Generation of MP-F2-specific TCC.
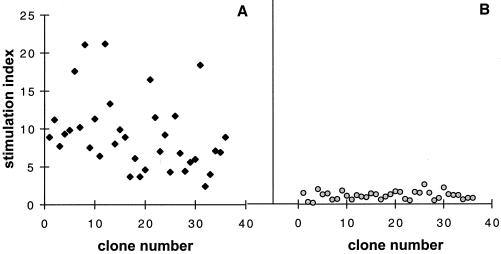
To generate specific human TCC, we used the highly immunogenic mannoprotein-rich fraction of C. albicans, MP-F2 (52), and PBMC from an HLA-DRB1*1101 (DR5) high-responder donor, as in previous experiments (32). Fifty-five clones from among 300 screened were selected for a specific proliferative response to MP-F2. All of the specific clones were tested for immunophenotyping by flow cytometry and were found to be alpha or beta T-cell receptor (TCR-α/β) CD4+/CD8− cells. The specificity of TCC for MP-F2 was verified by use of three proliferation assays with irradiated autologous PBMC prepulsed or not prepulsed with MP-F2. In addition, none of the clones tested proliferated in the presence of Sc-rMP65 or TT (Table 1). Furthermore, clonality was assessed via a subcloning test, which showed 100% MP-F2 specificity of growing subcloned cells. Interestingly, all TCC proliferated with MP-F2 in the presence of autologous PBMC as APC (Fig. 1A) but not with autologous LCL (Fig. 1B). Since T-cell-secreted cytokines are critical regulatory components of immunological responses, we also measured the production of IFN-γ and IL-4 in supernatants of MP-F2-stimulated TCC cultures with autologous PBMC as APC. All TCC tested secreted IFN-γ but not IL-4, suggesting that MP-F2 prevalently induces a typical T-helper type 1 (Th1) immune response and substantially confirming previous results from experimental animal models and PBMC proliferation assays (4, 38).
TABLE 1.
Antigen recognition of MP-F2-specific TCC in the presence of irradiated PBMC as APC
| Antigen | No. of clones tested | % of responding TCC | % Reactivitya
|
||
|---|---|---|---|---|---|
| Low | Intermediate | High | |||
| MP-F2 | 55 | 100 | 38.9 | 30.6 | 30.6 |
| M-sMP | 55 | 100 | 38.9 | 30.6 | 30.6 |
| MP65 | 55 | 94.5 | 33.3 | 16.6 | 50.0 |
| Ca-rMP65 | 55 | 94.5 | 33.3 | 16.6 | 50.0 |
| Sc-rMP65 | 10 | 0 | |||
| TT | 10 | 0 | |||
| M-sMP-P | 55 | 5.5 | 33.3 | 66.6 | |
| T1a | 55 | 7.3 | 25.0 | 75.0 | |
| T1bb | 55 | 94.5 | |||
| T2ac | 55 | 94.5 | |||
| T2bb | 55 | 94.5 | |||
Reactivity was considered low when the stimulation index (SI; counts per minute in the presence of antigen/counts per minute in the absence of antigen) of a TCC was between 2 and 10, intermediate when the SI was between 11 and 20, and high when the SI was >21.
Reactivity was high.
Reactivity was intermediate.
FIG. 1.
Proliferation of MP-F2-specific TCC in the presence of autologous PBMC (A) or LCL (B) as APC. APC were pulsed with MP-F2 at 5 μg/ml and then irradiated before incubation with specific clones. Data are expressed as the stimulation index (counts per minute in the presence of antigen/counts per minute in the absence of antigen). Data are from one experiment out of four independent experiments with similar results.
Identification of MP65 as an immunodominant antigen in MP-F2.
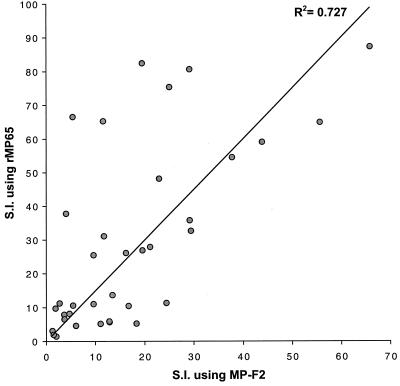
MP-F2 is a fraction from a crude mannoprotein extract of yeast cells of C. albicans separated from a whole cellular extract of the fungus by ion-exchange chromatography (52). MP65 is quantitatively a minor molecular constituent of MP-F2 and of other C. albicans fractions, such as the secretory hyphal constituent M-sMP, but is recognized as a main target of the human T-cell response against C. albicans (26). We therefore evaluated which fraction of MP-F2-specific clones recognized MP65 so as to have a direct measure of the relative immunodominance of this latter mannoprotein in the anti-C. albicans cellular response. As shown in Fig. 2, the majority of the MP-F2-specific TCC proliferated when tested with MP65-pulsed APC, suggesting that, although a minor molecular component of the preparation, MP65 represents its major antigen recognized by T cells.
FIG. 2.
Correlation between the proliferative responses of MP-F2-specific clones with MP-F2 or recombinant MP65 (rMP65) as the antigen. Autologous PBMC were pulsed with MP-F2 or MP65 at 5 μg/ml, irradiated, and then used as APC for specific clones. Data are expressed as the stimulation index (S.I.) (see the legend to Fig. 1). Data are from one experiment out of four independent experiments with similar results.
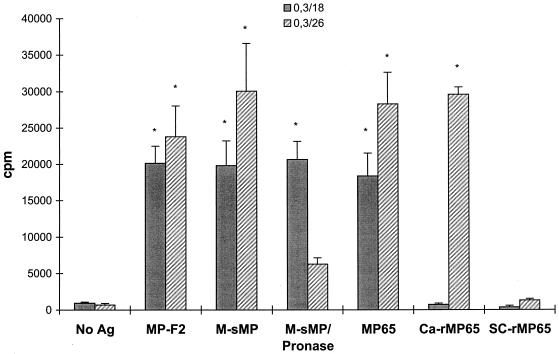
Since MP65 is amply O glycosylated (about one-third of the molecular mass [26]), we attempted to gain some insight into the influence of the polysaccharide moiety in MP65 T-cell epitope recognition. We therefore investigated the reactivities of all MP-F2-specific TCC toward the nonglycosylated recombinant (Ca-rMP65), natural MP65, untreated M-sMP, or M-sMP-P. The large majority of TCC recognized M-sMP, natural MP65, and Ca-rMP65 but not M-sMP-P. However, 3 out of 55 MP-F2-specific TCC recognized natural MP65, M-sMP, and M-sMP-P but not Ca-rMP65. Figure 3 shows representative responses of two TCC having these opposite reactivities. Figure 3 also shows that none of the TCC recognized Sc-rMP65, further attesting to the specificity of C. albicans MP65 epitope recognition.
FIG. 3.
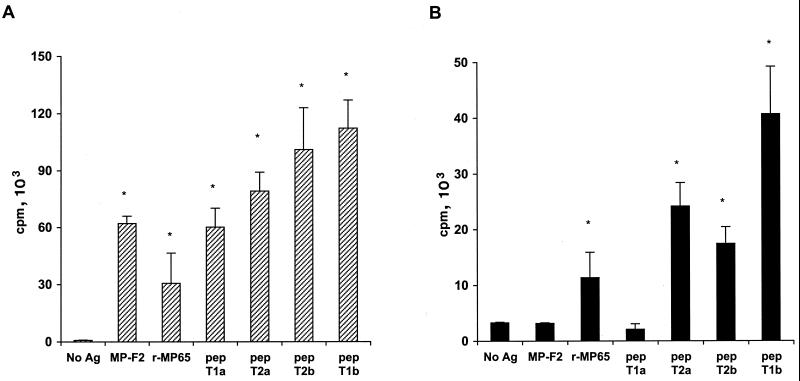
Proliferative responses of two representative clones to different preparations of C. albicans antigens, Ca-rMP65, or Sc-rMP65. Autologous PBMC were pulsed with or without antigens at 5 μg/ml, irradiated, and then used as APC for clones 0.3/18 and 0.3/26. Data are expressed as mean counts per minute for triplicate cultures from one experiment out of four independent experiments with similar results. Error bars indicate standard deviations. An asterisk indicates a significant difference (P < 0.01) between the mean counts per minute obtained in the presence of the indicated antigen and the mean counts per minute obtained in the absence of antigen (No Ag).
A motif sequence for MP65 peptide recognition by T cells.
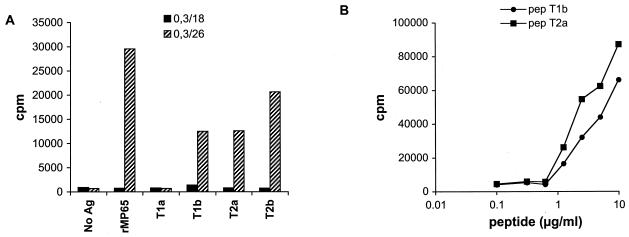
A previous study with MP65-stimulated PBMC identified two internal peptides as antigenic determinants of the molecule (26). Thus, in the attempt to identify major epitopes of MP65 in HLA-DRB1*1101 (DR5) subjects, we first investigated the TCC responses toward these two putative T-cell epitopes. Two pairs of peptides (T1a-T1b and T2a-T2b) were synthesized to reproduce the sequences of two previously described (26) tryptic peptides of MP65, derived from amino acids 142 to 164 (peptide T2) and 182 to 204 (peptide T1) of MP65 (32). Figure 4A shows two representative TCC with different reactivities toward natural MP65, Ca-rMP65, and synthetic peptides T1a, T1b, T2a, and T2b. Clone 0.3/26 reacted with both natural MP65 and recombinant MP65 as well as with peptides T1b, T2b, and T2a. The same results were obtained with the majority of the TCC tested, although different clones showed differences in the peptide resulting in the highest proliferation. Clone 0.3/18, on the other hand, showed a proliferative response when tested with natural MP65 but not with Ca-rMP65 or with the peptides used. Interestingly, this latter clone is one of the few which also proliferated in the presence of pronase-treated antigens (Fig. 3). The inability of this clone to respond to peptides or to Ca-rMP65 (which is nonglycosylated) and its ability to respond to protein-digested preparations suggest that the polysaccharide moiety of the molecule could be involved, at least in part, in the T-cell recognition of this C. albicans antigen.
FIG. 4.
(A) Proliferative responses of two representative clones (0.3/18 and 0.3/26) to recombinant MP65 (rMP65) and to synthetic peptides (T1a, T1b, T2a, and T2b). PBMC were pulsed with MP65 at 5 μg/ml or peptides at 10 μg/ml, irradiated, and then used as APC. Ag, antigen. (B) Dose-dependent proliferative responses of an MP-F2-specific clone (0/26) to two peptides (T1b and T2a) with different sequences. Data are expressed as mean counts per minute for triplicate cultures. The data reported in panel A are from one experiment out of four independent experiments with similar results; the data in panel B are representative of two independent experiments.
When tested for recognition of each peptide, the majority of our TCC recognizing Ca-rMP65 also did recognize the pair T2a-T2b and, surprisingly, the peptide T1b. Although there was a clear overlapping of sequences between each pair, no apparent homology exists between peptides T1 and T2 (Table 2). Only four TCC recognized peptide T1a (Fig. 4A). The proliferative responses to decreasing amounts of peptides T1b and T2a of a representative TCC are shown in Fig. 4B. It was clear that a single TCC recognized both peptides, although with slightly different affinities. The double reactivity with peptide T1a or T1b and peptide T2a or T2b was confirmed by using subclones of three different primary TCC (data not shown). Proliferation was inhibited by an anti-HLA class II MAb in a dose-dependent manner, indicating that MP-F2-specific TCC were class II restricted and excluding a mitogenic effect of possible peptide contaminants (Fig. 5). The most likely explanation is that most of our MP65-specific TCC recognize amino acid sequences characterized by the presence of fixed residues (I, S, I, and L) flanked by nonrelevant amino acids, according to the motif sequence reported in Table 2. Importantly, no such motif was present in any region of S. cerevisiae MP65, while two of them were present in MP65 of C. albicans (8, 32).
TABLE 2.
Amino acid sequences of Ca-MP65, Sc-MP65, and the peptides used to map Ca-MP65 epitopes
| Protein or peptide (amino acids) | Sequencea |
|---|---|
| Proteins | |
| Ca-MP65 (142–160) | SSSQIASEIAQLSGFNVIR |
| Sc-MP65 (152–170) | STAQVASDLEQLTGFDNIR |
| Ca-MP65 (182–204) | IFAGIFDVSSITSGIESLAEAVK |
| Sc-MP65 (192–214) | LFLGIYYVDKIQDAVDTIKSAVE |
| Peptides | |
| T1a | IFAGIFDVSSITSGI |
| T1b | SSITSGIESLAEAVK |
| T2a | SSSQIASEIAQLSGF |
| T2b | IASEIAQLSGFNVIR |
| Aligned peptides | |
| T1a | IFAGIFDVSSITSGI |
| T1b | SSITSGIESLAEAVK |
| T2a | SSSQIASEIAQLSGF |
| T2b | IASEIAQLSGFNVIR |
| Amino acid motifb | |
| Position | 12345678910 |
| Amino acid | XIXSXIXXLX |
Bold type indicates amino acids relevant for TCC recognition. Italic type indicates the Saccharomyces cerevisiae MP65 corresponding sequence.
Positions 1 to 10 indicate the amino acid position in the motif sequence. X indicates an amino acid not relevant for TCC recognition.
FIG. 5.
Proliferative response of an MP-F2-specific TCC in the presence of PBMC or LCL as APC. APC were pulsed with antigens at 5 μg/ml or peptides (pep) at 10 μg/ml and irradiated before incubation with specific clones. r-MP65, recombinant MP65. Data are expressed as mean counts per minute for triplicate cultures from one experiment out of four independent experiments with similar results. Error bars indicate standard deviations. An asterisk indicates a significant difference (P < 0.01) between the mean counts per minute obtained in the presence of the indicated antigen and the mean counts per minute obtained in the absence of antigen (No Ag).
Processing requirement of MP65.
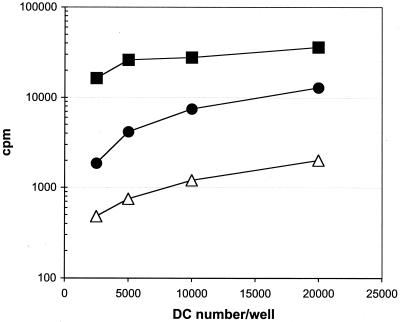
In screening our clones, we noted that autologous LCL were unable to act as MP-F2 APC; in fact, none of the clones was induced to proliferate with natural MP65-pulsed LCL (Fig. 1B). The same results were obtained when LCL from an HLA class II-compatible healthy subject were used (data not shown). We next assayed the capacity of autologous LCL to act as APC using different preparations of MP65, such as Ca-rMP65, MP-F2, and synthetic peptides, as sources of MP65 epitopes. As shown in Fig. 6, only Ca-rMP65 and peptides were efficiently presented by LCL to MP-F2-specific TCC. The failure of LCL to present natural MP65 might be related to the polysaccharide moiety of the glycoprotein, which could impair the uptake and/or the processing of natural MP65 in B cells. The possible lack of processing machinery to digest mannoproteins in B cells was investigated in cross-presentation experiments. LCL pulsed with or without MP-F2 were irradiated to induce apoptosis and then incubated with autologous DC. After incubation with apoptotic LCL, DC were induced to maturation with lipopolysaccharide and then used as APC for MP65-specific clones. Figure 6 shows that DC previously exposed to apoptotic LCL pulsed with MP-F2 were able to present MP65 epitopes to TCC.
FIG. 6.
Proliferative response of an MP-F2-specific TCC in the presence of autologous DC as APC. LCL were incubated with or without MP-F2 at 5 μg/ml and then irradiated. DC were incubated with MP-F2 (▪) or with irradiated, apoptotic LCL pulsed (●) or not pulsed (▵) with MP-F2 and then used as APC for an MP-F2-specific clone (0/26). Data are expressed as mean counts per minute for triplicate cultures and are representative of two independent experiments.
DISCUSSION
TCC are useful tools in the dissection of immune responses to self and non-self antigens. The isolation of antigen-specific TCC is crucial when the exact definition of antigenic specificity, as well as epitope dominance and functional assets of individual T-cell responses, is needed (14, 43, 54). Mucosal candidiasis relies upon some local or systemic deficiency in T-cell responses (6, 9, 10, 12, 18, 24, 37). Therefore, the analysis of these responses would greatly benefit from careful assessment of fine antigen recognition, phenotype, and function of TCC. Such an analysis would also provide potentially therapeutic tools for an attempt to reconstitute a protective response by administration of TCC which were deleted or altered in their specificity or function as a result of the immune deficiency.
Despite these promising perspectives, Candida-specific TCC have only sporadically been investigated (29). For instance, Sieck et al. showed that Candida-specific T-cell lines (antigen not specified) allowed fungus elimination from infected mice (50). More recently, Manca et al. demonstrated the preserved antigen specificity of Candida-reactive T-cell lines in AIDS subjects, despite the well-known numerical loss of specific clones (33). Overall, we are unaware of any published report specifically devoted to an understanding of defined antigen specificity in candidiasis through the use of human TCC.
One of the reasons for the paucity of TCC generation and usage in research on anticandidal immunity is the relatively low-grade definition of Candida antigens involved in the stimulation of T-cell responses and recognized as dominant T-cell epitopes. We have long been studying a 65-kDa mannoprotein of C. albicans, MP65, a structural and secretory component of the fungus that is particularly abundant in extracellular fractions of hyphal cells (4, 7, 26, 27). The reasons for focusing on this constituent were twofold. First, it is the main glycosylated member of a mannoprotein-rich fraction, MP-F2, capable of inducing an appreciable level of protection against murine candidiasis (10). Second, it was recognized as the main stimulating Candida antigen by peripheral blood lymphocytes from almost all healthy subjects tested (28, 32). Moreover, in animals immunized with MP-F2, the MP65 constituent was the principal one recognized by the animal splenocytes and was a strong stimulator of a delayed-type hypersensitivity reaction in vivo. More recently, the gene encoding the protein component of MP65 was cloned, and a recombinant product that was also strongly antigenic in human PBMC cultures was generated (32).
In order to understand more thoroughly the antigenic properties of MP65, we have generated human TCC using MP-F2 to stimulate PBMC from a healthy donor. It is generally believed that, when an antigen is added to PBMC in vitro, memory-experienced cells represent the population of CD4+ T cells that specifically proliferate. These cells originate from in vivo-primed lymphocytes upon presentation by professional APC of peptides with a high level of binding to MHC class II molecules, selected by enzymatic digestion of the antigen. The large majority of MP-F2-reactive clones generated were indeed reactive with MP65, a minor component of the MP-F2 mixture, indicating that in vivo MP65 is an immunodominant antigen that, more than others, is able to expand the specific T-lymphocyte population.
All of the clones tested were CD4+ and secreted IFN-γ but not IL-4 upon specific antigenic challenge, indicating a clear Th1 phenotype (4). It has been suggested that a Th1 response is an essential constituent of anti-Candida protection, although Th1 and Th2 responses may vary in different body sites (16, 22). A close cooperation between innate and adaptive responses appears to be required for anti-Candida protection, as also evidenced by the various strategies of immunoevasion adopted by the fungus (21). In this context, the clear definition of MP65-specific TCC as Th1 lymphocytes would indicate a potential role of these cells in the recognition of and possibly the defense against Candida invasion in humans. Experiments are in progress to assess the capacity of this constituent to induce protection in various experimental models of candidiasis.
We also wanted to investigate which epitopes were recognized by our MP65-specific TCC. TCC were isolated from an HLA-DRB1*1101 (DR5) donor whose PBMC responded to MP-F2 and MP65 peptides, T1 and T2, which have been previously shown to be inducers of human PBMC proliferation in several subjects tested (26). Peptides T1 and T2 were synthesized as two overlapping pairs (T1a-T1b and T2a-T2b) to reproduce the sequences of tryptic peptides of MP65 (Table 2). We noted that each clone recognizing the homologous pair T2a-T2b also recognized T1b. This result was rather surprising given that all lymphocytes of a TCC share the same TCR, thus virtually recognizing a single MHC-peptide complex. The possibility that every TCC was not a clone but was a mixture of two clones, one recognizing T1a or T1b and the other recognizing T2a or T2b peptides, exists in theory but is highly improbable. In fact, this reactivity was observed in all the Ca-rMP65-recognizing TCC, which have been isolated from different cultures grown after limiting dilution at 0.3 to 1 cell/well. The double reactivity with peptides T1a or T1b and T2a or T2b was confirmed by using subclones of three different primary TCC. A close insight into the peptide sequences suggested that T2a, T2b, and T1b have some residues (I, S, I, and L) at fixed positions in their sequences, flanked by other amino acids nonrelevant for peptide recognition. In the peptide sequence of a T-cell epitope, at least two amino acids are required to bind the MHC class II groove and two are required to engage the TCR of a specific T lymphocyte (36, 55). In this regard, it is of interest to note that peptide T1a has IXSXI but lacks the terminal lysine of the hypothesized motif, and it is not recognized by the majority of TCC which recognize the other three peptides. In addition, only a minority of other TCC recognize peptide T1a. The motif sequence is not present in any region of S. cerevisiae MP65, which is not recognized at all by our MP65-specific clones.
The availability of distinct MP65-specific TCC and both the natural (glycosylated) protein and the recombinant (nonglycosylated) protein allowed the analysis of the relevance of glycosylation in the specific recognition of this antigen by human T lymphocytes. First, we observed that three of our TCC were reactive with MP-F2, natural MP65, and M-sMP-P but not with recombinant MP65 or the synthetic peptides T1a, T1b, T2a, and T2b. These observations, although inconclusive, suggest possible T-cell recognition of the polysaccharide moiety, which accounts for about one-third of the whole molecule and is apparently composed of O-linked mannosyl residues only, given the absence in the molecule of a putative N-glycosidic site (17, 25, 32). Glycopeptides (25) and glycolipids (39, 49) have in fact been identified as defined T-cell epitopes presented in the context of classical MHC molecules and CD1 molecules, respectively. In addition, T-cell recognition of a glycosidic moiety has been suggested in the definition of epitopes in human allergens (20, 48). Thus, the possibility exists that complex polysaccharides linked to C. albicans proteins are involved in T-cell recognition of this pathogen. Given that most surface antigens of the fungus are indeed polysaccharidic in nature, studies aimed at a better definition of possible T-cell recognition of polysaccharides are currently in progress.
Second, we observed that LCL were unable to present natural MP65 but retained the ability to present Ca-rMP65 and peptides. However, DC were able to stimulate MP65-specific TCC after phagocytosis of natural MP65-pulsed apoptotic LCL (1, 2, 56). These data suggest that LCL can concentrate but not digest natural MP65. LCL have been previously described to have limitations in processing ability (35). These limitations have been ascribed to restriction or diversity in other APC in the proteolytic machinery used to cleave proteins into MHC class II binding peptides (13, 19). For MP65, however, LCL were able to digest the recombinant protein, revealing the efficiency of the protein-digesting machinery. Thus, it can be hypothesized that the enzymatic digestion of antigen in nonprofessional APC, such as LCL, is highly affected by the presence of O-linked mannoside chains that could hinder the cleavage of certain protein substrates. Interestingly, natural MP65 was demonstrated to be totally resistant to any protease (trypsin, chymotrypsin, or endoproteinase Asp-N) treatment used to partially or completely digest the molecule, unless it was previously denatured by heat (100°C) or sodium dodecyl sulfate (26).
The inability of nonprofessional APC to process natural MP65 could have functional consequences in AIDS patients, who have a high predisposition to mucosal candidiasis (6, 15). Because the availability of functional professional APC in lymph nodes decreases with the progression of the disease (23, 42), it can be hypothesized that the relative importance in activating T lymphocytes of nonprofessional APC, such as B lymphocytes, progressively increases. Thus, the inability of B cells to present a major antigen of C. albicans may contribute, together with the decrease in the level of specific CD4+ T cells, to the onset of disease.
In conclusion, in this paper we report the first characterization of C. albicans-specific human TCC and demonstrate the immunodominance of MP65 among C. albicans soluble products. We also provide evidence on the relevance of polysaccharides in the processing and T-cell recognition of MP65. Finally, we propose a motif sequence that, in HLA-DRB1*1101 (DR5) subjects, may represent the characterizing epitope of MP65 useful for possible immune intervention. Further studies using donors with other HLA class II haplotypes are required to extend the epitope mapping of MP65.
ACKNOWLEDGMENTS
This work was supported in part by Istituto Superiore di Sanità, Programma Nazionale AIDS, grants 50C/D and 50C/B.
REFERENCES
- 1.Albert L M, Pearce S F, Francisco L M, Sauter B, Roy P, Silverstein R L, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert L M, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello C M, Spagnoli G C, Boccanera M, Casalinuovo I, Malavasi F, Casciani C U, Cassone A. Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J Med Microbiol. 1986;22:195–202. doi: 10.1099/00222615-22-3-195. [DOI] [PubMed] [Google Scholar]
- 4.Ausiello C M, Urbani F, Gessani S, Spagnoli G C, Gomez M J, Cassone A. Cytokine gene expression in human peripheral blood mononuclear cells stimulated by mannoprotein constituents from Candida albicans. Infect Immun. 1993;61:4105–4111. doi: 10.1128/iai.61.10.4105-4111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoleschi C, Nisini R, Biselli R, Casciaro A, Fattorossi A. Flow cytometric analysis of cell function: cytosolic Ca++ modulation in human polymorphonuclear leukocytes and T lymphocytes. Eur J Histochem. 1994;38:47–52. [PubMed] [Google Scholar]
- 6.Brawner D L, Hovan A J. Oral candidiasis in HIV-infected patients. Curr Top Med Mycol. 1995;6:113–125. [PubMed] [Google Scholar]
- 7.Bromuro C, Torosantucci A, Gomez M J, Urbani F, Cassone A. Differential release of an immunodominant 65 kDa mannoprotein antigen from yeast and mycelial forms of Candida albicans. J Med Vet Mycol. 1994;32:447–459. doi: 10.1080/02681219480000601. [DOI] [PubMed] [Google Scholar]
- 8.Cappellaro C, Mrsa V, Tanner W. New potential cell wall glucanases of Saccharomyces cerevisiae and their involvement in mating. J Bacteriol. 1998;180:5030–5037. doi: 10.1128/jb.180.19.5030-5037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall A, Cassone A, Bistoni F, Cutler J E, Magliani W, Murphy J W, Polonelli L, Romani L. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med Mycol. 1998;36:95–105. [PubMed] [Google Scholar]
- 10.Cassone A, De Bernardis F, Ausiello C M, Gomez M J, Boccanera M, La Valle R, Torosantucci A. Immunogenic and protective Candida albicans constituents. Res Immunol. 1998;149:289–299. doi: 10.1016/s0923-2494(98)80753-0. [DOI] [PubMed] [Google Scholar]
- 11.Cassone A, Torosantucci A. Immunological moieties of the cell wall. In: Prasad R, editor. Candida albicans. Berlin, Germany: Springer-Verlag; 1991. pp. 89–107. [Google Scholar]
- 12.Chaffin W L, Lopez-Ribot J L, Casanova M, Gozalbo D, Martinez J P. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen B P, DeMars R, Sondel P M. Presentation of soluble antigen to human T cells by products of multiple HLA-linked loci: analysis of antigen presentation by a panel of cloned, autologous, HLA-mutant Epstein-Barr virus-transformed lymphoblastoid cell lines. Hum Immunol. 1987;18:75–91. doi: 10.1016/0198-8859(87)90114-5. [DOI] [PubMed] [Google Scholar]
- 14.Chiari R, Hames G, Stroobant V, Texier C, Maillere B, Boon T, Coulie P G. Identification of a tumor-specific shared antigen derived from an Eph receptor and presented to CD4 T cells on HLA class II molecules. Cancer Res. 2000;60:4855–4863. [PubMed] [Google Scholar]
- 15.Coleman D C, Bennett D E, Sullivan D J, Gallagher P J, Henman M C, Shanley D B, Russell R J. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit Rev Microbiol. 1993;19:61–82. doi: 10.3109/10408419309113523. [DOI] [PubMed] [Google Scholar]
- 16.De Bernardis F, Santoni G, Boccanera M, Spreghini E, Adriani D, Morelli L, Cassone A. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun. 2000;68:3297–3304. doi: 10.1128/iai.68.6.3297-3304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deck B M, Sjolin P, Unanue E R, Kihlberg J. MHC-restricted, glycopeptide-specific T cells show specificity for both carbohydrate and peptide residues. J Immunol. 1999;162:4740–4744. [PubMed] [Google Scholar]
- 18.Deepe G S., Jr Prospects for the development of fungal vaccines. Clin Microbiol Rev. 1997;10:585–596. doi: 10.1128/cmr.10.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demotz S, Matricardi P M, Irle C, Panina P, Lanzavecchia A, Corradin G. Processing of tetanus toxin by human antigen-presenting cells. Evidence for donor and epitope-specific processing pathways. J Immunol. 1989;143:3881–3886. [PubMed] [Google Scholar]
- 20.De Palma R, Wu S, Sallusto F, Di Felice G, Martucci P, Geraci D, Colombo P, Troise C, Sacerdoti G, Nocera A, Gorski J. Use of antagonist peptides to inhibit in vitro T cell responses to Par j1, the major allergen of Parietaria judaica pollen. J Immunol. 1999;162:1982–1987. [PubMed] [Google Scholar]
- 21.d'Ostiani C F, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elahi S, Pang G, Clancy R, Ashman R B. Cellular and cytokine correlates of mucosal protection in murine model of oral candidiasis. Infect Immun. 2000;68:5771–5777. doi: 10.1128/iai.68.10.5771-5777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fauci A S, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 24.Fidel P L, Jr, Sobel J D. The role of cell-mediated immunity in candidiasis. Trends Microbiol. 1994;2:202–206. doi: 10.1016/0966-842x(94)90112-i. [DOI] [PubMed] [Google Scholar]
- 25.Franco A, Yokoyama T, Huynh D, Thomson C, Nathenson S G, Grey H M. Fine specificity and MHC restriction of trinitrophenyl-specific CTL. J Immunol. 1999;162:3388–3394. [PubMed] [Google Scholar]
- 26.Gomez J M, Maras B, Barca A, La Valle R, Barra D, Cassone A. Biochemical and immunological characterization of MP65, a major mannoprotein antigen of the opportunistic human pathogen Candida albicans. Infect Immun. 2000;68:694–701. doi: 10.1128/iai.68.2.694-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez J M, Torosantucci A, Arancia S, Maras B, Parisi L, Cassone A. Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect Immun. 1996;64:2577–2584. doi: 10.1128/iai.64.7.2577-2584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossi P, Farina C, Fiocchi R, Dalla Gasperina D. Prevalence and outcome of invasive fungal infections in 1,963 thoracic organ transplant recipients: a multicenter retrospective study. Italian Study Group of Fungal Infections in Thoracic Organ Transplant Recipients. Transplantation. 2000;70:112–116. [PubMed] [Google Scholar]
- 29.Hermann E, Mayet W J, Klein O, Lohse A W, Trautwein C, Michiels I, Poralla T, Meyer zum Buschenfelde K H. Candida arthritis: cellular immune responses of synovial fluid and peripheral blood lymphocytes to Candida albicans. Ann Rheum Dis. 1991;50:697–701. doi: 10.1136/ard.50.10.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoppe J E, Klausner M, Klingebiel T, Niethammer D. Retrospective analysis of yeast colonization and infections in paediatric bone marrow transplant recipients. Mycoses. 1997;40:47–54. doi: 10.1111/j.1439-0507.1997.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan J E, Hanson D, Dworkin M S, Frederick T, Bertolli J, Lindegren M L, Holmberg S, Jones J L. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 32.La Valle R, Sandini S, Gomez M J, Mondello F, Romagnoli G, Nisini R, Cassone A. Generation of a recombinant 65-kilodalton mannoprotein (MP65), a major antigen target of cell-mediated immune response to Candida albicans. Infect Immun. 2000;68:6777–6784. doi: 10.1128/iai.68.12.6777-6784.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manca F, Fenoglio D, Franchin E, Saverino D, Li Pira G, Buffa F, Bignardi D, Del Pup L, Palu G. Anti-HIV genetic treatment of antigen-specific human CD4 lymphocytes for adoptive immunotherapy of opportunistic infections in AIDS. Gene Ther. 1997;4:1216–1224. doi: 10.1038/sj.gt.3300539. [DOI] [PubMed] [Google Scholar]
- 34.Manchado Lopez P, Ruiz de Morales J M, Ruiz Gonzalez I, Rodriguez Prieto M A. Cutaneous infections by papillomavirus, herpes zoster and Candida albicans as the only manifestation of idiopathic CD4+ T lymphocytopenia. Int J Dermatol. 1999;38:119–121. doi: 10.1046/j.1365-4362.1999.00364.x. [DOI] [PubMed] [Google Scholar]
- 35.Mandell R B, Hank J A, Chen B P, Robins H I, Sondel P M. Differential antigen presentation by heat-treated peripheral blood mononuclear cells and Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-LCL): heated EBV-LCL present alloantigen and soluble antigen but are deficient in the stimulation of autologous EBV-LCL primed T cells. Hum Immunol. 1987;19:163–177. doi: 10.1016/0198-8859(87)90067-x. [DOI] [PubMed] [Google Scholar]
- 36.Mazza G, Housset D, Piras C, Gregoire C, Lin S Y, Fontecilla-Camps J C, Malissen B. Glimpses at the recognition of peptide/MHC complexes by T-cell antigen receptors. Immunol Rev. 1998;163:187–196. doi: 10.1111/j.1600-065x.1998.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy G M. Host factors associated with HIV-related oral candidiasis. A review. Oral Surg Oral Med Oral Pathol. 1992;73:181–186. doi: 10.1016/0030-4220(92)90192-s. [DOI] [PubMed] [Google Scholar]
- 38.Mencacci A, Torosantucci A, Spaccapelo R, Romani L, Bistoni F, Cassone A. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect Immun. 1994;62:5353–5360. doi: 10.1128/iai.62.12.5353-5360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moody D B, Guy M R, Grant E, Cheng T Y, Brenner M B, Besra G S, Porcelli S A. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J Exp Med. 2000;192:965–976. doi: 10.1084/jem.192.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nisini R, Matricardi P M, Fattorossi A, Biselli R, D'Amelio R. Presentation of superantigen by human T cell clones: a model of T-T cell interaction. Eur J Immunol. 1992;22:2033–2039. doi: 10.1002/eji.1830220812. [DOI] [PubMed] [Google Scholar]
- 41.Nisini R, Paroli M, Accapezzato D, Bonino F, Rosina F, Santantonio T, Sallusto F, Amoroso A, Houghton M, Barnaba V. Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. J Virol. 1997;71:2241–2251. doi: 10.1128/jvi.71.3.2241-2251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 43.Pathan A A, Wilkinson K A, Wilkinson R J, Latif M, McShane H, Pasvol G, Hill A V, Lalvani A. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur J Immunol. 2000;30:2713–2721. doi: 10.1002/1521-4141(200009)30:9<2713::AID-IMMU2713>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Powderly W G, Mayer K H, Perfect J R. Diagnosis and treatment of oropharyngeal candidiasis in patients infected with HIV: a critical reassessment. AIDS Res Hum Retrovir. 1999;15:1405–1412. doi: 10.1089/088922299309900. [DOI] [PubMed] [Google Scholar]
- 45.Romani L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J Leukoc Biol. 2000;68:175–179. [PubMed] [Google Scholar]
- 46.Romani L, Bistoni F, Puccetti P. Initiation of T-helper cell immunity to Candida albicans by IL-12: the role of neutrophils. Chem Immunol. 1997;68:110–135. doi: 10.1159/000058688. [DOI] [PubMed] [Google Scholar]
- 47.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Poupot R, Clergue M, DePalma R, Fournie J J. A flavonoid sulfate antigen activates human alphabeta CD8+ Th2 lymphocytes in pollen allergy. Eur J Immunol. 2000;30:964–968. doi: 10.1002/1521-4141(200003)30:3<964::AID-IMMU964>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 49.Shamshiev A, Donda A, Prigozy T I, Mori L, Chigorno V, Benedict C A, Kappos L, Sonnino S, Kronenberg M, De Libero G. The alphabeta T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 2000;13:255–264. doi: 10.1016/s1074-7613(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 50.Sieck T G, Moors M A, Buckley H R, Blank K J. Protection against murine disseminated candidiasis mediated by a Candida albicans-specific T-cell line. Infect Immun. 1993;61:3540–3543. doi: 10.1128/iai.61.8.3540-3543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stearn B F, Polis M A. Prophylaxis of opportunistic infections in persons with HIV infection. Clevel Clin J Med. 1994;61:187–194. doi: 10.3949/ccjm.61.3.187. [DOI] [PubMed] [Google Scholar]
- 52.Torosantucci A, Bromuro C, Gomez M J, Ausiello C M, Urbani F, Cassone A. Identification of a 65-kDa mannoprotein as a main target of human cell-mediated immune response to Candida albicans. J Infect Dis. 1993;168:427–435. doi: 10.1093/infdis/168.2.427. [DOI] [PubMed] [Google Scholar]
- 53.Viviani A M, Tortorano A M, Malaspina C, Colledan M, Paone G, Rossi G, Bordone G, Pagano A. Surveillance and treatment of liver transplant recipients for candidiasis and aspergillosis. Eur J Epidemiol. 1992;8:433–436. doi: 10.1007/BF00158579. [DOI] [PubMed] [Google Scholar]
- 54.Vogt H M, Goulmy E, Kloosterboer F M, Blokland E, de Paus R A, Willemze R, Falkenburg J H. UTY gene codes for an HLA-B60-restricted human male-specific minor histocompatibility antigen involved in stem cell graft rejection: characterization of the critical polymorphic amino acid residues for T-cell recognition. Blood. 2000;96:3126–3132. [PubMed] [Google Scholar]
- 55.Young A C, Nathenson S G, Sacchettini J C. Structural studies of class I major histocompatibility complex proteins: insights into antigen presentation. FASEB J. 1995;9:26–36. doi: 10.1096/fasebj.9.1.7821756. [DOI] [PubMed] [Google Scholar]
- 56.Yrlid U, Wick M J. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J Exp Med. 2000;191:613–624. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]