Abstract
Introduction
Severe acute respiratory distress syndrome coronavirus disease-2 (SARS-CoV-2) can lead to acute hypoxemic respiratory failure (AHRF) with possible multisystemic involvement. Ventilation/perfusion mismatch and shunt increase are critical determinants of hypoxemia. Understanding hypoxemia and the mechanisms involved in its genesis is essential to determine the optimal therapeutic strategy. High flow nasal oxygen (HFNO) and awake prone positioning (APP) in patients with COVID-19 AHRF showed promising benefits. The aim of this systematic review was to depict current situation around the combined use of HFNO and APP in patients with COVID-19 AHRF. Particularly, to investigate and report the pathophysiological rationale for adopting this strategy and to evaluate the (1) criteria for initiation, (2) timing, monitoring and discontinuation, and to assess the (3) impact of HFNO/ APP on outcome.
Methods
We performed a systematic search collecting the articles present in PubMed, Scopus, EMBASE, and Cochrane databases with the following keywords: COVID-19 pneumonia, high flow nasal oxygen, awake prone position ventilation.
Results
Thirteen studies displayed inclusion criteria and were included, accounting for 1242 patients who received HFNO/ APP. The combination of HFNO/ APP has an encouraging pathophysiological rationale for implementing this technique. The recognition of patients who can benefit from HFNO/ APP is difficult and there are no validated protocols to start, monitoring, and discontinue HFNO/ APP therapy. The most used method to monitor the efficacy and failure of this combined technique are oxygenation indexes, but discontinuation techniques are inconsistently and poorly described limiting possible generatability. Finally, this technique provided no clear benefits on outcome.
Conclusions
Our systematic search provided positive feedbacks for improving the utilization of this combination technique, although we still need further investigation about methods to guide timing, management, and discontinuation, and to assess the intervention effect on outcome.
Keywords: Oxygen therapy; High flow nasal canula; Acute respiratory distress syndrome, awake prone position; Covid-19 pneumonia
1. Introduction
The severe acute respiratory distress syndrome coronavirus disease-2 (SARS-CoV-2) virus can lead to a disease characterized by multisystemic involvement, with acute hypoxemic respiratory failure (AHRF) and/or acute respiratory distress syndrome (ARDS) secondary to pneumonia being its predominant markers (Berlin et al., 2020). From a pathophysiological point of view, COVID-19 infection triggers a triad of deleterious events: inflammation, coagulation disorders, and dysregulation of the immune response (Robba et al., 2020). At the pulmonary level, hypoxemia is due to varied and complex mechanisms, among those which stand out: profound changes of the ventilation-perfusion (V’/Q’) relationship and the development of intrapulmonary shunt, which will appear to a greater or lesser extent, originating evolutionary stages quite well differentiated (Berlin et al., 2020, Gattinoni et al., 2020, Li et al., 2020). Understanding hypoxemia and the mechanisms involved in its genesis is essential, since it can determine the most optimal and convenient therapeutic strategy. For these reasons, oxygen therapy is of vital importance in this patient cohort. In pre-COVID 19 era prone positioning showed oxygenation improvement in patients with AHRF and reduced mortality in patients with moderate to severe ARDS (Guérin et al., 2013). During COVID-19 pandemic, patients complicated with severe hypoxemia usually required HFNO with or without combination of awake prone positioning (APP) (Schmid et al., 2022).
The aim of this manuscript is to depict current situation around the combined use of HFNO and APP in patients with COVID-19 AHRF. Particularly, to investigate and report the pathophysiological rationale for adopting this strategy and to evaluate the (1) criteria for initiation, (2) timing, monitoring and discontinuation, and to assess the (3) effect of HFNO/ APP on outcome.
2. Materials and methods
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)(Liberati et al., 2009; Page et al., 2021) and the Joanna Briggs Institute (JBI) Reviewers' Manual for Systematic Reviews of Literature and New Castle Ottawa Scale (Aromataris and Munn, 2020) (Supplementary material [SM] item S1).
2.1. Search strategy and study selection
For our purpose, three reviewers (DG, YL, BF) performed a systematic search of four databases (PubMed, Scopus, EMBASE, and Cochrane) to search and screen for relevant literature. Studies were also identified by citation searching from the bibliography of each relevant study. The search included MEdical Subject Heading [MESH] terms fitting with COVID-19 (coronavirus disease, COVID-19, coronavirus disease 2019, SARS-CoV-2, covid, coronavirus) and high flow oxygen (high flow nasal cannula, high flow nasal oxygen, high flow) and/ or awake prone positioning (prone positioning, prone position, prone) terms. The extended list of MeSH terms is reported in the SM item S2. Titles and abstracts of all identified studies were independently screened by three authors (DG, YL, BF) and retrieved for duplication checking. The full text of studies classified as relevant was reviewed by two investigators (DG and YL). Disagreement was resolved by a third reviewer (DB). No language restriction was applied. We included all the studies from January 1st, 2020, to September 1st, 2022. The inclusion criteria were as follow: (1) study population of adult patients (age 18 years of age or older) diagnosed with COVID-19 by positive real-time polymerase chain reaction assay, (2) studies that described the combined use of HFNO and APP as a therapy to improve hypoxemia, and (3) randomized controlled trials, observational studies, including case series, case-control studies, cohort studies were included. Exclusion criteria were (1) studies which reported data on non-invasive ventilatory techniques other than HFNO; (2) studies in which was impossible to retrieve individual data/ incomplete data/ absent data on HFNO/ APP combined technique from mixed patient-cohorts, after consulting the corresponding author asking for additional data; (3) studies with < 5 patients; (4) case reports; and (5) outside critical care setting.
2.2. Data extraction and risk-of-bias assessment
Two reviewers independently extracted data (DG and YL) on the use of combined HFNO and APP technique. The following data were extracted for each study: study design characteristics (case–control, cohort studies, or case series), study information (first author, date of publication, publication type, study site), COVID-19 population characteristics (number of patients, complications, survival), patient characteristics (age, country, gender, sample size, severity of COVID-19 AHRF and/or ARDS, death), and HFNO/ APP characteristics (criteria for starting HFNO [i.e., arterial partial pressure of oxygen/ fraction of inspired oxygen=PaO2/FiO2], discontinuation of HFNO, follow-up/ monitoring of HFNO, type of device for high flow oxygen administration, duration of prone positioning). When necessary, the corresponding authors of the included studies were contacted to obtain missing data related to trial demographics, methods, and outcomes.
For each study, two reviewers (DG and YL) independently assessed the risk of bias tool using the modified 8-item Newcastle–Ottawa scale (NOS)(Lo et al., 2014) (SM item S3). Disagreements amongst reviewers were discussed with a third author until a consensus was reached (DB).
2.3. Strategy for data synthesis
Findings from the included studies were reported as narrative and tabular synthesis, structured with the aim to assess the characteristics and outcomes of critically ill patients with COVID-19 undergoing HFNO and APP combination technique.
3. Results
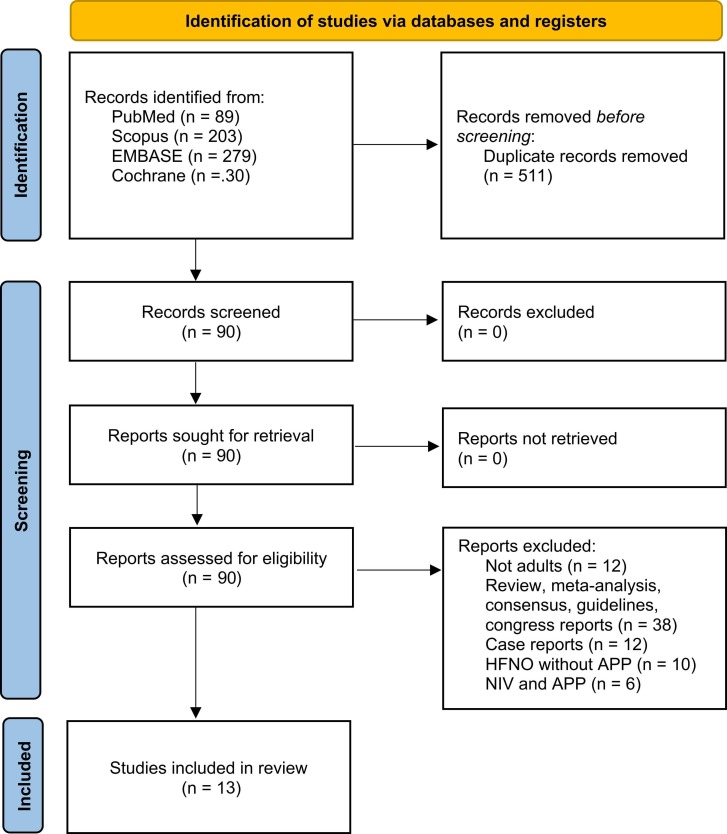
The search yielded thirteen relevant studies which fitted with our purpose (Ehrmann et al., 2021, Ferrando et al., 2020, Ibarra-Estrada et al., 2022, Jouffroy et al., 2021, Kaur et al., 2021, Kucukdemirci-Kaya et al., 2022, COVIDPREG Study Group, 2022, Vanderbilt Learning Healthcare System Platform Investigators, 2022, PROFLO Study Group, 2021, Tu et al., 2020, Vianello et al., 2021, Xu et al., 2020, Yang et al., 2020). The search and selection strategies are shown in Fig. 1. Overall, 1242 patients undergoing HFNO/ APP combination therapy in ICU setting were included. The risk of bias assessment of methodological quality of the studies according to the NOS revealed nine studies rated as high quality, four as moderate quality, and zero studies rated as low quality. The scoring process is presented in SM Item S4.
Fig. 1.
PRISMA flowchart.
The debates put forward and the preliminary data available, make it possible to generate hypotheses regarding the use of both techniques simultaneously, particularly when combining their effects on respiratory physiology, and enhance their benefits. Data on combination therapy with HFNO/ APP are presented in Table 1.
Table 1.
Studies that investigate the effect of prone position combined with HFNO compared to standard care in patients with COVID-19 pneumonia. HFNO=high flow nasal oxygen; APP=awake prone positioning; HR=hazard ratio; PaO2 =arterial partial pressure of oxygen; FiO2 =fraction of inspired oxygen; NA=not available; IMV=invasive mechanical ventilation; SpO2 =peripheral saturation of oxygen, OR=Odds Ratio, RR=Relative Risk.
| Reference | Country | Number of patients | HFNO Onset | Parameters of HFNO | APP Timing | APP/ HFNO discontinuation | Comments |
|---|---|---|---|---|---|---|---|
| (Ehrmann et al. (2021) | Canada France Ireland Mexico USA Spain |
1121 (HFNO/ APP 564, HFNO 557) | PaO2/FiO2 ≤ 300 or SpO2/FiO2 ≤ 315 | Maximum tolerated flow setting Titrated to SaO2 > 90–95 % |
As long as tollerated/ failure | Hospital discharge, intubation (respiratory rate >40 breaths min, fatigue, respiratory acidosis pH<7.25, copious tracheal secretions, SpO2 <90 % with FiO2 >0.8, hemodynamic instability, deteriorating mental status), death, improved oxygenation | Treatment failure (intubation or death) 40 % HFNO/ APP vs 46 % HFNO (RR 0.86), HR for intubation 0.75, for 28-day mortality 0.87. |
| (Xu et al., 2020) | China | 10 | PaO2/FiO2 < 300 | Flow NA SpO2 > 90 % |
> 16 h/day | NA | None of enrolled subject underwent IMV |
| (Tu et al., 2020) | China | 9 | HFNO > 2 days with PaO2/FiO2 < 150 | Initial flow rate 50–60 L/min targeted to SpO2 > 90 % | 2 h, 5 times/day | Scarce tolerance to APP. | Intubation rate 22.2 %, HFNO/ APP significantly increased SpO2 and PaO2 compared with HFNO |
| (Ferrando et al., 2020) | Spain | 1076 (55 HFNO/ APP) | Admission. SpO2 < 93% with oxygen 15 L/min. |
NA | > 16 h per day | NA | Intubation rate 40 %. HFNO/ APP did not reduce risk intubation (HR 0.87), but increased the time to intubation in comparison with HFNO alone. Similar length of ICU stay and 28-day mortality. |
| (Kaur et al., 2021) | USA | 125 | Admission PaO2/FiO2 < 240 |
Initial flow 50 L/min titrated to SaO2 > 90–95 % | As long as tollerated | FiO2 < 0.4 and HFNO flow 40 L/min. | Early APP lower mortality rate (26% vs 45%); no difference in intubation rate |
| (Ibarra-Estrada et al., 2022) | Mexico | 430 (216 HFNO/ APP, 214 HFNO) | Admission. SpO2 < 90 % with oxygen mask 15 L/min |
Max flow 40 L/min, FiO2 > 30 % Titrated to SpO2 90–95 % |
As long as tollerated. APP/HFNO success defined as respiratory rate< 25 breaths min, increased ROX> 1.25 after first session, APP> 8 h, lung ultrasound score reduced by 2 or more. | FiO2 ≤ 0.4 with flow ≤ 20 L/min to maintain SpO2 ≥ 90 % for 2 h, need for non-invasive mechanical ventilation, death. | HFNO/ APP group: lower intubation rate (30 %) than HFNO (43 %) RR= 0.7, shorter length of stay (11 % vs 13 %) |
| (Péju et al., 2022) | France, Belgium, Switzerland | 187 (8 HFNO/ APP) | According to the severity of respiratory failure and local protocol. | NA | NA | NA | Pregnant patients. Eight patients APP/ HFNO of whom 3 intubated. |
| (Kucukdemirci-Kaya et al., 2022) | Turkey | 35 (13 HFNO/ APP) | Admission. Respiratory rate > 30 breaths min with SpO2 < 92 % with oxygen 15 L/min, and/or PaO2/FiO2 < 150 | Flow 50–80 L/min, titrated to SpO2 > 88 % | 12 h for 1–2 times/day | Death, intubation. ROX index and PaO2/FiO2 at 2 h to predict intubation risk were higher in the treatment success group. |
No differences in treatment failure (intubation, death) between APP vs. non HFNO/ APP groups (OR 0.29), no difference in COVID-19 phenotype (OR 0.24). |
| (Qian et al. (2022) | USA | 501 (71 HFNO/ APP) | Admission, not specified. | Oxygen therapy targeted to SpO2 89 % | Admission 4 h/2 times day | NA | No clinical benefits on outcome of HFNO/ APP as compared to HFNO alone. |
| (Yang et al., 2020) | Canada | 51 (10 HFNO/ APP) | Admission, not specified. | At provider’s discretion | Daily If required FiO2 > 0.65 |
At provider’s discretion | 39 % not required intubation. |
| (Rosén et al., 2021) | Sweden | 75 (36 APP group of whom 31 HFNO/ APP, 39 control) | Admission. PaO2/FiO2 < 150 mmHg for more than 1 h |
Median flow 50 L/min | APP 16 h/day | Intubation, improvement (standard facemask with oxygen flow rate<5 L/min for 12 h), clincian discretion. | No differences. Intubation 13 patients control vs. 12 APP group (HR 1.01). |
| (Jouffroy et al., 2021) | France | 40 (37 HFNO/ APP) | Admission, not specified. | NA | 3–6 h, 2 times/ day | NA | No effects on intubation (HR 0.96) or 28-day mortality rates (HR 0.51) |
| (Vianello et al., 2021) | Italy | 93 | Admission, not specified. | FiO2 0.21–1.0 flow up to 60 L/min adjusted to maintain SpO2 > 93 % | > 2 h, 2 times/ day. | Non-invasive ventilation started when FiO2 > 0.6 needed to achieve SpO2 > 92%, intubation at provider’s discretion. APP failure was defined as inability to maintain APP> 2 h, refusal, inadequate cooperation, or lack of improvement/ deterioration in oxygenation. | Sixteen (17.2 %) patients were intubated, 27 (29 %) escalated to non-invasive ventilation, 9 died (9.7 %). In patients who passed the HFNO/ APP trial (around 50 %), survival benefits were observed without escalation of therapy. |
3.1. Criteria and rationale for initiation HFNO/ APP
The criteria for initiation of HFNO was not reported in three studies. Ten studies reported PaO2/FiO2 cut-off compatible with mild-moderate ARDS as criteria for HFNO/ APP initiation (Ehrmann et al., 2021, Kaur et al., 2021, Kucukdemirci-Kaya et al., 2022, PROFLO Study Group, 2021, Tu et al., 2020, Xu et al., 2020), one study used the modified Kigali definition of mild ARDS as possible alternative to the Berlin definition (Ehrmann et al., 2021), three studies reported a cut-off SpO2 < 90–93 % using oxygen flow 15 L/min through a non-rebreathing mask for HFNO/ APP initiation (Ferrando et al., 2020, Ibarra-Estrada et al., 2020, Kucukdemirci-Kaya et al., 2022).
All the studies did not report a local protocol for initiation and management of HFNO/ APP combination technique (Ehrmann et al., 2021, Ferrando et al., 2020, Ibarra-Estrada et al., 2022, Jouffroy et al., 2021, Kaur et al., 2021, Kucukdemirci-Kaya et al., 2022, COVIDPREG Study Group, 2022, Vanderbilt Learning Healthcare System Platform Investigators, 2022, PROFLO Study Group, 2021, Tu et al., 2020, Xu et al., 2020, Yang et al., 2020). Information on how to start HFNO were provided by seven studies. HFNO was mainly initiated at flow rates between 40 and 80 L/min, and tritated to SpO2 between 89 % and 95 % (Ehrmann et al., 2021, Kaur et al., 2021, Kucukdemirci-Kaya et al., 2022, Vanderbilt Learning Healthcare System Platform Investigators, 2022, PROFLO Study Group, 2021, Tu et al., 2020, Vianello et al., 2021). Criteria for initiation of APP were not clearly stated nor protocolised.
Delayed intubation and lower intubation rate were the most convincing rationale for initiation of HFNO/ APP in COVID-19 AHRF (Ferrando et al., 2020, Ibarra-Estrada et al., 2022, Tu et al., 2020, Yang et al., 2020), despite some studies did not confirmed this benefit (Kaur et al., 2021, PROFLO Study Group, 2021). In the study of Xu et al., no patient required invasive mechanical ventilation (Xu et al., 2020).
3.2. Timing, monitoring, and discontinuation of HFNO/ APP
Data on timing are heterogenous. Combined HFNO/ APP therapy was continued “as long as tolerated” (Ehrmann et al., 2021, Ibarra-Estrada et al., 2022, Kaur et al., 2021), “for > 16 h per day” (Ferrando et al., 2020, PROFLO Study Group, 2021, Xu et al., 2020), “12 h for 1–2 times per day”, or in some cases “for shorter periods of time” (2–6 h or more than 6 h for 1–5 times per day) (Jouffroy et al., 2021, Kucukdemirci-Kaya et al., 2022, Tu et al., 2020, Yang et al., 2020). One study did not report the timing of utilization of this technique (Péju et al., 2022).
Criteria for monitoring and discontinuation of HFNO/ APP were not clearly reported. However, four studies used the SpO2, ROX index and/or PaO2/FiO2 ratio to assess the efficacy and to discontinue the therapy and to predict the risk of HFNO/ APP failure (Kucukdemirci-Kaya et al., 2022, PROFLO Study Group, 2021). The main criteria for discontinuation of HFNO/ APP, were described as: FiO2 ≤ 0.4 with flow ≤ 20 L/min to maintain SpO2 > 90 % for 2 h (Ibarra-Estrada et al., 2020), FiO2 ≤ 0.4 using a flow ≤ 40 L/min (Kaur et al., 2021), hospital discharge, intubation, death, need for non-invasive mechanical ventilation, “at provider’s discretion”, or “intolerance” (Ehrmann et al., 2021, Kucukdemirci-Kaya et al., 2022, PROFLO Study Group, 2021, Tu et al., 2020, Yang et al., 2020).
3.3. Impact of combination HFNO/ APP therapy on outcome
The use of HFNO/ APP provided not clear benefits on outcome (Table 1). Survival benefits were observed in patients who passed the HFNO/ APP trial and early application of HFNO/ APP, although some other studies did not confirm these benefits (Kaur et al., 2021, Vianello et al., 2021). ICU length of stay was reduced in one study (Ibarra-Estrada et al., 2020). Therefore, there is still no consensus on the use of this technique to provide benefits on intubation rates and timings, ICU length of stay and mortality.
4. Discussion
To the best of our knowledge, this is the first systematic review investigating the use of combined HFNO/ APP in COVID-19 patients with AHRF. This combined technique is poorly defined and inconsistently applied in clinical practice, and recommendations on methods to start or discontinue HFNO/ APP are lacking. The main findings of our systematic search are that (1) the combination of HFNO/ APP has been inconsistently adopted during the pandemic but the pathophysiological rationale for implementing this technique is encouraging; (2) the recognition of patients who can benefit from HFNO/ APP is poorly defined and there are no validated protocols to start, monitoring, and discontinue HFNO/ APP therapy; (3) the most used method to monitor the failure of this combined technique are the PaO2/FiO2, SpO2, and ROX index, but methods to assess efficacy and discontinuation techniques are poorly described limiting possible generatability. Finally, (4) this technique provided no clear benefits on outcome. Therefore, the available literature is insufficient to provide recommendations on the applicability of this combined technique or on methods to assess its efficacy to recognize failure avoiding patients harm (Cammarota et al., 2021, Li et al., 2022). However, below we discuss the rationale for implementing the use of combined HFNO/ APP in patients with COVID-19 AHRF.
4.1. Criteria and rationale for initiation HFNO/ APP combination strategy
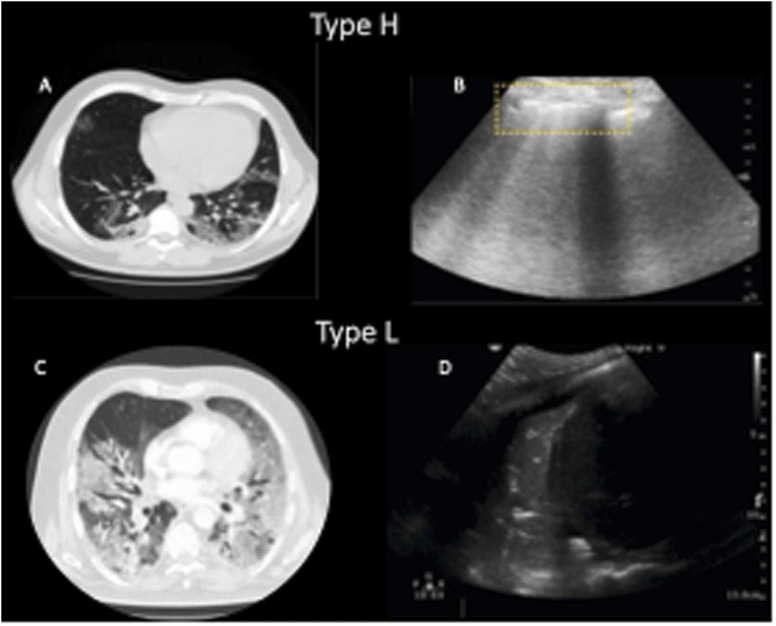
Our search did not provide clear criteria and rationale for initiation of HFNO/ APP therapy. The recognition of patients at risk of disease progression who may benefit from a combined strategy using HFNO/ APP is poorly described. However, the literature well-describes how to assess the severity of lung injury in patients with COVID-19 AHRF to identify those who may require intubation and invasive mechanical ventilation. These vary from gas exchange to lung images, scores of organ severity, and more recently also the adoption of biological markers (Gorman et al., 2022). COVID-19 pneumonia often meets the Berlin criteria of ARDS but manifests with peculiar features, which may need different treatment strategy according to radiological phenotype (Gattinoni et al., 2020, Robba et al., 2020). However, our search found that radiological criteria have not been considered to initiate HFNO/ APP therapy. Phenotype 1 (L) is characterized by a radiological aspect with ground-glass opacities, greater presence of aerated lung tissue, higher respiratory system compliance and relatively good respiratory mechanics (Gattinoni et al., 2020, Robba et al., 2020). This phenotype is typically seen during the initial stages of the disease, where hypoxia is driven by the predominance of low V’/Q’ areas (with decreased ventilation in poorly aerated lung areas or increased perfusion in normally or poorly aerated regions) (Gattinoni et al., 2020, Robba et al., 2020). Non-invasive respiratory support may be efficient at this stage, including the application of HFNO, continuous positive airway pressure (CPAP), or non-invasive mechanical ventilation (NIMV) as first-line therapeutic strategy with the use of high fraction of oxygen and low-moderate levels of positive end-expiratory pressure (PEEP) (Robba et al., 2021a). Phenotype 2 (H) is characterized mostly by non-perfused and poorly aerated lung regions with increased shunt, progressive reduction in the lung compliance with severe hypoxemia, and greater consolidation of lung regions (Gattinoni et al., 2020, Robba et al., 2020). In COVID-19 AHRF the distribution of perfusion follows a reverse gravitational pattern where the most blood volume is distributed mainly to the aerated areas (Pelosi et al., 2022). In this case, deterioration of gas exchange is frequent, and the patients may need invasive mechanical ventilation (Robba et al., 2021a) ( Fig. 2).
Fig. 2.
COVID-19 phenotypes at CT scan and LUS. A) CT Scan showed pattern type H, aerated lungs with subpleural effusions irregularly distributed. B) Lung Ultrasound (LUS) showed irregular and broken pleural lines with multiple B-lines. C) CT scan showed type L pattern, diffuse bilateral confluent patchy ground glass with bilateral consolidation; D) LUS showed large zone of consolidation with air bronchograms.
Accordingly, our search revealed that HFNO/ APP strategy can avoid or delay intubation in patients at risk, but intubation remains a clinicians’ decision based on individual patients’ factors (i.e., tiring, work of breathing, consolidation burden). The use of HFNO alone demonstrated lower intubation rates both in patients with and without COVID-19 AHRF (Agarwal et al., 2020, Demoule et al., 2020, Ospina-Tascón et al., 2021, Peng et al., 2022, Perkins et al., 2022). Additionally, HFNO facilitates the management of FiO2 reliably while concurrently modifying the conditions of the gas supplied such as temperature and humidity (Raoof et al., 2020, Suffredini and Allison, 2021). Physiologically, higher flows tend to meet inspiratory demands, decrease anatomical dead space, lower respiratory rate, and work of breathing. Additionally, high flows tend to generate positive pressure in the upper airways, thus, decreasing airway resistance and providing PEEP by means of 1 cmH2O for every 10 liters of flow administered. Benefits of its use include an increase in the end expiratory volume, decrease in the cardiac preload and easier management of secretions (Raoof et al., 2020, Suffredini and Allison, 2021). HFNO is a versatile device that can be used in every environment in the hospital including general wards and emergency rooms especially so when the capacity in critical care units is limited, such as the pandemic scenario. However, in low-income countries the availability of this device is low (Silva et al., 2020). The device is generally well tolerated by patients (Raoof et al., 2020, Suffredini and Allison, 2021), and guidelines and various clinical consensuses have recommended its use in clinical practice (Cinesi Gómez et al., 2020, Rochwerg et al., 2020).
In case of respiratory deterioration, a trial of APP may be considered before endotracheal intubation (Robba et al., 2021b). The aim is to implement HFNO/ APP for 12–16 h per day in 3–4 sessions depending on patient’s tolerance without delaying endotracheal intubation and mechanical ventilation if this fails or it is not tolerated. Over the last 30 years, APP has been used to improve oxygenation in ARDS, considered now standard of care (Guérin et al., 2020). The mechanisms with which it improves oxygenation varies. One theory supports the decrease of transpulmonary pressures by homogeneously distributing gravitational forces throughout the lung parenchyma rather than affecting only the lower lobes and posterior segments, thus, causing alveolar recruitment and improved ventilation of the aforementioned areas (Guérin et al., 2020). Additionally, it decreases the stress index and improves lung compliance (Guérin et al., 2020). Prone ventilation only partially changes pulmonary perfusion, but rather improves the V’/Q ‘mismatch and decreases the shunt component (Guérin et al., 2020). According to COVID-19 phenotypes, phenotype 1 in supine position presents a low V/Q with anti-gravitational distribution of pulmonary blood flow. In prone position, oxygenation can improve due to a partial redistribution of pulmonary blood flow that remains anti-gravitational. In phenotype 2, in supine position, the pulmonary blood flow is anti-gravitationally distributed and true shunt is increased, whereas in prone position, the partial redistribution of blood flow from dorsal to ventral lung regions may improve oxygenation regardless of effective alveolar recruitment. This can be also associated with decreased carbon dioxide washout (Pelosi et al., 2022). APP can be easily incorporated after the adequate training without increasing costs and can be initiated in patients that are spontaneously breathing outside the ICU (Guérin et al., 2020). Prior studies have demonstrated a clear benefit of APP in improving oxygen exchange in non-intubated patients with AHRF of different etiologies (Scaravilli et al., 2015). In SARS-CoV-2 pneumonia specifically, the APP is feasible and safe under clinical conditions (Jayakumar et al., 2021) and data has shown improved oxygenation (Coppo et al., 2020). As a result, various national societies and institutions have developed protocols and recommendations for the use of prone ventilation in COVID-19 patients. In conclusion, the available information does not detail timing and criteria to initiate APP, duration or when to discontinue, and how to safely combine this rescue strategy with HFNO. However, the rationale to implement this strategy is clearly highlighted by the literature. SM Item S5 presents a possible flowchart for HFNO/ APP initiation.
4.2. Timing, monitoring and discontinuation of HFNO/ APP
According to our systematic review, methods to assess timing of HFNO/APP were variable and poorly defined. Most studies did not report the duration of therapy, some studies applied this therapy for more than 16 h, while others for few hours but more than once/day. Therefore, there is no univocal information about methods to assess patient response to therapy nor to guide management. If the decision to use HFNO/ APP is made, the available literature suggest monitoring its failure via PaO2/FiO2, SpO2, ROX index (Prakash et al., 2021, Roca et al., 2016, Vega et al., 2022) or Single-Breath Counting Test (SBCT) evaluation (Longhitano et al., 2021). Prone positioning should be carefully monitored for possible hemodynamic instability. Other complications related to prolonged prone positioning have been reported, including bleeding of mouth and lips, nose, exit site vascular access, endobronchial, eyelid, and medical vascular displacement (Binda et al., 2021). Specific criteria based on HFNO parameters were provided by Ibarra-Estrada et al. who discontinued HFNO/ APP for FiO2 ≤ 0.4 with flow ≤ 20 L/min to maintain SpO2 greater than 90 % for 2 h (Ibarra-Estrada et al., 2020), and Kaur et al. (2021) who discontinued HFNO/ APP when achieving a FiO2 ≤ 0.4 using a flow ≤ 40 L/min. Other criteria for discontinuation were hospital discharge, intubation, death, need for non-invasive mechanical ventilation, “at provider’s discretion”, or “intolerance” (Ehrmann et al., 2021, Kucukdemirci-Kaya et al., 2022, PROFLO Study Group, 2021, Tu et al., 2020, Yang et al., 2020). Vianello et al. (2021) assessed a HFNO/ APP trial in each patient to investigate possible failure/ success of HFNO/ APP combination technique. Like timing and monitoring, also criteria for discontinuation of HFNO/ APP therapy is poorly reported by the literature and needs to be implemented. SM Item S5 presents a possible flowchart for HFNO/ APP discontinuation.
4.3. Impact of HFNO/ APP on outcome
According to our findings, there is no consensus on the potential benefits of HFNO/ APP on outcome. The literature available report contrasting findings. Ehrmann et al. (2021) reported no significant hazard for mortality. Kaur et al., (2021) found that early HFNO/ APP was associated with lower mortality rate in comparison with late HFNO/ APP (26 % vs 45 %). Jouffroy et al. (2021) found no significative reduction of mortality at 28-days in patients receiving HFNO/ APP therapy. In a post-hoc analysis of a randomized controlled trial, Kaur et al. evaluated the timing to start the awake prone position therapy. This sub-study includes 125 patients and evaluates early APP vs late APP (cut-off 24 h), all patients underwent HFNO oxygen therapy. The early APP group presented lower mortality (26 % vs 45 %, p = 0.039), despite no difference in terms of intubation rate (Kaur et al., 2021). Another recent multicentered randomized controlled trial evaluated patients with acute respiratory failure due to COVID-19 treated with HFNO oxygen therapy. They were divided in two groups those who underwent APP and those who did not. The results confirmed that APP reduces the intubation rate (30 % vs 43 %, relative risk [RR] 0.70; CI95 0.54–0.90, p = 0.006) and hospital length of stay (11 interquartile range [IQR, 9–14] vs 13 [IQR, 10–17] days, p = 0.001) (Ibarra-Estrada et al., 2022).
4.4. Limitations
This systematic review has some limitations that deserve to be addressed. First, the available literature was poor and highly heterogenous limiting the possibility of per-forming a meta-analysis. Second, data are insufficient to provide clear recommendations. Third, studies including mixed techniques other than HFNO in which individual patient data were impossible to extract after contacting the corresponding author were excluded.
5. Conclusions
In conclusion, the combined use of HFNO and APP is a valid and promising technique. Both modalities have attractive physiologic and noninvasive features that are well tolerated. With the available evidence, our systematic review was unable to provide clear recommendation on criteria for initiation, timing, monitoring, and discontinuation, and effect of HFNO/ APP on outcome. Further studies are necessary to promptly establish these principles.
Funding
None.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Daniel Agustin Godoy: Conceptualization, Methodology, Investigation, Project administration, Writing – original draft, Writing – review & editing. Yaroslava Longhitano: Data curation, Investigation, Writing – review & editing. Brigitta Fazzini: Data curation, Investigation, Writing – review & editing. Chiara Robba: Writing – review & editing. Denise Battaglini: Supervision, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing.
Conflicts of interest
None.
Acknowledgements
None.
Edited by Prof. Yu Ru Kou
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.resp.2023.104015.
Appendix A. Supplementary material
Supplementary material
.
Data availability
No data was used for the research described in the article.
References
- Agarwal A., Basmaji J., Muttalib F., Granton D., Chaudhuri D., Chetan D., Hu M., Fernando S.M., Honarmand K., Bakaa L., Brar S., Rochwerg B., Adhikari N.K., Lamontagne F., Murthy S., Hui D.S.C., Gomersall C., Mubareka S., Diaz J.V., Burns K.E.A., Couban R., Ibrahim Q., Guyatt G.H., Vandvik P.O. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can. J. Anesth. 2020;67:1217–1248. doi: 10.1007/s12630-020-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromataris, E., Munn, Z., 2020. JBI Manual for Evidence Synthesis.
- Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N. Engl. J. Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- Binda F., Galazzi A., Marelli F., Gambazza S., Villa L., Vinci E., Adamini I., Laquintana D. Complications of prone positioning in patients with COVID-19: a cross-sectional study. Intensive Crit. Care Nurs. 2021;67 doi: 10.1016/j.iccn.2021.103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G., Rossi E., Vitali L., Simonte R., Sannipoli T., Anniciello F., Vetrugno L., Bignami E., Becattini C., Tesoro S., Azzolina D., Giacomucci A., Navalesi P., De Robertis E. Effect of awake prone position on diaphragmatic thickening fraction in patients assisted by noninvasive ventilation for hypoxemic acute respiratory failure related to novel coronavirus disease. Crit. Care. 2021;25:305. doi: 10.1186/s13054-021-03735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinesi Gómez C., Peñuelas Rodríguez Ó., Luján Torné M., Egea Santaolalla C., Masa Jiménez J.F., García Fernández J., Carratalá Perales J.M., Heili-Frades S.B., Ferrer Monreal M., de Andrés Nilsson J.M., Lista Arias E., Sánchez Rocamora J.L., Garrote J.I., Zamorano Serrano M.J., González Martínez M., Farrero Muñoz E., Mediano San Andrés O., Rialp Cervera G., Mas Serra A., Hernández Martínez G., de Haro López C., Roca Gas O., Ferrer Roca R., Romero Berrocal A., Ferrando Ortola C. Recomendaciones de consenso respecto al soporte respiratorio no invasivo en el paciente adulto con insuficiencia respiratoria aguda secundaria a infección por SARS-CoV-2. Med. Intensiv. 2020;44:429–438. doi: 10.1016/j.medin.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppo A., Bellani G., Winterton D., Di Pierro M., Soria A., Faverio P., Cairo M., Mori S., Messinesi G., Contro E., Bonfanti P., Benini A., Valsecchi M.G., Antolini L., Foti G. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir. Med. 2020;8:765–774. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoule A., Vieillard Baron A., Darmon M., Beurton A., Géri G., Voiriot G., Dupont T., Zafrani L., Girodias L., Labbé V., Dres M., Fartoukh M., Azoulay E. High-flow nasal cannula in critically III patients with severe COVID-19. Am. J. Respir. Crit. Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann S., Li J., Ibarra-Estrada M., Perez Y., Pavlov I., McNicholas B., Roca O., Mirza S., Vines D., Garcia-Salcido R., Aguirre-Avalos G., Trump M.W., Nay M.-A., Dellamonica J., Nseir S., Mogri I., Cosgrave D., Jayaraman D., Masclans J.R., Laffey J.G., Tavernier E., Li J., Mirza S., Vines D., Elshafei A.A., Scott B.J., Weiss T., Kaur R., Harnois L.J., Miller A., Cerda F., Klein A., Burd J.R., Posa-Kearney K., Trump M., Jackson J., Oetting T., Greenwood M., Hazel L., Kingery L., Mogri I., Morris L., Moon J.Y., Garnett J., Jia S., Nelson K., McNicholas B., Cosgrave D., Giacomini C., Laffey J., Brennan A., Judge C., Kernan M., Kelly C., Ranjan R., Casey S., O’Connell K., Newell E., Gallagher D., Nichol A., Curley G., Estrada M.I., García-Salcido R., Vargas-Obieta A., Aguirre-Avalos G., Aguirre-Díaz S.A., Alcántar-Vallín L., Alvarado-Padilla M., Chávez-Peña Q., López-Pulgarín J.A., Mijangos-Méndez J.C., Marín-Rosales M., García-Alvarado J.E., Baltazar-González O.G., González-Guerrero M.C., Gutiérrez Ramírez P.G., Pavlov I., Gilman S., Plamondon P., Roy R., Jayaraman D., Shahin J., Ragoshai R., Kaur A., Campisi J., Dahine J., Perron S., Achouri S., Racette R., Kulenkamp A., Roca O., Pacheco A., García-de-Acilu M., Masclans J.R., Dot I., Perez Y., Bodet-Contentin L., Garot D., Ehrmann S., Mercier E., Salmon Gandonnière C., Morisseau M., Jouan Y., Darwiche W., Legras A., Guillon A., Tavernier E., Dequin P.-F., Tellier A.-C., Reignier J., Lascarrou J.-B., Seguin A., Desmedt L., Canet E., Guitton C., Marnai R., Callahan J.-C., Landais M., Chudeau N., Darreau C., Tirot P., Saint Martin M., Le Moal C., Nay M.-A., Muller G., Jacquier S., Prat G., Bailly P., Ferrière N., Thille A.W., Frat J.-P., Dellamonica J., Saccheri C., Buscot M., Plantefève G., Contou D., Roux D., Ricard J.-D., Federici L., Zucman N., Freita Ramos S., Amouretti M., Besset S., Gernez C., Delbove A., Voiriot G., Elabbadi A., Fartoukh M., Nseir S., Préau S., Favory R., Pierre A., Sement A., Terzi N., Sigaud F., Candille C., Turbil E., Maizel J., Brault C., Zerbib Y., Joret A., Daubin C., Lefebvre L., Giraud A., Auvet A., Vinsonneau C., Marzouk M., Quenot J.-P., Andreu P., Labruyère M., Roudaut J.-B., Aptel F., Boyer A., Boyer P., Lacherade J.-C., Hille H., Bouteloup M., Jeannot M., Feller M., Grillet G., Levy B., Kimmoun A. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir. Med. 2021;9:1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando C., Mellado-Artigas R., Gea A., Arruti E., Aldecoa C., Adalia R., Ramasco F., Monedero P., Maseda E., Tamayo G., Hernández-Sanz M.L., Mercadal J., Martín-Grande A., Kacmarek R.M., Villar J., Suárez-Sipmann F. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit. Care. 2020;24:597. doi: 10.1186/s13054-020-03314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes. Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman E.A., O’Kane C.M., McAuley D.F. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet. 2022;400:1157–1170. doi: 10.1016/S0140-6736(22)01439-8. [DOI] [PubMed] [Google Scholar]
- Guérin C., Albert R.K., Beitler J., Gattinoni L., Jaber S., Marini J.J., Munshi L., Papazian L., Pesenti A., Vieillard-Baron A., Mancebo J. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin C., Reignier J., Richard J.-C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., Clavel M., Chatellier D., Jaber S., Rosselli S., Mancebo J., Sirodot M., Hilbert G., Bengler C., Richecoeur J., Gainnier M., Bayle F., Bourdin G., Leray V., Girard R., Baboi L., Ayzac L. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- Ibarra-Estrada M., Li J., Pavlov I., Perez Y., Roca O., Tavernier E., McNicholas B., Vines D., Marín-Rosales M., Vargas-Obieta A., García-Salcido R., Aguirre-Díaz S.A., López-Pulgarín J.A., Chávez-Peña Q., Mijangos-Méndez J.C., Aguirre-Avalos G., Ehrmann S., Laffey J.G. Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. Crit. Care. 2022;26:84. doi: 10.1186/s13054-022-03950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Estrada M.Á., Marín-Rosales M., García-Salcido R., Aguirre-Díaz S.A., Vargas-Obieta A., Chávez-Peña Q., López-Pulgarín J.A., Mijangos-Méndez J.C., Aguirre-Avalos G. Prone positioning in non-intubated patients with COVID-19 associated acute respiratory failure, the PRO-CARF trial: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:940. doi: 10.1186/s13063-020-04882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar D., Ramachandran, DNB P., Rabindrarajan, DNB E., Vijayaraghavan, MD B.K.T., Ramakrishnan, AB N., Venkataraman, AB R. Standard care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection—a multicenter feasibility randomized controlled trial. J. Intensive Care Med. 2021;36:918–924. doi: 10.1177/08850666211014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouffroy R., Darmon M., Isnard F., Geri G., Beurton A., Fartoukh M., Tudesq J.-J., Nemlaghi S., Demoule A., Azoulay E., Vieillard-Baron A. Impact of prone position in non-intubated spontaneously breathing patients admitted to the ICU for severe acute respiratory failure due to COVID-19. J. Crit. Care. 2021;64:199–204. doi: 10.1016/j.jcrc.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Vines D.L., Mirza S., Elshafei A., Jackson J.A., Harnois L.J., Weiss T., Scott J.B., Trump M.W., Mogri I., Cerda F., Alolaiwat A.A., Miller A.R., Klein A.M., Oetting T.W., Morris L., Heckart S., Capouch L., He H., Li J. Early versus late awake prone positioning in non-intubated patients with COVID-19. Crit. Care. 2021;25:340. doi: 10.1186/s13054-021-03761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdemirci-Kaya P., Kilic I., Kaya M., Kelebek-Girgin N. Role and limitations of high-flow nasal oxygen therapy in COVID-19 patients: an observational study. Niger. J. Clin. Pract. 2022;25:1088–1093. doi: 10.4103/njcp.njcp_1646_21. [DOI] [PubMed] [Google Scholar]
- Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhou Q., Yang K., Luo Z., Chen Y. Rethinking the efficacy of awake prone positioning in COVID-19-related acute hypoxaemic respiratory failure. Lancet Respir. Med. 2022;10 doi: 10.1016/S2213-2600(22)00164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.K.-L., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhitano Y., Zanza C., Romenskaya T., Saviano A., Persiano T., Leo M., Piccioni A., Betti M., Maconi A., Pindinello I., Boverio R., Rello J., Franceschi F., Racca F. Single-breath counting test predicts non-invasive respiratory support requirements in patients with COVID-19 pneumonia. J. Clin. Med. 2021;11:179. doi: 10.3390/jcm11010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina-Tascón G.A., Calderón-Tapia L.E., García A.F., Zarama V., Gómez-Álvarez F., Álvarez-Saa T., Pardo-Otálvaro S., Bautista-Rincón D.F., Vargas M.P., Aldana-Díaz J.L., Marulanda Á., Gutiérrez A., Varón J., Gómez M., Ochoa M.E., Escobar E., Umaña M., Díez J., Tobón G.J., Albornoz L.L., Celemín Flórez C.A., Ruiz G.O., Cáceres E.L., Reyes L.F., Damiani L.P., Cavalcanti A.B., Rosso F., Moncada P.A., Carvajal S., Yara J., Jiménez A., Sotomayor A., Prieto M.I., López D., Medina C., Ángel A.M., Giraldo N., Watts F., Morell T., Revelo J., de Paz D., Villamil W., Orozco N., Rojas C.C., Martínez D.M., Sánchez Á.I., Vallecilla L., Sandoval J.A., Crispín A.M., Carvajal K., Romero L., Guarín N. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19. JAMA. 2021;326:2161. doi: 10.1001/jama.2021.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péju E., Belicard F., Silva S., Hraiech S., Painvin B., Kamel T., Thille A.W., Goury A., Grimaldi D., Jung B., Piagnerelli M., Winiszewski H., Jourdain M., Jozwiak M., COVIDPREG Study Group Management and outcomes of pregnant women admitted to intensive care unit for severe pneumonia related to SARS-CoV-2 infection: the multicenter and international COVIDPREG study. Intensive Care Med. 2022;48:1185–1196. doi: 10.1007/s00134-022-06833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P., Tonelli R., Torregiani C., Baratella E., Confalonieri M., Battaglini D., Marchioni A., Confalonieri P., Clini E., Salton F., Ruaro B. Different methods to improve the monitoring of noninvasive respiratory support of patients with severe pneumonia/ARDS due to COVID-19: an update. J. Clin. Med. 2022;11:1704. doi: 10.3390/jcm11061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Dai B., Zhao H., Wang W., Kang J., Hou H., Tan W. Comparison between high-flow nasal cannula and noninvasive ventilation in COVID-19 patients: a systematic review and meta-analysis. Ther. Adv. Respir. Dis. 2022;16 doi: 10.1177/17534666221113663. 175346662211136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins G.D., Ji C., Connolly B.A., Couper K., Lall R., Baillie J.K., Bradley J.M., Dark P., Dave C., De Soyza A., Dennis A.V., Devrell A., Fairbairn S., Ghani H., Gorman E.A., Green C.A., Hart N., Hee S.W., Kimbley Z., Madathil S., McGowan N., Messer Benjamin, Naisbitt J., Norman C., Parekh D., Parkin E.M., Patel J., Regan S.E., Ross C., Rostron A.J., Saim M., Simonds A.K., Skilton E., Stallard N., Steiner M., Vancheeswaran R., Yeung J., McAuley D.F., Duffy N., Kelly M., Concannon D., Ferguson K., McClintock D., Jha R., Krishnamurthy V., O’Farrell S., O’Kane C., Ross C., Turner R.D., Miodragovic S., Hawkins P., Welbourne J., Wells C., Lankester L., Waddy S.D., Lentaigne J., Nesbitt J., Clarke Sarah, Houghton C., O’Riordan D., Shepherd K., Turnpenny B., Joseph R., Steiner M., Rossall C., Mundin R., Boschi S., McAuley H.J.C., Russell R.J., Diver S., Elneima O., Ibrahim W., Yousuf A., Edwards S., Saim M., Hopkins B., Kelly L., Lenton D., Shackleford H., Thrasyvoulou L., Willis H., Fairbairn S., Green C., Patel Mamta, Linhartova L., Hayton E., Chue A., Collins B., Page M., Birkhamshaw E., Bellamy M., Bancroft H., Gallagher E., Antoine-Pitterson P., Jones B., Begum S., Dhani S., Crooks M., Brindle K., Faruqi S., Flockton R., Pinder E., Thackray-Nocera S., Dalemo K., Doidge J., Edwards J., Douse J., Bell S., Purewal B., Chabo C., Buckman C., Beeby D., Gray G., Francis R., Rivers V., Burton M., Innes N., Ghattas S., Rabbani R., Mahadevan Venkat, Mahadevan Venkateswaran, Green A., Burton B., Hacon C., Wilhelmsen E., Hughes P.R., Lee K., Lowsby R., Baker L., Board P., Chauhan V., Clarke Sheron, Fullerton D., Gabriel C., Houston T., Lees D., Normanton R., Pagett K., Thornley S., Wright H., McMillan A., Babores M., Lee X., Nagarajan T., Holland M., Sanctuary T., Innes R., Fletcher S., Sehgal N., Duncan T., Pooley J., Watkins E., Moudgil H., Carnahan M., Donaldson D., Rao D., Tey C.L., Linkson L., Buttle T., Vidler J., Griffiths N., Hicks A., Rupani H., Alfridi A., Barns D., Cowan E., David M., Darbyshire A., Giles B., Roberts C., Lameirinhas C., Neville D., Hossain E., Thompson F., Edwards H., Naftel J., Winter J., Burrows K., Wiffen L., Fox L., Murray L., Hawes L., Mamman M., Moon M., White M., Rowley M., Szarazova N., Gosling S., Cooper S., Baryschpolec S., Arndtz S., H-Davies Y., El Khaleq Y.A., Garner Z., Vythilingam S., Yang Y., Parekh D., Madathil S., Patel J., Bergin C., Bates M., McGhee C., Lynch D., Bhandal K., Tsakiridou K., Bamford A., Cooper L., Whitehouse T., Veenith T., Forster E., Lane S., Adams N., MacDonald S., Manan S., Lugg S., Shah P.A., McKemey E., Crowley L., Mussawar G., Gogokhia A., Gompertz S., Snelson C., Oelofse T., Wilson J., Bangash M., Huq S.S., Rauf F., Dosanjh D., Salmon N., Tengende J., Senior K.F., Cooper B., Sutton B., Woolhouse I., Crawshaw A., Thompson Richard, Glynn P., Naylor J., Alderman J., Chotalia M., Le Breuilly M., Talbot N., Packer G., Carlin C., Harvey D., Gray A., Gautam M., Welters I., Hamilton D.O., Burhan H., Hunter K., Johnston B., Lopez M., Lowe C., Mulla S., Roman J.F., Shaw D., Waite A., Waugh V., Williams K., Simonds A.K., Tatham K.C., Black E., Jhanji S., Ng Man Kwong G., Messer Ben, De-Soyza A., McAlinden P., West S.D., Anumakonda V., Dark P., McMorrow L., Marsden T., Proudfoot N., Charles B., Pendlebury J., Blackledge B., Harvey A., Knowles K., Doonan R., Lee S., Perez J., Slaughter M., Taylor M., Thomas V., Hardy E., Bakerly N., Catlow L., Majeed N., Horner D., Ali L., Hutchinson D., Fuller L., Dodd J., Bhatnagar R., Clive A., Adamali H., Bibby A., Higbee D., Welch H., Gendall E., Staddon L., Morley A., Clarke Sam, Smith K., Perry E., Rippon N., Jennings L., Solomon L., Alloway K., Lee H., Sandrey V., Bradburn K., Milne A., Goff E., Williams R., Ahmed M., Bloch S., Zaki A., Roy A., Rostron A., Woods L., Wakinshaw F., Bainbridge P., Hersey P., Carpenter M., Leech C., O’Connor L., Morrison A., Rodgers E., McAndrew P., Lear G., Coates J., Richardson M., Smith D., Green W., Murray S., Pennington C., De Wong H., Land D., Wheeler H., Harvey M., Watson M., Brown M., Irving B., Bigg J., Felongco M., Mackenzie J., Dhasmana D., Thompson Rob, Lui P., Adam F., Davey F., Penman J., McGregor A., Cochrane P., Shalan K., Bozic W., Brown J., Carey J., Daffern C., Dight E., Gane M., Ghuman B., Grummett J., Guck J., Hamilton Louisa, Hill C., Hill M., Muthiah C., Padfield E., Rai J., Raynes K., Scott G., Stimpson E., Strickland N., Willis A., Wood J., Attwood B., Atwal I., Parsons P., Vancheeswaran R., Konda S., Myint Y.M.M., Mehta M., Muhammad A., Navarro A., Rochester A., Sundayi S., Patel Manish, Smith A., Stewart C., Tate M., McGarry E., Pearson C. (Rebecca), Walsh B., Glass L., Black K., Clements S., Boyle R., MacDonald C., Hamilton Leigh, Moreland G., Hamill R., Reddy H., Smuts S., Bentley A. Vol. 327. 2022. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19; pp. 546–558. (JAMA). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash J., Bhattacharya P.K., Yadav A.K., Kumar A., Tudu L.C., Prasad K. ROX index as a good predictor of high flow nasal cannula failure in COVID-19 patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. J. Crit. Care. 2021;66:102–108. doi: 10.1016/j.jcrc.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian E.T., Gatto C.L., Amusina O., Dear M.L., Hiser W., Buie R., Kripalani S., Harrell F.E., Freundlich R.E., Gao Y., Gong W., Hennessy C., Grooms J., Mattingly M., Bellam S.K., Burke J., Zakaria A., Vasilevskis E.E., Billings F.T., Pulley J.M., Bernard G.R., Lindsell C.J., Rice T.W., Vanderbilt Learning Healthcare System Platform Investigators Assessment of awake prone positioning in hospitalized adults with COVID-19: a nonrandomized controlled trial. JAMA Intern. Med. 2022;182:612–621. doi: 10.1001/jamainternmed.2022.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoof S., Nava S., Carpati C., Hill N.S. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 With respiratory failure. Chest. 2020;158:1992–2002. doi: 10.1016/j.chest.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Ball L., Pelosi P., Rocco P.R.M. Ten things you need to know about intensive care unit management of mechanically ventilated patients with COVID-19. Expert Rev. Respir. Med. 2021;15:1293–1302. doi: 10.1080/17476348.2021.1906226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Ball L., Pelosi P., Rocco P.R.M. Ten things you need to know about intensive care unit management of mechanically ventilated patients with COVID-19. Expert Rev. Respir. Med. 2021 doi: 10.1080/17476348.2021.1906226. 17476348.2021.1906226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Pelosi P., Rocco R.M.P. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020;14:865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca O., Messika J., Caralt B., García-de-Acilu M., Sztrymf B., Ricard J.-D., Masclans J.R. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J. Crit. Care. 2016;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Rochwerg B., Einav S., Chaudhuri D., Mancebo J., Mauri T., Helviz Y., Goligher E.C., Jaber S., Ricard J.-D., Rittayamai N., Roca O., Antonelli M., Maggiore S.M., Demoule A., Hodgson C.L., Mercat A., Wilcox M.E., Granton D., Wang D., Azoulay E., Ouanes-Besbes L., Cinnella G., Rauseo M., Carvalho C., Dessap-Mekontso A., Fraser J., Frat J.-P., Gomersall C., Grasselli G., Hernandez G., Jog S., Pesenti A., Riviello E.D., Slutsky A.S., Stapleton R.D., Talmor D., Thille A.W., Brochard L., Burns K.E.A. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46:2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén J., von Oelreich E., Fors D., Jonsson Fagerlund M., Taxbro K., Skorup P., Eby L., Campoccia Jalde F., Johansson N., Bergström G., Frykholm P., PROFLO Study Group Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit. Care. 2021;25:209. doi: 10.1186/s13054-021-03602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaravilli V., Grasselli G., Castagna L., Zanella A., Isgrò S., Lucchini A., Patroniti N., Bellani G., Pesenti A. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J. Crit. Care. 2015;30:1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Schmid B., Griesel M., Fischer A.-L., Romero C.S., Metzendorf M.-I., Weibel S., Fichtner F. Awake prone positioning, high-flow nasal oxygen and non-invasive ventilation as non-invasive respiratory strategies in COVID-19 acute respiratory failure: a systematic review and meta-analysis. J. Clin. Med. 2022;11:391. doi: 10.3390/jcm11020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.B., Leite F.S., Colucci E., Eid R.A.C., Timenetsky K.T. In: A40. Critical Care: From HFNC TO ECMO. American Thoracic Society; 2020. High flow nasal Cannula in a low-income country: experience of a single center in Brazil. [DOI] [Google Scholar]
- Suffredini D.A., Allison M.G. A rationale for use of high flow nasal Cannula for select patients with suspected or confirmed severe acute respiratory syndrome coronavirus-2 infection. J. Intensive Care Med. 2021;36:9–17. doi: 10.1177/0885066620956630. [DOI] [PubMed] [Google Scholar]
- Tu G.-W., Liao Y.-X., Li Q.-Y., Dong H., Yang L.-Y., Zhang X.-Y., Fu S.-Z., Wang R.-L. Prone positioning in high-flow nasal cannula for COVID-19 patients with severe hypoxemia: a pilot study. Ann. Transl. Med. 2020;8:598. doi: 10.21037/atm-20-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega M.L., Dongilli R., Olaizola G., Colaianni N., Sayat M.C., Pisani L., Romagnoli M., Spoladore G., Prediletto I., Montiel G., Nava S. COVID-19 pneumonia and rox index: time to set a new threshold for patients admitted outside the ICU. Pulmonology. 2022;28:13–17. doi: 10.1016/j.pulmoe.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianello A., Turrin M., Guarnieri G., Molena B., Arcaro G., Turato C., Braccioni F., Bertagna De Marchi L., Lionello F., Subotic P., Masiero S., Giraudo C., Navalesi P. Prone positioning is safe and may reduce the rate of intubation in selected COVID-19 patients receiving high-flow nasal oxygen therapy. J. Clin. Med. 2021;10:3404. doi: 10.3390/jcm10153404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Wang T., Qin X., Jie Y., Zha L., Lu W. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit. Care. 2020;24:250. doi: 10.1186/s13054-020-02991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.S., Lipes J., Dial S., Schwartz B., Laporta D., Wong E., Baldry C., Warshawsky P., McMillan P., Hornstein D., de Marchie M., Jayaraman D. Outcomes and clinical practice in patients with COVID-19 admitted to the intensive care unit in Montréal, Canada: a descriptive analysis. C Open. 2020;8:E788–E795. doi: 10.9778/cmajo.20200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
No data was used for the research described in the article.