Abstract
Lyme arthritis is the most common complication following infection of human individuals with Borrelia burgdorferi sensu stricto. In mice, B. burgdorferi infection leads to arthritis of the tibiotarsal joints. Arthritis severity in mice is under host genetic control, as BALB/c mice developed mild arthritis but C3H/He mice developed severe disease following B. burgdorferi infection. To study the role of gamma interferon (IFN-γ) in arthritogenesis, targeted mutant mice lacking the IFN-γ receptor (IFN-γR) were infected by inoculation with B. burgdorferi. IFN-γR−/− and parental 129/SvEv mice developed mild arthritis of similar severity, as determined both by weekly tibiotarsal joint measurements and histopathology at 2 and 5 weeks postinfection. Both strains of mice had the same spirochetal burden in the joints, suggesting that the IFN-γR−/− mice were not impaired in controlling spirochetal expansion in vivo. The wild-type mice mounted a Th1 response, with a predominance of CD4+ IFN-γ+ T cells observed by flow cytometry. In contrast, the IFN-γR−/− mice mounted a Th2 response, with a predominance of CD4+ IL-4+ T cells. As expected given their cytokine profile, the IFN-γR−/− mice produced fewer CD8+ IFN-γ+ and MAC-1+ IL-12+ cells and less immunoglobulin G2a (IgG2a) than their wild-type counterparts. These results strongly suggest that IFN-γ is not required for arthritis resistance or as part of an effective immune response against B. burgdorferi.
Adaptive responses of T and B cells function primarily to control Borrelia burgdorferi expansion and only indirectly influence Lyme arthritis severity in the mouse. The natural reservoir for B. burgdorferi in the northeastern United States, Peromyscus leukopus, does not develop any signs of Lyme infection (25). However, various laboratory mice, including both immune-deficient and competent strains, developed signs following tick transmission of or inoculation with B. burgdorferi (4, 5, 35). These signs included periarticular edema, arthritis, and carditis. Arthritis severity is under host genetic control (45). C57BL/6J mice were very arthritis resistant, BALB/cJ (BALB/c) mice developed mild arthritis, and C3H/HeJ (C3H) mice developed severe arthritis (4, 5). The magnitudes and types of immune responses elicited were important determinants of disease pathogenesis. BALB/c congenic immunodeficient C.B-17-scid and C3H/HeSnSmn-scid mice developed severe arthritis, high spirochete burdens in the joint, and Lyme carditis following infection, demonstrating that adaptive antigen-specific T- and B-cell immune responses are protective in BALB/c and C3H mice (8, 10, 35). It was first suggested that inflammatory Th1 responses were pathogenic. C3H mice developed a Th1 immune response, and when this response was switched by administration of anti-gamma interferon (anti-IFN-γ) antibody, the spirochete burdens and disease severity were significantly reduced (22, 30). However, administration of anti-interleukin-12 (anti-IL-12) to C3H mice in doses that significantly suppressed IFN-γ production only slightly reduced acute arthritis and had little or no effect on joint edema (1). Spirochete burdens were significantly higher in the anti-IL-12-treated mice, suggesting that blockade of IL-12 weakened spirochete control by the immune system. Targeted mutant C3H IFN-γ−/− mice remained susceptible to severe arthritis (9). The majority of evidence for C3H mice suggests that IFN-γ is not responsible for arthritis susceptibility. In BALB/c mice arthritis resistance was linked to development of a Th2 immune response (22, 30). However, a protective function for IFN-γ in resistant mice was not ruled out.
Several independent studies have now demonstrated that IFN-γR−/− mice are immune competent and able to mount inflammatory immune responses in both the experimental allergic encephalomyelitis and collagen-induced arthritis models (19, 28, 34, 44, 46). IFN-γR−/− mice were more susceptible to Leishmania major (41). In contrast, our results demonstrate that IFN-γR−/− mice were able to control spirochete expansion as efficiently as their wild-type counterparts, with no change in arthritis resistance, in the context of a switch to a predominant Th2 response.
MATERIALS AND METHODS
Mice.
129-ifngrtml (IFN-γR−/−) mice (19) and wild-type control 129/SvEv mice were provided by Michel Aguet (Institute of Molecular Biology I, University of Zurich, Zurich, Switzerland), and mice were bred and housed in the barrier containment facility (BL-3) of the Division of Laboratory Animal Medicine, New England Medical Center, Boston, Mass. It is important to note that the 129/SvEv mice were not the contaminated, inbred, commercially available, 129/SvEvTac mice (36). BALB/cJ and C3H/HeJ mice were obtained from the Jackson Laboratory. All experimental protocols involving mice were reviewed and approved by the Institutional Review Board of the New England Medical Center.
B. burgdorferi infection.
Stephen Barthold (Yale University, New Haven, Conn.) kindly provided the N40 isolate of B. burgdorferi (7). Low-passage B. burgdorferi N40 organisms were frozen into single-experiment aliquots in Barbour-Stoenner-Kelly (BSK) II medium with 30% glycerol (Sigma Chemical Co., St. Louis, Mo.). Infections were performed as described previously (13). Briefly, frozen B. burgdorferi was thawed rapidly, transferred into 10 volumes of BSK II-medium, and cultured at 32°C overnight prior to the enumeration of motile spirochetes by dark-field microscopy. Motility, a minimal estimate of viability, was consistently greater than 75%. Five- to 6-week-old mice of an individual litter were divided into two groups. Mice in the first group were inoculated in the right hind footpad with 2 × 104 motile spirochetes in 50 μl of BSK II medium, and the remaining mice were mock infected with BSK II medium alone. Fifteen litters of mice were used for the reported experiments.
Culture of B. burgdorferi from tissue.
Infection was tested at the time of sacrifice by culture of ear punch biopsy material, joint tissue, or peripheral blood in BSK II medium. Infection was confirmed for a majority of B. burgdorferi-infected mice (range, 70 to 90%) in each experiment. Contaminated cultures were discarded and thus infection status was not confirmed by culture for some animals. Spirochetes from joint cultures were counted on culture day 7 using a Petroff-Hausser counting chamber (C. A. Hausser & Son, Philadelphia, Pa.). The initial number of spirochetes in the joint was extrapolated by comparison with counts from control 7-day cultures inoculated initially with 10-fold serially diluted (107 to 102) live spirochetes (23). Negative cultures were reexamined on days 10, 14, and 21.
Ankle measurement and histopathology.
Tibiotarsal joints were measured on a weekly basis with a spring-loaded microcaliper (Federal, Providence, R.I.). Tibiotarsal joints were surgically removed from infected mice after sacrifice and immediately fixed in 10% formalin (Sigma Chemical Co.). Thin section slides from decalcified and paraffin-embedded joints were prepared and stained with hematoxylin and eosin in the Department of Pathology, Tufts University School of Veterinary Medicine (Grafton, Mass.). Slides were numbered sequentially as sections were made, and this coding system was maintained until after the scoring was completed. Sections were examined microscopically for infiltrating cells and morphology. Based primarily on the amount of mononuclear cell infiltrate in synovium but also on other signs of arthritis, including synovial hypertrophy, tendonitis, and cartilage thickening, an integer score of 0 (no infiltrating cells were observed), 1 (one or several small and discrete areas of infiltrate), 2 (multiple infiltrates without other gross changes), or 3 (heavy contiguous infiltrates in joints, tendons, and muscle) was subjectively assigned to each sample. Each slide was scored by two investigators (J.D. and L.G.) independently, and the final score for each sample was the average of the two. If the independent scores for a given sample differed by more than one, both investigators reexamined the slide and one or both changed their scores to reduce the difference to one. Only six slides (6%) had to be rescored in this way. After all scoring and reconciliation was completed, a third investigator (M.E.) uncoded the results and calculated the group means. Data from culture-negative and unconfirmed mice in the infected group have been included in the figures and tables of the present paper.
Preparation of B. burgdorferi antigen.
B. burgdorferi soluble antigen was prepared from a 500-ml culture of spirochetes (109 spirochetes/ml). After centrifugation for 20 min at 15°C at 12,000 × g (10,000 rpm in an SS-34 rotor), the spirochete pellet was resuspended in 5 ml of phosphate-buffered saline (PBS) with Mg2 (Sigma Chemical Co.). The suspension was sonicated three times for 2 min each time (at 15-second intervals), on ice, at a cycle level of 50% on a Sonifier 450 (Branson Sonic Power Company, Danbury, Conn.). The sonicate was centrifuged again as described above and the supernatant of soluble antigens was removed and stored in aliquots at −70°C after being filtered through a 0.45-μm-pore-size filter (Millipore, Bedford, Mass.).
Antigen-specific recall response in vitro.
Draining lymph nodes were harvested from mice 10 to 14 days postinfection, at the onset of measurable arthritis (Fig. 1). Irradiated spleen cells (1,700 to 2,200 rad; 107 cells/ml) from mock-infected syngeneic mice were pulsed with B. burgdorferi soluble antigen (final concentration, 35 μl/ml) for 60 min and washed twice with complete RPMI medium (RPMI 1640 with 10% heat-inactivated fetal calf serum [Sigma Chemical Co.], 20 mM HEPES, 1 mM sodium pyruvate, a 1× concentration of nonessential amino acids, 2 mM l-glutamine, and 20 μg of gentamicin per ml [all constituents were obtained from BioWhittaker, Walkersville, Md.]) before use as antigen-presenting cells. Single-cell suspensions from pooled lymph nodes were washed twice with complete RPMI medium and plated at 5 × 105 cells/well in 96-well plates in the presence or absence of 106 antigen-pulsed irradiated antigen-presenting cells. After 4 to 10 days of in vitro restimulation, cells were activated for the final 5 h of culture with soluble anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) in the presence of 3 μM monensin (Sigma Chemical Co.) to retard cytokine export from the Golgi body.
FIG. 1.
Tibiotarsal joint diameters. IFN-γR−/− (RKO) mice developed arthritis similar to that of 129/SvEv mice following B. burgdorferi infection. Tibiotarsal joints of 129/SvEv and IFN-γR−/− mice were measured with calipers at weekly intervals after infection with B. burgdorferi (i) or injection with BSK II media. Each data point represents the mean sum of diameters for right and left hind joints for a minimum of 5, to a maximum of 24, mice (males and females). Standard deviations were greater for infected (0.02 to 0.03 mm) than for mock-infected (0.01 to 0.02 mm) mice (data not shown). IFN-γR−/− mice showed a significantly greater mean increase in diameter compared to 129/SvEv mice at day 7 only (P < 0.002). The infected mice showed significantly greater mean increases in diameter than the control mice for both strains on days 14 (P < 0.0004 for 129/SvEv mice; P < 0.0001 for IFN-γR−/− mice), 21 (P < 0.0002 for 129/SvEv mice; P < 0.0001 for IFN-γR−/− mice), 28 (P < 0.03 for 129/SvEv mice; P < 0.008 for IFN-γR−/− mice), and 35 (P < 0.003 for 129/SvEv mice; P < 0.0001 for IFN-γR−/− mice), but not day 7.
Antibodies.
Flow cytometry staining buffer I (FSB-I) consisted of PBS (BioWhittaker) with 1% heat-inactivated fetal calf serum (Sigma Chemical Co.) and 0.1% NaN3 (Sigma Chemical Co.), and FSB-II was PBS with 1% heat-inactivated fetal calf serum and 0.1% saponin (Sigma Chemical Co.). Cychrome–anti-CD4 (RM4-5) (working dilution, 1:100 in FSB-I), cychrome–anti-CD8 (53-6.7) (working dilution, 1:100 in FSB-I), phycoerythrin (PE)–anti-IL-4 (11B11) (working dilution, 1:50 in FSB-II), fluorescein isothiocyanate (FITC)–anti-IFN-γ (R4 6A2) (working dilution, 1:200 in FSB-II), PE–anti-IL-10 (JES5-16E3) (working dilution, 1:50 in FSB-II), PE–anti-IL-12 (C15.6) (working dilution, 1:50 in FSB-II), PE–anti-tumor necrosis factor alpha (TNF-α) (MP6-XT22) (working dilution, 1:50 in FSB-II), anti-CD3 (145.2C11), anti-CD28 (37.51), and Fc block (anti-CD16/32 [2.4G2]) were all purchased from PharMingen (San Diego, Calif.). FITC–anti-CD11b (MAC-1) (working dilution, 1:100 in FSB-I) was kindly provided by Henry Wortis (Tufts University School of Medicine, Boston, Mass.).
Intracellular cytokine analysis by flow cytometry.
Cells were washed in FSB-I and incubated for 5 min with Fc block at 4°C. These cells were then stained conventionally for cell surface markers (cychrome–anti-CD4, Cychrome–anti-CD8, and FITC–anti-MAC-1) and fixed in 4% paraformaldehyde (Sigma Chemical Co.) in PBS for 20 min. Fixed cells were washed and permeabilized with FSB-II and then stained with anti-cytokine antibodies. Cells stained in this manner were analyzed on a FACScan apparatus using LYSIS II software (Becton Dickinson, San Jose, Calif.). An analysis gate was limited to large activated blasts.
B. burgdorferi antigen-specific enzyme-linked immunosorbent assay (ELISA).
Flat-bottom microplates (96-well Immulon no. 1 plate; Dynatech Laboratories, Chantilly, Va.) were coated with 5 μg of soluble B. burgdorferi antigen sonicate per ml in coating buffer (50 mM sodium bicarbonate [Sigma Chemical Co.], 2 mM NaN3 [pH 9.6]) overnight at 4°C and then blocked with blocking buffer (PBS, 0.05% Tween 20 [Bio-Rad, Hercules, Calif.], 5% dried nonfat milk [pH 7.6; SACO Foods, Inc., Middleton, Wis.]) for 45 min at 37°C. Serum samples from individual mice were serially diluted with blocking buffer and incubated in the antigen-coated plates for 45 min at 37°C. Plates were washed with rinse buffer (PBS with 0.05% Tween 20). Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin M (IgM), IgG, IgG1, or IgG2a (all from Southern Biotechnology Associates, Birmingham, Ala.) were diluted 1:1,000 with blocking buffer and used as secondary antibodies; plates were incubated and washed as described above. Two hundred microliters of a solution containing 1-mg/ml pNpp and 8 μM ZnCl2 dissolved in alkaline buffer (0.1 M glycine, 1 mM MgCl2, 3 mM NaN3, [pH 10.5]; all reagents from Sigma Chemical Co.) were added to each well of the microtiter plate and incubated at 37°C for 1 to 5 min. Microplates were read with a microplate reader (model 550; Bio-Rad) at 405 nm. Titer was calculated by extrapolation from the linear regression of the absorbance from the 1:400, 1:2,000, and 1:10,000 dilutions of each serum sample. The titer was defined as the point where this line crossed that of the mean plus three standard deviations of a pool of negative control sera.
Statistical analysis.
Means of groups were compared using Student's t test (29). Probability (P) values that were less than or equal to 0.05 were considered significant.
RESULTS
Absence of the IFN-γ receptor (IFN-γR) did not change arthritis.
Mice were infected with B. burgdorferi sensu stricto and monitored for development of arthritis by weekly tibiotarsal joint measurements, and histopathological examination of joint tissue from mice sacrificed at 2 and 5 weeks postinfection. Increases in tibiotarsal joint diameter have been correlated with severe arthritis, although they do not directly demonstrate joint changes and primarily indicate edema and swelling of the tissues surrounding the joint itself (1, 22). Infected mice of both the IFN-γR−/− and parental 129/SvEv strains had significantly more arthritis than control mice, as indicated by the tibiotarsal diameters measured on days 14, 21, 28, and 35 and the arthritis scores for day 14 (129 only) and day 35 (Fig. 1; Table 1). The IFN-γR−/− mice developed measurable arthritis slightly faster than the 129/SvEv mice (at day 7), suggesting that IFN-γ or downstream effectors may have enhanced early immunity (Fig. 1). Overall, IFN-γR−/− mice developed arthritis of equal maximum severity as the parental 129/SvEv strain (Fig. 1). The arthritis in these strains was comparable to that observed in BALB/c mice but less severe than that seen in C3H mice (Fig. 1). Histopathological examination of joint tissue also demonstrated no significant difference between arthritis in the IFN-γR−/− mice and that in the 129/SvEv mice at either 2 or 5 weeks postinfection (Table 1).
TABLE 1.
Histopathological scoring of arthritis in B. burgdorferi-infected 129/SvEv and IFN-γR−/− mice
| Mouse strain | Week no. | Arthritis severity (n)a
|
|
|---|---|---|---|
| BSK | B. burgdorferi | ||
| 129/SvEv | 2 | 0.4 ± 0.5 (4) | 1.4 ± 0.8 (9)b |
| IFN-γR−/− | 2 | 0.8 ± 1.1 (2) | 2.0 ± 0.5 (3) |
| 129/SvEv | 5 | 1.3 ± 0.5 (6) | 2.3 ± 0.7 (14)b |
| IFN-γR−/− | 5 | 1.0 ± 0.7 (10) | 1.8 ± 0.8 (14)b |
Values are means ± standard deviations for histopathology scores of mock-infected (BSK) and infected (B. burgdorferi) mice. Arthritis scores ranged from 0 (no infiltrates or changes) to 3 (severe infiltrates with other joint changes). n, number of mice.
Significant difference (P < 0.05) from control value for same mouse strain and duration as calculated by Student's t test. Results reported for a single short-term experiment (litter) with IFN-γR−/− mice. The data suggest that the results might have been significant for this group had more mice been measured. Other groups include results for 2 to 4 experiments (litters).
IFN-γR−/− mice controlled spirochete growth.
Joints of infected mice were inoculated into BSK II medium at 2 weeks postinfection. Serial dilutions of spirochetes of known concentrations were inoculated into BSK II medium in parallel with the joint cultures. Cultures were checked on days 7 and 10, and viable (motile) spirochetes were counted. The number of spirochetes in the joints on day 0 was extrapolated from a standard curve prepared from the spirochete counts from the serially diluted cultures. There was no significant difference between the spirochete burden from the 129/SvEv (3.34 ± 0.27 log10 spirochetes/joint; n = 5) and IFN-γR−/− (3.26 ± 0.08 log10 spirochetes/joint; n = 7) mice (Table 2). The IFN-γR−/− mice controlled growth of organisms in the joints as well as wild-type mice.
TABLE 2.
TNF-α production by mononuclear cells in B. burgdorferi-infected 129/SvEv and IFN-γR−/− mice and mock-infected IFN-γR−/− mice
| Mouse strain | Cytokine-positive cells (%)a
|
||
|---|---|---|---|
| TNF+ | CD4+ TNF+ | IFN-γ+ TNF+ | |
| 129/SvEvb | 15.9 | 12.2 | 4.3 |
| IFN-γR−/−b | 13.9 | 12.0 | 2.1 |
| IFN-γR−/−c | 7.6 | 8.3 | 1.8 |
Percent blast cells on culture day 5 calculated from results of flow cytometry.
B. burgdorferi-infected mice.
Mock-infected mice.
Th2 response made by IFN-γR−/− mice.
It has been recognized for some time that naive CD4+ T cells differentiate upon activation into inflammatory (Th1) or helper (Th2) effectors. In experiments in which anti-IFN-γ reduced arthritis severity in C3H mice, this treatment also switched the immune response to a Th2 profile, defined as enhanced production of IL-4 (22). Arthritis resistance has been associated with Th2 effectors late in disease (21), as well as with high innate inflammatory responses and Th1 cells early in disease (13, 21, 51). The Th response phenotype was defined by determining the relative abundance of antigen-specific effector cells producing IL-4 (Th2) or IFN-γ (Th1). The draining lymph nodes were removed from mice 10 to 14 days postinfection, at the peak of the primary immune response, and the mononuclear cells were restimulated in vitro for 4 to 10 days with soluble B. burgdorferi antigen-pulsed irradiated syngeneic spleen cells as antigen-presenting cells. Few cells from mock-infected mice survived and proliferated under these conditions (data not shown). The T cells were then stimulated to produce and accumulate intracellular cytokine with a short, 5-h pulse with anti-CD3 and anti-CD28 in the presence of monensin, as described previously (13). These cells were then subjected to flow cytometry for the surface T-cell (CD4 or CD8) or macrophage (MAC-1) phenotype, and intracellular cytokine production (IL-4, IFN-γ, IL-10, TNF-α, IL-12). Analysis of CD4+ T cells showed clear evidence that, while the 129/SvEv mice produced a predominant Th1 response, the IFN-γR−/− mice produced a predominant Th2 response (Fig. 2). Loss of the IFN-γR enhanced production of IL-4, which was likely responsible for the diminished production of IFN-γ (Fig. 2). The skewed pattern was present after 4 days of culture, the earliest time point tested (Fig. 2B).
FIG. 2.
Flow cytometry of CD4+ T cells. CD4+ T cells producing IL-4 predominated in the IFN-γR−/− mice, while those producing IFN-γ predominated in the 129/SvEv mice at all time points tested. Mice were sacrificed 10 days postinfection and LN cells restimulated in vitro with B. burgdorferi antigen-pulsed irradiated syngeneic spleen cells. At the indicated times cells were stained for CD4, IL-4, and IFN-γ and analyzed by flow cytometry. (A) Log10 fluorescence of blast cells on day 8 is presented for a representative experiment of three; (B) Percent blast cells with the Th2 (CD4+ IL-4+) or Th1 (CD4+ IFN-γ+) phenotype for 129/SvEv and IFN-γR−/− mice, calculated from results of flow cytometry, as a function of time in vitro.
Despite the apparent switch from a predominance of Th1 effectors in the 129/SvEv mice to a predominance of Th2 effectors in the IFN-γR−/− mice, it was possible that other cell types, such as CD8+ T cells or macrophages, were present and producing cytokines characteristic of a Th1 response. The 129/SvEv mice had significantly more CD8+ IFN-γ+ T cells than the IFN-γR−/− animals (Fig. 3). Macrophages, including those producing IL-12, were present in small numbers but were more frequent in the 129/SvEv mice (Fig. 3). Taken together with the CD4 results, these criteria suggest that the IFN-γR−/− mice generated a Th2 rather than a Th1 response to infection with B. burgdorferi. Both Th responses were associated with arthritis resistance.
FIG. 3.
Flow cytometry of CD8+ T cells and macrophages. Levels of CD8+ cells producing IFN-γ and macrophages producing IL-12 were higher in the 129/SvEv than IFN-γR−/− mice. Restimulated cultures of mock-infected mice had few cells, and cultures from 129/SvEv and IFN-γR−/− mice generated approximately equivalent numbers of IL-12+ MAC-1+ cells (0.7% and 0.5%, respectively). Results presented are for culture day 8 and are from an experiment representative of three.
Anti-B. burgdorferi IgG2a production impaired in IFN-γR−/− mice.
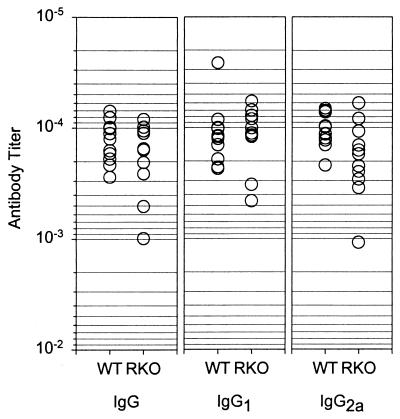
A final criterion for determining the predominance of the Th1 phenotype is the functional effect of the CD4+ effectors on antibody production. Class switching in the mouse, in particular, production of IgG2a, is dependent in part on IFN-γ (37). At 2 weeks postinfection, both strains of mice produced equivalent amounts of B. burgdorferi-specific IgM, and IgG levels were generally below the limit of detection of our ELISA assay (data not shown). We therefore examined the B. burgdorferi specific immunoglobulin in serum in both mouse strains 5 weeks postinfection. As predicted by their genetic deficit, many IFN-γR−/− mice had impaired production of IgG2a compared to the 129/SvEv mice (Fig. 4). Consistent with the apparent enhancement of IL-4 producing CD4+ Th cells in these mice (Fig. 2), there was a slight increase in the IgG1 titers. Total IgG titers were diminished in some animals (Fig. 4). This suggests that the IFN-γR−/− mice generated a weak Th2 immune response.
FIG. 4.
B. burgdorferi-specific antibody titers in infected IFN-γR−/− (RKO) and 129SvEv (WT) mice. There was a slight increase in IgG1 and a decrease in IgG2a in IFN-γR−/− mice compared with 129SvEv mice. Serum from mice sacrificed 5 weeks postinfection was diluted and used to detect B. burgdorferi antigen. The titer was calculated from the linear regression of three dilutions using as a cutoff the mean plus three standard deviations of the results for the negative controls. Each symbol represents one mouse.
Production of IL-10 and TNF-α equivalent in both mouse strains.
One of the major proinflammatory cytokines associated with arthritis in murine models and in human disease is TNF-α (20, 27, 32, 42, 47, 48). A large number of TNF-α+ cells, of which most were CD4+ TNF-α+ T cells, were identified in B. burgdorferi-stimulated cultures from infected mice of both strains (Table 2). In fact, a number of dual producing cells was observed. TNF-α production was observed in restimulated cultures from mock-infected IFN-γR−/− mice; values were slightly lower than those observed in cultures from infected mice. We also noted a similar number of IL-10+ cells in both 129/SvEv (10.9%) and IFN-γR−/− (11.3%) mouse strains, irrespective of the underlying Th response. IL-10 production was absent in restimulated cultures of mock infected 129/SvEv mice (1.1%). IL-10 generally has a strong inhibitory effect on Th1 cells and macrophages (15, 18, 26, 33).
DISCUSSION
Transmission of B. burgdorferi to humans by infected Ixodes ricinus complex ticks leads to a range of symptoms, from self-resolving erythema migrans with no sequelae to antibiotic treatment-resistant Lyme arthritis (39, 40). The observed variation reflects both differences in pathogen (12, 43) and differences in host susceptibility to infection and pathogenesis. For example, although even patients who developed severe destructive Lyme arthritis usually responded to antibiotic treatment, up to 10% of such patients had arthritis which continued for more than 3 months following 2 months of oral antibiotics and/or 1 month of intravenous antibiotic therapy (40). Some of these individuals were shown to possess a major histocompatibility complex molecule, DRB1∗0401 (38), which was capable of presenting both a peptide of B. burgdorferi OspA and a cross-reactive self-peptide, human LFA-1α332–340 (16). DRB1∗0401-positive individuals were thus at risk for developing a more severe disease course. Other risk factors for Lyme arthritis remain to be identified.
Arthritis has been associated with a proinflammatory cytokine milieu (49, 50). In a study of patients with Lyme arthritis, the Th1 to Th2 ratio correlated directly with arthritis severity, such that the higher the ratio, the greater the joint swelling (17). On the other hand, inflammatory immune responses were shown to be important for controlling infection, as demonstrated in the murine Lyme disease model. For example, genetically resistant mice with the beige mutation, which limits cell-mediated immunity, developed severe arthritis following B. burgdorferi infection (6). For C57BL/6 and DBA/2 strains, arthritis resistance was shown to be under the control of the innate immune system and independent of spirochete control by T and B cells (10). In C3H mice, in contrast, both innate and acquired immune responses failed to control spirochetes and mice developed severe disease (8, 10). BALB/c mice were shown to be unique in that their innate immune systems failed to control spirochetes, but they generated a robust T- and B-cell-mediated response. Thus the genetically related C.B-17 mice were inherently arthritis susceptible, as seen for C.B-17-scid mice, but T and B cells controlled spirochete expansion, carditis, and arthritis except in cases of extreme challenge (8, 10, 35). In BALB/c mice, arthritis resolution, but not carditis resolution or reduced spirochetemia, was induced by a humoral response to the B. burgdorferi arthritis-resolving protein (Arp) (14).
IFN-γ is produced by T cells and affects both T cells and macrophages. The role of IFN-γ in Lyme disease has been studied extensively in C3H mice, in which it was initially thought that pathogenic Th1 responses contributed to arthritis (22, 30). However, elimination of IFN-γ at the gene level in C3H mice had no effect on B. burgdorferi burden or arthritis severity (9). This ruled out a requirement for IFN-γ in pathogenesis. In fact the preponderance of evidence suggests that inflammatory responses are protective in mice infected with B. burgdorferi. In C3H-scid mice, blocking antibodies directed against the proinflammatory cytokine IL-12 limited innate responses, reduced spirochete control, and exacerbated arthritis severity (2). BALB/c mice generated a larger early Th1 response, and this correlated with arthritis resistance (21, 51).
Until the present study was undertaken, the role of IFN-γ had not yet been studied in genetically arthritis-resistant mice (9, 22), which left open the possibility of a protective role for this cytokine. In the present study, IFN-γR−/− mice had no gross defect in spirochete control, as determined by culture of organisms from joints of infected mice. This argues against a requirement for IFN-γ in immunity to B. burgdorferi in genetically arthritis-resistant mice. Loss of function also did not change arthritis susceptibility. This demonstrates that there is no protective requirement for IFN-γ in the murine Lyme disease model.
B. burgdorferi soluble antigens activated a large number of TNF-α-producing T cells in the 129/SvEv and IFN-γR−/− mice; TNF-α might have promoted arthritis in the presence and absence of IFN-γ (20, 27, 32, 42, 47, 48). In addition, a large number of IL-10-producing T cells were activated by B. burgdorferi antigen. Elimination of IL-10 in genetically resistant C57BL/6 mice enhanced spirochete control but nonetheless exacerbated arthritis (11). In vitro studies of human synovial infiltrating leukocytes showed that IL-10 suppressed production of inflammatory cytokines and possibly functionally inhibited synovial fluid macrophages (18). IL-10 may be produced as an anti-inflammatory cytokine (15, 18, 26) in a negative feedback response to TNF-α or other proinflammatory molecules. IL-10 has also been implicated in disregulated autoimmunity (3, 24, 31, 33).
In the murine L. major model, IFN-γR−/− mice were more susceptible to infection than wild-type mice, but they did not switch from a Th1 to Th2 immune response (41). However, in the present system dominance clearly changed from a Th1 to a Th2 response, using the criteria of cytokine production, accessory cell activation, and immunoglobulin isotype. The production of T-cell-derived IL-4 and IFN-γ was more dramatically affected than the B-cell response. These data demonstrate that the type of Th response is not related to genetic arthritis resistance in the 129/SvEv mice.
ACKNOWLEDGMENTS
This study was supported by grants from the Harold G. and Leila Y. Mathers Foundation (to L.G.) and National Institutes of Health training grant AR07570 (to J.Z.D.).
We thank Joseph Aroy for initial consultation on the histopathology and Allen Steere and Brigitte Huber for support and discussions.
REFERENCES
- 1.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J Clin Investig. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita J, Samanta S, Barthold S W, Fikrig E. Ablation of interleukin-12 exacerbates Lyme arthritis in SCID mice. Infect Immun. 1997;65:4334–4336. doi: 10.1128/iai.65.10.4334-4336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasa B, Sarvetnick N. The paradoxical effects of interleukin 10 in the immunoregulation of autoimmune diabetes. J Autoimmun. 1996;9:283–286. doi: 10.1006/jaut.1996.0036. [DOI] [PubMed] [Google Scholar]
- 4.Barthold S W. Lyme borreliosis in the laboratory mouse. J Spir Tick-borne Dis. 1996;3:22–44. [Google Scholar]
- 5.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Barthold S W, de Souza M. Exacerbation of Lyme arthritis in beige mice. J Infect Dis. 1995;172:778–784. doi: 10.1093/infdis/172.3.778. [DOI] [PubMed] [Google Scholar]
- 7.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 9.Brown C R, Reiner S L. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–3333. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown C R, Reiner S L. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect Immun. 1999;67:1967–1973. doi: 10.1128/iai.67.4.1967-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten R M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canica M M, Nato F, du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 13.Dong Z, Edelstein M D, Glickstein L J. CD8+ T cells are activated during the early Th1 and Th2 immune responses in a murine Lyme disease model. Infect Immun. 1997;65:5334–5337. doi: 10.1128/iai.65.12.5334-5337.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S, Hodzic E, Barthold S W. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect Immun. 2000;68:4169–4173. doi: 10.1128/iai.68.7.4169-4173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Villanueva P O, Zheng X X, Strom T B, Stadecker M J. Recombinant IL-10 and IL-10/Fc treatment down-regulate egg antigen-specific delayed hypersensistivity reactions and egg granuloma formation in schistosomiasis. J Immunol. 1996;156:3315–3320. [PubMed] [Google Scholar]
- 16.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 17.Gross D M, Steere A C, Huber B T. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J Immunol. 1998;160:1022–1028. [PubMed] [Google Scholar]
- 18.Hart P H, Ahern M J, Smith M D, Finlay-Jones J J. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84:536–542. [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 20.Joosten L A, Helsen M M, van de Loo F A, van den Berg W B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 21.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keane-Myers A, Nickell S P. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–2028. [PubMed] [Google Scholar]
- 23.Keane-Myers A, Nickell S P. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;154:1770–1776. [PubMed] [Google Scholar]
- 24.Lee M S, Mueller R, Wicker L S, Peterson L B, Sarvetnick N. IL-10 is necessary and sufficient for autoimmune diabetes in conjuction with NOD MHC homozygosity. J Exp Med. 1996;183:2663–2668. doi: 10.1084/jem.183.6.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine J F, Wilson M L, Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985;34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Elliott J F, Mosmann T R. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994;153:3967–3978. [PubMed] [Google Scholar]
- 27.Maini R N, Elliott M J, Brennan F M, Williams R O, Chu C Q, Paleolog E, Charles P J, Taylor P C, Feldmann M. Monoclonal anti-TNF alpha antibody as a probe of pathogenesis and therapy of rheumatoid disease. Immunol Rev. 1995;144:195–223. doi: 10.1111/j.1600-065x.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 28.Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, Huang S, Boissier M C, Fournier C. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 29.Matthews D E, Farewell V T. Using and understanding medical statistics. Basel, Switzerland: Karger; 1988. [Google Scholar]
- 30.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller R, Lee M S, Sawyer S P, Sarvetnick N. Transgenic expression of interleukin 10 in the pancreas renders resistant mice susceptible to low dose streptozotocin-induced diabetes. J Autoimmun. 1996;9:151–158. doi: 10.1006/jaut.1996.0018. [DOI] [PubMed] [Google Scholar]
- 32.Piguet P F, Grau G E, Vesin C, Loetscher H, Gentz R, Lesslauer W. Evolution of collagen arthritis in mice is arrested by treatment with anti-tumour necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. Immunology. 1992;77:510–514. [PMC free article] [PubMed] [Google Scholar]
- 33.Rennick D M, Fort M M, Davidson N J. Studies with IL-10−/− mice: an overview. J Leukoc Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 34.Sandberg J O, Benda B, Lycke N, Korsgren O. Xenograft rejection of porcine islet-like cell clusters in normal, interferon-gamma, and interferon-gamma receptor deficient mice. Transplantation. 1997;63:1446–1452. doi: 10.1097/00007890-199705270-00014. [DOI] [PubMed] [Google Scholar]
- 35.Schaible U E, Gay S, Museteanu C, Kramer M D, Zimmer G, Eichmann K, Museteanu U, Simon M M. Lyme borreliosis in the severe combined immunodeficiency (SCID) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 37.Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8:199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 38.Steere A C, Baxter-Lowe L A. Association of chronic, treatment-resistant Lyme arthritis with rheumatoid arthritis (RA) alleles. Arthritis Rheum. 1998;41:S81. [Google Scholar]
- 39.Steere A C, Malawista S E, Snydman D R, Shope R E, Andiman W A, Ross M R, Steele F M. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- 40.Steere A C, Schoen R T, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 41.Swihart K, Fruth U, Messmer N, Hug K, Behin R, Huang S, Del Giudice G, Aguet M, Louis J A. Mice from a genetically resistant backround lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med. 1995;181:961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorbecke G J, Shah R, Leu C H, Kuruvilla A P, Hardison A M, Palladino M A. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 44.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 45.Weis J J, McCracken B A, Ma Y, Fairbairn D, Roper R J, Morrison T B, Weis J H, Zachary J F, Doerge R W, Teuscher C. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162:948–956. [PubMed] [Google Scholar]
- 46.Willenborg D O, Fordham S, Bernard C C A, Cowden W B, Ramshaw I A. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune disease. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 47.Williams R O, Feldmann M, Maini R N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams R O, Mason L J, Feldmann M, Maini R N. Synergy between anti-CD4 and anti-tumor necrosis factor in the amelioration of established collagen-induced arthritis. Proc Natl Acad Sci USA. 1994;91:2762–2766. doi: 10.1073/pnas.91.7.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 50.Yssel H, Shanafelt M-C, Soderberg C, Schneider P V, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type I-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeidner N, Mbow M L, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]