Abstract
Background: Recently, the non-toxic properties of natural plant products have gained more focus as anticancer agents. Therefore, this study aimed to assess the apoptosis effects of the ethanolic extract of Oxalis corniculata on the MCF-7 breast cancer cell line.Materials and Methods: In this experimental study, aerial parts of O. corniculata were collected in Lahijan city (Iran), and after confirmation, they were dried and extracted with ethanol for 24 h. Then, the total phenolic and flavonoid contents of the extract were measured. The 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay was used to measure the antioxidant properties of the extract. Selected cell lines (MCF-7 and human dermal fibroblast) were cultured in 6-wells dishes (1×106 cells/well). After 72 h of treating the extract, cytotoxicity was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The expression of apoptotic genes (such as p53, bcl-2, bax, and CD95) was studied by real-time polymerase chain reaction (PCR). Results: The extract's total phenolic content was 31.30±02 μg of gallic acid equivalents/mg of dry extract, and the total flavonoid content was 49.61±04 μg of quercetin as equivalents/mg of extract. The antioxidant activity ofO. corniculata was measured at the dose of 619.2 μg/μl, indicating that it decreases cancer cell viability and enhances apoptosis. Within the half maximal inhibitory concentrations, real-time PCR revealed substantial increases in p53 (P<0.001), CD95 (P<0.05), and bcl-2 expression (P<0.05) in MCF-7 cells treated with O. corniculata. Conclusion: This study suggests that O. corniculata may cause apoptosis by oxidative stress in cancer cells.[GMJ.2022;11:e2484]
Keywords: Apoptosis, MCF-7, Breast Cancer, Oxalis Corniculate, bax, p53, bcl-2
Introduction
ancer cells have a high proliferative capacity that is unrelated to their physiological requirements. After cardiovascular disease, it is the world's second leading cause of death [1]. In 2018, 9.6 million people died, three-quarters of them occurring in low- and middle-income nations. Over the next ten years, this number is predicted to approach 11 million people [1]. Annually, 2,088,849 individuals worldwide and 112,000 people in Iran are diagnosed with cancer, which is expected to account for 80 % of deaths in Iran during the next ten years [2].
Chemotherapy, radiation therapy, and surgery are the most used treatments accessible in modern medicine. Chemotherapy is a significant problem for cancer patients. Because highly strong medications can be hazardous, only about 1% of the molecules injected reach the cells they are meant to reach [3]. Others, such as hydroxyl, peroxyl, and superoxide radicals could affect healthy cells and tissues [4], and lead to cancer, diabetes, and heart disease [5]. As a result, the effect of plants on cancer treatment must be considered while developing a new pharmacological molecule or its derivatives for cancer research.
The cell's innate mechanism for planned cell death is called apoptosis. It is especially important in long-lived mammals since it is essential for both development and homeostasis [6][7]. Both the mitochondrial (intrinsic) and the death receptor (extrinsic) pathways can be used to start the highly regulated process of apoptosis [8][9]. Numerous tumors have been shown to up-regulate anti-apoptotic or down-regulate pro-apoptotic proteins [10][11][12]. For instance, overexpression of B-cell lymphoma-2 (bcl-2) is frequently linked to several malignancies, such as colorectal adenocarcinomas, B-cell lymphomas, breast cancer, and prostate cancer [13].
The bcl-2 family is an essential intrinsic pathway apoptotic regulator. It can be divided into members that are pro- and anti-apoptotic (such as BCL2 Associated X [bax] and bcl-2) [14, 15]. A key element gene that promotes the production of the bax gene is p53, a pro-apoptotic tumor suppressor protein [16]. Direct induction and activation of the bax lead to bax transcription, which encourages cell cycle arrest or triggers apoptosis [17]. The extrinsic receptor pathway is triggered when members of the tumor necrosis factor (TNF) receptor superfamily's transmembrane cell surface receptors are stimulated. The first apoptosis signal (Fas; CD95), TNF-α, and TNF-related apoptosis-inducing ligand (TRAIL) receptors DR4 and DR5 are members of this family [18].
Breast cancer was the second most common cancer in 2018, with two million new cases. According to published statistics, the global breast cancer rate rises by 0.4% [19]. As a result, scientists and researchers have a significant problem in terms of prevention and treatment. In addition to the patients, growth in breast cancer rates has significant economic and social implications, necessitating the development of a new effective and helpful strategy [19]. Some chemotherapy medicines, such as paclitaxel and anthracycline, inhibit cancer cell development and trigger apoptosis in cancer cells [20]. However, these drugs are ineffective in some people and have harmful side effects on healthy cells [21]. As a result, among the natural substances, developing a potent, focused, and non-toxic agent to treat this condition appears important. Herbs have recently been touted as cancer-fighting medicine with fewer adverse effects [22][23][24].
Oxalis corniculata, often called reptile wood sure, is a subtropical plant (family Oxalidaceae) native to Iran [25][26]. The wetlands of northern Iran and sections of Khuzestan are where it thrives. It was traditionally used to treat diarrhea and anemia [27]. It is also utilized as an anti-inflammatory, cough suppressant, and anti-hypertensive herb [27][28][27,28]. According to the literature, inflammation and cancer are closely related, and many anticancer medications are also used to treat inflammatory conditions like rheumatoid arthritis [29]. In addition, chronic inflammation raises the chance of developing several malignancies, suggesting that reducing inflammation may be a sensible approach to both treating and preventing cancer [29].
However, there are very limited studies on the benefit of natural resources available for the treatment and prevention of cancer. Because O. corniculata is widely consumed in traditional foods in Guilan province and little research has been conducted to examine the anticancer potential of this extract [30], this study was aimed to evaluate the effect of O. corniculata extract on MCF-7 cell line survival and expression of the bax, p53, bcl-2, and CD95.
Materials and Methods
Plant Material
The aerial parts of O. corniculata were collected from villages around Lahijan city (Iran) with geographical coordinates (37 ° 13'59.4 "N 50 ° 02'39.1" E) on Google Maps and guided by three familiar natives in April 2017. Then, to confirm the genus and species, it was sent to the herbarium of the School of Pharmacy, Guilan University of Medical Sciences, Rasht, Iran, and after approval, the plant was dried in a dark and humid environment (10-15%) and ground in a hand mill. Then, for extraction, they were transferred to a ten-liter reactor equipped with speed and temperature control, and extraction was performed using ethanol for 24 hours. The solvent was evaporated by a rotary evaporator to obtain ethanol extract of O. corniculata. The extract was stored in a refrigerator until required.
Total Phenolic Content Measurements
As described previously, Folin-Ciocalteu method was used to determine the total phenolic content of extracts [31]. One mL of the extract (1 mg/mL) was combined with 5 mL of Folin-Ciocalteu reagent (which had previously been diluted 10-fold with distilled water) and allowed to stand at room temperature for 10 min. Then, the sodium bicarbonate solution was added to 4 mL (75 g/L). The combination was then allowed to rest at room temperature for another 30 minutes in the dark. A UV/VIS spectrophotometer (Lambda 25 PerkinElmer, USA) was used to measure the absorbance at 765 nm. The calibration curve was plotted using five different concentrations of the gallic acid standard (25, 50, 70, 100, and 200 g/mL). The calibration curve was then used to determine the total phenolic content of the samples. The number of gallic acid equivalents per gram of dried extract was calculated [32]. All the tests were done three times.
Total Flavonoid Content
The total flavonoid content was determined using Saeidnia and Gohari's method [33]. To 5 mL of extract (1 mg/mL), 5 mL of aluminum trichloride (AlCl3, 2% in methanol) was added. After 10 minutes, the mixture's absorbance was measured at 415 nm. Also, 5 mL extract and 5 mL methanol without AlCl3 made up the blank sample. A standard curve of quercetin (0-100 mg/L) was used to determine the total flavonoid content. The total flavonoid concentration was measured in milligrams of quercetin equivalents per gram of extract.
Radical Scavenging Activity
The 2,2'-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay was used to determine the antioxidant activity of the sample [31]. Briefly, 1 mL of various extract concentrations was mixed with 2 mL of DPPH methanol solution (40 g/mL). After 30 minutes, the absorbance was measured at 517 nm. For each concentration, the test was repeated three times. As a positive control, vitamin E was employed [34]. Inhibition percent =[(A0-As)/A0]100, where A0 is the absorbance of the control and As is the absorbance of the sample, was used to calculate the percentage of radical scavenging activity of the extract. From the graph plotting the scavenging percentage against extract concentration, the half maximal inhibitory concentration (IC50) values (showing the concentration of the extract [mg/mL] giving 50% radical scavenging) were calculated [34].
IC50 Calculation and Cytotoxicity Assay
The cytotoxic effect of an ethanolic extract from the plant was tested using a human breast cancer cell line (MCF-7) and human dermal fibroblast (HDF) as the control. All the cell lines were obtained from the Pasteur Institute (Tehran, Iran). The MCF-7 cell line was cultured in RPMI1640 medium (Gibco, Germany), and the HDF cell line was cultured in Dulbecco's modified Eagle's medium (Gibco, Germany), supplemented with 10% fetal bovine serum (FBS; Gibco, Germany), 100 U/mL penicillin (Sigma-Aldrich, Sweden), and 100 g/mL streptomycin (Sigma-Aldrich, Sweden), at 37 °C in humid. The ethanolic extract's cytotoxicity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Gibco, Germany) assay. In 96-well plates, 1×104 (cells of each cell line /well) were plated. The cells were incubated for 24 hours at 37 °C in 5% CO2 and a humidified environment.
The extracts were applied to cells at different concentrations (31.25, 62.5, 125, 250, 500, 1000, 1500, and 2000 g/mL) over 72 hours. Then, per well, MTT (0.5 mg/mL, 100 L) was applied and incubated for 4 hours in phosphate buffer saline (PBS; Gibco, Germany). The cells were then washed in PBS buffer after the MTT solution was removed. To dissolve the formazan crystals, 100 microliters of dimethyl sulfoxide (Gibco, Germany) were added to each well, and the plate was shaken at 100 rpm for 10 minutes. The cell survival was determined by measuring the absorbance at 570 nm using an ELISA reader instrument (Stat Fax 2100, Awareness, USA). The IC50 values were calculated using the concentration-response curve. All experiments were performed in triplicate.
RNA Extraction and cDNA Synthesis
The selected cell lines (MCF-7 and HDF) were grown in 6-well plates to get adequate RNA for cDNA synthesis and gene expression investigation by real-time polymerase chain reaction (PCR). Cell lines were grown at 37 ºC in humidified air with 5% CO2 in RPMI1640 medium, supplemented with 10% FBS, 100 U/mL penicillin, and 100 g/mL streptomycin. Briefly, 1×106 (cells of each cell line/well) were plated in 6-well plates, along with the determined IC50 for the plant's leaves and fruits. The cells were then incubated for 24 hours at 37 ºC in 5% CO2 in a humidified environment. One well from the same cell line was cultivated as a control adjacent to each of the extract wells (without adding the extract).
Microtubes were incubated at 55 ºC for 60 minutes after quick centrifugation. The reaction was completed by incubation for 5 minutes at 85 ºC. Finally, the samples were placed on ice for a short period before being stored at -20 ºC until needed.
Quantitative Real-Time PCR
The expression of the p53, bax, bcl-2, and CD95 genes, as well as GAPDH (a housekeeping gene), was measured using the Applied Biosystems Step OneTM Real-Time PCR System (USA) and SYBR Green Real-Time PCR dye (Yekta Tajhiz Azma, Iran). Primer3web (version 4, GenFanAvaran Co., Tehran, Iran) was applied to design the PCR primers. The Primer-BLAST system at the National Center for Biotechnology Information (NCBI) was used to assess the specificity of the prepared primers for the specified genes. Table-1 lists the sequences and product sizes of the primers. Then, 1 µl of each primer, 4 µl of diluted cDNA, 10 µl of SYBR Green Master Mix (Yekta Tajhiz Azma, Iran), and 4 µl nuclease-free water were used in the reaction. The PCR conditions were 15 minutes pre-activation at 95 ºC (first denaturation), 40 cycles of 15 seconds at 95 ºC, and 60 seconds at 60 ºC. For each sample, the reactions were carried out twice. Finally, using the 2-ΔΔCt equation, the difference in expression of p53, bax, bcl-2, and CD95 mRNAs in the samples was determined.
Table - 1. Sequences of Gene-Specific Primers.
| Primers | Sequence (5'-3') | Nucleotide count | Product size (bp) |
| GAPDH | F: GACAGTCAGCCGCATCTTCT | 20 | 104 |
| R: GCGCCCAATACGACCAAATC | 20 | ||
| p53 | F: GTGGAAGGAAATTTGGGTGTGG | 22 | 184 |
| R:CCAGTGTGATGATGGTGAGGATG | 20 | ||
| bax | F: TCTGACGGCAACTTCA | 16 | 186 |
| R: GAGGAGTCTCACCCAACCAC | 20 | ||
| bcl-2 | F: TGCACGTGACGCCCTTCAC | 19 | 293 |
| R: AGACAGCCAGGAGAAATCAAACAG | 24 | ||
| CD95 | F: TCAGTACGGAGTTGGGGAAG | 20 | 207 |
| R: CAGGCCTTCCAAGTTCTGAG | 20 |
Ethical Considerations
This study was approved by the Ethical Committee of Guilan University of Medical Sciences (approval code: IR.GUMS.REC.1396.228).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.3.0 software (GraphPad Software, USA). The normality of the data has been checked using the Shapiro-Wilk test. Differences between the two groups were evaluated by an unpaired t-test. P<0.05 was considered statistically significant.
Results
The Total Phenolic and Flavonoid Contents, and DPPH Radical Scavenging The total phenolic content of the extract was measured by the standard curve for gallic acid (y=0.00088x−0.0388, R²=0.997, Table-2). Also, the total flavonoid content was measured regarding the standard curve of quercetin (y=0.0178x−0.0119, R²=0.995, Table-2). The extract's antioxidant ability was tested using the DPPH radical scavenging assay (Table-2). IC50 Values The calculated IC50 values were 713.8 µg/ml) and 1397 µg/ml for MCF-7 and HDF cell lines, respectively.
Table - 2. Total Phenol and Flavonoid Contents and Antioxidant Activities of O. corniculata Extract.
| Sample | Total phenolic (%) | Total flavonoid (%) | DPPH IC50 (µg/µl) |
| O. corniculata | 49.61±0.04 | 31.3±0.02 | 619.207 |
| Vitamin E | - | - | 0.014 |
Results are expressed as mean±SD.
DPPH: 2,2'-diphenyl-1-picrylhydrazyl
Evaluation of mRNA Expression of p53, bax, bcl-2, and CD95After 24 hours, real-time PCR was used to assess the expression of apoptosis-related genes in MCF-7 and HDF cells that were treated with ethanolic extract of O. corniculata in IC50 values. In both cell lines, the expressions of p53 was increased significantly (Figure-1). While the expression of bax increased in both cell lines, the statistically was not significant (P˃0.05, Figure-2). In contrast, the expression of bcl-2 was decreased significantly in MCF-7 cells and insignificantly in HDF cells (Figure-3). Finally, CD95 expression was raised significantly in MCF-7 cells and decreased insignificantly in HDF cells (Figure-4).
Figure-1.
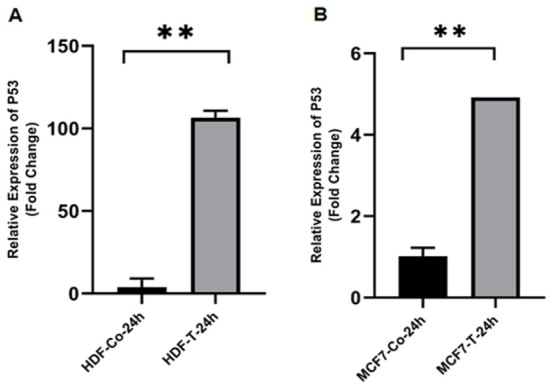
The effects of the ethanolic extract of O. corniculata on p53 expression in HDF (A) and MCF-7 (B) cell lines. Real-time PCR showed that the O. corniculata extract caused a significant increase in the p53 expression after 24 hours in both cell lines (compared to the control). Data are expressed as mean±SD. **P<0.0001 vs. control
Figure-2.
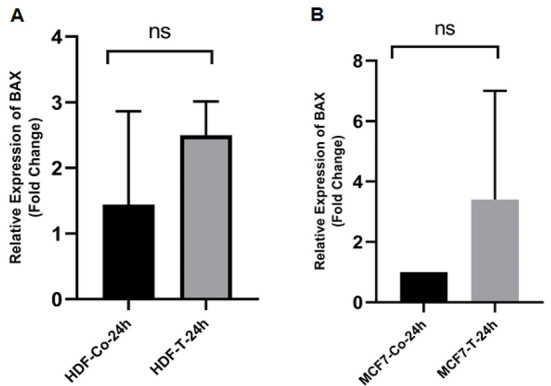
The effects of the ethanolic extract of O. corniculata on bax expression in HDF (A) and MCF-7 (B) cell lines. Real-time PCR showed that the O. corniculata extract caused an increase in the bax expression after 24 hours in both cell lines (compared to the control). However, this increase was not statistically significant. Data are expressed as mean±SD. ns: Not significant.
Figure-3.
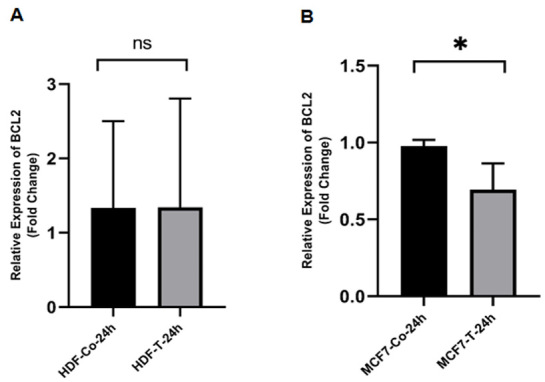
The effects of the ethanolic extract of O. corniculata on bcl-2 expression in HDF (A) and MCF-7 (B) cell lines. Real-time PCR showed that the O. corniculata extract caused a significant decrease in the bcl-2 expression after 24 hours in both cell lines (compared to the control).Data are expressed as mean±SD. *P<0.05 vs. control. ns: Not significant
Figure-4.
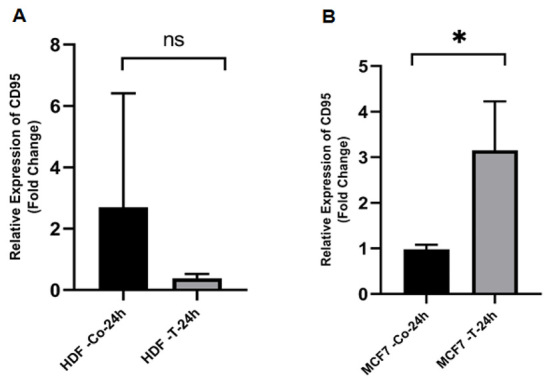
The effects of the ethanolic extract of O. corniculata on CD95 expression in HDF (A) and MCF-7 (B) cell lines. Real-time PCR showed that the O. corniculata extract caused a significant increase in the CD95 expression after 24 hours in MCF-7 cell lines (compared to the control). However, treated HDF cells with O. corniculata extract led to a significant decrease in the expression of CD95 after 24 hours. Data are expressed as mean±SD. *P<0.05 vs. control.ns: Not significant
Discussion
The O. corniculata extract had previously been thought to have a variety of activities, including antioxidants [35][36], potentially due to flavonoid chemicals [37][38] and phenolic acids [39]. Antioxidant chemicals such as flavonoids and phenolic acids are commonly found in plants. Flavonoid and phenolic compounds have phenolic hydrogen in their structures, which interacts with the hydrogen donor radical [40]. On the other hand, flavonoids vary in activity due to their more complicated structure than phenolic acids [41]. Plant flavonoids and phenolic acids have anticancer properties, and they do so by interfering with basic cellular functions such as cell cycle arrest, apoptosis induction, inflammation, angiogenesis inhibition, and antioxidant activity [42][43][44]. Furthermore, phenolic substances have been demonstrated to behave as peroxidants in cancer cells, inhibiting tumor cell proliferation by damaging DNA strands [45]. As a result, the phenol content of O. corniculata's whole extract has been determined. The results showed that the plant contains a high amount of phenolic chemicals. These findings are in line with a previous study, which found that the phenol concentration of O. corniculata extract was around 6.4 mg/g dry weight [35]. Also, O. corniculata contained phosphorus, iron, niacin, vitamin C, β-carotene, calcium, and oxalic acid. Antitumors, antioxidants, lipid peroxidation, and anti-inflammatory medications are just a few of the biological activities of these substances [3,35,46].
Moreover, several studies [47][48][49][50][51][52] looked at the anticancer potential of whole plant extracts with high phenolic content. Several compounds found in plants have been shown to inhibit the growth of cancer cells and prevent cancer by interrupting the cell cycle [1]. In the current study, the cell cycle was assumed to be inhibited by ethanolic extract via p53. Because one of the most critical functions of p53 is to interrupt the cell cycle in the G1, G2, or S phases to generate the conditions for DNA repair [53].
Studies have demonstrated that under tumor conditions, the amount of reactive oxygen species (ROS) increases because of increased metabolism and mitochondrial abnormalities [54], which favors cancer [55]. As a result, the cell adjusts to high amounts of ROS [56]. Anticancer drugs with antioxidant capabilities are thus thought to trigger cell apoptosis [57].
As a result, DPPH radical scanning activity must be used to assess antioxidant activity in MCF-7 cells treated with ethanolic extract of O. corniculata. The results of this approach revealed an increase in antioxidant activity in treated MCF-7 cells compared to control cells. The adsorption intensity corresponds to the level of ROS in the cytoplasm of the cell. Therefore, the ethanolic extract of
O. corniculata stimulates antioxidant activity in the IC50 dose. More researchs on the potential for mitochondrial membrane in MCF-7 cells treated with O. corniculata are needed to support this claim.
We used gene expression analysis to identify the major genes involved in cell death caused by the O. corniculata extract. Several genes implicated in innate apoptosis pathways have been examined, including bax, p53, and CD95 (Fas), and bcl-2 as anti-apoptotic [58,59]. The intrinsic mechanism of apoptosis has been demonstrated to be controlled by p53 activity [60]. Also, bcl-2 and bax are two genes that contribute to p53-induced apoptosis [61].
The extract regulates the production of pro-apoptotic genes, according to our study. In MCF-7 cells, bax and p53 lowered the expression of the anti-apoptotic gene
bcl-2, likely raising cytochrome c and caspase production and leading to greater apoptosis. Increased p53 expression may also cause cell cycle stages to be inhibited. Interestingly, some of our findings are in accordance with several earlier reports carried out on other plants. For example, bcl-2 down-regulation and bax up-regulation were observed in response to Euphorbia esula extract in human gastric carcinoma SGC-7901 cells [62]. Previous studies have shown triggering apoptosis in MCF-7 cells via significantly decreased bcl-2 gene expression by Calystegia sepium methanol extract [63] and significant up-regulation of bax and down-regulation of bcl-2 levels by the black turtle bean extract [64]. Patel et al. [65] indicated that Tribulus terrestris extract induced up-regulation in the expression of bax and p53 genes as well as down-regulation of bcl-2 expression.
FasL and Fas/CD95 receptor protein association mediates death receptor signaling, followed by caspase-8 activation [66][67][68]. We investigated whether Fas/CD95 plays a role in MCF-7-induced apoptosis. In MCF-7 cells, the quantity of the Fas/CD95 gene increased concentration-dependent manner.
Chen et al. demonstrated that Houttuynia cordata extract raised p27 expression, decreased cyclin D1, cyclin A, CDK 4, and CDK 2, and interrupted the G0/G1 cycle [69]. Also, they revealed that caspase-8/caspase-3 activation and regulation of Fas/CD95, caspase-8, and caspase-3 proteins were involved in H. cordata-induced apoptosis [69]. H. cordata growth inhibition caused by caspase-3 and -8 inhibitors was also significantly reduced [69]. HCT promotes apoptosis of A549 cells and activation of caspases-3 and -8 via the Fas/CD-95-mediated death receptor apoptosis pathway [69].
Our findings demonstrated that the ethanolic extract of O. corniculata regulates the expression of the pro-apoptotic p-53, which interrupts the cell cycle at the G0/G1 and G2/M stages. Furthermore, changing the gene expressions of members of the bcl-2 and bax families in the intrinsic pathway of apoptosis might reveal the role of MAPK family members in controlling the proliferation of cancer cells [70], which is consistent with our findings.
Conclusion
The ethanolic extract of O. corniculata produces cytotoxicity in the MCF-7 cell line; however, no cytotoxicity was seen in HDF cells. The extract has a regulatory influence on pro-apoptotic marker genes (p53, bax, and CD95) and the anti-apoptotic gene (bcl-2), which promotes apoptosis in breast cancer cells. This activity could be explained by the presence of flavonoids and related polyphenols. Hence, we recommended more studies to identify the extract's active constituent and establish the process.
Conflict of Interest
The authors declare that they have no competing interests.
Acknowledgments
The authors are grateful to the Guilan University of Medical Sciences for their support.
References
- Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005;6(26):318–26. doi: 10.1016/j.tips.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Nafissi N, Khayamzadeh M, Zeinali Z, Pazooki D, Hosseini M, Akbari ME. Epidemiology and histopathology of breast cancer in Iran versus other Middle Eastern countries. Middle East J Cancer. 2018;3(9):243–51. [Google Scholar]
- Kathiriya AK, Das K, Kumar E, Mathai K. Evaluation of Antitumor and Antioxidant Activity of Oxalis Corniculata Linn against Ehrlich Ascites Carcinoma on Mice. Int J Cancer Manag. 2010;4(3):e80710–e80710. [Google Scholar]
- Abdelghffar EA, El-Nashar HA, Al-Mohammadi AG, Eldahshan OA. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food Funct. 2021;19(12):9443–55. doi: 10.1039/d1fo01905h. [DOI] [PubMed] [Google Scholar]
- Liu Y-Q, Wang X-L, He D-H, Cheng Y-X. Protection against chemotherapy-and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine. 2021;(80):153402–153402. doi: 10.1016/j.phymed.2020.153402. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;2(116):205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;(2014):150845–150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chiu LC-M, Ho T-S, Wong EY-L, Ooi VE. Ethyl acetate extract of Patrinia scabiosaefolia downregulates anti-apoptotic Bcl-2/Bcl-XL expression, and induces apoptosis in human breast carcinoma MCF-7 cells independent of caspase-9 activation. J Ethnopharmacol. 2006;1-2(105):263–8. doi: 10.1016/j.jep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Pilane M, Bagla V, Mokgotho M, Mbazima V, Matsebatlela T, Ncube I, et al. Free radical scavenging activity: antiproliferative and proteomics analyses of the differential expression of apoptotic proteins in MCF-7 cells treated with acetone leaf extract of Diospyros lycioides (Ebenaceae) Evid Based Complement Alternat Med. 2015;(2015):534808–534808. doi: 10.1155/2015/534808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xiong J, Yi S, Zhang H, Zhou S, Gu L, et al. FKBP12 enhances sensitivity to chemotherapy-induced cancer cell apoptosis by inhibiting MDM2. Oncogene. 2017;12(36):1678–86. doi: 10.1038/onc.2016.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Li C, Huo K, Wang Q, Lu L, Zhang Q, et al. Luteolin prevents H2O2-induced apoptosis in H9C2 cells through modulating Akt-P53/Mdm2 signaling pathway. Biomed Res Int. 2016;0(2016):1–9. doi: 10.1155/2016/5125836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Wu Z, He Y, Xiao Y, Xia C. Single and dual target inhibitors based on Bcl-2: Promising anti-tumor agents for cancer therapy. Eur J Med Chem. 2020;(201):112446–112446. doi: 10.1016/j.ejmech.2020.112446. [DOI] [PubMed] [Google Scholar]
- Suvarna V, Singh V, Murahari M. Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur J Pharmacol. 2019;(862):172655–172655. doi: 10.1016/j.ejphar.2019.172655. [DOI] [PubMed] [Google Scholar]
- Kazemi N, Shahrestani SB. Effect of Saffron Extract on Expression of Bax and Bcl-2 Genes in Gastric Adenocarcinoma Cell Line (AGS) Gene, Cell and Tissue. 2018;3(5): e63608– e63608. [Google Scholar]
- Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;1(25):37–45. doi: 10.1038/cdd.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CF, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;3(10):1–12. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi H, Falahati-Pour SK, Mahmoodi M, Faramarz S, Maleki H, Nasab FB, et al. Nanoliposomal formulation of pistachio hull extract: preparation, characterization and anticancer evaluation through Bax/Bcl2 modulation. Mol Biol Rep. 2022;4(49):2735–43. doi: 10.1007/s11033-021-07083-5. [DOI] [PubMed] [Google Scholar]
- Knight T, Luedtke D, Edwards H, Taub JW, Ge Y. A delicate balance–The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem Pharmacol. 2019;0(162):250–61. doi: 10.1016/j.bcp.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;9(109):djx030–djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Hu X, Liu Z, Lin Y, Liang R, Zhang Y, et al. Synergistic action of microwave-induced mild hyperthermia and paclitaxel in inducing apoptosis in the human breast cancer cell line MCF-7. Oncol Lett. 2019;1(17):603–15. doi: 10.3892/ol.2018.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Mok TS, Lin X, Zhang W, Cui Y, Guo J, et al. SWATH-based proteomics identified carbonic anhydrase 2 as a potential diagnosis biomarker for nasopharyngeal carcinoma. Sci Rep. 2017;1(7):1–11. doi: 10.1038/srep41191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuasha N, Petros B, Asfaw Z. Plants used as anticancer agents in the Ethiopian traditional medical practices: a systematic review. Evid Based Complement Alternat Med. 2018;(2018 ):6274021–6274021. doi: 10.1155/2018/6274021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L-P, Wang A, Ye J-H, Zheng X-Q, Polito CA, Lu J-L, et al. Suppressive effects of tea catechins on breast cancer. Nutrients. 2016;8(8):458–458. doi: 10.3390/nu8080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajendran B, Durai P, Varier KM, Chinnasamy A. A novel phytosterol isolated from Datura inoxia, RinoxiaB is a potential cure colon cancer agent by targeting BAX/Bcl2 pathway. Bioorg Med Chem. 2020;2(28):115242–115242. doi: 10.1016/j.bmc.2019.115242. [DOI] [PubMed] [Google Scholar]
- Ghahremaninejad F, Gholamian F. A new record (Oxalis articulata) from Iran. Iran J Bot. 2006;1(12):55–6. [Google Scholar]
- Hosseini H, Handali S, Parishani M, Ghezelbash G, Ameri A. A Comparative Study of Antibacterial Effects of Aqueous Extract of Oxalis corniculata L with Antibacterial Effects of Common Atibiotics in Staphylococcus aureous and E coli Infections. J Med Plants. 2010;33(9):103–7. [Google Scholar]
- Madhava Chetty, Sivaji K, Tulasi Rao. Tirupati: Students Offset Printers; 200. [Google Scholar]
- Hsieh P-C, Mau J-L, Huang S-H. Antimicrobial effect of various combinations of plant extracts. Food Microbiol. 2001;1(18):35–43. [Google Scholar]
- Rayburn ER, Ezell SJ, Zhang R. Anti-inflammatory agents for cancer therapy. Mol Cell Pharmacol. 2009;1(1):29–29. doi: 10.4255/mcpharmacol.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin H, Mansoor Q, Batool R, Farooqi AA, Mahmood T, Ismail M. Anticancer activity of Cynodon dactylon and Oxalis corniculata on Hep2 cell line. Cell Mol Biol. 2016;5(62):60–3. [PubMed] [Google Scholar]
- Hamidi M, Ghasemi S, Bavafa Bighdilou, Eghbali Koohi. Evaluation of antioxidant, antibacterial and cytotoxic activity of methanol extract from leaves and fruits of Iranian squirting cucumber. Res J Pharmacogn. 2020;1(7):23–9. [Google Scholar]
- Ghasemi S, Koohi DE, Emmamzadehhashemi MSB, Khamas SS, Moazen M, Hashemi AK, et al. Investigation of phenolic compounds and antioxidant activity of leaves extracts from seventeen cultivars of Iranian olive (Olea europaea L.) J Food Sci Technol. 2018;11(55):4600–7. doi: 10.1007/s13197-018-3398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidnia S, Gohari AR. Germmany: Lap Lambert Academic Publ; 2012. [Google Scholar]
- Yousefbeyk F, Gohari AR, Hashemighahderijani Z, Ostad SN, Sourmaghi MHS, Amini M, et al. Bioactive terpenoids and flavonoids from Daucus littoralis Smith subsp hyrcanicus Rech f, an endemic species of Iran. Daru. 2014;1(22):1–6. doi: 10.1186/2008-2231-22-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah A, Yadav R, Unni B. Evaluation of antioxidant activity of different solvent extracts of Oxalis corniculata L. J Pharm Res. 2012;1(5):91–3. [Google Scholar]
- Khyadea MS, Kambleb SP, Wamanc MB, Padwala AD, Jadhava SD. Chemical profiling and free radical scavenging potential of Oxalis corniculata. Explorer. 2016;1(1):88–88. [Google Scholar]
- Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M, Calderon-Montano J. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;4(11):298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- Otake Y, Walle T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab Dispos. 2002;2(30):103–5. doi: 10.1124/dmd.30.2.103. [DOI] [PubMed] [Google Scholar]
- Lu X, Qian C-N, Mu Y-G, Li N-W, Li S, Zhang H-B, et al. Serum CCL2 and serum TNF-α–Two new biomarkers predict bone invasion, post-treatment distant metastasis and poor overall survival in nasopharyngeal carcinoma. Eur J Cancer. 2011;3(47):339–46. doi: 10.1016/j.ejca.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Jubair A, Hossain MA, Hossain MM, Azam MS, Biswas M. Free radical-scavenging capacity and HPLC-DAD screening of phenolic compounds from pulp and seed of Syzygium claviflorum fruit. J Agric Res Food Res. 2021;(6):100203–100203. [Google Scholar]
- Hosseini MM, Karimi A, Behroozaghdam M, Javidi MA, Ghiasvand S, Bereimipour A, et al. Cytotoxic and apoptogenic effects of cyanidin-3-glucoside on the glioblastoma cell line. World Neurosurg. 2017;(108):94–100. doi: 10.1016/j.wneu.2017.08.133. [DOI] [PubMed] [Google Scholar]
- Batra P, Sharma AK. Anticancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013;6(3):439–59. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar D, Rajora AK, Greco F, Osborn HM. Flavonoids as prospective compounds for anticancer therapy. Int J Biochem Cell Biol. 2013;12(45):2821–31. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Gomes CA, Girão da, Andrade JL, Milhazes N, Borges F, Marques MPM. Anticancer activity of phenolic acids of natural or synthetic origin: a structure− activity study. J Med Chem. 2003;25(46):5395–401. doi: 10.1021/jm030956v. [DOI] [PubMed] [Google Scholar]
- Azmi AS, Bhat SH, Hanif S, Hadi S. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties. FEBS Lett. 2006;2(580):533–8. doi: 10.1016/j.febslet.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Sakat S, Juvekar AR, Gambhire MN. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci. 2010;1(2):146–55. [Google Scholar]
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;1(12):1–12. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dabbagh B, Elhaty IA, Al Sakkaf, El-Awady R, Ashraf SS, Amin A. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement Altern Med. 2018;1(18):1–12. doi: 10.1186/s12906-018-2285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;10(15):7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchen J, Bortl L, Huml L, Miksatkova P, Doskocil I, Marsik P, et al. Phenolic composition, antioxidant and anti-proliferative activities of edible and medicinal plants from the Peruvian Amazon. Rev Bras Farmacogn. 2016;6(26):728–37. [Google Scholar]
- Nguyen NH, Ta QTH, Pham QT, Luong TNH, Phung VT, Duong T-H, et al. Anticancer activity of novel plant extracts and compounds from Adenosma bracteosum (bonati) in human lung and liver cancer cells. Molecules. 2020;12(25):2912–2912. doi: 10.3390/molecules25122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rimawi F, Rishmawi S, Ariqat SH, Khalid MF, Warad I, Salah Z. Anticancer activity, antioxidant activity, and phenolic and flavonoids content of wild Tragopogon porrifolius plant extracts. Evid Based Complement Alternat Med. 2016;(9612490):1–7. doi: 10.1155/2016/9612490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Zhu W-G. p53: structure, function and therapeutic applications. J Cancer Mol. 2006;4(2):141–53. [Google Scholar]
- Jangholi E, Sharifi ZN, Hoseinian M, Zarrindast MR, Rahimi HR, Mowla A, et al. Verapamil Inhibits Mitochondria-Induced Reactive Oxygen Species and Dependent Apoptosis Pathways in Cerebral Transient Global Ischemia/Reperfusion. Oxid Med Cell Longev. 2020;(2020):5872645–5872645. doi: 10.1155/2020/5872645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;1-3(10):1881–96. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;2(7):97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kong Q, Beel J, Lillehei K. A threshold concept for cancer therapy. Med Hypotheses. 2000;1(55):29–35. doi: 10.1054/mehy.1999.0982. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;4(35):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy. Pharmacol Ther. 2021:107943–107943. doi: 10.1016/j.pharmthera.2021.107943. [DOI] [PubMed] [Google Scholar]
- Wang Y-C, Wang L-T, Hung TI, Hong Y-R, Chen C-H, Ho C-J, et al. Severe cellular stress drives apoptosis through a dual control mechanism independently of p53. Cell Death Discov. 2022;1(8):1–5. doi: 10.1038/s41420-022-01078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. The role of P53 up-regulated modulator of apoptosis (PUMA) in ovarian development, cardiovascular and neurodegenerative diseases. Apoptosis. 2021;5(26):235–47. doi: 10.1007/s10495-021-01667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z-Y, Han X-D, Wang A-H, Liu X-B. Apoptosis of human gastric carcinoma cells induced by Euphorbia esula latex. World J Gastroenterol. 2016;13(22):3564–3564. doi: 10.3748/wjg.v22.i13.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezadoost MH, Kumleh HH, Ghasempour A. Cytotoxicity and apoptosis induction in breast cancer, skin cancer and glioblastoma cells by plant extracts. Mol Biol Rep. 2019;5(46):5131–42. doi: 10.1007/s11033-019-04970-w. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sharma VK, Yadav S, Dey S. Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem Cent J. 2017;1(11):1–10. doi: 10.1186/s13065-017-0281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Soni A, Siddiqi NJ, Sharma P. An insight into the anticancer mechanism of Tribulus terrestris extracts on human breast cancer cells. 3 Biotech. 2019;2(9):58–58. doi: 10.1007/s13205-019-1585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher P, Khadra N, Vacher A-M, Charles E, Bresson-Bepoldin L, Legembre P. Does calcium contribute to the CD95 signaling pathway? Anticancer Drugs. 2011;6(22):481–7. doi: 10.1097/CAD.0b013e32834433ea. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;1(19):42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz G, Hueber AO. The Fas/CD95 receptor regulates the death of autoreactive B cells and the selection of antigen-specific B cells. Front Immunol. 2012;(3):207–207. doi: 10.3389/fimmu.2012.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Yang JS, Chang WS, Tsai SC, Peng SF, Zhou YR. Houttuynia cordata Thunb extract modulates G0/G1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J Biomed Sci. 2013;1(20):18–18. doi: 10.1186/1423-0127-20-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrakhovitch EA, Cherian MG. Inhibition of extracellular signal regulated kinase (ERK) leads to apoptosis inducing factor (AIF) mediated apoptosis in epithelial breast cancer cells: the lack of effect of ERK in p53 mediated copper induced apoptosis. J Cell Biochem. 2005;6(95):1120–34. doi: 10.1002/jcb.20484. [DOI] [PubMed] [Google Scholar]


