Abstract
Introduction
TGF-β/Smad3 may be involved in the pathogenesis of acute kidney injury (AKI), but its functional role and mechanism of action in cisplatin-induced AKI are unclear. Here, we established a cisplatin-induced AKI mouse model to demonstrate that Smad3 may have roles in cisplatin nephropathy because of its potential effects on tubular epithelial cell (TEC) death and regeneration.
Methods
Using a cisplatin-induced AKI model, the expression levels of lncRNA Arid2-IR were measured by qRT-PCR and the location detected by FISH. Transfected with overexpression of lncRNA Arid2-IR by lentiviral vector in TECs, and the expression of cleaved caspase 3, Bax, Bcl-2, PCNA, p21, p27, transferrin receptor (TFRC), FTH, and FTL were measured by Western blot. Protein molecules bound to lncRNA Arid2-IR were identified by RIP, RNA pull-down assay, mass spectrometry.
Results
LncRNA Arid2-IR was significantly downregulated in vivo and in vitro. SIS3 decreased cell apoptosis and promoted cell regeneration by upregulating lncRNA Arid2-IR expression. LncRNA Arid2-IR regulated the cell cycle by decreasing expression of the cyclin-dependent kinase inhibitors p21 and p27. Finally, lncRNA Arid2-IR interacted with the TFRC, and overexpression of lncRNA Arid2-IR increased TFRC expression and decreased FTH and FTL.
Conclusion
Smad3 regulated lncRNA Arid2-IR via TFRC, thereby regulating the cell cycle, protecting against cell apoptosis, and promoting cell regeneration.
Keywords: Cisplatin, Acute kidney injury, Smad3, SIS3, LncRNA Arid2-IR
Introduction
Acute kidney injury (AKI) is a common clinical syndrome of renal dysfunction [1] that accounts for 10%–15% of inpatients [2] and more than 50% in intensive care units [3]. Approximately 20% of AKI is caused by acute tubular necrosis, due to nephrotoxic drugs [4]. Cisplatin is a common nephrotoxic drug used to treat solid tumors. It not only kills neoplastic cells, but also destroys other rapidly dividing cells, thereby affecting multiple organ systems [5]. The restricted prescription of cisplatin in clinical cases is due to its adverse effects such as nephrotoxicity. Therefore, it is necessary to explore the pathogenesis of cisplatin-induced AKI in the aim of discovering new therapeutic targets.
Cisplatin is a low molecular weight compound which freely filters through the glomeruli and enters the renal tubular epithelial cells (TECs). It is predominantly metabolized in the kidney. Cisplatin mainly accumulates in the renal cortex and the corticomedullary border, selectively within the location of the proximal and distal tubules; however, it is less distributed in the renal medulla [6]. After entering the cell, cisplatin is hydrolyzed into dichlorodiamine platinum, which forms a cisplatin-DNA complex. This complex then destructs the DNA double helix structure and further blocks the replication and transcription of DNA, leading to DNA damage. This consequently promotes cell cycle arrest and cell apoptosis, and affects cellular repair [7, 8].
The cell cycle is a necessary process for cell growth and division. It consists of four stages: G1, S, G2, and M phases. Under normal conditions, most of the renal TECs are in the G0 (resting phase) and do not proliferate. Under pathological conditions, for instance, when stimulated by injury, cells will enter the cell cycle. Abnormal repair of renal TECs is pivotal to the progression of AKI. Through therapeutic interventions in the cell cycle, renal injury can be alleviated, and patients' quality of life will be improved [9, 10, 11].
Smad3 plays an important role in various biological processes. Smad3 knockout not only reduces inflammation, fibrosis, and cell apoptosis, but also promotes the proliferation and repair of damaged renal TECs [12, 13]. Inhibition of Smad3 phosphorylation leads to a decrease in cell cycle arrest in the G1 phase [14]. Although Smad3 knockout has a certain protective effect on the kidney, Smad3 gene deletion can cause immune disorders, serious infections, and other adverse consequences. Hence, specific Smad3 therapeutic targets should be employed to provide better protection against diseases.
LncRNAs are a class of RNA molecules with more than 200 nucleotide (nt) length [15], which can regulate gene expression through epigenetics, or via transcription and post-transcription levels [16, 17, 18]. The lncRNA Arid2-IR is associated with kidney injury. Smad3 binds to the promoter region of lncRNA Arid2-IR and inhibits the interleukin-1-stimulated NF-γB-dependent inflammatory response [19]. A study has demonstrated that Arid2-IR affects AGE-induced human retinal endothelial cell injury by binding to Smad3 [20]. In a diabetic model, knockdown of lncRNA Arid2-IR reduced inflammatory factors, oxidative stress, and extracellular matrix [20, 21]. Hence, lncRNA Arid2-IR may serve as a specific Smad3 therapeutic target to provide better protection against AKI. However, the role of lncRNA Arid2-IR in cisplatin-induced AKI is still unclear, especially the regulation of Smad3 and the cell cycle. Therefore, this study intends to investigate the mechanism of Smad3 and lncRNA Arid2-IR in cisplatin-induced AKI to bring about innovative ideas for the treatment of AKI.
Materials and Methods
Mice
Experiments used sexually mature (approx. 10–12 weeks of age) male SV129 background mice (Vital River Laboratory Animal Technology Co., Beijing, China), weighing 20 to 25 gm. Mice were housed in the animal facility in a 12-h light/dark cycle at 22 ± 1°C ambient temperature. All animals were allowed 1 week to recover after arrival and had free access to rodent chow and tap water until the initiation of the experiment. Animal maintenance and experimental procedures were conducted in accordance with protocols approved by the Animal Care Committee of the Sun Yat-sen University (Guangzhou, China).
Mice were administered cisplatin (20 mg/kg; Selleck, Lanmu Chemical Co., Shanghai, China) or vehicle (saline) by a single intraperitoneal injection as previously reported [22, 23]. To investigate the effects of SIS3 on mice with cisplatin-induced AKI, the mice were pretreated with 5 mg/kg SIS3 dissolved in dimethyl sulfoxide per day by intraperitoneal injection 4 days before the cisplatin injection. At 72 h after cisplatin injection, the mice were anesthetized and executed. To identify cells entering S phase, we injected mice i.p. with bromodeoxyuridine (BrdU) (Sigma-Aldrich, Germany) at a dose of 50 mg/kg body weight 2 h before euthanasia. Cells entering into S phase were identified according to BrdU positivity through immunohistochemistry (IHC). Blood was taken from the eyeball for biochemical examination, and kidney tissue was taken for pathological examination.
Cell Culture
Mice TECs (MTECs) were donated by Prof. Huiyao Lan (Li Ka Shing Institute of Health, Hong Kong, China) and cultured in DMEM/F-12 medium (Gibco, USA) containing 10% fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin in a 5% CO2/95% air atmosphere at 37°C. When the cells reached 80–90% confluence, they were stimulated with cisplatin (5 μmol/L) in the presence or absence of SIS3 (5 μmol/L).
LncRNA Arid2-IR Overexpression and Cell Transfection
For lncRNA Arid2-IR analysis, overexpression and negative control lentiviruses were synthesized and constructed in the pHBLV-CMV-MCS-3FLAG-EF1-ZsGreen-T2A-PURO plasmid by Hanbio Biotechnology (Shanghai, China). The mTECs were seeded at a density of 1 × 104 per well in 24-well plates. After 24-h incubation, the cells were infected with lentivirus (50 μL recombinant lncRNA Arid2-IR lentiviral stocks or control lentiviral stocks plus 350 μL complete medium). Twelve hours post-infection, the medium was replaced with fresh complete medium. Three to 4 days later, the infection rate of the cells could be directly observed with green fluorescent protein under fluorescent microscopy. Then, 2 μg/mL puromycin was added into the medium with continuous subculture three times until stable clones became obvious. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis was employed to show the lncRNA Arid2-IR overexpression of cell lines (designated as Lnc). Control cell lines (designated as Vec) were generated in the same way described above with a control lentivirus.
Cell Counting Kit-8 Assay
To evaluate mTEC proliferation, the cell counting kit-8 (Dojindo, Kumamoto, Japan) was used according to the manufacturers' instructions. The mTECs were seeded at a density of 1,000 per well in 96-well plates and incubated overnight. Then, the cells were stimulated with cisplatin for 24 h. Next, cells were incubated with 10-μL cell counting kit-8 reagent for 3 h. Finally, the cells were detected with the multi-detection microplate reader (Molecular Devices, USA) at a wavelength of 450 nm.
RNA Extraction and qRT-PCR
Total RNA was extracted from mouse kidney or mTECs with a TRIzol reagent (Invitrogen, USA) and precipitated in isopropanol. Then, a PrimeScriptTM RT Reagent Kit with gDNA Eraser (Takara) was used for the total RNA reverse transcription. SYBRR Premix Ex TaqTM II (Takara) was used to amplify cDNA. The expression levels of the target genes were normalized by β-actin expression. The sequences of the primers are listed as follows: lncRNA Arid2-IR (mouse): forward, AGCAGGCTAAGTCAGGGTGA, reverse, GGGTTGAGCTGCCATGTATT; β-actin (mouse): forward, AGACCTTCAACACCCCAG, reverse, CACGATTTCCCTCTCAGC.
Western Blotting
Proteins from the kidney cortex and kidney cells were extracted in RNA immunoprecipitation (RIP) assay lysis buffer (Thermo Fisher) and subjected to Western blot analysis with a rabbit polyclonal antibody to phospho-Smad3, Smad3, p21, p27, Bax, Bcl2, ferritin heavy chain (FTH), and ferritin light chain (FTL) (Abcam, Cambridge, MA, USA); a rabbit polyclonal antibody to cleaved caspase 3 (CST, USA); a mouse monoclonal antibody to transferrin receptor (TFRC) (Invitrogen, USA), as described previously [23]. After being washed extensively, the membranes were incubated with a horseradish peroxidase-labeled goat anti-rabbit antibody or goat anti-mouse antibody (Bioworld, USA) for 1 h at room temperature in 5% bull serum albumin or Tris-buffered saline Tween. The signals were detected by enhanced chemiluminescence (Millipore) and quantitatively analyzed by normalizing to the level of β-actin using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Apoptosis Analysis
To evaluate mTEC apoptosis, the apoptosis kit (4A Biotech Co., Beijing, China) was used according to the manufacturers' instructions. The mTECs were gathered by trypsinization, washed in cold phosphate-buffered serum (PBS), and then incubated with Annexin V/Alexa Fluor 647. Next, the cells were analyzed by flow cytometry.
Cell Cycle Analysis
To evaluate mTEC cycle, the cell cycle kit (KeyGEN BioTECH, Nanjing, China) was used according to the manufacturers' instructions. The mTECs were gathered by trypsinization, washed in cold PBS, fixed in 70% cold ethanol for 4 h, and then incubated with PI/RNase A. Next, the cells were analyzed by flow cytometry.
Renal Function
Renal function was evaluated by measuring serum creatinine (Scr) and blood urea nitrogen (BUN). A Serum Creatinine Colorimetric Assay Kit (Cat #700460, Cayman Biochemical) was used to detect Scr levels. BUN was measured using a BUN assay kit (Cat# C013-2-1, Jiancheng Biotech). All procedures were performed according to the manufacturer's instructions.
Histology and IHC
The kidney samples were fixed in 4% paraformaldehyde, stained with periodic acid-Schiff staining, and observed by light microscopy. The percentage of tubular necrosis was scored from at least 10 randomly chosen fields by microscopy (×200) as previously reported [23].
IHC was performed on 5-mm kidney sections by incubating with sheep polyclonal antibody to BrdU (Abcam, Cambridge, UK), rabbit polyclonal antibody to proliferating cell nuclear antigen (PCNA), Ki67 or p-Smad2/3 (Abcam), followed by incubation with the relevant peroxidase-conjugated secondary antibodies. The quantification of positive tubular regions was performed by examining at least 10 fields (×200) for each mouse.
Fluorescence in situ Hybridization
LncRNA Arid2-IR probes (the sequences: 5′-CY3-CACAC CTAGA GAGCC CATTG GTCTC CTG-CY3-3′) were synthesized by Servicebio Company (Wuhan, China). Kidney tissues were fixed in 4% paraformaldehyde immediately after removal. After 72 h, the tissues were dehydrated in graded ethanol, cleared in dimethylbenzene, and embedded in paraffin. After dewaxing and hydration, the paraffin sections were boiled and denatured. After digestion with protein K for 30 min at 37°C, sections were washed with PBS thrice, and hybridization reaction solution (including probes) was added to the sections. The sections were hybridized overnight at 37°C. After that, the sections were washed with 2× saline sodium citrate twice and descending series of saline sodium citrate. Finally, the sections were stained with 4′,6-diamidino-2-phenylindole and subjected to fluorescent signal detection using an Olympus normal fluorescence microscope (Olympus, Japan).
RNA Pull-Down
RNAs were in vitro transcribed using T7 RNA polymerase (Invitrogen, Waltham, MA, USA), purified using a RNeasy Plus Mini Kit (Qiagen), and treated with RNase free DNase I (Qiagen). Transcribed RNAs were biotin labeled with Biotin RNA Labeling Mix (Roche, Switzerland). RNAs were mixed with magnetic beads and then incubated with protein lysate from mTECs 1 h at room temperature. Finally, beads were washed with washing buffer, and eluted proteins were examined by Coomassie brilliant blue staining and Western blot analysis.
RNA Immunoprecipitation
The mTECs were lysed in complete RIP lysis buffer (containing proteinase inhibitor and phosphatase inhibitor), and the cell extract was incubated with specific antibodies or control IgG overnight at 4°C. Then, protein A/G beads (Thermo, USA) were added and incubated for 2 h at 4°C. After washing, purified RNA was subjected to qRT-PCR analysis.
KEGG and Reactome Pathway Analysis
KEGG Orthology-Based Annotation System (KOBAS) was used to analyze the signaling pathway enrichment of proteins according to Kyoto encyclopedia of genes and genomes (KEGG) and Reactome, two popular and classic databases. The degree of enrichment was measured with the enrichment factor, p value, and the number of proteins enriched in the pathway. The enrichment factor was the ratio of the number of proteins enriched in the pathway to the number of annotation proteins. The greater the enrichment factor, the greater the degree of enrichment. The closer the p value to zero, the more reliable the enrichment significance of differentially expressed proteins in the pathway.
Statistical Analysis
Each experiment was repeated at least three times. All data were expressed as mean ± SEM. Statistical differences were carried out by using the unpaired t test between two groups and one-way analysis of variance between multiple groups. p < 0.05 indicated statistical significance in all tests. Statistical analysis was performed using the GraphPad Prism 6.0 (GraphPad Software).
Results
SIS3, a Specific Inhibitor of Smad3, Protects against Cisplatin-Induced AKI
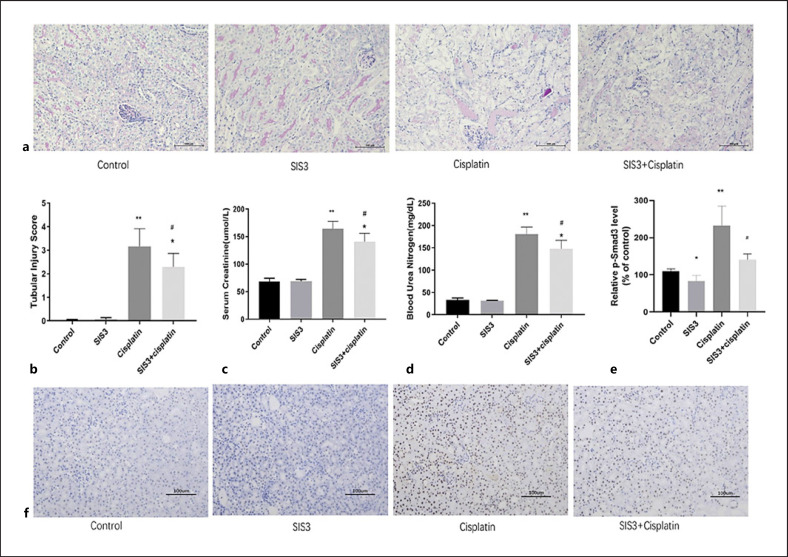
To assess the effects of SIS3 on cisplatin-induced AKI, mice were administered with a single intraperitoneal injection of 20 mg/kg cisplatin to induce AKI. The mice developed AKI in 3 days. To determine the role of SIS3 in cisplatin-induced AKI, the mice were pretreated with 5 mg/kg SIS3 dissolved in dimethyl sulfoxide per day by intraperitoneal injection 4 days before cisplatin injection. Periodic acid-Schiff staining revealed apparently normal kidney tubules in the control group. The cisplatin group exhibited severe kidney histological abnormalities, including swollen renal TECs and presence of vacuoles. However, the injury-promoting processes, including epithelial cell atrophy and necrosis, were significantly attenuated by pretreatment with SIS3 in the cisplatin-induced AKI model (Fig. 1a, b). BUN and Scr levels increased in the mice subjected to cisplatin-induced AKI. Treatment with SIS3 can significantly attenuate progressive renal functional injury, as compared with that in the cisplatin group (Fig. 1c, d). This protective effect was largely associated with inhibition of Smad3. IHC revealed that activated (phosphorylated) Smad3 increased in the kidneys of mice subjected to AKI and decreased in the SIS3 treatment group (Fig. 1e, f).
Fig. 1.
SIS3 protects against renal dysfunction and tubular necrosis and eliminates the exacerbation of cisplatin-induced acute kidney injury. a Periodic acid-Schiff (PAS) staining shows SIS3 decreases necrotic tubules in cisplatin-induced AKI. b Quantitative analysis of necrotic tubules. c SIS3 decreases serum creatinine in cisplatin-induced AKI. d SIS3 decreases blood urea nitrogen in cisplatin-induced AKI. e Quantitative analysis of p-Smad3-positivity. f Immunohistochemistry (IHC) showing that SIS3 decreases the level of p-Smad3 protein in cisplatin-induced AKI. Each bar represents the mean ± SEM. *p < 0.05, **p < 0.01, versus the control group. #p < 0.05, versus the cisplatin group.
SIS3 Promotes TEC Regeneration in Cisplatin-Induced AKI
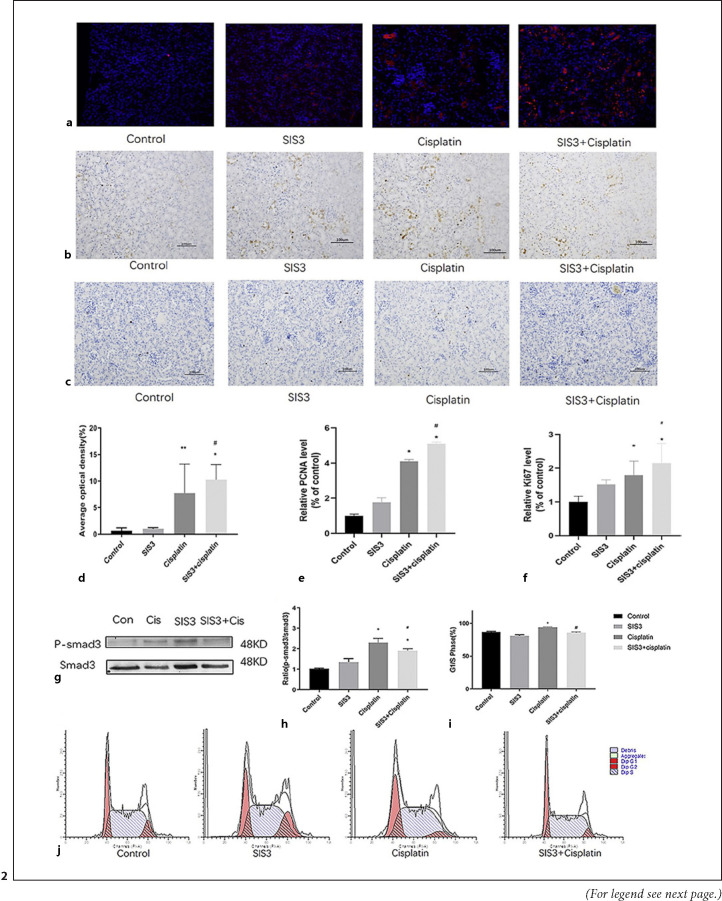
Because mTEC regeneration is crucial to the AKI repair process, we used BrdU uptake, PCNA, and the marker of proliferation Ki-67 staining to assess and compare proliferation of mTECs after AKI. The results showed that the cisplatin group was accompanied by an increase in the number of BrdU-positive (BrdU+), PCNA-positive (PCNA+), and Ki67-positive (Ki-67+) tubular cells, compared with the control group. However, the kidneys were largely protected from AKI-induced TEC necrosis, as indicated by the many BrdU+ (Fig. 2a, d), PCNA+ (Fig. 2b, e), and Ki67+ (Fig. 2c, f) positive mTECs in the SIS3 treatment group, compared with the cisplatin group. In vitro, cell cycle by flow cytometry revealed an increase in the proportion of cells in the G1/S phase in the cisplatin group, compared with the control group. And SIS3 decreased the proportion of cells in the G1/S phase in cisplatin-induced renal TECs (Fig. 2i, j). Simultaneously, SIS3 decreased the level of p-Smad3 protein in cisplatin-induced renal TECs (Fig. 2g, h). These observations suggest that cisplatin might foster an abnormal repair process in AKI by causing G1/S cell cycle arrest via a Smad3 pathway. Specific inhibition of Smad3 is expected to prevent this inhibitory effect.
Fig. 2.
SIS3 promotes tubular epithelial cell regeneration in cisplatin-induced acute kidney injury. a BrdU staining shows SIS3 promoted the proliferation of tubules in cisplatin-induced AKI. b IHC shows SIS3 increased the expression of PCNA protein in cisplatin-induced AKI. c IHC shows SIS3 increased the expression of Ki67 protein in cisplatin-induced AKI. d–f Quantitative analysis of BrdU+, PCNA+, and Ki67 + mTECs. g Western blot shows SIS3 decreases the level of p-Smad3 protein in cisplatin-induced renal tubular epithelial cell. h Quantitative analysis of p-Smad3 by Western blot. i Quantitative analysis of the proportion of cells in G1/S phase by flow cytometry. j Flow cytometry analysis detects that SIS3 decreased the proportion of cisplatin-induced renal tubular epithelial cell in G1/S phase. Each bar represents the mean ± SEM. *p < 0.05, **p < 0.01, versus the control group. #p < 0.05, ##p < 0.01, versus the cisplatin group.
SIS3 Upregulates LncRNA Arid2-IR Expression
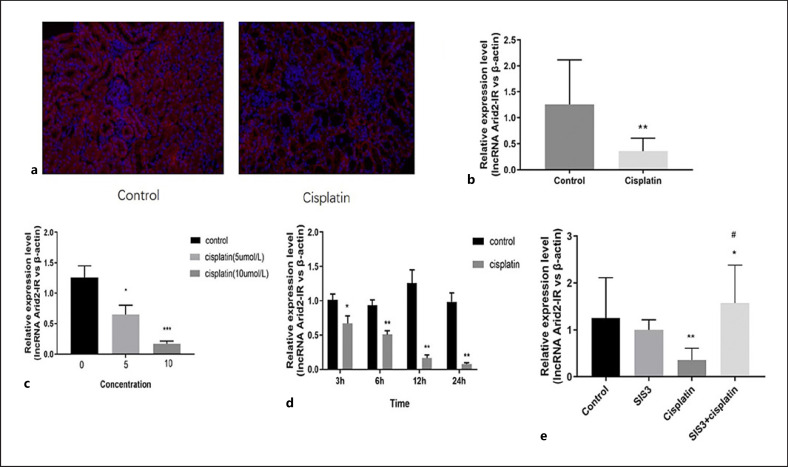
A recent study indicated that lncRNA Arid2-IR was a Smad3-associated lncRNA because a Smad3 binding site was found in the promoter region of lncRNA Arid2-IR in the diseased kidney [19]. In our study, fluorescence in situ hybridization showed that lncRNA Arid2-IR is mainly located in the cytoplasm of renal tubules (Fig. 3a). In vitro, PCR revealed that lncRNA Arid2-IR expression was clearly decreased in the cisplatin group (Fig. 3b–d), while pretreatment with SIS3 markedly increased lncRNA Arid2-IR expression (Fig. 3e).
Fig. 3.
SIS3 regulates the expression of lncRNA Arid2-IR in vitro. a FISH shows that lncRNA Arid2-IR is mainly located in the cytoplasm of renal tubules in cisplatin-induced AKI. b qRT-PCR shows cisplatin decreased the expression of lncRNA Arid2-IR in cisplatin-induced AKI. c, d RT-PCR shows cisplatin decreased the expression of lncRNA Arid2-IR in cisplatin-induced renal tubular epithelial cells. e RT-PCR shows SIS3 increased the expression of lncRNA Arid2-IR in cisplatin-induced AKI. Each bar represents the mean ± SEM. *p < 0.05, **p < 0.01, versus the control group. #p < 0.05, versus the cisplatin group.
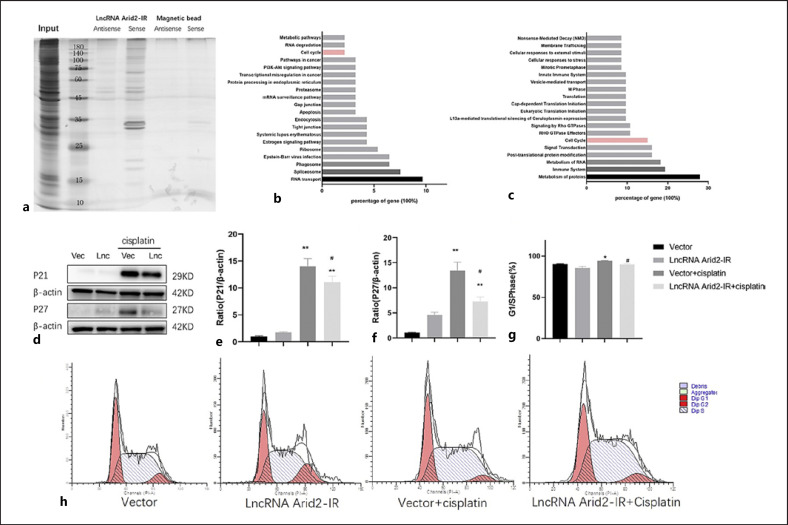
LncRNA Arid2-IR Reduces Tubular Cell Apoptosis and Promotes TEC Regeneration
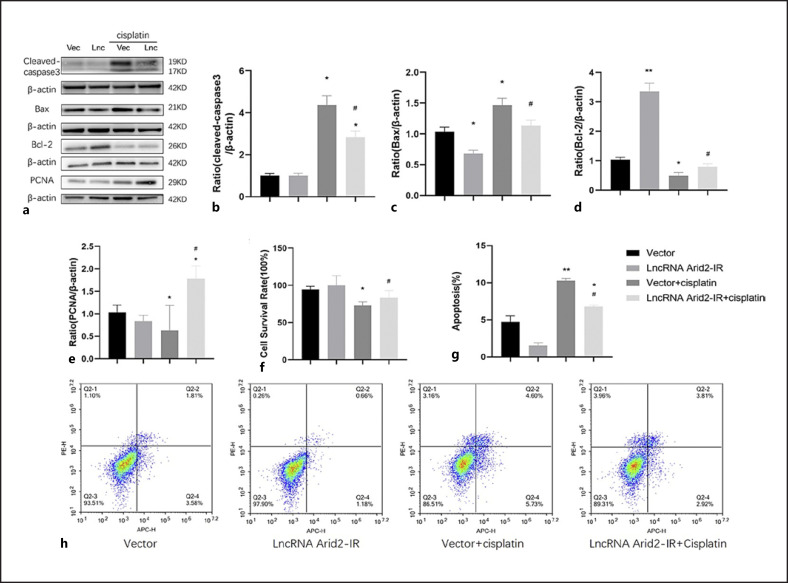
We next dissected the specific role of lncRNA-Arid2-IR by overexpression of lncRNA-Arid2-IR in mTECs. The Bcl-2/Bax/cleaved caspase 3 apoptotic signaling pathways regulate cell apoptosis and survival, and have been implicated in many diseases including kidney diseases [24, 25]. Western blotting indicated that cleaved caspase 3 and Bax expression was higher in cisplatin-treated cells than in the control group, but significantly lower in mTECs overexpressing lncRNA-Arid2-IR treated with cisplatin than in the empty vector control cells treated with cisplatin (Fig. 4a–c). In addition, overexpression of lncRNA Arid2-IR increased Bcl-2, PCNA expression, and proliferation activity of renal TECs beyond that in the vector group treated with cisplatin (Fig. 4a, d–f). Then apoptosis was examined by flow cytometry with Annexin V/Alexa Fluor 647. The staining revealed that cisplatin increased cell apoptosis compared vector group, but significantly lncRNA-Arid2-IR treated with cisplatin decreased cell apoptosis than in the empty vector control cells treated with cisplatin (Fig. 4g, h). These results indicated that overexpression of lncRNA Arid2-IR reduces tubular cell apoptosis and promotes TEC regeneration.
Fig. 4.
Overexpression of lncRNA Arid2-IR promotes tubular epithelial cell regeneration and reduces apoptosis. a Western blot shows overexpression of lncRNA Arid2-IR increases the expression of Bcl-2 and PCNA protein and decreases cleaved caspase 3 and Bax protein in cisplatin-induced renal tubular epithelial cell. b–e Quantitative analysis of cleaved caspase 3, Bax, Bcl-2, and PCNA by Western blot. f CCK8 shows overexpression of lncRNA Arid2-IR increases the proliferation activity of cisplatin-induced renal tubular epithelial cell. g Quantitative analysis of the proportion of cells in apoptosis by flow cytometry. h Flow cytometry shows overexpression of lncRNA Arid2-IR decreased the proportion of cisplatin-induced renal tubular epithelial cell apoptosis. Each bar represents the mean ± SEM. *p < 0.05, **p < 0.01, versus the control group. #p < 0.05, ##p < 0.01, versus the vector + cisplatin group.
LncRNA Arid2-IR Regulates the Cell Cycle in Renal TECs
Because lncRNAs often exert their functions through interaction with one or more RNA-binding proteins [26], we performed RNA pull-down assays to identify the proteins binding to lncRNA Arid2-IR in mTECs (Fig. 5a). We then performed mass spectrometry to further identify that 93 protein molecules bind to lncRNA Arid2-IR. After KEGG and Reactome pathway enrichment analyses of these binding proteins, it was concluded that lncRNA Arid2-IR regulated multiple signaling pathways involved in the cell cycle (Fig. 5b, c). In many cell types, including renal cells, the cell cycle is arrested in the middle to late G1 phase via induction of p21 and p27 [27, 28]. Therefore, we next examined whether lncRNA Arid2-IR regulation of the cell cycle after cisplatin treatment might involve p21/p27-dependent inhibition of cell regeneration. Our data from Western blot analysis showed that overexpression of lncRNA Arid2-IR reduced cyclin-dependent kinase inhibitor, p21 and p27 expression in cisplatin-treated cells (Fig. 5d–f). We also found that lncRNA Arid2-IR was involved in cell cycle through flow cytometry. The proportion of cells in the G1/S phase in the group of mTECs overexpressing lncRNA-Arid2-IR treated with cisplatin was decreased, compared with the cisplatin group (Fig. 5g, h). These results are consistent with cisplatin's inhibition of TEC regeneration by arresting cells in the G1/S phase.
Fig. 5.
LncRNA Arid2-IR regulates the cell cycle in cisplatin-induced renal tubular epithelial cells. a RNA pull-down assay detected the proteins specifically bound to lncRNA Arid2-IR. b KEGG pathway analyzed the binding proteins. c Reactome pathway analyzed the binding proteins. d Western blot shows overexpression of lncRNA Arid2-IR decreases p21 and p27 protein in cisplatin-induced renal tubular epithelial cell. e, f Quantitative analysis of p21 and p27 by Western blot. g Quantitative analysis of the proportion of cells in G1/S phase by flow cytometry. h Flow cytometry analysis detects that overexpression of lncRNA Arid2-IR decreased the proportion of cisplatin-induced renal tubular epithelial cell in G1/S phase. Each bar represents the mean ± SEM. *p < 0.05, **p < 0.01, versus the control group. #p < 0.05, ##p < 0.01, versus the vector + cisplatin group.
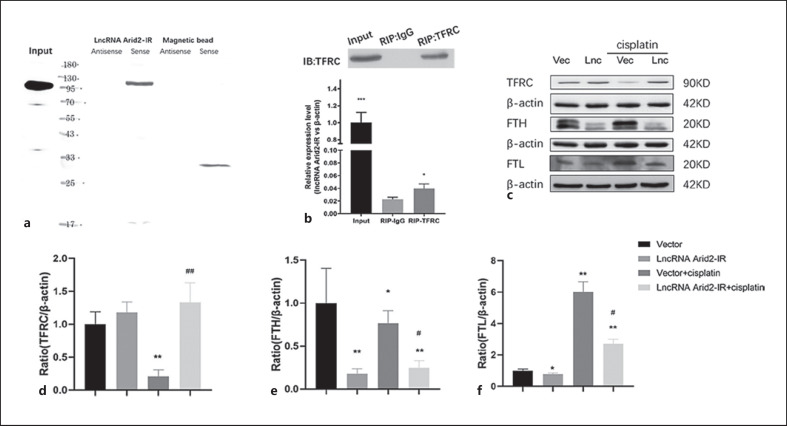
LncRNA Arid2-IR Binds to and Regulates the Transferrin Receptor Pathway
We then investigated the mechanism, whereby lncRNA Arid2-IR improved cell cycle disorder. Mass spectrometry showed that lncRNA Arid2-IR interacted with the TFRC. The interaction was further verified by RNA pull-down assay and Western blot (Fig. 6a), confirming the interaction of lncRNA Arid2-IR and TFRC. We also verified this result using RIP assay and found that lncRNA Arid2-IR could be enriched in TFRC precipitates, further proving that lncRNA Arid2-IR interacts with TFRC (Fig. 6b). We studied the effect of lncRNA Arid2-IR on TFRC and found that overexpression of lncRNA Arid2-IR increased TFRC expression in cisplatin-treated cells, whereas the expression of FTH and FTL decreased (Fig. 6c–f), thus suggesting that lncRNA Arid2-IR interacted with the TFRC pathway.
Fig. 6.
LncRNA Arid2-IR binds transferrin receptor. a RNA pull-down and Western blot show lncRNA Arid2-IR binding to TFRC. b RIP and RT-PCR show TFRC binding to lncRNA Arid2-IR. c Western blot shows overexpression of lncRNA Arid2-IR decreased TFRC, FTH, and FTL in cisplatin-induced renal tubular epithelial cells. d–f Quantitative analysis of TFRC, FTH, and FTL by Western blot. Each bar represents the mean ± SEM. *p < 0.05, **p < 0.01, versus the control group. #p < 0.05, ##p < 0.01, versus the vector + cisplatin group.
Discussion
Previous studies have shown that Smad3 plays an important role in AKI [13, 29]. The most important finding from the present study is the identification of mechanism through which lncRNA Arid2-IR regulates the cell cycle, decreases cell apoptosis, and promotes cell proliferation by targeting Smad3. This is a novel finding and is supported by our evidence showing that cisplatin can reduce the expression of lncRNA Arid2-IR in renal TECs, and the Smad3 inhibitor SIS3 increased the expression of lncRNA Arid2-IR in vivo and in vitro. Moreover, the findings of this study also provided the evidence of targeting Smad3 as a novel therapy for cisplatin-induced AKI, and SIS3 alleviates cisplatin-induced AKI by regulating the lncRNA Arid2-IR-TFRC pathway.
First, in this study, we explored the mechanisms of Smad3 in renal injury and cell proliferation. As a key mediator of the TGF-β signaling pathway, Smad3 plays an important role in maintaining normal cell growth by transferring signals from the cell membrane to the nucleus [30, 31]. Inhibiting Smad3 not only reduces inflammation, fibrosis, and cell apoptosis [32], but also promotes the proliferation and repair of damaged renal TECs [33]. SIS3 is a specific, permeable, and selective Smad3 inhibitor that inhibits Smad3 phosphorylation. In the unilateral ureteral obstruction mice model, the number of TUNEL-positive cells and cleaved caspase 3 expression was significantly reduced after SIS3 treatment [34]. Also, Smad3 reduced the expression of IL-6 and endothelin 1 [29], improved the decrease in renal TEC proliferation caused by C-reactive protein, and promoted the proliferation and repair of renal TECs with AKI injury [13]. The results of the current study showed that inhibition of Smad3 prevented cisplatin-induced renal functional injury by promoting TEC regeneration. This result indicated that Smad3 may play an important role in cell regeneration in cisplatin-induced AKI.
Second, we found that Smad3 plays a crucial role in cisplatin-induced renal injury and cell proliferation by regulating lncRNA Arid2-IR expression. Several lines of evidence indicate that Smad3 is involved in regulation of lncRNA [35, 36, 37, 38, 39, 40, 41]. It has been reported that 151 lncRNAs in the unilateral ureteral obstructive nephropathy kidney and 413 lncRNAs in kidneys with anti-glomerular basement membrane glomerulonephritis were significantly altered in Smad3 knockout mice [42]. The lncRNA Arid2-IR is enhanced under inflammatory conditions and promotes renal inflammation in UUO kidneys, whereas Arid2-IR expression decreases by 50% on day 1 in an ischemic reperfusion injury-induced AKI model [43]. Smad3 as a binding site was found in the promoter region of lncRNA Arid2-IR, silencing of which reduced inflammation, oxidative stress, and ECM production in the unilateral ureteral obstructive nephropathy kidney and diabetic kidney disease [19, 20, 21]. Our studies show that the lncRNA Arid2-IR was mainly expressed in the cytoplasm of renal tubule cells and decreased in cisplatin-induced AKI. This finding is consistent with those from a previous report indicating that Arid2-IR expression decreases in an AKI model [43]. Moreover, inhibiting Smad3 phosphorylation upregulated lncRNA Arid2-IR. LncRNA may play different roles in different diseases based on different pathophysiological processes in the occurrence and development of diseases in chronic kidney disease and AKI.
Finally, we identified a new function for lncRNA Arid2-IR, which was binding 93 proteins and regulating the expression of TFRC, FTH, and FTL by binding to TFRC protein, resulting in improved cell cycle disorder and cell regeneration. Iron is an important element in cell proliferation. Iron overload will activate the oxidative stress response, resulting in increased production of reactive oxygen species and other substances, and iron deficiency will lead to insufficient raw materials for cell metabolism, all of which will block cell proliferation, cause cell cycle arrest, and affect the expression of cell cycle-related protein p53, p21, p27, cyclinD1, and CDK2, leading to cell apoptosis [44]. TFRC which mediates cellular iron uptake through clathrin-dependent endocytosis of iron-loaded transferrin plays a key role in iron homeostasis and is highly expressed in cells with vigorous proliferation and metabolism [45]. TFRC is considered to be the receptor of IgA1, and IgA1 can promote the proliferation of glomerular mesangial cells by binding to TFRC in IgA nephropathy [46]. TFRC+/− mice exhibited attenuated renal fibrosis in obstructive nephropathy and diabetic kidney disease [47]. Also, silencing TFRC can upregulate p53 and p27 and downregulate cyclinD1, leading to cell cycle arrest [48]. TFRC may affect cell proliferation and repair by regulating the cell cycle. In the current study, our results provide new information indicating that lncRNA Arid2-IR may regulate the cell cycle by binding to TFRC, promoting cell proliferation and reducing cell apoptosis.
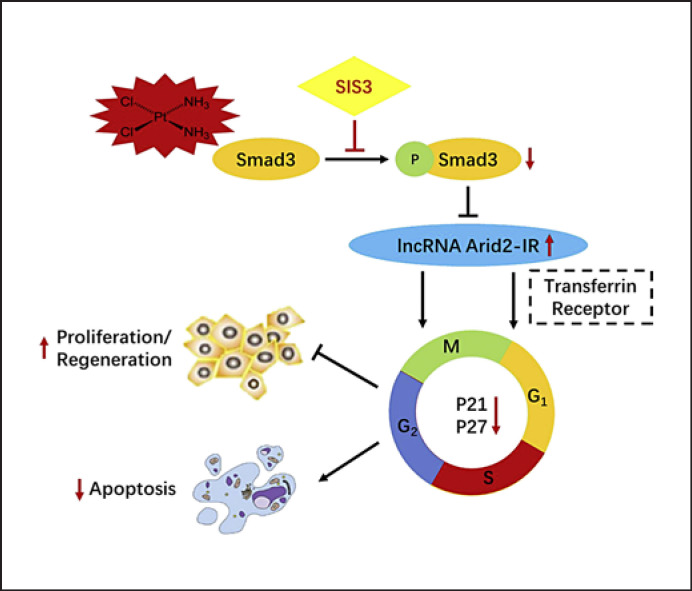
In conclusion, although there are probably multiple roles of Smad3 in AKI [12, 13], the results of our study suggest that one of the major means by which the Smad3 inhibitor SIS3 alleviates cisplatin-induced AKI is by directly interacting with Smad3-dependent lncRNA Arid2-IR to bind to the TFRC, which is required for the cell cycle (summarized in Fig. 7). Inhibition of Smad3 is able to block this pathway and, therefore, promotes TEC regeneration and protects against cisplatin-induced AKI. Smad3 gene deletion can cause a series of adverse consequences; however, lncRNA Arid2-IR may serve as a specific Smad3 therapeutic target to provide better protection against AKI. Thus, Smad3/lncRNA Arid2-IR/TFRC may represent a novel and effective therapeutic target for cisplatin-induced AKI.
Fig. 7.
Potential mechanisms for Smad3 to promote cisplatin-induced acute kidney injury. Cisplatin activates Smad3 phosphorylation and then decreases lncRNA Arid2-IR, thus causing tubular epithelial cell (TEC) growth arrest at the G1 cell cycle. Inhibiting Smad3 (SIS3) reduced Smad3 phosphorylation and then increased lncRNA Arid2-IR by binding to TFRC, improving cell cycle disorders, decreasing cell apoptosis, and increasing cell regeneration.
Statement of Ethics
This study was performed in accordance with the recommendations of the Animal Care Committee of the Sun Yat-sen University of guidelines and the Animal Care Committee of the Sun Yat-sen University of committee. The protocol was approved by the Institutional Animal Care and Use Committee of the Sun Yat-sen University (approval number: 2018000741).
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
The study was supported by the National Natural Science Foundation of China (Grant No. 81700598, 81070581, 81470954).
Author Contributions
Weiyan Lai, Jiayan Huang, Hui Peng, and Zengchun Ye conceived and designed the experiments. Jiayan Huang, Weiyan Lai, Ming Li, Canming Li, Tan-qi Lou, Zengchun Ye, and Hui Peng performed the experiments. Jiayan Huang wrote the paper. Weiyan Lai, Jiayan Huang, Zengchun Ye, and Hui Peng reviewed/edited the manuscript. Weiyan Lai and Jiayan Huang contributed equally to this work. All authors read and approved the final version of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We appreciate all participants in this work.
Funding Statement
The study was supported by the National Natural Science Foundation of China (Grant No. 81700598, 81070581, 81470954).
References
- 1.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394((10212)):1949–64. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA. Clinical decision support for in-hospital AKI. J Am Soc Nephrol. 2018;29((2)):654–60. doi: 10.1681/ASN.2017070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients the multinational AKI-EPI study. Intensive Care Med. 2015;41((8)):1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 4.Wen J, Cheng Q, Zhao J, Ma Q, Song T, Liu S, et al. Hospital-acquired acute kidney injury in Chinese very elderly persons. J Nephrol. 2013;26((3)):572–9. doi: 10.5301/jn.5000182. [DOI] [PubMed] [Google Scholar]
- 5.Astolfi L, Ghiselli S, Guaran V, Chicca M, Simoni E, Olivetto E, et al. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours a retrospective evaluation. Oncol Rep. 2013;29((4)):1285–92. doi: 10.3892/or.2013.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Gordaliza E, Giesen C, Lázaro A, Esteban-Fernández D, Humanes B, Cañas B, et al. Elemental bioimaging in kidney by LA-ICP-MS as a tool to study nephrotoxicity and renal protective strategies in cisplatin therapies. Anal Chem. 2011;83((20)):7933–40. doi: 10.1021/ac201933x. [DOI] [PubMed] [Google Scholar]
- 7.Zhu S, Pabla N, Tang C, He L, Dong Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch Toxicol. 2015;89((12)):2197–205. doi: 10.1007/s00204-015-1633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price PM, Megyesi J, Saf Irstein RL. Cell cycle regulation repair and regeneration in acute renal failure. Kidney Int. 2004;66((2)):509–14. doi: 10.1111/j.1523-1755.2004.761_8.x. [DOI] [PubMed] [Google Scholar]
- 9.Moonen L, D'Haese PC, Vervaet BA. Epithelial cell cycle behaviour in the injured kidney. Int J Mol Sci. 2018;19((7)):2038. doi: 10.3390/ijms19072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu YS, Liang S, Li DY, Wen JH, Tang JX, Liu HF. Cell cycle dysregulation and renal fibrosis. Front Cell Dev Biol. 2021;9:714320. doi: 10.3389/fcell.2021.714320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Gusella GL, He JC. Epithelial proliferation and cell cycle dysregulation in kidney injury and disease. Kidney Int. 2021;100((1)):67–78. doi: 10.1016/j.kint.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol. 2011;301((2)):F436–F442. doi: 10.1152/ajprenal.00162.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai W, Tang Y, Huang XR, Ming-Kuen Tang P, Xu A, Szalai AJ, et al. C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int. 2016;90((3)):610–26. doi: 10.1016/j.kint.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SG, Kim HA, Jong HS, Park JH, Kim NK, Hong SH, et al. The endogenous ratio of Smad2 and Smad3 influences the cytostatic function of Smad3. Mol Biol Cell. 2005;16((10)):4672–83. doi: 10.1091/mbc.E05-01-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S. Analysis of the mouse transcriptome based on functional annotation of 60, 770 full-length. cDNAs. Nature. 2002;420((6915)):563–73. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 16.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs functional surprises from the RNA world. Genes Dev. 2009;23((13)):1494–504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81((1)):145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kota SK, Kota SB. Noncoding RNA and epigenetic gene regulation in renal diseases. Drug Discov Today. 2017;22((7)):1112–22. doi: 10.1016/j.drudis.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Huang XR, Yu J, Yu X, Lan HY. Long noncoding RNA arid2-IR is a novel therapeutic target for renal inflammation. Mol Ther. 2015;23((6)):1034–43. doi: 10.1038/mt.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao H, Yang H, Zeng Y. Long non-coding RNA Arid2-IR affects advanced glycation end products-induced human retinal endothelial cell injury by binding to Smad3. Int Ophthalmol. 2020;40((5)):1123–33. doi: 10.1007/s10792-019-01277-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang YL, Hu F, Xue M, Jia YJ, Zheng ZJ, Li Y, et al. Early growth response protein-1 upregulates long noncoding RNA Arid2-IR to promote extracellular matrix production in diabetic kidney disease. Am J Physiol Cell Physiol. 2019;316((3)):C340–C352. doi: 10.1152/ajpcell.00167.2018. [DOI] [PubMed] [Google Scholar]
- 22.Li CM, Li M, Ye ZC, Huang JY, Li Y, Yao ZY, et al. Circular RNA expression profiles in cisplatin-induced acute kidney injury in mice. Epigenomics. 2019;11((10)):1191–207. doi: 10.2217/epi-2018-0167. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Li CM, Ye ZC, Huang J, Li Y, Lai W, et al. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J Cell Mol Med. 2020;24((9)):5109–21. doi: 10.1111/jcmm.15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27((50)):6398–406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 25.Mohan S, Abdelwahab SI, Kamalidehghan B, Syam S, May KS, Harmal NSM, et al. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound pyranocycloartobiloxanthone A. Phytomedicine. 2012;19((11)):1007–15. doi: 10.1016/j.phymed.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17((1)):47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 27.Robson CN, Gnanapragasam V, Byrne RL, Collins AT, Neal DE. Transforming growth factor-beta1 up-regulates p15, p21 and. p27 and blocks cell cycling in G1 in human prostate epithelium. J Endocrinol. 1999;160((2)):257–66. doi: 10.1677/joe.0.1600257. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430((6996)):226–31. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 29.Li XY, Chen HR, Zha XQ, Chen S, Pan LH, Li QM, et al. Prevention and possible mechanism of a purified Laminaria japonica polysaccharide on adriamycin-induced acute kidney injury in mice. Int J Biol Macromol. 2020;148:591–600. doi: 10.1016/j.ijbiomac.2020.01.159. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Yang Y, He K, Zhang F, Zhao L, Zhou W, et al. Single-molecule imaging reveals the activation dynamics of intracellular protein Smad3 on cell membrane. Sci Rep. 2016;6((1)):33469. doi: 10.1038/srep33469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh Y, Saitoh M, Miyazawa K. Smad3-STAT3 crosstalk in pathophysiological contexts. Acta Biochim Biophys Sin. 2018;50((1)):82–90. doi: 10.1093/abbs/gmx118. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Meng XM, Huang XR, Lan HY. The preventive and therapeutic implication for renal fibrosis by targetting TGF-β/Smad3 signaling. Clin Sci. 2018;132((13)):1403–15. doi: 10.1042/CS20180243. [DOI] [PubMed] [Google Scholar]
- 33.Wang HL, Wei B, He HJ, Huang XR, Sheng JY, Chen XC, et al. Smad3 deficiency improves islet-based therapy for diabetes and diabetic kidney injury by promoting β cell proliferation via the E2F3-dependent mechanism. Theranostics. 2022;12((1)):379–95. doi: 10.7150/thno.67034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji X, Wang H, Wu Z, Zhong X, Zhu M, Zhang Y, et al. Specific inhibitor of Smad3 (SIS3) attenuates fibrosis, apoptosis, and inflammation in unilateral ureteral obstruction kidneys by inhibition of transforming growth factor β (TGF-β)/Smad3 signaling. Med Sci Monit. 2018;24:1633–41. doi: 10.12659/MSM.909236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Q, Han L, Yan W, Ji X, Han R, Yang J, et al. miR-489 inhibits silica-induced pulmonary fibrosis by targeting MyD88 and Smad3 and is negatively regulated by lncRNA CHRF. Sci Rep. 2016;6((1)):30921. doi: 10.1038/srep30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun SF, Tang PM, Feng M, Xiao J, Huang XR, Li P, et al. Novel lncRNA erbb4-IR promotes diabetic kidney injury in db/db mice by targeting miR-29b. Diabetes. 2018;67((4)):731–44. doi: 10.2337/db17-0816. [DOI] [PubMed] [Google Scholar]
- 37.Lu C, Li Z, Hu S, Cai Y, Peng K. LncRNA PART-1 targets TGFBR2/Smad3 to regulate cell viability and apoptosis of chondrocytes via acting as miR-590-3p sponge in osteoarthritis. J Cell Mol Med. 2019;23((12)):8196–205. doi: 10.1111/jcmm.14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi M, Bucci G, Rizzotto D, Bordo D, Marzi MJ, Puppo M, et al. LncRNA EPR controls epithelial proliferation by coordinating Cdkn1a transcription and mRNA decay response to TGF-β. Nat Commun. 2019;10((1)):1969. doi: 10.1038/s41467-019-09754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Du YM, Li B. LncRNA BX357664 inhibits the proliferation and invasion of non-small cell lung cancer cells. Eur Rev Med Pharmacol Sci. 2019;23((2)):660–9. doi: 10.26355/eurrev_201901_16880. [DOI] [PubMed] [Google Scholar]
- 40.Yang Q, Cao K, Jin G, Zhang J. Hsa-miR-346 plays a role in the development of sepsis by downregulating SMAD3 expression and is negatively regulated by lncRNA MALAT1. Mol Cell Probes. 2019;47:101444. doi: 10.1016/j.mcp.2019.101444. [DOI] [PubMed] [Google Scholar]
- 41.Lang C, Dai Y, Wu Z, Yang Q, He S, Zhang X, et al. SMAD3/SP1 complex-mediated constitutive active loop between lncRNA PCAT7 and TGF-β signaling promotes prostate cancer bone metastasis. Mol Oncol. 2020;14((4)):808–28. doi: 10.1002/1878-0261.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Chung AC, Huang XR, Dong Y, Yu X, Lan HY. Identification of novel long noncoding RNAs associated with TGF-β/Smad3-mediated renal inflammation and fibrosis by RNA sequencing. Am J Pathol. 2014;184((2)):409–17. doi: 10.1016/j.ajpath.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Zhang P, Yu C, Yu J, Li Z, Lan HY, Zhou Q. Arid2-IR promotes NF-κB-mediated renal inflammation by targeting NLRC5 transcription. Cell Mol Life Sci. 2021;78((5)):2387–404. doi: 10.1007/s00018-020-03659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Y, Kovacevic Z, Richardson DR. Tuning cell cycle regulation with an iron key. Cell Cycle. 2007;6((16)):1982–94. doi: 10.4161/cc.6.16.4603. [DOI] [PubMed] [Google Scholar]
- 45.Gammella E, Buratti P, Cairo G, Recalcati S. The transferrin receptor the cellular iron gate. Metallomics. 2017;9((10)):1367–75. doi: 10.1039/c7mt00143f. [DOI] [PubMed] [Google Scholar]
- 46.Moura IC, Arcos-Fajardo M, Gdoura A, Leroy V, Sadaka C, Mahlaoui N, et al. Engagement of transferrin receptor by polymeric IgA1 evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. J Am Soc Nephrol. 2005;16((9)):2667–76. doi: 10.1681/ASN.2004111006. [DOI] [PubMed] [Google Scholar]
- 47.Yasumura S, Naito Y, Okuno K, Sawada H, Asakura M, Masuyama T, et al. Effects of heterozygous TfR1 (Transferrin Receptor 1) deletion in pathogenesis of renal fibrosis in mice. Hypertension. 2020;75((2)):413–21. doi: 10.1161/HYPERTENSIONAHA.119.13670. [DOI] [PubMed] [Google Scholar]
- 48.Campisi A, Bonfanti R, Raciti G, Bonaventura G, Legnani L, Magro G, et al. Gene silencing of transferrin-1 receptor as a potential therapeutic target for human follicular and anaplastic thyroid cancer. Mol Ther Oncolytics. 2020;16:197–206. doi: 10.1016/j.omto.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.