Abstract
Aims
Stroke is a leading cause of death and disability for Americans, and growing evidence suggests that air pollution may play an important role. To facilitate pollution control efforts, the National Academy of Sciences and the World Health Organization have prioritized determining which air pollutants are most toxic. However, evidence is limited for the simultaneous effects of multiple air pollutants on stroke.
Methods and results
We constructed a nationwide population-based cohort study, using the Medicare Chronic Conditions Warehouse (2000–2017) and high-resolution air pollution data, to investigate the impact of long-term exposure to ambient PM2.5, NO2, and ground-level O3 on incident stroke. Hazard ratios (HR) for stroke incidence were estimated using single-, bi-, and tri-pollutant Cox proportional hazards models. We identified ~2.2 million incident stroke cases among 17,443,900 fee-for-service Medicare beneficiaries. Per interquartile range (IQR) increase in the annual average PM2.5 (3.7 μg/m3), NO2 (12.4 ppb), and warm-season O3 (6.5 ppb) one-year prior to diagnosis, the HRs were 1.022 (95% CI: 1.017–1.028), 1.060 (95% CI: 1.054–1.065), and 1.021 (95% CI: 1.017–1.024), respectively, from the tri-pollutant model. There was strong evidence of linearity in concentration-response relationships for all three air pollutants in single-pollutant models. This linear relationship remained robust for NO2 and O3 in tri-pollutant models while the effect of PM2.5 attenuated at the lower end of concentrations.
Conclusion
Using a large nationwide cohort, our study suggests that long-term exposure to PM2.5, NO2, and O3 may independently increase the risk of stroke among the US elderly, among which traffic-related air pollution plays a particularly crucial role.
Keywords: Air pollution, Stroke, Incidence, Medicare
Introduction
Stroke is the number one cause of long-term disability and the second leading cause of death worldwide [1,2]. According to the Global Burden of Disease Study (GBD), there were approximately 11.9 million incidents, 104.2 million prevalent, and 6.2 million fatal cases of stroke globally in 2017, with 132.1 million stroke-related disability-adjusted life-years (DALYs) [3]. Since stroke is characterized by high mortality, high morbidity, and contributes to severe burden disease, identifying and intervening on modifiable risk factors of stroke is of great significance for public health.
An increasing number of epidemiologic studies have assessed the association between air pollution and stroke evaluated by hospital admission incidence, and mortality [[4], [5], [6]]. Recent studies examining relationship of air pollution on nonfatal stroke have shifted their focus from short-term to long-term exposures [[7], [8], [9], [10], [11], [12], [13], [14]]. However, the results were inconsistent, and the associations between exposure to air pollution and stroke have not been fully understood. Moreover, these studies mainly focused on particulate matter exposures (particulate matter with aerodynamic diameter < 2.5 μm or < 10 μm [PM2.5 or PM10]) [9,10,12,[14], [15], [16], [17]]; while investigations of gaseous air pollutants (such as nitrogen dioxide [NO2]; ground-level ozone [O3]) or simultaneous effects of multi-pollutants are scarce. Most of the studies that looked at multi-pollutants often explored a single pollutant at a time [8,11,13], and only a few evaluated air pollutants jointly in multi-pollutant models to investigate the potential independent associations between air pollution types and stroke risk [7,[18], [19], [20]]. To facilitate the targeting of pollution control efforts, the National Academy of Sciences (NAS) and the World Health Organization (WHO) have prioritized determining which air pollutants are most toxic.
To address this gap, we constructed a nationwide population-based cohort among the American Medicare population (aged ≥65 years), combined with high-resolution air pollution datasets, to examine the association of stroke incidence with annual average PM2.5, NO2, and warm-season O3 during the calendar year prior to diagnosis year. To better measure stroke incidence, we leveraged all Medicare claims across the contiguous United States (2000–2017) and further required a one-year “clean” period without events of interest to identify first diagnosis of stroke.
Methods
Study population
Health data were obtained from the two databases, namely the Medicare denominator file and the Medicare Chronic Conditions Warehouse (CCW), both from the Centers for Medicare and Medicaid Services (CMS). The Medicare denominator file (i.e., the enrollment file) contains enrollment records for each Medicare beneficiary in each year, including age, sex, race, Medicaid eligibility (a proxy for socioeconomic status - SES), the date of death (if any), and ZIP code of residence. Age, Medicaid eligibility, and ZIP code of residence are updated annually. The CCW database provides the date of the first occurrence with a stroke diagnosis code across the available Medicare claims. Based on the Medicare denominator file and the CCW database, we constructed an open cohort including all Medicare beneficiaries aged 65 and over with continuous enrollment [1] in Medicare Fee-for-Service (FFS) program; and [2] in both Medicare Part A (hospital insurance) and Part B (medical insurance) in the contiguous United States between 2000 and 2017. Namely, we excluded those with any time in Medicare Advantage Plan (Part C) over the study period since claim records are not available for these beneficiaries; and excluded those only enrolled in Medicare Part A or Part B. These exclusions were included because the CCW relies on FFS, Part A, and Part B to identify cases. If we relaxed these restrictions to broaden the cohort, the chance of missing stroke diagnoses among those additional participants brought into the analysis could be high.
In addition to the above-mentioned inclusion and exclusion criteria, we further required a “clean” period of one year after enrollment, during which there was no diagnosis code for stroke. For example, a participant entering Medicare in 2000 would be required to be stroke-free until 2011; follow-up for disease incidence began only then. We also excluded the one-year “clean” period from person-time to avoid immortal time bias. By removing potentially prevalent cases in their first years of follow-up, a diagnosis after that “clean” period more likely approximates disease “incidence”. We considered that a “clean” period of one year represent a reasonable time window to ensure a person was stroke diagnosis free prior to the Medicare diagnosis [21]; however, we also explored longer clean periods of 2 years and 4 years in sensitivity analyses. The person-time for the corresponding “clean” period was also excluded from the sub-cohorts. Therefore, study subjects entered the cohort on January 1st of the year following the “clean” period and were followed until first diagnosis of the outcome of interest across all Medicare claims, death, or end of follow-up. Cohort sizes and summary statistics for the study population with different clean periods are shown in Supplementary Table S1. Our research is approved by Emory's IRB (#STUDY00000316) and the Centers for Medicare & Medicaid Services (CMS) under the data use agreement (#RSCH-2020-55,733). The Medicare dataset was stored and analyzed in Emory Rollins School secure cluster environment (HPC), with Health Insurance Portability and Accountability Act (HIPAA) compliance.
Outcome classification
The primary outcome of interest for this study was the time to stroke. The CCW database includes a pre-defined indicator for stroke, which is identified using an algorithm that incorporates information from all available Medicare claims (such as inpatient and outpatient claims, Carrier file, skilled nursing facility, and home health-care claims) indicating that an individual was diagnosed with stroke (Chronic condition algorithms) [22]. We extracted the date of the first occurrence with a stroke diagnosis code for each study subject from the CCW database and defined the outcome stroke as the first occurrence of stroke diagnosis (reoccurrences of stroke were not considered in this study).
Exposure assessment
High-resolution ambient PM2.5, NO2, and O3 concentrations for the entire contiguous United States were derived using spatiotemporal ensemble models that integrated three different machine learning algorithms, including a neural network, a random forest, and a gradient boosting machine [[23], [24], [25]]. The ensemble-based model used over one hundred predictors, including satellite measurements, land-use terms, meteorological variables, and chemical transport model predictions to estimate daily levels of the pollutants on a scale of 1 km × 1 km [[26], [27], [28]]. The quality of the estimates was assessed using 10-fold cross-validation against monitoring measurements values from the Environmental Protection Agency (EPA), Air Quality Systems (AQS) across the United States. The resulting R2 values for annual predictions of PM2.5, NO2, and O3 were 0.89, 0.84, and 0.86, respectively, yielding strong model performance [[26], [27], [28]]. We averaged these 1-km resolution predictions for each pollutant at ZIP code scale, because ZIP code is the smallest level of geography in the Medicare data and ZIP code of residence is updated annually. We then calculated annual averages for PM2.5, NO2, and warm-season O3 (May 1 to October 31) concentrations in each ZIP code, for each calendar year and defined long-term exposure in our study as the annual averages of air pollutants. All Medicare beneficiary's outcome data for each calendar year were linked to the annual averaged exposures one year ago (i.e., one-year lag exposure) according to the residential ZIP code in the previous year such that any annual residential mobility changes by ZIP code were considered. We also explored different lagged exposures, including two-year, one-year, and zero-year lag exposure, in sensitivity analyses.
Covariates
We obtained individual-level covariates, including sex, race, age at entry, and Medicaid eligibility, from the Medicare denominator file. Based on individual age at entry, a 5-year age at entry group variable was created. We also included neighborhood-level covariates which have both been associated with ambient air pollution and implicated in cerebrovascular diseases into the analyses. These included ZIP code-level SES variables (population density, proportion of the population > 65 years of age living below the poverty line, proportion of the population listed as Black, median household income, proportion of housing units occupied by the owner, and proportion of the population > 65 years of age who had not graduated from high school), meteorological variables (annual mean temperature and annual mean relative humidity), land-use variable normalized difference vegetation index (NDVI) values, and county-level behavioral risk factors (mean body mass index and smoking rate) and health care capacity variables (number of hospitals and active medical doctors), as well as a geographical region (categorized as five U.S. region: West, Southwest, Midwest, Northeast, and Southeast). Specifically, SES variables were derived from the 2000 U.S. Census, 2010 U.S. Census, and the American Community Survey for 2005–2012; meteorological data were acquired from the North American Regional Reanalysis data (NARR) for 2000–2017; NDVI values were retrieved from NASA's MODIS satellite (MOD13C2) for 2000–2017; behavioral risk factors were obtained from the Behavioral Risk Factor Surveillance System (BRFSS) between 2000 and 2016; and health care capacity data were obtained from the 2010, 2015, and 2018 American Hospital Association Annual Survey Database. We linearly interpolated or extrapolated the missing values based on the data available [29]. We also obtained co-morbidity conditions (hypertension and diabetes) from the CCW database. These covariates have been suggested to associate with air pollution and stroke [30,31], and we included them as candidate confounders in sensitivity analyses.
Statistical analysis
We estimated the relationship between long-term exposure to PM2.5, NO2, O3, and incident stroke using a series of stratified Cox proportional-hazards models with a generalized estimating equation (GEE). The coefficient for the exposure variable was the parameter of interest, and years of follow-up were the time scale. Specifically, we fitted single-pollutant, bi-pollutant, and tri-pollutant models and estimated hazard ratios (HRs) per interquartile-range (IQR) increase in the annual average of PM2.5, NO2, and warm-season O3 concentrations in the one-year period prior to diagnosis. All three pollutants were of interest because some prior studies have shown associations between each of them and stroke [4,[32], [33], [34]]. GEE was used to adjust for residual autocorrelation within ZIP code with the use of robust standard errors (and 95% CI confidence intervals). To allow for flexible strata-specific baseline hazard functions, we stratified all models on four individual characteristics, including sex, race (white, black, other), Medicaid eligibility, and 5-year categories of age at study entry. We included 13 ZIP code-level and county-level time-varying covariates, including SES, behavioral risk factors, health care capacity, land-use, and meteorological variables in the model to adjust for potential confounding. Potential residual temporal and spatial trends were controlled by respectively including indicator variables for calendar years and geographical regions.
To assess the shape of the concentration-response (C-R) relationship between each air pollutant and stroke, we respectively fitted penalized splines [35] for PM2.5, NO2, and O3, adjusting for all covariates included in the tri-pollutant models. We also compared the C-R relationship using the single-pollutant models for each air pollutant without adjustment for co-pollutants. To identify subpopulations who might be more vulnerable than others, we examined six potential effect modifiers (gender, race (white, black), Medicaid eligibility, age (<75, ≥75), neighborhood-level household income, and education) by stratification.
Additionally, we estimated the attributable fraction (AF) of stroke cases due to PM2.5, NO2, and O3 air pollution, for those in the US exposed to an additional IQR of PM2.5 (a difference of 3.7 μg/m3), NO2 (12.4 ppb), and O3 (6.5 ppb), beyond current levels in US cities with relatively low exposure (i.e., 7 μg/m3 for PM2.5, 4 ppb for NO2, and 30 ppb for O3, the counterfactual) [36], using results from the tri-pollutant model, and using standard AF calculations when the entire population is exposed (RR-1)/RR (see Steenland and Armstrong 2006 [37]).
We conducted a series of sensitivity analyses to test the robustness of our main findings. First, we assessed the alternative exposure time windows by comparing the results using different lags (2-, 1-, and 0-year lags), in which exposure was assigned either as the annual exposure at 2 years prior to case, or 1 year prior, or 0 year prior. Second, we repeated the analyses using a sub-cohort of a longer “clean” period of 2 years and 4 years, i.e., thinking that excluding cases with a diagnosis during their first 2 years or 4 years of enrollment would increase the probability that we are capturing the first diagnosis and thus more closely estimating disease incidence, albeit at the cost of a smaller number of years of subjects and cases. Additionally, to evaluate whether the associations we observed were robust to different levels of confounding adjustment, we fitted the models with different set of covariates and modeled the potential residual temporal trend as a linear term for calendar years instead of categorical year. We also tested how sensitive our models might be to adjusting for space with a spatial smoother and with a state-level adjustment. Lastly, to evaluate whether the associations we observed can be attributed to comorbidities also linked to air pollution, we additionally adjusted for the comorbidities (including diabetes and hypertension), and restricted analyses to subjects without the comorbidities.
All computational analyses were run on the Rollins High-Performance Computing (HPC) Cluster at Emory University. R software, version 4.0.2, was used for all analyses.
Results
Study population characteristics
Table 1 provides descriptive information on the stroke cohort for main analysis. Data were analyzed for Medicare beneficiaries who were 65 years and older between Jan 1, 2000, and Dec 31, 2017, and met the inclusion and exclusion criteria (see Methods). The cohort was followed requiring a one-year “clean” period without stroke events to better capture stroke incidence. A total of 2,240,586 incident stroke cases were identified in 2001–2017 (as we applied a one-year “clean” period) among 17,443,900 studied subjects with 144,611,520 person-years of follow-up (Table 1). Most of the participants (88.2%) entered the cohort between ages 65 and 74, and the mean age at entry was 69.1 years (SD 4.8). The median follow-up was 8 years. Supplementary Table S2 summarized the new study subjects that entered the cohort serially over time and the mean age of the cohort increased gradually from 73.3 to 75.8 years. There were slightly more women than men, and most participants were white. More than 90% were not Medicaid-eligible, indicating that most participants were above the poverty level. Most of the observations came from the southern and midwestern regions of the contiguous United States.
Table 1.
Descriptive statistics for the study population.
| Variables | Full cohort |
|
|---|---|---|
| Number | % | |
| Years of clean period | 1 | |
| Total population | 17,443,900 | 100 |
| Total Stroke cases | 2,240,586 | 12.8 |
| Total person-years | 144,611,520 | |
| Median follow-up year | 8 | |
| Age at entry (years) | ||
| 65–74 | 15,378,824 | 88.2 |
| 75–115 | 2,065,076 | 11.8 |
| Mean (SD) | 69.1 (4.8) | |
| Sex | ||
| Male | 7,291,664 | 41.8 |
| Female | 10,152,236 | 58.2 |
| Race | ||
| White | 15,405,416 | 88.3 |
| Black | 1,039,110 | 6.0 |
| Othera | 999,374 | 5.7 |
| Medicaid eligibility | ||
| Dual-eligible | 1,177,587 | 6.8 |
| Non-dual eligible | 16,266,313 | 93.2 |
| Region | ||
| Northeast | 3,672,246 | 21.1 |
| Southeast | 4,781,586 | 27.4 |
| Midwest | 4,130,490 | 23.7 |
| West | 2,934,175 | 16.8 |
| Southwest | 1,921,509 | 11.0 |
| Body-mass index (kg/m2) | 27.5 (1.0) | |
| Ever smoked (%) | 46.2 (7.4) | |
| Below poverty level (%) | 12.4 (7.3) | |
| Not graduated from high school (%) | 13.8 (8.2) | |
| Median household income (US$1000) | 57.1 (23.7) | |
| Air pollutantsb | ||
| Annual PM2.5 (μg/m3) | 9.4 (3.7) | |
| Annual NO2 (ppb) | 17.5 (12.4) | |
| Warm-season O3 (ppb) | 42.8 (6.5) | |
Note: Data are n (%) or mean (SD), except otherwise specified.
Other included Asian, Hispanic, American Indian or Alaskan Native, and unknown.
Presented as mean concentration (interquartile range) over the study period.
Air pollution levels
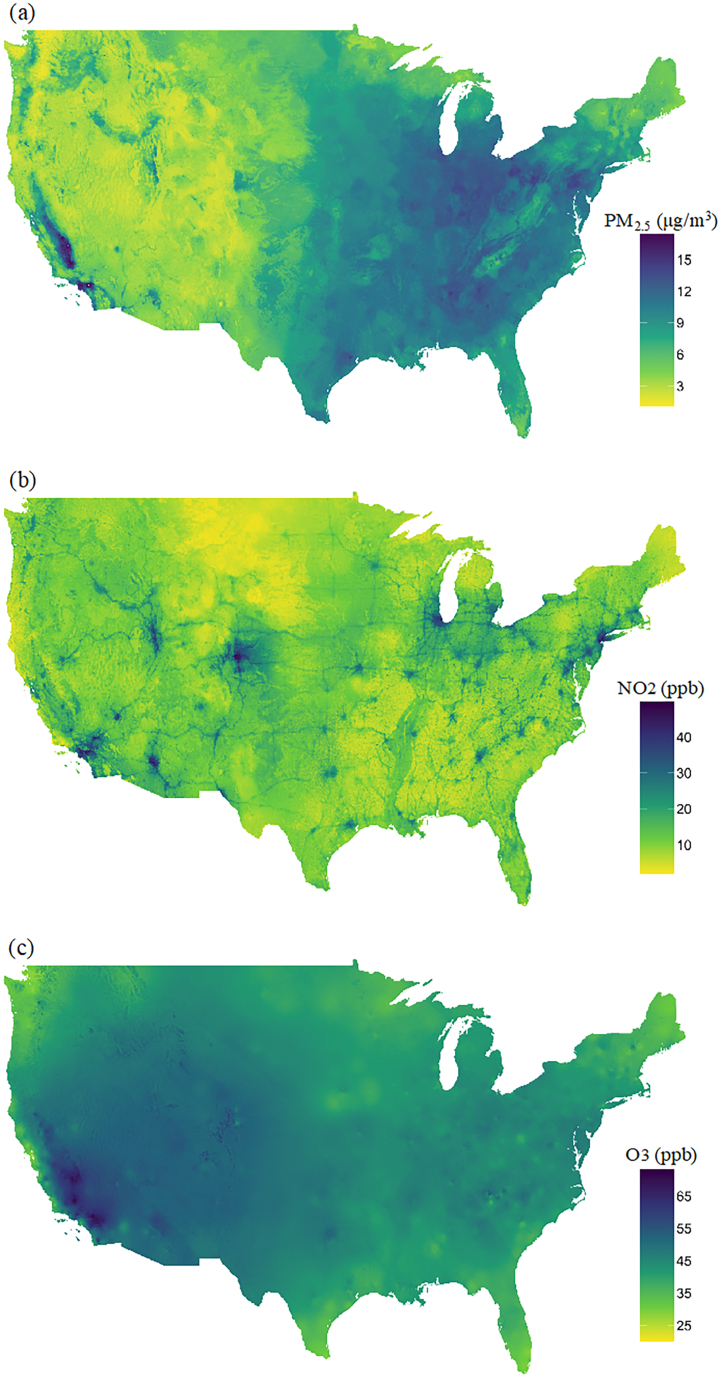
The annual average levels of PM2.5 and NO2 were 9.4 μg/m3 and 17.5 ppb across the contiguous United States from 2000 to 2016, which were both below the U.S. Environmental Protection Agency (EPA) annual standards of 12 μg/m3 and 53 ppb. About 66% and 99% of the participants were always exposed to annual PM2.5 and NO2 below the national standards over the follow-up period. The annual warm-season (May–October) average O3 was 42.8 ppb. We examined warm-season O3 because O3 is a temperature-dependent variable (i.e. the level of O3 rises significantly as the weather warms) and warm-season O3 is often used in epidemiological studies for assessing long-term health effects [38]. While the EPA does not have an annual standard for ozone, the daily 8-h period standard for ozone is 70 ppb [39], indicating the warm-season O3 levels in the cohort were generally below the EPA daily standard. Fig. 1 showed the distribution of the three air pollutants across the contiguous United States over the study period, estimated by the exposure models used in our analysis. PM2.5 was the highest in the eastern region and West Coast of the United States, primarily reflected pollution transported over large distances. Warm-season O3 was highest in the western region. High NO2 was concentrated in urban centers, suggesting it mainly indicated local fossil fuel combustion sources, especially motorized traffic. Supplementary Table S3 shows the detailed distribution of the exposure levels. The three pollutants in this cohort were modestly correlated with the highest correlation observed between PM2.5 and NO2. The Spearman's rank correlations between pollutants (average annual exposure at ZIP code level) were PM2.5 and NO2 0.38, PM2.5 and O3 0.28, NO2 and O3 0.21.
Fig. 1.
Average concentrations of (a) annual PM2.5 (μg/m3), (b) annual NO2 (ppb), and (c) warm-season O3 (ppb) across the contiguous United States over the study period (2000–2016).
Health effect estimates
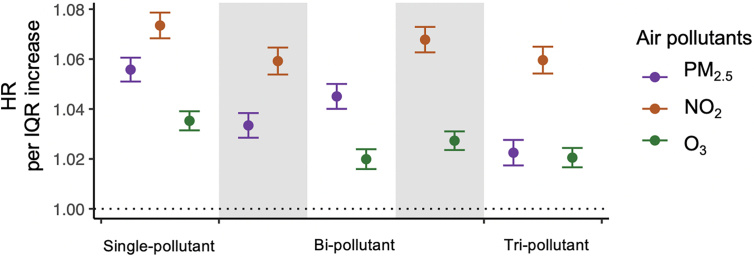
Fig. 2 provides our main analysis results from the Cox proportional hazards models stratified by individual characteristics, adjusting for neighborhood-level socioeconomic status (SES), behavioral risk factors, health care capacity variables, land-use variables, and residual temporal and spatial trends (see Methods). Long-term exposure to PM2.5, NO2, and warm-season O3 was associated with an increased risk of incident stroke in all models with or without adjusting for co-pollutants. An interquartile range (IQR) increase in the annual average NO2 (12.4 ppb) preceding one-year diagnosis was associated with an increased risk of stroke (HR = 1.073, 95% CI: 1.068–1.079) in the single pollutant model, with effect sizes changing little in models adjusting for other pollutants. An IQR increase in annual average PM2.5 (3.7 μg/m3) was associated with an HR of 1.056 (95% CI: 1.051–1.061) in the single pollutant model, dropping to 1.022 (1.017–1.028) in the tri-pollutant model. An IQR increase in annual average warm-season O3 (6.5 ppb) was associated with an HR of 1.035 (95% CI: 1.031–1.039) in the single-pollutant model, dropping to 1.021 (1.017–1.024) in the tri-pollutant model.
Fig. 2.
Hazard ratios of stroke incidence associated with annual PM2.5 (a), or annual NO2 (b), or warm-season O3 (c) concentration expressed per IQR increment. The estimated hazard ratios were obtained using single-pollutant, bi-pollutant, and tri-pollutant models, respectively. IQR, interquartile range. HR, hazard ratio.
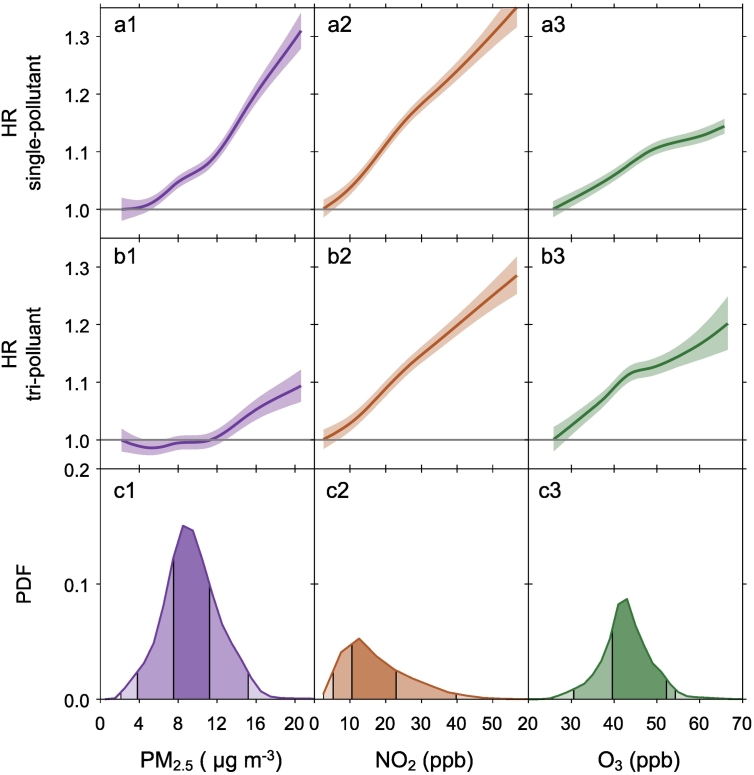
Concentration-response relationships
Fig. 3 showed the concentration-response (C-R) relationships between long-term PM2.5, NO2, and warm-season O3 exposures and stroke incidence derived from the single-pollutant and the tri-pollutant models and the distribution of each pollutant in the cohort. The C-R relationships of NO2 for stroke were approximately linear across the exposure distribution from both the single- and tri-pollutant models (Fig. 3, a2 and b2), although a slightly steeper slope was observed in the single-pollutant model. For PM2.5, the C-R curve from the single-pollutant model showed a linear relationship for increasing annual mean PM2.5 concentrations (Fig. 3, a1). When controlling for multi-pollutants, however, we observed a positive linear relationship for PM2.5 and stroke incidence HR at concentrations above 11 μg/m3 with an attenuated slope (Fig. 3, a2). For concentrations below 11 μg/m3 (representing 75% of the distribution of the PM2.5), the C-R curve from the tri-pollutant model is close to the null (Fig. 3, a2). The C-R relationship of O3 for stroke from the single-pollutant model showed a linear relationship across the exposure distribution and the associations level off at higher concentrations (Fig. 3, a3). This C-R relationship of O3 persisted when controlling for multi-pollutants (Fig. 3, b3). Across the 0.1th to 99.9th percentile of the exposure distribution, NO2 showed the strongest and most robust effect on stroke among all pollutants. The C-R relationship of NO2 for stroke was almost linear, with no signal of threshold down to 4 ppb (1st percentile) in both single- and tri-pollutant models.
Fig. 3.
Concentration–response relationships between long-term PM2.5, NO2, and O3 exposures and stroke incidence from (a) single pollutant models and (b) tri-pollutant models, and (c) probability distribution functions (PDF) of long-term PM2.5, NO2, and O3 exposures. (a, b) The concentration-response curves, derived from the single-pollutant or tri-pollutant models, are shown for the concentration ranges between 0.1th to 99.9th percentiles of the pollutants, i.e. with 0.2% poorly constrained extreme values excluded. (c) The PDF shows the distribution of each exposure in the cohort and the shading areas (from the darkest to the lightest) represent pollutant concentration ranges of the IQR (i.e., 25th to 75th percentiles), 95% (2.5th to 97.5th), and 99.8% (0.1th to 99.9th), respectively. IQR, interquartile range. HR, hazard ratio.
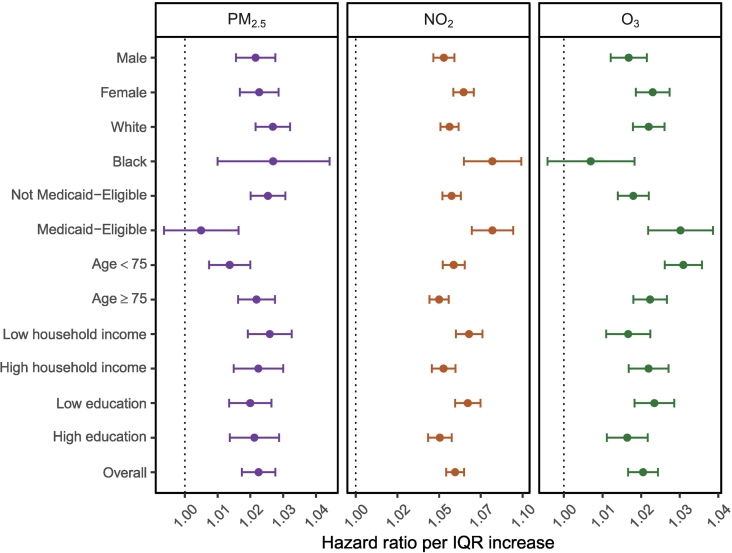
Effect modifications
We examined six potential effect modifiers [gender, race (white, black), Medicaid eligibility, age (<75, ≥75), neighborhood-level household income, and education]. Fig. 4 showed subgroup-specific hazard ratios for each pollutant from the tri-pollutant model, based on stratification of the potential effect modifiers. The same analyses derived from the single-pollutant models are shown in Fig. S1. Overall, the magnitude of HR differences between subgroups was small. We observed an increased hazard of stroke for women vs. men, Blacks vs. Whites, and Medicaid eligible vs. Medicaid ineligible in relation to NO2. At the same time, those living in areas with lower median household incomes and lower education were found to have a higher association between stroke and NO2. All these effect modifiers except race were robust to both the tri-pollutant and single-pollutant modeling (Fig. S1). We did not observe such effect modification patterns in relation to PM2.5 or O3. Regarding differences in HR by gender, although non-significant for PM2.5 and O3 in both the tri-pollutant and single-pollutant models, females had a general stronger association between stroke for all air pollutant exposures than males. Finally, we found little evidence of an interaction between air pollutant exposures and age in relation to stroke.
Fig. 4.
Hazard ratios of stroke incidence associated with an IQR increase in annual PM2.5, annual NO2, and warm-season O3 concentration by study subgroups. All results were derived from the tri-pollutant models. The grey shades indicate the 95% CIs of the overall effect estimates. “Household income” denotes median household income at zip-code level, “Low household income” represents the subgroup with median household income lower than the population median while “High household income” represents the subgroup with median household income higher than the population median. “Education” denotes the proportion of the population > 65 years of age who had not graduated from high school at zip-code level, “Low education” represents the subgroup with the proportion of less educated people higher than the population proportion median while “High education” represents the subgroup with the proportion of less educated people lower than the population proportion median. IQR, interquartile range. HR, hazard ratio. CI, confidence interval.
Attributable fraction
The strongest relationship we found with stroke was for NO2 among the three pollutants. If the U.S. NO2 levels could be lowered by 12.4 ppb, which is the IQR, then the attributable fraction (AF) for stroke due to current exposure levels, based on our main results from tri-pollutant models assuming a linear relationship, would be 6%. Namely, assuming there is a causal relationship, an estimated 6% of stroke cases would be avoided if NO2 levels decreased by 12.4 ppb, which is approximately the difference between our large cities like Los Angeles and New York, and smaller cities like Portland and New Haven [36]. Likewise, if the U.S. PM2.5 and O3 levels could be reduced by 3.7 μg/m3 (IQR) and 6.5 ppb (IQR), an estimated 2% and 2% of stroke cases would be avoided assuming a causal relationship.
Sensitivity analyses
Associations between long-term exposure to PM2.5, NO2, O3, and stroke were robust to a series of sensitivity analyses. First, the use of alternative exposure windows (annual exposure 2, 1, or 0 years prior to stroke diagnosis, i.e., lags 2, 1, or 0) all supported a positive association with PM2.5, NO2, and O3, though HRs varied in magnitude and attenuated with increasing lag periods (Supplementary Table S4). Second, two sub-cohorts with more stringent “clean” periods were constructed by excluding anyone who had a diagnosis for stroke in their first 2 years or 4 years of follow-up. Descriptive information of the sub-cohorts is summarized in Supplementary Table S1 and these new sub-cohorts yielded similar results to the main analyses (Supplementary Table S4). Third, the observed associations with stroke were robust to different levels of confounding adjustment (Supplementary Table S5). Except for O3, the association with stroke became negative when the model did not adjust for meteorological variables, including ZIP code level annual temperature, and relative humidity. Lastly, the additional adjustment for comorbidities such as diabetes and hypertension attenuated the associations between air pollutants and stroke to some extent, while the effects of PM2.5, NO2, O3 remained robust in the single- and multi-pollutant models (Supplementary Table S6). However, these pollutants have been associated with hypertension and diabetes, so this may be controlling for a mediator.
Discussion
In this large, national population-based Medicare cohort, we found long-term exposure to PM2.5, NO2, and warm-season O3 were associated with an increased risk of first incident stroke, in both single-pollutant models and models adjusting for co-pollutants. Among the three air pollutants studied, NO2 had a larger effect on stroke risk than PM2.5 or O3 at the scale of interquartile range increase in concentrations in all models. NO2 also had the most robust association with an increased risk of stroke, while the effect estimates of both PM2.5 and O3 attenuated after adjustment for co-pollutants.
The shapes of the concentration-response relationship between air pollutant exposure and stroke were investigated in both single- and tri-pollutant models. In the single-pollutant models, risks of first diagnosis with stroke linearly increased with increasing PM2.5, NO2, and O3 concentrations, suggesting no safe threshold for harmful pollution. The concentration-response (C-R) shapes remained robust for NO2 and O3 in the tri-pollutant models while the effect of PM2.5 attenuated at the lower end of concentrations. Similar results about the C-R shapes of PM2.5 and NO2 were observed in a pooled analysis of six European cohorts within the ELAPSE (the multicenter study Effects of Low-level Air Pollution: A study in Europe) project [40]. The reduction of the PM2.5 hazard ratio at low concentrations did not imply that particles had no effect, as adjustment for NO2 might also have adjusted for particles co-emitted with NO2, including those from motorized traffic and other fossil fuel combustion sources [40,41]. While for the robust association for NO2, this may reflect the direct effects of NO2 or correlated combustion-related particles such as ultrafine particles [40,41]. The study population had low annual NO2 exposures relative to the current national standard (53 ppb) and low warm-season O3 exposures relative to the daily 8-h period standard (70 ppb). Nevertheless, our data suggest that lowering levels of pollution would have substantial public health benefits. In the subgroup analyses, female, black people, lower-income, and lower educated populations were susceptible subgroups for the effect of NO2, reflecting a general pattern of increased susceptibility among those of lower SES [19,20]. In single-pollutant models, females had a higher risk of stroke to PM2.5 and O3, consistent with previous studies suggesting that the association between air pollution and cardiovascular diseases are stronger in women than in men [42,43]. Since the magnitude of HR differences between subgroups were relatively small, the subgroup health effects should be interpreted cautiously.
Most of the evidence on the effects of air pollution on stroke comes from time-series and case-crossover studies of cerebrovascular mortality [[4], [5], [6],34]. Previous studies on the long-term health effects have reported mixed results. Two prior Medicare population-specific studies of air pollutant exposure and cardiovascular outcomes are generally consistent with our results. Danesh Yazdi et al. (2019) [20] focused on the Medicare beneficiaries in the southeastern region of the United States and found both PM2.5 and O3 to be risk factors for stroke hospitalizations on the multiplicative scale, reporting a HR of 1.031 (95%CI: 1.030–1.032) and 1.012 (1.012–1.013) per unit increase in annual PM2.5 and O3 in the bi-pollutant models. A more recent study that expanded the study population to the entire Medicare cohort across the contiguous United States and studied the simultaneous effects for PM2.5, NO2, and O3 found only PM2.5 and NO2 to be positive for ischemic stroke risk on an additive scale [19]. The discrepancies about the effect of O3 on stroke could be due to the difference in cohort construction and outcome assessments. Our study included hospitalizations, physician's visits, and nursing home data to better ascertain stroke incidence, while the previous study only used hospital admission data [19]. In addition, that study only examined ischemic stroke, whereas we examined all strokes. A national sample cohort study in South Korea also found a significantly increased risk of stroke with long-term exposure to PM2.5 and NO2, in both single and multi-pollutant models [44]. A pooled analysis of six population-based European cohort studies within ELAPSE also found incidence of stroke was associated with PM2.5 and NO2 [40]. Conversely, the South Korea study [44] found a significant negative relationship between O3 and stroke incidence while the ELAPSE study [40] found no association with O3, in contrast with our results that found O3 to be harmful. But we noticed that the study population in both the South Korean cohort [44] and the pooled European cohort [40] are younger, the mean age at baseline (SD) was 42(SD 15) and 54 (SD 9), respectively. Researchers looking at long-term exposure to ambient air pollutants and cerebrovascular disease within the European Study of Cohorts of Air Pollution Effects (ESCAPE) data found marginally significant associations between exposure to PM2.5 and the incidence of stroke, with a significant relationship seen for those 60 or above [13]. Although they found non-significant associations for NO2 in their single-pollutant models, a study in the Danish, Diet, Cancer and Health cohort that was included in the ESCAPE study found a borderline significant association between NO2 and incident stroke [45]. Last, three meta-analyses reported per 10-μg/m3 increase in long-term exposure to PM2.5 levels were associated with 5% (95% CI: 2–8%) [4], 13% (11–15%) [5], and 23% (10–26%) [6] increase in the hazard of stroke incidence, which are comparable to our effect estimates of 6% (5–8%) increased hazard per 10-μg/m3 increase in PM2.5. One meta-analysis evaluated the effect for NO2 and found a significant but much smaller increased hazard of stroke incidence per 10 ppb increases in NO2 compared with our results (0.4% (95% CI: 0.0–0.6%) vs. 4.8% (4.3–5.2%)) [4]. Furthermore, they did not find a significant association between O3 and stroke incidence [4]. These inconsistent results compared to our study might be due to the relatively limited number of studies focusing on the health effects of gaseous air pollutants (NO2 and O3) [7,8,11,13,18,45] compared to particulate matter [9,10,12,[14], [15], [16], [17]].
Air pollution has been shown to harm respiratory and cardiovascular systems through mechanisms including systemic inflammation, oxidative stress, and altered cardiac autonomic and vascular function [[46], [47], [48], [49]]. However, the effects of exposure to multiple air pollutants on cerebral vessels are more uncertain and the mechanism by which any effect on cerebrovascular disease is mediated is yet to be elucidated. Postmortem examination has reported evidence of particle-associated inflammation in the brain [50,51]. Although subclinical outcomes could not be examined in our Medicare cohort, these mechanisms underscore the biological plausibility of our finding that long-term exposure to PM2.5, NO2, and O3 is independently associated with incident stroke. Hypertension and diabetes, established risk factors for stroke and also linked to air pollution, are hypothesized to be on a biological pathway of the air pollution effects leading to stroke, with systemic inflammation providing a common link [30,31]. In our study, diabetes and hypertension attenuated the associations between air pollutants and stroke to some extent while the effects of PM2.5, NO2, O3 remained positive in the single- and multi-pollutant models (Table S5), suggesting hypertension and diabetes could be mediators. However, a formal mediation analysis would be important to confirm these findings.
Our study has several strengths. First, our study accounted for multiple air pollutants (PM2.5, NO2, and O3), which were estimated from prediction models on a final scale [[26], [27], [28]], allowing us to compare the dependent and independent associations between stroke and air pollutant groups. Second, the population-based Medicare cohort has a large sample size that gave us ample statistical power to detect effects even though they are small, which is often the case in environmental studies. The use of all available Medicare claims not restricting to hospital admissions is likely to capture cases better and reduce the chance for outcome misclassification. In addition, we used a conservative method by requiring a one-year “clean” period and restricting the analysis to Medicare beneficiaries with continuous enrollment in Medicare FFS, and Part A (hospital insurance) and Part B (medical insurance) programs throughout the study period. We also excluded those who enrolled in the Medicare Part C plan since claims in Part C were inaccessible for us [52]. These stringent inclusion and exclusion criteria can increase the possibility that the cases were newly diagnosed and thus better approximate disease incidence. Lastly, we were able to control for a comprehensive set of individual- and neighborhood-level covariates. We controlled potential residual temporal trends by including indicator variables for calendar years to better account for the potential nonlinear trends in cerebrovascular disease incidences [[53], [54], [55]].
Our study also had several limitations. The first limitation is the potential for exposure error since we assessed only ambient outdoor air pollution concentrations based on participants' ZIP code of residence, individual-level exposure was not feasible. However, the exposure prediction model we used has excellent predictive accuracy, which may reduce the exposure error [[26], [27], [28]]. Furthermore, a possible exposure misclassification is likely to be non-differential with respect to stroke incidence and have a net bias towards the null [[56], [57], [58]]. Second, our study is subject to unmeasured and residual confounding. We could not control for risk factors for cardiovascular diseases at the individual level such as smoking, physical activity, socioeconomic deprivation, and education, which may confound the association between air pollution and stroke [33]. However, individual smoking and other predictors have been shown in personal exposure studies [59] to be uncorrelated with ambient exposure levels, and are only correlated through neighborhood level covariates, and we believe we have controlled for a rich set of those covariates. Third, outcome misclassification is possible because Medicare is an administrative claims database primarily collected for payment purposes [52]. We expect this misclassification to be non-differential and should bias the results to the null because such errors are unlikely to be related to air pollution exposure. Fourth, we assessed overall stroke only and were not able to study stroke further in its subtypes, such as ischemic stroke and hemorrhagic stroke. In sensitivity analyses, we controlled two common stroke risk factors (hypertension and diabetes) and observed attenuation in effect estimates, but we neglected that the risk factors profiles for stroke subtypes can be different [60,61]. Epidemiological studies that evaluated the heterogeneous effects of air pollution on stroke subtypes are still limited [[62], [63], [64], [65]]. Given the etiological heterogeneity in stroke, future study of subtype-specific effects would be warranted. Furthermore, we did not consider the possible impact of loss to follow-up due to dropout or death on our findings. Air pollution exposure is an established cause of mortality [66,67], so that older participants are likely to represent a population that is “selected” for characteristics that place them at lower risk for stroke, resulting in underestimation of the relation of exposure with stroke risk [13]. Finally, we only studied the Medicare FFS population who continuously enrolled in both Part A and Part B programs, and never enrolled in Part C program. Some population characteristics differences were observed between our cohort and other Medicare cohorts with different inclusion and exclusion criteria (Supplementary Table S7), suggesting our results may not be generalizable to the entire US Medicare population and further work is needed to determine how study population composition can impact the association [68].
Conclusion
In conclusion, our study provides strong epidemiological evidence that long-term exposure to PM2.5, NO2, and O3 are independently associated with a higher risk of incident stroke among the US elderly population. The strongest relationship we found with incident stroke was for NO2 among the three pollutants, suggesting that traffic-related air pollution may play a particularly significant role in increased risks of stroke.
Data availability
The rules governing the Medicare dataset prohibit any sharing of the health datasets being used for our epidemiologic research. Restricted by our Data Use Agreement with the U.S. Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available. Academic and non-profit researchers who are interested in using Medicare data should contact the U.S. Centers for Medicare & Medicaid Services directly to obtain their own datasets upon completion of a Data Use Agreement.
Author contributions
L.S. designed research and directed its implementation; T.M. and L.S. analyzed data, T.M. and P.L. made the figures and tables, T.M., L.S., M.D.·Y, P.L., W.R., Q.D., Y.W., and J.S. prepared datasets. T.M. lead the writing of the manuscript, with input from all authors.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by Emory HERCULES Center (P30 ES019776), the National Institute of Environmental Health Sciences (R21 ES032606) and the Emory Climate and Health Research Incubator. The authors acknowledge Dr. Joel Schwartz's lab for providing us with access to their estimated air pollution data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gloepi.2022.100073.
Contributor Information
Tszshan Ma, Email: tsz.shan.ma@emory.edu.
Liuhua Shi, Email: liuhua.shi@emory.edu.
Appendix A. Supplementary data
Supplementary Data
References
- 1.Mukherjee D., Patil C.G. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6):S85–S90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnamurthi R.V., Ikeda T., Feigin V.L. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54(2):171–179. doi: 10.1159/000506396. [DOI] [PubMed] [Google Scholar]
- 4.Niu Z., Liu F., Yu H., Wu S., Xiang H. Association between exposure to ambient air pollution and hospital admission, incidence, and mortality of stroke: an updated systematic review and meta-analysis of more than 23 million participants. Environ Health Prevent Med. 2021;26(1):1–14. doi: 10.1186/s12199-021-00937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexeeff S.E., Liao N.S., Liu X., Van Den Eeden S.K., Sidney S. Long-term PM2. 5 exposure and risks of ischemic heart disease and stroke events: review and meta-analysis. J Am Heart Assoc. 2021;10(1):e016890. doi: 10.1161/JAHA.120.016890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan S., Wang J., Jiang Q., He Z., Huang Y., Li Z., et al. Long-term exposure to PM2. 5 and stroke: a systematic review and meta-analysis of cohort studies. Environ Res. 2019;177:108587. doi: 10.1016/j.envres.2019.108587. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson R.W., Carey I.M., Kent A.J., van Staa T.P., Anderson H.R., Cook D.G. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology. 2013;44-53 doi: 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- 8.Crichton S., Barratt B., Spiridou A., Hoang U., Liang S.F., Kovalchuk Y., et al. Associations between exhaust and non-exhaust particulate matter and stroke incidence by stroke subtype in South London. Sci Total Environ. 2016;568:278–284. doi: 10.1016/j.scitotenv.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Huang K., Liang F., Yang X., Liu F., Li J., Xiao Q., et al. Long term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China-PAR project. BMJ. 2019;367 doi: 10.1136/bmj.l6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H., Guo Y., Di Q., Zheng Y., Kowal P., Xiao J., et al. Ambient PM2. 5 and stroke: effect modifiers and population attributable risk in six low-and middle-income countries. Stroke. 2017;48(5):1191–1197. doi: 10.1161/STROKEAHA.116.015739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipsett M.J., Ostro B.D., Reynolds P., Goldberg D., Hertz A., Jerrett M., et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184(7):828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu H., Sun S., Tsang H., Wong C.-M., RSY Lee, Schooling C.M., et al. Fine particulate matter exposure and incidence of stroke: a cohort study in Hong Kong. Neurology. 2017;88(18):1709–1717. doi: 10.1212/WNL.0000000000003903. [DOI] [PubMed] [Google Scholar]
- 13.Stafoggia M., Cesaroni G., Peters A., Andersen Z.J., Badaloni C., Beelen R., et al. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect. 2014;122(9):919–925. doi: 10.1289/ehp.1307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockfelt L., Andersson E.M., Molnár P., Gidhagen L., Segersson D., Rosengren A., et al. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg. Sweden Environ Res. 2017;158:61–71. doi: 10.1016/j.envres.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann B., Weinmayr G., Hennig F., Fuks K., Moebus S., Weimar C., et al. Air quality, stroke, and coronary events: results of the Heinz Nixdorf recall study from the Ruhr region. Dtsch Arztebl Int. 2015;112(12):195. doi: 10.3238/arztebl.2015.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korek M.J., Bellander T.D., Lind T., Bottai M., Eneroth K.M., Caracciolo B., et al. Traffic-related air pollution exposure and incidence of stroke in four cohorts from Stockholm. J Expo Sci Environ Epidemiol. 2015;25(5):517–523. doi: 10.1038/jes.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To T., Zhu J., Villeneuve P.J., Simatovic J., Feldman L., Gao C., et al. Chronic disease prevalence in women and air pollution—a 30-year longitudinal cohort study. Environ Int. 2015;80:26–32. doi: 10.1016/j.envint.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Chen G., Wang A., Li S., Zhao X., Wang Y., Li H., et al. Long-term exposure to air pollution and survival after ischemic stroke: the China national stroke registry cohort. Stroke. 2019;50(3):563–570. doi: 10.1161/STROKEAHA.118.023264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danesh Yazdi M., Wang Y., Di Q., Wei Y., Requia W.J., Shi L., et al. Long-term association of air pollution and hospital admissions among medicare participants using a doubly robust additive model. Circulation. 2021;143(16):1584–1596. doi: 10.1161/CIRCULATIONAHA.120.050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazdi M.D., Wang Y., Di Q., Zanobetti A., Schwartz J. Long-term exposure to PM2. 5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int. 2019;130:104879. doi: 10.1016/j.envint.2019.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahmeyer J.T., Stubenrauch S., Geyer S., Weissenborn K., Eberhard S. The frequency and timing of recurrent stroke: an analysis of routine health insurance data. Dtsch Arztebl Int. 2019;116(42):711. doi: 10.3238/arztebl.2019.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CCW 2015. https://www2.ccwdata.org/web/guest/condition-categories Available from.
- 23.Di Q., Wei Y., Shtein A., Hultquist C., Xing X., Amini H., et al. NASA Socioeconomic Data and Applications Center (SEDAC); Palisades, NY: 2021. Daily and annual PM2.5 concentrations for the contiguous United States, 1-km Grids, v1 (2000–2016) [Google Scholar]
- 24.Di Q., Wei Y., Shtein A., Hultquist C., Xing X., Amini H., et al. NASA Socioeconomic Data and Applications Center (SEDAC); Palisades, NY: 2021. Daily and annual NO2 concentrations for the contiguous United States, 1-km Grids, v1 (2000–2016) [Google Scholar]
- 25.Requia W.J., Wei Y., Shtein A., Hultquist C., Xing X., Di Q., et al. NASA Socioeconomic Data and Applications Center (SEDAC); Palisades, NY: 2021. Daily 8-hour maximum and annual O3 concentrations for the contiguous United States, 1-km Grids, v1 (2000–2016) [Google Scholar]
- 26.Di Q., Amini H., Shi L., Kloog I., Silvern R., Kelly J., et al. An ensemble-based model of PM(2.5) concentration across the contiguous United States with high spatiotemporal resolution. Environ Int. 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Q., Amini H., Shi L., Kloog I., Silvern R., Kelly J., et al. Assessing NO(2) concentration and model uncertainty with high spatiotemporal resolution across the contiguous United States using ensemble model averaging. Environ Sci Technol. 2020;54(3):1372–1384. doi: 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Requia W.J., Di Q., Silvern R., Kelly J.T., Koutrakis P., Mickley L.J., et al. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol. 2020;54(18):11037–11047. doi: 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junninen H., Niska H., Tuppurainen K., Ruuskanen J., Kolehmainen M. Methods for imputation of missing values in air quality data sets. Atmos Environ. 2004;38(18):2895–2907. [Google Scholar]
- 30.Henrotin J.-B., Zeller M., Lorgis L., Cottin Y., Giroud M., Béjot Y. Evidence of the role of short-term exposure to ozone on ischaemic cerebral and cardiac events: the Dijon vascular project (DIVA) Heart. 2010;96(24):1990–1996. doi: 10.1136/hrt.2010.200337. [DOI] [PubMed] [Google Scholar]
- 31.O’donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 32.Newell K., Kartsonaki C., Lam K.B.H., Kurmi O. Cardiorespiratory health effects of gaseous ambient air pollution exposure in low and middle income countries: a systematic review and meta-analysis. Environ Health. 2018;17(1):1–14. doi: 10.1186/s12940-018-0380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Requia W.J., Adams M.D., Arain A., Papatheodorou S., Koutrakis P., Mahmoud M. Global association of air pollution and cardiorespiratory diseases: a systematic review, meta-analysis, and investigation of modifier variables. Am J Public Health. 2018;108(S2):S123–S130. doi: 10.2105/AJPH.2017.303839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang W.-S., Wang X., Deng Q., Fan W.-Y., Wang W.-Y. An evidence-based appraisal of global association between air pollution and risk of stroke. Int J Cardiol. 2014;175(2):307–313. doi: 10.1016/j.ijcard.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 35.Meyer M.C. Constrained penalized splines. Canad J Stat. 2012;40(1):190–206. [Google Scholar]
- 36.USEPA Air Quality - Citiees and Counties. 2020. https://www.epa.gov/air-trends/air-quality-cities-and-counties Available from.
- 37.Steenland K., Armstrong B. An overview of methods for calculating the burden of disease due to specific risk factors. Epidemiology. 2006;512-9 doi: 10.1097/01.ede.0000229155.05644.43. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J.J., Wei Y., Fang Z. Ozone pollution: a major health hazard worldwide. Front Immunol. 2019;10:2518. doi: 10.3389/fimmu.2019.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EPA 2015. https://www.epa.gov/sites/default/files/2015-10/documents/overview_of_2015_rule.pdf cited 2021. Available from:
- 40.Wolf K., Hoffmann B., Andersen Z.J., Atkinson R.W., Bauwelinck M., Bellander T., et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet Health. 2021;5(9):e620–e632. doi: 10.1016/S2542-5196(21)00195-9. [DOI] [PubMed] [Google Scholar]
- 41.Strak M., Weinmayr G., Rodopoulou S., Chen J., De Hoogh K., Andersen Z.J., et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ. 2021;374 doi: 10.1136/bmj.n1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L.H., Knutsen S.F., Shavlik D., Beeson W.L., Petersen F., Ghamsary M., et al. The association between fatal coronary heart disease and ambient particulate air pollution: are females at greater risk? Environ Health Perspect. 2005;113(12):1723–1729. doi: 10.1289/ehp.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ljungman P.L., Mittleman M.A. Ambient air pollution and stroke. Stroke. 2014;45(12):3734–3741. doi: 10.1161/STROKEAHA.114.003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H., Kim J., Kim S., Kang S.H., Kim H.J., Kim H., et al. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J Am Heart Assoc. 2017;6(11) doi: 10.1161/JAHA.117.007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen Z.J., Kristiansen L.C., Andersen K.K., Olsen T.S., Hvidberg M., Jensen S.S., et al. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke. 2012;43(2):320–325. doi: 10.1161/STROKEAHA.111.629246. [DOI] [PubMed] [Google Scholar]
- 46.Brook R.D., Rajagopalan S., Pope C.A., III, Brook J.R., Bhatnagar A., Diez-Roux A.V., et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 47.Chuang K.-J., Chan C.-C., Su T.-C., Lee C.-T., Tang C.-S. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176(4):370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 48.Mirowsky J.E., Dailey L.A., Devlin R.B. Differential expression of pro-inflammatory and oxidative stress mediators induced by nitrogen dioxide and ozone in primary human bronchial epithelial cells. Inhal Toxicol. 2016;28(8):374–382. doi: 10.1080/08958378.2016.1185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Zhu T., Kipen H., Wang G., Huang W., Rich D., et al. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst. 2013;174:5. [PMC free article] [PubMed] [Google Scholar]
- 50.Calderon-Garciduenas L., Solt A.C., Henriquez-Roldan C., Torres-Jardon R., Nuse B., Herritt L., et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36(2):289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 51.Calderon-Garciduenas L., Azzarelli B., Acuna H., Garcia R., Gambling T.M., Osnaya N., et al. Air pollution and brain damage. Toxicol Pathol. 2002;30(3):373–389. doi: 10.1080/01926230252929954. [DOI] [PubMed] [Google Scholar]
- 52.Mues K.E., Liede A., Liu J., Wetmore J.B., Zaha R., Bradbury B.D., et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol. 2017;9:267. doi: 10.2147/CLEP.S105613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane D.A., Skjøth F., Lip G.Y., Larsen T.B., Kotecha D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modig K., Talbäck M., Ziegler L., Ahlbom A. Temporal trends in incidence, recurrence and prevalence of stroke in an era of ageing populations, a longitudinal study of the total Swedish population. BMC Geriatr. 2019;19(1):1–9. doi: 10.1186/s12877-019-1050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vangen-Lønne A.M., Wilsgaard T., Johnsen S.H., Carlsson M., Mathiesen E.B. Time trends in incidence and case fatality of ischemic stroke: the Tromsø study 1977–2010. Stroke. 2015;46(5):1173–1179. doi: 10.1161/STROKEAHA.114.008387. [DOI] [PubMed] [Google Scholar]
- 56.Kioumourtzoglou M.-A., Spiegelman D., Szpiro A.A., Sheppard L., Kaufman J.D., Yanosky J.D., et al. Exposure measurement error in PM 2.5 health effects studies: a pooled analysis of eight personal exposure validation studies. Environ Health. 2014;13(1):1–11. doi: 10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X., Braun D., Kioumourtzoglou M.-A., Choirat C., Di Q., Dominici F. Causal inference in the context of an error prone exposure: air pollution and mortality. Ann Appl Stat. 2019;13(1):520. doi: 10.1214/18-AOAS1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeger S.L., Thomas D., Dominici F., Samet J.M., Schwartz J., Dockery D., et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisskopf M.G., Webster T.F. Trade-offs of personal vs. more proxy exposure measures in environmental epidemiology. Epidemiology. 2017;28(5):635. doi: 10.1097/EDE.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell B.C., De Silva D.A., Macleod M.R., Coutts S.B., Schwamm L.H., Davis S.M., et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):1–22. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 61.Chen R., Ovbiagele B., Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci. 2016;351(4):380–386. doi: 10.1016/j.amjms.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maheswaran R., Pearson T., Beevers S.D., Campbell M.J., Wolfe C.D. Outdoor air pollution, subtypes and severity of ischemic stroke–a small-area level ecological study. Int J Health Geogr. 2014;13(1):1–9. doi: 10.1186/1476-072X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maheswaran R., Pearson T., Beevers S.D., Campbell M.J., Wolfe C.D. Air pollution and subtypes, severity and vulnerability to ischemic stroke—a population based case-crossover study. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0158556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verhoeven J.I., Allach Y., Vaartjes I.C., Klijn C.J., de Leeuw F.-E. Ambient air pollution and the risk of ischaemic and haemorrhagic stroke. Lancet Planet Health. 2021;5(8):e542–e552. doi: 10.1016/S2542-5196(21)00145-5. [DOI] [PubMed] [Google Scholar]
- 65.Yorifuji T., Kashima S., Tsuda T., Ishikawa-Takata K., Ohta T., Tsuruta K.-i., et al. Long-term exposure to traffic-related air pollution and the risk of death from hemorrhagic stroke and lung cancer in Shizuoka. Jpn Sci Total Environ. 2013;443:397–402. doi: 10.1016/j.scitotenv.2012.10.088. [DOI] [PubMed] [Google Scholar]
- 66.Beelen R., Raaschou-Nielsen O., Stafoggia M., Andersen Z.J., Weinmayr G., Hoffmann B., et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383(9919):785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 67.Scheers H., Jacobs L., Casas L., Nemery B., Nawrot T.S. Long-term exposure to particulate matter air pollution is a risk factor for stroke: meta-analytical evidence. Stroke. 2015;46(11):3058–3066. doi: 10.1161/STROKEAHA.115.009913. [DOI] [PubMed] [Google Scholar]
- 68.Neuman P., Jacobson G.A. Medicare advantage checkup. N Engl J Med. 2018;379(22):2163–2172. doi: 10.1056/NEJMhpr1804089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Data Availability Statement
The rules governing the Medicare dataset prohibit any sharing of the health datasets being used for our epidemiologic research. Restricted by our Data Use Agreement with the U.S. Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available. Academic and non-profit researchers who are interested in using Medicare data should contact the U.S. Centers for Medicare & Medicaid Services directly to obtain their own datasets upon completion of a Data Use Agreement.