Abstract
BACKGROUND & AIMS:
As many as one-half of all patients with suspected gastroesophageal reflux disease (GERD) do not derive benefit from acid suppression. This review outlines a personalized diagnostic and therapeutic approach to GERD symptoms.
METHODS:
The Best Practice Advice statements presented here were developed from expert review of existing literature combined with extensive discussion and expert opinion to provide practical advice. Formal rating of the quality of evidence or strength of recommendations was not the intent of this clinical practice update.
BEST PRACTICE ADVICE 1:
Clinicians should develop a care plan for investigation of symptoms suggestive of GERD, selection of therapy (with explanation of potential risks and benefits), and long-term management, including possible de-escalation, in a shared-decision making model with the patient.
BEST PRACTICE ADVICE 2:
Clinicians should provide standardized educational material on GERD mechanisms, weight management, lifestyle and dietary behaviors, relaxation strategies, and awareness about the brain-gut axis relationship to patients with reflux symptoms.
BEST PRACTICE ADVICE 3:
Clinicians should emphasize safety of proton pump inhibitors (PPIs) for the treatment of GERD.
BEST PRACTICE ADVICE 4:
Clinicians should provide patients presenting with troublesome heartburn, regurgitation, and/ or non-cardiac chest pain without alarm symptoms a 4- to 8-week trial of single-dose PPI therapy. With inadequate response, dosing can be increased to twice a day or switched to a more effective acid suppressive agent once a day. When there is adequate response, PPI should be tapered to the lowest effective dose.
BEST PRACTICE ADVICE 5:
If PPI therapy is continued in a patient with unproven GERD, clinicians should evaluate the appropriateness and dosing within 12 months after initiation, and offer endoscopy with prolonged wireless reflux monitoring off PPI therapy to establish appropriateness of long-term PPI therapy.
BEST PRACTICE ADVICE 6:
If troublesome heartburn, regurgitation, and/or non-cardiac chest pain do not respond adequately to a PPI trial or when alarm symptoms exist, clinicians should investigate with endoscopy and, in the absence of erosive reflux disease (Los Angeles B or greater) or long-segment (≥3 cm) Barrett’s esophagus, perform prolonged wireless pH monitoring off medication (96-hour preferred if available) to confirm and phenotype GERD or to rule out GERD.
BEST PRACTICE ADVICE 7:
Complete endoscopic evaluation of GERD symptoms includes inspection for erosive esophagitis (graded according to the Los Angeles classification when present), diaphragmatic hiatus (Hill grade of flap valve), axial hiatus hernia length, and inspection for Barrett’s esophagus (graded according to the Prague classification and biopsied when present).
BEST PRACTICE ADVICE 8:
Clinicians should perform upfront objective reflux testing off medication (rather than an empiric PPI trial) in patients with isolated extra-esophageal symptoms and suspicion for reflux etiology.
BEST PRACTICE ADVICE 9:
In symptomatic patients with proven GERD, clinicians should consider ambulatory 24-hour pHimpedance monitoring on PPI as an option to determine the mechanism of persisting esophageal symptoms despite therapy (if adequate expertise exists for interpretation).
BEST PRACTICE ADVICE 10:
Clinicians should personalize adjunctive pharmacotherapy to the GERD phenotype, in contrast to empiric use of these agents. Adjunctive agents include alginate antacids for breakthrough symptoms, nighttime H2 receptor antagonists for nocturnal symptoms, baclofen for regurgitation or belch predominant symptoms, and prokinetics for coexistent gastroparesis.
BEST PRACTICE ADVICE 11:
Clinicians should provide pharmacologic neuromodulation, and/or referral to a behavioral therapist for hypnotherapy, cognitive behavioral therapy, diaphragmatic breathing, and relaxation strategies in patients with functional heartburn or reflux disease associated with esophageal hypervigilance reflux hypersensitivity and/or behavioral disorders.
BEST PRACTICE ADVICE 12:
In patients with proven GERD, laparoscopic fundoplication and magnetic sphincter augmentation are effective surgical options, and transoral incisionless fundoplication is an effective endoscopic option in carefully selected patients.
BEST PRACTICE ADVICE 13:
In patients with proven GERD, Roux-en-Y gastric bypass is an effective primary anti-reflux intervention in obese patients, and a salvage option in non-obese patients, whereas sleeve gastrectomy has potential to worsen GERD.
BEST PRACTICE ADVICE 14:
Candidacy for invasive anti-reflux procedures includes confirmatory evidence of pathologic GERD, exclusion of achalasia, and assessment of esophageal peristaltic function.
Keywords: Ambulatory Reflux Monitoring, Gastroesophageal Reflux Disease, Proton Pump Inhibitors
The prevalence of symptomatic gastro-esophageal reflux disease (GERD) is rising, with more than 30% of United States adults reporting at least weekly symptoms.1,2 Symptoms of GERD encompass heartburn or regurgitation (typical esophageal symptoms), non-cardiac chest pain (atypical esophageal symptom), and a myriad of extra-esophageal symptoms which include cough, dysphonia, sore throat, and globus.3 Further, symptoms can arise from coexisting or confounding pathophysiology such as mechanical defects, physiologic abnormalities, heightened nociception, and hypervigilance. Despite heterogeneous presentations and pathogeneses, patients with GERD have historically been managed in a similar catch-all fashion, often in the absence of objective abnormalities. Up to 50% of patients, however, do not derive adequate relief with empirical proton pump inhibitor (PPI) therapy.4–6 Drivers of inadequate response include absence of pathologic GERD to begin with or symptom pathophysiology that is insufficiently targeted with acid suppression.7 In recognition of this problem, the current care paradigm has shifted towards a personalized approach to the evaluation and management of GERD symptoms.8 This Clinical Practice Update (CPU) provides best practice advice for a personalized diagnostic and therapeutic approach to GERD.
Methods
This expert review was commissioned jointly by the American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee, the AGA Center for GI Innovation and Technology (CGIT), and the AGA Governing Board to provide timely guidance on a topic of high clinical importance to the AGA membership. The AGA CGIT Consensus Conferences bring together content experts, stakeholders (industry, regulatory, and payor), along with a patient advocate to discuss current needs and gaps in innovation relevant to the topic. This is an exhaustive, comprehensive didactic and discussion session created to provide a novel interactive environment to foster the AGA CGIT mission. The topic of this CPU was thoroughly discussed by expert faculty contributors selected by AGA CGIT, industry representatives and patient advocates at the conference organized and hosted by AGA CGIT. The content of this expert review was generated, discussed, and voted upon by the expert faculty contributors at a closed-door meeting during the AGA CGIT conference. All faculty contributors provided up-to-date declaration of conflicts of interest to ensure credibility of this document, and signed off on the final manuscript, which underwent internal peer review by the AGA Institute Clinical Practice Updates Committee as well as external peer review through standard procedures of Clinical Gastroenterology and Hepatology.
Approaching GERD Symptoms in the Clinic
Care Plan
Patients with GERD symptoms seek care from a spectrum of health care providers including primary care physicians, gastroenterologists, otolaryngologists, pulmonologists, and surgeons. Health care providers and patients alike have questions and concerns regarding treatment of choice, need for objective testing, concerns about GERD complications over time, and risks of long-term treatments. Consistent, standardized approaches across health care teams are essential to streamline GERD evaluation and management. Clinicians should develop a care plan for investigation of symptoms suggestive of GERD, selection of therapy (with explanation of potential risks and benefits), and long-term management, including possible de-escalation, in a shared decision making model with the patient (Best Practice Advice [BPA] 1).
To develop a care plan, providers need to ascertain the likelihood of pathologic GERD and discern which mechanisms may be driving symptoms. Symptom characterization is an essential first step. Typical esophageal symptoms of heartburn and regurgitation are approximately 70% sensitive and specific for objective GERD, providing the rationale for first-line PPI trials with high therapeutic gain for symptom relief despite lack of prior objective testing.6 Conversely, an empiric PPI trial is not optimal for isolated extra-esophageal symptoms because mechanisms other than GERD frequently contribute to symptom generation, making likelihood of PPI non-response high.9,10 Additional clinical factors that can explain symptom generation include central obesity and/ or a known hiatal hernia pointing to a mechanical etiology of gastro-esophageal reflux, anxiety, or stress-induced symptoms suggesting visceral hypersensitivity and/or hypervigilance, behavioral disorders including rumination and supragastric belching, or mixed connective tissue disorder raising suspicion for esophageal dysmotility and reduced refluxate clearance.11–13
Patient Education
During the initial clinic visit, it is essential that clinicians provide standardized educational material on GERD mechanisms, weight management, lifestyle and dietary behaviors, relaxation strategies, and awareness about the brain-gut axis relationship to patients with reflux symptoms (BPA 2). Patient education should emphasize that gastro-esophageal reflux is a physiologic process, commonly mediated through transient lower esophageal sphincter relaxations and controlled by protective factors such as the anti-reflux barrier, effective esophageal peristalsis and salivation, and downstream gastric motility.14 This discussion frames patient expectations in terms of response to acid suppression and potential need for adjunctive strategies. For instance, appreciating the role of the crural diaphragm may facilitate adherence to diaphragmatic breathing.15 Further, understanding the intra-abdominal to intra-thoracic pressure gradient may improve acceptance of weight management and modified dietary/nighttime routines.16–19 For patients with a known hiatal hernia and/or symptom burden following meals or during sleep, reduction of supine GERD by elevating the head of the bed and avoiding meals within 3 hours of bedtime are useful.20 An introductory discussion about the brain-gut axis can also empower and encourage the patient to integrate stress-reducing activities such as mindfulness into their daily lives, and can open the door for future psychological interventions.21 The supplemental document available with this update is a handout that can be provided to patients with suspected GERD (Supplemental Figure 1).
PPI Trial
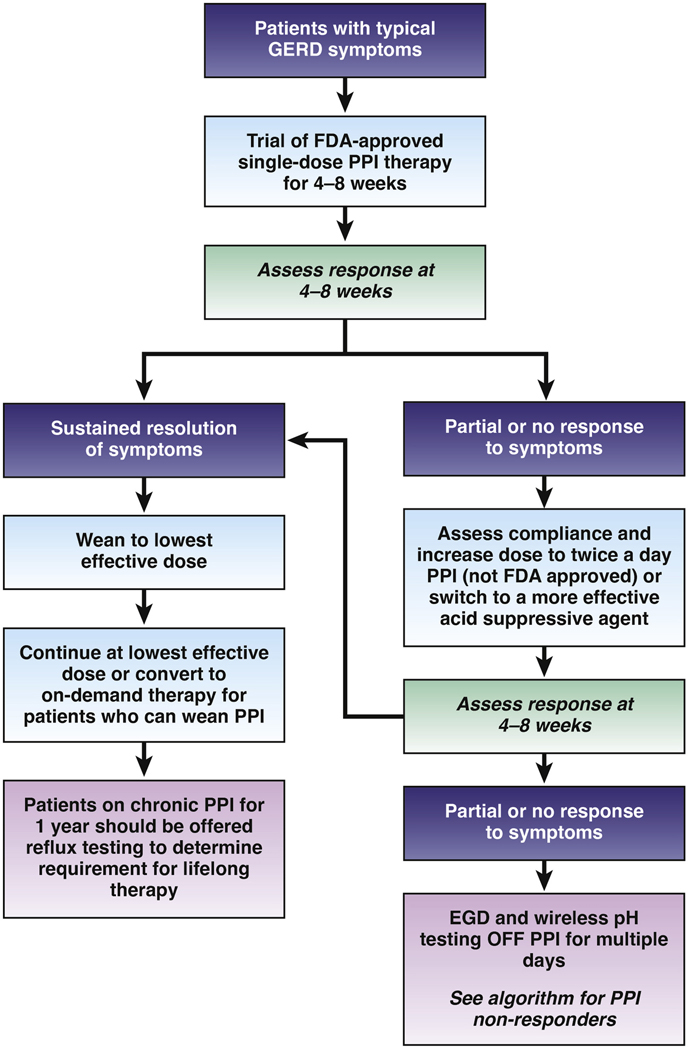
Clinicians should provide patients presenting with troublesome heartburn, regurgitation, and/or non-cardiac chest pain without alarm symptoms a 4- to 8-week trial of single-dose PPI therapy (BPA 4). Any commercially available PPI can be used for the trial, the choice of which may be guided by payor coverage, out-of-pocket costs, and prior experiences with a particular PPI. Patients should be counseled to take the PPI 30 to 60 minutes prior to a meal. Education and literature emphasizing safety of PPIs for the treatment of GERD should be provided (BPA 3).22 Patient symptoms should be reassessed after a 4- to 8-week trial (Figure 1). With inadequate response, dosing can be increased to twice a day or switched to a more effective acid suppressive agent once a day (BPA 4). These can include PPIs that are more potent,23 less metabolized through the CYP2C19 pathway (eg, rabeprazole, esomeprazole), or available in an extended release formulation (eg, dexlansoprazole),24 as well as potassium competitive acid blockers when available. Routine re-evaluation of treatment should be performed, and the PPI should be tapered to the lowest effective dose when there is adequate response (BPA 4) (Figure 1). Best practices surrounding PPI de-prescribing are further elaborated in a separate AGA CPU.
Figure 1.
Utilization of empiric PPI therapy in suspected gastroesophageal reflux disease. Patients with typical reflux symptoms (heartburn, acid regurgitation) without alarm symptoms can be offered a trial of single dose PPI therapy, and response assessed in 4 to 8 weeks. Responders can be weaned down to the lowest effective dose, and if symptoms remain controlled, titrated further to on demand therapy if possible. Patients who need to remain on chronic PPI therapy can be offered reflux testing at the 1-year time point to determine appropriateness of long term therapy. Dose increase to twice a day or a switch to a more efficacious PPI can be offered to non- or partial responders to single-dose PPI trial. If response remains suboptimal, esophageal testing is suggested (see Figure 3). Patients with isolated extra-esophageal GERD symptoms benefit most from upfront esophageal testing rather than an empiric PPI trial.
Personalized Diagnostic Approach to GERD Symptoms
Indications for Objective Testing
Particular clinical scenarios warrant objective evaluation. If troublesome heartburn, regurgitation, and/or non-cardiac chest pain do not respond adequately to a PPI trial or if alarm symptoms exist, clinicians should investigate with endoscopy and, in the absence of erosive reflux disease (Los Angeles B or greater) or long-segment (≥3 cm) Barrett’s esophagus, perform prolonged wireless pH monitoring off medication (96-hour preferred if available) to confirm and phenotype or to rule out GERD (BPA 6). In addition, clinicians should perform upfront objective reflux testing (rather than an empiric PPI trial) in patients with isolated extra-esophageal symptoms and suspicion of reflux etiology (BPA 8).
Another indication for objective testing may include patients with unproven GERD that have a symptom response to empiric PPI therapy, in order to establish the appropriateness of long-term PPI therapy (Figure 1). Thus, if PPI therapy is continued in a patient with unproven GERD, clinicians should evaluate the appropriateness and dosing within 12 months after initiation, and offer endoscopy with prolonged wireless reflux monitoring off PPI therapy to establish appropriate use of long-term PPI therapy (BPA 5). In this context, endoscopy with prolonged reflux monitoring is optimally performed after withholding PPI for 2 to 4 weeks whenever possible.25 This is an important consideration in terms of shared decision-making as many patients want to understand why they may need chronic lifelong maintenance therapy.
Upper Endoscopy
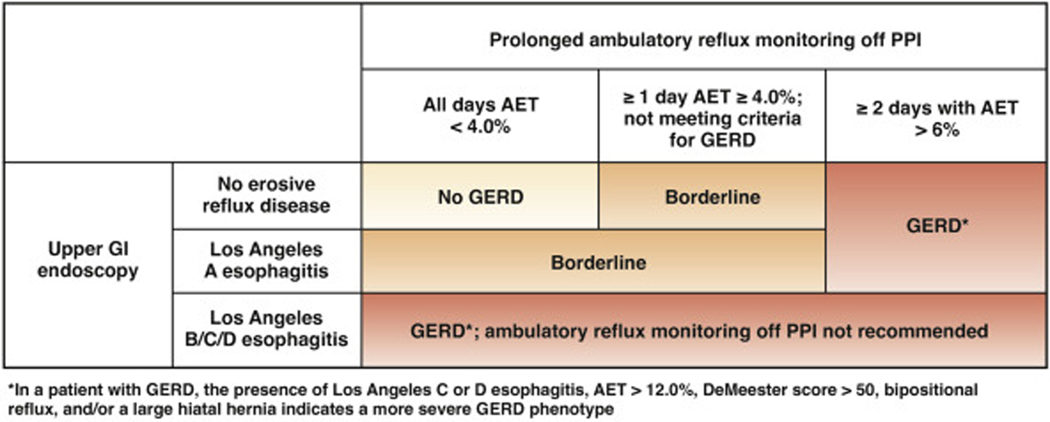
Complete endoscopic evaluation of GERD symptoms includes inspection for erosive esophagitis (graded according to the Los Angeles classification when present), diaphragmatic hiatus (Hill grade of flap valve), axial hiatus hernia length, and inspection for Barrett’s esophagus (with grading according to the Prague classification and biopsy when present) (BPA 7).26,27 Confirmatory evidence of erosive reflux on endoscopy is found in a minority of patients. These findings include esophagitis (Los Angeles B or greater) and/or the presence of long-segment (≥3 cm) Barrett’s esophagus, with Los Angeles C or D esophagitis constituting severe erosive disease. However, up to 80% of symptomatic patients will not have objective reflux evidence on endoscopy.28 Of note, Los Angeles A esophagitis can be seen in healthy asymptomatic volunteers and is not considered evidence of erosive reflux disease (Figure 2).
Figure 2.
Utilization of prolonged reflux monitoring off PPI therapy to characterize severity of GERD. Reflux monitoring is offered in patients without higher grades of reflux esophagitis on endoscopy. Absence of pathologic acid exposure on ambulatory reflux monitoring (AET <4.0% on all 4 days of the prolonged wireless pH study) with a normal endoscopy rules out GERD. Erosive esophagitis of Los Angeles Grade B or higher, and/or AET ≥6.0% on 2 or more days constitutes conclusive GERD evidence. Patients with LA grade A esophagitis, and/or AET ≥4.0% but otherwise not meeting criteria for conclusive GERD are considered to have borderline GERD.
Ambulatory Reflux Monitoring
Ambulatory reflux monitoring is available in 2 configurations. Wireless pH monitoring (Bravo) uses a pH capsule introduced via a trans-oral catheter during sedated esophagogastroduodenoscopy that adheres to the distal esophagus (6-cm proximal to the endoscopically identified squamocolumnar junction) using a vacuum suction mechanism.29 Wireless pH monitoring measures acid exposure in the distal esophagus for up to 96 hours (based on recorder battery life) and assesses the relationship between patient reported symptoms and acid reflux episodes.30 Catheter-based pH monitoring uses a trans-nasal catheter placed without sedation, which measures acid exposure in the distal esophagus as well as reflux-symptom association for up to 24 hours. Ideally, catheter-based pH monitoring is combined with multiple pairs of intraluminal impedance electrodes to assess air and liquid movement along the esophagus irrespective of pH.29 Based on advantages in assessing acid exposure over a prolonged period of time to account for day-to-day variability, ease of placement during sedated upper endoscopy, and patient tolerance, wireless pH monitoring is the preferred ambulatory reflux monitoring method to objectively assess for GERD in a symptomatic patient.31,32 Outcome data from a recent prospective study demonstrated that normal acid exposure time (<4.0%) on all 4 days of a 96-hour wireless study had an odds ratio of 10.0 (95% confidence interval, 2.70–43.32) in predicting successful PPI withdrawal, and abnormal acid exposure time on ≥2 days had an odds ratio of 5.3 (95% confidence interval, 2.91–13.44) in predicting need for continuing PPI treatment.25 If wireless pH monitoring is not available, 24-hour impedance-pH monitoring off PPI therapy can be utilized when expertise in frame-by-frame interpretation is available.9,33 In particular, 24-hour impedance-pH monitoring off PPI may be preferred in the evaluation of extra-esophageal symptoms,34 and is the optimal reflux monitoring system in symptomatic patients with previously proven GERD with the test performed on twice-a-day PPI therapy.35
Precision Management Approach Based on Ambulatory Reflux Monitoring and Upper Gastrointestinal Endoscopy
Esophageal acid exposure time (AET), the percent time spent at pH of 4.0 or less, is a key physiomarker for phenotyping patients with GERD.25,28,30 Reflux symptom association on ambulatory reflux monitoring (symptom association probability >95% and symptom index >50%) increase confidence that symptoms are truly associated with reflux when AET is increased, and indicate reflux hypersensitivity (a functional esophageal disorder) when AET is physiologic.21 In addition to acid exposure, other key determinants that need to be considered in planning GERD management include reflux-symptom association on ambulatory reflux monitoring, integrity of the anti-reflux barrier, central obesity, esophageal physiology, visceral sensitivity, hypervigilance, and downstream gastrointestinal (GI) motility.
Absence of Erosive Findings on Upper GI Endoscopy and Physiologic Acid Exposure
In general, absence of erosive reflux disease on upper GI endoscopy and findings of a physiologic AET of less than 4.0% across all days of wireless pH monitoring reflects normal gastro-esophageal reflux physiology.25,28 Patients with normal acid exposure are not considered to have GERD and have a high likelihood of a functional esophageal disorder.21 PPI therapy should be weaned off in these patients unless symptoms demonstrate a clear escalation off therapy and improve with PPI, a pattern seen in some patients with reflux hypersensitivity. Strong consideration should be given to referral to a GI psychologist for cognitive behavioral therapy (CBT), esophageal directed hypnotherapy, and/or pharmacologic neuromodulation, as detailed below. High-resolution manometry may be considered to evaluate patients with suspected rumination syndrome or an esophageal motor disorder.28
Erosive Findings on Upper GI Endoscopy and/ or Elevated Acid Exposure
The presence of erosive reflux disease and/or an AET of greater than 4.0% across at least 1 day of wireless pH monitoring performed off PPI reflects elevated acid burden. The presence of Los Angeles B or greater esophagitis and/or ≥2 days with AET >6% support a GERD diagnosis.28 Specifically, the presence of Los Angeles C or D esophagitis, bi-positional reflux, extreme levels of acid exposure (such as AET >12% or DeMeester score >50), and/or a large hiatal hernia represents a more severe manifestation of GERD.36 At the other end of the spectrum, Los Angeles A esophagitis and/or elevated AET not meeting GERD criteria defined above identifies a borderline GERD group.
Lifestyle Optimization
Most patients with non-severe GERD typically improve with optimization of lifestyle, PPI therapy, and adjunctive pharmacotherapy when appropriate. Aggressive lifestyle modifications and weight management, as outlined in the supplemental material, should be utilized.
PPI Optimization
Optimization of PPI includes ensuring adequate timing of dose, considering escalation to double dose, and/or switching to a different PPI.34 When symptoms are adequately controlled, acid suppression should be weaned down to the lowest effective dose, or switched to H2 receptor antagonists (H2RAs) or other antacids for most patients. Exceptions to weaning acid suppression include patients with erosive esophagitis (Los Angeles B or greater), biopsy proven Barrett’s esophagus, and/or peptic stricture, who will require at least single-dose, long-term PPI therapy.34 Patients with severe GERD require indefinite long-term PPI therapy and/or an invasive anti-reflux procedure.
Adjunctive Pharmacotherapy
Clinicians should personalize adjunctive pharmacotherapy to the GERD phenotype, in contrast to empiric use of these agents. Adjunctive agents include alginate antacids for breakthrough symptoms, night-time H2RAs for nocturnal symptoms, baclofen for regurgitation or belch predominant symptoms, and prokinetics for coexistent gastroparesis (BPA 10). Alginates are useful in neutralizing the post-prandial acid pocket, and may be particularly useful for patients with post-prandial and/or nighttime symptoms, and in those with a known hiatal hernia.34,37 H2RAs may be helpful for breakthrough and/ or night-time symptoms; however, use is limited by tachyphylaxis.38–40 Transient lower esophageal sphincter relaxation inhibition with baclofen, a GABA-B agonist, may be effective for belch predominant symptoms and mild regurgitation, although often limited by central nervous system and GI side effects.28,41 Prokinetics have not been shown to be useful in GERD, but may have a role in patients with concomitant gastroparesis.28,34
As highlighted by the Rome IV update, esophageal hypervigilance and visceral hypersensitivity can augment symptom burden across the entire spectrum of acid exposure, from normal to severe.21 Adjunctive pharmacotherapy can include neuromodulation with low-dose anti-depressants, which requires familiarity and comfort with prescribing and following patients treated with these agents.42 With the recognition of the role of esophageal hypersensitivity, hypervigilance, behavioral disorders including supragastric belching and rumination, and other psychosocial factors in esophageal symptomatology, behavioral interventions to target these underlying mechanisms are becoming increasingly utilized.34,43 The most researched behavioral interventions for esophageal disorders include CBT, esophageal-directed hypnotherapy, and diaphragmatic breathing.44–47 Treatments are typically administered by clinical health psychologists or other mental health professionals that have specialized training in treating a variety of chronic GI disorders. Thus, clinicians should provide pharmacologic neuromodulation, and/or referral to a behavioral therapist for hypnotherapy, CBT, diaphragmatic breathing, and relaxation strategies in patients with functional heartburn or reflux disease associated with esophageal hypervigilance, reflux hypersensitivity, and/or behavioral disorders (BPA 11).
Inadequate Symptom Response Despite Optimization
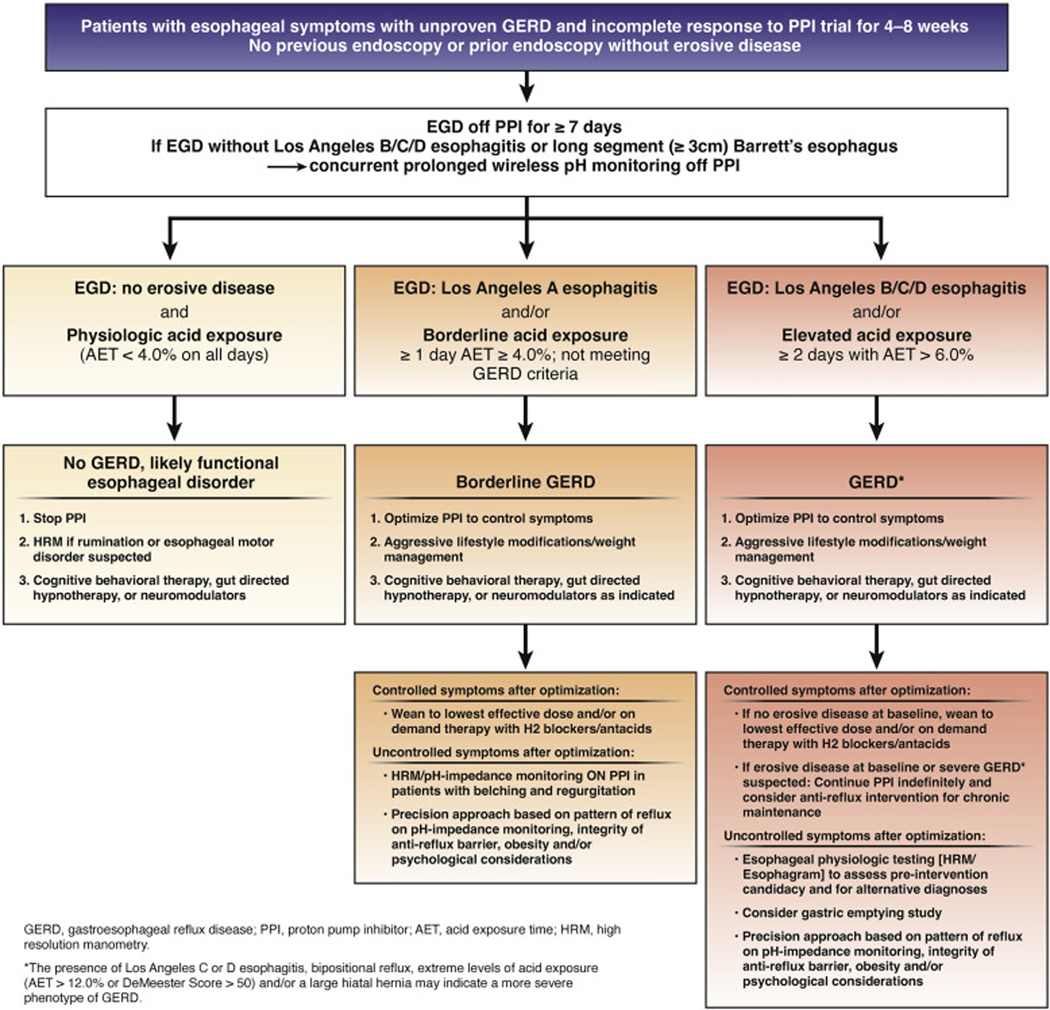
If symptoms are inadequately controlled following lifestyle and pharmacotherapy optimization, additional testing can be useful, including assessment of esophageal peristaltic function and exclusion of achalasia (with high-resolution manometry, for instance) and gastric emptying testing if delayed gastric emptying is suspected.48 Clinicians should consider ambulatory 24-hour pH-impedance monitoring on PPI as an option to determine the mechanism of persisting esophageal symptoms despite therapy (BPA 9), particularly in patients without a known major abnormality in the anti-reflux barrier, to confirm PPI refractory GERD and exclude other etiologies of ongoing symptoms such as an overlap with reflux hypersensitivity, rumination syndrome, or a belching disorder.35 Clinicians should then escalate therapy via a precision approach based on the pattern of reflux on impedance-pH monitoring, integrity of the anti-reflux barrier, presence of obesity, and/or psychological considerations (Figure 3).34,35
Figure 3.
Personalized approach to diagnosis and GERD based on findings on endoscopy and prolonged ambulatory wireless pH monitoring. Patients with no GERD likely have an alternate explanation for symptoms, which can be a functional disorder; hence, PPIs can be discontinued, and other management options explored. Patients with borderline GERD may need PPIs but these are titrated to the lowest dose or frequency that controls symptoms, or replaced with H2RAs. Adjunctive approaches include lifestyle and behavior modification. Patients with GERD have Los Angeles grade B esophagitis or higher, and/or AET ≥6.0% on 2 or more days on prolonged wireless pH monitoring performed off PPI therapy. Within patients with GERD, a severe GERD phenotype exists characterized by advanced grade esophagitis (Los Angeles grade C or D), and/or AET >12.0%, bipositional reflux or Demeester score >50, which requires either continuous long-term PPI therapy or invasive anti-reflux procedures, in addition to optimization of lifestyle measures. Medical management may be adequate for patients with GERD who respond to therapy, whereas escalation to anti-reflux procedures can be considered after appropriate esophageal physiologic testing for non-responders despite optimization of therapy.
Endoscopic and Surgical Anti-Reflux Procedures
Laparoscopic fundoplication is often utilized in the non-obese patient. Type of fundoplication may be tailored, with partial fundoplication preferred in patients with known esophageal hypomotility or impaired peristaltic reserve when there is concern of postoperative dysphagia.49–51 Magnetic sphincter augmentation is another option, often combined with a crural repair in the setting of known hiatal hernia.52 Transoral incisionless fundoplication is an endoscopic anti-reflux procedure that is increasingly performed for carefully selected patients with GERD in the absence of a hiatal hernia.53 These approaches have demonstrable value in patients with regurgitation-predominant GERD.53,54 Recent data suggest efficacy of transoral incisionless fundoplication with a combined laparoscopic hiatal hernia and crural repair in patients with a minor crural defect.55 Further research into risks/benefits, durability, effectiveness, and treatment outcomes will enhance optimal utilization of these newer endoscopic and surgical options. In patients with proven GERD, laparoscopic fundoplication and magnetic sphincter augmentation are effective surgical options, and transoral incisionless fundoplication is an effective endoscopic option in carefully selected patients (BPA 12). In patients with proven GERD, Roux-en-Y gastric bypass is an effective primary anti-reflux intervention in obese patients, and a salvage option in non-obese patients, while sleeve gastrectomy has potential to worsen GERD (BPA 13). Candidacy for invasive antireflux procedures includes confirmatory evidence of pathologic GERD, exclusion of achalasia, and assessment of esophageal peristaltic function (BPA 14).
Conclusion
For patients presenting with GERD symptoms, a stepwise diagnostic approach will identify mechanisms driving symptoms for a precision management approach. Patients should receive education on GERD pathophysiology and lifestyle modifications, and be involved in a shared decision-making model. A 4- to 8-week trial of single-dose PPI is considered safe and appropriate for patients with typical reflux symptoms and no alarm symptoms, with escalation to twice-a-day dosing or switching to a more potent acid suppressive agent if symptoms persist. Symptom response should prompt PPI titration to the lowest effective dose. When long-term PPI therapy is planned, objective reflux testing should be offered to establish a diagnosis of GERD and a long-term management plan. Objective testing with upper GI endoscopy is warranted in PPI non-response, presence of alarm signs/symptoms, isolated extra-esophageal symptoms, or in patients who meet criteria to undergo screening for Barrett’s esophagus. In the absence of confirmed erosive disease or Barrett’s esophagus on endoscopy, prolonged wireless pH monitoring off PPI therapy is utilized to assess esophageal acid exposure. Patients without erosive disease on endoscopy and with physiologic acid exposure often have a functional esophageal disorder. In these patients, neuromodulation or behavioral interventions can be utilized, and PPI therapy can be titrated off as tolerated. Patients with non-severe GERD often respond well to optimization of lifestyle and pharmacotherapy, and may ultimately be able to wean pharmacotherapy down to the lowest effective dose (unless erosive reflux disease or Barrett’s esophagus exists). On the other hand, patients with severe GERD will generally require long-term anti-reflux management. A precision approach to escalation of management is suggested for patients with ongoing symptoms despite these measures, which should be driven by integrity of the anti-reflux barrier, presence of visceral hypersensitivity and hypervigilance, confirmation of PPI refractory-GERD, symptom profile, body mass index, and esophageal (as well as gastric) motor function.
| Best Practice Advice (BPA) Statements |
|---|
|
|
| Approaching GERD Symptoms in Clinic |
| BPA #1. Clinicians should develop a care plan for investigation of symptoms suggestive of gastroesophageal reflux disease (GERD), selection of therapy (with explanation of potential risks and benefits), and long-term management, including possible de-escalation, in a shared decision-making model with the patient. |
| BPA #2. Clinicians should provide standardized educational material on GERD mechanisms, weight management, lifestyle and dietary behaviors, relaxation strategies, and awareness about the brain-gut axis relationship to patients with reflux symptoms. |
| BPA #3. Clinicians should emphasize safety of proton pump inhibitors (PPIs) for the treatment of GERD. |
| BPA #4. Clinicians should provide patients presenting with troublesome heartburn, regurgitation, and/or non-cardiac chest pain without alarm symptoms a 4- to 8-week trial of single-dose PPI therapy. With inadequate response, dosing can be increased to twice a day or switched to a more effective acid suppressive agent once a day. When there is adequate response, PPI should be tapered to the lowest effective dose. |
| Personalized Diagnostic Approach to GERD Symptoms |
| BPA #5. If PPI therapy is continued in a patient with unproven GERD, clinicians should evaluate the appropriateness and dosing within 12 months after initiation, and offer endoscopy with prolonged wireless reflux monitoring off PPI therapy to establish appropriate use of long-term PPI therapy. |
| BPA #6. If troublesome heartburn, regurgitation, and/or non-cardiac chest pain do not respond adequately to a PPI trial or when alarm symptoms exist, clinicians should investigate with endoscopy and, in the absence of erosive reflux disease (Los Angeles B or greater) or long-segment (≥3cm) Barrett’s esophagus, perform prolonged wireless pH monitoring off medication (96-hour preferred if available) to confirm and phenotype or to rule out GERD. |
| BPA #7. Complete endoscopic evaluation of GERD symptoms includes inspection for erosive esophagitis (graded according to the Los Angeles classification when present), diaphragmatic hiatus (Hill grade of flap valve), axial hiatus hernia length, and inspection for Barrett’s esophagus (with grading according to the Prague classification and biopsy when present). |
| BPA #8. Clinicians should perform upfront objective reflux testing off medication (rather than an empiric PPI trial) in patients with isolated extra-esophageal symptoms and suspicion of reflux etiology. |
| BPA #9. In symptomatic patients with proven GERD, clinicians should consider ambulatory 24-hour pH-impedance monitoring on PPI as an option to determine the mechanism of persisting esophageal symptoms despite therapy (if adequate expertise exists for interpretation). |
| Precision Management Approach to GERD |
| BPA# 10 Clinicians should personalize adjunctive pharmacotherapy to the GERD phenotype, in contrast to empiric use of these agents. Adjunctive agents include alginate antacids for breakthrough symptoms, nighttime H2 receptor antagonists for nocturnal symptoms, baclofen for regurgitation or belch predominant symptoms, and prokinetics for coexistent gastroparesis. |
| BPA #11 Clinicians should provide pharmacologic neuromodulation, and/or referral to a behavioral therapist for hypnotherapy, cognitive behavioral therapy, diaphragmatic breathing, and relaxation strategies in patients with functional heartburn or reflux disease associated with esophageal hypervigilance, reflux hypersensitivity, and/or behavioral disorders. |
| BPA #12 In patients with proven GERD, laparoscopic fundoplication and magnetic sphincter augmentation are effective surgical options, and transoral incisionless fundoplication is an effective endoscopic option in carefully selected patients. |
| BPA #13 In patients with proven GERD, Roux-en-Y gastric bypass is an effective primary anti-reflux intervention in obese patients, and a salvage option in non-obese patients, whereas sleeve gastrectomy has potential to worsen GERD. |
| BPA #14 Candidacy for invasive anti-reflux procedures includes confirmatory evidence of pathologic GERD, exclusion of achalasia, and assessment of esophageal peristaltic function. |
Supplementary Material
Funding
Rena Yadlapati, C. Prakash Gyawali, and John E. Pandolfino are supported by National Institutes of Health R01 DK092217-04 (PI: Pandolfino); Rena Yadlapati is supported by National Institutes of Health K23 DK125266 (PI: Yadlapati).
Conflicts of interest
The authors disclose the following: Rena Yadlapati is a consultant for Medtronic (Institutional), Ironwood Pharmaceuticals (Institutional), and Phathom Pharmaceuticals; receives research support from Ironwood Pharmaceuticals; and serves on the advisory board with stock options for RJS Mediagnostix. C. Prakash Gyawali is a consultant for Medtronic, Diversatek, Ironwood, and Takeda. John E. Pandolfino is a consultant for Medtronic, Ironwood Pharmaceuticals, Diversatek; receives research support from Ironwood Pharmaceuticals and Takeda; serves on the advisory board for Medtronic and Diversatek; and receives stock options from Crospon Inc.
Disclosures for the CGIT GERD Consensus Conference Participants
These participants disclose the following: Kenneth Chang: Apollo, Boston Scientific, Cook, ERBE, Endogastric Solutions, Mauna Kea, Medtronic, Olympus, Ovesco, Pentax (consultant). Peter Kahrilas: Ironwood (research/educational grants, advisory board), Reckitt Benckiser (advisory board), patent (functional lumen imaging probe). Philip Katz: Diversatek (research/education grants); Phathom Pharmaceuticals, Medtronic (consultant). David Katzka: Celgene, Takeda, Phathom, Adare (advisory board). Sri Komanduri: Medtronic, Boston Scientific, Motus GI (consultant). John Lipham: Ethicon/Torax (consultant). V. Raman Muthusamy: Medtronic, Boston Scientific, Interpace Diagnostics, Medivators (consultant), Boston Scientific (research support), Motus GI, Endogastric Solutions (advisory board), Torax Medical/Ethicon (honoraria), Stock: Capsovision (stock ownership). Joel Richter: Medtronic (consultant). Virendra K. Sharma: AquaMedical, Attract Medical, EndoStim, AquaHeart, aGUaRx, Aqua Therapeutic, Libero Health (founder/ownership). Michael Vaezi: Ironwood, Medtronic (consultant). Sachin Wani: Lucid Diagnostics, Ambu and CDx Diagnostics (research/education grants), Exact Sciences, Interpace Diagnostics, Advisory Board: Cernostics (consultant). Paul Menard-Katcher discloses no conflicts.
Abbreviations used in this paper:
- AET
acid exposure time
- AGA
American Gastroenterological Association
- CBT
cognitive behavioral therapy
- CGIT
AGA Center for GI Innovation and Technology
- BPA
Best Practice Advice
- CPU
Clinical Practice Update
- GERD
gastro-esophageal reflux disease
- GI
gastrointestinal
- H2RA
Histamine-2 receptor antagonist
- PPI
proton pump inhibitor
Footnotes
CGIT GERD Consensus Conference Participants
Kenneth Chang, Peter J. Kahrilas, Philip O. Katz, David Katzka, Sri Komanduri, John Lipham, Paul Menard-Katcher, V. Raman Muthusamy, Joel Richter, Virender K. Sharma, Michael F. Vaezi, and Sachin Wani.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2022.01.025.
References
- 1.Delshad SD, Almario CV, Chey WD, et al. Prevalence of gastroesophageal reflux disease and proton pump inhibitor-refractory symptoms. Gastroenterology 2020;158:1250–1261. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015;149:1731–1741.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920, quiz: 1943. [DOI] [PubMed] [Google Scholar]
- 4.Sigterman KE, van Pinxteren B, Bonis PA, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2013;CD002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Boeckxstaens G, Smout AJ. Management of the patient with incomplete response to PPI therapy. Best Pract Res Clin Gastroenterol 2013;27:401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent J, Vakil N, Jones R, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond Study. Gut 2010;59:714–721. [DOI] [PubMed] [Google Scholar]
- 7.Bytzer P, Jones R, Vakil N, et al. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2012;10:1360–1366. [DOI] [PubMed] [Google Scholar]
- 8.Yadlapati R, Pandolfino JE. Personalized approach in the workup and management of gastroesophageal reflux disease. Gastrointest Endosc Clin N Am 2020;30:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyawali CP, Carlson DC, Chen JW, et al. Esophageal physiologic testing: American College of Gastroenterology Clinical Guideline. Am J Gastroenterol 2020;115:1412–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bortoli N, Nacci A, Savarino E, et al. How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related? World J Gastroenterol 2012;18:4363–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel DA, Sharda R, Choksi YA, et al. Model to select on-therapy vs off-therapy tests for patients with refractory esophageal or extraesophageal symptoms. Gastroenterology 2018;155:1729–1740.e1. [DOI] [PubMed] [Google Scholar]
- 12.Guadagnoli L, Yadlapati R, Taft T, et al. Esophageal hypervigilance is prevalent across gastroesophageal reflux disease presentations. Neurogastroenterol Motil 2021;e14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawada A, Guzman M, Nikaki K, et al. Identification of different phenotypes of esophageal reflux hypersensitivity and implications for treatment. Clin Gastroenterol Hepatol 2021; 19:690–698.e2. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Yadlapati R. Pathophysiology and treatment options for gastroesophageal reflux disease: looking beyond acid. Ann N Y Acad Sci 2021;1486:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong AM, Chua LT, Khor CJ, et al. Diaphragmatic breathing reduces belching and proton pump inhibitor refractory gastro-esophageal reflux symptoms. Clin Gastroenterol Hepatol 2018; 16:407–416.e2. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006;354:2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadlapati R, Pandolfino JE, Alexeeva O, et al. The Reflux Improvement and Monitoring (TRIM) program is associated with symptom improvement and weight reduction for patients with obesity and gastroesophageal reflux disease. Am J Gastroenterol 2018;113:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindam A, Ness-Jensen E, Jansson C, et al. Gastroesophageal reflux and sleep disturbances: a bidirectional association in a population-based cohort study: the HUNT Study. Sleep 2016; 39:1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ness-Jensen E, Lindam A, Lagergren J, et al. Weight loss and reduction in gastroesophageal reflux. A prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2013; 108:376–382. [DOI] [PubMed] [Google Scholar]
- 20.Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2016;14:175–182.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aziz Q, Fass R, Gyawali CP, et al. Functional esophageal disorders. Gastroenterology 2016;150:1368–1379. [DOI] [PubMed] [Google Scholar]
- 22.Moayyedi P. How to advise patients on the risk of chronic proton pump inhibitor therapy. Curr Opin Gastroenterol 2020; 36:317–322. [DOI] [PubMed] [Google Scholar]
- 23.Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol 2018;16:800–808.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima JJ, Franciosi JP. Pharmacogenomic testing: the case for CYP2C19 proton pump inhibitor gene-drug pairs. Pharmacogenomics 2014;15:1405–1416. [DOI] [PubMed] [Google Scholar]
- 25.Yadlapati R, Masihi M, Gyawali CP, et al. Ambulatory reflux monitoring guides proton pump inhibitor discontinuation in patients with gastroesophageal reflux symptoms: a clinical trial. Gastroenterology 2021;160:174–182.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie C, Li Y, Zhang N, et al. Gastroesophageal flap valve reflected EGJ morphology and correlated to acid reflux. BMC Gastroenterol 2017;17:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010;71:28–34. [DOI] [PubMed] [Google Scholar]
- 28.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017; 29:1–15. [DOI] [PubMed] [Google Scholar]
- 30.Hasak S, Yadlapati R, Altayar O, et al. Prolonged wireless pH monitoring in patients with persistent reflux symptoms despite proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2020; 18:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarpulla G, Camilleri S, Galante P, et al. The impact of prolonged pH measurements on the diagnosis of gastroesophageal reflux disease: 4-day wireless pH studies. Am J Gastroenterol 2007;102:2642–2647. [DOI] [PubMed] [Google Scholar]
- 32.Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005;3:329–334. [DOI] [PubMed] [Google Scholar]
- 33.Gyawali CP, Rogers B, Frazzoni M, et al. Inter-reviewer variability in interpretation of pH-impedance studies: the Wingate Consensus. Clin Gastroenterol Hepatol 2021;19:1976–1978.e1. [DOI] [PubMed] [Google Scholar]
- 34.Zerbib F, Bredenoord AJ, Fass R, et al. ESNM/ANMS consensus paper: diagnosis and management of refractory gastro-esophageal reflux disease. Neurogastroenterol Motil 2021;33: e14075. [DOI] [PubMed] [Google Scholar]
- 35.Gyawali CP, Tutuian R, Zerbib F, et al. Value of pH impedance monitoring while on twice-daily proton pump inhibitor therapy to identify need for escalation of reflux management. Gastroenterology 2021;161:1412–1422. [DOI] [PubMed] [Google Scholar]
- 36.Krill JT, Naik RD, Higginbotham T, et al. Association between response to acid-suppression therapy and efficacy of antireflux surgery in patients with extraesophageal reflux. Clin Gastroenterol Hepatol 2017;15:675–681. [DOI] [PubMed] [Google Scholar]
- 37.De Ruigh A, Roman S, Chen J, et al. Gaviscon double action liquid (antacid & alginate) is more effective than antacid in controlling post-prandial oesophageal acid exposure in GERD patients: a double-blind crossover study. Aliment Pharmacol Ther 2014;40:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdul-Hussein M, Freeman J, Castell D. Concomitant administration of a histamine2 receptor antagonist and proton pump inhibitor enhances gastric acid suppression. Pharmacotherapy 2015;35:1124–1129. [DOI] [PubMed] [Google Scholar]
- 39.Mainie I, Tutuian R, Castell DO. Addition of a H2 receptor antagonist to PPI improves acid control and decreases nocturnal acid breakthrough. J Clin Gastroenterol 2008; 42:676–679. [DOI] [PubMed] [Google Scholar]
- 40.Gyawali CP, Fass R. Management of gastroesophageal reflux disease. Gastroenterology 2018;154:302–318. [DOI] [PubMed] [Google Scholar]
- 41.Cossentino MJ, Mann K, Armbruster SP, et al. Randomised clinical trial: the effect of baclofen in patients with gastro-oesophageal reflux–a randomised prospective study. Aliment Pharmacol Ther 2012;35:1036–1044. [DOI] [PubMed] [Google Scholar]
- 42.Dickman R, Maradey-Romero C, Fass R. The role of pain modulators in esophageal disorders - no pain no gain. Neurogastroenterol Motil 2014;26:603–610. [DOI] [PubMed] [Google Scholar]
- 43.Roman S, Keefer L, Imam H, et al. Majority of symptoms in esophageal reflux PPI non-responders are not related to reflux. Neurogastroenterol Motil 2015;27:1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riehl ME, Kinsinger S, Kahrilas PJ, et al. Role of a health psychologist in the management of functional esophageal complaints. Dis Esophagus 2015;28:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riehl ME, Chen JW. The proton pump inhibitor nonresponder: a behavioral approach to improvement and wellness. Curr Gastroenterol Rep 2018;20:34. [DOI] [PubMed] [Google Scholar]
- 46.Halland M, Bharucha AE, Crowell MD, et al. Effects of diaphragmatic breathing on the pathophysiology and treatment of upright gastroesophageal reflux: a randomized controlled trial. Am J Gastroenterol 2021;116:86–94. [DOI] [PubMed] [Google Scholar]
- 47.Sawada A, Anastasi N, Green A, et al. Management of supragastric belching with cognitive behavioural therapy: factors determining success and follow-up outcomes at 6–12 months post-therapy. Aliment Pharmacol Ther 2019;50:530–537. [DOI] [PubMed] [Google Scholar]
- 48.Gyawali CP, Roman S, Bredenoord AJ, et al. , International GERD Consensus Working Group. Classification of esophageal motor findings in gastro-esophageal reflux disease: conclusions from an international consensus group. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 49.Dallemagne B, Weerts J, Markiewicz S, et al. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc 2006;20:159–165. [DOI] [PubMed] [Google Scholar]
- 50.Du X, Hu Z, Yan C, et al. A meta-analysis of long follow-up outcomes of laparoscopic Nissen (total) versus Toupet (270 degrees) fundoplication for gastro-esophageal reflux disease based on randomized controlled trials in adults. BMC Gastroenterol 2016;16:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaker A, Stoikes N, Drapekin J, et al. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013; 108:1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bell R, Lipham J, Louie BE, et al. Magnetic sphincter augmentation superior to proton pump inhibitors for regurgitation in a 1-year randomized trial. Clin Gastroenterol Hepatol 2020; 18:1736–1743.e2. [DOI] [PubMed] [Google Scholar]
- 53.Hunter JG, Kahrilas PJ, Bell RC, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology 2015;148: 324–333.e5. [DOI] [PubMed] [Google Scholar]
- 54.Bell R, Lipham J, Louie B, et al. Laparoscopic magnetic sphincter augmentation versus double-dose proton pump inhibitors for management of moderate-to-severe regurgitation in GERD: a randomized controlled trial. Gastrointest Endosc 2019; 89:14–22.e1. [DOI] [PubMed] [Google Scholar]
- 55.Gawron AJ, Bell R, Abu Dayyeh BK, et al. Surgical and endoscopic management options for patients with GERD based on proton pump inhibitor symptom response: recommendations from an expert U.S. panel. Gastrointest Endosc 2020;92:78–87.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.